Kinetics of Heavy Reformate Conversion to Xylenes over MCM-41 on Zeolite Beta Composite Catalyst
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Characterization of Catalyst
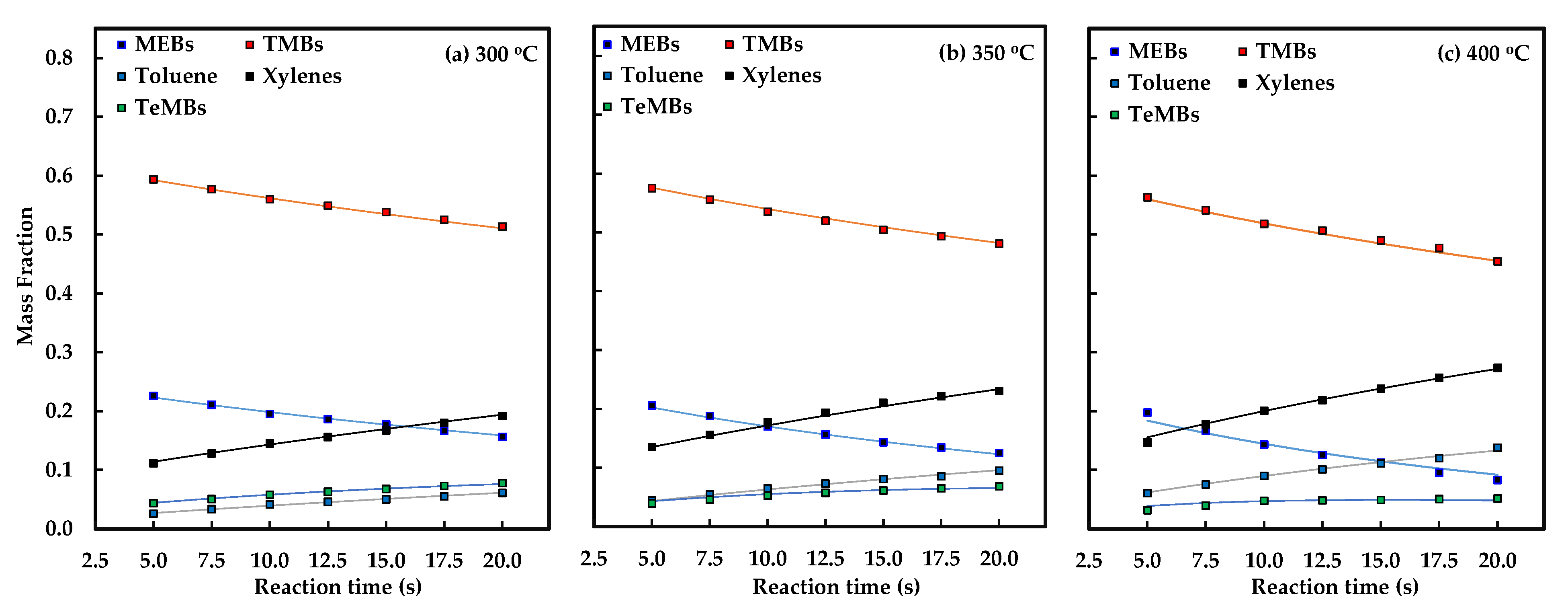
2.2. Conversion of Heavy Reformate
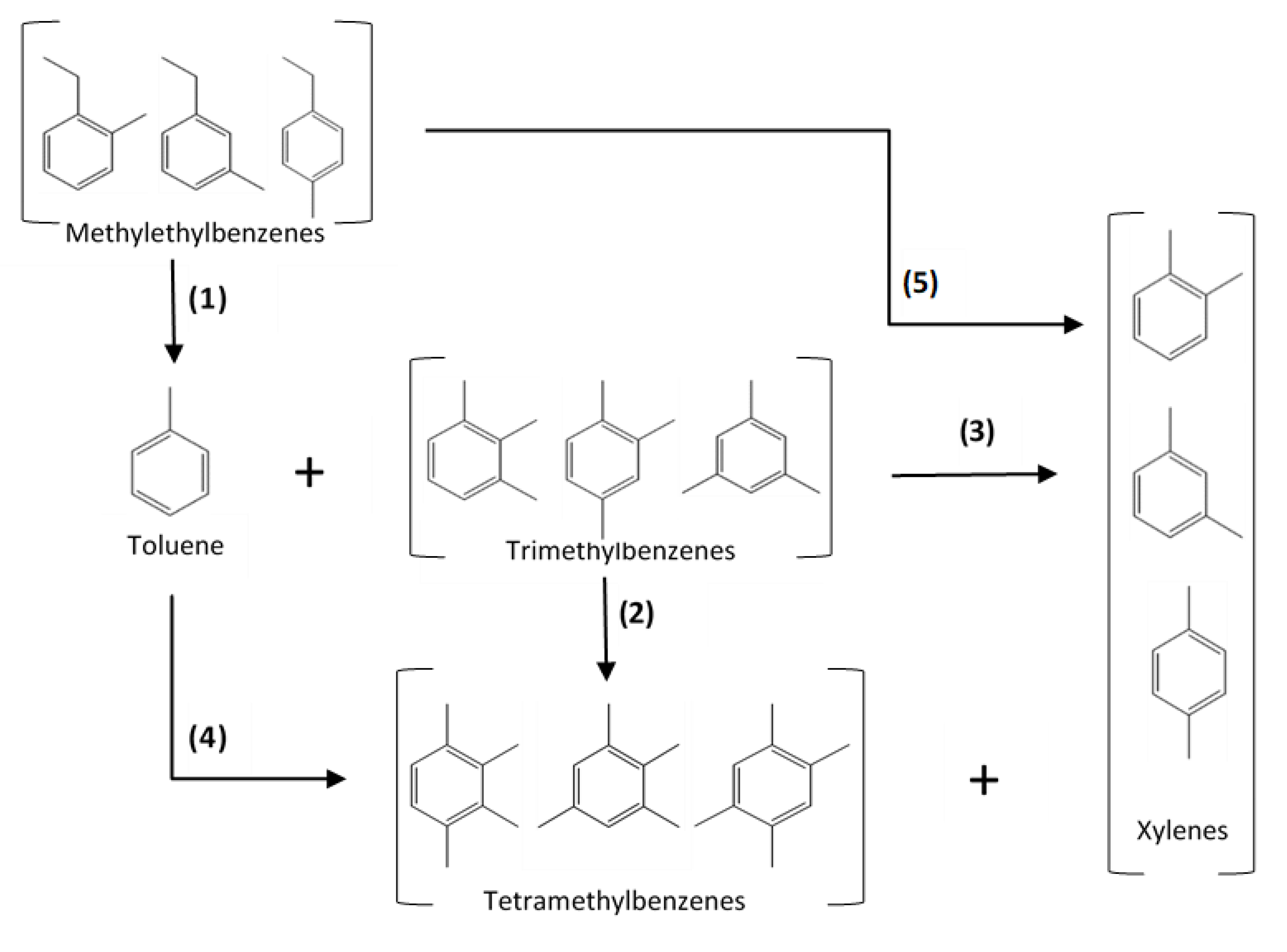
2.3. Model Formulation
- (i)
- dealkylation of MEBs to form toluene;
- (ii)
- disproportionation of TMBs to form TeMBs and xylenes;
- (iii)
- transalkylation of toluene and TMBs to form xylenes;
- (iv)
- paring reaction of TeMBs to form toluene; and
- (v)
- dealkyation of MEBs to form xylenes.
- (a)
- The five reactions are elementary;
- (b)
- The catalyst deactivation is defined by a single function for all of the reactions taking place; and
- (c)
- The reaction was conducted under isothermal conditions due to the fluidization of the reactants and catalyst particles.
- (a)
- The kinetic model presumes the occurrence of only catalytic reactions, and thermal conversion is not taken into consideration.
- (b)
- Considering the short reaction time (maximum 20 s) and low C9 aromatics conversions, the change in temperature during each run is negligible.
- (c)
- At any given instant, the concentrations of chemical species are effectively uniform due to the high intensity of gas recirculation and a short reaction time.
- (d)
- The concentration of coke is uniform due to the high degree of fluidization at any given time.
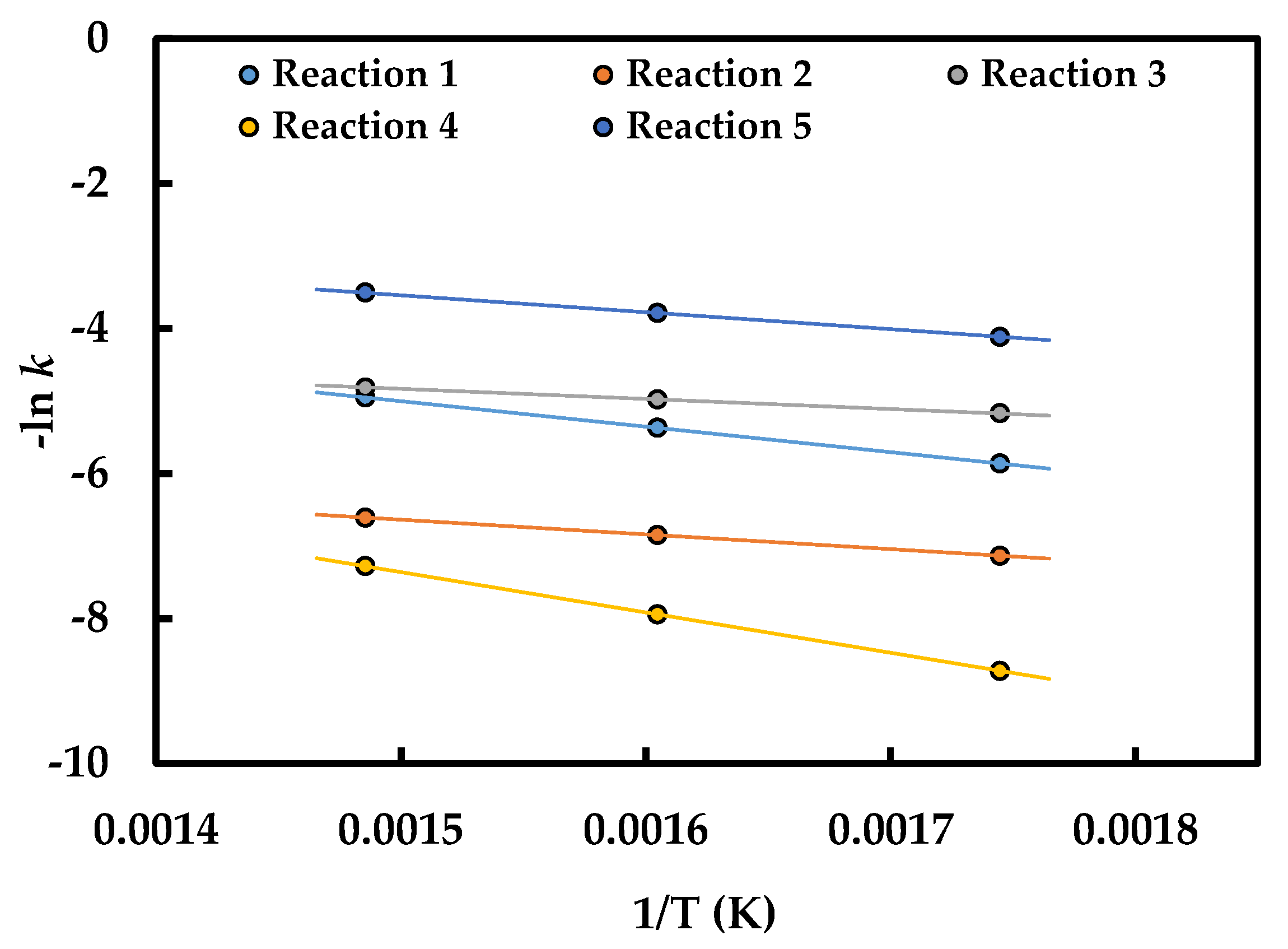
2.4. Estimation of Kinetic Parameters and Model Evaluation
- (i)
- The specific reaction rate and activation energy for each reaction should agree with the physical–chemical principles.
- (ii)
- The regression coefficient (R2) should be close to unity.
- (iii)
- The sum of squares of the residuals (SSR) should be lower than an acceptable minimum value.
- (iv)
- The kinetic parameters should display low confidence interval spans.
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Catalyst Synthesis
4.3. Catalyst Characterization
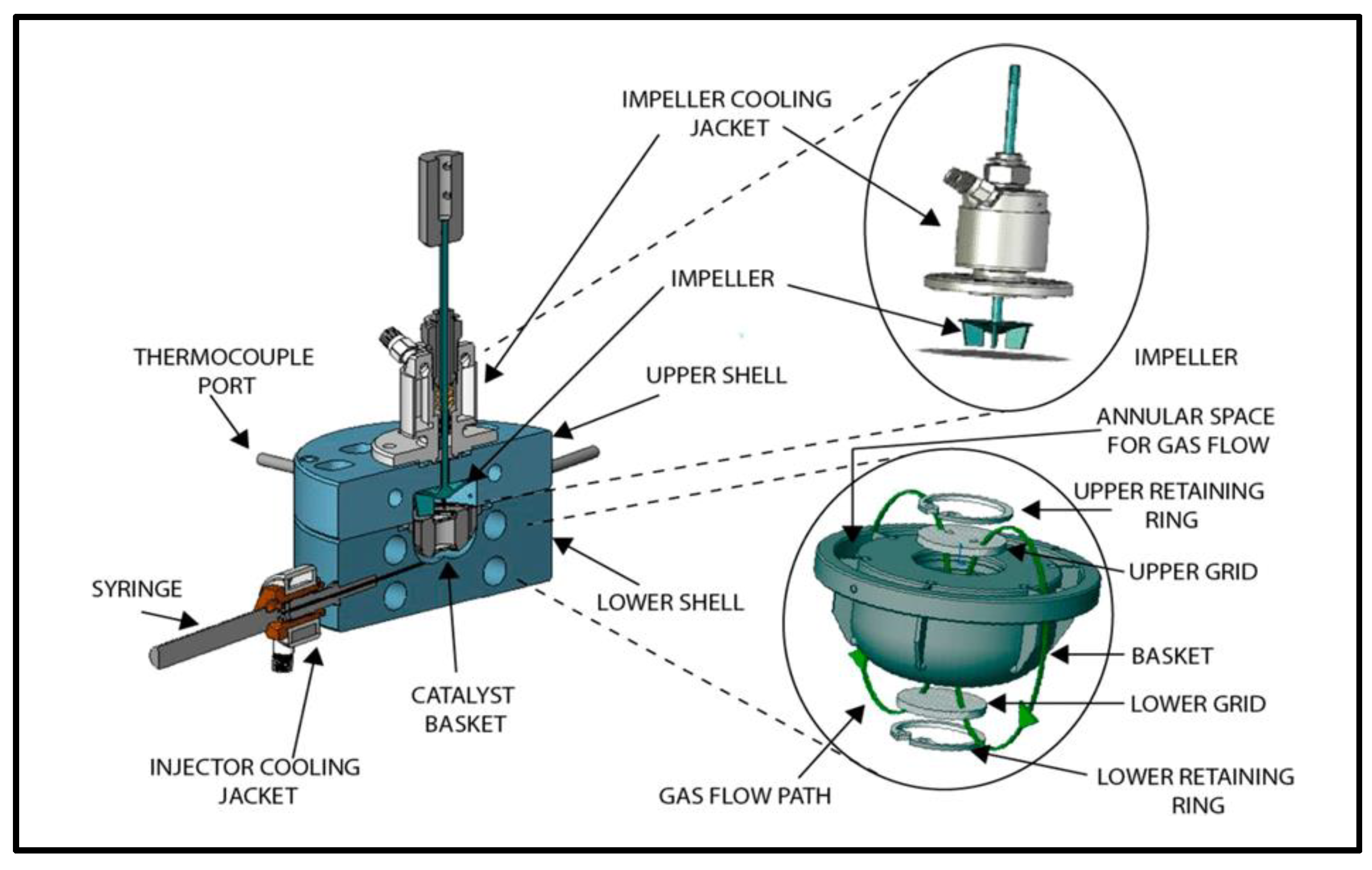
4.4. Generation of Catalyst Performance Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.A.; Al-Nawad, K.; Ercan, C.; Wang, Y. Parametric study of dealkylation-transalkylation reactions over mordenite-based bi-functional catalysts. App. Catal. A Gen. 2010, 393, 96–108. [Google Scholar] [CrossRef]
- Wang, I.; Tsai, T.C.; Huang, S.T. Disproportionation of toluene and of trimethylbenzene and their transalkylation over zeolite beta. Ind. Eng. Chem. Res. 1990, 29, 2005–2012. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Dong, M.; Li, J.; Qin, Z.; Wang, J.; Fan, W. Effect of zeolite pore structure on the diffusion and catalytic behaviors in the transalkylation of toluene with 1,2,4-trimethylbenzene. RSC Adv. 2015, 5, 66301–66310. [Google Scholar] [CrossRef]
- Cha, S.; Hong, S. 1,2,4-Trimethylbenzene disproportionation over large-pore zeolites: An experimental and theoretical study. J. Catal. 2018, 357, 145–157. [Google Scholar] [CrossRef]
- Almulla, F.M.; Ali, S.A.; Aldossary, M.R.; Alnaimi, E.I.; Jumah, A.B.; Garforth, A. Transalkylation of 1, 2, 4-trimethylbenzene with toluene over large pore zeolites: Role of pore structure and acidity. Appl. Catal. A Gen. 2020, 608, 117886. [Google Scholar] [CrossRef]
- Krejcı, A.; Al-Khattaf, S.; Ali, M.A.; Bejblova, M. Cejka, Transalkylation of toluene with trimethylbenzenes over large-pore zeolite. Appl. Catal. A Gen. 2010, 377, 99–106. [Google Scholar] [CrossRef]
- Serra, J.; Guillon, E.; Corma, A. Optimizing the conversion of heavy reformate streams into xylenes with zeolite catalysts by using knowledge base high-throughput experimentation techniques. J. Catal. 2005, 232, 342–354. [Google Scholar] [CrossRef]
- Tsai, T.C.; Liu, S.; Wang, I. Supported zeolite for heavy aromatics transalkylation process. Catal. Surv. Asia 2009, 13, 94–103. [Google Scholar] [CrossRef]
- Al-Khattaf, S.; Ali, S.A.; Aitani, A.; Al-Nawad, K.; Chiu, C.; Tsai, T. Catalysis of metal supported zeolites for dealkylation-transalkylation of alkyl-aromatics. App. Catal. A Gen. 2016, 514, 154–163. [Google Scholar] [CrossRef]
- Altındas¸, C.; Sher, F.; Smjecanin, N.; Lima, E.; Rashid, T.; Hai, I.; Karaduman, A. Synergistic interaction of metal loaded multifactorial nanocatalysts over bifunctional transalkylation for environmental applications. Environ. Res. 2023, 216, 114479. [Google Scholar] [CrossRef]
- Margarit, V.J.; Portilla, M.T.; Navarro, M.T.; Abudawoud, R.; Al-Zahrani, I.M.; Shaikh, S.; Martínez, C.; Corma, A. One-pot co-crystallization of beta and pentasil nanozeolites for the direct conversion of a heavy reformate fraction into xylenes. Appl. Catal. A Gen. 2019, 581, 11–22. [Google Scholar] [CrossRef]
- Ali, S.A.; Almulla, F.M.; Jermy, B.R.; Aitani, A.M.; Abudawoud, R.H.; AlAmer, M.A.; Qureshi, Z.S.; Mohammad, T.; Alasiri, H.S. Hierarchical composite catalysts of MCM-41 on zeolite beta for conversion of heavy reformate to xylenes. J. Ind. Eng. Chem. 2021, 98, 189–199. [Google Scholar] [CrossRef]
- Salama, R.S.; El-Bahy, S.M.; Mannaa, M.A. Sulfamic acid supported on mesoporous MCM-41 as a novel, efficient and reusable heterogenous solid acid catalyst for synthesis of xanthene, dihydropyrimidinone and coumarin derivatives. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127261. [Google Scholar] [CrossRef]
- El-Hakam, S.A.; Samra, S.E.; El-Dafrawy, S.M.; Ibrahim, A.A.; Salama, R.S. Surface acidity and catalytic activity of sulfated titania supported on mesoporous MCM-41. Int. J. Mod. Chem. 2013, 5, 55–70. [Google Scholar]
- Waziri, S.; Aitani, A.M.; Al-Khattaf, S. Transformation of toluene and 1,2,4-trimethylbenzene over ZSM-5 and mordenite catalysts: A comprehensive kinetic model with reversibility. Ind. Eng. Chem. Res. 2010, 49, 6376–6387. [Google Scholar] [CrossRef]
- Thakur, R.; Barman, S.; Gupta, R.K. Kinetic investigation in transalkylation of 1,2,4 trimethylbenzene with toluene over rare earth metal-modified large pore zeolite. Chem. Eng. Commun. 2017, 204, 254–264. [Google Scholar] [CrossRef]
- Ali, S.A.; Ogunronbi, K.; Al-Khattaf, S. Kinetics of dealkylation-transalkylation of c9 alkyl-aromatics over zeolites of different structures. Chem. Eng. Res. Des. 2013, 91, 2601–2616. [Google Scholar] [CrossRef]
- Al-Mubaiyedh, U.; Ali, S.A.; Al-Khattaf, S. Kinetic modeling of heavy reformate conversion into xylenes over mordenite-ZSM-5 based catalysts. Chem. Eng. Res. Des. 2012, 90, 1943–1955. [Google Scholar] [CrossRef]
- Khaksar, S.A.N.; Farsi, M.; Khaksar, S.A. Kinetic modeling of gas-phase disproportionation and transalkylation of C9 and C10 aromatics over industrial zeolite catalyst. React. Kin. Mech. Catal 2021, 32, 1075–1093. [Google Scholar] [CrossRef]
- Hamedi, N.; Iranshahi, D.; Rahimpour, M.R.; Raeissi, S.; Rajaei, H. Development of a detailed reaction network for industrial upgrading of heavy reformates to xylenes using differential evolution technique. J. Taiwan Inst. Chem. Eng. 2015, 48, 56–72. [Google Scholar] [CrossRef]
- Almulla, F.; Zholobenko, V.I.; Tedstone, A.A.; Jumah, A.B.; Aldossary, M.R.; Garforth, A.A. Effect of hydrogenative regeneration on the activity of beta and Pt-beta zeolites during the transalkylation of toluene with 1,2,4-trimethylbenzene. Microporous Mesoporous. Mater. 2020, 293, 109737. [Google Scholar] [CrossRef]
- De Lasa, H.I. Riser Simulator for Catalytic Cracking Studies. U.S. Patent No. 5,102,628, 7 April 1992. [Google Scholar]
- De Lasa, H.I. The CREC Fluidized Riser Simulator a Unique Tool for Catalytic Process Development. Catalysts 2022, 12, 888. [Google Scholar] [CrossRef]




| Property | Parent Zeolite β | Catalyst |
|---|---|---|
| BET Surface Area (m2/g) | 567 | 714 |
| Total Pore Volume (cm3/g) | 0.242 | 0.598 |
| t-Plot Micropore Volume (cm3/g) | 0.182 | 0.167 |
| Mesopore Volume (cm3/g) | 0.060 | 0.431 |
| Average Pore Diameter (nm) | 2.67 | 3.35 |
| Total Acidity (NH3 μmol/g) | 768 | 668 |
| Reaction | Reactant(s) | Product(s) |
|---|---|---|
| Dealkylation | MEB | Toluene |
| MEB | Xylene | |
| Disproportionation | Toluene + Toluene | Benzene + Xylene |
| MEB + MEB | Toluene + MDEB | |
| TMB + TMB | Xylene + TeMB | |
| Transalkylation | Toluene + TMB | Xylene + Xylene |
| Toluene + TMB | Benzene + TeMB | |
| Paring | TeMB | Toluene + propylene |
| Reaction Temperature (°C) | Reaction Time (s) | Feed/Product Composition (wt.%) | Conversion (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MEBs | TMBs | Toluene | Xylenes | TeMBs | MEBs | TMBs | C9 | ||
| Heavy Reformate Feed | 31.08 | 67.35 | 0.00 | 0.91 | 0.65 | - | - | - | |
| 300 | 5.0 | 22.58 | 59.38 | 2.57 | 11.11 | 4.35 | 27.34 | 11.83 | 16.73 |
| 7.5 | 21.07 | 57.71 | 3.35 | 12.80 | 5.06 | 32.20 | 14.32 | 19.96 | |
| 10.0 | 19.54 | 56.01 | 4.14 | 14.52 | 5.79 | 37.13 | 16.83 | 23.24 | |
| 12.5 | 18.63 | 54.91 | 4.57 | 15.60 | 6.29 | 40.05 | 18.47 | 25.28 | |
| 15.0 | 17.72 | 53.80 | 5.01 | 16.69 | 6.78 | 42.99 | 20.12 | 27.34 | |
| 17.5 | 16.66 | 52.52 | 5.54 | 18.01 | 7.27 | 46.38 | 22.01 | 29.71 | |
| 20.0 | 15.63 | 51.35 | 6.08 | 19.17 | 7.77 | 49.72 | 23.76 | 31.95 | |
| 350 | 5.0 | 20.58 | 57.53 | 4.40 | 13.54 | 3.95 | 33.77 | 14.58 | 20.64 |
| 7.5 | 18.82 | 55.54 | 5.43 | 15.60 | 4.61 | 39.43 | 17.54 | 24.45 | |
| 10.0 | 17.03 | 53.51 | 6.49 | 17.69 | 5.28 | 45.21 | 20.54 | 28.33 | |
| 12.5 | 15.68 | 51.98 | 7.28 | 19.35 | 5.71 | 49.55 | 22.82 | 31.26 | |
| 15.0 | 14.32 | 50.43 | 8.08 | 21.03 | 6.14 | 53.93 | 25.12 | 34.22 | |
| 17.5 | 13.44 | 49.35 | 8.54 | 22.17 | 6.51 | 56.76 | 26.73 | 36.21 | |
| 20.0 | 12.51 | 48.09 | 9.51 | 23.03 | 6.86 | 59.75 | 28.60 | 38.43 | |
| 400 | 5.0 | 19.75 | 56.34 | 6.08 | 14.67 | 3.15 | 36.44 | 16.34 | 22.69 |
| 7.5 | 16.67 | 54.14 | 7.53 | 17.72 | 3.94 | 46.38 | 19.61 | 28.07 | |
| 10.0 | 14.34 | 51.83 | 9.02 | 20.07 | 4.74 | 53.85 | 23.04 | 32.77 | |
| 12.5 | 12.53 | 50.68 | 10.10 | 21.85 | 4.83 | 59.67 | 24.75 | 35.77 | |
| 15.0 | 11.23 | 49.00 | 11.10 | 23.78 | 4.88 | 63.87 | 27.24 | 38.80 | |
| 17.5 | 9.52 | 47.73 | 12.01 | 25.68 | 5.06 | 69.38 | 29.13 | 41.84 | |
| 20.0 | 8.28 | 45.45 | 13.78 | 27.36 | 5.13 | 73.36 | 32.52 | 45.41 | |
| Frequency Factors * | Activation Energy (kJ/mol) | ||
|---|---|---|---|
| k1,0 | 1.31 ± 0.06 | E1 | 29.25 ± 2.43 |
| k2,0 | 0.03 ± 0.00 | E2 | 16.82 ± 1.56 |
| k3,0 | 0.06 ± 0.01 | E3 | 11.46 ± 9.14 |
| k4,0 | 2.71 ± 0.15 | E4 | 46.33 ± 3.83 |
| k5,0 | 0.98 ± 0.04 | E5 | 19.54 ± 2.41 |
| λ (deactivation) = 0.41 ± 0.15 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.A.; Hossain, M.M. Kinetics of Heavy Reformate Conversion to Xylenes over MCM-41 on Zeolite Beta Composite Catalyst. Catalysts 2023, 13, 335. https://doi.org/10.3390/catal13020335
Ali SA, Hossain MM. Kinetics of Heavy Reformate Conversion to Xylenes over MCM-41 on Zeolite Beta Composite Catalyst. Catalysts. 2023; 13(2):335. https://doi.org/10.3390/catal13020335
Chicago/Turabian StyleAli, Syed Ahmed, and Mohammad Mozahar Hossain. 2023. "Kinetics of Heavy Reformate Conversion to Xylenes over MCM-41 on Zeolite Beta Composite Catalyst" Catalysts 13, no. 2: 335. https://doi.org/10.3390/catal13020335
APA StyleAli, S. A., & Hossain, M. M. (2023). Kinetics of Heavy Reformate Conversion to Xylenes over MCM-41 on Zeolite Beta Composite Catalyst. Catalysts, 13(2), 335. https://doi.org/10.3390/catal13020335








