Copper Oxide Nanoparticles over Hierarchical Silica Monoliths for Continuous-Flow Selective Alcoholysis of Styrene Oxide
Abstract
:1. Introduction
2. Results and Discussion
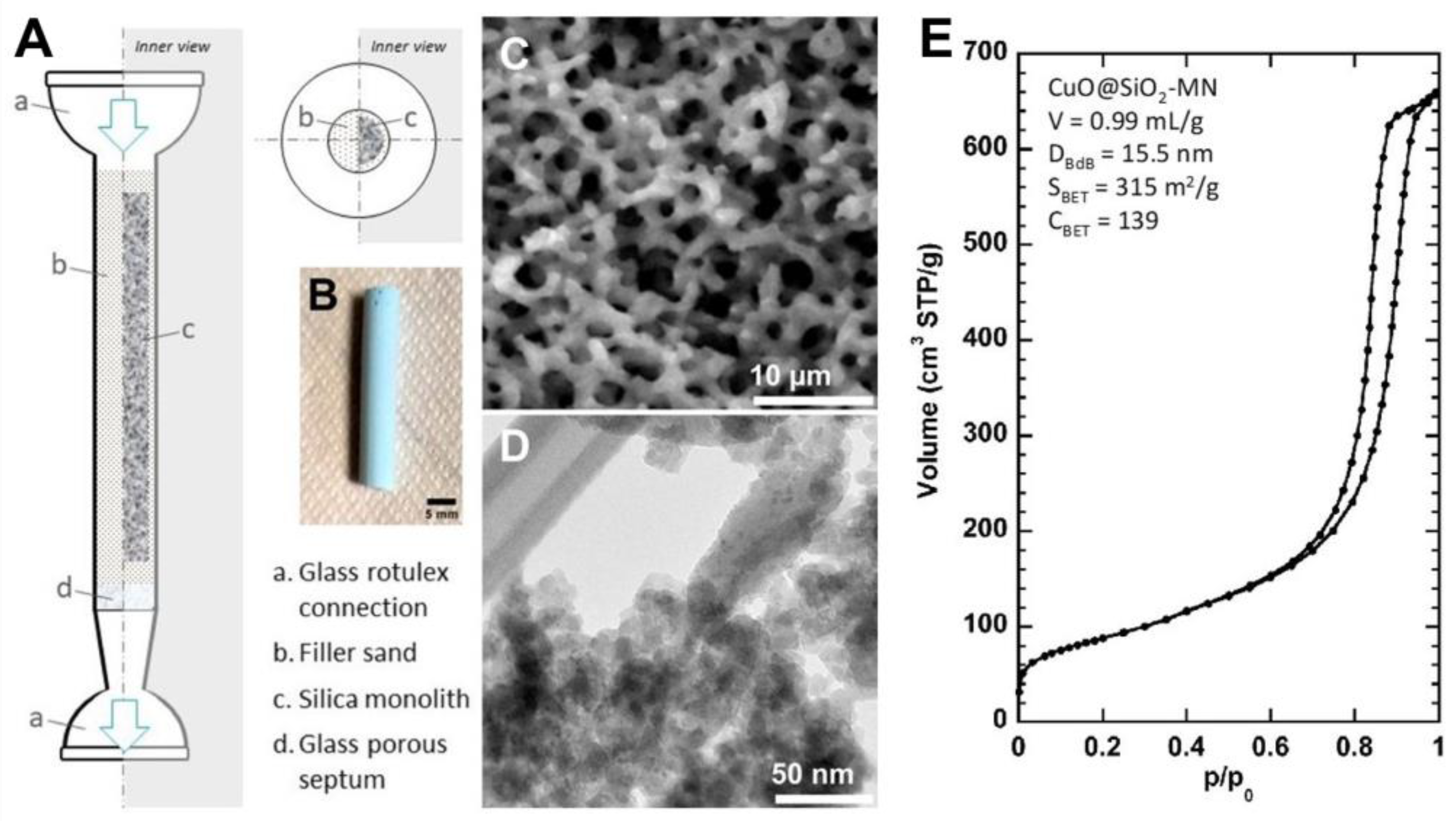
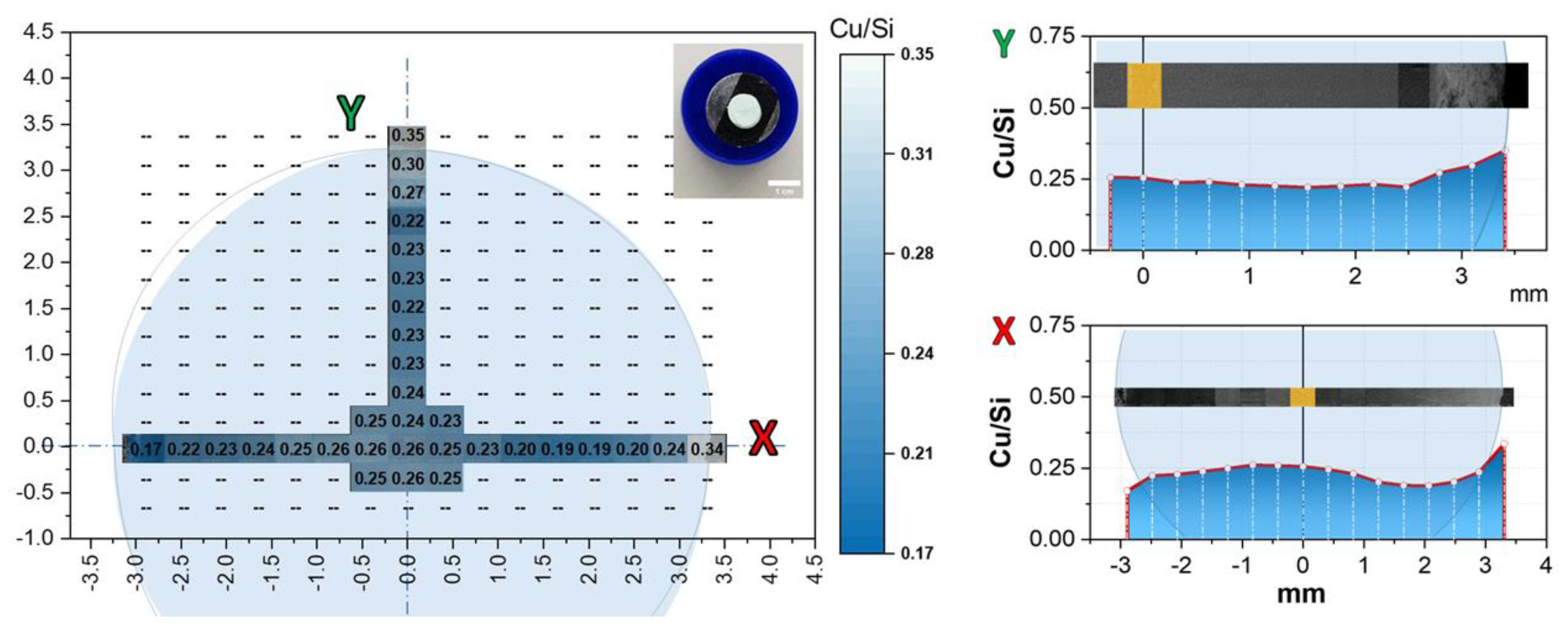
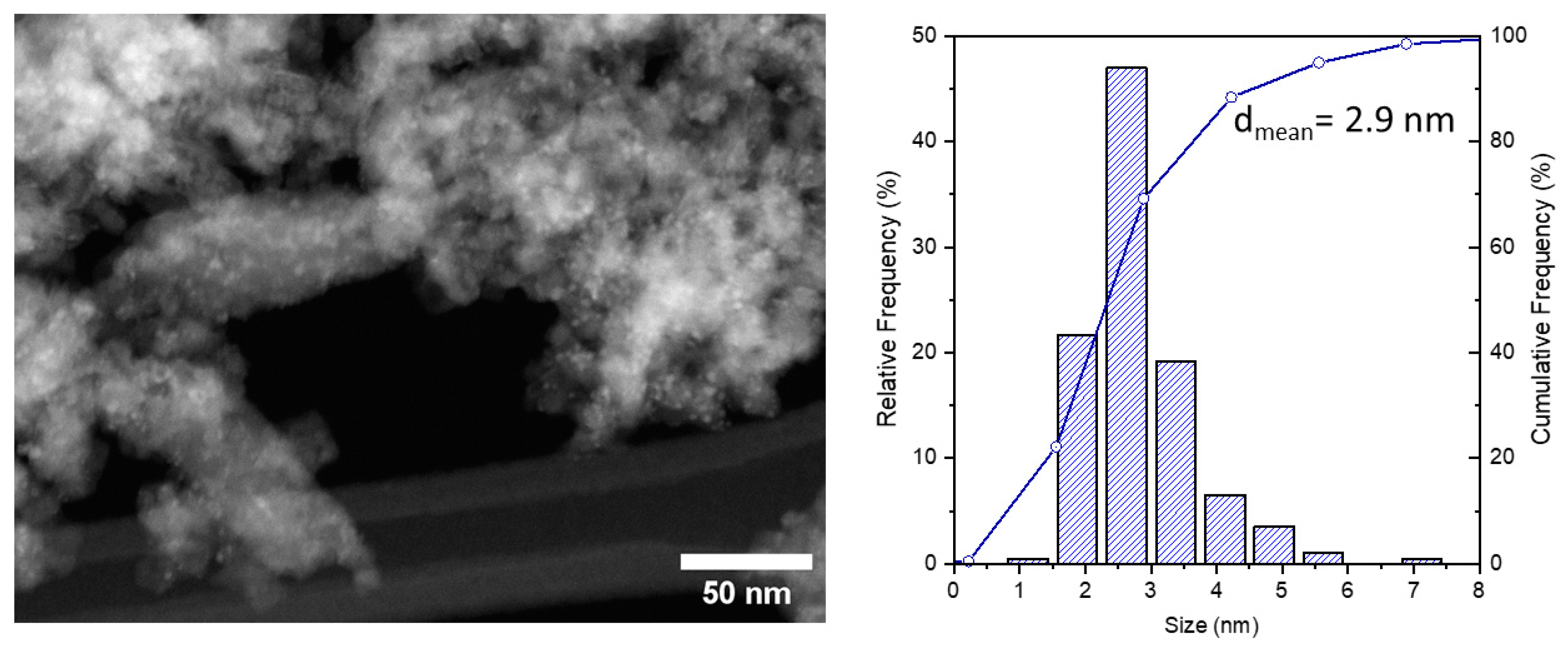
2.1. Preparation and Characterization of CuO@SiO2-MN
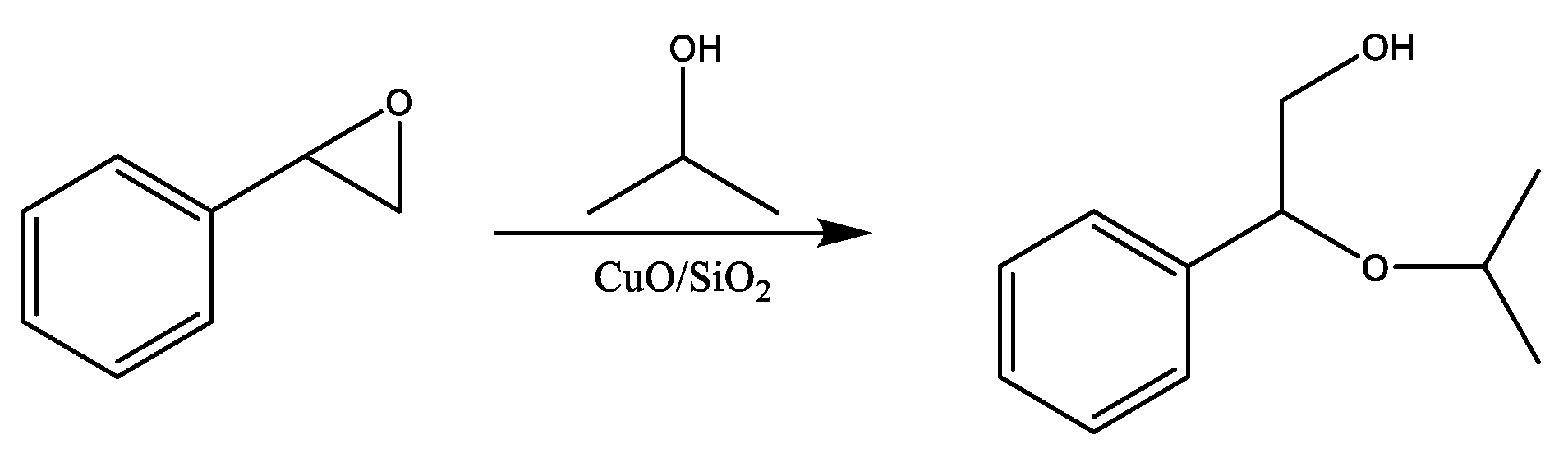
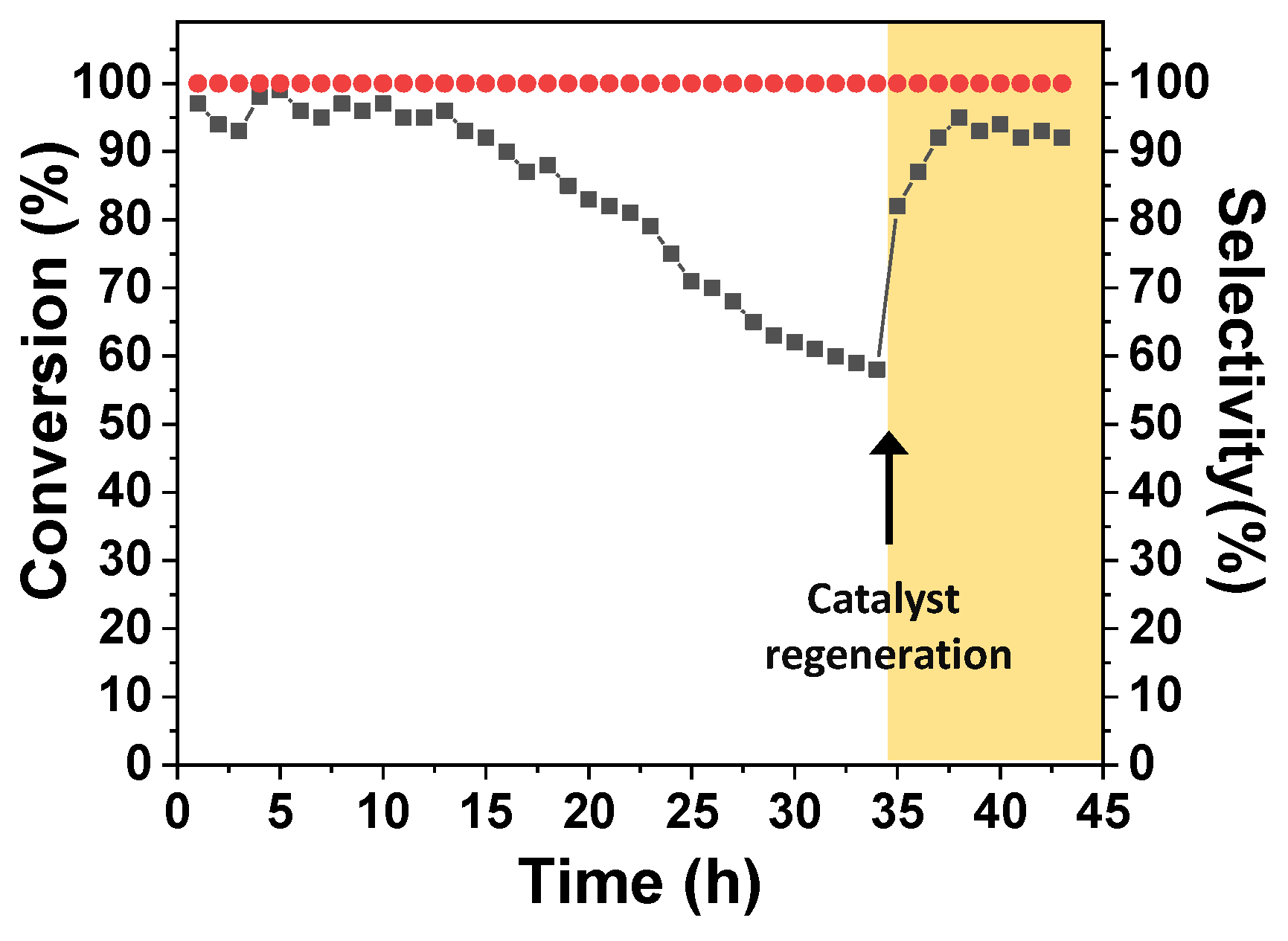
2.2. Catalytic Tests
3. Materials and Methods
3.1. General
3.2. Monolith Synthesis
3.3. CuO NP Deposition into SiO2 Monolith
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hommes, A.; Heeres, H.J.; Yue, J. Catalytic Transformation of Biomass Derivatives to Value-Added Chemicals and Fuels in Continuous Flow Microreactors. ChemCatChem 2019, 11, 4671–4708. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous Flow Reactors: A Perspective. Green Chem. 2012, 14, 38–54. [Google Scholar] [CrossRef]
- Noêl, T.; Buchwald, S.L. Cross-Coupling in Flow. Chem. Soc. Rev. 2011, 40, 5010–5029. [Google Scholar] [CrossRef]
- Su, B.L.; Sanchez, C.; Yang, X.Y. Hierarchically Structured Porous Materials: From Nanoscience to Catalysis, Separation, Optics, Energy, and Life Science; John Wiley & Sons: New York, NY, USA, 2011; ISBN 9783527327881. [Google Scholar]
- Feinle, A.; Elsaesser, M.S.; Hüsing, N. Sol-Gel Synthesis of Monolithic Materials with Hierarchical Porosity. Chem. Soc. Rev. 2016, 45, 3377–3399. [Google Scholar] [CrossRef]
- Munirathinam, R.; Huskens, J.; Verboom, W. Supported Catalysis in Continuous-Flow Microreactors. Adv. Synth. Catal. 2015, 357, 1093–1123. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Haas, C.P.; Müllner, T.; Kohns, R.; Enke, D.; Tallarek, U. High-Performance Monoliths in Heterogeneous Catalysis with Single-Phase Liquid Flow. React. Chem. Eng. 2017, 2, 498–511. [Google Scholar] [CrossRef]
- Poupart, R.; Le Droumaguet, B.; Guerrouache, M.; Carbonnier, B. Copper Nanoparticles Supported on Permeable Monolith with Carboxylic Acid Surface Functionality: Stability and Catalytic Properties under Reductive Conditions. Mater. Chem. Phys. 2015, 163, 446–452. [Google Scholar] [CrossRef]
- Galarneau, A.; Sachse, A.; Said, B.; Pelisson, C.H.; Boscaro, P.; Brun, N.; Courtheoux, L.; Olivi-Tran, N.; Coasne, B.; Fajula, F. Hierarchical Porous Silica Monoliths: A Novel Class of Microreactors for Process Intensification in Catalysis and Adsorption. Comptes Rendus Chim. 2016, 19, 231–247. [Google Scholar] [CrossRef]
- Sachse, A.; Galarneau, A.; Fajula, F.; Di Renzo, F.; Creux, P.; Coq, B. Functional Silica Monoliths with Hierarchical Uniform Porosity as Continuous Flow Catalytic Reactors. Microporous Mesoporous Mater. 2011, 140, 58–68. [Google Scholar] [CrossRef]
- Didi, Y.; Said, B.; Cacciaguerra, T.; Nguyen, K.L.; Wernert, V.; Denoyel, R.; Cot, D.; Sebai, W.; Belleville, M.P.; Sanchez-Marcano, J.; et al. Synthesis of Binderless FAU-X (13X) Monoliths with Hierarchical Porosity. Microporous Mesoporous Mater. 2019, 281, 57–65. [Google Scholar] [CrossRef]
- Sebai, W.; Ahmad, S.; Belleville, M.-P.; Boccheciampe, A.; Chaurand, P.; Levard, C.; Brun, N.; Galarneau, A.; Sanchez-Marcano, J. Biocatalytic Elimination of Pharmaceutics Found in Water With Hierarchical Silica Monoliths in Continuous Flow. Front. Chem. Eng. 2022, 4, 823877. [Google Scholar] [CrossRef]
- Ou, J.; Liu, Z.; Wang, H.; Lin, H.; Dong, J.; Zou, H. Recent Development of Hybrid Organic-Silica Monolithic Columns in CEC and Capillary LC. Electrophoresis 2015, 36, 62–75. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Haswell, S.J.; Fletcher, P.D.I.; Kelly, S.M.; Mansfield, A. Scaling up of Continuous-Flow, Microwave-Assisted, Organic Reactions by Varying the Size of Pd-Functionalized Catalytic Monoliths. Beilstein J. Org. Chem. 2011, 7, 1150–1157. [Google Scholar] [CrossRef]
- El Kadib, A.; Chimenton, R.; Sachse, A.; Fajula, F.; Galarneau, A.; Coq, B. Functionalized Inorganic Monolithic Microreactors for High Productivity in Fine Chemicals Catalytic Synthesis. Angew. Chemie-Int. Ed. 2009, 48, 4969–4972. [Google Scholar] [CrossRef]
- Sachse, A.; Galarneau, A.; Coq, B.; Fajula, F. Monolithic Flow Microreactors Improve Fine Chemicals Synthesis. N. J. Chem. 2011, 35, 259–264. [Google Scholar] [CrossRef]
- Song, G.Q.; Lu, Y.X.; Zhang, Q.; Wang, F.; Ma, X.K.; Huang, X.F.; Zhang, Z.H. Porous Cu-BTC Silica Monoliths as Efficient Heterogeneous Catalysts for the Selective Oxidation of Alkylbenzenes. RSC Adv. 2014, 4, 30221–30224. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, C.K.; Ahn, C.H.; Kim, G.J. Syntheses and Application of Silica Monolith with Bimodal Meso/Macroscopic Pore Structure. J. Porous Mater. 2006, 13, 201–205. [Google Scholar] [CrossRef]
- Ciemięga, A.; Maresz, K.; Malinowski, J.J.; Mrowiec-Białoń, J. Continuous-Flow Monolithic Silica Microreactors with Arenesulphonic Acid Groups: Structure–Catalytic Activity Relationships. Catalysts 2017, 7, 255. [Google Scholar] [CrossRef]
- Sachse, A.; Ameloot, R.; Coq, B.; Fajula, F.; Coasne, B.; De Vos, D.; Galarneau, A. In Situ Synthesis of Cu–BTC (HKUST-1) in Macro-/Mesoporous Silica Monoliths for Continuous Flow Catalysis. Chem. Commun. 2012, 48, 4749–4751. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Kundu, D.; Uyama, H. One-Pot Fabrication of Palladium Nanoparticles Captured in Mesoporous Polymeric Monoliths and Their Catalytic Application in C-C Coupling Reactions. J. Colloid Interface Sci. 2015, 451, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Jumde, R.P.; Marelli, M.; Scotti, N.; Mandoli, A.; Psaro, R.; Evangelisti, C. Ultrafine Palladium Nanoparticles Immobilized into Poly(4-Vinylpyridine)-Based Porous Monolith for Continuous-Flow Mizoroki-Heck Reaction. J. Mol. Catal. A Chem. 2016, 414, 55–61. [Google Scholar] [CrossRef]
- Moitra, N.; Matsushima, A.; Kamei, T.; Kanamori, K.; Ikuhara, Y.H.; Gao, X.; Takeda, K.; Zhu, Y.; Nakanishi, K.; Shimada, T. A New Hierarchically Porous Pd@HSQ Monolithic Catalyst for Mizoroki-Heck Cross-Coupling Reactions. N. J. Chem. 2014, 38, 1144–1149. [Google Scholar] [CrossRef]
- Pélisson, C.H.; Nakanishi, T.; Zhu, Y.; Morisato, K.; Kamei, T.; Maeno, A.; Kaji, H.; Muroyama, S.; Tafu, M.; Kanamori, K.; et al. Grafted Polymethylhydrosiloxane on Hierarchically Porous Silica Monoliths: A New Path to Monolith-Supported Palladium Nanoparticles for Continuous Flow Catalysis Applications. ACS Appl. Mater. Interfaces 2017, 9, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Liguori, F.; Barbaro, P.; Said, B.; Galarneau, A.; Santo, V.D.; Passaglia, E.; Feis, A. Unconventional Pd@Sulfonated Silica Monoliths Catalysts for Selective Partial Hydrogenation Reactions under Continuous Flow. ChemCatChem 2017, 9, 3245–3258. [Google Scholar] [CrossRef]
- Linares, N.; Hartmann, S.; Galarneau, A.; Barbaro, P. Continuous Partial Hydrogenation Reactions by Pd@unconventional Bimodal Porous Titania Monolith Catalysts. ACS Catal. 2012, 2, 2194–2198. [Google Scholar] [CrossRef]
- Troncoso, F.D.; Tonetto, G.M. Highly Stable Platinum Monolith Catalyst for the Hydrogenation of Vegetable Oil. Chem. Eng. Process.-Process Intensif. 2022, 170, 108669. [Google Scholar] [CrossRef]
- Moitra, N.; Kanamori, K.; Ikuhara, Y.H.; Gao, X.; Zhu, Y.; Hasegawa, G.; Takeda, K.; Shimada, T.; Nakanishi, K. Reduction on Reactive Pore Surfaces as a Versatile Approach to Synthesize Monolith-Supported Metal Alloy Nanoparticles and Their Catalytic Applications. J. Mater. Chem. A 2014, 2, 12535–12544. [Google Scholar] [CrossRef]
- Russell, M.G.; Veryser, C.; Hunter, J.F.; Beingessner, R.L.; Jamison, T.F. Monolithic Silica Support for Immobilized Catalysis in Continuous Flow. Adv. Synth. Catal. 2020, 362, 314–319. [Google Scholar] [CrossRef]
- Alotaibi, M.T.; Taylor, M.J.; Liu, D.; Beaumont, S.K.; Kyriakou, G. Selective Oxidation of Cyclohexene through Gold Functionalized Silica Monolith Microreactors. Surf. Sci. 2016, 646, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Barluenga, J.; Vàzquez-Villa, H.; Ballesteros, A.; Gonza, M. Copper (II) Tetrafluoroborate Catalyzed Ring-Opening Reaction of Epoxides With. Org. Lett. 2002, 4, 2817–2819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Liu, Q.S.; Zhang, Z.H. Amberlyst-15 as a New and Reusable Catalyst for Regioselective Ring-Opening Reactions of Epoxides to β-Alkoxy Alcohols. J. Mol. Catal. A Chem. 2008, 296, 42–46. [Google Scholar] [CrossRef]
- Robinson, M.W.C.; Buckle, R.; Mabbett, I.; Grant, G.M.; Graham, A.E. Mesoporous Aluminosilicate Promoted Alcoholysis of Epoxides. Tetrahedron Lett. 2007, 48, 4723–4725. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, C.; Mei, W.; Zhang, J.; Cheng, L.; Xue, N.; Ding, W.; Chen, J.; Hou, W. Highly Efficient Sulfated Zr-Doped Titanoniobate Nanoplates for the Alcoholysis of Styrene Epoxide at Room Temperature. Appl. Surf. Sci. 2015, 357, 1951–1957. [Google Scholar] [CrossRef]
- Zaccheria, F.; Scotti, N.; Marelli, M.; Psaro, R.; Ravasio, N. Unravelling the Properties of Supported Copper Oxide: Can the Particle Size Induce Acidic Behaviour? Dalt. Trans. 2013, 42, 1319–1328. [Google Scholar] [CrossRef]
- Scotti, N.; Dangate, M.; Gervasini, A.; Evangelisti, C.; Ravasio, N.; Zaccheria, F. Unraveling the Role of Low Coordination Sites in a Cu Metal Nanoparticle: A Step toward the Selective Synthesis of Second Generation Biofuels. ACS Catal. 2014, 4, 2818–2826. [Google Scholar] [CrossRef]
- Scotti, N.; Finocchio, E.; Evangelisti, C.; Marelli, M.; Psaro, R.; Ravasio, N.; Zaccheria, F. Some Insight on the Structure/Activity Relationship of Metal Nanoparticles in Cu/SiO2 Catalysts. Chin. J. Catal. 2019, 40, 1788–1794. [Google Scholar] [CrossRef]
- Zaccheria, F.; Santoro, F.; Psaro, R.; Ravasio, N. CuO/SiO2: A Simple and Efficient Solid Acid Catalyst for Epoxide Ring Opening. Green Chem. 2011, 13, 545–548. [Google Scholar] [CrossRef]
- Zaccheria, F.; Shaikh, N.I.; Scotti, N.; Psaro, R.; Ravasio, N. New Concepts in Solid Acid Catalysis: Some Opportunities Offered by Dispersed Copper Oxide. Top. Catal. 2014, 57, 1085–1093. [Google Scholar] [CrossRef]
- Scotti, N.; Zaccheria, F.; Evangelisti, C.; Psaro, R.; Ravasio, N. Dehydrogenative Coupling Promoted by Copper Catalysts: A Way to Optimise and Upgrade Bio-Alcohols. Catal. Sci. Technol. 2017, 7, 1386–1393. [Google Scholar] [CrossRef]
- Scotti, N.; Ravasio, N.; Zaccheria, F.; Psaro, R.; Evangelisti, C. Epoxidation of Alkenes through Oxygen Activation over a Bifunctional CuO/Al2O3 Catalyst. Chem. Commun. 2013, 49, 1957–1959. [Google Scholar] [CrossRef] [PubMed]
- Galarneau, A.; Desplantier, D.; Dutartre, R.; Di Renzo, F. Micelle-Templated Silicates as a Test Bed for Methods of Mesopore Size Evaluation. Microporous Mesoporous Mater. 1999, 27, 297–308. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marelli, M.; Zaccheria, F.; Ravasio, N.; Pitzalis, E.; Didi, Y.; Galarneau, A.; Scotti, N.; Evangelisti, C. Copper Oxide Nanoparticles over Hierarchical Silica Monoliths for Continuous-Flow Selective Alcoholysis of Styrene Oxide. Catalysts 2023, 13, 341. https://doi.org/10.3390/catal13020341
Marelli M, Zaccheria F, Ravasio N, Pitzalis E, Didi Y, Galarneau A, Scotti N, Evangelisti C. Copper Oxide Nanoparticles over Hierarchical Silica Monoliths for Continuous-Flow Selective Alcoholysis of Styrene Oxide. Catalysts. 2023; 13(2):341. https://doi.org/10.3390/catal13020341
Chicago/Turabian StyleMarelli, Marcello, Federica Zaccheria, Nicoletta Ravasio, Emanuela Pitzalis, Youcef Didi, Anne Galarneau, Nicola Scotti, and Claudio Evangelisti. 2023. "Copper Oxide Nanoparticles over Hierarchical Silica Monoliths for Continuous-Flow Selective Alcoholysis of Styrene Oxide" Catalysts 13, no. 2: 341. https://doi.org/10.3390/catal13020341







