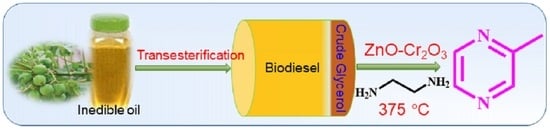
Synthesis of 2-Methylpyrazine Using Crude Glycerol over Zn-Cr-O Catalyst: A Value Addition Process for the Utilization of Biodiesel By-Product
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts with Different Zn/Cr Mole Ratio
2.1.1. XRD Analysis and BET Surface Area of Zn-Cr Samples
2.1.2. FT-IR Analysis of Calcined Zn-Cr Samples
2.1.3. H2-TPR and O2 Pulse Chemisorption Results of Zn-Cr Samples
2.1.4. UV-DRS Analysis of Zn-Cr Samples
2.1.5. X-ray Photoelectron Spectroscopic Studies of Zn-Cr Samples
2.1.6. DTA-TGA Analysis of Zn-Cr Samples
2.1.7. Catalytic Activity Measurements
3. Materials and Methods
3.1. Catalyst Preparation
Preparation of Zn-Cr Samples with Varied Zn/Cr Mole Ratios
3.2. Catalyst Characterization
3.3. Catalyst Activity Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parate, R.; Mane, R.; Dharne, M.; Rode, C. Mixed bacterial culture mediated direct conversion of bio-glycerol to diols. Bioresour. Technol. 2018, 250, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.B.; Ramírez de la Piscina, P.; Homs, N. Efficient hydrogen production from ethanol and glycerol by vapour-phase reforming processes with new cobalt-based catalysts. Bioresour. Technol. 2011, 102, 3419–3423. [Google Scholar] [CrossRef] [PubMed]
- Akula, V.; Sarkari, R.; Chatla, A.; Vankudoth, K.; Mandari, K.K. Synthesize 2-methylpyrazine using aqueous glycerol and ethylenediamine over zinc oxide–zinc chromite catalysts: Structure–activity relationship. Appl. Catal. A Gen. 2012, 441–442, 108–118. [Google Scholar] [CrossRef]
- Krishna, V.; Kumar, S.N.; Reema, S.; Padmasri, A.H.; Chary, K.V.R.; Venugopal, A. Bio-glycerol utilization: Synthesis of 2,6-dimethylpyrazine over MxOy–MCr2O4 (M = Mg, Fe, Co, Ni, Cu and Zn) catalysts. Appl. Catal. A Gen. 2014, 488, 275–284. [Google Scholar] [CrossRef]
- Krishna, V.; Naresh, G.; Kumar, V.V.; Sarkari, R.; Padmasri, A.H.; Venugopal, A. Synthesis of 2,6-dimethylpyrazine by dehydrocyclization of aqueous glycerol and 1,2-propanediamine over CuCrO catalyst: Rationalization of active sites by pyridine and formic acid adsorbed IR studies. Appl. Catal. B Environ. 2016, 193, 58–66. [Google Scholar] [CrossRef]
- Forni, L. Structural analysis of ZnO/ZnCr2O4/Pd catalyst. J. Catal. 1988, 111, 199–209. [Google Scholar] [CrossRef]
- Vankudoth, K.; Gutta, N.; Velisoju, V.K.; Mutyala, S.; Aytam, H.P.; Akula, V. CuCr2O4 derived by the sol-gel method as a highly active and selective catalyst for the conversion of glycerol to 2,6-dimethylpyrazine: A benign and eco-friendly process. Catal. Sci. Technol. 2017, 7, 3399–3407. [Google Scholar] [CrossRef]
- Rani, V.R.; Srinivas, N.; Kishan, M.R.; Kulkarni, S.; Raghavan, K.V. Zeolite-catalyzed cyclocondensation reaction for the selective synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Green Chem. 2001, 3, 305–306. [Google Scholar] [CrossRef]
- Vankudoth, K.; Padmasri, A.H.; Sarkari, R.; Velisoju, V.K.; Gutta, N.; Sathu, N.K.; Rohita, C.N.; Akula, V. The role of Lewis acid-base pair sites in ZnO-ZnCr2O4 catalysts for cyclization: Via dehydrogenative condensation of crude glycerol and 1,2-propanediamine for the synthesis of 2,6-dimethylpyrazine. New J. Chem. 2017, 41, 9875–9883. [Google Scholar] [CrossRef]
- Sarkari, R.; Anjaneyulu, C.; Krishna, V.; Kishore, R.; Sudhakar, M.; Venugopal, A. Vapor phase synthesis of methylpyrazine using aqueous glycerol and ethylenediamine over ZnCr2O4 catalyst: Elucidation of reaction mechanism. Catal. Commun. 2011, 12, 1067–1070. [Google Scholar] [CrossRef]
- Rojas Delgado, R.; Arandigoyen Vidaurre, M.; De Pauli, C.P.; Ulibarri, M.A.; Avena, M.J. Surface-charging behavior of Zn-Cr layered double hydroxide. J. Colloid Interface Sci. 2004, 280, 431–441. [Google Scholar] [CrossRef] [PubMed]
- del Arco, M.; Rives, V.; Trujillano, R.; Malet, P. Thermal behaviour of Zn–Cr layered double hydroxides with hydrotalcite-like structures containing carbonate or decavanadate. J. Mater. Chem. 1996, 6, 1419–1428. [Google Scholar] [CrossRef]
- Simard, F.; Sedran, U.A.; Sepúlveda, J.; Fígoli, N.S.; de Lasa, H.I. ZnO Cr2O3 + ZSM-5 catalyst with very low Zn/Cr ratio for the transformation of synthesis gas to hydrocarbons. Appl. Catal. A Gen. 1995, 125, 81–98. [Google Scholar] [CrossRef]
- Dhas, N.A.; Koltypin, Y.; Gedanken, A. Sonochemical Preparation and Characterization of Ultrafine Chromium Oxide and Manganese Oxide Powders. Chem. Mater. 1997, 9, 3159–3163. [Google Scholar] [CrossRef]
- Murakami, Y.; Sawata, A.; Tsuru, Y. Crystallization behavior of amorphous solid solutions and phase separation in the Cr2O3-Fe2O3 system. J. Mater. Sci. 1999, 34, 951–955. [Google Scholar] [CrossRef]
- Cheng, R.; Borca, C.N.; Dowben, P.A.; Stadler, S.; Idzerda, Y.U. Potential phase control of chromium oxide thin films prepared by laser-initiated organometallic chemical vapor deposition. Appl. Phys. Lett. 2001, 78, 521–523. [Google Scholar] [CrossRef]
- Steger, W.E.; Landmesser, H.; Boettcher, U.; Schubert, E. Infrared spectra of amorphous oxides. J. Mol. Struct. 1990, 217, 341–346. [Google Scholar] [CrossRef]
- Carta, G.; Natali, M.; Rossetto, G.; Zanella, P.; Salmaso, G.; Restello, S.; Rigato, V.; Kaciulis, S.; Mezzi, A. A comparative study of Cr2O3 thin films obtained by MOCVD using three different precursors. Chem. Vap. Depos. 2005, 11, 375–380. [Google Scholar] [CrossRef]
- Menon, R.; Gupta, V.; Tan, H.H.; Sreenivas, K.; Jagadish, C. Origin of stress in radio frequency magnetron sputtered zinc oxide thin films. J. Appl. Phys. 2011, 109, 064905. [Google Scholar] [CrossRef]
- Ashkenov, N.; Mbenkum, B.N.; Bundesmann, C.; Riede, V.; Lorenz, M.; Spemann, D.; Kaidashev, E.M.; Kasic, A.; Schubert, M.; Grundmann, M.; et al. Infrared dielectric functions and phonon modes of high-quality ZnO films. J. Appl. Phys. 2002, 93, 126–133. [Google Scholar] [CrossRef]
- Itina, G.V.; Davydov, A.A.; Osipova, M.A.; Kurina, L.N. Promoting effect of potassium on catalytic and surface properties and phase commposition of catalysts for synthesis of alcohols. React. Kinet. Catal. Lett. 1991, 45, 243–249. [Google Scholar] [CrossRef]
- Venugopal, A.; Sarkari, R.; Anjaneyulu, C.; Krishna, V.; Kumar, M.K.; Narender, N.; Padmasri, A.H. Influence of acid-base sites on ZnO–ZnCr2O4 catalyst during dehydrocyclization of aqueous glycerol and ethylenediamine for the synthesis of 2-methylpyrazine: Kinetic and mechanism studies. Appl. Catal. A Gen. 2014, 469, 398–409. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Brito, J.L.F.; Dieguez, L.C. Characterization of chromium species in catalysts for dehydrogenation and polymerization. J. Mol. Catal. A Chem. 2003, 203, 251–266. [Google Scholar] [CrossRef]
- Park, S.W.; Joo, O.S.; Jung, K.D.; Kim, H.; Han, S.H. Development of ZnO/Al2O3 catalyst for reverse-water-gas-shift reaction of CAMERE (carbon dioxide hydrogenation to form methanol via a reverse-water-gas-shift reaction) process. Appl. Catal. A Gen. 2001, 211, 81–90. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Zeńczak, K. Activity of chromium oxide deposited on different silica supports in the dehydrogenation of propane with CO2—A comparative study. J. Mol. Catal. A Chem. 2011, 349, 185–192. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Visuvamithiran, P.; Tharmaraj, V.; Pitchumani, K. Clay encapsulated ZnO nanoparticles as efficient catalysts for N-benzylation of amines. Catal. Commun. 2011, 16, 15–19. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Schoonheydt, R.A.; Jehng, J.-M.; Wachs, I.E.; Cho, S.J.; Ryoo, R.; Kijlstra, S.; Poels, E. Combined DRS–RS–EXAFS–XANES–TPR study of supported chromium catalysts. J. Chem. Soc. Faraday Trans. 1995, 91, 3245–3253. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Verberckmoes, A.A.; De Baets, A.R.; Schoonheydt, R.A. Diffuse Reflectance Spectroscopy of Supported Chromium Oxide Catalysts: A Self-Modeling Mixture Analysis. J. Catal. 1997, 166, 160–171. [Google Scholar] [CrossRef]
- Rodrigues, S.; Ranjit, K.T.; Uma, S.; Martyanov, I.N.; Klabunde, K.J. Visible-light photooxidation of trichloroethylene by Cr–Al-MCM-41. J. Catal. 2005, 230, 158–165. [Google Scholar] [CrossRef]
- Allen, G.C.; Curtis, M.T.; Hooper, A.J.; Tucker, P.M. X-Ray photoelectron spectroscopy of chromium–oxygen systems. J. Chem. Soc. Dalt. Trans. 1973, 23, 1675–1683. [Google Scholar] [CrossRef]
- Allen, G.C.; Tucker, P.M. Multiplet splitting of X-ray photoelectron lines of chromium complexes. The effect of covalency on the 2p core level spin-orbit separation. Inorg. Chim. Acta 1976, 16, 41–45. [Google Scholar] [CrossRef]
- Cimino, A.; De Angelis, B.A.; Luchetti, A.; Minelli, G. The characterization of CrOxSiO2 catalysts by photoelectron spectroscopy (XPS), X-ray and optical measurements. J. Catal. 1976, 45, 316–325. [Google Scholar] [CrossRef]
- Yasuaki, O.; Masaki, F.; Toshinobu, I.; Shiichiro, T. X-Ray Photoelectron Spectroscopic Studies of Catalysts—Chromia–Alumina Catalysts. Bull. Chem. Soc. Jpn. 2006, 49, 859–863. [Google Scholar] [CrossRef]
- Jagannathan, K.; Srinivasan, A.; Rao, C.N.R. An XPS study of the surface oxidation states of metals in some oxide catalysts. J. Catal. 1981, 69, 418–427. [Google Scholar] [CrossRef]
- Best, S.A.; Squires, R.G.; Walton, R.A. The X-ray photoelectron spectra of heterogeneous catalysts: II. The chromia-silica catalyst system. J. Catal. 1977, 47, 292–299. [Google Scholar] [CrossRef]
- Grünert, W.; Feldhaus, R.; Anders, K.; Shpiro, E.S.; Antoshin, G.V.; Minachev, K.M. Reduction and aromatization activity of chromia-alumina catalysts: II. An XPS investigation of chromia-alumina catalysts. J. Catal. 1986, 100, 138–148. [Google Scholar] [CrossRef]
- Hoflund, G.B.; Epling, W.S.; Minahan, D.M. Reaction and surface characterization study of higher-alcohol synthesis catalysts XII: K- and Pd-promoted Zn/Cr/Mn spinel. Catal. Today 1999, 52, 99–109. [Google Scholar] [CrossRef]
- Parhi, P.; Manivannan, V. Microwave metathetic approach for the synthesis and characterization of ZnCr2O4. J. Eur. Ceram. Soc. 2008, 8, 1665–1670. [Google Scholar] [CrossRef]
- Venugopal, A.; Palgunadi, J.; Deog, J.K.; Joo, O.S.; Shin, C.H. Hydrotalcite derived Cu-Zn-Cr catalysts admixed with γ-Al2O3 for single step dimethyl ether synthesis from syngas: Influence of hydrothermal treatment. Catal. Today 2009, 147, 94–99. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. The effect of varying synthesis conditions on zinc chromium hydrotalcite: A spectroscopic study. Mater. Chem. Phys. 2005, 89, 99–109. [Google Scholar] [CrossRef] [Green Version]






| Zn:Cr Mole Ratio | a SBET (m2 g−1) | b H2 Uptake (µmol g−1) | c O2 Uptake | d Crystallite Size (nm) | |||
|---|---|---|---|---|---|---|---|
| (µmol g−1) | (µmol m−2) | ZnO | ZnCr2O4 | Cr2O3 | |||
| 4:1 | 78.0 | 40.5 | 23.2 | 0.29 | 15.4 | 7.68 | nf |
| 3:1 | 68.0 | 74.9 | 38.7 | 0.57 | 17.3 | 8.64 | nf |
| 2:1 | 38.9 | 102.5 | 36.8 | 0.94 | 19.8 | 14.5 | nf |
| 1:1 | 43.9 | 101.6 | 46.5 | 1.06 | nf | 9.2 | nf |
| 1:2 | 56.9 | 124.6 | 68.3 | 1.20 | nf | 13.8 | nf |
| 1:3 | 45.3 | 78.9 | 55.9 | 1.23 | nf | 9.8 | nf |
| 1:4 | 62.3 | 54.8 | 37.2 | 0.60 | nf | 9.9 | 19.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkari, R.; Mandari, K.K.; Medak, S.; Ramineni, K.; Aytam, H.P.; Akula, V. Synthesis of 2-Methylpyrazine Using Crude Glycerol over Zn-Cr-O Catalyst: A Value Addition Process for the Utilization of Biodiesel By-Product. Catalysts 2023, 13, 318. https://doi.org/10.3390/catal13020318
Sarkari R, Mandari KK, Medak S, Ramineni K, Aytam HP, Akula V. Synthesis of 2-Methylpyrazine Using Crude Glycerol over Zn-Cr-O Catalyst: A Value Addition Process for the Utilization of Biodiesel By-Product. Catalysts. 2023; 13(2):318. https://doi.org/10.3390/catal13020318
Chicago/Turabian StyleSarkari, Reema, Kotesh Kumar Mandari, Sudhakar Medak, Kishore Ramineni, Hari Padmasri Aytam, and Venugopal Akula. 2023. "Synthesis of 2-Methylpyrazine Using Crude Glycerol over Zn-Cr-O Catalyst: A Value Addition Process for the Utilization of Biodiesel By-Product" Catalysts 13, no. 2: 318. https://doi.org/10.3390/catal13020318
APA StyleSarkari, R., Mandari, K. K., Medak, S., Ramineni, K., Aytam, H. P., & Akula, V. (2023). Synthesis of 2-Methylpyrazine Using Crude Glycerol over Zn-Cr-O Catalyst: A Value Addition Process for the Utilization of Biodiesel By-Product. Catalysts, 13(2), 318. https://doi.org/10.3390/catal13020318