Lipase B from Candida antarctica in Highly Saline AOT-Water-Isooctane Reverse Micelle Systems for Enhanced Esterification Reaction
Abstract
:1. Introduction
2. Results and Discussions
2.1. Size Characterization of AOT/Water/Isooctane Reverse Micellar Systems
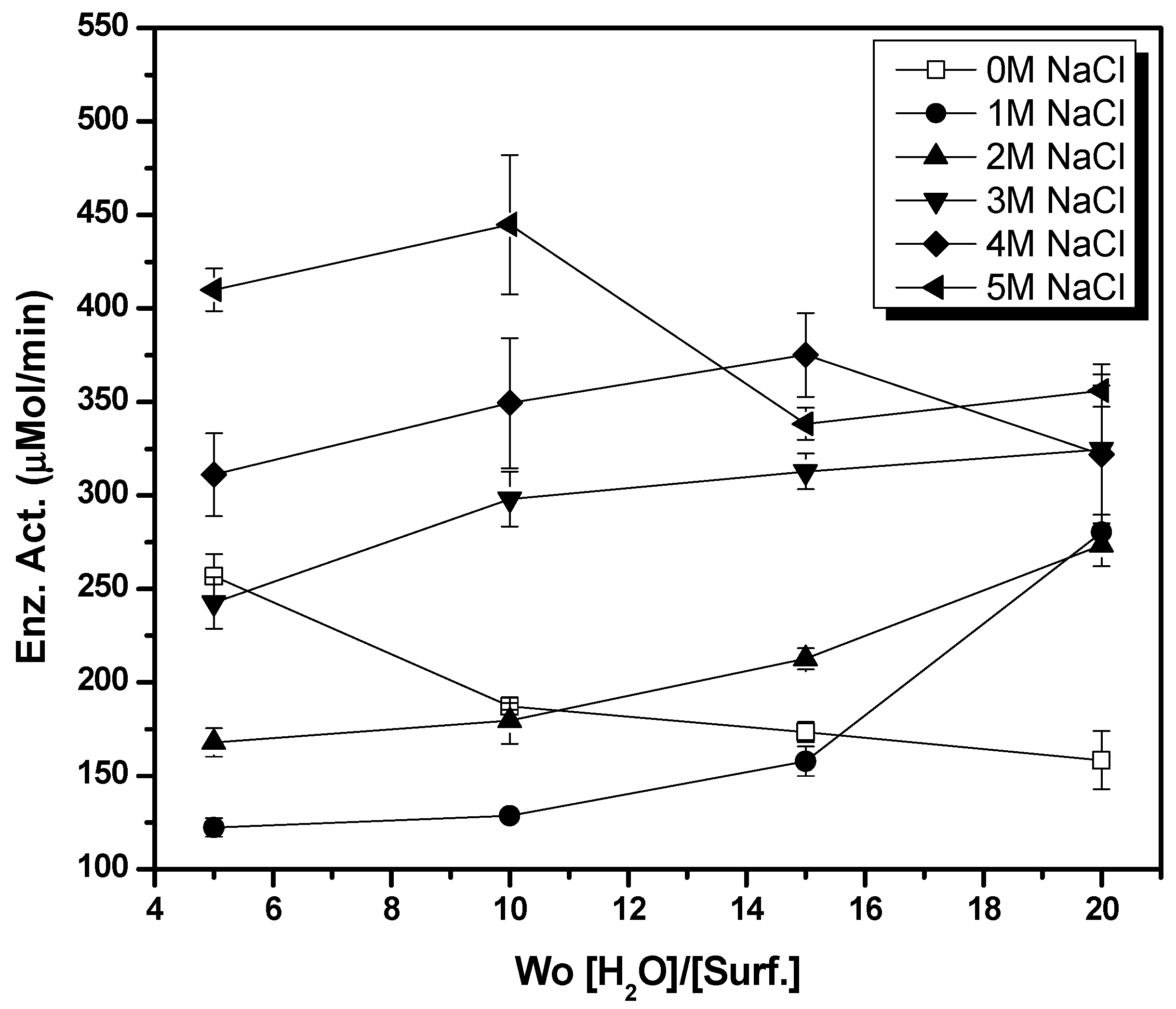
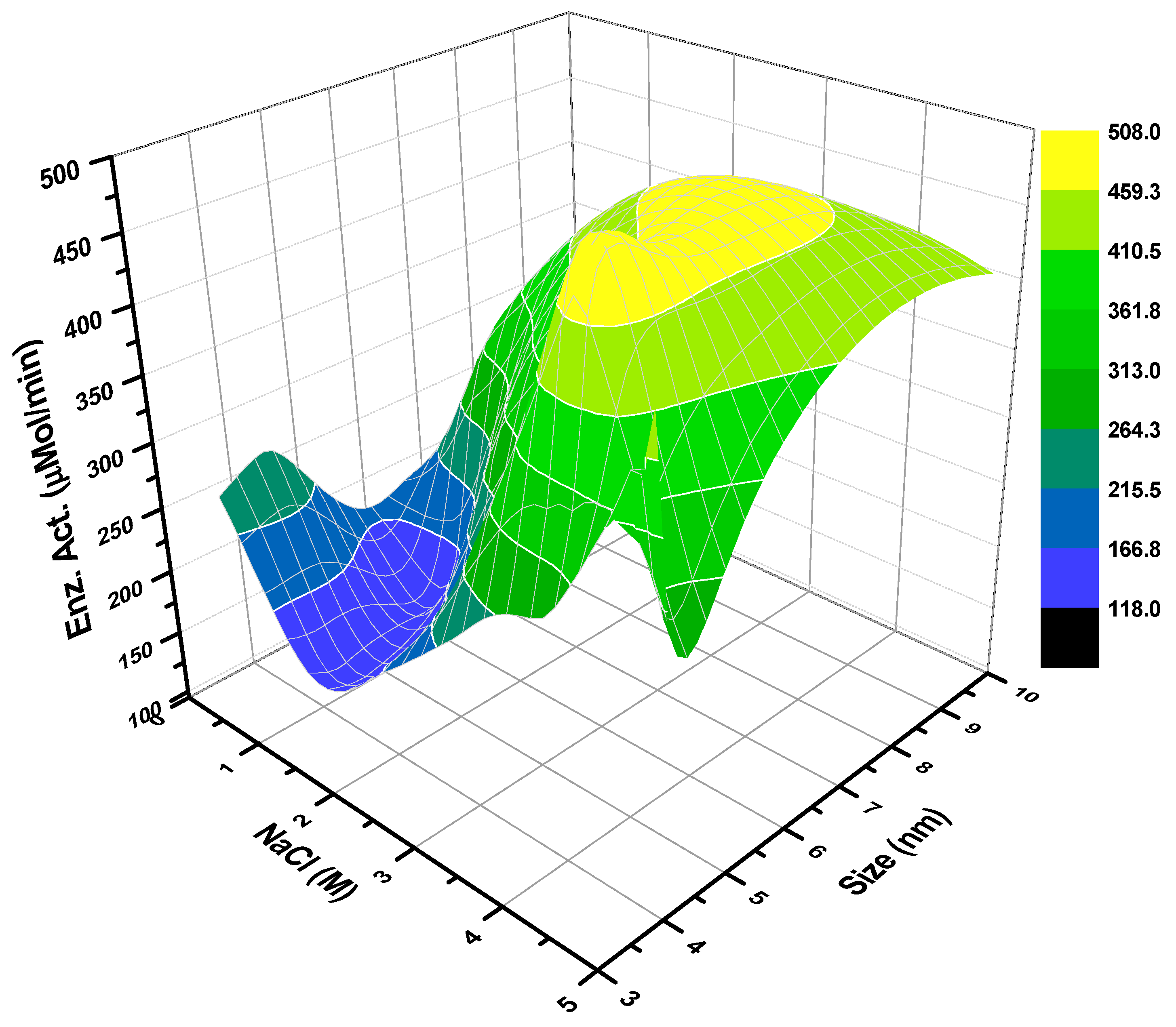
2.2. Enzymatic Esterification Reactions in Reverse Micellar AOT/Water/Isooctane
3. Materials and Methods
3.1. Preparation of AOT/Water/Isooctane Reverse Micellar Systems with the Injection Method
3.2. Size Determination of AOT/Water/Isooctane Reverse Micellar Systems using Dynamic Light Scattering (DLS)
3.3. Characterization of Synthesis Reactions in Reverse Micellar AOT/Water/Isooctane Systems
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mateos-Diaz, J.C.; Cordova, J.; Baratti, J.; Carriere, F.; Abousalham, A. Effect of Nonionic Surfactants on Rhizopus homothallicus Lipase Activity: A Comparative Kinetic Study. Mol. Biotechnol. 2007, 35, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Casas-Godoy, L.; Gasteazoro, F.; Duquesne, S.; Bordes, F.; Marty, A.; Sandoval, G. Lipases: An overview. In Lipases and Phospholipase; Sandoval, G., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1835, pp. 3–38. ISBN 9781493986729. [Google Scholar]
- Taipa, M.A.; Aires-Barros, M.R.; Cabral, J.M.S. Purification of lipases. J. Biotechnol. 1992, 26, 111–142. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzyme Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Dumorné, K.; Córdova, D.C.; Astorga-Eló, M.; Renganathan, P. Extremozymes: A potential source for industrial applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250. [Google Scholar] [CrossRef]
- Stergiou, P.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Verger, R. “Interfacial activation” of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Messaoudi, A.; Belguith, H.; Ghram, I.; Hamida, J. Ben LIPABASE: A database for “true” lipase family enzymes. Int. J. Bioinform. Res. Appl. 2011, 7, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-López, C.; Godoy, C.; de las Rivas, B.; Fernández-Lorente, G.; Palomo, J.M.; Guisán, J.M.; Fernández-Lafuente, R.; Martínez-Ripoll, M.; Hermoso, J.A. Activation of Bacterial Thermoalkalophilic Lipases is Spurred by Dramatic Structural Rearrangements. J. Biol. Chem. 2009, 284, 4365–4372. [Google Scholar] [CrossRef] [Green Version]
- Brito e Cunha, D.A.; Bartkevihi, L.; Robert, J.M.; Cipolatti, E.P.; Ferreira, A.T.S.; Oliveira, D.M.P.; Gomes-Neto, F.; Almeida, R.V.; Fernandez-Lafuente, R.; Freire, D.M.G.; et al. Structural differences of commercial and recombinant lipase B from Candida antarctica: An important implication on enzymes thermostability. Int. J. Biol. Macromol. 2019, 140, 761–770. [Google Scholar] [CrossRef]
- Stauch, B.; Fisher, S.J.; Cianci, M. Open and closed states of Candida antarctica lipase B: Protonation and the mechanism of interfacial activation. J. Lipid Res. 2015, 56, 2348–2358. [Google Scholar] [CrossRef] [Green Version]
- de Miranda, A.S.; Miranda, L.S.M.; de Souza, R.O.M.A. Lipases: Valuable catalysts for dynamic kinetic resolutions. Biotechnol. Adv. 2015, 33, 372–393. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T. Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 2002, 6, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, P.; Muderhwa, J.M.; Graille, J.; Haas, M.J. Customizing lipases for biocatalysis: A survey of chemical, physical and molecular biological approaches. J. Mol. Catal.-B Enzym. 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Fatima, S.; Faryad, A.; Ataa, A.; Joyia, F.A.; Parvaiz, A. Microbial lipase production: A deep insight into the recent advances of lipase production and purification techniques. Biotechnol. Appl. Biochem. 2021, 68, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Singh, T.; Pandey, A. Microbial Enzymes—An Overview. In Advances in Enzyme Technology; Singh, R.S., Singhania, R.R., Pandey, A., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–40. ISBN 9780444641144. [Google Scholar]
- Liu, X.; Kokare, C. Microbial Enzymes of Use in Industry. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Academic Press: Cambridge, UK, 2017; pp. 267–298. ISBN 9780128037256. [Google Scholar]
- Borrelli, G.M.; Trono, D. Recombinant Lipases and Phospholipases and Their Use as Biocatalysts for Industrial Applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Dias, S.; Sandoval, G.; Plou, F.; Valero, F. The potential use of lipases in the production of fatty acid derivatives for the food and nutraceutical industries. Electron. J. Biotechnol. 2013, 16, 12. [Google Scholar] [CrossRef] [Green Version]
- Persson, M.; Costes, D.; Wehtje, E.; Adlercreutz, P. Effects of solvent, water activity and temperature on lipase and hydroxynitrile lyase enantioselectivity. Enzyme Microb. Technol. 2002, 30, 916–923. [Google Scholar] [CrossRef]
- Sharma, S.; Kanwar, S.S. Organic Solvent Tolerant Lipases and Applications. Sci. World J. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hayes, D.G.; Gulari, E. Formation of polyol–fatty acid esters by lipases in reverse micellar media. Biotechnol. Bioeng. 1992, 40, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Chahinian, H.; Ben, Y.; Abousalham, A.; Petry, S.; Mandrich, L.; Manco, G.; Canaan, S.; Sarda, L. Substrate specificity and kinetic properties of enzymes belonging to the hormone-sensitive lipase family: Comparison with non-lipolytic and lipolytic carboxylesterases. Biochim. Biophys. Acta 2005, 1738, 29–36. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Mendoza, L.D.; Pezzotti, F.; Vanthyne, N.; Leclaire, J.; Verger, R.; Buono, G.; Carriere, F.; Fotiadu, F. Novel chromatographic resolution of chiral diacylglycerols and analysis of the stereoselective hydrolysis of triacylglycerols by lipases. Anal. Biochem. 2008, 375, 196–208. [Google Scholar] [CrossRef]
- Camacho, R.M.; Mateos-Díaz, J.C.; Diaz-Montaño, D.M.; González-Reynoso, O.; Córdova, J. Carboxyl ester hydrolases production and growth of a halophilic archaeon, Halobacterium sp. NRC-1. Extremophiles 2010, 14, 99–106. [Google Scholar] [CrossRef]
- Yin, J.; Chen, J.; Wu, Q.; Chen, G. Halophiles, coming stars for industrial biotechnology. Biotechnol. Adv. 2014, 33, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhang, X.; Kuang, Y.; Du, H.; Song, L.; Han, X.; Liang, X. Optimized microemulsion production of biodiesel over lipase-catalyzed transesterification of soybean oil by response surface methodology. Green Process. Synth. 2014, 3, 471–478. [Google Scholar] [CrossRef]
- Badenes, S.M.; Lemos, F.; Cabral, J.M.S. Transesterification of oil mixtures catalyzed by microencapsulated cutinase in reversed micelles. Biotechnol. Lett. 2010, 32, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Badenes, S.M.; Lemos, F.; Cabral, J.M.S. Stability of cutinase, wild type and mutants, in AOT reversed micellar system—Effect of mixture components of alkyl esters production. J. Chem. Technol. Biotechnol. 2011, 86, 34–41. [Google Scholar] [CrossRef]
- Tonova, K.; Lazarova, Z.; Nemestothy, N.; Gubicza, L.; Belafi-Bako, K. Lipase-catalyzed esterification in a reversed micellar reaction system. Chem. Ind. Chem. Eng. Q. 2006, 12, 175–179. [Google Scholar] [CrossRef]
- Márquez-Villa, J.M.; Mateos-Díaz, J.C.; Rodríguez-González, J.A.; Camacho-Ruíz, R.M. Reverse micellar systems as a versatile tool on halophilic biocatalysts. In Extremozymes and Their Industrial Applications; Arora, N.K., Agnihotri, S., Mishra, J., Eds.; Elsevier Academic Press: London, UK, 2022; pp. 353–373. ISBN 9780323902748. [Google Scholar]
- Mohd-Setapar, S.H.; Mohamad-aziz, S.N.; Harun, N.H.; Mohd-azizi, C.Y. Review on the extraction of biomolecules by biosurfactant reverse micelles. Procedia APCBEE 2012, 3, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Bhavya, S.G.; Priyanka, B.S.; Rastogi, N.K. Reverse Micelles-Mediated Transport of Lipase in Liquid Emulsion Membrane for Downstream Processing. Biotechnol. Prog. 2012, 28, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Pileni, M.P. Reverse micelles as microreactors. J. Phys. Chem. 1993, 97, 6961–6973. [Google Scholar] [CrossRef]
- Su, E.; Wei, D. Production of fatty acid butyl esters using the low cost naturally immobilized Carica papaya lipase. J. Agric. Food Chem. 2014, 62, 6375–6381. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.L.M.; Krieger, N.; Baron, A.M.; Zamora, P.P.; Ramos, L.P.; Mitchell, D.A. Hydrolysis and synthesis reactions catalysed by Thermomyces lanuginosa lipase in the AOT /Isooctane reversed micellar system. J. Mol. Catal. B Enzym. 2004, 30, 43–49. [Google Scholar] [CrossRef]
- Tsai, S.-W.; Chiang, C.-L. Kinetics, Mechanism, and Time Course Analysis of Lipase-Catalyzed Hydrolysis of High Concentration Olive Oil in AOT-Isooctane Reversed Micelles. Biotechnol. Bioeng. 1991, 38, 206–211. [Google Scholar] [CrossRef]
- Pileni, M.P. Structure and Reactivity in Reverse Micelles; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Gangadharappa, B.S.; Dammalli, M.; Rajashekarappa, S.; Pandurangappa, K.M.T.; Siddaiah, G.B. Reverse micelles as a bioseparation tool for enzymes. J. Proteins Proteom. 2017, 8, 105–120. [Google Scholar] [CrossRef]
- Mitra, R.K.; Paul, B.K. Effect of NaCl and temperature on the water solubilization behavior of AOT/nonionics mixed reverse micellar systems stabilized in IPM oil. Colloids Surf. A Physicochem. Eng. Asp. 2005, 255, 165–180. [Google Scholar] [CrossRef]
- Debnath, S.; Das, D.; Das, P.K. Unsaturation at the surfactant head: Influence on the activity of lipase and horseradish peroxidase in reverse micelles. Biochem. Biophys. Res. Commun. 2007, 356, 163–168. [Google Scholar] [CrossRef]
- Stamatis, H.; Xenakis, A.; Kolisis, F.N. Bioorganic reactions in microemulsions: The case of lipases. Biotechnol. Adv. 1999, 17, 293–318. [Google Scholar] [CrossRef]
- Luisi, P.L.; Giomini, M.; Pileni, M.P.; Robinson, B.H. Reverse micelles as hosts for proteins and small molecules. Biochim. Biophys. Acta 1988, 947, 209–246. [Google Scholar] [CrossRef]
- Sankaran, R.; Bong, J.H.; Chow, Y.H.; Wong, F.W.F.; Ling, T.C.; Show, P.L. Reverse Micellar System in Protein Recovery—A Review of the Latest Developments. Curr. Protein Pept. Sci. 2019, 20, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Yeung, P.S.; Eskici, G.; Axelsen, P.H. Infrared spectroscopy of proteins in reverse micelles. Biochim. Biophys. Acta 2012, 1828, 2314–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, S.; Waks, M.; Urbach, W.; Marchi, M. Structure, Stability, and Hydration of a Polypeptide in AOT Reverse Micelles. J. Am. Chem. Soc. 2006, 128, 382–383. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; García, A.E. Simulations of the confinement of ubiquitin in self-assembled reverse micelles. J. Chem. Phys. 2011, 134, 225101. [Google Scholar] [CrossRef] [Green Version]
- Vasquez, V.R.; Williams, B.C.; Graeve, O.A. Stability and Comparative Analysis of AOT /Water /Isooctane Reverse Micelle System Using Dynamic Light Scattering and Molecular Dynamics. J. Phys. Chem. B 2011, 115, 2979–2987. [Google Scholar] [CrossRef]
- Naoe, K.; Yoshimoto, S.; Naito, N.; Kawagoe, M.; Imai, M. Preparation of protein nanoparticles using AOT reverse micelles. Biochem. Eng. J. 2011, 55, 140–143. [Google Scholar] [CrossRef]
- Maitra, A. Determination of Size Parameters of Water-Aerosol OT-Oil Reverse Micelles from Their Nuclear Magnetic Resonance Data. J. Phys. Chem. 1984, 88, 5122–5125. [Google Scholar] [CrossRef]
- Abel, S.; Sterpone, F.; Bandyopadhyay, S.; Marchi, M. Molecular Modeling and Simulations of AOT-Water Reverse Micelles in Isooctane: Structural and Dynamic Properties. J. Phys. Chem. B 2004, 108, 19458–19466. [Google Scholar] [CrossRef] [Green Version]
- Baruah, B.; Roden, J.M.; Sedgwick, M.; Correa, N.M.; Crans, D.C.; Levinger, N.E. When Is Water Not Water? Exploring Water Confined in Large Reverse Micelles Using a Highly Charged Inorganic Molecular Probe. J. Am. Chem. Soc. 2006, 128, 12758–12765. [Google Scholar] [CrossRef]
- Cao, H.; An, B.; Wang, Y.; Zhou, K.; Lu, N. Investigation of Surfactant AOT Mediated Charging of PS Particles Dispersed in Aqueous Solutions. Coatings 2019, 9, 471. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Vishal, G.; Gandhi, K.S.; Ayappa, K.G. Ion Exchange in Reverse Micelles. Langmuir 2005, 21, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Voth, G.A. Computer Simulation of Proton Solvation and Transport in Aqueous and Biomolecular Systems. Acc. Chem. Res. 2006, 39, 143–150. [Google Scholar] [CrossRef]
- Bru, R.; Sanchez-Ferrer, A.; Garcia-Carmona, F. A theoretical study on the expression of enzymic activity in reverse micelles. Biochem. J. 1989, 259, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Piera-Velázquez, S.; Marhuenda-Egea, F.; Cadenas, E. The dependence of a halophilic malate dehydrogenase on Wo and surfactant concentration in reverse micelles. J. Mol. Catal. B Enzym. 2001, 13, 49–55. [Google Scholar] [CrossRef]
- Dekker, M.; Hilhorst, R.; Laane, C. Isolating enzymes by reversed micelles. Anal. Biochem. 1989, 178, 217–226. [Google Scholar] [CrossRef]
- Opawale, F.O.; Burgess, D.J. Influence of Interfacial Properties of Lipophilic Surfactants on Water- in-Oil Emulsion Stability. J. Colloid Interface Sci. 1998, 197, 142–150. [Google Scholar] [CrossRef]
- Hou, M.J.; Kim, M.; Shah, D.O. A light scattering study on the droplet size and interdroplet interaction in microemulsions of AOT-Oil-Water system. J. Colloid Interface Sci. 1988, 123, 398–412. [Google Scholar] [CrossRef]
- Li, Q.; Li, T.; Wu, J. Water solubilization capacity and conductance behaviors of AOT and NaDEHP systems in the presence of additives. Colloids Surf. A Physicochem. Eng. Asp. 2002, 197, 101–109. [Google Scholar] [CrossRef]
- Rabie, H.R.; Helou, D.; Weber, M.E.; Vera, J.H. Comparison of the Titration and Contact Methods for the Water Solubilization Capacity of AOT Reverse Micelles in the Presence of a Cosurfactant. J. Colloid Interface Sci. 1997, 189, 208–215. [Google Scholar] [CrossRef]
- Derouiche, A.; Tondre, C. Correlation between maximum water/electrolyte solubilization and conductivity percolation in AOT reversed micelles. J. Dispers. Sci. Technol. 1991, 12, 517–530. [Google Scholar] [CrossRef]
- Mazzini, V.; Craig, V.S.J. What is the fundamental ion-specific series for anions and cations? Ion specificity in standard partial molar volumes of electrolytes and electrostriction in water and non-aqueous solvents. Chem. Sci. 2017, 8, 7052–7065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamukcu, S.; Hannum, L.; Wittle, J.K. Delivery and activation of nano-iron by DC electric field. J. Environ. Sci. Health Part A 2008, 43, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Electrostriction in Electrolyte Solutions. Chem. Rev. 2011, 111, 2761–2783. [Google Scholar] [CrossRef]
- Brooks, H.B.; Geeganage, S.; Kahl, S.D.; Montrose, C.; Sittampalam, S.; Smith, M.C.; Weidner, J.R. Basics of Enzymatic Assays for HTS. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Eds.; Eli Lilly & Company: Chicago, IL, USA, 2004; pp. 93–104. [Google Scholar]
- Khmelnitsky, Y.L.; Hilhorst, R.; Visser, A.J.W.G.; Veeger, C. Enzyme inactivation and protection during entrapment in reversed micelles. Eur. J. Biochem. 1993, 211, 73–77. [Google Scholar] [CrossRef]
- Marhuenda-Egea, F.C.; Piera-Velázquez, S.; Cadenas, C.; Cadenas, E. Kinetic studies of an extremely halophilic enzyme entrapped in reverse micelles. Biocatal. Biotransform. 1999, 18, 201–222. [Google Scholar] [CrossRef]
- Verhaert, R.M.D.; Hilhorst, R. Enzymes in reversed micelles: 4. Theoretical analysis of a one-substrate/one-product conversion and suggestions for efficient application. Recl. Trav. Chim. Pays-Bas. 1991, 110, 236–246. [Google Scholar] [CrossRef]
- Pire, C.; Marhuenda-Egea, F.C.; Esclapez, J.; Alcaraz, L.; Ferrer, J.; Bonete, M.J. Stability and Enzymatic Studies of Glucose Dehydrogenase from the Archaeon Haloferax mediterrranei in reverse micelles. Biocatal. Biotransform. 2004, 22, 17–23. [Google Scholar] [CrossRef]
- Van Rantwijk, F.; Sheldon, R.A. Biocatalysis in Ionic Liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar] [CrossRef]
- Patel, M.T.; Nagarajan, N.; Kilara, A. Characteristics of lipase-catalysed hydrolysis of triacylglycerols in Aerosol-OT/iso-octane reverse-micellar media. Biotechnol. Appl. Biochem. 1995, 22, 1–14. [Google Scholar]
- Rupley, J.A.; Gratton, E.; Careri, G. Water and globular proteins. Trends Biochem. Sci. 1983, 8, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Marhuenda-Egea, F.C.; Piera-Velázquez, S.; Cadenas, C.; Cadenas, E. Reverse micelles in organic solvents: A medium for the biotechnological use of extreme halophilic enzymes at low salt concentration. Archaea 2002, 1, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Bru, R.; Sanchez-Ferrer, A.; Garcia-Carmona, F. Kinetic models in reverse micelles. Biochem. J. 1995, 310, 721–739. [Google Scholar] [CrossRef] [Green Version]
- Marhuenda-Egea, F.C.; Bonete, M.J. Extreme halophilic enzymes in organic solvents. Protein Technol. Commer. Enzym. 2002, 13, 385–389. [Google Scholar] [CrossRef]
- Klibanov, A.M. Why are enzymes less active in organic solvents than in water? Trends Biotechnol. 1997, 15, 97–101. [Google Scholar] [CrossRef]
- Maiangwa, J.; Ali, M.S.M.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Normi, Y.M.; Shariff, F.M.; Leow, T.C. Lid opening and conformational stability of T1 lipase is mediated by increasing chain length polar solvents. PeerJ 2017, 5, 1–32. [Google Scholar] [CrossRef] [Green Version]
- DasSarma, S.; DasSarma, P. Halophiles; John Wiley & Sons, Ltd.: Chichester, UK, 2017; ISBN 9780470015902. [Google Scholar]
- Fukuchi, S.; Yoshimune, K.; Wakayama, M.; Moriguchi, M.; Nishikawa, K. Unique Amino Acid Composition of Proteins in Halophilic Bacteria. J. Mol. Biol. 2003, 327, 347–357. [Google Scholar] [CrossRef] [PubMed]
- DasSarma, S.; DasSarma, P. Halophiles and their enzymes: Negativity put to good use. Curr. Opin. Microbiol. 2015, 25, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dym, O.; Mevarech, M.; Sussman, J.L. Structural Features That Stabilize Halophilic Malate Dehydrogenase from an Archaebacterium. Science 1995, 267, 1344–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madern, D.; Ebel, C.; Zaccai, G. Halophilic adaptation of enzymes. Extremophiles 2000, 4, 91–98. [Google Scholar] [CrossRef]
- Zaccai, G.; Eisenberg, H. Halophilic proteins and the influence of solvent on protein stabilization. Trends Biochem. Sci. 1990, 15, 333. [Google Scholar] [CrossRef]
- Skjøt, M.; Maria, L.; de Chatterjee, R.; Svendsen, A.; Patkar, S.A.; Østergaard, P.R.; Brask, J. Understanding the Plasticity of the a/b Hydrolase Fold: Lid Swapping on the Candida antarctica Lipase B Results in Chimeras with Interesting Biocatalytic Properties. Chembiochem 2009, 10, 520–527. [Google Scholar] [CrossRef]
- Luan, B.; Zhou, R. A Novel Self-Activation Mechanism of Candida antarctica Lipase B. Phys. Chem. Chem. Phys. 2017, 19, 15709–15714. [Google Scholar] [CrossRef]
- Britton, K.L.; Stillman, T.J.; Yip, K.S.P.; Forterre, P.; Engel, P.C.; Rice, D.W. Insights into the Molecular Basis of Salt Tolerance from the Study of Glutamate Dehydrogenase from Halobacterium salinarum. J. Biol. Chem. 1998, 273, 9023–9030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marhuenda-Egea, F.C.; Piera-Velázquez, S.; Cadenas, C.; Cadenas, E. Enzymatic activity of an extremely halophilic phosphatase from the Archaea Halobacterium salinarum in reversed micelles. J. Mol. Catal. B Enzym. 2000, 10, 555–563. [Google Scholar] [CrossRef]
- Gupta, S.; Mukhopadhyay, L.; Moulik, S.P. Kinetics in microemulsion medium 2. Hydrolysis of p-nitrophenyl phosphate with alkaline phosphatase in w/o microemulsion medium using the surfactant AOT. Colloids Surf. B Biointerfaces 1994, 3, 191–201. [Google Scholar] [CrossRef]
- Jia, H.; Zhu, G.; Wang, P. Catalytic Behaviors of Enzymes Attached to Nanoparticles: The Effect of Particle Mobility. Biotechnol Bioeng. 2003, 84, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Betancor, L.; Luckarift, H.R. Bioinspired enzyme encapsulation for biocatalysis. Trends Biotechnol. 2008, 26, 566–572. [Google Scholar] [CrossRef]
- Luisi, P.L. Enzymes Hosted in Reverse Micelles in Hydrocarbon Solution. Angew. Cheme 1985, 24, 439–450. [Google Scholar] [CrossRef]
- Matzke, S.F.; Creagh, A.L.; Haynes, C.A.; Prausnitz, J.M.; Blanch, H.W. Mechanisms of Protein Solubilization in Reverse Micelles. Biotechnol. Bioeng. 1992, 40, 91–102. [Google Scholar] [CrossRef]
- Melo, E.P.; Aires-Barros, M.R.; Cabral, J.M.S. Reverse micelles and protein biotechnology. Biotechnol. Annu. Rev. 2001, 7, 87–129. [Google Scholar] [CrossRef]
- Novozymes Biopharma. Lipases for Biocatalysts (Brochure); Novozymes Biopharma: Bagsvaerd, Debamark, 2016; pp. 1–11. [Google Scholar]
- Malvern Instruments Ltd. Zetasizer Nano Series User Manual; Malvern Instruments Ltd.: Malvern, UK, 2004. [Google Scholar]
- Lawrie, A.S.; Albanyan, A.; Cardigan, R.A.; MacKie, I.J.; Harrison, P. Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox Sang. 2009, 96, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Rhee, J.S. A simple and Rapid Colorimetric Method for Determination of Free Fatty Acids for Lipase Assay. J. Am. Oil Chem. Soc. 1986, 63, 89–92. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Cavalcanti-Oliveira, E.D.; De Castro, A.M.; Langone, M.A.P.; Freire, D.M.G. Biodiesel production from Acrocomia aculeata acid oil by (enzyme/enzyme) hydroesterification process: Use of vegetable lipase and fermented solid as low-cost biocatalysts. Fuel 2014, 135, 315–321. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Villa, J.M.; Mateos-Díaz, J.C.; Rodríguez, J.A.; Camacho-Ruíz, R.M. Lipase B from Candida antarctica in Highly Saline AOT-Water-Isooctane Reverse Micelle Systems for Enhanced Esterification Reaction. Catalysts 2023, 13, 492. https://doi.org/10.3390/catal13030492
Márquez-Villa JM, Mateos-Díaz JC, Rodríguez JA, Camacho-Ruíz RM. Lipase B from Candida antarctica in Highly Saline AOT-Water-Isooctane Reverse Micelle Systems for Enhanced Esterification Reaction. Catalysts. 2023; 13(3):492. https://doi.org/10.3390/catal13030492
Chicago/Turabian StyleMárquez-Villa, José Martín, Juan Carlos Mateos-Díaz, Jorge A. Rodríguez, and Rosa María Camacho-Ruíz. 2023. "Lipase B from Candida antarctica in Highly Saline AOT-Water-Isooctane Reverse Micelle Systems for Enhanced Esterification Reaction" Catalysts 13, no. 3: 492. https://doi.org/10.3390/catal13030492
APA StyleMárquez-Villa, J. M., Mateos-Díaz, J. C., Rodríguez, J. A., & Camacho-Ruíz, R. M. (2023). Lipase B from Candida antarctica in Highly Saline AOT-Water-Isooctane Reverse Micelle Systems for Enhanced Esterification Reaction. Catalysts, 13(3), 492. https://doi.org/10.3390/catal13030492








