Abstract
Three-dimensionally printed materials show great performance and reliable stability in the removal of refractory organic pollutants in Fenton-like reactions. In this work, hierarchically porous zero-valent copper (3DHP-ZVC) was designed and fabricated via 3D printing and applied as a catalyst for the degradation of tetracycline (TC) through heterogeneous Fenton-like processes. It was found that the 3DHP-ZVC/H2O2 system could decompose over 93.2% of TC within 60 min, which is much superior to the homogeneous Cu2+/H2O2 system under similar conditions. The leaching concentration of Cu2+ ions in the 3DHP-ZVC/H2O2 system is 2.14 times lower than that in the Cu powder/H2O2 system in a neutral environment, which could be ascribed to the unique hierarchically porous structure of 3DHP-ZVC. Furthermore, 3DHP-ZVC exhibited compelling stability in 20 consecutive cycles. The effects of co-existing inorganic anions, adaptability, and pH resistance on the degradation of TC were also investigated. A series of experiments and characterizations revealed that Cu0 and superoxide radicals as reducing agents could facilitate the cycling of Cu(II)/Cu(I), thus enhancing the generation of hydroxyl radicals to degrade TC. This study provides new insights into employing promising 3D printing technology to develop high-reactivity, stable, and recycling-friendly components for wastewater treatment.
1. Introduction
Antibiotics are chemical medicines that possess antibacterial activity and are widely employed for the treatment of bacterial infections [1,2,3,4,5]. Recently, antibiotics have been extensively detected in aquatic systems owing to the overuse of antibiotics and the incomplete metabolism of humans and animals [6,7]. The long-term accumulation of antibiotics in water bodies may increase antibiotic resistance, which seriously threatens human health [8,9,10]. Conventional techniques such as adsorption, biological treatment, and membrane separation are limited by their low and insufficient removal efficiency [11]. Therefore, the development of an economic and efficient route for antibiotic elimination is urgently needed.
Fenton processes are frequently applied to decompose hazardous organic pollutants, which can produce highly oxidized hydroxyl radicals (•OH) (1.9–2.7 V vs. NHE) through the catalytic decomposition of hydrogen peroxide (H2O2) by Fe2+ or other transition metals [12,13,14,15]. However, the practical application of the conventional Fenton reaction is hindered by iron-sludge generation, the impossibility of recycling, and a narrow operational pH range [16]. Heterogeneous Fenton or Fenton-like catalysts have received increasing attention, which greatly overcome the above drawbacks of conventional Fenton reactions [17,18,19,20]. Among various developed heterogeneous catalysts, zero-valent copper (ZVC) arouses ever-growing interest due to its unique merits of excellent electrical conductivity and high stability. As reported, ZVC frequently serves as an intermediate of electronic transmission, which is responsible for the better activation of H2O2 [21]. In addition, ZVC demonstrates more reliable stability than zero-valent iron (ZVI) owing to the drawbacks of easy agglomeration and oxidation nature [22]. To date, a series of ZVC catalysts have been synthesized due to these advantages, but they are generally in the form of nanoparticles, which is inconvenient in separation and recovery from an aqueous environment, raising the risk of secondary contamination [23]. It is desired to break through the rigid concepts of nanoparticles to manufacture stable, high-reactivity, and recycling-friendly catalysts via a feasible method for practical application.
Three-dimensional (3D) printing technology is an additive manufacturing process used to manufacture specialized functional structures directly guided by a 3D model, which greatly optimizes structural properties and simplifies manufacturing processes, further minimizing production costs [24]. Three-dimensional printing technology is highly appropriate in the field of catalysis owing to its distinctive functional structures and favorable control of the target catalysts, which is of vital significance for the performance of catalytic materials [25]. Among the multifarious 3D printing techniques, selective laser melting (SLM) is a preferred technology to fabricate metallic components with sufficient accuracy to lattice structures from powder [26]. Recent studies have indicated that SLM-produced catalysts act as effective and stable activators for H2O2. For example, Yang et al. designed a hierarchical porous metallic glass/copper composite to activate H2O2 for wastewater treatment, with a rate constant of removing rhodamine B (RhB) 620 times higher than commercial zero-valent iron particles [27]. In our previous work, hierarchically micro- and nanoporous Cu catalysts were prepared for H2O2 activation through the combination of SLM and chemical dealloying techniques [28]. However, the obtained Cu catalysts encounter the dilemmas of having a complicated process, high dissolution, and excessive oxidant consumption.
In this work, ZVC with hierarchically porous structures (3DHP-ZVC) was fabricated as an efficient Fenton-like catalyst to decompose multiple organic pollutants via 3D printing technology, which displayed excellent reactivity for H2O2 activation. The morphology, catalytic performance, stability, and universality of the printed 3DHP-ZVC were thoroughly illustrated. The influence of essential factors such as initial pH, H2O2 concentration, and co-existing inorganic anions was evaluated. Finally, a possible activation mechanism was proposed and confirmed by experiments and characterization analysis. Our study provides a novel strategy to develop stable and recycling-friendly catalysts in Fenton-like systems for wastewater purification.
2. Results and Discussion
2.1. Characterization
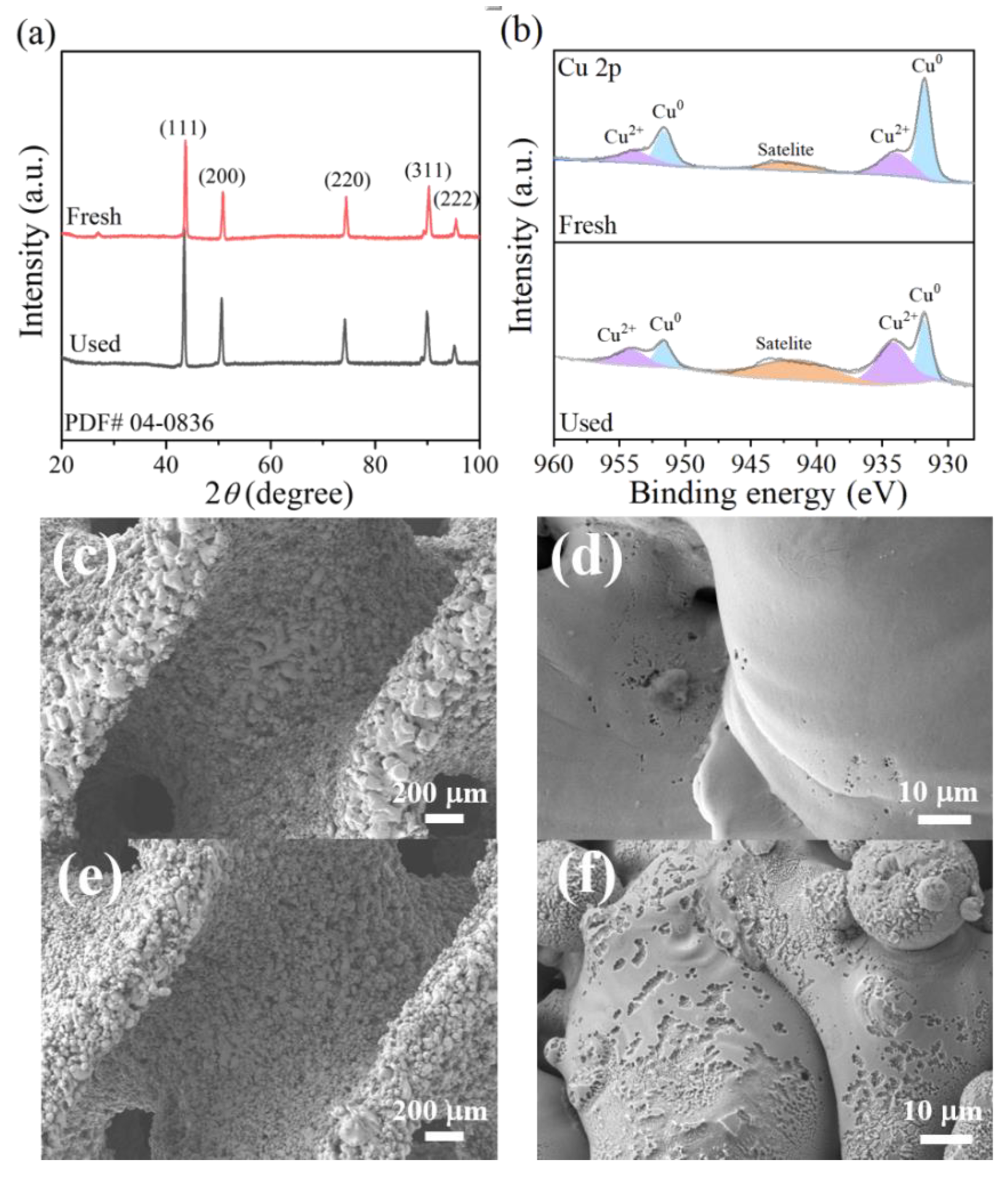
The X-ray diffraction (XRD) patterns of the printed 3DHP-ZVC are presented in Figure 1a. The characteristic peaks at 2θ of 43.3°, 50.4°, 74.1°, 89.9°, and 95.1° could be ascribed to the (111), (200), (220), (311), and (222) lattice planes of Cu0 (PDF#04–0836), respectively [29]. This result indicated that the developed 3DHP-ZVC samples were composed of Cu0. X-ray photoelectron spectroscopy (XPS) provides available element information on the surface of 3DHP-ZVC. As depicted in Figure 1b, Cu 2p peaks located at 931.8 and 951.6 eV were consistent with the binding energies of Cu0 [30,31]. Meanwhile, the relatively weak peaks at 934.2, 954.2, and 941.6 eV were ascribed to the appearance of a trace amount of Cu2+ due to the inevitable surface oxidation during the XPS determination [31]. Similar phenomena that the characteristic peaks of Cu2+ were also detected on the surface of Cu0 in XPS measurements were observed in previous studies [32]. The surface images of the 3DHP-ZVC samples were revealed using a scanning electron microscopy (SEM) instrument (Figure 1c–f). 3HDP-ZVC possesses hierarchically porous structures and a coarse surface, which is beneficial to the exposure of more active sites (Figure 1c) [33]. Moreover, Brunauer–Emmett–Teller (BET) analysis was applied to measure the specific surface area of the samples. The surface area of 3DHP-ZVC (2.5 m2/g) is comparable to that of Cu powder (3.1 m2/g), suggesting that the specific surface area characteristic undergoes no significant change during the printing process.
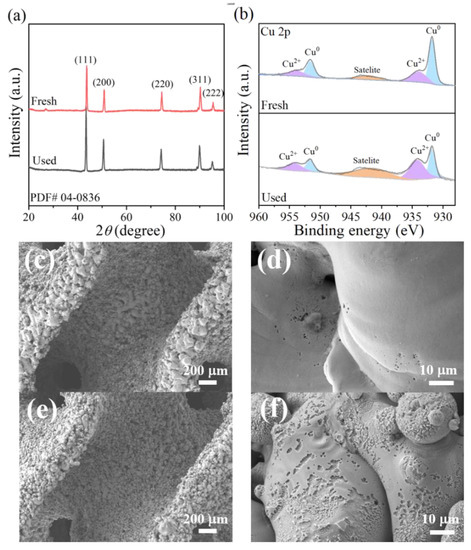
Figure 1.
(a) XRD patterns of 3DHP-ZVC, (b) Cu 2p XPS spectra of 3DHP-ZVC before and after reactions, and SEM images of (c,d) 3DHP-ZVC, and (e,f) used 3DHP-ZVC.
2.2. Catalytic Performance of 3DHP-ZVC
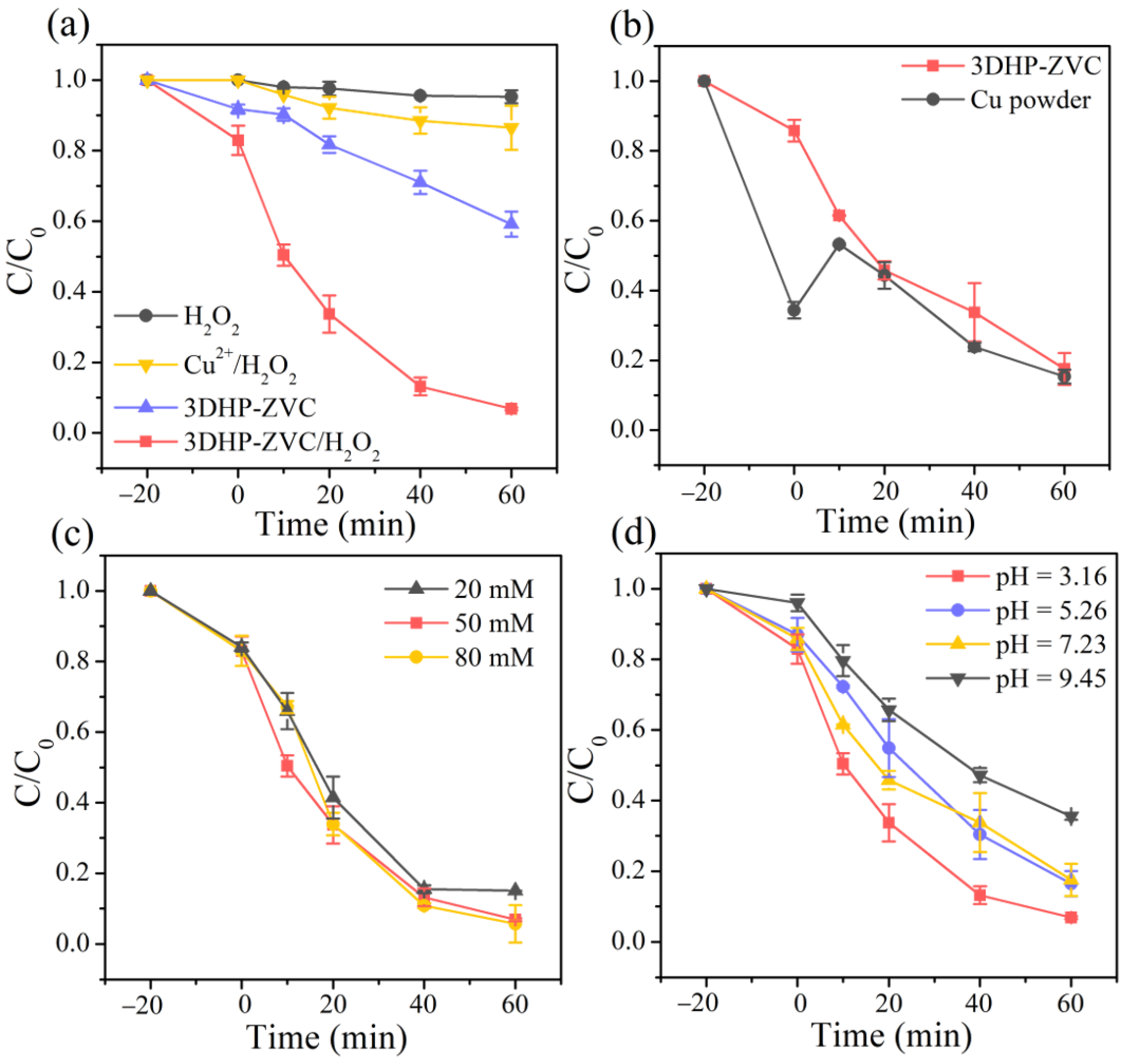
The Fenton-like performance of the developed 3DHP-ZVC samples was evaluated using tetracycline (TC, a typical antibiotic) as a target pollutant. As presented in Figure 2a,b, H2O2 decomposed a negligible amount of TC, while 40.8% of TC was removed by sole 3DHP-ZVC within 60 min. Nevertheless, the removal efficiency of TC was remarkably improved to 93.2% in the 3DHP-ZVC/H2O2 system. Furthermore, the concentration of Cu2+ ions was determined to be 0.7 mg/L during the reaction process under a neutral condition, which was much lower than the permissible limit of the World Health Organization (2 mg/L) [34]. The homogeneous leaching copper ions contributed only 13.5% of TC degradation with the addition of H2O2, suggesting the removal of TC was mainly invoked via a heterogeneous catalytic reaction of 3DHP-ZVC. Figure 2b presents the comparison of different catalyst-H2O2 systems under neutral conditions. Although the form of powder was easily dispersed in the solution, the aggregation of Cu powder would inevitably prevent contact among H2O2 and TC molecules, resulting in comparable catalytic performance to 3DHP-ZVC. However, the leaching concentration of Cu2+ ions in the Cu powder/H2O2 system (1.5 mg/L) was 2.14 times that in the 3DHP-ZVC/H2O2 system (0.7 mg/L), implying the merit of the hierarchically porous structures.
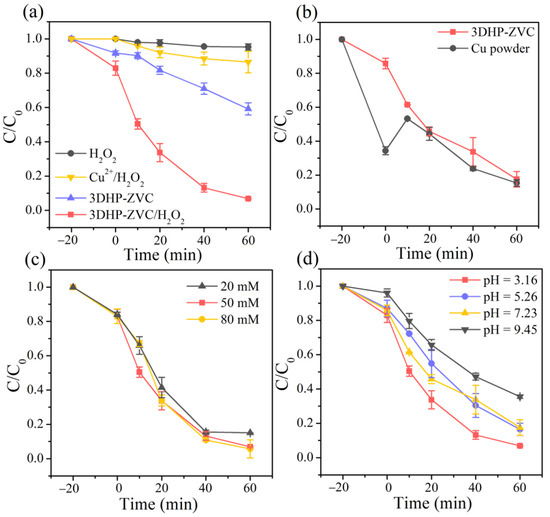
Figure 2.
TC degradation (a) in different systems under optimal conditions, (b) by different catalyst-H2O2 systems at pH = 7.23, effects of (c) H2O2 dosage, and (d) initial pH value on the degradation of TC.
The concentration of the leaching metal ions was measured to be approximately 10 mg/L in the ZVI/H2O2 system under identical conditions, which was 14.3 times higher than that of the 3DHP-ZVC/H2O2 system. These results indicate the excellent catalytic activity and reliable stability of 3DHP-ZVC in activating H2O2. Furthermore, 3DHP-ZVC exhibits comparable catalytic performance in activating H2O2 and demonstrates better recycling performance compared to those of many other Cu-based catalysts (Table 1).

Table 1.
Comparison of catalytic activity of various Cu-based catalysts in references.
2.3. Influence of Experimental Conditions
2.3.1. Effect of H2O2 Concentration
The concentration of H2O2 can significantly affect the degradation of TC in the 3DHP-ZVC/H2O2 system. As illustrated in Figure 2c, the decomposition of TC was enhanced by raising the H2O2 concentration from 20 to 50 mM, which was attributed to the generation of more active radicals [41]. Nevertheless, further increasing the H2O2 amount to 80 mM did not significantly improve the TC removal owing to the radical scavenging effect of excessive H2O2 [42]. Thus, the optimal H2O2 dosage was fixed at 50 mM.
2.3.2. Effect of Initial pH
The initial pH of the solution plays an essential role during Fenton-like processes [43]. Therefore, various experiments were performed over a pH range from 3.16 to 9.45 to investigate the catalytic activity of the 3DHP-ZVC/H2O2 system. As illustrated in Figure 2d, the highest degradation efficiency of TC reached 93.2% at pH = 3.16 and slightly decreased to 83.5% and 82.4% with increasing pH to 5.25 and 7.23, respectively. Such an observation might be ascribed to the fact that more active intermediates were released under acidic conditions [44]. However, when the solution pH was further raised to 9.45, the degradation activity significantly decreased, which was assigned to a decreased redox potential of •OH and the appearance of Cu(OH)2 [45,46]. Moreover, an alkaline environment favors the existence of more carbonate and bicarbonate, which are typical •OH quenches [45]. It is noted that the efficient degradation efficiency was maintained at over 82% under acidic and neutral pH conditions (3.16–7.23), implying its great potential for practical application.
2.4. Environmental Applications
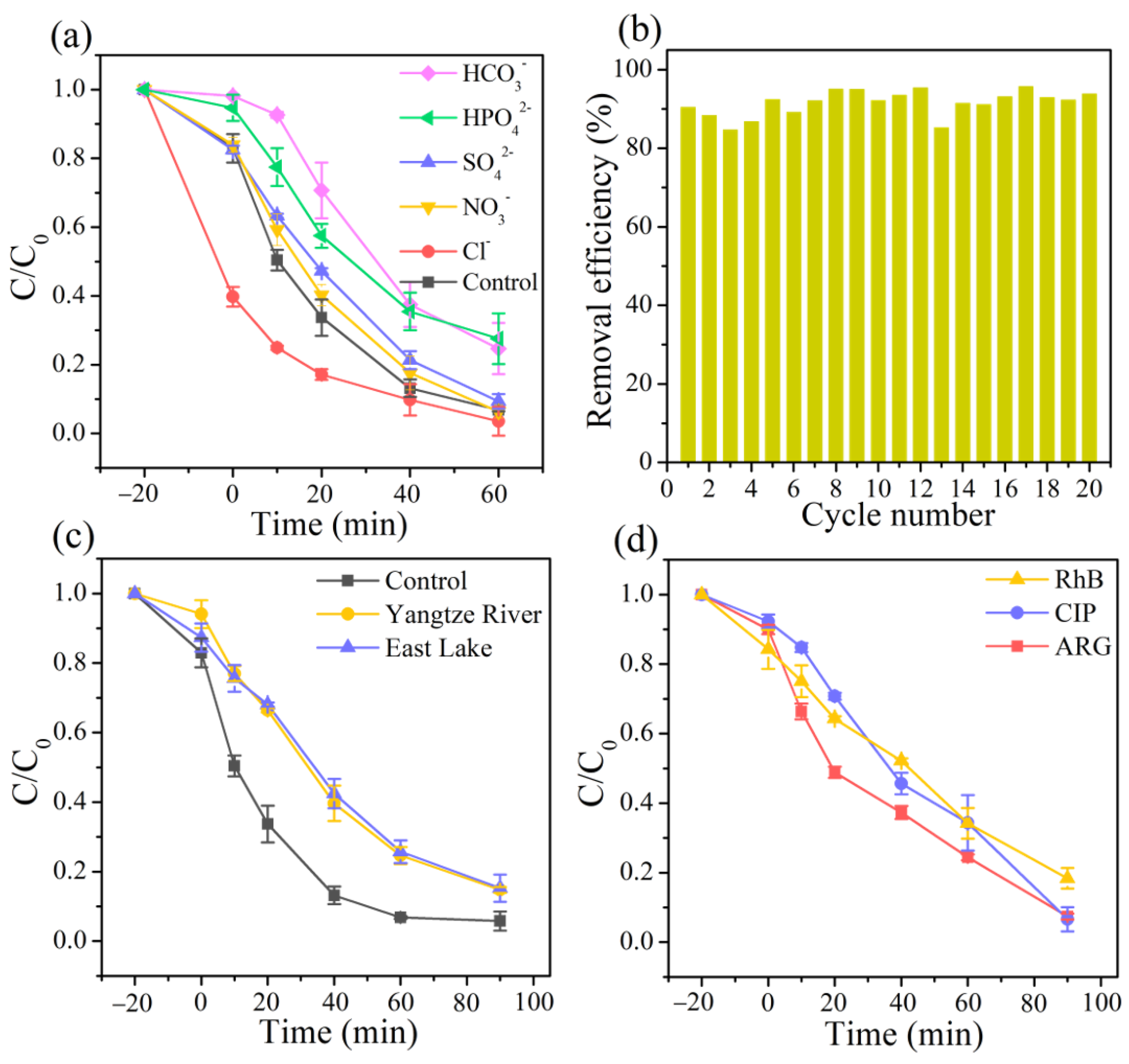
Inorganic anions, widespread in actual water environments, are capable of competing for possible reactive oxygen species with target pollutants, which shows their significant impact on the oxidation process [47,48]. As revealed in Figure 3a, the addition of HCO3− and HPO42− to the reaction drastically delayed the removal efficiency of TC from 93.2% to 75.3% and 72.4%, respectively, which was attributed to the scavenging effect of HCO3− and HPO42− for •OH [49]. Comparatively, SO42− and NO3− exhibited a negligible inhibitory effect in the process owing to the slow reaction with reactive oxygen species, which was consistent with previous reports [50]. In contrast, adding 10 mM of Cl− to the reaction solution significantly promoted the decomposition of TC due to the formation of various chlorine active species in the reaction, such as Cl•−, ClOH•−, and Cl2−• [51].
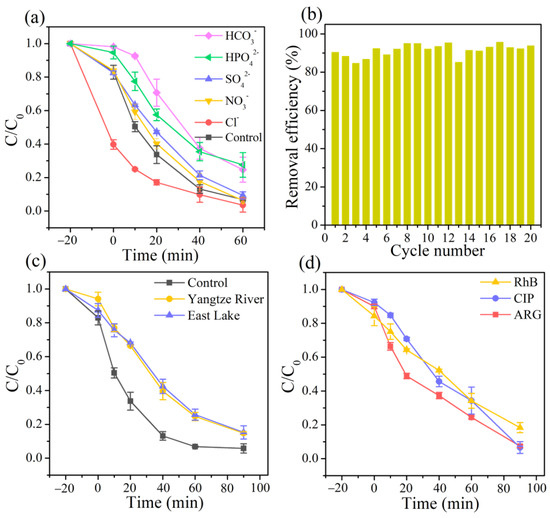
Figure 3.
(a) Effects of co-existing inorganic anions (10 mM), (b) the reusability of the 3DHP-ZVC samples, (c) various water systems on the TC removal, and (d) degradation of different organic pollutants including RhB, CIP, and ARG.
The stability of the obtained 3DHP-ZVC samples was also evaluated. As displayed in Figure 3b, the catalytic activity of 3DHP-ZVC for TC degradation had no significant decay during 20 continuous cycles, which exceeds most of the conventional Cu-based nanoparticle catalysts. This result may be due to the advantages of hierarchically porous structures of 3DHP-ZVC, which facilitate separation and recovery from water bodies and reduce the dissolution of ions, decreasing the risk of secondary pollution. In addition, no change had been observed in the XRD spectra of the 3DHP-ZVC sample after the reaction process, indicating its excellent structural stability (Figure 1a).
To explore the adaptation of 3DHP-ZVC for further practical applications, the reactions in different actual water matrixes were conducted and the catalytic performance toward various organic contaminants was investigated. The removal efficiency of TC achieved 85.3% and 84.7% in the Yangtze River and East Lake water, respectively, implying the great application potential of 3DHP-ZVC in actual water treatment (Figure 3c). In addition, RhB (a cationic dye), ciprofloxacin (CIP, an antibiotic), and acid red G (ARG, an azo dye) were selected to prove the applicability of the 3DHP-ZVC/H2O2 system. Figure 3d demonstrates that all the selected contaminants could be decomposed by over 82% in 90 min, indicating that 3DHP-ZVC is potentially effective in Fenton-like systems for wastewater purification.
2.5. Activation Mechanism
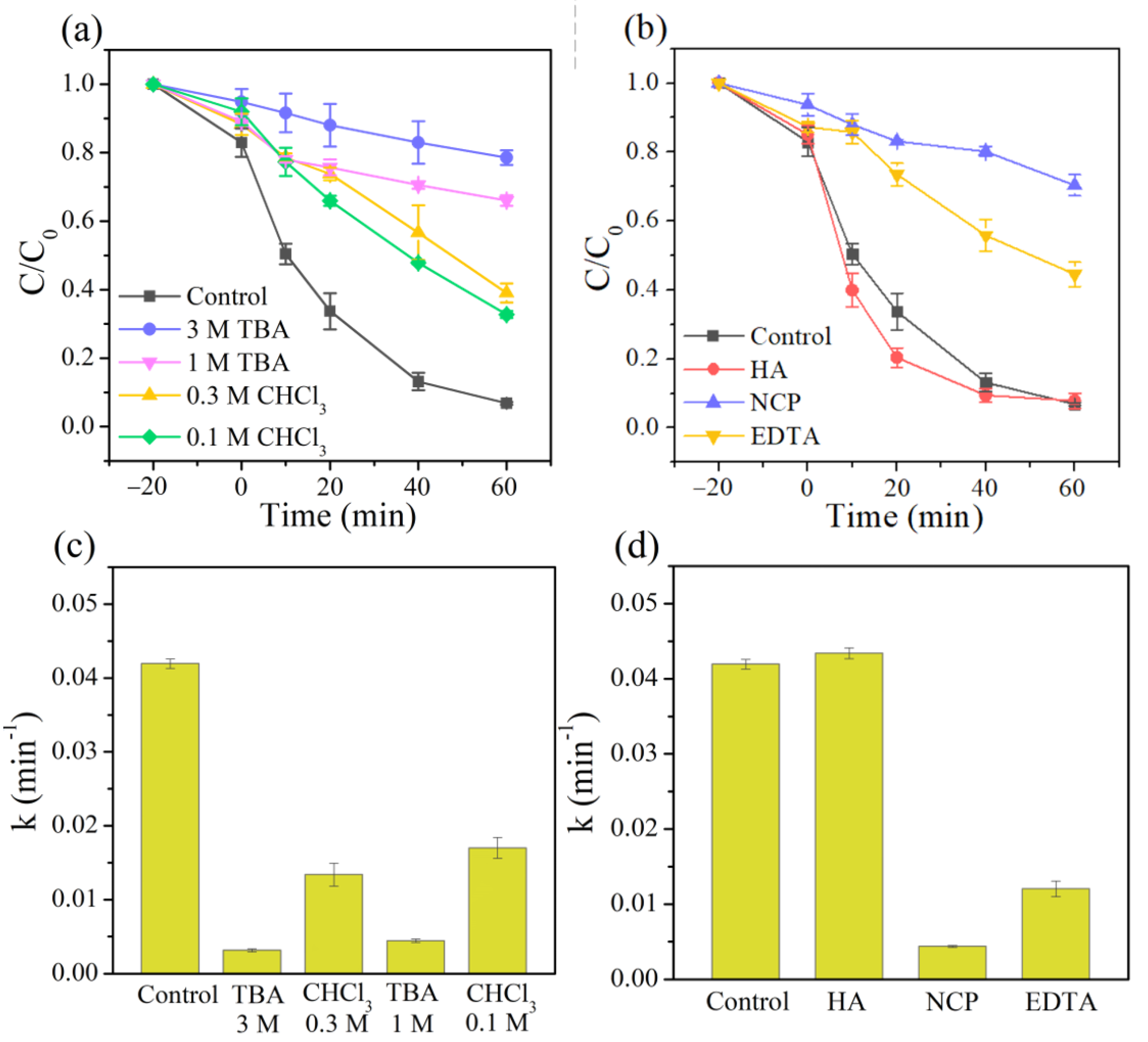
Numerous studies have confirmed that •OH and superoxide radicals (O2•−) play essential roles in heterogeneous Fenton systems [52,53,54]. Therefore, tert-butanol (TBA, a typical scavenger for •OH) and chloroform (CHCl3, an effective scavenger of O2•−) were used to investigate the contribution of •OH and O2•− radicals in the 3DHP-ZVC/H2O2 system [41,55]. As shown in Figure 4a, adding 1 M of TBA to the reaction solution dropped the degradation efficiency of TC from 93.2% to 32.9% within 60 min and significantly suppressed it to 21.4% with the TBA concentration further increasing to 3 M. Meanwhile, the reaction rate in the existence of TBA (3 M) was dramatically decreased over 13 times compared to the control groups (Figure 4c). These results indicated the dominant role of •OH in the 3DHP-ZVC/H2O2 system. The introduction of 0.1–0.3 M of CHCl3 resulted in a significant inhibition effect on the TC removal, suggesting that O2•− also participated in the reaction. Recent reports indicate that the cycle of Cu(II)/Cu(I) is responsible for the generation of •OH for TC degradation [56]. To ascertain this conclusion, neocuproine (NCP) and ethylene diamine tetraacetic acid (EDTA) were employed to identify the role of Cu(I) and Cu(II) during the reaction, respectively [57]. As shown in Figure 4b,d, the presence of NCP and EDTA greatly prevented the removal of TC, strongly confirming the involvement of Cu(I) and Cu(II) in the reaction. In addition, it is widely accepted that the reduction of high-valent species is the rate-limiting step in the oxidation process. Hydroxylamine (HA) served as a reducing agent that significantly accelerated the degradation of TC (Figure 4b,d), indicating the cycle of Cu(II)/Cu(I) [58]. Besides, compared to fresh 3DHP-ZVC, the relative ratio of Cu2+ and Cu0 (Figure 1b) increased significantly for the used 3DHP-ZVC samples, and slight corrosion was observed after the oxidation process (Figure 1e,f), which elucidated the transformation of Cu0 to Cu2+ in the TC degradation process.
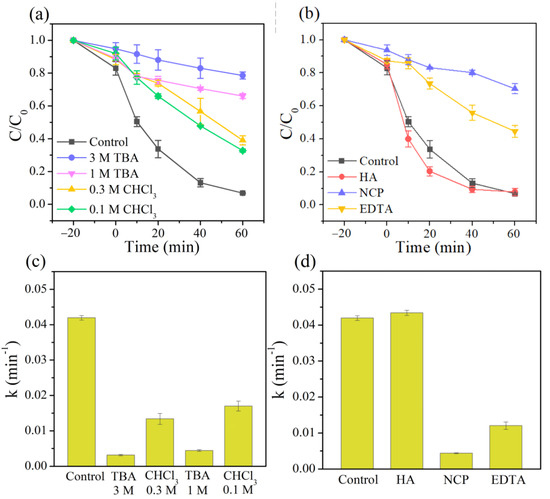
Figure 4.
Effects of (a) different scavengers (TBA and CHCl3), (b) HA, NCP, and EDTA for TC removal in the 3DHP-ZVC/H2O2 system, and (c,d) their corresponding reaction rate constant under different conditions.
According to the above discussion, a feasible mechanism for TC removal in the 3DHP-ZVC/H2O2 system was proposed. First, the surface of 3DHP-ZVC was corroded by H+ and H2O2 to release Cu(I) (Equation (1)) [59]. The released Cu(I) was responsible for the H2O2 activation to generate •OH, as described by Equation (2) [60]. Subsequently, Cu(I) was further oxidized by •OH to generate Cu(II), and O2•− was produced with the reaction between Cu(II) and H2O2 (Equations (3) and (4)) [61,62]. Then, O2•− and Cu0 could serve as reducing agents for the regeneration of Cu(I) via Equations (5) and (6) [62,63]. Finally, the removal of TC was achieved by the generation of •OH in the 3DHP-ZVC/H2O2 system.
2 Cu0 + 2 H+ + H2O2 → 2 Cu(I) + 2 H2O
Cu(I) + H2O2 → Cu(II) + •OH + OH-
Cu(I) + •OH → Cu(II) + OH-
Cu(II) + H2O2 → Cu(I) + O2•− + 2 H+
Cu(II) + O2•− → Cu(I) + O2
Cu(II) + Cu0 → 2 Cu(I)
2.6. Analysis of TC Removal by 3D-EEMs
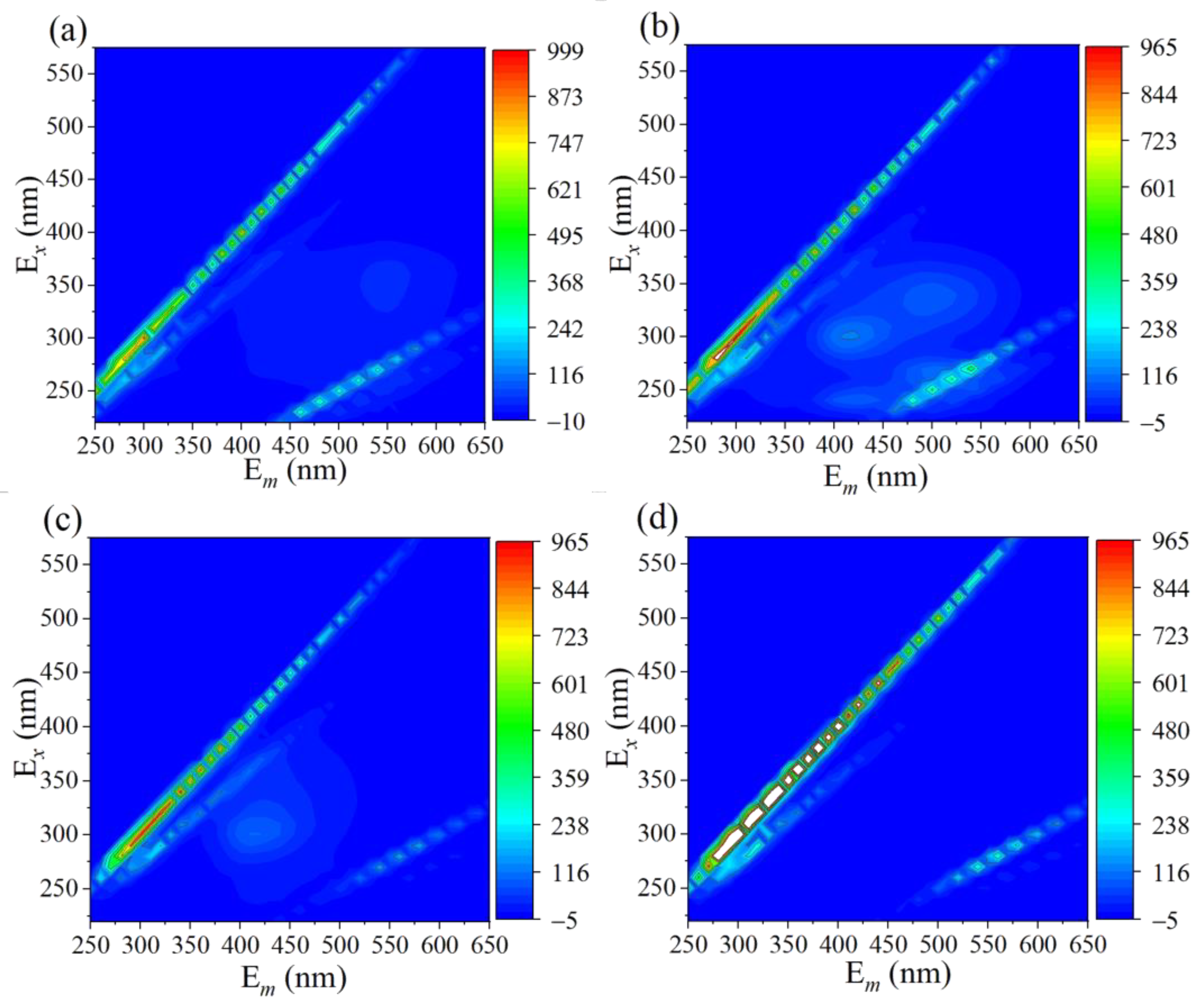
Three-dimensional excitation-emission matrix fluorescence spectroscopy (EEMS) technology was applied to explain the overall removal and mineralization of TC in the 3DHP-ZVC/H2O2 system. As revealed in Figure 5a, no fluorescence peak was observed in the initial TC solution due to the electron-withdrawing groups of TC, which was consistent with previous reports [64]. Two maxima fluorescence peaks belonging to the areas of fulvic acids-like (Ex/Em = 260–320/400–460) and humic acid (Ex/Em = 300–360/460–550) were found after degradation for 30 min (Figure 5b) [65]. When the degradation time was increased to 60 min, the intensities of the fluorescence signals were significantly weakened, and the signals disappeared when increasing the reaction time to 24 h, implying that the generated intermediate substances were finally mineralized into smaller fragments (Figure 5c,d). The change in the intensity of fluorescence signals during the reaction reflects the removal pathway of TC to some extent.
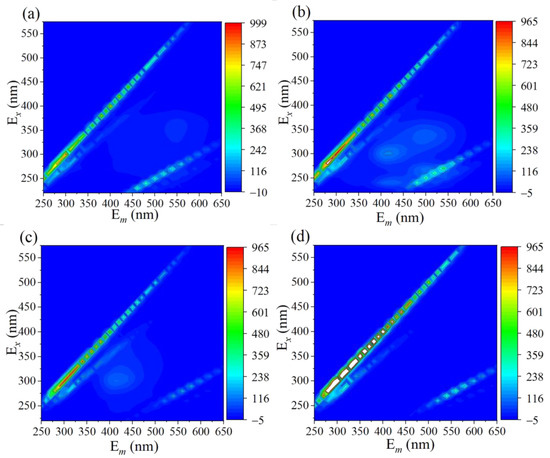
Figure 5.
Three-dimensional EEM fluorescence spectra of TC solution after (a) 0 min, (b) 30 min, (c) 60 min, and (d) 24 h during the reaction.
3. Materials and Methods
3.1. Materials and Reagents
NaOH, HNO3, RhB, H2O2, HA, EDTA, Na2HPO4, NaCl, Na2SO4, NaHCO3, NaNO3, and CHCl3 were purchased from China Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). TBA, NCP, and TC were supplied by China Aladdin Chemistry Co., Ltd. (Shanghai, China). ARG and CIP were obtained from China Macklin in Biochemical Reagent Co., Ltd. (Shanghai, China). All reagents used in this experiment were analytical grade and used without further purification. Pure copper powder was supplied from VilWory Advanced Materials Technology Co., Ltd. (Jiangsu, China).
3.2. SLM Processing
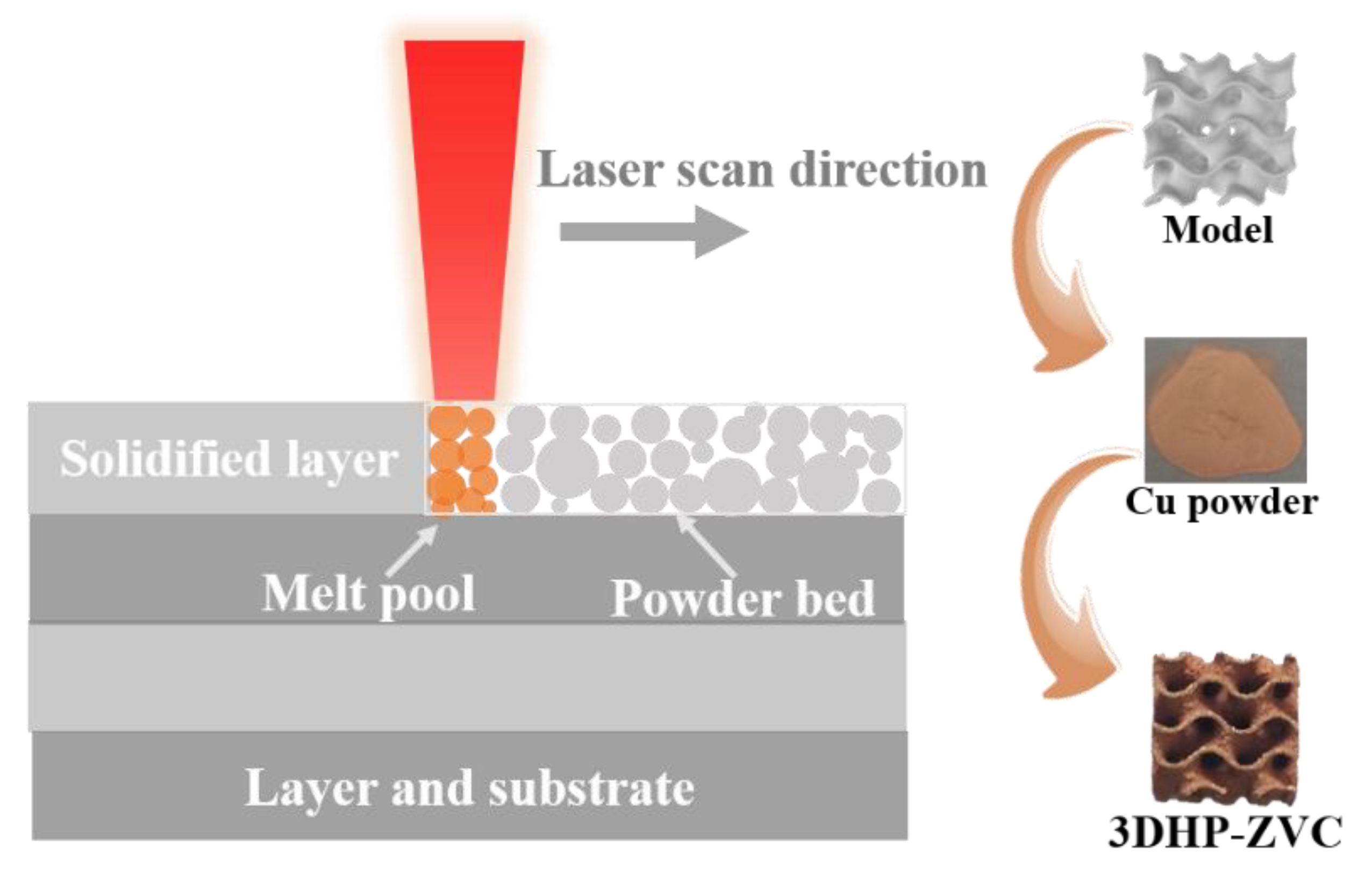
Cu powder particles (15–40 μm) with spherical shapes were sieved out for SLM processing. The samples were fabricated through a self-developed SLM-150 machine (ZRapid Tech, Suzhou, China), where a 500 W fiber laser was equipped. The chamber was full of high-purity argon to prevent oxygen. The laser scanning speed and power play a vital role in the quality of the SLM-produced samples [66]. To minimize the possible defects, the optimized parameters are listed in Table 2, and the SLM process is illustrated in Figure 6.

Table 2.
Optimized parameters in this work and the weight and dimension of 3DHP-ZVC.
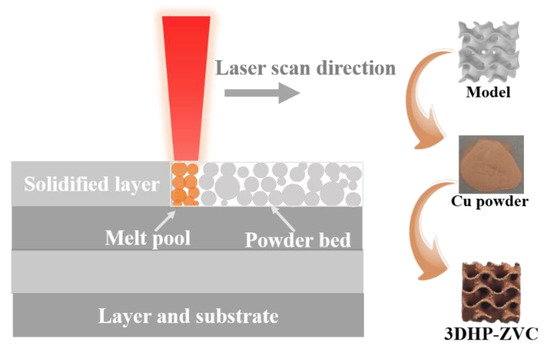
Figure 6.
Schematic illustration of printing 3DHP-ZVC through SLM technology.
3.3. Characterization
The microstructure of the 3DHP-ZVC catalysts was characterized via X-ray diffraction (XRD, XRD 6100, Kratos, Kyoto, Japan), and the data were determined from 20° to 100°. The surface morphology information was obtained via Scanning Electron Microscopy (SEM, Gemini 300, ZEISS, Oberkochen, Germany). The chemical state of the fresh and used 3DHP-ZVC was determined using an X-ray photoelectron spectroscopy (XPS, ESCALAB Xl+, Thermo, Waltham, MA, USA) instrument. Three-dimensional excitation-emission matrix fluorescence spectra (3D EEMs, F-2700, HITACHI, Tokyo, Japan) were used to investigate the degradation pathway of TC. The leached Cu2+ ions were obtained through an SP−3520AA atomic absorption spectrometer (SP−3520AA, Shanghai Spectrum Instruments, Shanghai, China). The specific surface area of the samples was measured through nitrogen adsorption on an ASAP2020 HD88 nitrogen adsorption apparatus (Micromeritics, Shanghai, China).
3.4. Experimental Procedure
All the degradation reactions were performed in 250 mL glass beakers in a water bath under continuous magnetic stirring. Firstly, 2.1 g of 3DHP-ZVC was sunk into 100 mL of TC reaction solution (10 mg/L) for 30 min to achieve an adsorption equilibrium. The reaction was initiated by the addition of a certain amount of H2O2. At fixed intervals, the reaction solution (approximately 2 mL) was taken out for immediate measurement using a UV–vis spectrophotometer (UV−1990PC, AOE Instruments, Shanghai, China). The maximum detection wavelength of ARG, RhB, CIP, and TC was 505, 554, 275, and 356 nm, respectively. In typical experiments, the dosage of H2O2 was 50 mM, and the solution of initial pH was adjusted to 3 unless otherwise stated. NaOH or HNO3 solution (0.1 M) was applied to adjust the pH of the TC solution. After each cycle, the 3DHP-ZVC catalyst was extracted from the solution and rinsed using an ultrasonic cleaning instrument (KM-400KDE, Meimei Ultrasonic Instrument Co., Ltd., Kunshan, China) with deionized water for 30 min.
4. Conclusions
In summary, a novel 3DHP-ZVC catalyst with hierarchically porous structures was developed via 3D printing technology and served as an efficient H2O2 activator to decompose various organic pollutants (e.g., TC, RhB, CIP, and ARG). The obtained hierarchically porous 3DHP-ZVC samples not only facilitated recycling but reduced ionic leaching compared to Cu powder particles for H2O2 activation to remove TC. Under optimal conditions, the 3DHP-ZVC/H2O2 system could remove over 93.2% of TC, which is much superior to the homogeneous Cu2+/H2O2 system. The catalyst presented excellent stability for 20 continuous cycles without significant decay. In addition, the 3DHP-ZVC/H2O2 system demonstrated general applicability under a pH range from 3.16 to 7.23 and actual water environments. Trapping experiments indicated that •OH radicals were the dominant active species and O2•− were involved in the 3DHP-ZVC/H2O2 system. The cycle of Cu(II)/Cu(I) was proved to be responsible for the generation of •OH and O2•−. This work paves a new avenue to the development of stable and recycling-friendly catalysts in Fenton-like systems for environmental remediation.
Author Contributions
Conceptualization, S.G.; funding acquisition, S.G. and C.C.; formal analysis, Y.H.; investigation, Y.W., Q.W. and J.A.; project administration, S.G.; supervision, S.G. and C.C.; software, Y.W. and J.A.; validation, C.C. and Q.W.; visualization, M.C. and Y.H.; writing—original draft preparation, M.C.; writing—review and editing, S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from the National Natural Science Foundation of China (Nos. 51604194 and 51905192) and the Natural Science Foundation of Hubei Province of China (No. 2021CFB296).
Data Availability Statement
The data presented in this study are openly available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, Y.W.; Zhu, Y.; Li, T.; Chen, Z.H.; Jiang, Q.K.; Zhao, Z.Y.; Liang, X.Y.; Hu, C. Unraveling the high-activity origin of single-atom iron catalysts for organic pollutant oxidation via peroxymonosulfate activation. Environ. Sci. Technol. 2021, 55, 8318–8328. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Pang, H.W.; Liu, X.W.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M.Q.; Hu, B.W.; Yang, H.; Wang, X.K. Orderly porous covalent organic frameworks-based materials: Superior adsorbents for pollutants removal from aqueous solutions. Innovation 2021, 2, 100076. [Google Scholar] [CrossRef] [PubMed]
- Kouvelis, K.; Kampioti, A.A.; Petala, A.; Frontistis, Z. Degradation of sulfamethoxazole using a hybrid CuOx–BiVO4/SPS/solar system. Catalysts 2022, 12, 882. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Chen, H.; Ni, B.J. Recent advances in waste-derived functional materials for wastewater remediation. EEH 2022, 1, 86–104. [Google Scholar] [CrossRef]
- Wang, T.Z.; Cao, X.J.; Jiao, L.F. Ni2P/NiMoP heterostructure as a bifunctional electrocatalyst for energy-saving hydrogen production. EScience 2021, 1, 69–74. [Google Scholar] [CrossRef]
- Zhu, L.L.; Ji, J.H.; Liu, J.; Mine, S.Y.; Matsuoka, M.Y.; Zhang, J.L.; Xing, M.Y. Designing 3D-MoS2 sponge as excellent cocatalysts in advanced oxidation processes for pollutant control. Angew. Chem. Int. Ed. 2020, 132, 14072–14080. [Google Scholar] [CrossRef]
- Zhang, X.K.; Liu, J.H.; Zheng, X.C.; Chen, R.; Zhang, M.; Liu, Z.Y.; Wang, Z.Y.; Li, J. Activation of oxalic acid via dual-pathway over single-atom Fe catalysts: Mechanism and membrane application. Appl. Catal. B—Environ. 2023, 321, 122068. [Google Scholar] [CrossRef]
- Yin, H.L.; Cai, Y.W.; Li, G.Y.; Wang, W.J.; Wong, P.K.; An, T.C. Persistence and environmental geochemistry transformation of antibiotic-resistance bacteria/genes in water at the interface of natural minerals with light irradiation. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2270–2301. [Google Scholar] [CrossRef]
- Qin, D.Y.; Zhang, C.; Zhou, Y.; Qin, F.Z.; Wang, H.; Wang, W.J.; Yang, Y.; Zeng, G.M. Dual optimization approach to Mo single atom dispersed g-C3N4 photocatalyst: Morphology and defect evolution. Appl. Catal. B—Environ. 2022, 303, 120904. [Google Scholar]
- Li, C.Q.; Huang, Y.; Dong, X.B.; Sun, Z.M.; Duan, X.D.; Ren, B.X.; Zheng, S.L.; Dionysiou, D.D. Highly efficient activation of peroxymonosulfate by natural negatively−charged kaolinite with abundant hydroxyl groups for the degradation of atrazine. Appl. Catal. B—Environ. 2019, 247, 10–23. [Google Scholar] [CrossRef]
- Chen, G.Y.; Yu, Y.; Liang, L.; Duan, X.G.; Li, R.; Lu, X.K.; Yan, B.B.; Li, N.; Wang, S.B. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef]
- Chen, F.X.; Lv, H.X.; Chen, W.; Chen, R. Catalytic wet peroxide oxidation of anionic pollutants over fluorinated Fe3O4 microspheres at circumneutral pH values. Catalysts 2022, 12, 1564. [Google Scholar] [CrossRef]
- Yang, Z.X.; Wang, Z.Z.; Wang, J.T.; Li, Y.; Zhang, G.K. Facet-dependent activation of oxalic acid over magnetic recyclable Fe3S4 for efficient pollutant removal under visible light irradiation: Enhanced catalytic activity, DFT calculations, and mechanism insight. Environ. Sci. Technol. 2022, 56, 18008–18017. [Google Scholar] [CrossRef]
- Van Scoy, A.R.; Tjeerdema, R.S. Environmental fate and toxicology of chlorothalonil. Rev. Environ. Contam. Toxicol. 2014, 232, 89–105. [Google Scholar]
- Tian, X.K.; Gao, P.P.; Nie, Y.L.; Yang, C.; Zhou, Z.X.; Li, Y.; Wang, Y.X. A novel singlet oxygen involved peroxymonosulfate activation mechanism for degradation of ofloxacin and phenol in water. Chem. Commun. 2017, 53, 6589–6592. [Google Scholar] [CrossRef]
- Guo, S.; Yang, W.; You, L.M.; Li, J.; Chen, J.Y.; Zhou, K. Simultaneous reduction of Cr(VI) and degradation of tetracycline hydrochloride by a novel iron-modified rectorite composite through heterogeneous photo-Fenton processes. Chem. Eng. J. 2020, 393, 124758. [Google Scholar] [CrossRef]
- Kaushal, S.; Singh, P.P.; Kaur, N. Metal organic framework-derived Zr/Cu bimetallic photocatalyst for the degradation of tetracycline and organic dyes. Environ. Nanotechnol. Monit. Manag. Environ. 2022, 18, 100727. [Google Scholar] [CrossRef]
- Jawad, A.; Chen, Z.Q.; Yin, G.C. Bicarbonate activation of hydrogen peroxide: A new emerging technology for wastewater treatment. Chin. J. Catal. 2016, 37, 810–825. [Google Scholar] [CrossRef]
- Su, C.P.; Cheng, M.X.; Tian, F.; Chen, F.X.; Chen, R. Anti-oil-fouling Au/BiOCl coating for visible light-driven photocatalytic inactivation of bacteria. J. Colloid Interface Sci. 2022, 628, 955–967. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.L.; Li, J.; Xiang, Q.J.; Liu, Z.Y.; Liu, B. Identification of the active sites on metallic MoO2-x nano-sea-urchin for atmospheric CO2 photoreduction under UV, visible, and near-infrared light illumination. Angew. Chem. Int. Ed. 2022, 62, e202213124. [Google Scholar]
- Zarif, F.; Khurshid, S.; Muhammad, N.; Qureshi, M.Z.; Shah, N.S. Colorimetric sensing of hydrogen peroxide using ionic-liquid-sensitized zero-valent copper nanoparticle (nZVCu). Chem. Sel. 2020, 5, 6066–6074. [Google Scholar] [CrossRef]
- Li, C.Q.; Yang, S.S.; Bian, R.Z.; Tan, Y.; Zhang, X.W.; Zheng, S.L.; Sun, Z.M. Efficient catalytic degradation of bisphenol A coordinated with peroxymonosulfate via anchoring monodispersed zero-valent iron on natural kaolinite. Chem. Eng. J. 2022, 448, 137746. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Ahmed, B.; Sushkova, S.; Singh, R.; Soldatov, M.; Laratte, B.; Fedorenko, A.; Mandzhieva, S.; Blicharska, E.; et al. Interaction of copper-based nanoparticles to soil, terrestrial, and aquatic systems: Critical review of the state of the science and future perspectives. Rev. Environ. Contam. Toxicol. 2019, 252, 51–96. [Google Scholar]
- Guo, S.; Chen, M.; You, L.M.; Wei, Y.; Cai, C.; Wei, Q.S.; Zhang, H.L.; Zhou, K. 3D printed hierarchically porous zero-valent copper for efficient pollutant degradation through peroxymonosulfate activation. Sep. Purif. Technol. 2023, 305, 122437. [Google Scholar] [CrossRef]
- Zhou, X.T.; Liu, C.J. Three-dimensional printing for catalytic applications: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1701134. [Google Scholar] [CrossRef]
- Maconachie, T.; Leary, M.; Lozanovski, B.; Zhang, X.Z.; Qian, M.; Faruque, Q.; Brandt, M. SLM lattice structures: Properties, performance, applications and challenges. Mater. Design 2019, 183, 108137. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, C.; Chen, Z.J.; Li, Y.; Yan, W.Y.; Yu, H.B.; Liu, L. Three-dimensional hierarchical porous structures of metallic glass/copper composite catalysts by 3D printing for efficient wastewater treatments. ACS Appl. Mater. Interfaces 2021, 13, 7227–7237. [Google Scholar] [CrossRef]
- Cai, C.; Guo, S.; Li, B.Y.; Tian, Y.J.; Qiu, J.C.D.; Sun, C.N.; Yan, C.Z.; Qi, H.J.; Zhou, K. 3D printing and chemical dealloying of a hierarchically micro-and nanoporous catalyst for wastewater purification. ACS Appl. Mater. Interfaces 2021, 13, 48709–48719. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Y.S.; Wang, Z.R.; Zhou, G.F.; Zhou, R.Y.; Liu, Y.Q. Removal of diclofenac in water using peracetic acid activated by zero valent copper. Sep. Purif. Technol. 2021, 276, 119319. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.; Ma, S.Y.; Shang, Z.F. Effect factors, kinetics and thermodynamics of remediation in the chromium contaminated soils by nanoscale zero valent Fe/Cu bimetallic particles. Chem. Eng. J. 2016, 302, 663–669. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Zhang, L.; Guan, J.; Qian, S.Y.; Zhang, Z.X.; Ngaw, C.K.; Wan, S.L.; Wang, S.; Lin, J.D.; Wang, Y. Controlled synthesis of Cu0/Cu2O for efficient photothermal catalytic conversion of CO2 and H2O. ACS Sustain. Chem. Eng. 2021, 9, 1754–1761. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Wang, J.L. MOF-derived Cu0/C activation of molecular oxygen for efficient degradation of sulfamethazine. Chem. Eng. J. 2022, 427, 131961. [Google Scholar] [CrossRef]
- Shen, H.Y.; Sun, P.J.; Meng, X.; Wang, J.L.; Liu, H.Y.; Xu, L.J. Nanoscale Fe0/Cu0 bimetallic catalysts for Fenton-like oxidation of the mixture of nuclear-grade cationic and anionic exchange resins. Chemosphere 2021, 269, 128763. [Google Scholar] [CrossRef]
- Kong, A.Q.; Ji, Y.H.; Ma, H.H.; Song, Y.F.; He, B.Q.; Li, J.X. A novel route for the removal of Cu(II) and Ni(II) ions via homogeneous adsorption by chitosan solution. J. Clean. Prod. 2018, 192, 801–808. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.K.; Wang, S.W.; Li, X.; Liu, B.M.; Xu, Y.H.; Yu, P.; Sun, Y.J. High-density dispersion of CuNx sites for H2O2 activation toward enhanced photo-Fenton performance in antibiotic contaminant degradation. J. Hazard. Mater. 2022, 423, 127039. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Gu, Y.Y.; Xin, S.S.; Lu, L.L.; Huang, Z.W.; Li, M.Y.; Cui, Y.F.; Fu, R.B.; Wang, S.B. CuxNiyCo-LDH nanosheets on graphene oxide: An efficient and stable Fenton-like catalyst for dual-mechanism degradation of tetracycline. Chem. Eng. J. 2022, 434, 134574. [Google Scholar] [CrossRef]
- Liu, M.G.; Xia, H.; Yang, W.X.; Liu, X.Y.; Xiang, J.; Wang, X.M.; Hu, L.S.; Lu, F.S. Novel Cu-Fe bimetal oxide quantum dots coupled g-C3N4 nanosheets with H2O2 adsorption-activation trade-off for efficient photo-Fenton catalysis. Appl. Catal. B—Environ. 2022, 301, 120765. [Google Scholar] [CrossRef]
- Huang, G.Y.; Chang, W.J.; Lu, T.W.; Tsai, I.L.; Wu, S.J.; Ho, M.H.; Mi, F.L. Electrospun CuS nanoparticles/chitosan nanofiber composites for visible and near-infrared light-driven catalytic degradation of antibiotic pollutants. Chem. Eng. J. 2022, 431, 134059. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, Y.Q.; Ge, Y.J.; Wu, H.D.; Li, Q.S.; Gao, N.Y.; Deng, J. Ultrasound-enhanced nanosized zero-valent copper activation of hydrogen peroxide for the degradation of norfloxacin. Ultrason. Sonochem. 2018, 40, 763–772. [Google Scholar] [CrossRef]
- Lyu, L.; Cao, W.R.; Yu, G.F.; Yan, D.B.; Deng, K.L.; Lu, C.; Hu, C. Enhanced polarization of electron-poor/rich micro-centers over nZVCu-Cu(II)-rGO for pollutant removal with H2O2. J. Hazard. Mater. 2020, 383, 121182. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Zhao, J.; Li, Y.; Wang, Y.; Qiu, T.; Wu, Y.L.; Dong, W.B.; Mailhot, G. Improving Fenton-like system with catechin, an environmental-friendly polyphenol: Effects and mechanism. Chem. Eng. J. 2021, 426, 127946. [Google Scholar] [CrossRef]
- Hou, L.W.; Wang, L.G.; Royer, S.; Zhang, H. Ultrasound-assisted heterogeneous Fenton-like degradation of tetracycline over a magnetite catalyst. J. Hazard. Mater. 2016, 302, 458–467. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, L.J.; Chen, M.; Ahmad, F.; Fida, H.; Zhang, H.L. Heterogeneous activation of peroxymonosulfate by a spinel CoAl2O4 catalyst for the degradation of organic pollutants. Catalysts 2022, 12, 847. [Google Scholar] [CrossRef]
- Xu, L.J.; Yang, Y.J.; Li, W.Y.; Tao, Y.J.; Sui, Z.G.; Song, S.; Yang, J. Three-dimensional macroporous graphene-wrapped zero-valent copper nanoparticles as efficient micro-electrolysis-promoted Fenton-like catalysts for metronidazole removal. Sci. Total Environ. 2019, 658, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Gao, N.Y.; Deng, Y.; Chu, W.C.; Rong, W.L.; Zhou, S.D. Factors affecting ultraviolet irradiation/hydrogen peroxide (UV/H2O2) degradation of mixed N-nitrosamines in water. J. Hazard. Mater. 2012, 231, 43–48. [Google Scholar] [CrossRef]
- Li, W.; Chen, C.; Zhu, J.Y.; Zhou, L.X.; Lan, Y.Q. Efficient removal of aniline by micro-scale zinc-copper (mZn/Cu) bimetallic particles in acidic solution: An oxidation degradation mechanism via radicals. J. Hazard. Mater. 2019, 366, 482–491. [Google Scholar] [CrossRef]
- Yu, B.W.; Li, Z.J.; Zhang, S.L. Zero-valent copper-mediated peroxymonosulfate activation for efficient degradation of azo dye orange G. Catalysts 2022, 12, 700. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.J.; Wang, X.; Ji, Q.Y.; Li, T.Z.; He, H.; Song, H.O.; Yang, S.G.; Li, S.Y.; Yan, S.C.; et al. Identifying the role of oxygen vacancy on cobalt-based perovskites towards peroxymonosulfate activation for efficient iohexol degradation. Appl. Catal. B—Environ. 2022, 319, 121901. [Google Scholar] [CrossRef]
- Guo, S.; Yang, Z.X.; Zhang, H.L.; Yang, W.; Li, J.; Zhou, K. Enhanced photocatalytic degradation of organic contaminants over CaFe2O4 under visible LED light irradiation mediated by peroxymonosulfate. J. Mater. Sci. Technol. 2020, 62, 34–43. [Google Scholar] [CrossRef]
- Guo, S.; Liu, M.D.; You, L.M.; Cheng, G.; Li, J.; Zhou, K. Oxygen vacancy induced peroxymonosulfate activation by Mg-doped Fe2O3 composites for advanced oxidation of organic pollutants. Chemosphere 2021, 279, 130482. [Google Scholar] [CrossRef]
- Zhen, Y.F.; Sun, Z.Q.; Jia, Z.Y.; Liu, C.H.; Zhu, S.S.; Li, X.Y.; Wang, W.; Ma, J. Facile preparation of α-MnO2 nanowires for assembling free-standing membrane with efficient Fenton-like catalytic activity. Chin. Chem. Lett. 2023, 34, 107664. [Google Scholar] [CrossRef]
- Shi, J.G.; Ai, Z.H.; Zhang, L.Z. Fe@Fe2O3 core-shell nanowires enhanced Fenton oxidation by accelerating the Fe(III)/Fe(II) cycles. Water Res. 2014, 59, 145–153. [Google Scholar] [CrossRef]
- Peng, W.; Niu, W.J.; Paerhati, S.; Guo, W.J.; Ma, J.G.; Hou, J.W. Facile synthesis of n-Fe3O4/ACF functional cathode for efficient dye degradation through heterogeneous E-Fenton process. Catalysts 2022, 12, 879. [Google Scholar] [CrossRef]
- Katagi, T. Photodegradation of pesticides on plant and soil surfaces. Rev. Environ. Contam. Toxicol. 2004, 182, 1–78. [Google Scholar]
- Qi, J.J.; Yang, X.Y.; Pan, P.Y.; Huang, T.B.; Yang, X.D.; Wang, C.C.; Liu, W. Interface engineering of Co(OH)2 nanosheets growing on the KNbO3 perovskite based on electronic structure modulation for enhanced peroxymonosulfate activation. Environ. Sci. Technol. 2022, 56, 5200–5212. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, P.F.; Ding, D.D.; Yang, Z.X.; Wang, W.Z.; Xin, H.; Xu, J.; Han, Y.F. Revealing the active species of Cu-based catalysts for heterogeneous Fenton reaction. Appl. Catal. B—Environ. 2019, 258, 117985. [Google Scholar] [CrossRef]
- Liu, T.C.; Yin, K.; Li, N.; Liu, H.; Chen, J.B.; Zhou, X.F.; Zhang, Y.L. Efficient activation of peroxymonosulfate by copper supported on polyurethane foam for contaminant degradation: Synergistic effect and mechanism. Chem. Eng. J. 2022, 427, 131741. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, P.; Sun, Y.M.; Zhang, P.; Zhou, C.Y.; Xiong, Z.K.; Zhang, H.; Liang, J.; Lai, B. Reducing agents enhanced Fenton-like oxidation (Fe (III)/Peroxydisulfate): Substrate specific reactivity of reactive oxygen species. Water Res. 2022, 218, 118412. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Lqbal, J.; Khan, Z.U.H.; Muhammad, N.; Polychronopoulou, K.; Hussain, S.; Imran, M.; Murtaza, B.; et al. Nano-zero valent copper as a Fenton-like catalyst for the degradation of ciprofloxacin in aqueous solution. J. Water Process Eng. 2020, 37, 101325. [Google Scholar] [CrossRef]
- Xu, Z.; Shan, C.; Xie, B.H.; Liu, Y.; Pan, B.C. Decomplexation of Cu(II)-EDTA by UV/persulfate and UV/H2O2: Efficiency and mechanism. Appl. Catal. B—Environ. 2017, 200, 439–447. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, H.; Zhao, Y.X.; Wan, J.; Li, L.L.; Wang, L.Y. Facile synthesis of copper-based bimetallic oxides for efficient removal of bisphenol a via Fenton-like degradation. Sep. Purif. Technol. 2021, 279, 119724. [Google Scholar]
- Lin, C.C.; Zhong, Y.H. Performance of nZVC/H2O2 process in degrading chloramphenicol in water. J. Taiwan Inst. Chem. E. 2021, 129, 112–117. [Google Scholar] [CrossRef]
- Shen, M.X.; Huang, Z.J.; Luo, X.W.; Ma, Y.J.; Chen, C.Y.; Chen, X.; Cui, L.H. Activation of persulfate for tetracycline degradation using the catalyst regenerated from Fenton sludge containing heavy metal: Synergistic effect of Cu for catalysis. Chem. Eng. J. 2020, 396, 125238. [Google Scholar] [CrossRef]
- Cui, Y.L.; Zheng, J.; Zhu, Z.J.; Hu, C.Y.; Liu, B.J. Preparation and application of Bi4O7/Cu-BiOCl heterojunction photocatalyst for photocatalytic degradation of tetracycline under visible light. J. Mol. Struct. 2023, 1274, 134486. [Google Scholar] [CrossRef]
- Li, B.S.; Lai, C.; Xu, P.; Zeng, G.M.; Huang, D.L.; Qiu, L.; Yi, H.; Cheng, M.; Wang, L.L.; Huang, F.L.; et al. Facile synthesis of bismuth oxyhalogen-based Z-scheme photocatalyst for visible-light-driven pollutant removal: Kinetics, degradation pathways and mechanism. J. Clean. Prod. 2019, 225, 898–912. [Google Scholar] [CrossRef]
- Sun, S.S.; Teng, Q.; Xie, Y.; Liu, T.; Ma, R.; Bai, J.; Cai, C.; Wei, Q.S. Two-step heat treatment for laser powder bed fusion of a nickel-based superalloy with simultaneously enhanced tensile strength and ductility. Addit. Manuf. 2021, 46, 102168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).