Intensification of Photo-Assisted Advanced Oxidation Processes for Water Treatment: A Critical Review
Abstract
:1. Introduction
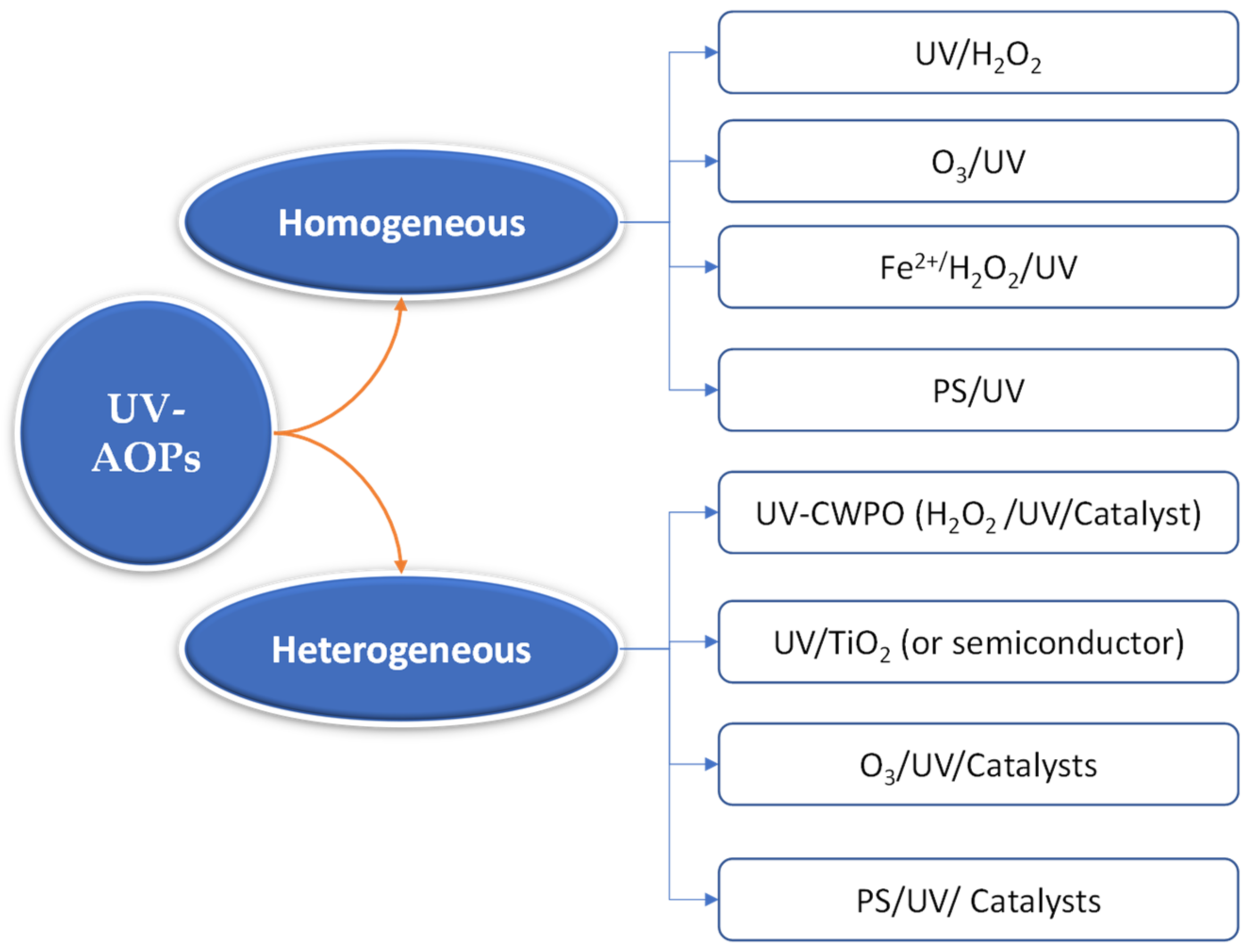
2. Intensification of Operational Conditions in UV-AOPs
2.1. Catalyst Loading
| Photocatalytical System | Target Pollutant | Catalysts Loading Conditions | Remarks | Experimental Remarks | Ref. |
|---|---|---|---|---|---|
| TiO2/UV-A | Methylene Blue (Dye) | [catalyst] = 0.5, 1, 1.5 g/L | Optimum achieved at 1 g/L (k = 0.0801 min−1) | MB0 = 60 mg/L; pH neutral; T = 20 °C, UV-A = 40 W/m2 Immersion Reactor, Ø = 76 mm | [11] |
| TiO2/Solar | Methylene Blue, Dichloroacetic acid, 4-Chlorophenol | [catalyst] = 0.2, 0.25, 0.3, 0.35 g/L. | Optimal photocatalyst loading 0.25 g/L. 99% MB degradation at 8000 J/m2. | [MB]0 = 10 ppm, flow rate = 24 L/min, pH = natural (7.45). Solar Pilot-scale Offset Multi Tubular Photoreactor (OMTP), Ø = 32 mm | [12] |
| 1% Mg-ZnO-Al2O3/UV-A/B | Caffeine | [catalyst] = 0.1–1.5 g/L | Optimal photocatalyst loading 0.3 g/L (1% Mg-ZnO-Al2O3) Caffeine degradation was 98.9% | [Caffeine] = 20 mg/L. Cylindrical Pyrex immersion photoreactor (2 L). UV Hg lamp (400 W). T = 25 °C | [13] |
| TiO2/UV-A | Diclofenac (DIC) | [catalyst] = 0.1–2 g/L | kapp, (0.35 min−1) was optimal at a mass of catalyst of 1.0 g/L | [DIC]0 = 8 mg/L. T = 293 K, pH = 6. 800 mL glass immersion photoreactor (Ø = 7.5 cm) TQ 150 mp Hg lamp (λexc < 366 nm) | [16] |
| TiO2/UV | Cefotaxime | [catalyst] = 1.0–2.5 g/L | ≈84.2% of Cefotaxime removal optimized at 2.3 g/L of TiO2 | Sunlight sim. Xe lamp (300–800 nm) 250 W/m2 [Cefotaxime] = 20 mg/L, T = 35 °C | [15] |
| ZnO/UV | and around 1.45 g L−1 of ZnO. | ||||
| TiO2/UV-LED | Ibuprofen (municipal and pharmaceutical spiked MWW) | [catalyst] = 0.5–4 g/L | 100% removal of IBU and 55% removal of DOC. kapp, (0.024 min−1) was optimal at 2.5 g/L | Lab-scale photoreactor: 10 W UVhigh LEDs (λmax = 382 nm); V = 250 mL, IBUP (up to 213 mg/L) | [14] |
| Na2S2O8/TiO2/Solar | Mixture of 8 pesticides in agro-phitosanitary wastewater | [catalyst] = 100 to 500 mg/L | The degradation rate increases up to 300 mg/L of catalyst loading | [Pesticides]: 0.21 mg/L (hexythiazox) to 5.97 mg/L (thiamethoxam) CPC photoreactor, Ø 14.6 cm, V = 180 L (75 L illuminate V) | [17] |
| PS/Cu0-Cu2O/UVA | sulfamerazine | [catalyst] = 0.06 to 1 g/L | SMZ removal increases to 0.2 g/L | [SMZ]0 = 50 mg/L; [PS]0 = 0.8 g/L, λmax = 382 nm, initial; pH = 7, 25 °C; photo-reactor with Hg lamp (800 W) | [18] |
| g-C3N4/PMS/Vis system | Acid Orange 7 (AO7) | [catalyst] = 0.05 to 1.2 g/L | The degradation rate increased with catalyst load to 0.8 g/L | 500 W xenon lamp, [PMS] = 0.4 g/L, [AO7] = 20 mg/L, T = 25 °C | [19] |
| UV/TiO2 | MeOH | Radiation field was simulated. [TiO2] = 0.005–5 g/L | Maximum at 0.2 g/L Radiative model was confirmed with MeOH oxidation experiments | Differential photoreactor (ODPR). V = 3.2 mL, quartz cell (Hellma QS-130), optical path = 1 cm; 36 UV-A LEDs (365 nm, Rad flux = 410 mW at 700 mA) | [20] |
| P25, P25/20 and P90 (TiO2)/UV | Phenol (Model) | Radiation field was simulated. [TiO2] = 0.1–1.5 g/L | P25 and P90 optimum catalyst load (0.25–0.50 g/L) was 2 times P25/20 (∼0.7–1.0 g/L) The kinetic model was successfully validated by experimental data (phenol oxidation) | 1L Pyrex slurry photoreactor (Ø = 76 mm), 15 W × 6 Black Light Blue lamps(15W) [phenol]0 = 50 mg/L, pH0 = 6.0, irradiance = 38.4 W/m2 | [21,22] |
| TiO2, Ag-TiO2, goethite/UV–Vis | 2-hydroxybenzoic acid (2-HBA) | Initial rate of photon absorption (IRPA) was correlated to optimum catalyst Concentration [TiO2] = 0.1–2 g/L | The apparent optical thickness: τapp = 4.1–4.4 provides optimum catalyst and reactor performance. Intrinsic kinetic parameters of 2-HBA photocatalytic oxidation were determined. | Flat plane photo-reactor (thickness L = 1 cm) or annular reactor (Ø int = 0.054 m), cylindrical lamp mounted axially [2-HBA] = 0.2 mmol/dm3; T = 22; pH = 4.0 UVA to natural light (UVA up to 89.4 W/m2 | [23,24] |
| Fe2+/H2O2/UV–Vis | 5-fluorouracil | [Fe2+] = 0–100 mg/L | Performance increased to a ferric ion concentration of 4.5 mg/L At higher concentrations, the degradation rate increases marginally | double-walled Pyrex glass reactor, thermostated in a solar with a xenon lamp (1.5 kW, 500 W/m2) 100 mL of 5-FU solution (10 mg/L); pH = 3; H2O2 = 0–90 mg/L | [25] |
| Fe2+/H2O2/UV-B | Sourwater from petroleum refineries | [FeSO4] = 0.13–0.4 g/L | DOC removal was optimised with the highest amount of iron. | 2 reactors operating in continuous mode. Fenton (400 mL) and Photo-Fenton (1600 mL), with UV-B lamp (15 W) [COD] = 850–1020 mg/L; [H2O2]0 = 4 g/L, [FeSO4]0 = 0.1–0.4 g/L. | [27] |
| Fe2+/H2O2/UVA | pyrimethanil | [Fe2+] = 8, 20 and 32 mg/L | At 20 mg/L, the treatment always improved with irradiance (process was photo-limited). At 32 mg/L, the excess of iron was counter-productive. | Lab-scale raceway photo-reactor in a SolarBox equipped with Xenon lamp (300–800 nm) [H2O2] = 35 mM, pH = 2.8 | [28] |
2.2. pH
| Photocatalytical System | Target Pollutant | pH Conditions | Remarks | Experimental Remarks | Ref. |
|---|---|---|---|---|---|
| 1% Mg-ZnO-Al2O3/UV-A/B | Caffeine | pH = 3.5, 4.5, 9.5 | Photocatalytic activity was enhanced at pH of 9.5 and dramatically decreased at pH of 3.5 At pH > 8.41 (surface negatively charged), favours cationic adsorption | [Caffeine] = 20 mg/L. Cylindrical Pyrex immersion photoreactor (2 L). UV Hg lamp (400 W). T = 25 °C | [13] |
| Immobilized TiO2/UVA | Nitrosamines in MWTP, river or eutrophic matrices | pH = 3.0, 5.5, 7.0, 9.5 | Optimum pH highly dependent on the proportion and speciation of intermediates during oxidation of each nitrosamine | UVA photocatalytic reactor (Blacklight λ = 315–400 nm) = 0.67 mW/cm2; T = 22 °C | [30] |
| TiO2 NPs/UVA | Methylene blue | Synthesis pH = 1.6, 7.0 and 10 | Synthesis pH determined pHPZC photocatalyst Optimum degradation (97%, k = 0.018 min−1) was achieved with TiO2 NPs prepared at pH 10 | 1 L photoreactor with 6W Lamp (365 nm) The cell was filled with 0.6 L of 10 mg/L of MB and 100 mg/L of the photocatalyst; T = 25 °C | [31] |
| TiO2/Solar | Phenol, dichloroacetic acid, pyrimethanil | Natural pH | At pH 2.7, titania (positively charged) and CHCl2COO− (pKa = 1.26) interaction favoured direct DCA degradation. Conversely, indirect ∙OH attack govern phenol removal (pKa = 9.9) at natural pH | CPC (Compound Parabolic Collectors) tubes (3.2 m2 irradiated area) under turbulent flow conditions and solar light. VT = 35 L | [32] |
| H2O2/Fe2+/UV | Antibiotics (amoxicillin, ampicillin and cloxacillin) | pH = 2.0, 2.5, 3.0, 3.5 and 4. | Maximum degradation was achieved at pH 3 | 600 mL Pyrex reactor equipped with a UV lamp (6 W) emitting at 365 nm. [AMX, AMP, CLX]0 = ~100 mg/L, [COD] = 520 mg/L; [H2O2] = 16.25 mM | [36] |
| H2O2 or S2O82−/UV/Fe-complex (NTA, FeEDTA or FeCit, or FeOx) | Naproxen in a wastewater effluent collected in a MWWT | pH natural | Photo-Fenton at neutral pH was efficient for naproxen degradation in the presence of all iron complexes | Vis Xe high-intensity discharge lamp (X-HID) [NAP] = 1 μmol/L; [H2O2] = 16.3 mmol/L or [S2O82−] = 4.9 mmol;/Ligand [Fe3+] = 21.4 μmol/L, pH = 7.5 (natural pH). | [42] |
| ZnFe2O4/UV/H2O2 | Orange II | Initial at pH0 = 3, 6, 7 and 9 | Decolourization efficiency increased slightly with pH, optimum at pH = 6 | Xe high intensity discharge lamp (X-HID) (454 nm and 150 W) 50 mL glass beaker [Orange II] = 100 mg/L, [H2O2] = 5 mM, [catalyst] = 0.5 g/L, T = 20 °C, pH0 = 6) | [43] |
| Metallurgical slag as a Fenton-type photocatalyst | Diclofenac | Natural pH = 7 | Complete depletion and a partial mineralization were achieved with the COB/H2O2/sunlight system at pH 7 | [Diclofenac] = 500 mg/L, Sunlight simulator with Xe arc lamp (500 W/m2) pH = 7, [H2O2]0 = 180 mg/L, 1:18 mass ratio of Fe/H2O2. | [45] |
| Fe3+/H2O2/UV | Diuron and amoxicillin | Pollutants exhibited a strong degradation keeping the circumneutral pH | Presence of anions (HCO32−, HCO3-, humic acids…) leads to photo-chemical reactions (dissolved ferric–humic acid complexes, colloidal iron…) at circumneutral pH | Solar simulator (300 W/m2) [amoxicillin] = 10 mg/L humic acids (HA) = 2.0 mg/L; [carbonates] = 100 mg/L; [Fe3+] = 0.3 mg/L, [H2O2] = 15.2 mg/L, pH0 = 7.0 | [46] |
| UV/H2O2 and neutral photo-Fenton | 22 micropollutants (including 15 pharmaceuticals) in MWTP | Natural pH | Fe addition to the reactor did not improve the process; degradation was higher using uniquely the Fe present in water (1.6 mg/L) | Continuous operation Reactor 5 LP Hg lamps (254 nm, 150 W each); V = 37 L | [47] |
2.3. Temperature
2.4. Oxidants
| Photocatalytical System | Target Pollutant | Temperature Conditions | Remarks | Experimental Remarks | Ref. |
|---|---|---|---|---|---|
| TiO2/Ag | Procion red MX-5B | R.T. to 50 °C | Operating temperature increased decolourization efficiency from R.T. to 40 °C but decreased at 50 °C as e−/h+ recombination accelerates | Photoreactor VT = 0.5 L; Lamp: 10-W UVA (0.7 μW/cm2); [MX-5B] = 30 ppm | [49] |
| TiO2, Pd/TiO2 or Cu/TiO2 | Methylene blue | 0 to 70 °C | At 0–50 °C, TiO2 and Pd/TiO2 activity increased with temperature; at 70 °C, rate dropped slightly or became less effective due to recombination rate increase | UVC lamps (λ = 254 nm), TUV PL-L 18 W. VT = 20 mL; [MB] = 10 mg/L | [50] |
| FeTiO3 | NO3− in saline water | range of 20–80 °C | 73% total nitrogen reduction was reached at 420′ An increase in the temperature enhanced reaction kinetics. At high T, N2 bubbling to maintain inert conditions is avoided (lowering O2 solubility) | Set-up: Magnetically stirred glass jacketed batch reactor (VT = 700 mL). Lamp: 150 W M.P. Hg lamp (30 W/cm2) Working at [C2O42−] = 180 mg/L, [FeTiO3] = 450 mg/L; [HCl] = 13 mM,) | [51] |
| UV–Vis/H2O2/Fe(II) | Textile effluents | 25 to 70 °C | Temperatures above 25 °C and up to 70 °C show a beneficial effect on organic load reduction | Lamps: 6 W Black-light and 250 W Xe and Solar light. | [55] |
| Solar/H2O2/Fe(II) | Alachlor | 20 to 50 °C | At best operating conditions (maximal iron concentration 2.6 mM, maximal temperature 70 °C) an increase reaction rate 5-fold by raising temperature from 20 to 50 °C | Pilot-plant CPC sunlight operated in batch mode. Collector (CF = 1): 20 Pyrex tubes (Øin = 46.4 mm). Acollector = 4.16 m2, Vi = 44.6 L | [58] |
| Solar/H2O2/Fe(II) | commercial pesticide mixture | 25 to 50 °C | Photo-Fenton efficiency gradually rose with temperature; nevertheless, at 50 °C, efficiency decreases | Pilot-plant CPC with sunlight operated in batch mode. Collector (CF = 1): (Øin = 50.0 mm). Acollector = 1.04 m2 20 Pyrex tubes; Vi = 44.6 L [DOC] = 200 mg/L (40 mg/L of each commercial pesticide); pH = 2.7–2.9; [H2O2] = 100 to 300 mg/L | [61] |
| UV/H2O2/Fe(II) | Phenolic and landfill leachate wastewater | 25 to 90 °C | Time to achieve maximum TOC and COD removals (80%) was reduced from 180 to 45 min from 50 to 90 °C. Irradiation efficiency increased 4-fold within this range | immersion-wall batch jacketed 1 L photoreactor; Lamp: 150 W MP Hg. pH = 2.7–2.9; [H2O2] = 100 to 300 mg/L; Fe2+ = 10 ppm | [62] |
| Photocatalytical System | Target Pollutant | Oxidant Type and Conditions | Remarks | Experimental Remarks | Ref. |
|---|---|---|---|---|---|
| UV–Vis/H2O2/Fe(II) | mixture of 6 emerging pollutants | Stoichiometric H2O2 to mineralize mixture (146 mg/L) | Data show that photo-Fenton in high-salinity wastewater at pH = 2.8 and pH = 5.0 was capable to remove all pollutants in 1 h | 50 W xenon lamp on open glass reactor; borosilicate glass [Emerging pollutants] = 5 mg·L−1 each; [Fe] = 5 mg·L−1 | [63] |
| UV–Vis/H2O2/Fe(II) | sertraline | [H2O2] = 10–100% Sub-stoichiometric amount | TOC removal up to 90% was achieved at a hydrogen peroxide dose as low as 40% of the stoichiometric amount for mineralization | Lamp: Xenon 550 W m−2 (300 to 800 nm). Vr = 500 mL; [Sertraline] = 50 mgL−1. The [Fe2+] = 1–10 mgL−1; [H2O2] = 10–100% stoich amount | [66] |
| UV–Vis/H2O2/Fe(II) | Acetylsalicylic acid | [H2O2] = Up to 9-fold the stoichiometric Amount | Mineralization around 90% is reached at 10 min with 4.5-fold excess of H2O2 | 2 Parabolic tubular modules in series, Lamp: Black-light UVA (40 W) [Fe2+] = 1.5 mM; [H2O2] = 45 Mm [Acetylsalicylic acid]0 = 100 ppm | [69] |
| UV–Vis/H2O2/Fe(II) | Orange II (OII) | Continuous addition of H2O2 | H2O2 continuous dosage optimize photocatalytic efficiency (scavenger effect is minimized); 100% decolouration (95% TOC removal) with continuous addition of peroxide | Solar reactor (50 L); Ai = 2 m2 (CF = 1); 16 borosilicate-glass tubes (OD = 32 mm) Fe(II) = 2 ppm; Orange II = 20 ppm, | [72] |
| H2O2 or S2O82−/UV/ Fe-complex (NTA, FeEDTA or FeCit, or FeOx) | Naproxen in a sewage effluent collected at a MWWT | H2O2 compared to S2O82− | H2O2 best performed in ultrapure water, while S2O82− best performed with real WW | Lamp: Xe high int. discharge [NAP] = 1 μmol/L; [H2O2] = 16.3 mmol/L or [S2O82−] = 4.9 mmol; Ligand [Fe3+] = 21.4 μmol/L, pH = 7.5 (natural pH). | [42] |
| TiO2/g-C3N4 | Acetaminophen | PS dosage increases from 0.5 mM to 2 mM | The addition of PS greatly improved the degradation efficiency (5 mg/L AAP almost degraded; at 30 min; k = 0.061 min−1, XTOC = 82.5%) | Lamp: Xe (300 W, 400 nm cutoff filter) [TiO2/g-C3N4] = 500 mg/L, [AAP] = 5 mg/L [PS] = up to 2 mM | [82] |
| TiO2/Fe-TiO2 | Ethidium bromide | [H2O2] = 80–160 mg/L, 1–2-fold stoichiom, and continuous dosification was evaluated | 196 mg/L H2O2 addition was optimized throughout; several dosages maintaining H2O2/TOC ratio; performance was maximized, raising 84% of TOC conversion | Pyrex photoreactor with a Hg MP lamp (500 W); Vr = 1 L, [EtBr] = 20 mg/L, [Fe-TiO2] = 500 mg/L, pHo = 3, [H2O2] Total = 196 mg/L | [83] |
3. Catalysts Engineering
- (a)
- Photocatalyst design is orientated to reduce the limitations in mass and photon transfer. This strategy can be achieved via three steps as follows:
- Improving photocatalyst activation and preventing deactivation. This fact is related to the shift of the absorption edge from the ultraviolet to the visible range. This item has been extensively studied; thus, it will not be developed as a separate alternative in this review unless combined with other sorts of intensifications.
- Promotion of the adsorption of reagent onto the catalyst surface: the initial step of a Langmuir–Hinshelwood model.
- Promotion of the desorption of reactions products from the catalyst: The last step of a Langmuir–Hinshelwood model.
- (b)
- Design of bifunctional catalysts favouring the increase in oxidant species generation yield (mainly HOx·). In the same way, two steps have been performed for this purpose:
- The combination of several single processes without oxidant addition.
- The extra-addition of oxidant agent.
3.1. Design Oriented to Reduce the Physical Limitations
| Catalyst | Processes Involved | Light Spectra | Pollutant | Conversions | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| B/Bi2WO6 | Adsorption/photocatalysis | Solar | Rhodamine B | B/Bi2WO6 = 8.8 k Bi2WO6 XRhB = 100% (180 min) | Mesoporous favours adsorption. B presence creates higher pore volume related to mesopores B acts also as electron trap | No TOC | [84] |
| LaFeO3/Zeolite | Adsorption/photo-Fenton | Visible | Rhodamine B | Higher adsorption and active sites | No evaluation of TOC parameter | [88] | |
| LaFeO3/Silica | Adsorption/photo-Fenton | Rhodamine B | [87] | ||||
| Mesoporous Au/TiO2 | Adsorption/photocatalysis | Visible | AO7 (dye) | [89] | |||
| SO42−/Fe2−xZrxO3 | Adsorption/photo-Fenton | Visible | [90] | ||||
| LaFeO3/Resin | Adsorption/photo-Fenton/photocatalysis | Solar | Caffeine (CECs) | 80% removal caff in 3 h 60% TOC | Reusability until 6th cycle | Not a complete mineralization | [91] |
| Mesoporous Fe2O3-TiO2 | Adsorption/photo-Fenton/photocatalysis | Visible | Norfloxacin antibiotic | 100% removal and 97% mineralization in 120 min | Good reusability. New reactor designed with LED light | Low iron leached. | [85] |
| Mesoporous Ga2O3-TiO2 | Adsorption/photocatalysis | UV | Imazapyr pesticide | 98%removal in 180 min | 10 and 3 times more activity than Ga2O3 and UV100 | Loss of activity in 5 cycles | [86] |
3.2. Design of (Bifunctional) Catalysts Favouring the Increase in Oxidant Species Generation Yield (Mainly HOx)
3.3. Wave-Assisted Photocatalysis
3.4. Synergistic Effect: Photocatalysis/Photo-Fenton
3.5. Persulfate Addition to the Photo-Assisted Process
| Catalyst | Processes | Conditions | Pollutant | Yield | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| Fe2O3 doped-C3N4 | Photocatalysis-photo-Fenton | Visible light | Dicamba pesticide | LEDs light | No TOC measured | [106] | |
| FeVO4@BiOCl | Photocatalysis-photo-Fenton-sonophotocatalysis | UV light | p-nitrophenol | 89% mineralization in 40 min | Additional OH· by the V5+/V4+ cycle | Stability decreased from the 6th cycle | [98] |
| LaFeO3/BiOBr | Photocatalysis-photo-Fenton | Sunlight | Rhodamine B | 99.6% removal RhB in 30 min | Kinetic constant rate is 21 times higher than LFO | Stable just until 4th cycle | [99] |
| FeOOH/Bi2MoO6 | Photocatalysis-photo-Fenton | Visible light | Phenol | 100% removal phenol (3 h) | Synergy: removal 1.54 higher than photocatalysis 1.33 higher than Fenton O vacancies improved photocatalysis | After 5th cycle a step decrease in activity Iron leached detected. 48% TOC removal | [100] |
| Carbon quantum dots/α-FeOOH | Photocatalysis-photo-Fenton | Sunlight | tetracycline | 94% removal in 60 min | Oxidation at different pH values; Removal drastically higher than in separate processes | Less H2O2 consumption, but necessary for oxidation; stability decreases after 5th cycle | [101] |
| TiO2-graphene oxide-Fe3O4 | Photocatalysis-photo-Fenton | Visible light | Amoxicillin antibiotic | 90% TOC pH = 3 50% TOC pH = 5 in 120 min | Magnetic recovery properties | TOC degradation is kept constant until 5th run; leached iron detected in all runs (<1 mg/L) | [102] |
| Fe-Cu oxide/diamond | Photocatalysis-photo-Fenton | Visible light | Phenol | 100% phenol removal in 120 min pH = 4 | Iron and copper cycle involved in Fenton reactions | Not good reusability; Expensive support | [103] |
| Zn1−1.5xFexS/g-C3N4 | Photocatalysis-photo-Fenton | Visible light | p-nitrophenol | 96% removal and 55% TOC in 60 min | Incorporation of Fe in crystal lattice improves degradation rates | Not photocatalytic contribution; Ow reusability of the catalysts | [109] |
| FeTiO3 | Photocatalysis-photo-Fenton | Solar light, visible light | Phenol and sulfonamides | 100% removal and 98% mineralization | Synergy between processes; low cost of catalyst. | Small iron concentration leached | [104,111,112] |
| FeTiO3 | Photocatalysis-photo-Fenton | UV–Vis; high temperature | Real hospital wastewater | 80% TOC in 300 min | Synergy between processes; low cost of catalyst | Small iron concentration leached | [113] |
| La1−xTixFeO3 | Photocatalysis-photo-Fenton | UV, solar and visible light | 4-Cl-phenol | 100% and 100% mineralization in 120 min | One single-phase catalyst; the substitution lattice improved redox properties | No iron detected | [107,108,114] |
| Graphene/Fe3O4 | Photocatalysis-photo-Fenton | UV light | Methyl orange | 99% removal of dye | Synergy with respect to Fe3O4 | Slight decrease in activity | [105] |
| Catalyst | Processes | Conditions | Pollutant | Yield | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| TiO2/g-C3N4 | PS/photocatalysis | Vis light | Acetaminophen | 100% removal in 30 min 86% TOC in 60 min | Persulfate got 13 times activity than single photocatalyst | Reduction of 5 percent of activity after 5 cycles | [81] |
| ZnO | PS/photocatalysis | Sunlight | Pesticides | 92% TOC in 1000 min | Improvement reaction rate | Generation of big loads of sulphate in solution | [115] |
| Ag/AgBr/ ZnFe2O4 | PS/photocatalysis | Visible light | Carbamazepine | 53% removal; double of activity with PS. | LED light; magnetic separation | When using H2O2 the Z-scheme was inhibited and a reduction of the degradation | [116] |
| Co-doped Bi2Fe4O9 | PS/photocatalysis | Visible light | Levofloxacin | LED light; doped materials have 3.52 times higher than that non-doped; | Iron and cobalt leaching | [117] | |
| Ilmenite | PS/photocatalysis | UV light | Azo dye | >95% of mineralization | improvement reaction rate; no iron leached | Generation of big loads of sulphate in solution | [118] |
4. Catalyst Immobilisation: Intensification of Reactor Design
| Catalyst/ Support | Conditions | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| TiO2/β-SiC | Pesticide pyrimethanil /photocatalysis | Titania coating optimized; no active phase release | [121] | |
| TiO2/β-SiC | Nanoplastic pollutants /photocatalysis | Titania coating optimized; no active phase release | [120] | |
| TiO2/ZrO2 | CEC and disinfection /photocatalysis | No evaluation of active phase release | [126] | |
| TiO2@β-SiC | Diuron pesticide /photocatalysis | Single unique phase; no separation between the active phase and the support. | No active phase release | [122,123] |
| Perovskite/monolith | Methylparaben /photo-Fenton | Photo-Fenton at pH0 = 7 | [125] | |
| TiO2-FeSO4/metallic foam | Malachite green dye/photo-Fenton | LED employment | Small amount of Fe leached | [124] |
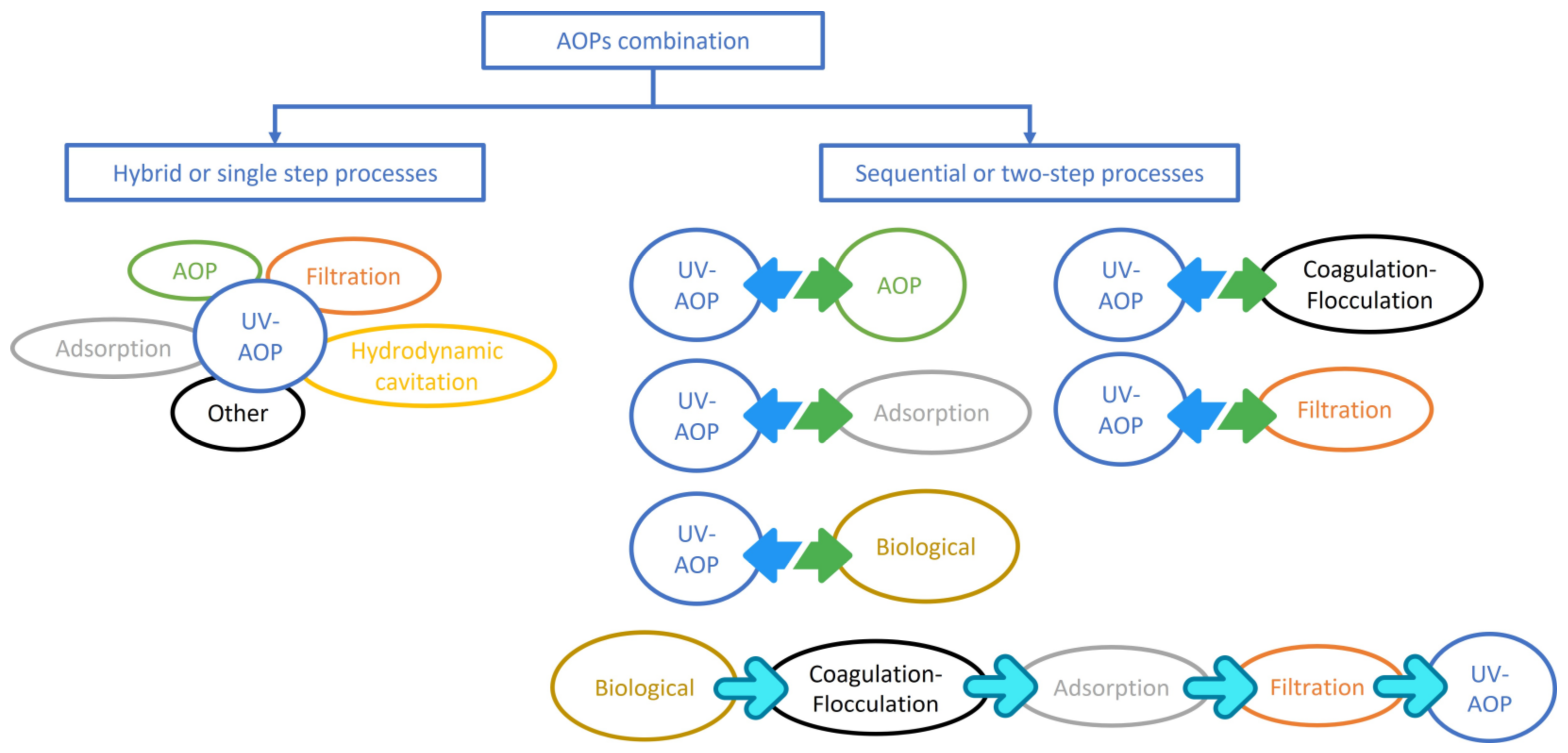
5. Process Combination
5.1. Hybrid/Integrated Systems including at Least One Photo-Assisted AOP
5.2. Sequential or Two-Step Combination Processes
6. Concluding Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. Chem. Eng. 2022, 6, 8. [Google Scholar] [CrossRef]
- Ushani, U.; Lu, X.; Wang, J.; Zhang, Z.; Dai, J.; Tan, Y.; Wang, S.; Li, W.; Niu, C.; Cai, T.; et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the–art review. Chem. Eng. J. 2020, 402, 126232. [Google Scholar] [CrossRef]
- Brienza, M.; Katsoyiannis, I.A. Sulfate Radical Technologies as Tertiary Treatment for the Removal of Emerging Contaminants from Wastewater. Sustainability 2017, 9, 1604. [Google Scholar] [CrossRef]
- Salazar, L.M.; Grisales, C.M.; Garcia, D.P. How does intensification influence the operational and environmental performance of photo-Fenton processes at acidic and circumneutral pH. Environ. Sci. Pollut. Res. 2018, 26, 4367–4380. [Google Scholar] [CrossRef] [PubMed]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Kiss, A.A. Process Intensification. Comput. Aided Chem. Eng. 2014, 397–448. [Google Scholar] [CrossRef]
- Huang, K.; Wang, S.-J.; Shan, L.; Zhu, Q.; Qian, J. Seeking synergistic effect—A key principle in process intensification. Sep. Purif. Technol. 2007, 57, 111–120. [Google Scholar] [CrossRef]
- Mesones, S.; Mena, E.; López-Muñoz, M.J.; Adán, C.; Marugán, J. Synergistic and antagonistic effects in the photoelectrocatalytic disinfection of water with TiO2 supported on activated carbon as a bipolar electrode in a novel 3D photoelectrochemical reactor. Sep. Purif. Technol. 2020, 247, 117002. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Photocatalysis fundamentals revisited to avoid several misconceptions. Appl. Catal. B Environ. 2010, 99, 461–468. [Google Scholar] [CrossRef]
- Azzaz, A.; Jellali, S.; Hamed, N.; El Jery, A.; Khezami, L.; Assadi, A.; Amrane, A. Photocatalytic Treatment of Wastewater Containing Simultaneous Organic and Inorganic Pollution: Competition and Operating Parameters Effects. Catalysts 2021, 11, 855. [Google Scholar] [CrossRef]
- Ochoa-Gutiérrez, K.S.; Tabares-Aguilar, E.; Mueses, M.; Machuca-Martínez, F.; Puma, G.L. A Novel Prototype Offset Multi Tubular Photoreactor (OMTP) for solar photocatalytic degradation of water contaminants. Chem. Eng. J. 2018, 341, 628–638. [Google Scholar] [CrossRef]
- Elhalil, A.; Elmoubarki, R.; Farnane, M.; Machrouhi, A.; Sadiq, M.; Mahjoubi, F.; Qourzal, S.; Barka, N. Photocatalytic degradation of caffeine as a model pharmaceutical pollutant on Mg doped ZnO-Al2O3 heterostructure. Environ. Nanotechnol. Monit. Manag. 2018, 10, 63–72. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.; Faria, J.L.; Hentati, O.; Silva, A.M.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- León, D.E.; Zúñiga-Benítez, H.; Peñuela, G.A.; Mansilla, H.D. Photocatalytic Removal of the Antibiotic Cefotaxime on TiO2 and ZnO Suspensions Under Simulated Sunlight Radiation. Water Air Soil Pollut. 2017, 228, 361. [Google Scholar] [CrossRef]
- Martínez, C.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials. Appl. Catal. B Environ. 2011, 107, 110–118. [Google Scholar] [CrossRef]
- Garrido, I.; Flores, P.; Hellín, P.; Vela, N.; Navarro, S.; Fenoll, J. Solar reclamation of agro-wastewater polluted with eight pesticides by heterogeneous photocatalysis using a modular facility. A case study. Chemosphere 2020, 249, 126156. [Google Scholar] [CrossRef]
- Wang, B.; Fu, T.; An, B.; Liu, Y. UV light-assisted persulfate activation by Cu0-Cu2O for the degradation of sulfamerazine. Sep. Purif. Technol. 2020, 251, 117321. [Google Scholar] [CrossRef]
- Tao, Y.; Ni, Q.; Wei, M.; Xia, D.; Li, X.; Xu, A. Metal-free activation of peroxymonosulfate by g-C3N4 under visible light irradiation for the degradation of organic dyes. RSC Adv. 2015, 5, 44128–44136. [Google Scholar] [CrossRef]
- Casado, C.; Marugán, J.; Timmers, R.; Muñoz, M.; van Grieken, R. Comprehensive multiphysics modeling of photocatalytic processes by computational fluid dynamics based on intrinsic kinetic parameters determined in a differential photoreactor. Chem. Eng. J. 2017, 310, 368–380. [Google Scholar] [CrossRef] [Green Version]
- Carbajo, J.; Tolosana-Moranchel, A.; Casas, J.A.; Faraldos, M.; Bahamonde, A. Analysis of photoefficiency in TiO2 aqueous suspensions: Effect of titania hydrodynamic particle size and catalyst loading on their optical properties. Appl. Catal. B Environ. 2018, 221, 1–8. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, A.; Casas, J.; Carbajo, J.; Faraldos, M.; Bahamonde, A. Influence of TiO2 optical parameters in a slurry photocatalytic reactor: Kinetic modelling. Appl. Catal. B Environ. 2017, 200, 164–173. [Google Scholar] [CrossRef]
- Acosta-Herazo, R.; Mueses, M.; Puma, G.L.; Machuca-Martínez, F. Impact of photocatalyst optical properties on the efficiency of solar photocatalytic reactors rationalized by the concepts of initial rate of photon absorption (IRPA) dimensionless boundary layer of photon absorption and apparent optical thickness. Chem. Eng. J. 2018, 356, 839–849. [Google Scholar] [CrossRef]
- Grčić, I.; Puma, G.L. Six-flux absorption-scattering models for photocatalysis under wide-spectrum irradiation sources in annular and flat reactors using catalysts with different optical properties. Appl. Catal. B Environ. 2017, 211, 222–234. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Antonopoulou, M.; Sykiotou, M.; Εvgenidou, E.; Konstantinou, I.; Lambropoulou, D. Photo-Fenton and Fenton-like processes for the treatment of the antineoplastic drug 5-fluorouracil under simulated solar radiation. Environ. Sci. Pollut. Res. 2016, 24, 4791–4800. [Google Scholar] [CrossRef] [PubMed]
- Villota, N.; Ferreiro, C.; Qulatein, H.; Lomas, J.; Lombraña, J. Turbidity Changes during Carbamazepine Oxidation by Photo-Fenton. Catalysts 2021, 11, 894. [Google Scholar] [CrossRef]
- Coelho, A.; Castro, A.V.; Dezotti, M.; Sant’Anna, G. Treatment of petroleum refinery sourwater by advanced oxidation processes. J. Hazard. Mater. 2006, 137, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Reina, A.; Miralles-Cuevas, S.; López, J.C.; Pérez, J.S. Pyrimethanil degradation by photo-Fenton process: Influence of iron and irradiance level on treatment cost. Sci. Total Environ. 2017, 605-606, 230–237. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Seid, M.G.; Byun, J.; Kim, W.; Cho, K.; Hong, S.W. Changes in levels of N-nitrosamine formed from amine-containing compounds during chloramination via photocatalytic pretreatment with immobilized TiO2: Effect of source water and pH. J. Hazard. Mater. 2021, 424, 127398. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef] [PubMed]
- Carbajo, J.; Jiménez, M.; Miralles, S.; Malato, S.; Faraldos, M.; Bahamonde, A. Study of application of titania catalysts on solar photocatalysis: Influence of type of pollutants and water matrices. Chem. Eng. J. 2016, 291, 64–73. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; Sandim, L.D.R.; Bogo, D.; Barbosa, A.M.J.; Osugi, M.E.; Blanco, M.; De Oliveira, S.C.; Matos, M.D.F.C.; Machulek, A.; Ferreira, V.S. Application of Fenton, photo-Fenton, solar photo-Fenton, and UV/H2O2 to degradation of the antineoplastic agent mitoxantrone and toxicological evaluation. Environ. Sci. Pollut. Res. 2012, 20, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Governo, M.; Santos, M.S.F.; Alves, A.; Madeira, L.M. Degradation of the cytostatic 5-Fluorouracil in water by Fenton and photo-assisted oxidation processes. Environ. Sci. Pollut. Res. 2016, 24, 844–854. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of the antibiotics amoxicillin, ampicillin and cloxacillin in aqueous solution by the photo-Fenton process. J. Hazard. Mater. 2009, 172, 1476–1481. [Google Scholar] [CrossRef]
- El Shahawy, A.; Mohamadien, R.H.; El-Fawal, E.M.; Moustafa, Y.M.; Dawood, M.M.K. Hybrid Photo-Fenton oxidation and biosorption for petroleum wastewater treatment and optimization using Box–Behnken Design. Environ. Technol. Innov. 2021, 24, 101834. [Google Scholar] [CrossRef]
- Tawfik, A.; Alalm, M.G.; Awad, H.M.; Islam, M.; Qyyum, M.A.; Al-Muhtaseb, A.H.; Osman, A.I.; Lee, M. Solar photo-oxidation of recalcitrant industrial wastewater: A review. Environ. Chem. Lett. 2022, 20, 1839–1862. [Google Scholar] [CrossRef]
- Arzate, S.; Campos-Mañas, M.; Miralles-Cuevas, S.; Agüera, A.; Sánchez, J.G.; Pérez, J.S. Removal of contaminants of emerging concern by continuous flow solar photo-Fenton process at neutral pH in open reactors. J. Environ. Manag. 2020, 261, 110265. [Google Scholar] [CrossRef] [PubMed]
- Prada-Vásquez, M.A.; Estrada-Flórez, S.E.; Serna-Galvis, E.A.; Torres-Palma, R.A. Developments in the intensification of photo-Fenton and ozonation-based processes for the removal of contaminants of emerging concern in Ibero-American countries. Sci. Total Environ. 2020, 765, 142699. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Silva, G.D.; Marson, E.O.; Batista, L.L.; Ueira-Vieira, C.; Starling, M.C.V.; Trovó, A.G. Contrasting the performance of photo-Fenton at neutral pH in the presence of different organic iron-complexes using hydrogen peroxide or persulfate as oxidants for naproxen degradation and removal of antimicrobial activity. Process. Saf. Environ. Prot. 2021, 147, 798–807. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Liu, J.; Shan, N.; Zhang, H.; Dionysiou, D.D. Visible light-assisted heterogeneous Fenton with ZnFe2O4 for the degradation of Orange II in water. Appl. Catal. B Environ. 2016, 182, 456–468. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Q.; Wen, Y.; Liu, W. Fe-g-C3N4/graphitized mesoporous carbon composite as an effective Fenton-like catalyst in a wide pH range. Appl. Catal. B Environ. 2017, 201, 232–240. [Google Scholar] [CrossRef]
- Arzate-Salgado, S.-Y.; Morales-Pérez, A.-A.; Solís-López, M.; Ramírez-Zamora, R.-M. Evaluation of metallurgical slag as a Fenton-type photocatalyst for the degradation of an emerging pollutant: Diclofenac. Catal. Today 2016, 266, 126–135. [Google Scholar] [CrossRef]
- Buitrago, J.L.; Sanabria, J.; Gútierrez-Zapata, H.M.; Urbano-Ceron, F.J.; García-Barco, A.; Osorio-Vargas, P.; Rengifo-Herrera, J.A. Photo-Fenton process at natural conditions of pH, iron, ions, and humic acids for degradation of diuron and amoxicillin. Environ. Sci. Pollut. Res. 2019, 27, 1608–1624. [Google Scholar] [CrossRef]
- De la Cruz, N.; Esquius, L.; Grandjean, D.; Magnet, A.; Tungler, A.; de Alencastro, L.; Pulgarín, C. Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res. 2013, 47, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Tambat, S.; Umale, S.; Sontakke, S. Photocatalytic degradation of Milling Yellow dye using sol–gel synthesized CeO. Mater. Res. Bull. 2016, 76, 466–472. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lee, H.-S. Effects of TiO2 coating dosage and operational parameters on a TiO2/Ag photocatalysis system for decolorizing Procion red MX-5B. J. Hazard. Mater. 2010, 179, 462–470. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Hsu, Y.-H. Effects of Reaction Temperature on the Photocatalytic Activity of TiO2 with Pd and Cu Cocatalysts. Catalysts 2021, 11, 966. [Google Scholar] [CrossRef]
- Silveira, J.E.; Garcia-Costa, A.L.; Carbajo, J.; Ribeiro, A.R.; Pliego, G.; Paz, W.S.; Zazo, J.A.; Casas, J.A. Nitrate removal in saline water by photo-reduction using natural FeTiO3 as catalyst. Chem. Eng. J. Adv. 2022, 12, 100387. [Google Scholar] [CrossRef]
- Bloh, J.Z. Intensification of Heterogeneous Photocatalytic Reactions Without Efficiency Losses: The Importance of Surface Catalysis. Catal. Lett. 2021, 151, 3105–3113. [Google Scholar] [CrossRef]
- Prieto-Rodríguez, L.; Spasiano, D.; Oller, I.; Fernández-Calderero, I.; Agüera, A.; Malato, S. Solar photo-Fenton optimization for the treatment of MWTP effluents containing emerging contaminants. Catal. Today 2013, 209, 188–194. [Google Scholar] [CrossRef]
- Pérez, M.; Torrades, F.; Domènech, X.; Peral, J. Fenton and photo-Fenton oxidation of textile effluents. Water Res. 2001, 36, 2703–2710. [Google Scholar] [CrossRef]
- Torrades, F.; Pérez, M.; Mansilla, H.D.; Peral, J. Experimental design of Fenton and photo-Fenton reactions for the treatment of cellulose bleaching effluents. Chemosphere 2003, 53, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Sagawe, G.; Lehnard, A.; Lübber, M.; Bahnemann, D. The insulated solar fenton hybrid process: Fundamental investi-gations. Helv. Chim. Acta 2001, 84, 3742–3759. [Google Scholar] [CrossRef]
- Gernjak, W.; Fuerhacker, M.; Fernández-Ibañez, P.; Blanco, J.; Malato, S. Solar photo-Fenton treatment—Process parameters and process control. Appl. Catal. B Environ. 2006, 64, 121–130. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wang, Y. Degradation of phenol by a heterogeneous photo-Fenton process using Fe/Cu/Al catalysts. RSC Adv. 2016, 6, 13168–13176. [Google Scholar] [CrossRef]
- Expósito, A.J.; Monteagudo, J.M.; Díaz, I.; Durán, A. Photo-fenton degradation of a beverage industrial effluent: Intensification with persulfate and the study of radicals. Chem. Eng. J. 2016, 306, 1203–1211. [Google Scholar] [CrossRef]
- Zapata, A.; Oller, I.; Bizani, E.; Sánchez-Pérez, J.; Maldonado, M.; Malato, S. Evaluation of operational parameters involved in solar photo-Fenton degradation of a commercial pesticide mixture. Catal. Today 2009, 144, 94–99. [Google Scholar] [CrossRef]
- Carbajo, J.; Silveira, J.; Pliego, G.; Zazo, J.; Casas, J. Increasing Photo-Fenton process Efficiency: The effect of high temperatures. Sep. Purif. Technol. 2021, 271, 118876. [Google Scholar] [CrossRef]
- Vallés, I.; Santos-Juanes, L.; Amat, A.; Moreno-Andrés, J.; Arques, A. Effect of Salinity on UVA-Vis Light Driven Photo-Fenton Process at Acidic and Circumneutral pH. Water 2021, 13, 1315. [Google Scholar] [CrossRef]
- Pliego, G.; Xekoukoulotakis, N.; Venieri, D.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J.; Mantzavinos, D. Complete degradation of the persistent anti-depressant sertraline in aqueous solution by solar photo-Fenton oxidation. J. Chem. Technol. Biotechnol. 2014, 89, 814–818. [Google Scholar] [CrossRef]
- Hermosilla, D.; Merayo, N.; Ordóñez, R.; Blanco, Á. Optimization of conventional Fenton and ultraviolet-assisted oxidation processes for the treatment of reverse osmosis retentate from a paper mill. Waste Manag. 2012, 32, 1236–1243. [Google Scholar] [CrossRef]
- Pouran, S.R.; Aziz, A.A.; Daud, W.M.A.W. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Sirtori, C.; Zapata, A.; Gernjak, W.; Malato, S.; Lopez, A.; Agüera, A. Solar photo-Fenton degradation of nalidixic acid in waters and wastewaters of different composition. Analytical assessment by LC–TOF-MS. Water Res. 2011, 45, 1736–1744. [Google Scholar] [CrossRef]
- Hermosilla, D.; Cortijo, M.; Huang, C.P. Optimizing the treatment of landfill leachate by conventional Fenton and photo-Fenton processes. Sci. Total Environ. 2009, 407, 3473–3481. [Google Scholar] [CrossRef]
- Cunha-Filho, F.J.; Mota-Lima, A.; Ratkievicius, L.A.; Silva, D.J.; Silva, D.N.; Chiavone-Filho, O.; Nascimento, C.A.O.D. Rapid mineralization rate of acetylsalicylic acid in a tubular photochemical reactor: The role of the optimized excess of H2O2. J. Water Process. Eng. 2019, 31, 100856. [Google Scholar] [CrossRef]
- Batista, A.P.S.; Nogueira, R.F.P. Parameters affecting sulfonamide photo-Fenton degradation–Iron complexation and substituent group. J. Photochem. Photobiol. A Chem. 2012, 232, 8–13. [Google Scholar] [CrossRef]
- Chu, W.; Chan, K.; Kwan, C.; Choi, K. Degradation of atrazine by modified stepwise-Fenton’s processes. Chemosphere 2007, 67, 755–761. [Google Scholar] [CrossRef]
- Monteagudo, J.; Durán, A.; Martín, I.S.; Aguirre, M. Effect of continuous addition of H2O2 and air injection on ferrioxalate-assisted solar photo-Fenton degradation of Orange II. Appl. Catal. B Environ. 2009, 89, 510–518. [Google Scholar] [CrossRef]
- Prieto-Rodríguez, L.; Oller, I.; Zapata, A.; Agüera, A.; Malato, S. Hydrogen peroxide automatic dosing based on dissolved oxygen concentration during solar photo-Fenton. Catal. Today 2011, 161, 247–254. [Google Scholar] [CrossRef]
- Yu, X.; Somoza-Tornos, A.; Graells, M.; Pérez-Moya, M. An experimental approach to the optimization of the dosage of hydrogen peroxide for Fenton and photo-Fenton processes. Sci. Total Environ. 2020, 743, 140402. [Google Scholar] [CrossRef] [PubMed]
- Saldaña-Flores, K.E.; Flores-Estrella, R.A.; Alcaraz-Gonzalez, V.; Carissimi, E.; de Souza, B.G.; Ruotolo, L.A.M.; Urquieta-Gonzalez, E. Regulation of Hydrogen Peroxide Dosage in a Heterogeneous Photo-Fenton Process. Processes 2021, 9, 2167. [Google Scholar] [CrossRef]
- Silva, T.F.; Fonseca, A.; Saraiva, I.; Boaventura, R.A.; Vilar, V.J. Scale-up and cost analysis of a photo-Fenton system for sanitary landfill leachate treatment. Chem. Eng. J. 2015, 283, 76–88. [Google Scholar] [CrossRef]
- Devi, L.G.; Srinivas, M.; ArunaKumari, M. Heterogeneous advanced photo- Fenton process using peroxymonosulfate and peroxydisulfate in presence of zero valent metallic iron: A comparative study with hydrogen peroxide photo-Fenton process. J. Water Process. Eng. 2016, 13, 117–126. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2020, 406, 127083. [Google Scholar] [CrossRef]
- Ge, M.; Hu, Z.; Wei, J.; He, Q.; He, Z. Recent advances in persulfate-assisted TiO2-based photocatalysis for wastewater treatment: Performances, mechanism and perspectives. J. Alloys Compd. 2021, 888, 161625. [Google Scholar] [CrossRef]
- Ismail, L.; Ferronato, C.; Fine, L.; Jaber, F.; Chovelon, J.-M. Elimination of sulfaclozine from water with SO4−radicals: Evaluation of different persulfate activation methods. Appl. Catal. B Environ. 2017, 201, 573–581. [Google Scholar] [CrossRef]
- Ding, H.; Hu, J. Degradation of ibuprofen by UVA-LED/TiO2/persulfate process: Kinetics, mechanism, water matrix effects, intermediates and energy consumption. Chem. Eng. J. 2020, 397, 125462. [Google Scholar] [CrossRef]
- Du, X.; Bai, X.; Xu, L.; Yang, L.; Jin, P. Visible-light activation of persulfate by TiO2/g-C3N4 photocatalyst toward efficient degradation of micropollutants. Chem. Eng. J. 2019, 384, 123245. [Google Scholar] [CrossRef]
- Carbajo, J.; Adán, C.; Rey, A.; Martínez-Arias, A.; Bahamonde, A. Optimization of H2O2 use during the photocatalytic degradation of ethidium bromide with TiO2 and iron-doped TiO2 catalysts. Appl. Catal. B Environ. 2010, 102, 85–93. [Google Scholar] [CrossRef]
- Cuervo Lumbaque, E.; Sirtori, C.; Vilar, V.J. Heterogeneous photocatalytic degradation of pharmaceuticals in synthetic and real matrices using a tube-in-tube membrane reactor with radial addition of H2O2. Sci. Total Environ. 2020, 743, 140629. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, C.; Chen, P.; Chu, X.; Zhu, L. Enhanced photocatalytic performance of boron doped Bi2WO6 nanosheets under simulated solar light irradiation. J. Hazard. Mater. 2013, 254, 185–192. [Google Scholar] [CrossRef]
- García-Muñoz, P.; Zussblatt, N.P.; Pliego, G.; Zazo, J.A.; Fresno, F.; Chmelka, B.F.; Casas, J.A. Evaluation of photoassisted treatments for norfloxacin removal in water using mesoporous Fe2O3-TiO2 materials. J. Environ. Manag. 2019, 238, 243–250. [Google Scholar] [CrossRef]
- Ismail, A.A.; Abdelfattah, I.; Faisal, M.; Helal, A. Efficient photodecomposition of herbicide imazapyr over mesoporous Ga2O3-TiO2 nanocomposites. J. Hazard. Mater. 2018, 342, 519–526. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Nikoloski, A.N.; Bahri, P.A.; Li, D. Adsorption and photo-Fenton catalytic degradation of organic dyes over crystalline LaFeO3-doped porous silica. RSC Adv. 2018, 8, 36181–36190. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Nikoloski, A.N.; Bahri, P.A.; Li, D. Enhanced removal of organic using LaFeO3-integrated modified natural zeolites via heterogeneous visible light photo-Fenton degradation. J. Environ. Manag. 2018, 233, 471–480. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Liu, J.; Sun, X. Mesoporous Au/TiO2 Nanocomposite Microspheres for Visible-Light Photocatalysis. Chem. Eur. J. 2012, 18, 5361–5366. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, L.; Zhang, G.; Chen, L.; Guo, X.; Liu, M. Porous Solid Superacid SO42–/Fe2–xZrxO3 Fenton Catalyst for Highly Effective Oxidation of X-3B under Visible Light. Ind. Eng. Chem. Res. 2013, 52, 16698–16708. [Google Scholar] [CrossRef]
- Simsek, E.B.; Tuna, Ö.; Balta, Z. Construction of stable perovskite-type LaFeO3 particles on polymeric resin with boosted photocatalytic Fenton-like decaffeination under solar irradiation. Sep. Purif. Technol. 2019, 237, 116384. [Google Scholar] [CrossRef]
- Barik, A.J.; Gogate, P.R. Degradation of 4-chloro 2-aminophenol using combined strategies based on ultrasound, photolysis and ozone. Ultrason. Sonochem. 2016, 28, 90–99. [Google Scholar] [CrossRef]
- Sheydaei, M.; Zangouei, M.; Vatanpour, V. Coupling visible light sono-photocatalysis and sono-enhanced ultrafiltration processes for continuous flow degradation of dyestuff using N-doped titania nanoparticles. Chem. Eng. Process. Process. Intensif. 2019, 143. [Google Scholar] [CrossRef]
- Kakavandi, B.; Bahari, N.; Kalantary, R.R.; Fard, E.D. Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: A new hybrid system. Ultrason. Sonochem. 2019, 55, 75–85. [Google Scholar] [CrossRef]
- Babu, S.G.; Karthik, P.; John, M.C.; Lakhera, S.K.; Ashokkumar, M.; Khim, J.; Neppolian, B. Synergistic effect of sono-photocatalytic process for the degradation of organic pollutants using CuO-TiO2/rGO. Ultrason. Sonochem. 2018, 50, 218–223. [Google Scholar] [CrossRef]
- Geng, N.; Chen, W.; Xu, H.; Lin, T.; Ding, M.; Wang, Y.; Tao, H.; Hu, K. Preparation of Fe3O4/TiO2-N-GO sonocatalyst and using for humic acid removal with the assist of ultrasound. Mater. Sci. Semicond. Process. 2019, 102, 104593. [Google Scholar] [CrossRef]
- Isari, A.A.; Hayati, F.; Kakavandi, B.; Rostami, M.; Motevassel, M.; Dehghanifard, E. N, Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: An effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem. Eng. J. 2019, 392, 123685. [Google Scholar] [CrossRef]
- Eshaq, G.; Wang, S.; Sun, H.; Sillanpää, M. Core/shell FeVO4@BiOCl heterojunction as a durable heterogeneous Fenton catalyst for the efficient sonophotocatalytic degradation of p-nitrophenol. Sep. Purif. Technol. 2019, 231, 115915. [Google Scholar] [CrossRef]
- Guan, S.; Yang, H.; Sun, X.; Xian, T. Preparation and promising application of novel LaFeO3/BiOBr heterojunction photocatalysts for photocatalytic and photo-Fenton removal of dyes. Opt. Mater. 2019, 100, 109644. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Cui, J.; An, W.; Liu, L.; Liang, Y.; Cui, W. Surface oxygen vacancies enriched FeOOH/Bi2MoO6 photocatalysis- fenton synergy degradation of organic pollutants. J. Hazard. Mater. 2019, 384, 121399. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Q.; Liu, P.; Ma, S.; Xie, B.; Yang, K.; Zhao, Y. Novel up-conversion carbon quantum dots/α-FeOOH nanohybrids eliminate tetracycline and its related drug resistance in visible-light responsive Fenton system. Appl. Catal. B Environ. 2019, 263, 118336. [Google Scholar] [CrossRef]
- Li, Q.; Kong, H.; Li, P.; Shao, J.; He, Y. Photo-Fenton degradation of amoxicillin via magnetic TiO2-graphene oxide-Fe3O4 composite with a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). J. Hazard. Mater. 2019, 373, 437–446. [Google Scholar] [CrossRef]
- Manickam-Periyaraman, P.; Espinosa, J.C.; Ferrer, B.; Subramanian, S.; Álvaro, M.; García, H.; Navalón, S. Bimetallic iron-copper oxide nanoparticles supported on nanometric diamond as efficient and stable sunlight-assisted Fenton photocatalyst. Chem. Eng. J. 2020, 393, 124770. [Google Scholar] [CrossRef]
- García-Muñoz, P.; Pliego, G.; Zazo, J.; Bahamonde, A.; Casas, J. Ilmenite (FeTiO3) as low cost catalyst for advanced oxidation processes. J. Environ. Chem. Eng. 2016, 4, 542–548. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Ahmad, I.; Israr, M. Graphene/Fe3O4 nanocomposite: Interplay between photo-Fenton type reaction, and carbon purity for the removal of methyl orange. Ceram. Int. 2018, 44, 2643–2648. [Google Scholar] [CrossRef]
- Desipio, M.M.; Van Bramer, S.E.; Thorpe, R.; Saha, D. Photocatalytic and photo-fenton activity of iron oxide-doped carbon nitride in 3D printed and LED driven photon concentrator. J. Hazard. Mater. 2019, 376, 178–187. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Lefevre, C.; Robert, D.; Keller, N. Ti-substituted LaFeO3 perovskite as photoassisted CWPO catalyst for water treatment. Appl. Catal. B Environ. 2019, 248, 120–128. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Fresno, F.; Lefevre, C.; Robert, D.; Keller, N. Highly robust La1−xTixFeO3 dual catalyst with combined photocatalytic and photo-CWPO activity under visible light for 4-chlorophenol removal in water. Appl. Catal. B Environ. 2019, 262, 118310. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Xu, P.; Li, Y.; Duan, J.; Zhang, G.; Hu, L.; Wang, X.; Zhang, W. Visible-light-driven photo-Fenton reactions using Zn1-1.5Fe S/g-C3N4 photocatalyst: Degradation kinetics and mechanisms analysis. Appl. Catal. B Environ. 2020, 266, 118653. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- García-Muñoz, P.; Pliego, G.; Zazo, J.A.; Casas, J.A. Photocatalytic wet peroxide oxidation process at circumneutral pH using ilmenite as catalyst. J. Environ. Chem. Eng. 2018, 6, 7312–7317. [Google Scholar] [CrossRef]
- García-Muñoz, P.; Pliego, G.; Zazo, J.; Bahamonde, A.; Casas, J. Sulfonamides photoassisted oxidation treatments catalyzed by ilmenite. Chemosphere 2017, 180, 523–530. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, P.; Pliego, G.; Zazo, J.; Barbero, B.; Bahamonde, A.; Casas, J. Modified ilmenite as catalyst for CWPO-Photoassisted process under LED light. Chem. Eng. J. 2017, 318, 89–94. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Fresno, F.; Lefevre, C.; Robert, D.; Keller, N. Synergy effect between photocatalysis and heterogeneous photo-Fenton catalysis on Ti-doped LaFeO3 perovskite for high efficiency light-assisted water treatment. Catal. Sci. Technol. 2020, 10, 1299–1310. [Google Scholar] [CrossRef]
- Grilla, E.; Matthaiou, V.; Frontistis, Z.; Oller, I.; Polo, I.; Malato, S.; Mantzavinos, D. Degradation of antibiotic trimethoprim by the combined action of sunlight, TiO2 and persulfate: A pilot plant study. Catal. Today 2018, 328, 216–222. [Google Scholar] [CrossRef]
- Yentür, G.; Dükkancı, M. Fabrication of magnetically separable plasmonic composite photocatalyst of Ag/AgBr/ZnFe2O4 for visible light photocatalytic oxidation of carbamazepine. Appl. Surf. Sci. 2020, 510, 145374. [Google Scholar] [CrossRef]
- Zhong, X.; Zou, Z.-S.; Wang, H.-L.; Huang, W.; Zhou, B.-X. Enhanced Activation of Persulfate by Co-Doped Bismuth Ferrite Nanocomposites for Degradation of Levofloxacin Under Visible Light Irradiation. Materials 2019, 12, 3952. [Google Scholar] [CrossRef]
- Silveira, J.E.; Paz, W.S.; Garcia-Muñoz, P.; Zazo, J.A.; Casas, J.A. UV-LED/ilmenite/persulfate for azo dye mineralization: The role of sulfate in the catalyst deactivation. Appl. Catal. B Environ. 2017, 219, 314–321. [Google Scholar] [CrossRef]
- Robert, D.; Keller, V.; Keller, N. Immobilization of a Semiconductor Photocatalyst on Solid Supports: Methods, Materials, and Applications. In Photocatalysis and Water Purification: From Fundamentals to Recent Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 145–178. [Google Scholar] [CrossRef]
- Allé, P.H.; García-Muñoz, P.; Adouby, K.; Keller, N.; Robert, D. Efficient photocatalytic mineralization of polymethylmethacrylate and polystyrene nanoplastics by TiO2/β-SiC alveolar foams. Environ. Chem. Lett. 2020, 19, 1803–1808. [Google Scholar] [CrossRef]
- M’Bra, I.C.; García-Muñoz, P.; Drogui, P.; Keller, N.; Trokourey, A.; Robert, D. Heterogeneous photodegradation of Pyrimethanil and its commercial formulation with TiO2 immobilized on SiC foams. J. Photochem. Photobiol. A Chem. 2019, 368, 1–6. [Google Scholar] [CrossRef]
- Rico-Santacruz, M.; García-Muñoz, P.; Marchal, C.; Batail, N.; Pham, C.; Robert, D.; Keller, N. Coating-free TiO2@β-SiC alveolar foams as a ready-to-use composite photocatalyst with tunable adsorption properties for water treatment. RSC Adv. 2020, 10, 3817–3825. [Google Scholar] [CrossRef] [PubMed]
- Rico-Santacruz, M.; García-Muñoz, P.; Keller, V.; Batail, N.; Pham, C.; Robert, D.; Keller, N. Alveolar TiO2-β-SiC photocatalytic composite foams with tunable properties for water treatment. Catal. Today 2018, 328, 235–242. [Google Scholar] [CrossRef]
- Abdi, P.; Farzi, A.; Karimi, A. Application of a hybrid enzymatic and photo-fenton process for investigation of azo dye decolorization on TiO2/metal-foam catalyst. J. Taiwan Inst. Chem. Eng. 2017, 71, 137–144. [Google Scholar] [CrossRef]
- Orak, C.; Atalay, S.; Ersöz, G. Photocatalytic and photo-Fenton-like degradation of methylparaben on monolith-supported perovskite-type catalysts. Sep. Sci. Technol. 2017, 52, 1310–1320. [Google Scholar] [CrossRef]
- Martín-Sómer, M.; Pablos, C.; de Diego, A.; van Grieken, R.; Encinas, A.; Monsalvo, V.M.; Marugán, J. Novel macroporous 3D photocatalytic foams for simultaneous wastewater disinfection and removal of contaminants of emerging concern. Chem. Eng. J. 2019, 366, 449–459. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef]
- Spuhler, D.; Rengifo-Herrera, J.A.; Pulgarin, C. The effect of Fe2+, Fe3+, H2O2 and the photo-Fenton reagent at near neutral pH on the solar disinfection (SODIS) at low temperatures of water containing Escherichia coli K12. Appl. Catal. B Environ. 2010, 96, 126–141. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Aliste, M.; Vela, N.; Garrido, I.; Fenoll, J.; Navarro, S. Decline of fluroxypyr and triclopyr residues from pure, drinking and leaching water by photo-assisted peroxonation. Process. Saf. Environ. Prot. 2020, 137, 358–365. [Google Scholar] [CrossRef]
- Martini, J.; Orge, C.A.; Faria, J.L.; Pereira, M.F.R.; Soares, O.S.G. Sulfamethoxazole degradation by combination of advanced oxidation processes. J. Environ. Chem. Eng. 2018, 6, 4054–4060. [Google Scholar] [CrossRef]
- Gmurek, M.; Gomes, J.F.; Martins, R.C.; Quinta-Ferreira, R.M. Comparison of radical-driven technologies applied for paraben mixture degradation: Mechanism, biodegradability, toxicity and cost assessment. Environ. Sci. Pollut. Res. 2019, 26, 37174–37192. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Álvarez, P.M.; Rey, A.; Contreras, S.; Beltrán, F.J. Application of solar photocatalytic ozonation for the degradation of emerging contaminants in water in a pilot plant. Chem. Eng. J. 2015, 260, 399–410. [Google Scholar] [CrossRef]
- Roccamante, M.; Salmerón, I.; Ruiz, A.; Oller, I.; Malato, S. New approaches to solar Advanced Oxidation Processes for elimination of priority substances based on electrooxidation and ozonation at pilot plant scale. Catal. Today 2019, 355, 844–850. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Rey, A.; Mena, E.; Beltrán, F.J. Application of solar photocatalytic ozonation in water treatment using supported TiO2. Appl. Catal. B Environ. 2019, 254, 237–245. [Google Scholar] [CrossRef]
- Buyukada, M. Removal, potential reaction pathways, and overall cost analysis of various pollution parameters and toxic odor compounds from the effluents of turkey processing plant using TiO2–assisted UV/O3 process. J. Environ. Manag. 2019, 248, 109298. [Google Scholar] [CrossRef] [PubMed]
- Fathinia, M.; Khataee, A.; Naseri, A.; Aber, S. Monitoring simultaneous photocatalytic-ozonation of mixture of pharmaceuticals in the presence of immobilized TiO2 nanoparticles using MCR-ALS: Identification of intermediates and multi-response optimization approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1275–1290. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Gągol, M.; Makoś, P.; Khan, J.A.; Boczkaj, G. Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds. Sep. Purif. Technol. 2019, 224, 1–14. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Alonso, E.; Singh, D.N. Photocatalytic Mechanisms for Peroxymonosulfate Activation through the Removal of Methylene Blue: A Case Study. Int. J. Environ. Res. Public Health 2019, 16, 198. [Google Scholar] [CrossRef]
- Talwar, S.; Verma, A.K.; Sangal, V.K.; Štangar, U.L. Once through continuous flow removal of metronidazole by dual effect of photo-Fenton and photocatalysis in a compound parabolic concentrator at pilot plant scale. Chem. Eng. J. 2020, 388, 124184. [Google Scholar] [CrossRef]
- Salmerón, I.; Plakas, K.V.; Sirés, I.; Oller, I.; Maldonado, M.I.; Karabelas, A.J.; Malato, S. Optimization of electrocatalytic H2O2 production at pilot plant scale for solar-assisted water treatment. Appl. Catal. B Environ. 2018, 242, 327–336. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Degradation of methylene blue and congo-red dyes using Fenton, photo-Fenton, sono-Fenton, and sonophoto-Fenton methods in the presence of iron(II,III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol. 2018, 210, 563–573. [Google Scholar] [CrossRef]
- Xu, L.; Chu, W.; Graham, N. Degradation of di-n-butyl phthalate by a homogeneous sono–photo–Fenton process with in situ generated hydrogen peroxide. Chem. Eng. J. 2014, 240, 541–547. [Google Scholar] [CrossRef]
- Kouvelis, K.; Kampioti, A.A.; Petala, A.; Frontistis, Z. Degradation of Sulfamethoxazole Using a Hybrid CuOx–BiVO4/SPS/Solar System. Catalysts 2022, 12, 882. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Wang, F.; Xia, M.; Chen, Q.; Ju, X. Peroxymonosulfate activation through 2D/2D Z-scheme CoAl-LDH/ /BiOBr photocatalyst under visible light for ciprofloxacin degradation. J. Hazard. Mater. 2021, 420, 126613. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. Chem. Eng. J. 2021, 430, 132806. [Google Scholar] [CrossRef]
- Marjanovic, M.; Giannakis, S.; Grandjean, D.; de Alencastro, L.F.; Pulgarin, C. Effect of μM Fe addition, mild heat and solar UV on sulfate radical-mediated inactivation of bacteria, viruses, and micropollutant degradation in water. Water Res. 2018, 140, 220–231. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Giannakis, S.; Marjanovic, M.; Kohantorabi, M.; Gholami, M.R.; Grandjean, D.; de Alencastro, L.F.; Pulgarín, C. Solar-assisted bacterial disinfection and removal of contaminants of emerging concern by Fe2+-activated HSO5-vs. S2O82− in drinking water. Appl. Catal. B Environ. 2019, 248, 62–72. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Mesones, S.; Marugán, J. Hybrid UV-C/microfiltration process in membrane photoreactor for wastewater disinfection. Environ. Sci. Pollut. Res. 2018, 26, 36080–36087. [Google Scholar] [CrossRef] [PubMed]
- Aydiner, C.; Mert, B.K.; Dogan, E.C.; Yatmaz, H.C.; Dagli, S.; Aksu, S.; Tilki, Y.M.; Goren, A.Y.; Balci, E. Novel hybrid treatments of textile wastewater by membrane oxidation reactor: Performance investigations, optimizations and efficiency comparisons. Sci. Total Environ. 2019, 683, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Moslehyani, A.; Mobaraki, M.; Matsuura, T.; Ismail, A.; Othman, M.; Chowdhury, M. Novel green hybrid processes for oily water photooxidation and purification from merchant ship. Desalination 2016, 391, 98–104. [Google Scholar] [CrossRef]
- Kakavandi, B.; Ahmadi, M. Efficient treatment of saline recalcitrant petrochemical wastewater using heterogeneous UV-assisted sono-Fenton process. Ultrason. Sonochem. 2019, 56, 25–36. [Google Scholar] [CrossRef]
- Raut-Jadhav, S.; Saharan, V.K.; Pinjari, D.V.; Saini, D.R.; Sonawane, S.H.; Pandit, A.B. Intensification of degradation of imidacloprid in aqueous solutions by combination of hydrodynamic cavitation with various advanced oxidation processes (AOPs). J. Environ. Chem. Eng. 2013, 1, 850–857. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Y.; Xiang, Y.; Shang, C. Influence of pre-ozonation of DOM on micropollutant abatement by UV-based advanced oxidation processes. J. Hazard. Mater. 2020, 391, 122201. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Wu, J.; Khatebasreh, M.; Ding, D.; Lin, K.-Y.A. Efficient treatment for landfill leachate through sequential electrocoagulation, electrooxidation and PMS/UV/CuFe2O4 process. Sep. Purif. Technol. 2020, 242, 116828. [Google Scholar] [CrossRef]
- Webler, A.D.; Moreira, F.C.; Dezotti, M.W.; Mahler, C.F.; Segundo, I.D.B.; Boaventura, R.A.; Vilar, V.J. Development of an integrated treatment strategy for a leather tannery landfill leachate. Waste Manag. 2019, 89, 114–128. [Google Scholar] [CrossRef]
- Changotra, R.; Rajput, H.; Dhir, A. Treatment of real pharmaceutical wastewater using combined approach of Fenton applications and aerobic biological treatment. J. Photochem. Photobiol. A Chem. 2019, 376, 175–184. [Google Scholar] [CrossRef]
- Bener, S.; Atalay, S.; Ersöz, G. The hybrid process with eco-friendly materials for the treatment of the real textile industry wastewater. Ecol. Eng. 2020, 148. [Google Scholar] [CrossRef]
- Giannakis, S.; Gamarra Vives, F.A.; Grandjean, D.; Magnet, A.; de Alencastro, L.F.; Pulgarin, C. Effect of advanced oxidation processes on the micropollutants and the effluent organic matter contained in municipal wastewater previously treated by three different secondary methods. Water Res. 2015, 84, 295–306. [Google Scholar] [CrossRef]
- Ly, Q.V.; Kim, H.C.; Hur, J. Tracking fluorescent dissolved organic matter in hybrid ultrafiltration systems with TiO2/UV oxidation via EEM-PARAFAC. J. Membr. Sci. 2018, 549, 275–282. [Google Scholar] [CrossRef]
- Piras, F.; Santoro, O.; Pastore, T.; Pio, I.; de Dominicis, E.; Gritti, E.; Caricato, R.; Lionetto, M.G.; Mele, G.; Santoro, D. Controlling micropollutants in tertiary municipal wastewater by O3/H2O2, granular biofiltration and UV254/H2O2 for potable reuse applications. Chemosphere 2020, 239, 124635. [Google Scholar] [CrossRef]
- Ponce-Robles, L.; Oller, I.; Polo-López, M.I.; Rivas-Ibáñez, G.; Malato, S. Microbiological evaluation of combined advanced chemical-biological oxidation technologies for the treatment of cork boiling wastewater. Sci. Total Environ. 2019, 687, 567–576. [Google Scholar] [CrossRef]
- De Oliveira Gonçalves, L.; Starling, M.C.V.M.; Leal, C.D.; Oliveira, D.V.M.; Araújo, J.C.; Leão, M.M.D.; Amorim, C.C. Enhanced biodiesel industry wastewater treatment via a hybrid MBBR combined with advanced oxidation processes: Analysis of active microbiota and toxicity removal. Environ. Sci. Pollut. Res. 2019, 26, 4521–4536. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Amor, C.; Fernandes, J.R.; Tavares, P.B.; Lucas, M.S.; Peres, J.A. Treatment of crystallized-fruit wastewater by UV-A LED photo-Fenton and coagulation-flocculation. Chemosphere 2016, 145, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.M.; Golbini Mofrad, M.M.; Pourzamani, H.; Sebaradar, S.M.; Ebrahim, K. Treatment of industrial wastewater contaminated with recalcitrant metal working fluids by the photo-Fenton process as post-treatment for DAF. J. Ind. Eng. Chem. 2017, 45, 412–420. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Morales, M.; Mosteo, R.; Ormad, M.P.; Ovelleiro, J.L. Inactivation of Enterococcus faecalis, Pseudomonas aeruginosa and Escherichia coli present in treated urban wastewater by coagulation-flocculation and photo-Fenton processes. Photochem. Photobiol. Sci. 2013, 12, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Laski, E.; García-Cañibano, C.; Martín de Vidales, M.J.; Encinas, B.; Kuch, J. Marugán, Micropollutants removal by full-scale UV-C/sulfate radical based Advanced Oxidation Processes. Sci. Total Environ. 2018, 630, 1216–1225. [Google Scholar] [CrossRef] [PubMed]




| Catalyst | Processes | Conditions | Pollutant | Yield | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| CuO-TiO2/rGO | Ultrasound/photocatalysis | UV light | Methyl orange | 99% oxidation in 90 min | Synergy of 3.7 times | No TOC evaluated. Reduction of activity with the reuse | [95] |
| Fe3O4/TiO2–N-GO | Ultrasound/photocatalysis | Visible light | Humic acids | 93% removal | Surface cleaning, improved mass transfer. 26% higher removal compared to single photocatalysis | [96] | |
| N-Cu co-doped TiO2@CNTs | Ultrasound/photocatalysis | Xenon lamp | Sulfamethoxazole in real pharmaceutical wastewater | 100% antibiotic 93% COD 89% TOC | Real pharmaceutical water | Reuse until 6th cycle | [97] |
| TiO2 decorated on magnetic activated carbon | Ultrasound/photocatalysis | UV light | tetracycline | 93% removal 50% TOC in 180 min | Fe leached measured | [94] | |
| N-doped titania | Ultrasound/photocatalysis/ filtration | Visible light | Dye | Synergetic effect higher than 20% | Ultrasonic cleans the membrane | [93] | |
| TiO2 | Microwave/photocatalysis | UV | 4-chloro-2-aminophenol | Improvement in removal of more than 50% | Reaction rates of more than one order of magnitude | High energy consumption | [92] |
| Combination | Target Pollutant | Water Matrix | Maximum Efficiency | Remarks | Ref. |
|---|---|---|---|---|---|
| O3/H2O2/UV-C | Fluroxypyr and Triclopyr (herbicides) | Drinking water (DW) Leaching water (LW) | 92% Fluroxypyr in DW; 62% Triclopyr in DW, reached with O3/H2O2/UV-C system (120 min) | H2O2 = 75 mg/L; O3 = 500 mg/h; pH = 7.3 (LW); 8.2 (DW) | [129] |
| ZnO/Na2S2O8/UV-C | ZnO = 300 mg/L; Na2S2O2 = 300 mg/L; pH = 7.3 (LW); 8.2 (DW) | ||||
| TiO2/Na2S2O8/UV-C | TiO2 = 300 mg/L; Na2S2O2 = 300 mg/L; pH = 7.3 (LW); 8.2 (DW) | ||||
| O3/H2O2/UV-A | Sulfamethoxazole (antibiotic) | Ultrapure water (UW) | 100% (15 min) | H2O2 = 45.3 μL; O3 = 50g/m3; pH = 5.1 | [130] |
| O3/H2O2/UV-A | Methylparaben, ethylparaben, propylparaben, butylparaben and benzylparaben (parabens) | Ultrapure water (UW) | 100% (90 min) | H2O2 = 13.5 mg/L; O3 = 0.2 mg/L; pH = 5.7 | [131] |
| Solar Photo-Fenton/O3 | Acetaminophen (drug), antipyrine (analgesic), bisphenol A (additive), caffeine (drug abuse), metoprolol (β-blocker) and testosterone (hormone) | Milli-Q water (UW) | ≈100% in average (10 min) | Fe(III) = 2.79 mg/L; H2O2/Fe(III) = 6.09 (mass); average incident UV-A solar radiation 41.2 W/m2; O3 = 13 mg/L; pH = 3 | [132] |
| TiO2/Solar radiation/O3 | ≈100% in average (15–20 min) | TiO2 = 200 mg/L; average incident UV-A solar radiation 41.2 W/m2; Ozone = 13 mg/L; pH = 7 | |||
| Solar photo-Fenton/O3 | Pentachlorophenol (plaguicide), Terbutryn (herbicide), Chlorofenvinphos (insecticide), Diclofenac (drug) | Ultrapure water (UW) Natural water (NW) Simulated wastewater (SW) | 80% in average (4.4 kJ/L) in SW | H2O2 = 1.5 mM; Fe(II) and Fe(III) = 0.1 mM; O3 = 0.2–0.6 mg/L; pH = 8 | [133] |
| Solar H2O2/O3 | |||||
| Solar Fe/O3 | |||||
| Solar TiO2/O3 | DEET (insecticide) | Ultrapure water (UW) Real Wastewater (WW) | ≈90% (10 min) | TiO2 = 150 g/L (supported); pH = 8; O3 = 15 mg/L; Isolar =550 W/m2 | [134] |
| TiO2/UV/O3 | Dimethyl silanediol, acetic acid; diisobutyl phthalate (odour compounds) | Industrial wastewater (IW) | 63.8% Dimethyl silanediol; 41.5% acetic acid; 74.2% diisobutyl phthalate (25 min) | O3 = 16 mg/L; pH = 7.5; TiO2 = 3 g/L | [135] |
| TiO2/O3/UV-A | Methyl-dopa and famotidine (drugs), and nalidixic acid (antibiotic) | Ultrapure water (UW) | 84.93–99.15% (30 min) | O3 = 6 L/h; TiO2 (supported) = 12.5 g/L | [136] |
| TiO2/UV/O3/H2O2 | Volatile Organic Compounds mix | Simulated wastewater | 98% (30 min) | TiO2 = 100 mg/L; O3 = 5.92 g; H2O2 = 1.78 g | [137] |
| PMS/TiO2/UV-A | Methylene Blue (dye) | Ultrapure water (UW) | >90% (60 min) | PMS = 0.32 mM; TiO2 = 5 mM; pH = 7 | [138] |
| Solar photo-Fenton/TiO2 | Metronidazole (antibiotic) | Real Wastewater (WW) | ≈60% (15 min) | H2O2 = 450 mg/L; pH = 3.0–3.5; Fe and TiO2 supported | [139] |
| Solar photoelectron-Fenton (Fe3O4/ZnO/graphene) | Pyrimethanil and Methomyl mix (pesticides) | Ultrapure water (UW) | >50% (5 min) | Na2SO4 = 50 mM; pH = 3.0; j = 74 mA/cm2; Q = 5.6 L/min; Qair = 10 L/min; Fe(II) = 0.5 mM; 64.9 mg H2O2/min | [140] |
| Sono photo-Fenton | Methylene blue and Congo red (dyes) | Ultrapure water (UW) | 100% (<60 min) | 40 W UV-C lamps; 40 kHz of US; Fe3O4/ZnO/graphene nanocomposites; Ph = 3 and 13; H2O2 = 4 mL | [141] |
| Sono photo-Fenton | Di-n-butyl phthalate (plasticizer) | Ultrapure water (UW) | 80% (30 min) | Fe(II) = 0.1 mM; 400 kHz; UV-C; pH = 2–9; H2O2 = 0.025–0.2 mM | [142] |
| CuOx–BiVO4/SPS/Solar System | Sulfamethoxazole | Ultrapure water (UW), bottled water (BW) secondary wastewater (WW) | UW: 100% (30 min) BW: 100% (60 min) WW: 60% (120 min) | Solar radiation; [SPS] = 500 mg/L; [Catalyst] = 500 mg/L; 0.75, 3.0 and 10.0 Cu.BVO | [143] |
| CoAl-LDH/ BiOBr/PMS/Visible | Ciprofloxacin | Ultrapure water (UW) | 96% (30 min) | 300 W Xenon lamp; 8wt% CoAl-LDH-BiOBr; [PMS] = 60–100 mg/L; [Catalyst] = 30–50 mg/L | [144] |
| WO3/BiOBr/PMS/visible | Tetracycline and enrofloxacin | Ultrapure water (UW) | Tetracycline: 98% (60 min) Enrofloxacin: 87% | 300 W Xenon lamp; 2 WO3 + BiOBr; [PMS] = 30 mg/L; [Catalyst] = 20–60 mg | [145] |
| PDS/H2O2/Fe/Solar radiation | E. coli (bacteria); MS2 (bacteriophage); 13 micropollutants mix | Ultrapure water (UW) | >6-Log for E. coli (30 min) and MS2 (10 min); >90% for micropollutants (30 min) | H2O2 = 10 ppm; 40°CM Fe(II) = 1 ppm; PDS = 9 × 10−5 M; 900 W/m2 | [146] |
| PMS/H2O2/Fe/Solar radiation | E. coli (bacteria) 13 micropollutants mix | Natural water (NW) | >6-Log for E. coli (20 min); >90% for micropollutants (15 min) | H2O2 = 10 ppm; 40°CM Fe(II) = 1 ppm; PMS = 3.6 × 10−5 M; 900 W/m2 | [147] |
| Combination | Target Pollutant | Water Matrix | Maximum Efficiency | Remarks | Ref. |
|---|---|---|---|---|---|
| TiO2/UV-C/Microfiltration | E. coli (bacteria), Enterococcus sp. (bacteria), Candida albicans (fungi) | Simulated wastewater (SW) | 4-Log | 316-L porous stainless-steel membranes; 0.2 gTiO2/membrane; UV-C (254 nm) | [148] |
| Photo-Fenton/Ultrafiltration | Chemical Oxygen Demand (COD); Total Organic Carbon (TOC) | Industrial wastewater (IW) | 85.9% COD; 74.5% TOC | Under UV-A: pH = 4.00; H2O2/Fe2+ (g/g) = 10.75 Under UV-C: pH = 4.44; H2O2/Fe2+ (g/g) = 7.27 | [149] |
| TiO2/UV/Ultrafiltration | Chemical Oxygen Demand (COD); Total Organic Carbon (TOC) | Industrial wastewater (IW) | 87.4% COD; 70% TOC (360 min) | UV-A (340 nm); 10 g TiO2 immobilized to Halloysite nanotubes; Hollow fibre ultrafiltration membrane | [150] |
| Activated Carbon/US/UV/H2O2 | Chemical Oxygen Demand (COD) | Petrochemical wastewater (IW) | 87% (80 min) | pH = 4.0 ± 0.2; Activated Carbon = 0.4 g/L; H2O2 8.0 mM; 320 W US | [151] |
| Hydrodynamic cavitation/photo-Fenton | Imidacloprid (insecticide) | Ultrapure water (DW) | 99.23% (15 min) | H2O2 = 3.91 mM; Fe(II):H2O2 = 1:40; UV-A (364 nm); inlet pressure from 5 to 20 bar | [152] |
| Hydrodynamic cavitation/UV-A | 45.56% (120 min) | UV-A (364 nm); inlet pressure from 5 to 20 bar | |||
| Hydrodynamic cavitation/photocatalysis | 55.18% (120 min) | Nb2O5 = 200 mg/L; UV-A (364 nm); inlet pressure from 5 to 20 bar |
| Combination | Target Pollutant | Water Matrix | Efficiency | Remarks | Ref. |
|---|---|---|---|---|---|
| Ozonation/UV/H2O2 | N,N-Diethyl-p- phenylenediamine sulfate, nitrobenzene, benzoic acid | Ultrapure water (UW) with Dissolved Organic Matter | Benzoic acid 80% (30 min) | O3 = 5 mg/L; UV-C (254 nm); H2O2 = 1 mM; pH = 7 | [153] |
| Ozonation/UV/S2O82− | Benzoic acid > 90% (30 min) | O3 = 5 mg/L; UV-C (254 nm); S2O82− = 1 mM; pH = 7 | |||
| Ozonation/UV/HClO | Benzoic acid > 20% (20 min) | O3 = 5 mg/L UV-C (254 nm); HClO = 5 mg/L; pH = 7 | |||
| Electrocoagulation/Electrooxidation/ PMS/UV/CuFe2O4 | Chemical Oxygen Demand (COD); Total Organic Carbon (TOC); Biochemical Oxygen Demand (BOD), Ammonia (NH4+) | Landfill leachate (LL) | COD 95.6%; TOC 90.5%; BOD 91.6%; NH4+ 99.8% | Current density = 50 mA/cm2; PbO2 anode; pH = 5; PMS = 15 mM; CuFe2O4 = 0.15 g/L | [154] |
| Combination | Target Pollutant | Water Matrix | Efficiency | Remarks | Ref. |
|---|---|---|---|---|---|
| Biological/Coagulation–flocculation/Photo-Fenton/Biological | Chemical Oxygen Demand (COD); Biochemical Oxygen Demand (BOD); Ammonium; Alkalinity; chromium; total suspended solids (TSS); recalcitrant organic compounds | Leather tannery landfill leachate (LL) | The efficiency for each target pollutant is not reported in the global system | Continuous-flow SBR: 2.0 g/L < MLVSS < 4.0 g/L; sludge volume index (SVI) of 53 mL/g; 200–400 mg/L FeCl3 as coagulant at pH 3; H2O2 = 400 mg/L; Dissolved iron = 150 mg/L | [155] |
| Biological/Coagulation–flocculation/Photo-electroFenton/Biological | Continuous-flow SBR: 2.0 g/L < MLVSS < 4.0 g/L; sludge volume index (SVI) of 53 mL/g; 200–400 mg/L FeCl3 as coagulant at pH 3; H2O2 = 400 mg/L; Dissolved iron = 150 mg/L; Current density = 300 mA/cm2 | ||||
| Solar photo-Fenton/Activated sludge | Chemical Oxygen Demand (COD); Total Organic Carbon (TOC) | Low strength Industrial wastewater (IW) | 84% (COD) | pH = 3; H2O2 = 0.25 M; Fe(II) = 0.05 M; 20% (v/v) sludge concentration | [156] |
| High strength Industrial wastewater (IW) | 82% (COD) | pH = 3; H2O2 = 1 M; Fe(II) = 0.1 M; 25% (v/v) sludge concentration | |||
| Electrocoagulation/Adsorption/photo-Fenton-like | Chemical Oxygen Demand (COD); Total Organic Carbon (TOC); Turbidity; Colour; Suspended Solids (SS) | Textile Wastewater (IW) | 87% (TOC); 49% (COD); 96% (Turbidity); 90% (Colour); 95–97% (SS) | Electrocoagulation: Al Electrode; current density = 25 mA/cm2; pH = 5. Adsorption: 1 g/L corncob. Photo-Fenton-like: BiNiO3 = 0.75 g/L; H2O2 = 2–8 mM; pH = 7–7.5 | [157] |
| Activated sludge/H2O2/UV-C | Carbamazepine; Clarithromycin; Diclofenac; Metoprolol; Benzotriazole; Mecoprop. | Urban Wastewater (WW) | Average removal micropollutants: 25% (activated sludge) + 100% (10 min) | Activated sludge: hydraulic retention time = 4 h; sludge retention time = 2 d; H2O2 = 25 mg/L | [158] |
| Activated sludge/solar photo-Fenton | Average removal micropollutants: 25% (activated sludge) + 28% (60 min) | Activated sludge: hydraulic retention time = 4 h; sludge retention time = 2 d Fe(II) = 5 mg/L; H2O2 = 25 mg/L | |||
| Moving bed bioreactor/H2O2/UV-C | Average removal micropollutants: 40% (moving bed bioreactor) + 100% (10 min) | Moving bed bioreactor: no remarks; H2O2 = 25 mg/L | |||
| Moving bed bioreactor/solar photo-Fenton | Average removal micropollutants: 40% (moving bed bioreactor) + 31% (60 min) | Moving bed bioreactor: no remarks; Fe(II) = 5 mg/L; H2O2 = 25 mg/L | |||
| Coagulation–Flocculation/H2O2/UV-C | Average removal micropollutants: 20% (coagulation–flocculation) + 100% (30 min) | Coagulation–flocculation = FeCl3 (40%) as coagulant. H2O2 = 25 mg/L | |||
| Coagulation–Flocculation/solar photo-Fenton | Average removal micropollutants: 20% (coagulation–flocculation) + 11% (60 min) | Coagulation–flocculation = FeCl3 (40%) as coagulant. Fe(II) = 5 mg/L; H2O2 = 25 mg/L | |||
| TiO2/UV/Ultrafiltration | Dissolved Organic Carbon (DOC) | Simulated fresh water | >80% (120 min) | 10 kDa-flat sheet polyethersulfone (PES) membrane 41.8 cm2; TiO2 = 0.4–0–6 g/L; UV-A (354 nm) | [159] |
| O3/H2O2/Carbon based biofilter/UV-C/H2O2 | 13 detected micropollutants | Treated real wastewater (WW) | O3/H2O2 = 78%; Carbon based biofilter = 87%; UV/H2O2 = 43% | O3 = 13 ± 0.5 mg/L, H2O2 = 11 ± 0.4 mg/L for the O3/H2O2 process, and UV = 410 ± 63.5 mJ/cm2, H2O2 = 5 mg/L for the UV-C/H2O2 process | [160] |
| O3/H2O2/Limestone Based Biofilter/UV-C/H2O2 | O3/H2O2 = 78%; Limestone based biofilter = 67%; UV/H2O2 = 43% | ||||
| O3/H2O2/Ultrafiltration/UV-C/H2O2/Reverse Osmosis | O3/H2O2 = 78%; Ultrafiltration = 0%; UV/H2O2 = 43%; Reverse osmosis = 99% | ||||
| Coagulation–flocculation/solar photo-Fenton/aerobic bio-treatment | Chemical Oxygen Demand (COD); Dissolved Organic Carbon (DOC); Total polyphenol content (TPC) | Cork boiling wastewater (IW) | Coagulation–flocculation/solar photo-Fenton = 93,4% (COD); 92,8% (DOC); 94,5% (TPC) | Coagulation–flocculation: FeCl3 as coagulant (3 min 100 rpm + 30 min 30 rpm + 30 min). Solar photo-Fenton: Fe(III) from coagulation = 46–80 mg/L; H2O2 = 1–2.5 g/L; Sequencing Batch Bioreactor (Activated sludge) | [161] |
| Moving bed bioreactor/photo-Fenton | Chemical Oxygen Demand (COD); Total Organic Carbon (TOC); Oil and grease | Industrial Wastewater (IW) | >95% (COD) | Fe(II):H2O2 = 250:800 (mg/L) | [162] |
| Coagulation–flocculation/UV-A-LED/Photo-Fenton | Chemical Oxygen Demand (COD) | Industrial wastewater (IW) | 74% (360 min) | H2O2 = 5459 mg/L; Fe(III) = 286 mg/L; UV-A LED 85 W/m2 | [163] |
| Photo-Fenton/Chemical Addition Dissolved Air Flotation (CA-DAF) | Chemical Oxygen Demand (COD); Total Petroleum Hydrocarbon (TPH) | Industrial wastewater (IW) | 99.85% for COD and 98.9% for TPH | pH = 3; FeSO4 = 100 mg/L; H2O2 = 17.8 g/L. For DAF unit volume and loading rate were 7 m3 and 35–40 L/min. Aeration rate 15–20 L/min, and pressure was set at 3 bar and the saturation time of 30 min | [164] |
| Coagulation–flocculation/Photo-Fenton | E. coli; Enterococcus sp.; Pseudomonas aeruginosa | Simulated wastewater (SW) | >4 log in all bacteria (210 min) | pH = 5; Fe(III) = 5 mg/L; H2O2 = 25 mg/L | [165] |
| Coagulation–Flocculation/H2O2/UV-C | Detected micropollutants | Treated urban wastewater (WW) | Average removal: Coagulation–flocculation < 10%; 55% (H2O2/UV-C) | Coagulation–flocculation = 1.1 kg/m3 of polyelectrolyte; H2O2 = 0.5 mM; pH = natural | [166] |
| Coagulation–Flocculation/PDS/UV-C | Average removal: Coagulation–flocculation < 10%; <20% (PDS/UV-C) | Coagulation–flocculation = 1.1 kg/m3 of polyelectrolyte; PDS = 0.5 mM; pH = natural | |||
| Coagulation–Flocculation/PMS/UV-C | Average removal: Coagulation–flocculation < 10%; 48% (H2O2/UV-C) | Coagulation–flocculation = 1.1 kg/m3 of polyelectrolyte; PMS = 0.5 mM; pH = natural |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Chueca, J.; Carbajo, J.; García-Muñoz, P. Intensification of Photo-Assisted Advanced Oxidation Processes for Water Treatment: A Critical Review. Catalysts 2023, 13, 401. https://doi.org/10.3390/catal13020401
Rodríguez-Chueca J, Carbajo J, García-Muñoz P. Intensification of Photo-Assisted Advanced Oxidation Processes for Water Treatment: A Critical Review. Catalysts. 2023; 13(2):401. https://doi.org/10.3390/catal13020401
Chicago/Turabian StyleRodríguez-Chueca, Jorge, Jaime Carbajo, and Patricia García-Muñoz. 2023. "Intensification of Photo-Assisted Advanced Oxidation Processes for Water Treatment: A Critical Review" Catalysts 13, no. 2: 401. https://doi.org/10.3390/catal13020401
APA StyleRodríguez-Chueca, J., Carbajo, J., & García-Muñoz, P. (2023). Intensification of Photo-Assisted Advanced Oxidation Processes for Water Treatment: A Critical Review. Catalysts, 13(2), 401. https://doi.org/10.3390/catal13020401








