Abstract
Fossil fuels are still the main source of energy in today’s society, so emissions of CO2 are inevitable, but when the CO2 level in the atmosphere is too high, many environmental problems will arise, such as the greenhouse effect, among others. Electrocatalytic reduction of CO2 is one of the most important methods that one can use to reduce the amount of CO2 in the atmosphere. This paper reviews bimetallic catalysts prepared on the basis of copper materials, such as Ag, Au, Zn and Ni. The effects of different ratios of metal atoms in the bimetallic catalysts on the selectivity of CO2RR were investigated and the effects of bimetallic catalysts on the CO2RR of different ligands were also analysed. Finally, this paper points out that the real reaction of CO2RR still needs to be studied and analysed, and the effect of the specific reaction environment on selectivity has not been thoroughly studied. This article also describes some of the problems encountered so far.
1. Introduction
Global carbon dioxide emissions are increasing due to the widespread use of fossil fuels in current society. This has brought about a series of environmental challenges, such as the energy crisis and greenhouse effect, which have seriously affected the environmental conditions on which human beings depend [1]. To solve these problems, scientists continue to explore ways to reduce carbon dioxide emissions, among which catalytic reduction of carbon dioxide stands out. Catalytic reduction can not only reduce the concentration of carbon dioxide, but also convert it into other carbon-based fuels with high added value. Due to the relatively stable chemical properties of carbon dioxide and its low reactivity, the common methods for converting carbon dioxide into energy are photocatalysis and electrocatalytic reduction of carbon dioxide.
Photocatalytic reduction is clean. It requires no external energy, instead using solar energy directly to reduce carbon dioxide to usable energy, making the resource available. Since the first report by Inove and Fujishima et al. and up to now there have been many studies on how to improve the reduction efficiency of CO2 [2]. Many catalysts have been used to reduce carbon dioxide, including zinc oxide (ZnO) [3], gallium oxide (Ga2O3) [4], gallium phosphide (GaP) [5], tungsten oxide (WO3) [6], zirconium oxide (ZrO2) [7], zinc sulphide (ZnS) [8], cadmium sulphide (CdS) [8], lead selenide (PbSe) [9], bismuth sulphide (Bi2S3) [10], graphitic carbon nitride (g-C3N4) [11] and titanium dioxide (TiO2) [12]. Recently, Bharath et al. [13] designed a plasma Ag NPs-modified TiO2/RGO photocatalyst (Ag-TiO2/RGO), which has stable structural, optical and photoelectrochemical properties with good selectivity for CH3OH. This work provides a new avenue for the development of stable high-performance photocatalysts for sustainable liquid fuel production.
The electrochemical reduction of carbon dioxide has the advantages of relatively simple operating conditions, easy control of the reaction process, high conversion rates, simple catalytic reduction of raw materials, modularity of the electrocatalytic system, easy scale-up of production and easy industrialisation. However, due to the low solubility of CO2 in water, competition with hydrogen precipitation reactions and high activation energy, difficulties arise. As well as, larger problems arise on the way to industrialisation. For this reason, scientists have been hoping to develop economically viable and sustainable high selective electrocatalytic systems that can be put into practical uses.
Monometallic catalysts have become favourable candidates for catalytic CO2 reduction due to their simple structure and easy operation. After analysing the main reduction products of CO2 over different monometallic catalysts, it is found that the main products are CO over Zn monometallic catalysts [14]; HCOOH over Pb monometallic catalysts [15]; hydrocarbons over Cu monometallic catalysts [16] and H2 over Pt monometallic catalysts [17]. In recent years, the reduction of CO2 to valuable end products such as ethanol and ethylene has become a major area of research. Among all monometallic CO2 electrocatalysts, Cu is the only monometallic that can deeply reduce CO2 to hydrocarbon products. However, since CO intermediate on the surface of the Cu catalyst has the best binding energy and exhibits positive binding energy for H, CO poisoning occurs in the HER process. Although CO adsorption on the surface allows the reaction to continue, it requires a high overpotential. With the large product selectivity of Cu, the ultimate goal of sustainability can be achieved through the modification of Cu catalysts.
This paper focuses on how to use copper-based bimetallic materials to reduce CO2 to a utilisation-worthy end product. In this paper, the research progress of several copper-based bimetallic catalysts (Cu-Ag, Cu-Au, Cu-Zn and Cu-Ni) in recent years is reviewed, and the effects of Cu metal catalysts, Cu metal–modified substrates and Cu cluster–modified substrates on CO2RR are discussed. Finally, the effects of different preparation methods of the same bimetallic material and different ligand environments on the selectivity of the final product are emphatically studied.
2. Electrocatalytic Reduction of CO2
2.1. Transition Metal Catalytic Reduction
The most common CO2 electrocatalysts are transition metals and their related compounds, which have vacant orbitals and active d-electrons, and are thought to strongly facilitate bonding between the metal and CO2 to promote desorption of the final reduction product. The history of electrocatalytic reduction of CO2 dates back to the 1950s. The first systematic analysis of the products of CO2RR on different metals was carried out by Hori et al. in 1985, when it was found that CO2 possessed reduction products on different metal surfaces. For example, the products on the surface of Ag and Au electrodes are mainly CO; on the surface of Cd, In, Sn and Pb electrodes are formate; on the electrode surface of Cu is the deep reduction product methane, while the partial two-carbon product ethylene was also found [18].
Garza et al. [19] investigated possible pathways for the generation of C2 products on the surface of Cu. The difference in C2 product selectivity between the (100) and (111) faces without any chemical or physical modification of Cu was revealed. They hope to determine as much as possible the mechanism of CO2RR on the Cu surface. The free energy of each step was calculated from first principles using density function theory and the model of the double-layer potential at the cathode. The surface of Cu(100) was found because of its high selectivity for C2 products. The constant electrode potential (CEP) model was used in this study. Under this model, Goodpaster et al. [20] found that at high potentials, CO is reduced to CHO, which then reacts with CO to form COCHO. The dimerisation of CO as a first step and subsequent reduction of the C2 product proposed by Calle-Vallejo and Koper [21] is more favourable. The CHO pathway was calculated to be thermodynamically favourable to the CO dimer pathway, and therefore CHO is a common intermediate between ethylene and methane. According to calculations by Goodpaster et al. [20], on Cu surfaces, the kinetic barriers to the CO dimerisation reaction on Cu(100) were found to increase with the increase of voltage. The kinetic barrier to C-C dimer formation by CHO was also found to decrease with the increase of voltage, further suggesting that the ethylene pathway occurs on the Cu(100) surface.
Li et al. [22] calculated that CO dimerisation occurs on Cu(100) because of the square symmetry on its surface. Due to the low coordination number and the rather large atoms of Cu, the preferred geometry of COCO on Cu(100) has C atoms adsorbed at bridge sites and interconnected in cavity positions. Whereas this structure in Cu(111) would allow the CO dimer to adopt the more unstable, shorter-length C-C bond, such a structure is not possible on this surface.
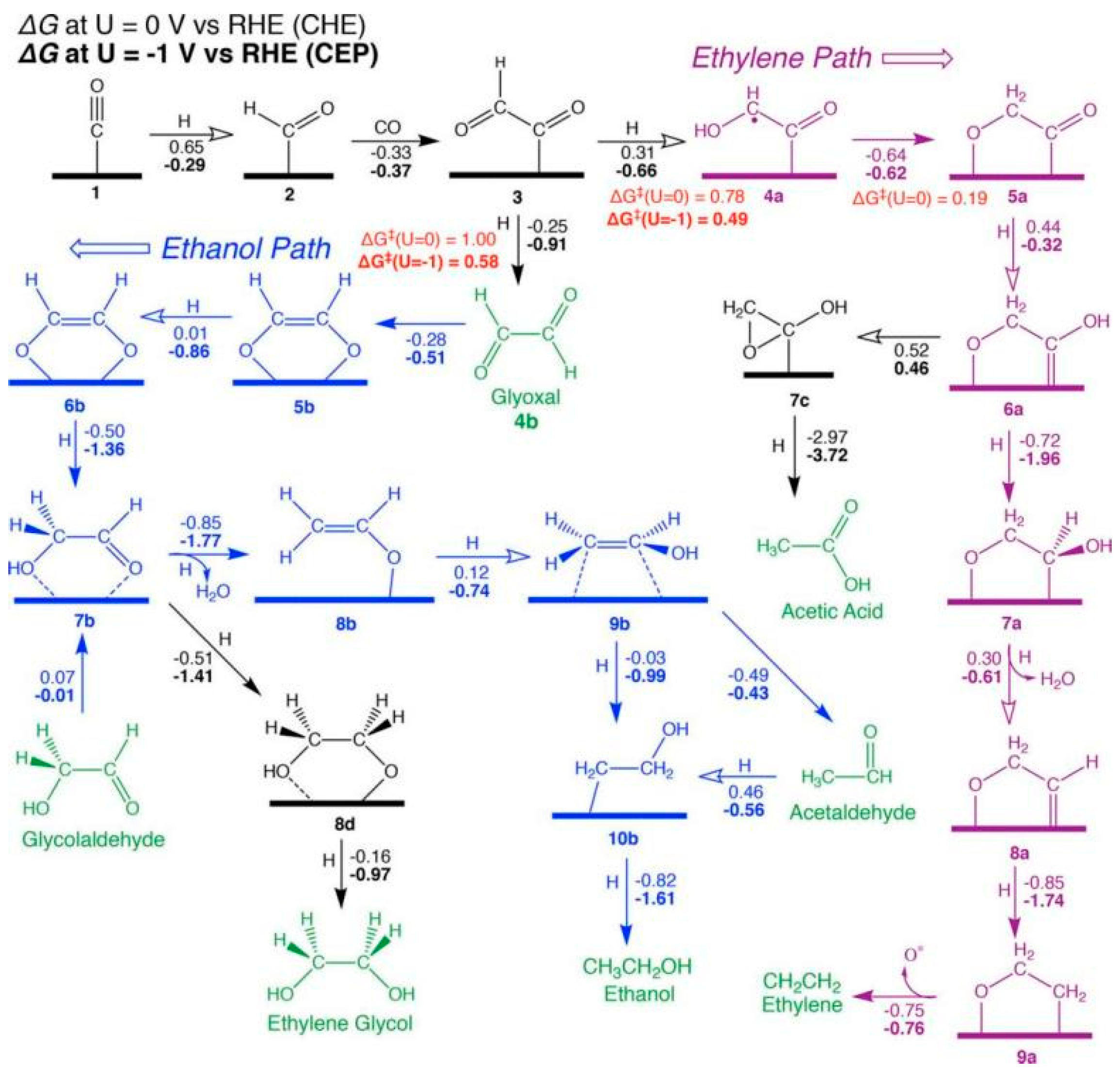
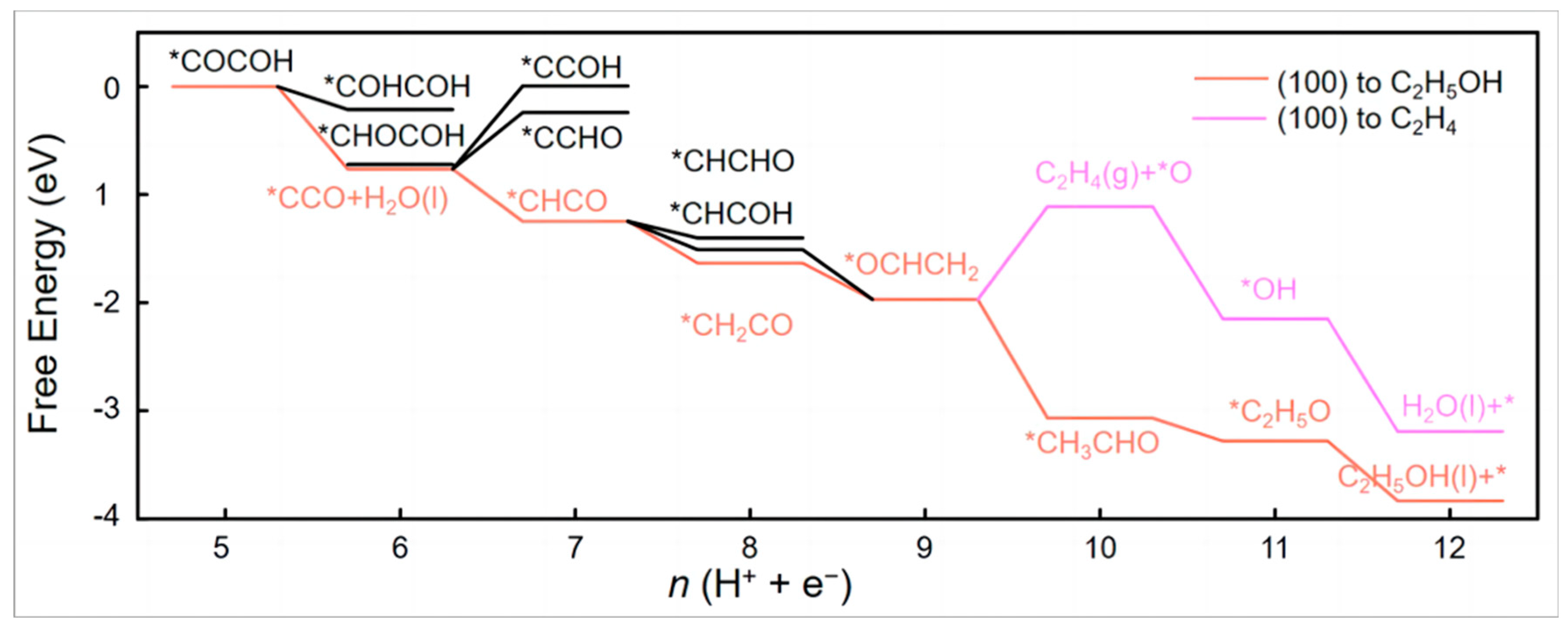
As shown in Figure 1, free energies (always in eV) excluding the effects of applied potential (at 0 V vs. RHE using the CHE) are written next to reaction arrows; those computed at −1 V using the CEP are highlighted in bold. At the same time, the solid and hollow arrows are used to represent exergonic and endergonic reactions, respectively, at 0 V vs. the RHE. The reduction pathway of CO on the Cu(100) surface is discussed and it is concluded that the surface *COCHO is critical in the generation of C2 compounds. It is also a key intermediate in determining the selectivity of the two main C2 products of the reaction, ethylene and ethanol. In Figure 1, the pathway leading to COCHOH eventually leads to ethylene.
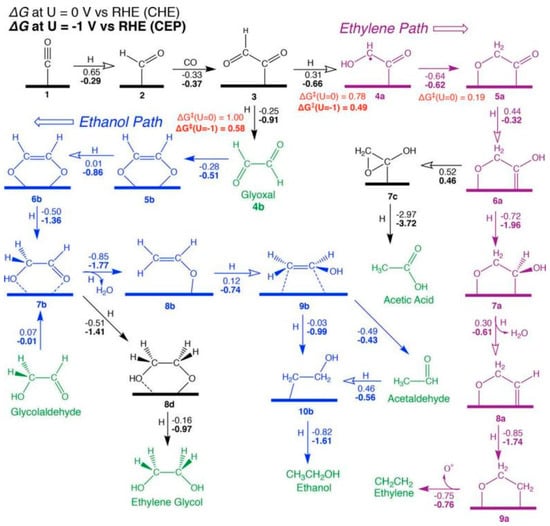
Figure 1.
Proposed mechanism for the reduction of CO to C2 products at high potentials on Cu(100). Calculated free energies (eV) are the numbers parallel to reaction arrows, where ΔG values at U = 0 V using the CHE appear in standard font (steps involving H+ + e− can be corrected to U = −1 V by subtracting 1 eV). ΔG values at U = −1 V using the CEP (at pH = 7) appear in bold font, reproduced with permission from [19]. Copyright American Chemical Society, 2018.
Whereas the pathway for glyoxal produces acetaldehyde at low potentials, it produces ethanol at high potentials. The mechanism is confirmed by experiments, and the calculated potential correctly predicted that the selectivity of ethylene would be higher than that of ethanol. It also mechanistically explains that glyoxal is readily consumed because the post-reaction step is thermodynamically favourable. As no significant amounts of glyoxal were detected, it is believed that most of the glyoxal generated does not leave the surface during the course of the reaction. The calculated adsorption energy of −0.28 eV also supports this assumption. However, as competition from hydrogen precipitation reactions leads to higher overpotentials and lower Faraday efficiencies, other strategies need to be explored to improve the performance of Cu as a catalyst [19].
2.2. Cu Atom Modified Substrate Catalysis
In previous work, copper has been used directly as a catalyst for the electrochemical reduction of CO2. As research into nanoparticles (NPs) has progressed, copper nanocatalysts have shown superior reduction activity and selectivity for CO2 [23]. Studies on Cu-based catalysts have focused on the shape [24], size [25,26,27] and Cu nanoporous catalysts [28,29] of Cu NPs. However, only certain Cu-based catalysts have been found to be sufficient to reduce CO2 to advanced hydrocarbons and oxides. In particular, CuO is prone to deactivation and oxidation at room temperature, severely hindering its progress in electrocatalysis.
The low-dimensional Cu-containing material differs from Cu particles in that it has a large surface area and no mixing surface. Such properties allow for higher conversion frequencies and higher product selectivity. Li et al. [30] investigated the effect of first-row transition metal doping on graphene on the electrocatalytic reduction of CO2. A single vacant graphene with two adjacent vacancies was chosen as the substrate, and then transition metals were used to fill the vacancies. It was found that the Cu atoms filling the corresponding vacancies was the only metal that could possibly convert CO2 to CO, while other metals would further reduce CO. At the same time, embedding Cu metal in graphene substrates not only reduces the overpotential and power, but also improves the selectivity of the product.
Zhu et al. [31] investigate the effect of graphene nanoribbon edges modified with copper on the electrocatalytic reduction of CO2. After comparing armchair GNRs of three different widths (n), graphene nanoribbons in serrated and armchair edges were synthesised by a bottom-up approach. After comparison, it was found that the lowest applied voltage was required for the reaction to occur at n = 5. The handrail edge structure and the Cu-Cu spacing prevented excessive adsorption of *OH, while the structure thermodynamically hindered the formation of formic acid. Therefore, the catalyst was considered to have good performance.
2.3. Cu Cluster–Modified Substrate Catalysis
Since the small clusters as catalysts showed strong reactivity towards water–gas conversion, it was found that such small clusters adsorbed on a carrier had a high concentration of reactive sites and a high activity-to-catalyst-loading ratio. It has been found recently that the synthesis of loaded monometallic atomic catalysts facilitates the reduction reaction of CO2. Passalacqua et al. [32] studied the effect of Cu5 and Cu20 clusters on CO2 and found that Cu5 clusters could effectively coordinate CO2 (hydrogen carbonate) in solution, while Cu20 clusters could reduce the potential required for redox processes. Iyemperumal et al. [33] studied the effect of loading copper clusters on TiO2 on CO2 reduction and found that TiO2-loaded Cu2 clusters is a very active catalyst. It has also been found that it is possible to break the scalar relationship by loading Cu atomic chains onto β-borophene sheets to provide secondary adsorption sites, which can effectively reduce the overpotential for the electrocatalytic reduction of CO2 to methanol [34]. By studying the electrocatalytic reduction of CO2 by copper clusters modified with defective graphene [35], it was found that an inverted triangular shape of Cu3 adsorbed on defective graphene significantly reduces the overpotential for the reduction of CO2 to methane, that the structure is stable and that the side reactions do not hinder the catalytic activity of CO2RR.
3. Cu-Based Bimetallic Electrocatalytic Reduction of CO2
Bimetallic catalysts are considered to be good catalysts for improving the activity, selectivity and stability of CO2RR due to their unique electronic structure, strain effect and geometric effect. Furthermore, the composition, distribution, structure and shape of bimetallic atoms have a strong influence on their catalytic properties. Therefore, a feature of bimetallic catalysts is that the binding strength of the reaction intermediates can be varied to modulate product selectivity and activity [36]. Therefore Cu-based bimetallic catalysts are considered excellent catalysts for the reduction of CO2 to C2 products.
3.1. Cu-Ag
Single-atom alloys provide a way to tune the electronic structure and properties of bimetallic catalysts [37]. A nano-Ag-Cu single-atom alloy catalyst with an atomic ratio of Cu:Ag = 1:110 was prepared by a chemical dealloying process. It was found that it exhibited enhanced selectivity for CO2 reduction and inhibited hydrogen precipitation reactions. The structure has a Faraday efficiency (FE) of 97.5% for CO, as well as a very long-lasting catalytic effect.
Steady-state CO2 consumption rates were found to be lower than the diffusion-limited consumption rates on polycrystalline Ag at bimetallic electrodes alloyed with Cu-Ag surfaces. The FE for H2 on this bimetallic catalyst is significantly lower than that on polycrystalline Cu, but the maximum FE formed on this catalyst for polycarbonate oxygenates is 35%, which is more than twice the total oxygenate FE on pure Cu. Acetic acid and acetaldehyde make up the majority of the polycarbonate oxygenates in all the products, reaching a combined FE of 15%, whereas the FE of these polycarbonate products over pure copper does not even reach 1%. It can be seen that the selectivity of the polycarbon product at Cu-Ag bimetallic electrodes is much greater than that at pure copper. It was also found that the enhanced selectivity of the bimetallic electrode for multicarbon products was due to the inhibition of the hydrogen precipitation reaction rather than the enhancement of CO2RR. It is thought that the precipitation of Ag inhibits the hydrogen precipitation reaction, thereby increasing the coverage of *CO in Cu adsorption [38]. The activity of the Cu phase in the Cu-Ag bimetal is independent of the near-surface composition, with Ag acting as a surface promoter for Cu. Strain effect (incorporation of Ag atoms into the Cu surface results in compressive strain on adjacent Cu atoms, shifting the valence band density of Cu to a deeper level, thereby inhibiting the hydrogen precipitation reaction and allowing enhanced selectivity for multicarbon products.) can cause the surface to evolve, often leading to a deterioration in its activity and durability.
Ishimaru et al. [39] found that the Ag-Cu bulk alloy was selective for C2H5OH compounds under pulsed conditions. Huang et al. [40] investigated Ag-Cu nanodimers (NDs) with different Cu domain sizes (Ag1-Cu0.4, Ag1-Cu1.1 and Ag1-Cu3.2) and found that compressive strain was unlikely to occur in the case of NDs. What is observed in this case is a clean interface, rather than an alloy interface, where very little, if any, diffusion of Cu and Ag atoms into each other takes place.
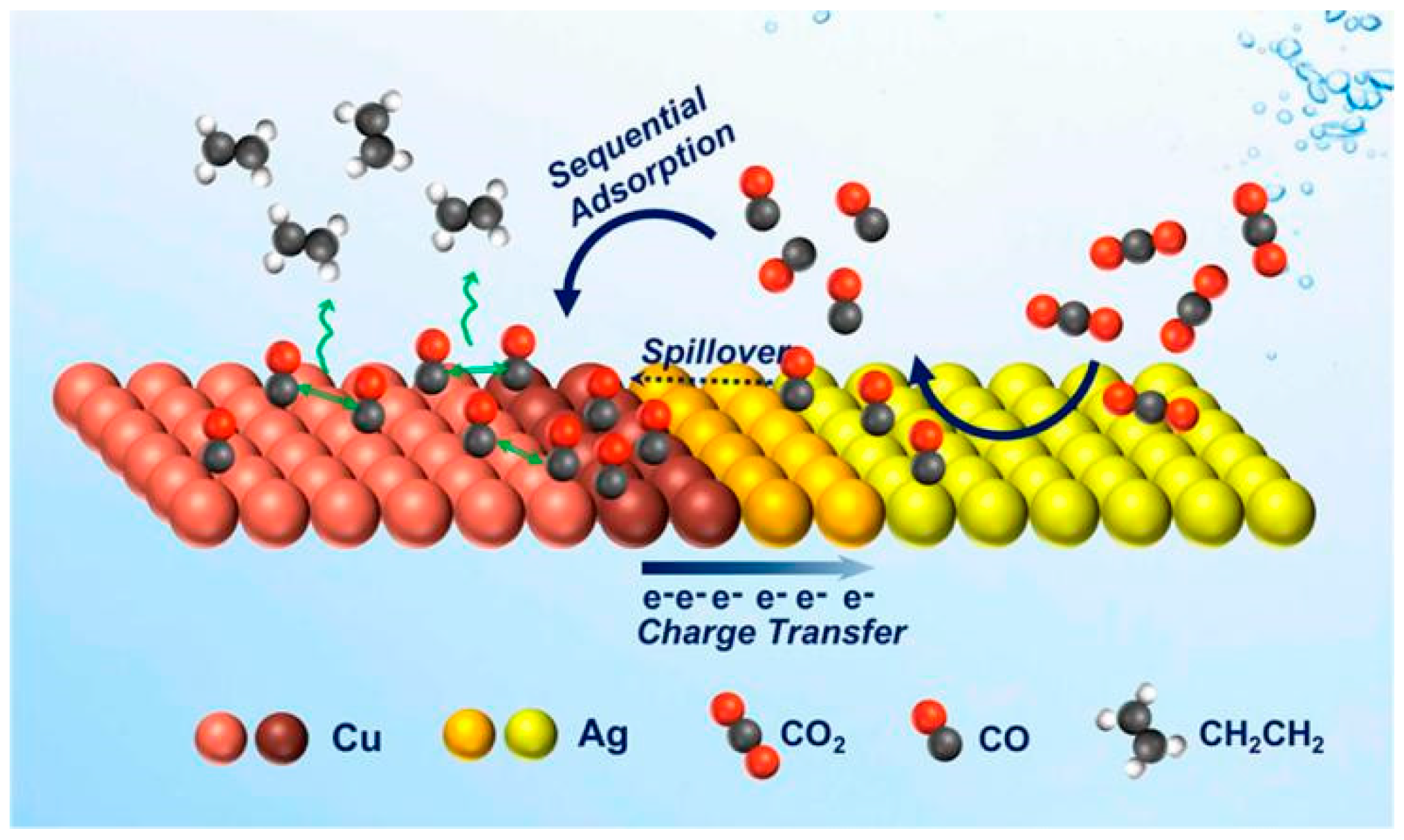
A schematic diagram depicting the modelling of the electrocatalytic activity of Ag-Cu NDs is shown in Figure 2. They synthesise Ag-Cu NDs with tunable domain size by developing a seeded-growth approach through colloidal chemistry. When Ag and Cu are mixed at the nanoscale, sequential catalytic and electronic effects are combined. It is suggested that tandem catalysis and electronic effects are responsible for the enhanced C2H4 selectivity and CO2 reduction activity of Ag-Cu NDs.
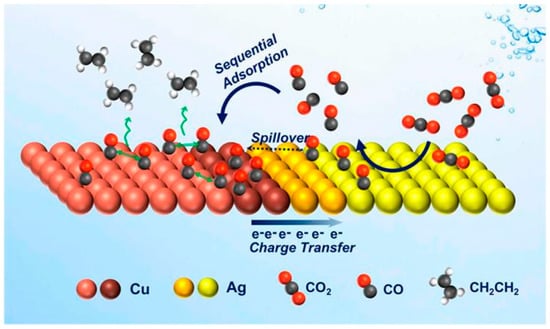
Figure 2.
Schematic representation of the proposed mechanism of C2H4 promotion in the Ag-Cu NDs which couples the tandem catalysis (CO spillover or sequential adsorption), reproduced with permission from [40]. Copyright American Chemical Society, 2019.
Cu-Ag bimetallic nanowire arrays were used as catalysts and were found to exhibit stronger catalytic performance than Cu nanowires (NW) [41], which is thought to be due to the fact that the incorporation of Ag onto the CuNW inhibits the hydrogen precipitation reaction. The structural transformation was shown by using the Ag@Cu core–shell system [42], through geometrical effects that tune and optimise the binding energy of the metal and intermediate, which leads to changes in the activity and selectivity of the CO2RR, resulting in different final products.
Jeon et al. [43] prepared Ag-Cu NPs by current displacement using the surface of Cu NPs prepared by anodic oxidation, which showed a significant increase in CO FE and CO partial current density compared to Cu NPs. It was also found that the FE of CO increased as the coverage of Ag increased. Finally, it was concluded that the Ag coverage of the Cu surface has a decisive influence on the catalytic activity of bimetallic Ag-Cu NPs.
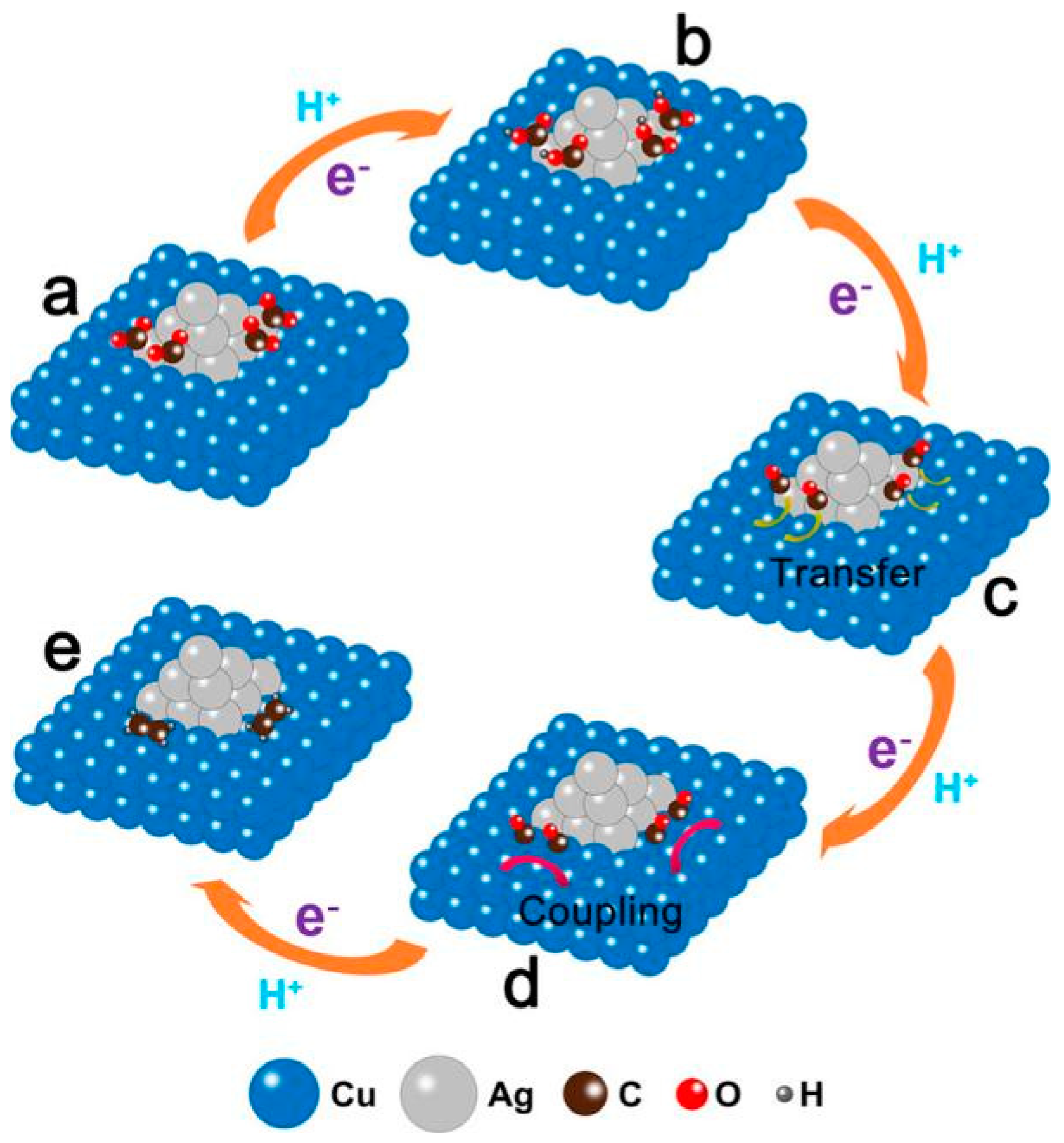
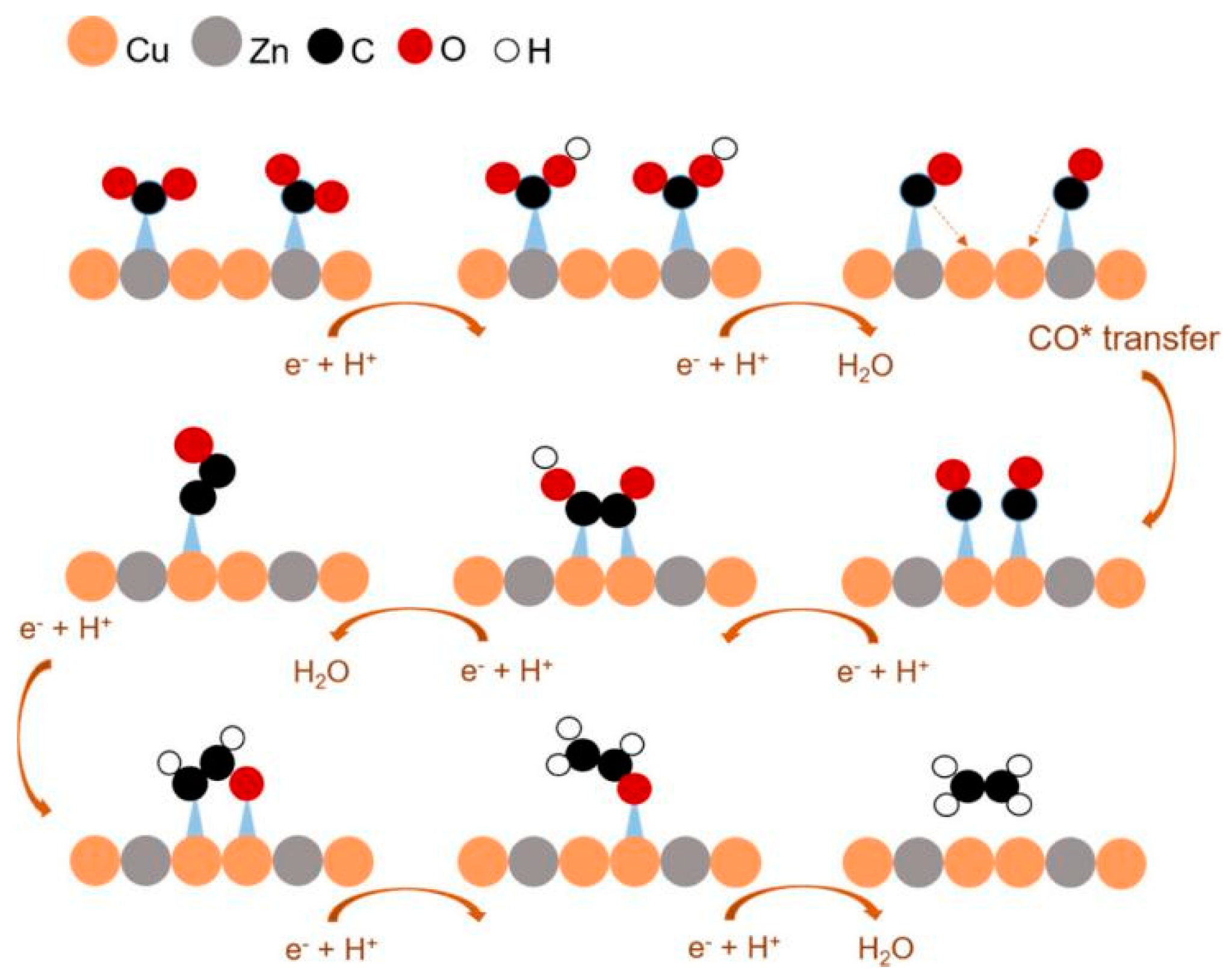
Wang et al. [44] constructed Cu-Ag bimetallic catalysts using continuous precipitation of Ag/Cu2O heterostructures followed by electroreduction, with tiny Ag NPs evenly distributed on the surface of large Cu NPs. By analysing its product composition, this interface was found to show excellent selectivity towards ethylene and remain stable over 30 h. Figure 3 shows the mechanism by which carbon dioxide is reduced to ethylene on this surface. The rate-determining step in the reaction is the *CO dimerisation to form a C-C bond.
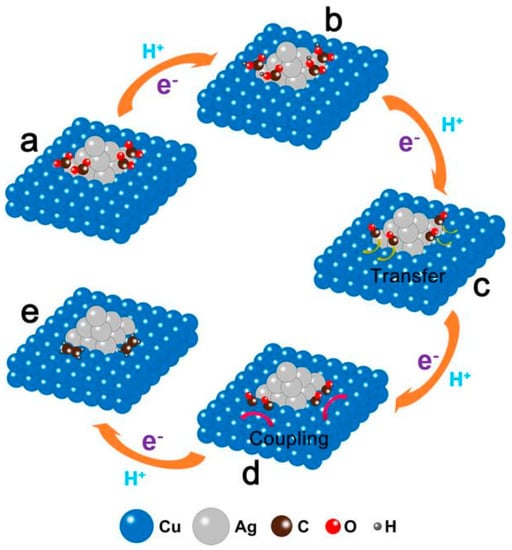
Figure 3.
Proposed mechanism for the electroreduction of CO2 to ethylene at Ag/Cu interface. (a) CO2 molecules adsorbed on Ag nanoparticle. (b) Formation of *COOH intermediate on Ag nanoparticle by transferring a proton and an electron to a CO2 molecule. (c) Formation of *CO intermediate on Ag nanoparticle by transferring an electron and a proton to *COOH. (d) Transfer of CO intermediate from Ag nanoparticle to Cu side. (e) Formation of ethylene by coupling two CO molecules. The orange arrows indicate proton and electron transfer. The yellow arrow indicates the migration of CO molecule from Ag side to Cu side. The pink arrows indicate the coupling two CO molecules, reproduced with permission from [44]. Copyright American Chemical Society, 2019.
Jeon et al. [45] synthesised Cu-Ag bimetallic catalysts with different Cu-Ag ratios by using ultrasonic spray pyrolysis. By comparison, Cu90Ag10 was found to be 1.5 times more selective for C2H4 compared to Cu100. In addition, the Cu90Ag10 exhibited stable CO2RR performances over 46.5 h. The adjacent Ag and Cu on the surface of this catalyst act as storage for *CO and raise additional *CO to the surface, leading to the selective conversion of CO2RR to ethylene. The reason for the enhanced selectivity of this structure of CO2RR is thought to be the synergistic effect of Cu and Ag, as well as the appropriate ratio of Cu and Ag.
3.2. Cu-Au
The rapid oxidation of Cu forms CuO and Cu2O, thereby impairing its catalytic activity. One solution is to alloy Cu and Au to form a bulk Au-Cu alloy, where Au can limit the growth of Cu2O to protect the Cu from oxidation [46]. To investigate the effect of electrode treatment on the surface selectivity and poisoning during the reaction, an alloy of Cu-Au was studied. Pace et al. [47] synthesised a polycrystalline alloy of Cu-Au, but found that oxidation of the Cu-Au alloy led to copper depletion and surface roughening. When comparing different ratios of Cu-Au alloys, it was found that CO2 showed a similar trend on the surface of Au1Cu99 and polycrystalline pure copper, both exhibiting an inhibitory response at potentials above −1.3 V. However, as the content of gold in the alloy increases, the inhibition reaction will gradually weaken, and it is believed that the Au on the surface contributes to the CO2 reduction reaction. The Au50Cu50 alloy is considered to be the most suitable substrate for the conversion of CO2 into carbon-containing compounds [48].
The FE of each product was studied in order to confirm which catalyst was more effective for CO2 reduction [49]. It was found that the Cu-Au bimetallic catalyst particles were not only capable of producing the product produced on the Cu catalyst, but that the FE of their product varied with the ratio of Cu to Au, and the bimetallic nanoparticles exhibited significant antioxidant stability in the ambient atmosphere. As the Cu content increases, so does the total number of products. The highest-yield types are found on pure copper NPs, while FE of methane and ethylene decreases significantly with the increase of Au content and is eventually lost completely at high Au nanoparticle concentrations. It was found that when the proportions of catalyst components varied, the FE of CO and H2 underwent opposite trends, with the FE of H2 increasing with the increase of Cu content, while the opposite was true for CO. By adjusting the composition of Au-Cu bimetallic NPs, the yield of CO can be increased. It is thought that the effect on CO activity at bimetallic surfaces is due to two factors, one being the electronic effects of different surface compositions that can modulate the binding of intermediates, and the other being the geometric effects associated with the local atomic arrangement of the active site. The authors suggest that their product distribution is controlled by intermediates in the reaction pathway, which can be described in terms of electronic and geometric effects, inferring interactions between intermediates and specific catalysts that modify their catalytic activity.
The catalytic activity of certain reactions can be enhanced due to the high surface area of the nanoparticle bilayers. By investigating the oxidation of Cu-Au NPs as a function of composition, Xu et al. [50] found that the oxidation rate of Cu depends on the composition of the NP. They found that improving the stability of Au when increasing its content reduced the overpotential for electrochemical reduction of CO2. Another approach is to confine Au and Cu to Santa Barbara Amorphous-15 (SBA-15) type nanospaces, limiting their growth to large particles under high temperature treatment [51]. Cu-Au alloy NPs such as these are highly active in CO oxidation due to geometrical and electronic effects.
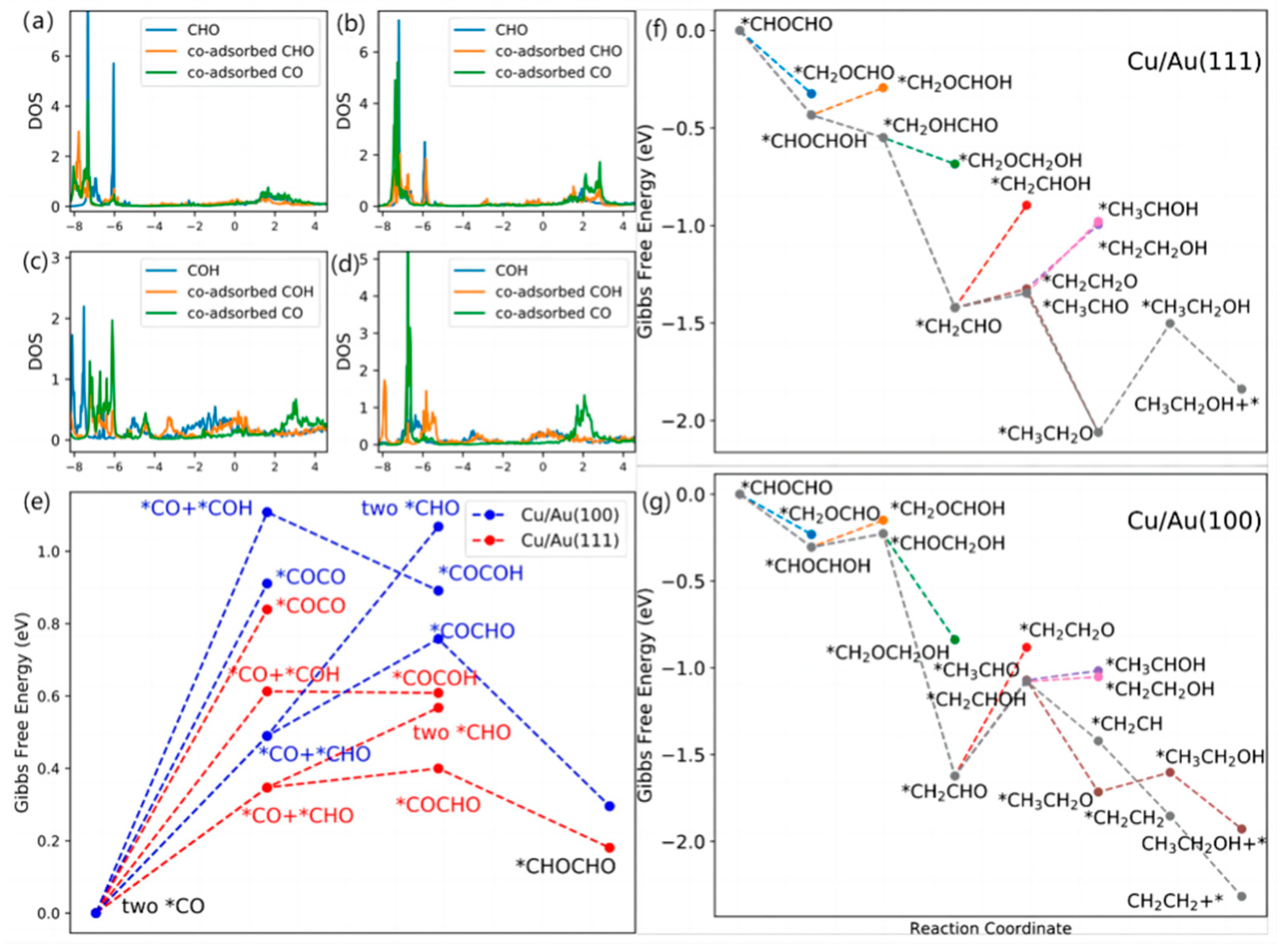
In order to investigate the relationship between surface properties and catalytic performance, different bimetallic catalysts were constructed using sub-monoatomic layers of Cu and different Au surfaces. Zhang et al. [52] chose Cu-Au(111) and Cu-Au(100) bimetallic catalyst systems in order to investigate the variation of CO2RR with the interfacial stress of Cu crystals, and found that the CO2RR was enhanced and the hydrogen precipitation reaction was inhibited on the Cu-Au surface after calculations. By comparing it with pure copper, it was found that the altered morphology contributed to enhanced molecular and surface interactions. As shown in Figure 4a–d, studies of their intermediates have revealed greater orbital overlap between CHO and CO on both surfaces, with strong surface CHO and CO interactions favouring the formation of C-C bonds, which is a key step in the formation of C2 compounds. Meanwhile, the free energy of C-C coupling formed by different intermediates on different surfaces was calculated (as shown in Figure 4e), and it was found that *CO and *CHO had the lowest energy barrier for C-C coupling. It is considered that the coupling of *CO and *CHO to *COCHO is the main way to form C2 compounds. A detailed reaction mechanism diagram of *CHOCHO on a Cu-Au surface is given in Figure 4f–g. The formation of ethanol is the determining step of reaction rate for the Cu-Au(111) bimetallic electrode, and the reduction of *CH2CHO is the determining step of reaction rate for the Cu-Au(100) bimetallic electrode. This result shows the influence of surface stress on the performance of CO2RR.
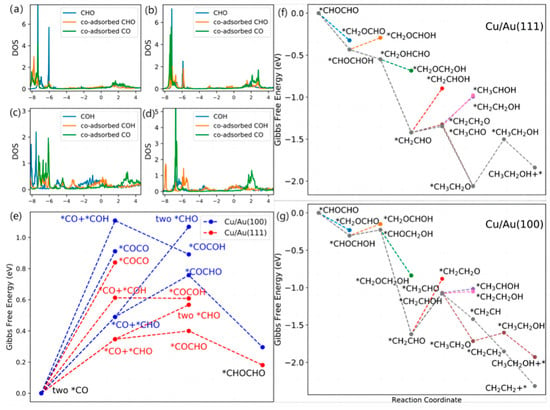
Figure 4.
DOS of *C atoms of adsorption of intermediates on (a,c) Cu/Au(111) and (b,d) Cu/Au(100) and (e) Gibbs free energy diagrams for C-C coupling. The energies are provided relative to two *CO molecules vs. RHE. Gibbs free energy diagrams of the reaction of the reduction of *CHOCHO to alcohol and ethylene on (f) Cu/Au(111) and (g) Cu/Au(100) surfaces. The energies are provided relative to *CHOCHO vs. RHE, reproduced with permission from [52]. Copyright American Chemical Society, 2020.
It was also found that different Cu-Au ratios have different effects on C1/C2 product selectivity. Feng et al. [53] constructed different Cu-Au ratios (3:1, 1:1, 1:3) by selecting the two most exposed crystal surfaces, the (111) and (100) faces of Cu, in order to investigate the mechanism of their influence. They found that bridge sites on the (100) face of the alloy helped to stabilise CO and that the adsorption of *COOH and *CO increased with increasing Au content. The best selectivity for CO is achieved when the ratio of Cu to Au on the (100) side is 1:1. In contrast, the most favourable path for CC coupling is *CO + *COH to generate *COCOH, and the best coupling performance is found for Cu3Au1 in the (100) plane. The highest selectivity was also found for ethanol in the Cu3Au1(100) face and Cu3Au1(111). Figure 5 shows the reaction pathway for the production of the C2 product from Cu3Au1 on the (100) side. *COCOH is used as a precursor for the C2 product.
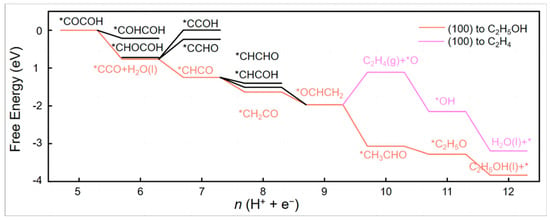
Figure 5.
Mechanism of the C2 product on the surface of the Cu3Au1 alloy. C2 pathway on the Cu3Au1(100) surface, reproduced with permission from [53]. Copyright American Chemical Society, 2022.
The graph shows that *CH3CHO is exothermic on the (100) surface Cu3Au1 while C2H4 is exothermic, which means that C2H5OH is more selective than C2H4 on this alloy. Therefore, ethanol is the main reduction product on this surface.
3.3. Cu-Zn
Cu and Zn are both low-cost and environmentally friendly materials, which is why Cu-Zn bimetallic materials are of interest. Zn is even more abundant and cheaper than Cu, and with the low activity of Zn towards HER [54], it is expected that Cu-Zn bimetallic catalysts will inhibit the production of H2, while being selective for the formation of CO due to the nanoparticulate Zn [14]. In order to improve the selectivity of CO2 reduction to ethanol, Ren et al. [55] prepared Cu-Zn alloys with different ratios (10:1, 4:1, 2:1). A study of the selectivity of ethanol and ethylene on the surface of the alloy showed that Zn and Cu synergistically promoted the formation of ethanol and that the formation of formate was inhibited on the surface. In Cu-Zn bimetallic catalysts, the role of Zn is to increase the concentration of CO on the surface, but too much Zn leads to fewer sites for Cu, resulting in inhibited selectivity for ethanol. Ethanol is highly selective and stable on the surface of the Cu4Zn bimetallic catalyst. Yin et al. [56] constructed a Cu5Zn8 bimetallic catalyst and found that the selectivity of HCOOH on the surface was higher than that of pure Cu and pure Zn catalysts.
Yi Feng et al. [57] prepared homogeneous CuZn bimetallic catalyst nanomaterials (NP) using the pulsed laser ablation in liquid (PLAL) technique. After evaluation it was found that its total current density, as well as FE of C2H4 and CO, did not decrease significantly after 15 h, and the catalyst was considered to be stable. They found the catalyst to have excellent selectivity for ethylene; the CuZn alloys with different contents (1:1, 3:1, 4:1, 5:1, 7:1) were also compared and Cu4Zn was found to have the highest FE for C2H4, and this catalyst was considered to be the best catalyst for producing the C2H4 product. In the Cu4Zn catalyst, the Zn site produces a large amount of CO intermediate, which is subsequently transferred to the Cu site where it forms a C-C dimer, resulting in the formation of C2H4.
This reaction path is shown in Figure 6, where CO2 adsorbs onto Zn and then reduces to *CO, but with the adsorption site shifting to Cu, it then dimerises to form *COCOH and eventually C2H4. It is considered that the homogeneous distribution of Cu and Zn in the CuZn alloy NP prepared using the laser facilitates the adsorption of *CO species at their active sites, thus reducing barriers to C-C dimerisation and promoting the formation of C2 products. Zeng et al. [58] prepared Cu-Zn bimetallic catalysts by a microwave-assisted solvothermal method and designed catalysts with various Cu-Zn atomic ratios (CuZn0.1, CuZn0.25, CuZn0.4, CuZn0.5). All of these catalysts were found to display two different particle types: ZnO NPs and submicron Cu particles. CuZn electrodes possess higher CO selectivity relative to monometallic electrodes, and it is thought that this phenomenon is due to the selective active site of ZnO and the high conductivity of Cu.
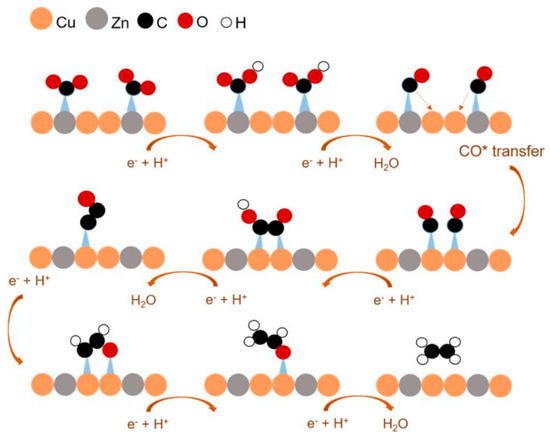
Figure 6.
Mechanism of catalytic CO2 reduction on the surface of metallic CuZn NPs, reproduced with permission from [57]. Copyright American Chemical Society, 2018.
It is necessary to investigate the effect of surface modification of Cu-Zn bimetallic catalysts on CO2RR products, and that annealing treatment can change the surface properties of Cu and Zn metals, which can improve the efficiency, selectivity and stability of CO2RR [59,60]. Therefore Hu et al. [61] prepared in situ grown nanoporous Cu-Zn bimetallic catalysts by annealing copper foils. A significant increase in the FE of both CO and HCOOH was found over this catalyst. In order to investigate the correlation between the basic structure and catalytic performance of bimetallic catalysts, Cu-Zn bimetallic NPs were prepared using an inverse micelle encapsulation method [62]. This study found that Cu90Zn10, Cu70Zn30 and Cu50Zn50 all showed enhanced selectivity for CH4 relative to pure Cu, but when the Zn content was greater than 70%, the selectivity for CH4 was suddenly inhibited, while the selectivity for CO increased. Under CO2RR conditions, the alloying of Cu50Zn50 and Cu30Zn70 nanoatoms gradually changes to resemble bulk CuZn brass, a result that suggests that the ratio of Cu to Zn and their oxidation state are critical to the change in their alloying level after the start of CO2RR. It is suggested that product selectivity in CuZnNP is controlled by ligand effects, leading to increased selectivity of CO.
The reduction of CO2 to high-value chemicals and fuels, particularly C2 products, has been investigated using one-step carbonisation with a metal-organic framework (MOF). Juntrapirom et al. [63] synthesised Cu-Zn catalysts loaded on conductive porous carbon by altering its metal-organic framework thereby change the Cu-Zn ratio. Of all the Cu-Zn catalysts, formate was found to be selectivity on Cu-C, while Zn-C exhibited high selectivity for CO. When forming the Cu90Zn10 and Cu75Zn25 bimetallic catalysts, C-C coupling was facilitated by the close contact of the Cu and Zn atoms for the C2 product. Though, the Cu50Zn50 bimetallic catalyst has excellent CO selectivity, its ability to form C-C coupling is weak due to the large distance between Cu and Zn atoms, which inhibits the formation of C2 products. The analysis of its specific products revealed that Cu75Zn25-C preferentially produces ethanol at lower overpotentials and ethylene at higher potentials, and the total current density and FE of ethylene were found to be relatively stable after 7 h of reaction. The steps for the formation of C-C coupling are also discussed, and Cu3Zn(111) and Cu(111) surfaces are selected for the study of active sites on their surfaces. The energy barrier on the Cu3Zn(111) surface was also found to be only 0.36 eV, which is lower than the 0.49 eV for Cu(100), suggesting a significant increase in selectivity for C2 products on the secondary surface. The intermediate forming the C-C coupling is located in the Cu site, not in the Zn site.
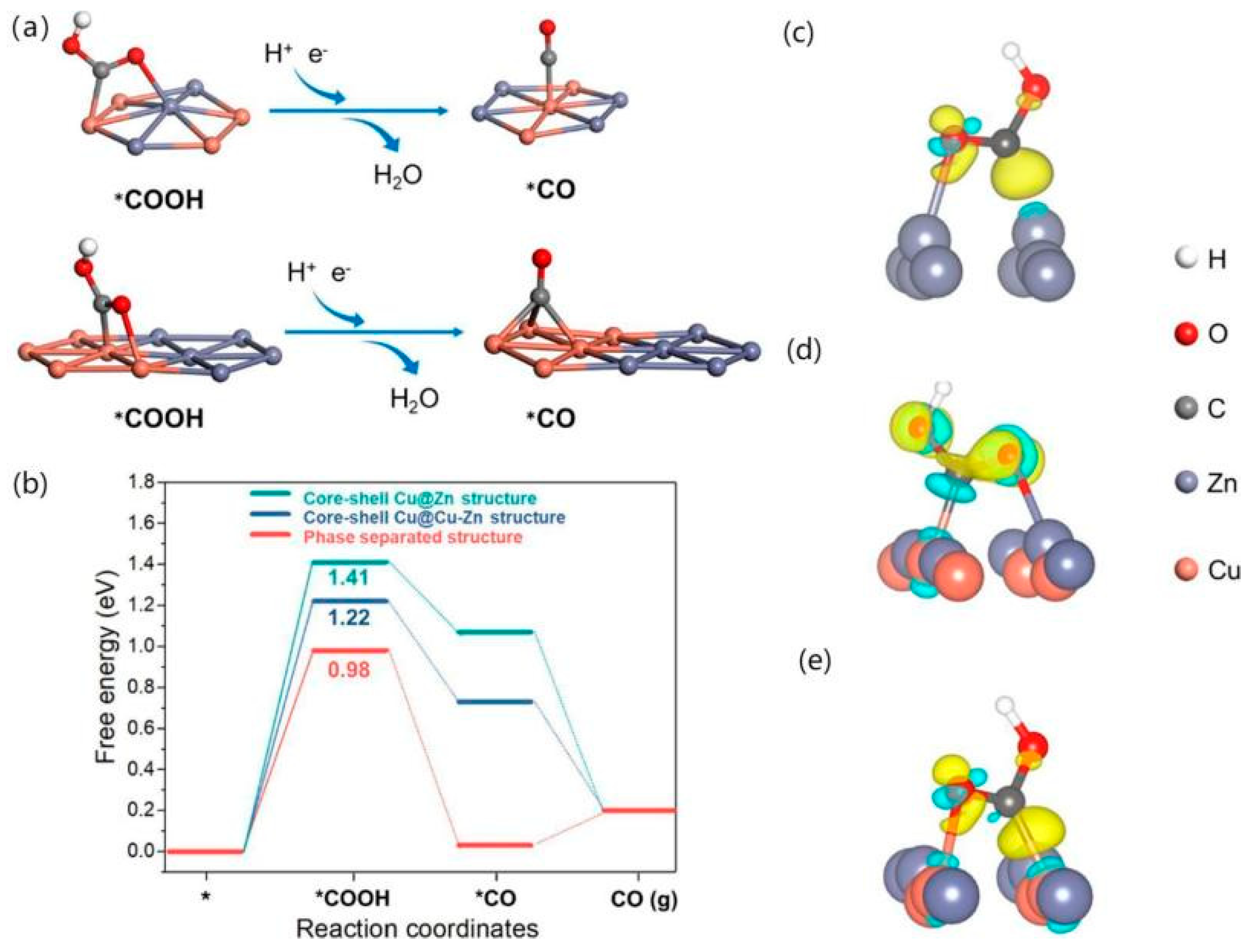
Although the bimetallic effect of Cu-Zn provides a synergistic effect for the reduction of CO2 to CO, the activity of CO2 over Cu-Zn bimetallic catalysts is still unclear. Wan et al. [64] constructed two types of CuOx-ZnO NW structures, one with a core–shell structure and the other with a phase-separated structure, which were electrochemically reduced to Cu-Zn bimetallic NW. The phase-separated sample demonstrated a higher FECO and durability than the core–shell sample. However, no significant redistribution of elements was observed on the phase-separated samples. To investigate the CO2RR mechanism of this structure, a Cu(111) surface was used as the basis for building three structures, namely core–shell Cu@Cu-Zn structures, core–shell Cu@Zn structures, and phase-separated structures. The reaction pathway starting from the COOH intermediate explores the adsorption properties of *COOH and *CO on its surface, as shown in Figure 7a, which exhibits the adsorption sites of *COOH and *CO on different structures. Figure 7b shows that the phase-separation structure has a low energy barrier and that it has a low hydrogen precipitation reactivity. The structure showed no significant elemental redistribution and structural distortion by stability tests (as shown in Figure 7c–e), indicating good stability, and suggesting that its high CO2RR activity is due to its high CO2RR activity between the Cu and Zn interfaces.
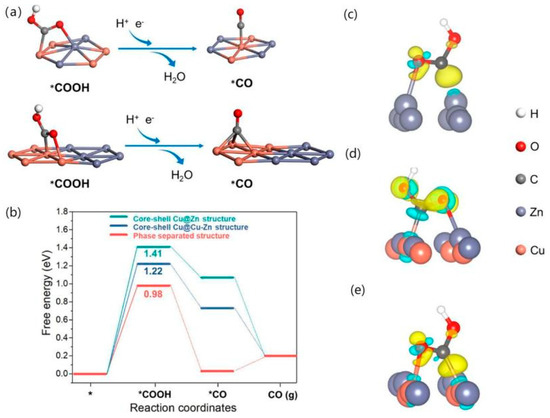
Figure 7.
Theoretical calculations. Simulated probable pathways of *COOH to *CO on the optimised atomistic configuration model of the (a) core–shell Cu@Cu–Zn structure, and phase-separated structure; (b) Calculated free energy diagrams of CO2RR to CO, the charge density difference after *COOH adsorption on the (c) core–shell Cu@Zn structure, (d) core–shell Cu@Cu–Zn structure and (e) phase-separated structure. The yellow and baby-blue colours represent the charge accumulation and depletion areas, respectively. The isosurface value is 0.01 e/Å3, reproduced with permission from [64]. Copyright American Chemical Society, 2022.
3.4. Cu-Ni
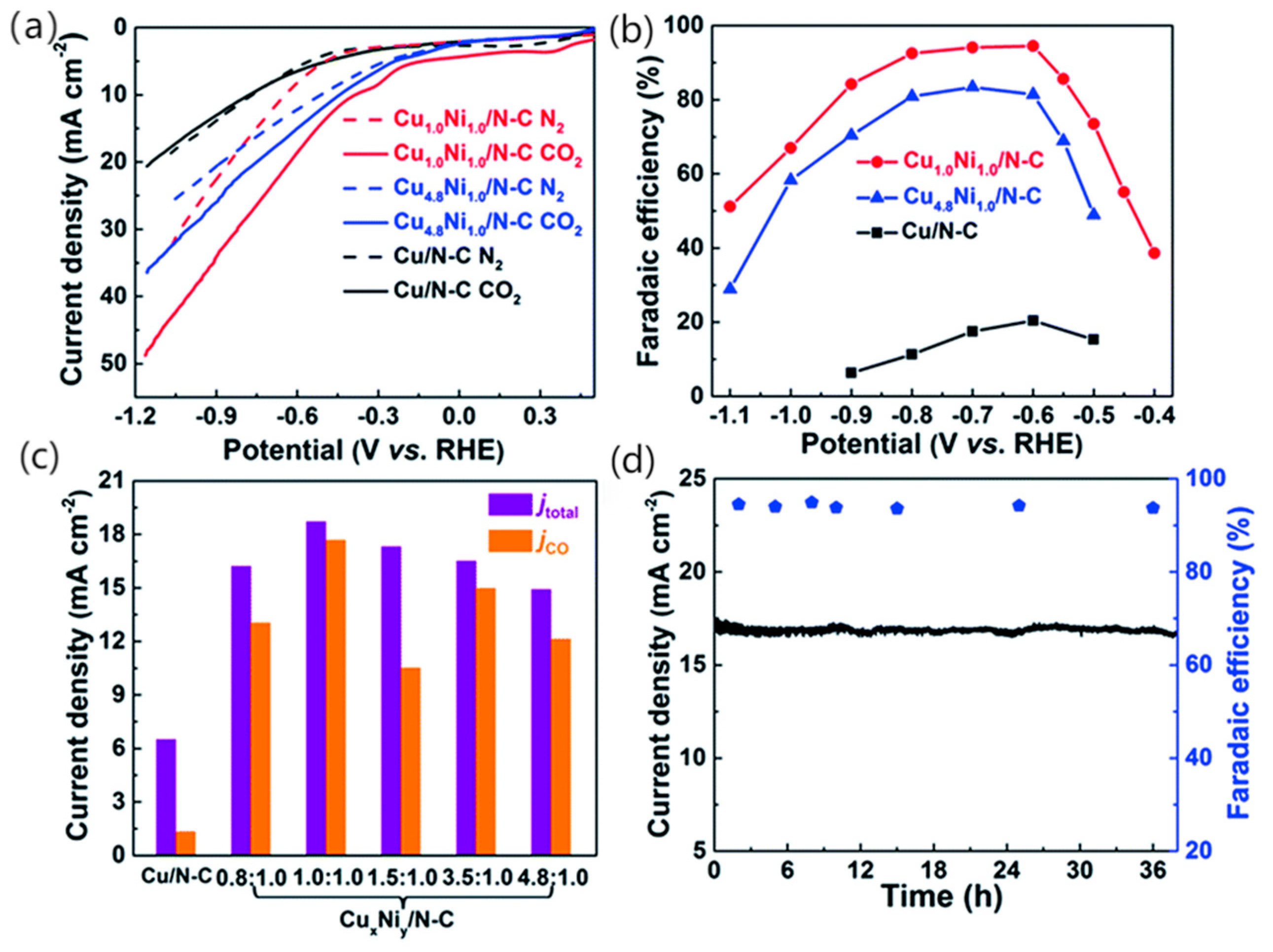
As a metal that can strongly combine with CO, Ni can also be used for CO2RR, but as the binding capacity to CO is too strong and can lead to surface poisoning, it has been proposed that Ni can be alloyed with other metals to form a bimetallic catalyst, thereby destroying the adsorption capacity of CO on the Ni surface. Tan et al. [65] synthesised a Cu-Ni nanoparticle alloy embedded in a three-dimensional nitrogen-carbon network by a hydrothermal method using copper powder and nickel nitrate hexahydrate as precursors to investigated the electrocatalytic activity of this alloy. The CO2 electroreduction activity of CuxNiy/N-C was investigated (shown in Figure 8a) and linear scanning voltammetry measurements were carried out in N2 and CO2 saturated 0.5 M KHCO3, Cu1.0Ni1.0/N-C and Cu4.8Ni1.0/N-C were found to be more favourable for CO2RR. Figure 8b shows the CO Faraday efficiencies at different potentials, and it is considered that Cu1.0Ni1.0/N-C and Cu4.8Ni1.0/N-C are more favourable for CO2RR than for HER. Throughout the electrolysis process, CuxNiy/N-C produced CO and H2 and no liquid, while Cu/N-C produced mainly H2 with small amounts of CO and HCOOH. Cu1.0Ni1.0/N-C has excellent catalytic properties and is thought to combine high CO FE and current density at relatively low potentials, accelerating the reaction rate of CO2 reduction in the presence of an applied high potential. The current density for the reduction of different ratios of bimetallic catalysts to CO at −0.6 V (shown in Figure 8c) was also examined, and the highest catalyst was found to be Cu1.0Ni1.0/N-C. At the same time, the catalytic performance of Cu1.0Ni1.0/N-C is very stable and remains constant under constant potential electrolysis for 38 h (shown in Figure 8d). The CuxNiy/N-C catalyst has excellent performance in reducing CO2 to CO. In particular, the enhanced electrocatalytic activity compared to monometallic catalysts is thought to be due to the more stable *COOH adsorption of the CuNi alloy and its low overpotential. Meanwhile, compared with the Ni surface, the *CO binding energy of the Cu-Ni surface is lower, making it easier to release *CO. This Cu-Ni alloy not only improves the CO2 adsorption capacity, but also its stability during long-term electrode processes.
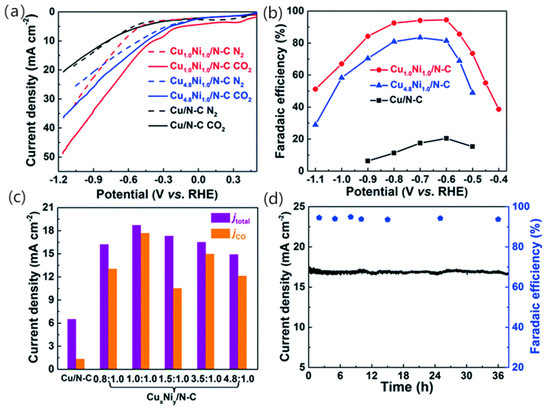
Figure 8.
LSV curves acquired in N2-saturated (dotted line) or CO2-saturated (solid line) 0.5 M KHCO3 solution at a scan rate of 50 mV s−1 (a). CO faradaic efficiency at various potentials (b). Total current density and partial current density of different catalysts at an applied potential of −0.60 V vs. RHE (c). Stability of Cu1.0Ni1.0/N-C at a potential of −0.60 V vs. RHE during 38 h (d). Catalyst loading: 0.5 mg cm−2, reproduced with permission from [65]. Copyright The Royal Society of Chemistry, 2019.
To date, many homogeneous molecular catalysts have been shown to have excellent performance for CO2RR, but most of them require the use of expensive ionic liquid promoters in the organic phase [66,67,68,69], which greatly enhances their reaction costs. Due to the presence of the unit point metal-N4 (M-N4) skeleton and suitable ligands, CO2RR also showed significant activity. Ding et al. [70] embedded Ni-C3N4 molecular scaffolds on carbon nanotubes (CNTs) and used Cu to enhance their catalytic activity, and then probed the CO2 reduction activity by controlled potential electrolysis.
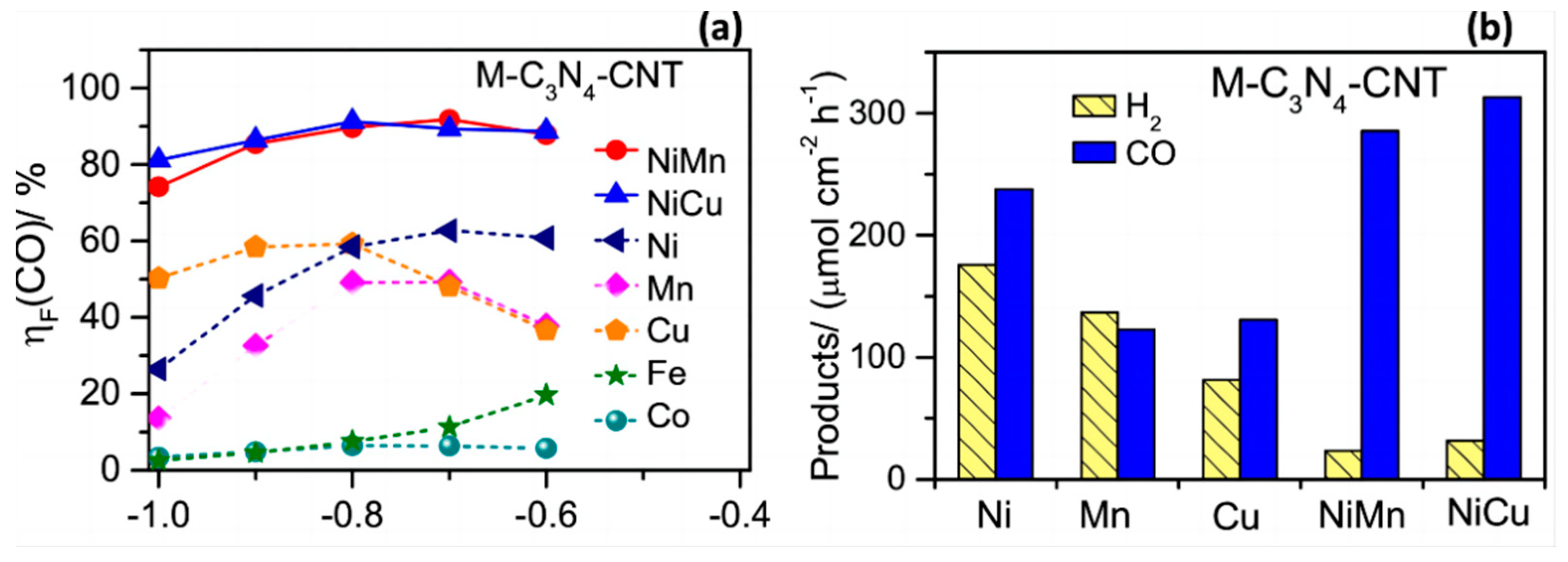
After the CO2 reduction activity of different materials in saturated CO2 electrolyte at different potentials, as shown in Figure 9a, it was found that the FE of CO could reach 80% in the presence of a Ni-Cu bimetallic catalyst and the hydrogen precipitation reaction was inhibited (Figure 9b). Also, the source of CO was found to be the reduction of the solvent molecule CO2 rather than the ionisation of the carbonate.
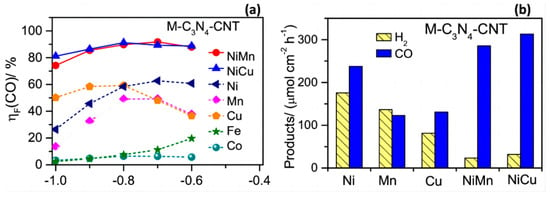
Figure 9.
(a) The Faradic efficiency (ηF) of CO evolution at different potentials. (b) Hydrogen and CO evolution rates at −0.8 V vs. RHE on M-C3N4-CNT (M = Fe, Co, Ni, Mn, Cu, NiMn, and NiCu) catalysts in 0.5 M KHCO3 saturated with CO2, reproduced with permission from [70]. Copyright Elsevier B.V, 2019.
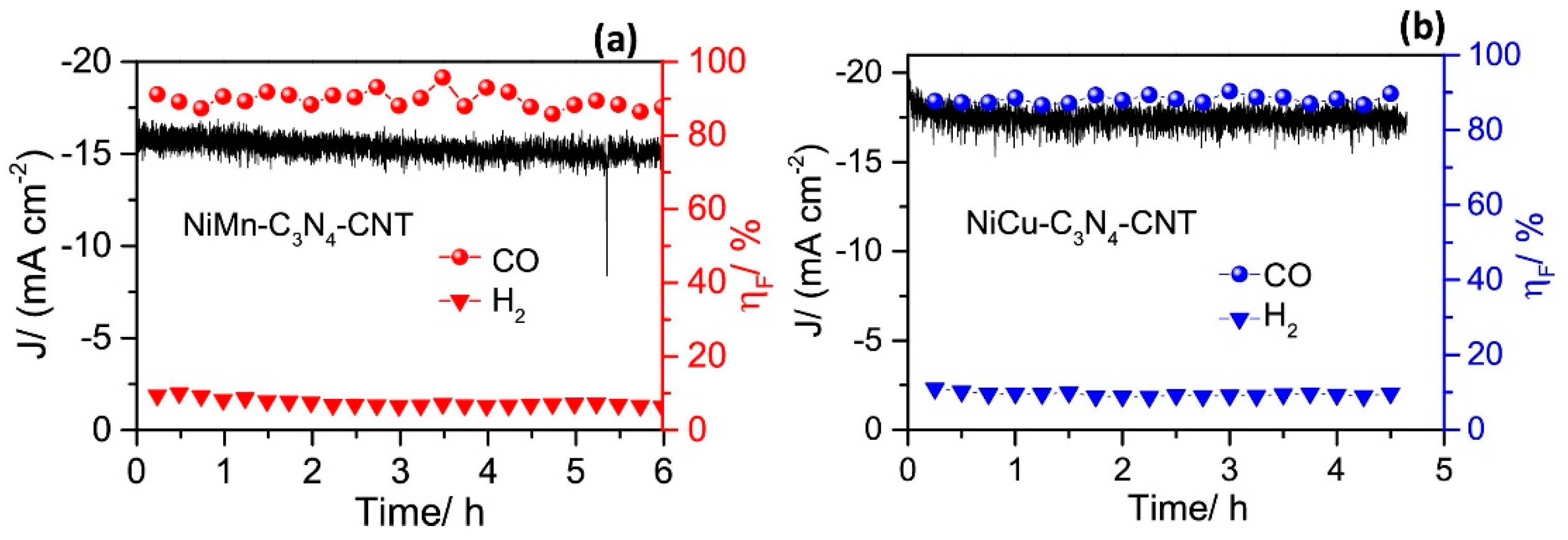
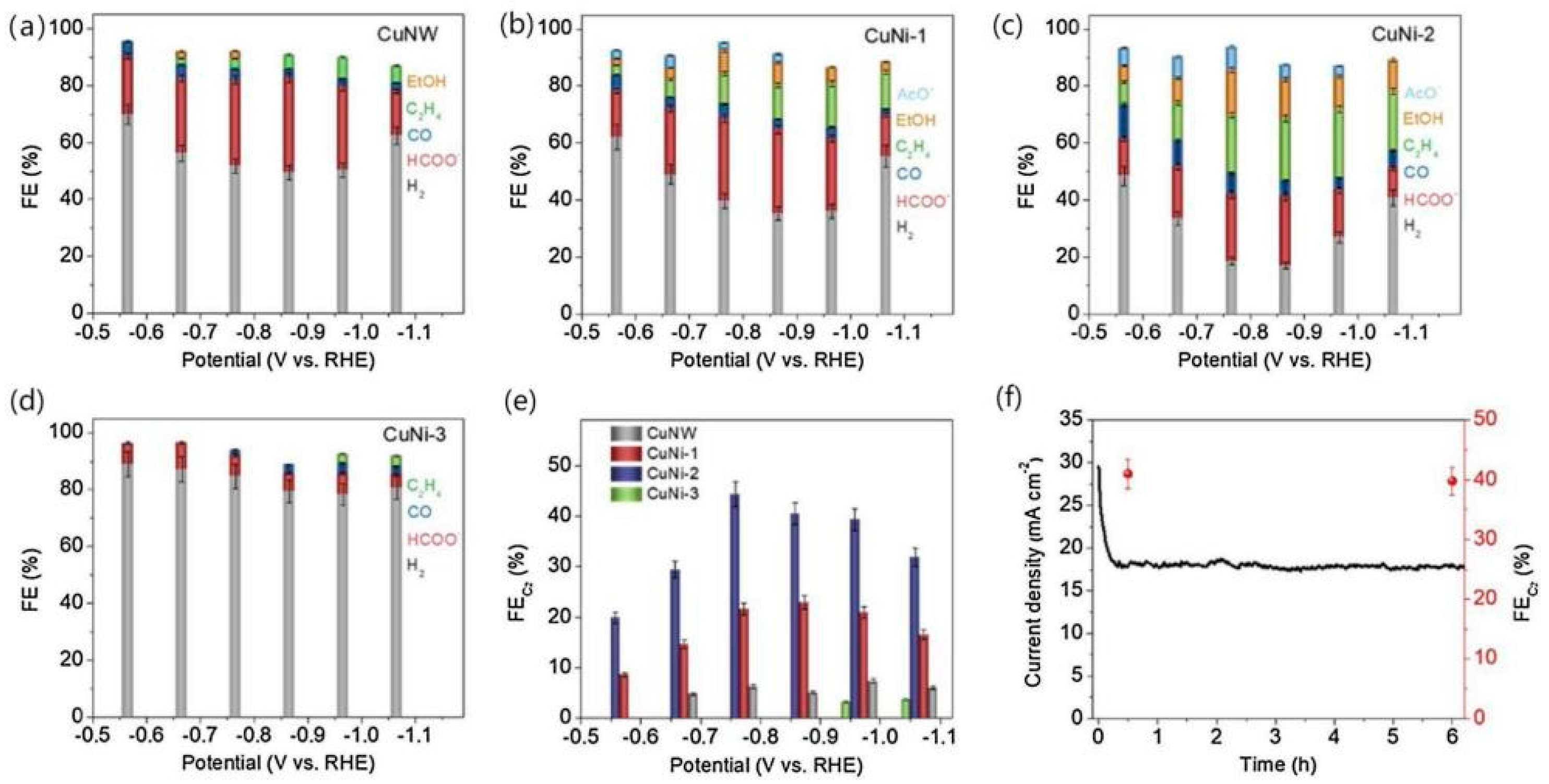
Analysis of its structure revealed that the bimetallic structure of Cu-Ni-Nx is stable. Compared with the single metal centre, this structure greatly improves the binding ability of the CO2 and bimetal structure, suggesting that the synergistic effect of surface bimetal structure is strong. Meanwhile, its stability was tested, as shown in Figure 10, which tells that its stability is good. It is believed that its high activity against CO2 stems from the synergistic effect of the two metal species and the unsaturated coordination of the atomic dispersion between the metal and the C3N4 matrix. In this process, C3N4 not only acts as a scaffold, but also provides a favourable environment for CO2 reduction. In contrast, the Ni surfaces in the above two alloys are subject to segregation, leading to an uneven distribution of Ni, which weakens the advantages of their bimetallic system [71]. Zhang et al. [72] believed that if Ni was highly dispersed on the Cu surface, the C-C coupling could be enhanced and the production of productive products could be promoted. Uniform distribution of Ni clusters were found on the defect-rich CuNW surface using a ligand-activated electrocouple replacement method As shown in Figure 11, CuNi-1 (0.13%), CuNi-2 (0.82%), CuNi-3 (1.3%) and CuNi-4 (1.5%) were divided according to the content of Ni atoms on the surface. The CO2RR activity and product selectivity of CuNW and Ni-CuNW were studied.
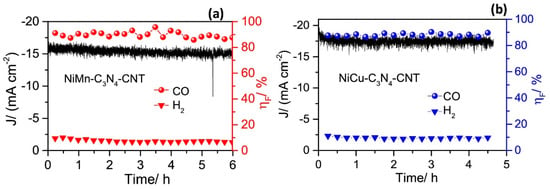
Figure 10.
The amperometric J-t curves, and the ηF for CO and H2 evolution of (a) NiMn-C3N4-CNT, and (b) NiCu-C3N4-CNT catalysts at −0.8 V vs. RHE in 0.5 M KHCO3 saturated with CO2, reproduced with permission from [70]. Copyright Elsevier B.V, 2019.
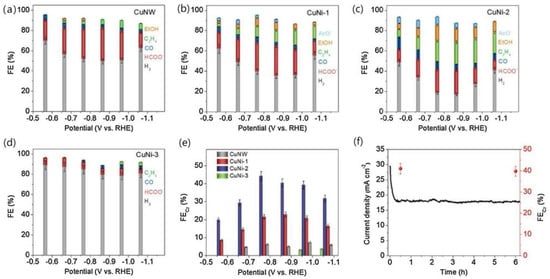
Figure 11.
Bulk electrolysis performed in an H-type cell containing a CO2 saturated 0.50 M NaHCO3 solution. Product faradaic efficiency distributions obtained on (a) CuNW, (b) CuNi-1, (c) CuNi-2 and (d) CuNi-3. (e) Comparison of overall C2+ faradaic efficiency on the CuNW and Ni-CuNW samples. (f) i-t curve (black trace) and overall C2+ faradaic efficiency (red spheres) obtained with CuNi-2 at an applied potential of −0.86 V vs. RHE during a 6-h bulk electrolysis experiment. No IRu correction was applied to the potential values, reproduced with permission from [72]. Copyright Elsevier B.V, 2021.
It was found that for CuNW, at all potentials, the formate was mainly generated with H2. In the case of CuNi-1 and CuNi-2, the hydrogen precipitation reaction was significantly suppressed and CuNi-2, at a more negative potential than −0.67 V vs. RHE, produced a significant amount of C2 product. Therefore, when CuNW is modified with a small amount of Ni, it is more likely to produce C2 products. Further, its content determines the CO2RR performance of the Ni-CuNW catalyst.
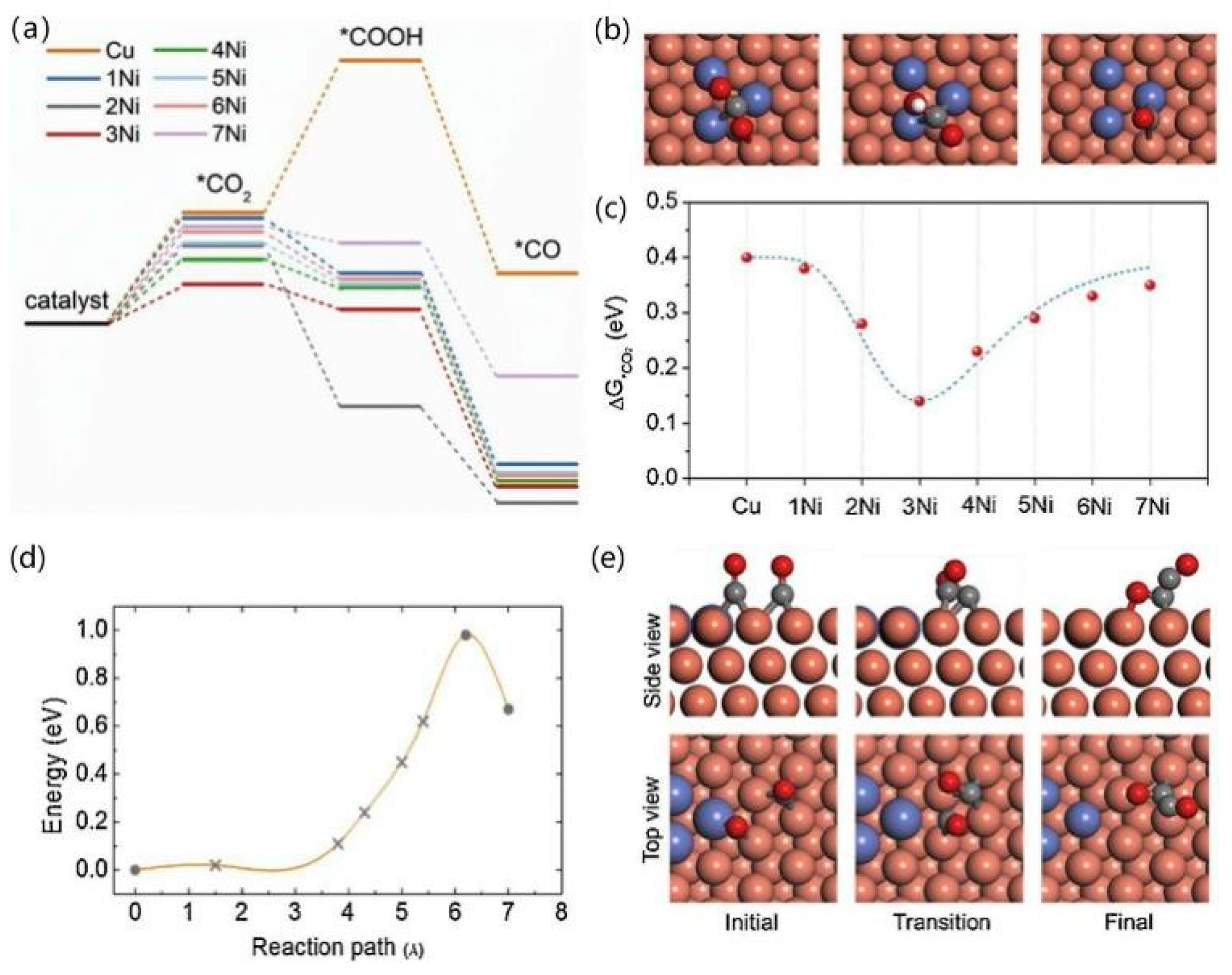
As shown in Figure 12, after comparison, Cu-Ni-2 was more selective for the C2 product than Cu-Ni-1, while the main product of Cu-Ni-3 was H2, therefore showing that Cu-Ni-2 was more selective for the C2 product than the other catalysts. In order to explain the role of Ni atoms in Cu-Ni bimetallic catalysts, four layers of Cu atoms were used as a substrate, and different numbers of Ni atoms (0–7) were doped on the Cu(111) surface. The three important intermediates were analysed, and the decisive step in the reduction of CO2 to CO was considered to be the chemisorption of CO2 onto its surface. The formation of *COOH on the Ni-Ni bridge site by CO2 adsorption is considered preferable, and further hydrogenation to *CO facilitates local accumulation of CO and C-C dimerisation at the Cu site. When doped with three Ni atoms, the doping of 0.87% is close to the Cu-Ni-2 (0.82%) model, and its *C2O2 adsorption with O and C atoms combined with three Cu atoms on the surface is in agreement with the results obtained by Calle- Vallejo and Koper [21].
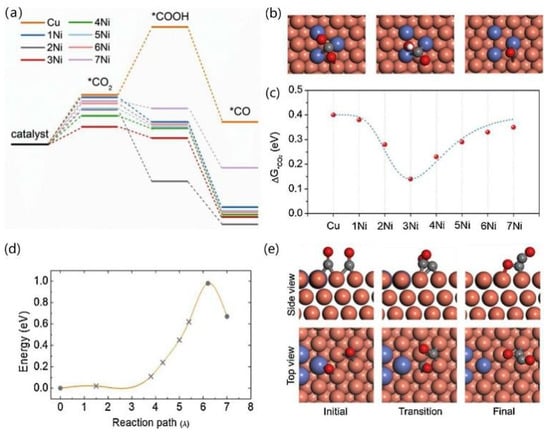
Figure 12.
DFT mechanistic study. (a) Free energy profiles for CO2 activation on the pristine Cu(111) and Ni decorated Cu surfaces. (b) Optimised structures of *CO2, *COOH and *CO on the 3Ni model surface (Cu atoms in orange and Ni atoms in navy). (c) The plot of the Gibbs free energy of *CO2 as a function of Ni substitute number on a Cu surface. (d) Kinetic barrier for the formation of a CO dimer from two adsorbed CO species. (e) Side view (up) and top view (down) of the initial, transition and final states of the C-C coupling process on the 3Ni model surface, reproduced with permission from [72]. Copyright Elsevier B.V, 2021.
Yin et al. [73] reported a hybrid catalyst that mixed single-atom Ni and nano-Cu catalysts to improve C-C coupling and increase the selectivity of C2H4. The stability of this catalyst was evaluated in 10 M KOH, and it was found that the FE of C2H4 decreased from 65 to 53% after 10 h, and the total current density was stable.
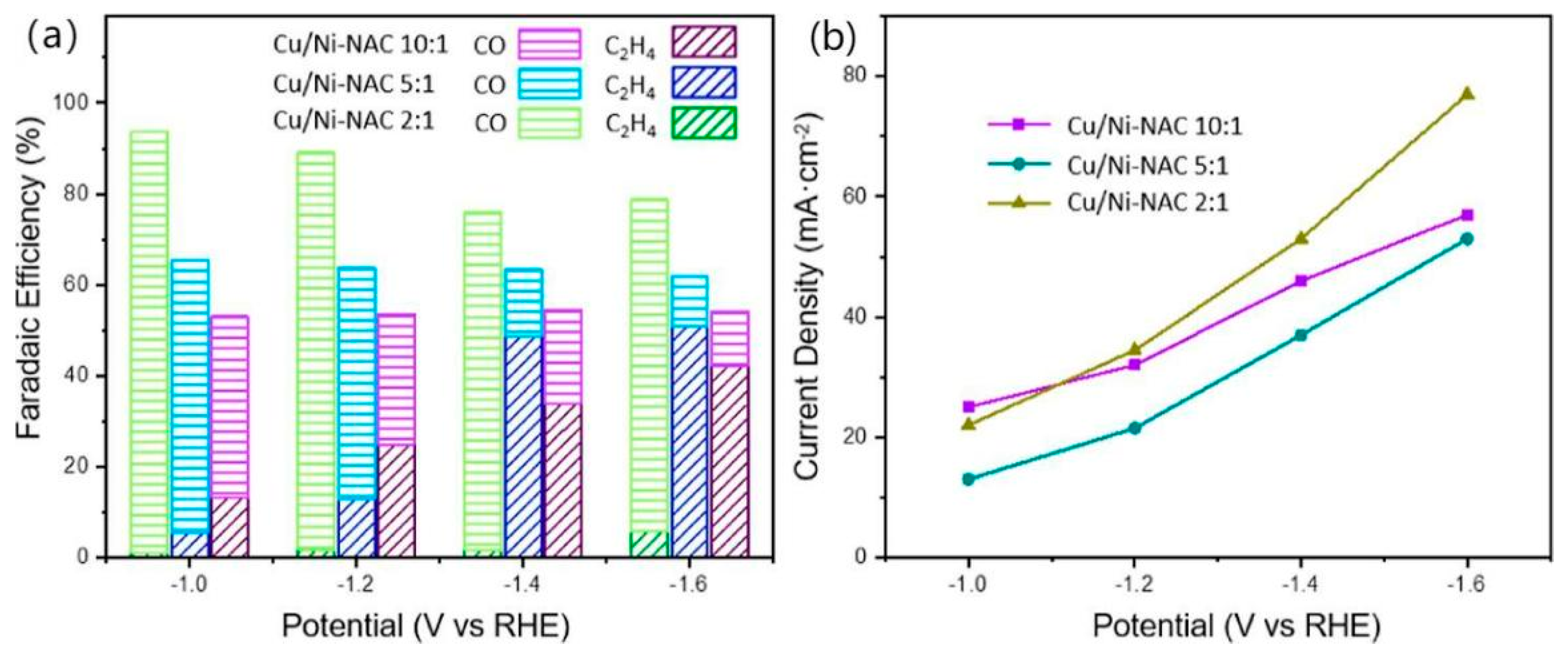
Anchoring a single atom of Ni to nitrogen-assembled carbon (Ni-NAC), the catalyst can convert CO2 to CO over a wide range of potentials and with a FE of over 90% [74,75]. In order to investigate the bicomponent effect while controlling the CO content, different ratios of CuNW and Ni-NAC (2:1, 5:1, 10:1) were prepared for analysis of the products and current densities, as shown in Figure 13a,b. Of the three samples, Cu-Ni-NAC 2:1 showed the highest CO FE, and it is thought that when the ratio of Ni-NAC is higher, it leads to a higher FE of CO. The higher the CuNW content, the higher the FE of C2H4. Considering the FE and current density of C2H4, a Cu-Ni-NAC 10:1 catalyst was considered to be optimal. This hybrid catalyst allows the final product to be selected as a C2 product, and it has excellent selectivity for C2H4.
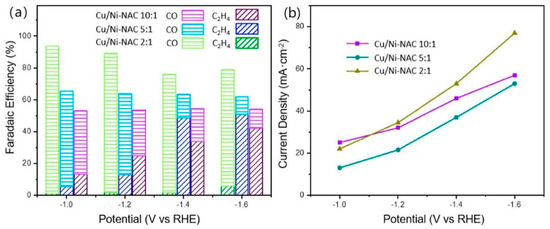
Figure 13.
eCO2RR (a) product distribution, and (b) current density of Cu/Ni-NAC hybrid catalysts in different potentials, reproduced with permission from [73]. Copyright American Chemical Society, 2022.
In summary, Cu-Ag bimetallic catalysts constructed using continuous precipitation of Ag/Cu2O heterostructures and subsequent electroreduction exhibited excellent selectivity for ethylene, and Cu-Ag bimetallic catalysts with different Cu-Ag ratios synthesised using ultrasonic spray pyrolysis were found to exhibit excellent selectivity for C2H4 by Cu90Ag10. The (111) and (100) faces of Cu were chosen to constitute bimetallic alloys with different Cu-Au ratios, and ethanol was found to exhibit the highest selectivity in Cu3Au1(100) face and Cu3Au1(111). Among the different ratios of Cu-Zn bimetals, ethanol was found to exhibit the highest selectivity in Cu4Zn, and the homogeneous Cu-Zn bimetallic catalyst NP prepared using the PLAL technique exhibited excellent selectivity for C2H4, loaded on conductive porous carbon, with Cu75Zn25-C preferentially producing ethanol at lower overpotentials and ethylene at higher potentials. The hybrid catalysts integrating single-atom nickel and nanoscale copper catalytic components exhibited excellent selectivity for ethylene.
4. Conclusions and Prospects
As fossil fuels will remain the main source of energy for some time to come, the production of CO2 can only be moderately controlled. Therefore the question of how to reduce CO2 remains a key issue. The electrocatalytic reduction of CO2 is still an area worth investigating as a method that can reduce CO2 in large quantities. Electrocatalytic reduction of CO2 is a method to convert CO2 into fuel or other chemical products by using renewable energy. This method allows the storage of high-density chemical energy while reducing CO2 emissions in the use of fossil fuels. Meanwhile Cu is the only metal that can reduce CO2 to polycarbonate products, but because of its high potential over and uncontrollable selectivity of the product, a great deal of research has been carried out to solve these problems, and this has led to a great deal of interest in bimetallic catalysts, where changes in the composition, structure and shape of the atoms can lead to a consequent change in their catalytic properties. Bimetallic catalysts made up of Cu and other different metal elements (Ag, Au, Zn, Ni) are therefore used to achieve low overpotentials and control the selectivity of the C2 product by controlling the combination, atomic ratio and assembly on different substrates.
Although the prepared copper-based bimetallic catalysts have shown significant progress in the preparation of C2 products from electrochemical CO2RR, their overpotential is less than ideal and their selectivity for specific C2 products is less than satisfactory. Therefore, bimetallic catalysts with different atomic ratios can be tried for the purpose of promoting C-C coupling. At the same time, different Cu surfaces can have an effect on the final C2 product selectivity, so the same metal can form a bimetallic catalyst with different Cu surfaces for the purpose of enhancing the target C2 product.
Due to the complex structure of bimetallic catalysts, the active sites of some bimetallic catalysts are not well studied. However, most mechanisms are currently obtained by DFT calculations and should be investigated more often by an operando scanning tunneling microscope, operando transmission electron microscope, operando scanning electron microscope, operando X-ray diffraction, etc., to study the real reaction of CO2RR on catalysts, while other techniques can also be used to measure and characterise the reaction intermediates. These calculations also do not take into account the influence of the catalysts in a particular environment, such as local pH and CO2 concentration, on the CO2RR production.
At the same time, Cu-based bimetallic catalysts also encounter some problems in the catalytic process. For example, in Cu-Ag, the Ag atom shifts the valence band density of Cu to a deeper level to achieve enhanced selectivity for multicarbon products, but its surface usually evolves, making it less catalytically active and durable. In Cu-Au, as the proportion of Au atoms increases, the d-band centre of the alloy surface is closer to the Fermi energy level, leading to more stable adsorption of *CO and *COOH on its surface, making it difficult to form C-C coupling and thus becoming less selective for C2 products. In Cu-Zn, when there are more Zn atoms, this distances the Cu-Zn atoms further from the original close contact. When a certain degree is reached, this decreases the C-C coupling ability, thus inhibiting the formation of C2 products. Finally, catalyst stability is important for the industrial application of electrocatalytic CO2RR, but the time available for testing the stability of bimetallic catalysts is very short and far less than that required for industrial use. The cost of the catalyst is also a major issue, and it is necessary to develop a low-cost, buildable and economical catalyst for large-scale use.
Author Contributions
Conceptualization, H.Z. and Y.L.; formal analysis, X.H. and H.Z.; investigation, X.H.; resources, X.H.; writing—original draft preparation, X.H.; writing—review and editing, D.D. and Q.Z.; funding acquisition, H.Z. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by grants from the National Natural Science Foundation of China (21803041 and 51572219); Natural Science Foundation of Shaanxi Province of China (No. 2018JM1010 and 2019JM-592); the Graduate’s Innovation Fund of the Northwest University of China (No. YJG15007) and the Double First-class University Construction project of Northwest University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, K.; Peng, B.; Peng, T.Y. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Xu, Z.W.; Chen, X.X.; Gong, B.G.; Zhao, Y.C.; Zhao, H.B.; Zhang, J.Y.; Zheng, C.G. Flame spray pyrolysis synthesized ZnO/CeO2 nanocomposites for enhanced CO2 photocatalytic reduction under UV–Vis light irradiation. J. CO2 Util. 2017, 18, 53–61. [Google Scholar] [CrossRef]
- Pan, Y.X.; Sun, Z.Q.; Cong, H.P.; Men, Y.L.; Xin, S.; Song, J.; Yu, S.H. Photocatalytic CO2 reduction highly enhanced by oxygen vacancies on Pt-nanoparticle-dispersed gallium oxide. Nano Res. 2016, 9, 1689–1700. [Google Scholar] [CrossRef]
- Senftle, T.P.; Lessio, M.; Carter, E.A. Interaction of pyridine and water with the reconstructed surfaces of GaP(111) and CdTe(111) photoelectrodes: Implications for CO2 reduction. Chem. Mater. 2016, 28, 5799–5810. [Google Scholar] [CrossRef]
- Yang, Y.H.; Zhan, F.Q.; Li, H.; Liu, W.H.; Yu, S. In Situ Sn-doped WO3 films with enhanced photoelectrochemical performance for reducing CO2 into formic acid. J. Solid State Electrochem. 2017, 21, 2231–2240. [Google Scholar] [CrossRef]
- Lo, C.C.; Hung, C.H.; Yuan, C.S.; Wu, J.F. Photoreduction of carbon dioxide with H2 and H2O over TiO2 and ZrO2 in a circulated photocatalytic reactor. Sol. Energy Mater. Sol. Cells 2007, 91, 1765–1774. [Google Scholar] [CrossRef]
- Kočí, K.; Praus, P.; Edelmannová, M.; Ambrožová, N.; Troppová, L.; Fridrichová, D.; Słowik, G.; Ryczkowski, J. Photocatalytic reduction of CO2 over CdS, ZnS and core/shell CdS/ZnS nanoparticles deposited on montmorillonite. J. Nanosci. Nanotechnol. 2017, 17, 4041–4047. [Google Scholar] [CrossRef]
- Ali, A.; Oh, W.C. A simple ultrasono-synthetic route of PbSe-graphene-TiO2 ternary composites to improve the photocatalytic reduction of CO2. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 449–458. [Google Scholar] [CrossRef]
- Wang, Y.W.; Xin, F.; Chen, J.S.; Xiang, T.Y.; Yin, X.H. Photocatalytic reduction of CO2 in isopropanol on Bi2S3 quantum dots/TiO2 nanosheets with exposed {001} facets. J. Nanosci. Nanotechnol. 2017, 17, 1863–1869. [Google Scholar] [CrossRef]
- Ohno, T.; Murakami, N.; Koyanagi, T.; Yang, Y. Photocatalytic reduction of CO2 over a hybrid photocatalyst composed of WO3 and graphitic carbon nitride (g-C3N4) under visible light. J. CO2 Util. 2014, 6, 17–25. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Indium-doped TiO2 nanoparticles for photocatalytic CO2 reduction with H2O vapors to CH4. Appl. Catal. B Environ. 2015, 162, 98–109. [Google Scholar] [CrossRef]
- Bharath, G.; Prakash, J.; Rambabu, K.; Devanand Venkatasubbu, G.; Kumar, A.; Lee, S.; Theerthagiri, J.; Choi, M.Y.; Banat, F. Synthesis of TiO2/RGO with plasmonic Ag nanoparticles for highly efficient photoelectrocatalytic reduction of CO2 to methanol toward the removal of an organic pollutant from the atmosphere. Environ. Pollut. 2021, 281, 116990. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Forest, R.V.; Moore, A.; Jiao, F. Electrodeposited Zn dendrites with enhanced CO selectivity for electrocatalytic CO2 reduction. ACS Catal. 2015, 5, 4586–4591. [Google Scholar] [CrossRef]
- Back, S.; Kim, J.H.; Kim, Y.T.; Jung, Y. On the mechanism of high product selectivity for HCOOH using Pb in CO2 electroreduction. Phys. Chem. Chem. Phys. 2016, 18, 9652–9657. [Google Scholar] [CrossRef]
- Weng, Z.; Zhang, X.; Wu, Y.S.; Huo, S.J.; Jiang, J.B.; Liu, W.; He, G.J.; Liang, Y.Y.; Wang, H.L. Self-cleaning catalyst electrodes for stabilized CO2 reduction to hydrocarbons. Angew. Chem. 2017, 129, 13315–13319. [Google Scholar] [CrossRef]
- Hussain, J.; Jónsson, H.; Skúlason, E. Faraday efficiency and mechanism of electrochemical surface reactions: CO2 reduction and H2 formation on Pt(111). Faraday Discuss. 2016, 195, 619–636. [Google Scholar] [CrossRef]
- Hori, Y.; Kikuchi, K.; Suzuki, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem. Lett. 1985, 14, 1695–1698. [Google Scholar] [CrossRef]
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 reduction at copper surfaces: Pathways to C2 products. ACS Catal. 2018, 8, 1490–1499. [Google Scholar] [CrossRef]
- Goodpaster, J.D.; Bell, A.T.; Head-Gordon, M. Identification of possible pathways for C–C bond formation during electrochemical reduction of CO2: New theoretical insights from an improved electrochemical model. J. Phys. Chem. Lett. 2016, 7, 1471–1477. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Koper, M.T.M. Theoretical considerations on the electroreduction of CO to C2 species on Cu(100) electrodes. Angew. Chem. Int. Ed. 2013, 125, 7423–7426. [Google Scholar] [CrossRef]
- Li, H.J.; Li, Y.D.; Koper, M.T.M.; Calle-Vallejo, F. Bond-making and breaking between carbon, nitrogen, and oxygen in electrocatalysis. J. Am. Chem. Soc. 2014, 136, 15694–15701. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.F.; Zhao, M.; Zhang, G.X.; Zhang, X.G.; Gao, G.; Jiang, Q. Electroreduction of CO2 on Cu clusters: The effects of size, symmetry, and temperature. ChemElectroChem 2019, 6, 1831–1837. [Google Scholar] [CrossRef]
- Wang, Z.N.; Yang, G.; Zhang, Z.R.; Jin, M.S.; Yin, Y.D. Selectivity on etching: Creation of high-energy facets on copper nanocrystals for CO2 electrochemical reduction. ACS Nano 2016, 10, 4559–4564. [Google Scholar] [CrossRef]
- Reske, R.; Mistry, H.; Behafarid, F.; Cuenya, B.R.; Strasser, P. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. J. Am. Chem. Soc. 2014, 136, 6978–6986. [Google Scholar] [CrossRef]
- Li, Q.; Mahmood, N.; Zhu, J.H.; Huo, Y.L.; Sun, S.H. Graphene and its composites with nanoparticles for electrochemical energy applications. Nano Today 2014, 9, 668–683. [Google Scholar] [CrossRef]
- Manthiram, K.; Beberwyck, B.J.; Alivisatos, A.P. Enhanced electrochemical methanation of carbon dioxide with a dispersible nanoscale copper catalyst. J. Am. Chem. Soc. 2014, 136, 13319–13325. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Ma, S.; Gold, J.I.; Kenis, P.J.A.; Gewirth, A.A. Nanoporous copper films by additive-controlled electrodeposition: CO2 reduction catalysis. ACS Catal. 2017, 7, 3313–3321. [Google Scholar] [CrossRef]
- Huan, T.N.; Simon, P.; Rousse, G.; Génois, I.; Artero, V.; Fontecave, M. Porous dendritic copper: An electrocatalyst for highly selective CO2 reduction to formate in water/ionic liquid electrolyte. Chem. Sci. 2017, 8, 742–747. [Google Scholar] [CrossRef]
- Li, Y.; Cui, F.; Ross, M.B.; Kim, D.; Sun, Y.; Yang, P. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 2017, 17, 1312–1317. [Google Scholar] [CrossRef]
- Zhu, G.Z.; Li, Y.W.; Zhu, H.Y.; Su, H.B.; Chan, S.H.; Sun, Q. Enhanced CO2 electroreduction on armchair graphene nanoribbons edge-decorated with copper. Nano Res. 2017, 10, 1641–1650. [Google Scholar] [CrossRef]
- Passalacqua, R.; Parathoner, S.; Centi, G.; Halder, A.; Tyo, E.C.; Yang, B.; Seifert, S.; Vajda, S. Electrochemical behaviour of naked sub-nanometre sized copper clusters and effect of CO2. Catal. Sci. Technol. 2016, 6, 6977–6985. [Google Scholar] [CrossRef]
- Iyemperumal, S.K.; Deskins, N.A. Activation of CO2 by supported Cu clusters. Phys. Chem. Chem. Phys. 2017, 19, 28788–28807. [Google Scholar] [CrossRef]
- Shen, H.M.; Li, Y.W.; Sun, Q. Cu atomic chains supported on β-borophene sheets for effective CO2 electroreduction. Nanoscale 2018, 10, 11064–11071. [Google Scholar] [CrossRef]
- Du, D.C.; Zhu, H.Y.; Guo, Y.N.; Hong, X.L.; Zhang, Q.S.; Sou, B.B.; Zou, W.L.; Li, Y.W. Anchoring Cu Clusters over Defective Graphene for Electrocatalytic Reduction of CO2. J. Phys. Chem. C 2022, 126, 11611–11618. [Google Scholar] [CrossRef]
- Ni, Z.Y.; Liang, H.M.; Yi, Z.Y.; Guo, R.; Liu, C.M.; Liu, Y.G.; Sun, H.Y.; Liu, X.W. Research progress of electrochemical CO2 reduction for copper-based catalysts to multicarbon products. Coord. Chem. Rev. 2021, 441, 213983. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhou, X.J.; Yu, T.S.; Lu, X.L.; Qian, L.H.; Liu, P.; Lei, P.X. Surface restructuring in AgCu single-atom alloy catalyst and self-enhanced selectivity toward CO2 reduction. Electrochim. Acta 2022, 426, 140774. [Google Scholar] [CrossRef]
- Clark, E.L.; Hahn, C.; Jaramillo, T.F.; Bell, A.T. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 2017, 139, 15848–15857. [Google Scholar] [CrossRef]
- Ishimaru, S.; Shiratsuchi, R.; Nogami, G. Pulsed Electroreduction of CO2 on Cu-Ag Alloy Electrodes. J. Electrochem. Soc. 2000, 147, 1864. [Google Scholar] [CrossRef]
- Huang, J.F.; Mensi, M.; Oveisi, E.; Mantella, V.; Buonsanti, R. Structural sensitivities in bimetallic catalysts for electrochemical CO2 reduction revealed by Ag–Cu nanodimers. J. Am. Chem. Soc. 2019, 141, 2490–2499. [Google Scholar] [CrossRef]
- Wang, Y.X.; Niu, C.L.; Zhu, Y.C. Copper–silver bimetallic nanowire arrays for electrochemical reduction of carbon dioxide. Nanomaterials 2019, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.Y.; Huo, S.J.; Zhang, W.; Fang, J.H.; Wang, H.L. The tunable and highly selective reduction products on Ag@Cu bimetallic catalysts toward CO2 electrochemical reduction reaction. J. Phys. Chem. C 2017, 121, 11368–11379. [Google Scholar] [CrossRef]
- Jeon, Y.; Choi, I.; Kim, J.J. Facile electrochemical fabrication of Ag/Cu bi-metallic catalysts and the dependence of their selectivity for electrochemical CO2 reduction on the surface composition. Thin Solid Films 2021, 726, 138674. [Google Scholar] [CrossRef]
- Wang, J.Q.; Li, Z.; Dong, C.K.; Feng, Y.; Yang, J.; Liu, H.; Du, X.W. Silver/copper interface for relay electroreduction of carbon dioxide to ethylene. ACS Appl. Mater. Interfaces 2019, 11, 2763–2767. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.E.; Ko, Y.N.; Kim, J.; Choi, H.; Lee, W.; Kim, Y.E.; Lee, D.; Kim, H.Y.; Park, K.T. Selective production of ethylene from CO2 over CuAg tandem electrocatalysts. J. Ind. Eng. Chem. 2022, 116, 191–198. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.C. Enhanced nucleation and decreased growth rates of Cu2O in Cu0.5Au0.5 (001) thin films during In Situ oxidation. J. Mater. Res. 2005, 20, 1902–1909. [Google Scholar] [CrossRef]
- Pace, S.; Hoof, T.V.; Hou, M.; Buess-Herman, C.; Reniers, F. Surface composition of CuAu single crystal electrodes determined by a coupled UHV–electrochemical approach and a Monte-Carlo simulation. Surf. Interface Anal. 2004, 36, 1078–1082. [Google Scholar] [CrossRef]
- Christophe, J.; Doneux, T.; Buess-Herman, C. Electroreduction of carbon dioxide on copper-based electrodes: Activity of copper single crystals and copper–gold alloys. Electrocatalysis 2012, 3, 139–146. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P.D. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef]
- Xu, Z.C.; Lai, E.; Shao-Horn, Y.; Hamad-Schifferli, K. Compositional dependence of the stability of AuCu alloy nanoparticles. Chem. Commun. 2012, 48, 5626–5628. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, A.Q.; Wang, X.D.; Mou, C.Y.; Zhang, T. Au–Cu alloy nanoparticles confined in SBA-15 as a highly efficient catalyst for CO oxidation. Chem. Commun. 2008, 27, 3187–3189. [Google Scholar] [CrossRef]
- Zhang, X.G.; Feng, S.S.; Zhan, C.; Wu, D.Y.; Zhao, Y.; Tian, Z.Q. Electroreduction reaction mechanism of carbon dioxide to C2 products via Cu/Au bimetallic catalysis: A theoretical prediction. J. Phys. Chem. Lett. 2020, 11, 6593–6599. [Google Scholar] [CrossRef]
- Feng, H.S.; Chen, C.Y.; Wang, S.; Zhang, M.; Ding, H.; Liang, Y.J.; Zhang, X. Theoretical Investigation of Cu–Au Alloy for Carbon Dioxide Electroreduction: Cu/Au Ratio Determining C1/C2 Selectivity. J. Phys. Chem. Lett. 2022, 13, 8002–8009. [Google Scholar] [CrossRef]
- Won, D.H.; Shin, H.; Koh, J.; Chung, J.; Lee, H.S.; Kim, H.; Woo, S.I. Highly efficient, selective, and stable CO2 electroreduction on a hexagonal Zn catalyst. Angew. Chem. Int. Ed. 2016, 128, 9443–9446. [Google Scholar] [CrossRef]
- Ren, D.; Ang, B.S.H.; Yeo, B.S. Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide-derived CuxZn catalysts. ACS Catal. 2016, 6, 8239–8247. [Google Scholar] [CrossRef]
- Yin, G.; Abe, H.; Kodiyath, R.; Ueda, S.; Srinivasan, N.; Yamaguchi, A.; Miyauchi, M. Selective electro-or photo-reduction of carbon dioxide to formic acid using a Cu–Zn alloy catalyst. J. Mater. Chem. A 2017, 5, 12113–12119. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Liu, H.; Dong, C.K.; Wang, J.Q.; Kulinich, S.A.; Du, X.W. Laser-prepared CuZn alloy catalyst for selective electrochemical reduction of CO2 to ethylene. Langmuir 2018, 34, 13544–13549. [Google Scholar] [CrossRef]
- Zeng, J.Q.; Rino, T.; Bejtka, K.; Castellino, M.; Sacco, A.; Farkhondehfal, M.A.; Chiodoni, A.; Drago, F.; Pirri, C.F. Coupled Copper–Zinc Catalysts for Electrochemical Reduction of Carbon Dioxide. ChemSusChem 2020, 13, 4128–4139. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhong, H.X.; Qiu, Y.L.; Li, X.F.; Zhang, H.M. Zn electrode with a layer of nanoparticles for selective electroreduction of CO2 to formate in aqueous solutions. J. Mater. Chem. A 2016, 4, 16670–16676. [Google Scholar] [CrossRef]
- Hu, H.J.; Tang, Y.; Hu, Q.; Wan, P.Y.; Dai, L.M.; Yang, X.J. In-situ grown nanoporous Zn-Cu catalysts on brass foils for enhanced electrochemical reduction of carbon dioxide. Appl. Surf. Sci. 2018, 445, 281–286. [Google Scholar] [CrossRef]
- Jeon, H.S.; Timoshenko, J.; Scholten, F.; Sinev, I.; Herzog, A.; Haase, F.T.; Cuenya, B.R. Operando insight into the correlation between the structure and composition of CuZn nanoparticles and their selectivity for the electrochemical CO2 reduction. J. Am. Chem. Soc. 2019, 141, 19879–19887. [Google Scholar] [CrossRef] [PubMed]
- Juntrapirom, S.; Santatiwongchai, J.; Watwiangkham, A.; Suthirakun, S.; Butburee, T.; Faungnawakij, K.; Chakthranont, P.; Hirunsit, P.; Rungtaweevoranit, B. Tuning CuZn interfaces in metal–organic framework-derived electrocatalysts for enhancement of CO2 conversion to C2 products. Catal. Sci. Technol. 2021, 11, 8065–8078. [Google Scholar] [CrossRef]
- Wan, L.L.; Zhang, X.L.; Cheng, J.S.; Chen, R.; Wu, L.X.; Shi, J.W.; Luo, J.S. Bimetallic Cu–Zn Catalysts for Electrochemical CO2 Reduction: Phase-Separated versus Core–Shell Distribution. ACS Catal. 2022, 12, 2741–2748. [Google Scholar] [CrossRef]
- Tan, D.X.; Zhang, J.L.; Cheng, X.Y.; Tan, X.N.; Shi, J.B.; Zhang, B.X.; Han, B.X.; Zheng, L.R.; Zhang, J. CuxNiy alloy nanoparticles embedded in a nitrogen–carbon network for efficient conversion of carbon dioxide. Chem. Sci. 2019, 10, 4491–4496. [Google Scholar] [CrossRef]
- Ding, C.M.; Li, A.L.; Lu, S.M.; Zhang, H.F.; Li, C. In Situ electrodeposited indium nanocrystals for efficient CO2 reduction to CO with low overpotential. ACS Catal. 2016, 6, 6438–6443. [Google Scholar] [CrossRef]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470. [Google Scholar] [CrossRef]
- Oh, Y.; Hu, X.L. Organic molecules as mediators and catalysts for photocatalytic and electrocatalytic CO2 reduction. Chem. Soc. Rev. 2013, 42, 2253–2261. [Google Scholar] [CrossRef]
- Varela, A.S.; Sahraie, N.R.; Steinberg, J.; Ju, W.; Oh, H.S.; Strasser, P. Metal-doped nitrogenated carbon as an efficient catalyst for direct CO2 electroreduction to CO and hydrocarbons. Angew. Chem. Int. Ed. 2015, 54, 10758–10762. [Google Scholar] [CrossRef]
- Ding, C.M.; Feng, C.C.; Mei, Y.H.; Liu, F.Y.; Wang, H.; Dupuis, M.; Li, C. Carbon nitride embedded with transition metals for selective electrocatalytic CO2 reduction. Appl. Catal. B Environ. 2020, 268, 118391. [Google Scholar] [CrossRef]
- Sachtler, W.M.H.; Dorgelo, G.J.H. The surface of copper-nickel alloy films: I. Work function and phase composition. J. Catal. 1965, 4, 654–664. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, C.W.; Zhao, Y.; Li, L.B.; Chen, Y.; Raziq, F.; Qiao, L.; Guo, S.X.; Wang, C.Y.; Wallace, G.G.; et al. Atomic nickel cluster decorated defect-rich copper for enhanced C2 product selectivity in electrocatalytic CO2 reduction. Appl. Catal. B Environ. 2021, 291, 120030. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Yu, J.Q.; Xie, Z.H.; Yu, S.W.; Zhang, L.Y.; Akauola, T.; Chen, J.G.; Huang, W.Y.; Qi, L.; Zhang, S. Hybrid Catalyst Coupling Single-Atom Ni and Nanoscale Cu for Efficient CO2 Electroreduction to Ethylene. J. Am. Chem. Soc. 2022, 144, 20931–20938. [Google Scholar] [CrossRef]
- Luo, Z.C.; Yin, Z.Y.; Yu, J.Q.; Yu, Y.; Hu, B.; Nie, R.F.; Kolln, A.F.; Wu, X.; Behera, R.K.; Chen, M.; et al. General Synthetic Strategy to Ordered Mesoporous Carbon Catalysts with Single-Atom Metal Sites for Electrochemical CO2 Reduction. Small 2022, 18, 2107799. [Google Scholar] [CrossRef]
- Luo, Z.C.; Nie, R.F.; Nguyen, V.T.; Biswas, A.; Behera, R.K.; Wu, X.; Kobayashi, T.; Sadow, A.; Wang, B.; Huang, W.Y.; et al. Transition metal-like carbocatalyst. Nat. Commun. 2020, 11, 4091. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).