Self-Assembly of Pt3Co Superlattice as a Catalyst for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results
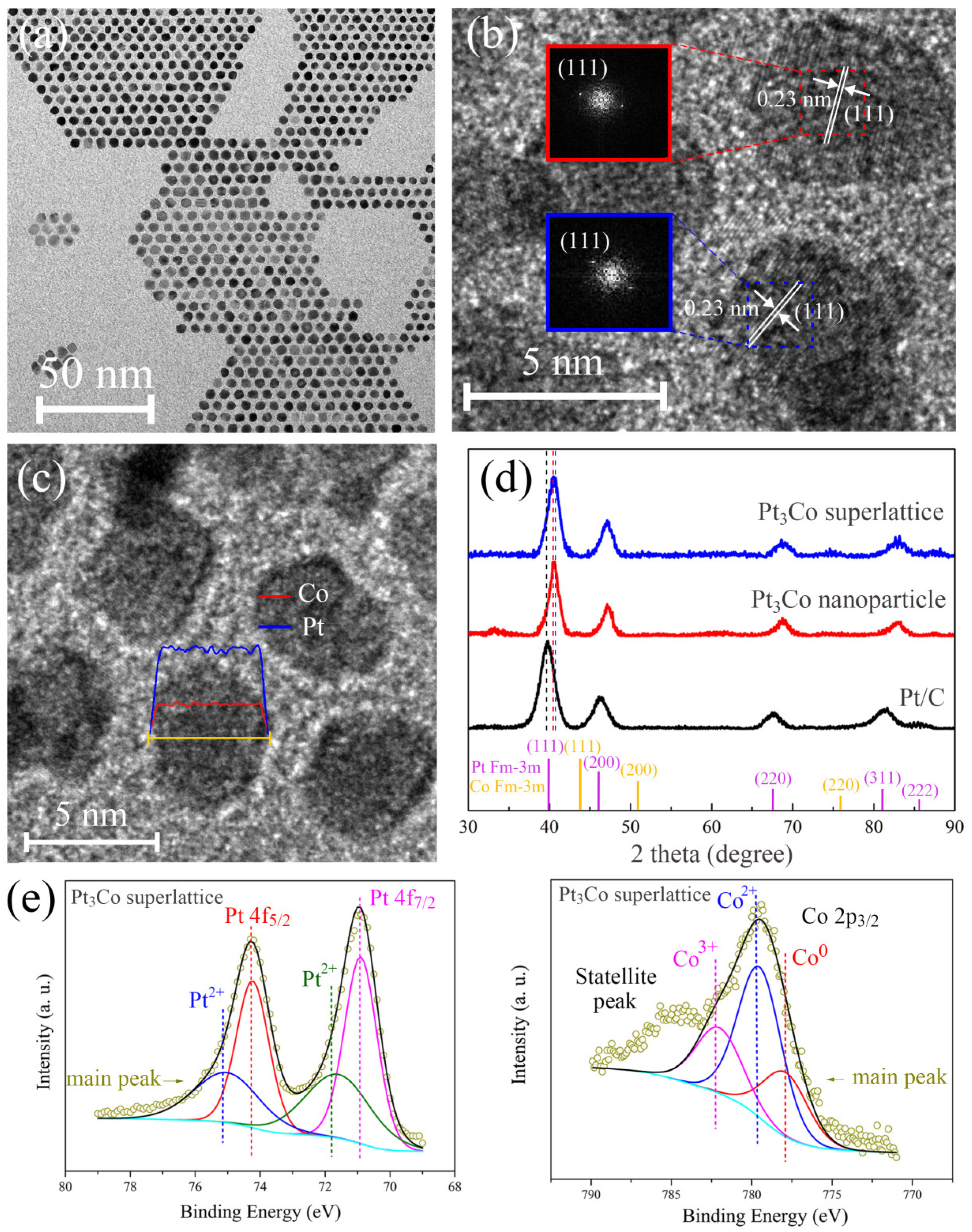
2.1. Structure and Charaterization of Pt3Co Superlattice
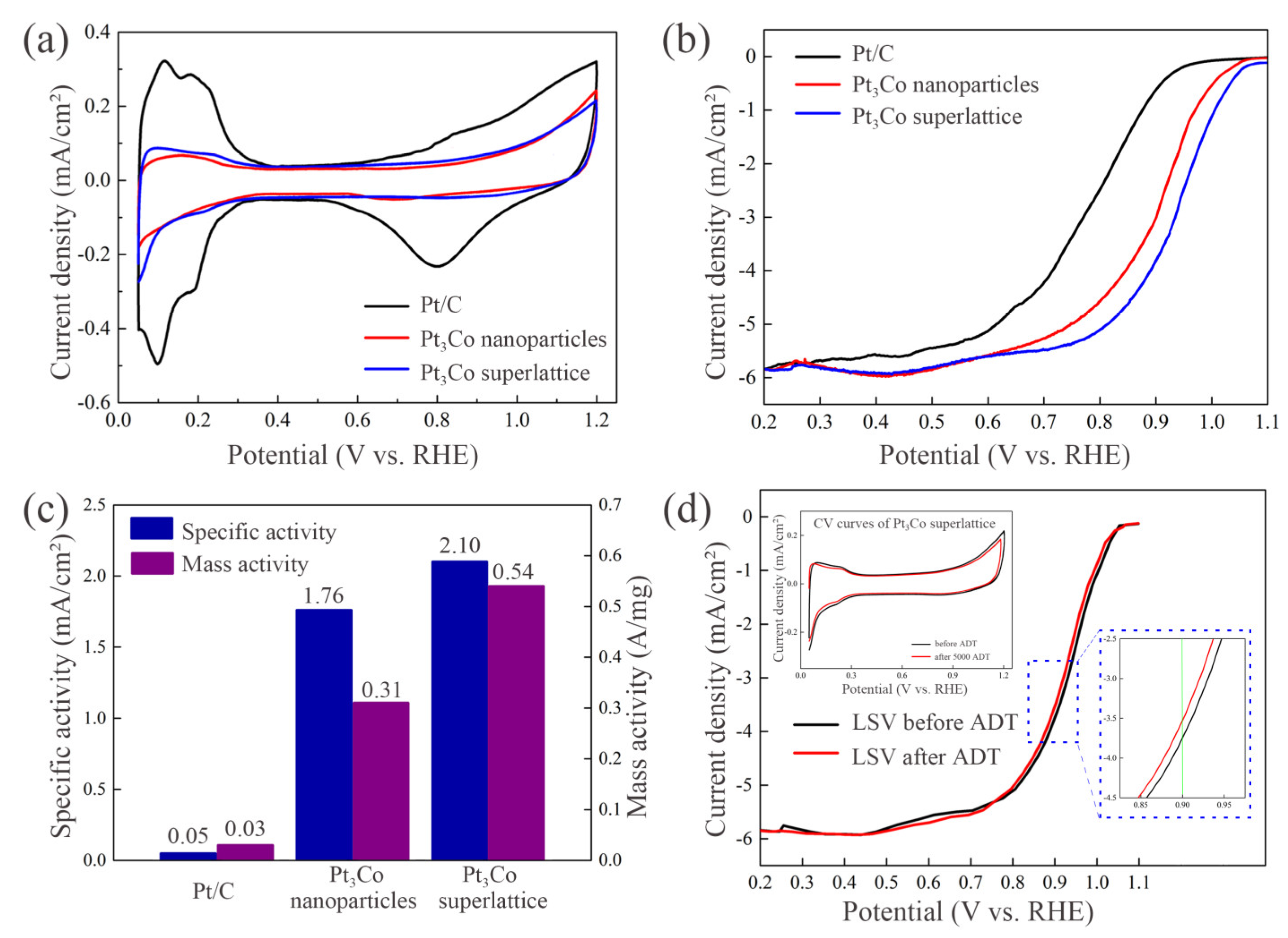
2.2. Electrochemical Characterization Analysis
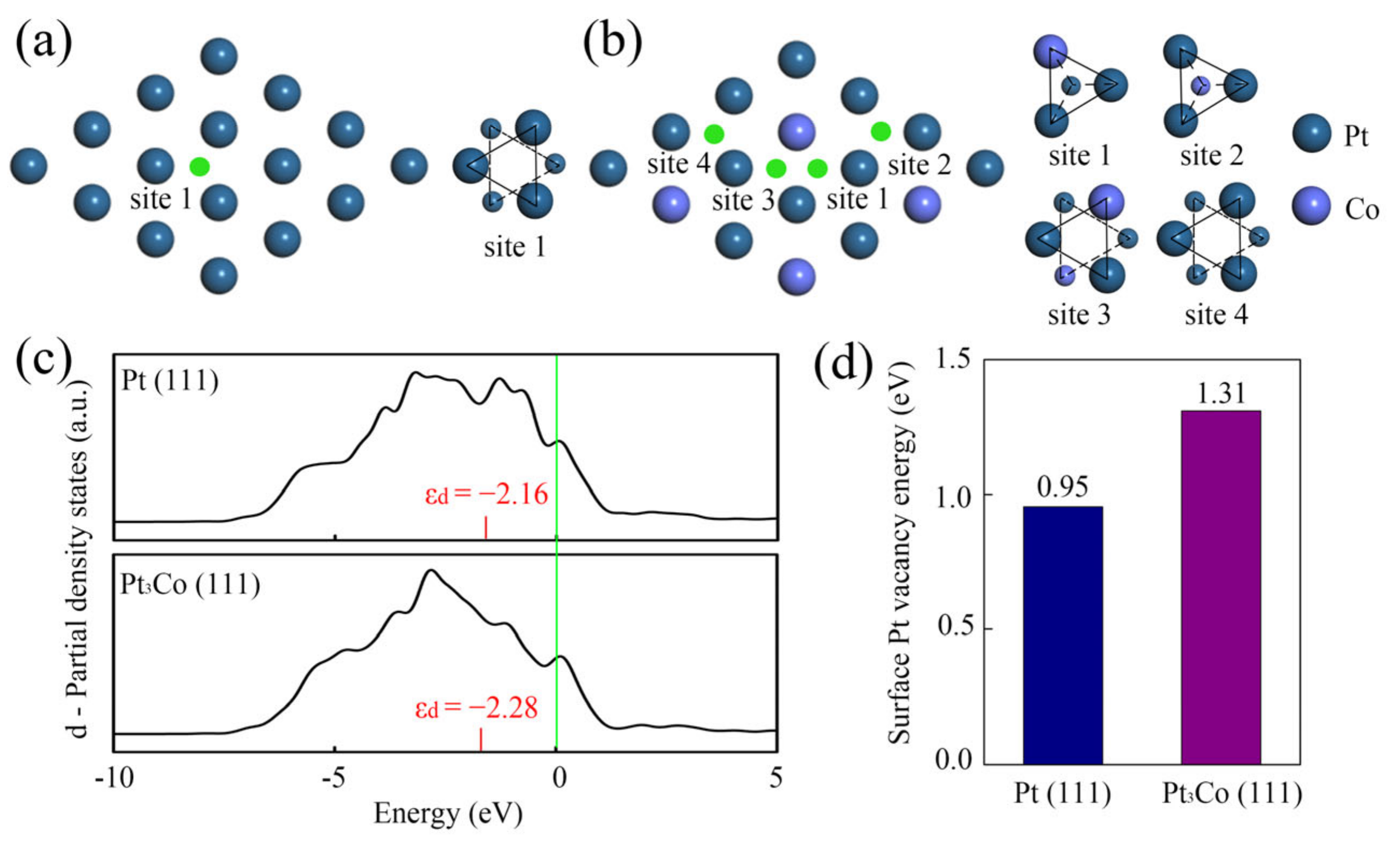
2.3. DFT Calculation of Pt3Co Superlattice Catalyst
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of Pt3Co Superlattice Catalyst
3.3. Electrochemical Characterization
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saibuathong, N.; Saejeng, Y.; Pruksathorn, K.; Hunsom, M.; Tantavichet, N. Catalyst electrode preparation for PEM fuel cells by electrodeposition. J. Appl. Electrochem. 2010, 40, 903–910. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Markovic, N.M. Just a Dream—Or Future Reality? Science 2009, 324, 48–49. [Google Scholar] [CrossRef]
- Toda, T.; Igarashi, H.; Uchida, H.; Watanabe, M. Enhancement of the electroreduction of oxygen on Pt alloys with Fe, Ni, and Co. J. Electrochem. Soc. 1999, 146, 3750–3756. [Google Scholar] [CrossRef]
- Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Core-shell compositional fine structures of dealloyed Pt(x)Ni(1-x) nanoparticles and their impact on oxygen reduction catalysis. Nano Lett. 2012, 12, 5423–5430. [Google Scholar] [CrossRef]
- Huang, X.Q.; Zhao, Z.P.; Cao, L.; Chen, Y.; Zhu, E.B.; Lin, Z.Y.; Li, M.F.; Yan, A.M.; Zettl, A.; Wang, Y.M.; et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef]
- Shen, C.; Li, X.; Wei, Y.; Cao, Z.; Li, H.; Jiang, Y.; Xie, Z. PtCo-excavated rhombic dodecahedral nanocrystals for efficient electrocatalysis. Nanoscale Adv. 2020, 2, 4881–4886. [Google Scholar] [CrossRef]
- Guo, S.J.; Li, D.G.; Zhu, H.Y.; Zhang, S.; Markovic, N.M.; Stamenkovic, V.R.; Sun, S.H. FePt and CoPt Nanowires as Efficient Catalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Edit. 2013, 52, 3465–3468. [Google Scholar] [CrossRef]
- Sievers, G.; Mueller, S.; Quade, A.; Steffen, F.; Jakubith, S.; Kruth, A.; Brueser, V. Mesoporous Pt-Co oxygen reduction reaction (ORR) catalysts for low temperature proton exchange membrane fuel cell synthesized by alternating sputtering. J. Power Sources 2014, 268, 255–260. [Google Scholar] [CrossRef]
- Bu, L.Z.; Zhang, N.; Guo, S.J.; Zhang, X.; Li, J.; Yao, J.L.; Wu, T.; Lu, G.; Ma, J.Y.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef]
- Wang, Q.; Mi, B.S.; Zhou, J.; Qin, Z.W.; Chen, Z.; Wang, H.B. Hollow-Structure Pt-Ni Nanoparticle Electrocatalysts for Oxygen Reduction Reaction. Molecules 2022, 27, 2524. [Google Scholar] [CrossRef]
- Zhang, X.R.; Xu, X.M.; Yao, S.X.; Hao, C.; Pan, C.; Xiang, X.; Tian, Z.Q.; Shen, P.K.; Shao, Z.P.; Jiang, S.P. Boosting Electrocatalytic Activity of Single Atom Catalysts Supported on Nitrogen-Doped Carbon through N Coordination Environment Engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Wang, W.; Zhou, W.; Shao, Z.P. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Watanabe, M.; Tsurumi, K.; Mizukami, T.; Nakmura, T.; Stonehart, P. Activity and Stability of Ordered and Disordered Co-Pt Alloys for Phosphoric Acid Fuel Cells. J. Electrochem. Soc. 1994, 141, 2659–2668. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhao, N.N.; Fang, B.Z.; Li, H.; Bi, X.T.T.; Wang, H.J. Carbon-Supported Pt-Based Alloy Electrocatalysts for the Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells: Particle Size, Shape, and Composition Manipulation and Their Impact to Activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef]
- Roldan Cuenya, B. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Rao, C.V.; Viswanathan, B. ORR Activity and Direct Ethanol Fuel Cell Performance of Carbon-Supported Pt-M (M = Fe, Co, and Cr) Alloys Prepared by Polyol Reduction Method. J. Phys. Chem. C 2009, 113, 18907–18913. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Talapin, D.V.; Kotov, N.A.; O’Brien, S.; Murray, C.B. Structural diversity in binary nanoparticle superlattices. Nature 2006, 439, 55–59. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef]
- Xu, G.R.; Si, R.; Liu, J.Y.; Zhang, L.Y.; Gong, X.; Gao, R.; Liu, B.C.; Zhang, J. Directed self-assembly pathways of three-dimensional Pt/Pd nanocrystal superlattice electrocatalysts for enhanced methanol oxidation reaction. J. Mater. Chem. A 2018, 6, 12759–12767. [Google Scholar] [CrossRef]
- Feng, J.J.; He, L.L.; Fang, R.; Wang, Q.L.; Yuan, J.H.; Wang, A.J. Bimetallic PtAu superlattice arrays: Highly electroactive and durable catalyst for oxygen reduction and methanol oxidation reactions. J. Power Sources 2016, 330, 140–148. [Google Scholar] [CrossRef]
- Sahoo, L.; Gautam, U.K. Boosting Bifunctional Oxygen Reduction and Methanol Oxidation Electrocatalytic Activity with 2D Superlattice-Forming Pd Nanocubes Generated by Precise Acid Etching. ACS Appl. Nano. Mater. 2020, 3, 8117–8125. [Google Scholar] [CrossRef]
- Wang, C.Y.; Siu, C.; Zhang, J.; Fang, J.Y. Understanding the forces acting in self-assembly and the implications for constructing three-dimensional (3D) supercrystals. Nano Res. 2015, 8, 2445–2466. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Wilmer, C.E.; Kim, J.; Browne, K.P.; Bishop, K.J.M. Self-assembly: From crystals to cells. Soft Matter 2009, 5, 1110–1128. [Google Scholar] [CrossRef]
- Bian, B.R.; Chen, G.X.; Zheng, Q.; Du, J.; Lu, H.M.; Liu, J.P.; Hu, Y.; Zhang, Z.D. Self-Assembly of CoPt Magnetic Nanoparticle Arrays and its Underlying Forces. Small 2018, 14, e1801184. [Google Scholar] [CrossRef]
- Talapin, D.V.; Shevchenko, E.V.; Murray, C.B.; Titov, A.V.; Kral, P. Dipole-dipole interactions in nanoparticle superlattices. Nano Lett. 2007, 7, 1213–1219. [Google Scholar] [CrossRef]
- Cheng, W.L. Free-standing nanoparticle superlattice sheets: From design to applications. EPL Europhys. Lett. 2017, 119, 48004. [Google Scholar] [CrossRef]
- Jiao, Y.C.; Han, D.D.; Ding, Y.; Zhang, X.F.; Guo, G.N.; Hu, J.H.; Yang, D.; Dong, A.G. Fabrication of three-dimensionally interconnected nanoparticle superlattices and their lithium-ion storage properties. Nat. Commun. 2015, 6, 6420. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.C.; Zhou, T.; Zhang, H.; Sun, X.H.; Tang, J.; Zhang, L.J.; Al-Enizi, A.M.; Yang, Z.Q.; Zheng, G.F. Nanoparticle Superlattices as Efficient Bifunctional Electrocatalysts for Water Splitting. J. Am. Chem. Soc. 2015, 137, 14305–14312. [Google Scholar] [CrossRef]
- Janet, C.M.; Navaladian, S.; Viswanathan, B.; Varadarajan, T.K.; Viswanath, R.P. Heterogeneous Wet Chemical Synthesis of Superlattice-Type Hierarchical ZnO Architectures for Concurrent H-2 Production and N-2 Reduction. J. Phys. Chem. C 2010, 114, 2622–2632. [Google Scholar] [CrossRef]
- Zeng, Z.P.; Yan, Y.B.; Chen, J.; Zan, P.; Tian, Q.H.; Chen, P. Boosting the Photocatalytic Ability of Cu2O Nanowires for CO2 Conversion by MXene Quantum Dots. Adv. Funct. Mater. 2019, 29, 201806500. [Google Scholar] [CrossRef]
- Sial, M.A.Z.G.; Lin, H.F.; Zulfiqar, M.; Ullah, S.; Ni, B.; Wang, X. Trimetallic PtCoFe Alloy Monolayer Superlattices as Bifunctional Oxygen-Reduction and Ethanol-Oxidation Electrocatalysts. Small 2017, 13, 201700250. [Google Scholar] [CrossRef]
- Zhang, J.T.; Mao, X.N.; Wang, S.L.; Liang, L.L.; Cao, M.F.; Wang, L.; Li, G.; Xu, Y.; Huang, X.Q. Superlattice in a Ru Superstructure for Enhancing Hydrogen Evolution. Angew. Chem. Int. Edit. 2022, 61, e20211686. [Google Scholar] [CrossRef]
- Park, J.I.; Cheon, J. Synthesis of “solid solution” and “core-shell” type cobalt-platinum magnetic nanoparticles via transmetalation reactions. J. Am. Chem. Soc. 2001, 123, 5743–5746. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef]
- Mukerjee, S.; Srinivasan, S.; Soriage, M.P. Effect of Preparation Conditions of Pt Alloys on Their Electronic, Structural, and Electrocatalytic Activities for Oxygen Reduction—XRD, XAS, and Electrochemical Studies. J. Phys. Chem. 1995, 99, 4577–4589. [Google Scholar] [CrossRef]
- Duong, H.T.; Rigsby, M.A.; Zhou, W.P.; Wieckowski, A. Oxygen reduction catalysis of the Pt3Co alloy in alkaline and acidic media studied by x-ray photoelectron Spectroscopy and electrochemical methods. J. Phys. Chem. C 2007, 111, 13460–13465. [Google Scholar] [CrossRef]
- Mavrikakis, M.; Hammer, B.; Norskov, J.K. Effect of strain on the reactivity of metal surfaces. Phys. Rev. Lett. 1998, 81, 2819–2822. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Gasteiger, H.A.; Stäb, G.D.; Urban, P.M.; Kolb, D.M.; Behm, R.J. Characterization of high-surface-area electrocatalysts using a rotating disk electrode configuration. J. Electrochem. Soc. 1998, 145, 2354–2358. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Blizanac, B.B.; Arenz, M.; Stamenkovic, V.R.; Ross, P.N.; Markovic, N.M. The impact of geometric and surface electronic properties of Pt-catalysts on the particle size effect in electocatalysis. J. Phys. Chem. B 2005, 109, 14433–14440. [Google Scholar] [CrossRef]
- Keeling, D.L.; Oxtoby, N.S.; Wilson, C.; Humphry, M.J.; Champness, N.R.; Beton, P.H. Assembly and processing of hydrogen bond induced supramolecular nanostructures. Nano Lett. 2003, 3, 9–12. [Google Scholar] [CrossRef]
- Paulus, U.A.; Wokaun, A.; Scherer, G.G.; Schmidt, T.J.; Stamenkovic, V.; Radmilovic, V.; Markovic, N.M.; Ross, P.N. Oxygen reduction on carbon-supported Pt-Ni and Pt-Co alloy catalysts. J. Phys. Chem. B 2002, 106, 4181–4191. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.X.; Yao, Z.Y.; Bai, H.J.; Lu, Y.F.; Ma, C.; Lu, S.F.; Peng, Z.M.; Yang, J.L.; Pan, A.L.; et al. Unconventional p-d Hybridization Interaction in PtGa Ultrathin Nanowires Boosts Oxygen Reduction Electrocatalysis. J. Am. Chem. Soc. 2019, 141, 18083–18090. [Google Scholar] [CrossRef]
- Li, M.F.; Zhao, Z.P.; Cheng, T.; Fortunelli, A.; Chen, C.Y.; Yu, R.; Zhang, Q.H.; Gu, L.; Merinov, B.V.; Lin, Z.Y.; et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef]
- Yan, D.X.; Kristoffersen, H.H.; Pedersen, J.K.; Rossmeisl, J. Rationally Tailoring Catalysts for the CO Oxidation Reaction by Using DFT Calculations. ACS Catal. 2022, 12, 116–125. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Norskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Theoretical surface science and catalysis—Calculations and concepts. Adv. Catal. 2000, 45, 71–129. [Google Scholar] [CrossRef]
- Norskov, J.K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 937–943. [Google Scholar] [CrossRef]
- Li, K.; Li, X.X.; Huang, H.W.; Luo, L.H.; Li, X.; Yan, X.P.; Ma, C.; Si, R.; Yang, J.L.; Zeng, J. One-Nanometer-Thick PtNiRh Trimetallic Nanowires with Enhanced Oxygen Reduction Electrocatalysis in Acid Media: Integrating Multiple Advantages into One Catalyst. J. Am. Chem. Soc. 2018, 140, 16159–16167. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-Principles Simulation: Ideas, Illustrations and the CASTEP Code. J. Phys. Condens. Mater 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Anglada, J.M.; Bofill, J.M. How good is a Broyden–Fletcher–Goldfarb–Shanno-like update Hessian formula to locate transition structures? Specific reformulation of Broyden–Fletcher–Goldfarb–Shanno for optimizing saddle points. J. Comput.Chem. 1998, 19, 349–362. [Google Scholar] [CrossRef]
- Chen, S.; Ferreira, P.J.; Sheng, W.C.; Yabuuchi, N.; Allard, L.F.; Shao-Horn, Y. Enhanced activity for oxygen reduction reaction on “Pt3Co” nanoparticles: Direct evidence of percolated and sandwich-segregation structures. J. Am. Chem. Soc. 2008, 130, 13818–13819. [Google Scholar] [CrossRef] [PubMed]
- Pavlets, A.S.; Alekseenko, A.A.; Tabachkova, N.Y.; Safronenko, O.I.; Nikulin, A.Y.; Alekseenko, D.V.; Guterman, V.E. A novel strategy for the synthesis of Pt–Cu uneven nanoparticles as an efficient electrocatalyst toward oxygen reduction. Int. J. Hydrog. Energ. 2021, 46, 5355–5368. [Google Scholar] [CrossRef]
- Komanicky, V.; Menzel, A.; You, H. Investigation of Oxygen Reduction Reaction Kinetics at (111)−(100) Nanofaceted Platinum Surfaces in Acidic Media. J. Phys. Chem. B 2005, 109, 23550–23557. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Gan, L.; Li, H.H.; Yu, S.H.; Heggen, M.; Strasser, P. Octahedral PtNi Nanoparticle Catalysts: Exceptional Oxygen Reduction Activity by Tuning the Alloy Particle Surface Composition. Nano Lett. 2012, 12, 5885–5889. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, E.; Baltruschat, H. Apparent transfer coefficient for ORR at polycrystalline platinum under convection conditions: A potential modulation study. J. Solid State Electrochem. 2013, 17, 1861–1867. [Google Scholar] [CrossRef]
- Ashok, P.; Ramaprabhu, S. Journal of colloid and interface science investigation of catalytic activity towards oxygen reduction reaction of pt dispersed on boron doped graphene in acid medium. J. Colloid Interface Sci. 2016, 479, 260–270. [Google Scholar]
- Liu, L.S.; Shi, X.; Wang, W.; Pei, M.F.; Hong, C.X.; Xue, Y.L.; Xu, Z.W.; Tian, F.; Guo, X.F. Carbon nitride/positive carbon black anchoring PtNPs assembled by gamma-rays as ORR catalyst with excellent stability. Nanotechnology 2021, 32, 345601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Jiang, C.; Mi, B.; Wang, H. Self-Assembly of Pt3Co Superlattice as a Catalyst for Oxygen Reduction Reaction. Catalysts 2023, 13, 406. https://doi.org/10.3390/catal13020406
Wang Q, Jiang C, Mi B, Wang H. Self-Assembly of Pt3Co Superlattice as a Catalyst for Oxygen Reduction Reaction. Catalysts. 2023; 13(2):406. https://doi.org/10.3390/catal13020406
Chicago/Turabian StyleWang, Quan, Chang Jiang, Baosen Mi, and Hongbin Wang. 2023. "Self-Assembly of Pt3Co Superlattice as a Catalyst for Oxygen Reduction Reaction" Catalysts 13, no. 2: 406. https://doi.org/10.3390/catal13020406
APA StyleWang, Q., Jiang, C., Mi, B., & Wang, H. (2023). Self-Assembly of Pt3Co Superlattice as a Catalyst for Oxygen Reduction Reaction. Catalysts, 13(2), 406. https://doi.org/10.3390/catal13020406




