Catalytic Low-Temperature Thermolysis of Heavy Oil in the Presence of Fullerene C60 Nanoparticles in Aquatic and N2 Medium
Abstract
:1. Introduction
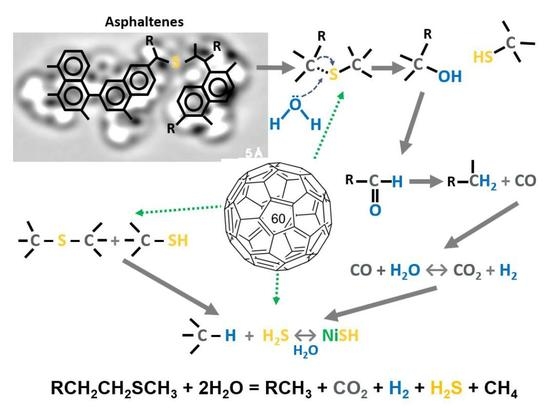
2. Results and Discussions
3. Materials and Methods
3.1. Experiments in Autoclave
3.2. SARA Analysis
3.3. Total Sulfur Analysis
3.4. Viscosity Measurements
3.5. Atmospheric Distillation of Crude Oil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maity, S.; Ancheyta, J.; Marroqun, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Babadagli, T. Viscosity reduction of heavy oil/bitumen using micro and nano metal Particles during aqueous and non-aqueous thermal applications. J. Petrol. Sci. Eng. 2014, 119, 210–220. [Google Scholar] [CrossRef]
- Petrukhina, N.N.; Kayukova, G.P.; Romanov, G.V.; Tumanyan, B.P.; Foss, L.E.; Kosachev, I.P.; Musin, R.Z.; Ramazanova, A.I.; Vakhin, A.V. Conversion processes for high-viscosity heavy crude oil in catalytic and noncatalytic aquathermolysis. Chem. Technol. Fuels Oils 2014, 50, 315–326. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Mukhamatdinov, I.I.; Shmeleva, E.I.; Aliev, F.A.; Vakhin, A.V. Influence of nanosized iron oxides (II, III) on conversion of biodegradated oil. Pet. Sci. Technol. 2019, 37, 971–976. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; He, J.; Li, P.; Yang, C. Mechanism of catalytic aquathermolysis: Influences on heavy oil by two types of efficient catalytic ions: Fe3+ and Mo6+. Energy Fuels 2010, 24, 1502–1510. [Google Scholar] [CrossRef]
- Chao, K.; Chen, Y.; Li, J.; Zhang, X.; Dong, B. Upgrading and visbreaking of super-heavy oil by catalytic aquathermolysis with aromatic sulfonic copper. Fuel Process. Technol. 2012, 104, 174–180. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Zhang, L.; Deng, W.; Que, G. Phase Separation and Colloidal Stability Change of Karamay Residue Oil during Thermal Reaction. Energy Fuels 2009, 23, 3002–3007. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Musin, R.Z.; Nasyrova, Z.R.; Aliev, F.A.; Vakhin, A.V. Hydrothermal Impact on Hydrocarbon Generation from Low-Permeable Domanic Sedimentary Rocks with Different Lithofacies. Energy Fuels 2021, 35, 11223–11238. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Ismael, M.; Aliev, F.A.; Davletshin, R.R.; Vakhin, A.V. Influence of Naphthenic Hydrocarbons and Polar Solvents on the Composition and Structure of Heavy-Oil Aquathermolysis Products. Ind. Eng. Chem. Res. 2021, 60, 13191–13203. [Google Scholar] [CrossRef]
- Aliev, F.A.; Akhunov, A.; Mirzaev, O.; Vakhin, A.V. Development of New Amphiphilic Catalytic Steam Additives for Hydrothermal Enhanced Oil Recovery Techniques. Catalysts 2022, 12, 921. [Google Scholar] [CrossRef]
- Mukhamed’yarova, A.N.; Gareev, B.I.; Nurgaliev, D.K.; Aliev, F.A.; Vakhin, A.V. A review on the role of amorphous aluminum compounds in catalysis: Avenues of investigation and potential application in petrochemistry and oil refining. Processes 2021, 9, 1811. [Google Scholar] [CrossRef]
- Clark, P.D.; Clarke, R.A.; Hyne, J.B.; Lesage, K.L. Studies on the effect of metal species on oil sands undergoing steam treatments. AOSTRA J. Res. 1990, 6, 53−64. [Google Scholar]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Extra-heavy oil aquathermolysis using nickel-based catalyst: Some aspects of in-situ transformation of catalyst precursor. Catalysts 2021, 11, 189. [Google Scholar] [CrossRef]
- Shen, Z.; Huang, Z.; Li, Y.; Gu, X.; Li, B.; Qu, C.; Song, H. MeOH Enhanced Aquathermolysis of Heavy Oil Catalyzed by Hydroxamic Acid-Ni (II) Complex at Low Temperature. J. Chem. Soc. Pak. 2018, 40, 487–494. [Google Scholar]
- Vakhin, A.V.; Mukhamatdinov, I.I.; Aliev, F.A.; Feoktistov, D.F.; Sitnov, S.A.; Gafurov, M.R.; Minkhanov, I.F.; Varfolomeev, M.A.; Nurgaliev, D.K.; Simakov, I.O.; et al. Industrial Application of Nickel Tallate Catalyst During Cyclic Steam Stimulation in Boca De Jaruco Reservoir. In Proceedings of the SPE Russian Petroleum Technology Conference, Virtual, 12 October 2021. [Google Scholar]
- Fu, H.; Xu, T.; Zhu, S.; Zhu, Y. Photocorrosion inhibition and enhancement of photocatalytic activity for ZnO via hybridization with C60. Environ. Sci. Technol. 2008, 42, 8064–8069. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, Y.; Park, S.-J. Recent advances in carbonaceous photocatalysts with enhanced photocatalytic performances: A mini review. Materials 2019, 12, 1916. [Google Scholar] [CrossRef]
- Quo, Y.; Karasawa, N.; Goddard, W.A., III. Prediction of fullerene packing in C60 and C70 crystals. Nature 1991, 351, 464–467. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Xu, Y. Recent progress on graphene-based photocatalysts: Current status and future perspectives. Nanoscale 2012, 4, 5792–5813. [Google Scholar] [CrossRef]
- Sepahvand, S.; Farhadi, S. Fullerene-modified magnetic silver phosphate (Ag3PO4/Fe3O4/C60) nanocomposites: Hydrothermal synthesis, characterization and study of photocatalytic, catalytic and antibacterial activities. RSC Adv. 2018, 8, 10124–10140. [Google Scholar] [CrossRef]
- Ito, O.; D’souza, F. Recent advances in photoinduced electron transfer processes of fullerene-based molecular assemblies and nanocomposites. Molecules 2012, 17, 5816–5835. [Google Scholar] [CrossRef] [PubMed]
- Imahori, H.; Sakata, Y. Donor-linked fullerenes: Photoinduced electron transfer and its potential application. Adv. Mater. 1997, 9, 537–546. [Google Scholar] [CrossRef]
- Toshifumi, K.; Mamoru, F.; Osamu, I.; Yasumasa, T.; Usui, Y. C60 as photosensitizing electron-transfer mediator for ion-pair charge-transfer complexes between borate anions and methyl viologen dication. J. Phys. Chem. A 1999, 103, 9938–9942. [Google Scholar]
- Deguchi, S.; Alargova, R.G.; Tsujii, K. Stable dispersions of fullerenes, C60 and C70, in water. Preparation and characterization. Langmuir 2001, 17, 6013–6017. [Google Scholar] [CrossRef]
- Zhong, Y.; Munir, R.; Balawi, A.H.; Sheikh, A.D.; Yu, L.; Tang, M.; Hu, H.; Laquai, F.; Amassian, A. Mesostructured fullerene electrodes for highly efficient n-i-p perovskitesolar cells. ACS Energy Lett. 2016, 1, 1049–1056. [Google Scholar] [CrossRef]
- Bai, W.; Krishna, V.; Wang, J.; Moudgil, B.; Koopman, B. Enhancement of nano titanium dioxide photocatalysis in transparent coatings by polyhydroxy fullerene. Appl. Catal. B Environ. 2012, 125, 128–135. [Google Scholar] [CrossRef]
- Djordjevic, A.; Sojic Merkulov, D.; Lazarevic, M.; Borisev, I.; Medic, I.; Pavlovic, V.; Miljevic, B.; Abramovic, B. Enhancement of nano titanium dioxide coatings by fullerene and polyhydroxy fullerene in the photocatalytic degradation of the herbicide mesotrione. Chemosphere 2018, 196, 145–152. [Google Scholar] [CrossRef]
- Abe, T.; Taira, N.; Tanno, Y.; Kikuchi, Y.; Nagai, K. Decomposition of hydrazine by an organic fullerene-phthalocyanine p-n bilayer photocatalysis system over the entire visible-light region. Chem. Commun. 2014, 50, 1950–1952. [Google Scholar] [CrossRef]
- Arunachalam, P.; Zhang, S.; Abe, T.; Komura, M.; Iyoda, T.; Nagai, K. Weak visible light (~mW/cm2) organophotocatalysis for mineralization of amine, thiol and aldehyde by biphasic cobalt phthalocyanine/fullerene nanocomposites prepared by wet process. Appl. Catal. B Environ. 2016, 193, 240–247. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, Z.; Ao, Y.; Li, B.; Chen, L.; Shen, L.; Feng, X.; Yang, Y.; Yuan, H.; Mi, Y. Demulsification of water-in-crude oil emulsion driven by a carbonaceous demulsifier from natural rice husks. Chemosphere 2022, 288 Pt 3, 132656. [Google Scholar] [CrossRef]
- Ma, L.; Slaný, M.; Guo, R.; Du, W.; Li, Y.; Chen, G. Study on synergistic catalysis of ex-situ catalyst and in-situ clay in aquathermolysis of water-heavy oil-ethanol at low temperature. Chem. Eng. 2023, 453 Pt 2, 139872. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Q.; Zhou, Y.; Zheng, L.; Jin, H.; Bawaa, B.; Guo, L. Kinetic study of asphaltenes phase separation in supercritical water upgrading of heavy oil. Fuel Process. Technol. 2023, 241, 107588. [Google Scholar] [CrossRef]
- Wang, C.; Gao, L.; Liu, M.; Xia, S.; Han, Y. Viscosity reduction mechanism of functionalized silica nanoparticles in heavy oil-water system. Fuel Process. Technol. 2022, 237, 107454. [Google Scholar] [CrossRef]
- Guo, R.; Fu, W.; Qu, L.; Li, Y.; Yuan, W.; Chen, G. Methanol-Enhanced Fe(III) Oleate-Catalyzed Aquathermolysis of Heavy Oil. Processes 2022, 10, 1956. [Google Scholar] [CrossRef]
- Wang, J.; Tang, X.; Li, J.; Dai, L.; Deng, G.; Zhang, Z. Sawdust biomass promotes aquathermolysis of extra-heavy oil. FuelProcess. Technol. 2022, 238, 107522. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Li, Y.; Dong, S.; Peng, S.; Chen, G. Viscosity Reduction and Mechanism of Aquathermolysis of Heavy Oil Co-Catalyzed by Bentonite and Transition Metal Complexes. Catalysts 2022, 12, 1383. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, Q.; Chen, L.; Guo, L.; Miao, Y.; Wang, Y.; Jin, H. Experimental investigation of enhanced oil recovery and in-situ upgrading of heavy oil via CO2- and N2-assisted supercritical water flooding. Chem. Eng. Sci. 2023, 268, 118378. [Google Scholar] [CrossRef]
- Tongcheng, S. Insights into the Structure–Performance Relationship and Viscosity Reduction Performance of Recyclable Magnetic Fe/Zeolite for Crude Oil Aquathermolysis. ACS Omega 2022, 7, 40267–40274. [Google Scholar]
- Xiong, P.; Yang, H.; Wu, P.; Liao, Y.; Tan, D.; Ma, Z.; Yan, X. Study on catalytic aquathermolysis of heavy oil by simple synthesis of highly dispersed nickel-loaded nitrogen-doped carbon catalysts. Mol. Catal. 2022, 529, 112528. [Google Scholar] [CrossRef]
- Abdelsalam, Y.I.I.; Khamidullin, R.F.; Karalin, E.A. Catalytic thermolysis as a method of upgrading heavy and high-viscosity oil. Bull. Technol. Univ. 2021, 24, 39–42. [Google Scholar]






| Samples | i.b.p., °C | Fraction (i.b.p-200 °C), %wt. | Fraction 200–300 °C | Total sulfur, %wt. |
|---|---|---|---|---|
| Initial crude oil | 170 | 1.02 | 12.07 | 4.72 |
| Crude oil + N2 | 140 | 1.85 | 14.40 | 4.27 |
| Crude oil + C60 + N2 | 110 | 2.60 | 13.35 | 4.25 |
| Crude oil + H2O (10%) | 142 | 2.15 | 16.40 | 4.28 |
| Crude oil + H2O (20%) | 130 | 2.50 | 16.91 | 4.22 |
| Crude oil + C60 + H2O (20%) | 98 | 4.1 | 13.01 | 4.25 |
| Characteristics | Values |
|---|---|
| Dynamic viscosity at 20 °C, mPa.s | 3110 |
| Density at 20 °C, kg/m3 | 970 |
| Total sulfur content, wt.% | 4.72 |
| Resins, wt.% | 26.2 |
| Asphaltenes, wt.% | 6.1 |
| Initial boiling point, °C | 175 |
| Yield of fractions, wt.%: | |
| Up to 200 °C | 1.0 |
| Up to 300 °C | 12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsalam, Y.I.I.; Aliev, F.A.; Khamidullin, R.F.; Dengaev, A.V.; Katnov, V.E.; Vakhin, A.V. Catalytic Low-Temperature Thermolysis of Heavy Oil in the Presence of Fullerene C60 Nanoparticles in Aquatic and N2 Medium. Catalysts 2023, 13, 347. https://doi.org/10.3390/catal13020347
Abdelsalam YII, Aliev FA, Khamidullin RF, Dengaev AV, Katnov VE, Vakhin AV. Catalytic Low-Temperature Thermolysis of Heavy Oil in the Presence of Fullerene C60 Nanoparticles in Aquatic and N2 Medium. Catalysts. 2023; 13(2):347. https://doi.org/10.3390/catal13020347
Chicago/Turabian StyleAbdelsalam, Yasser I. I., Firdavs A. Aliev, Renat F. Khamidullin, Aleksey V. Dengaev, Vladimir E. Katnov, and Alexey V. Vakhin. 2023. "Catalytic Low-Temperature Thermolysis of Heavy Oil in the Presence of Fullerene C60 Nanoparticles in Aquatic and N2 Medium" Catalysts 13, no. 2: 347. https://doi.org/10.3390/catal13020347
APA StyleAbdelsalam, Y. I. I., Aliev, F. A., Khamidullin, R. F., Dengaev, A. V., Katnov, V. E., & Vakhin, A. V. (2023). Catalytic Low-Temperature Thermolysis of Heavy Oil in the Presence of Fullerene C60 Nanoparticles in Aquatic and N2 Medium. Catalysts, 13(2), 347. https://doi.org/10.3390/catal13020347