Molten Salt-Assisted Catalytic Preparation of MoS2/α-MoO3/Graphene as High-Performance Anode of Li-Ion Battery
Abstract
:1. Introduction
2. Results
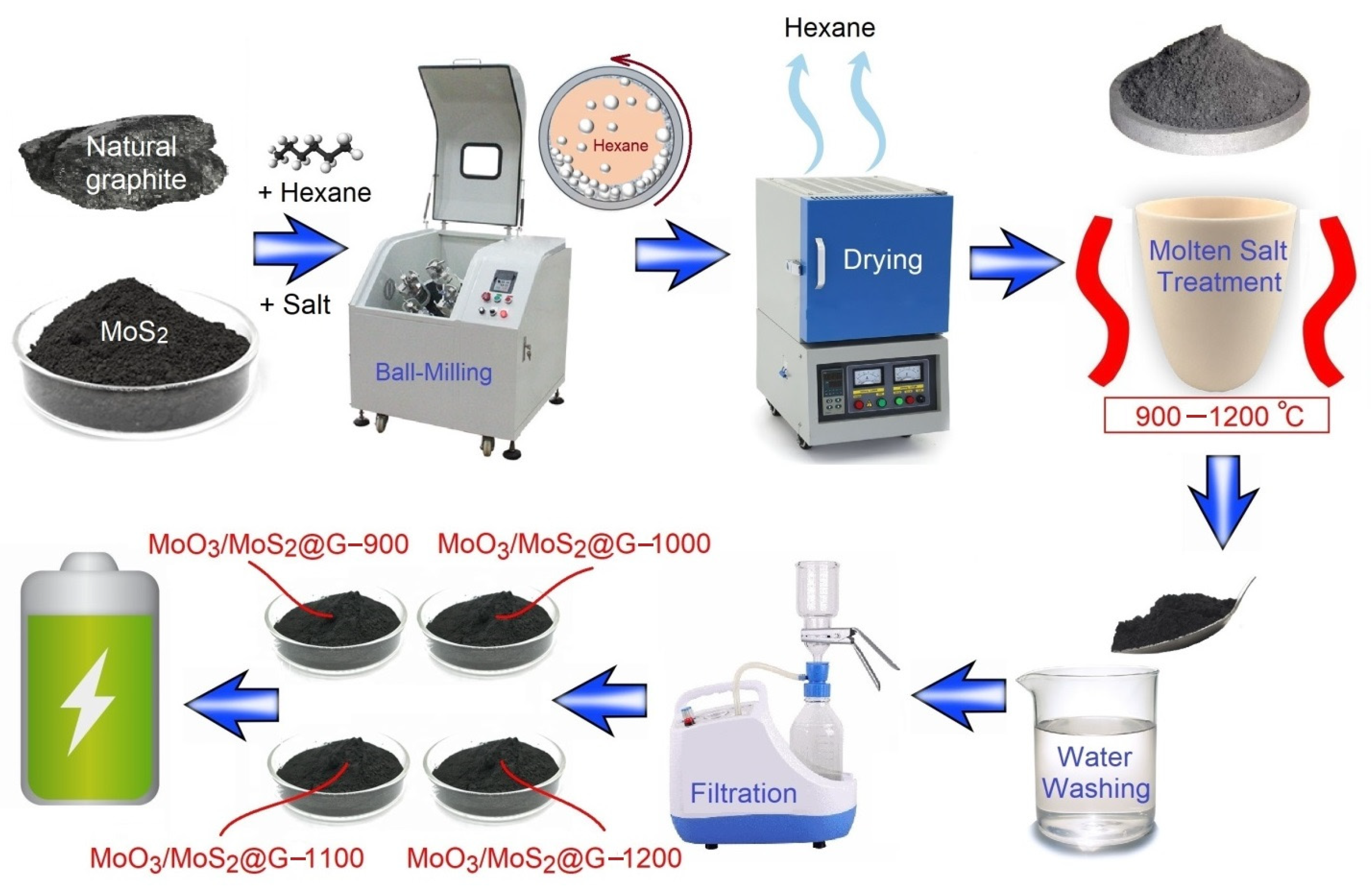
2.1. Preparation of Materials
2.2. Characterization of the Natural Graphite Mineral
2.3. Catalytic Phase Transitions during the Molten Salt Treatment
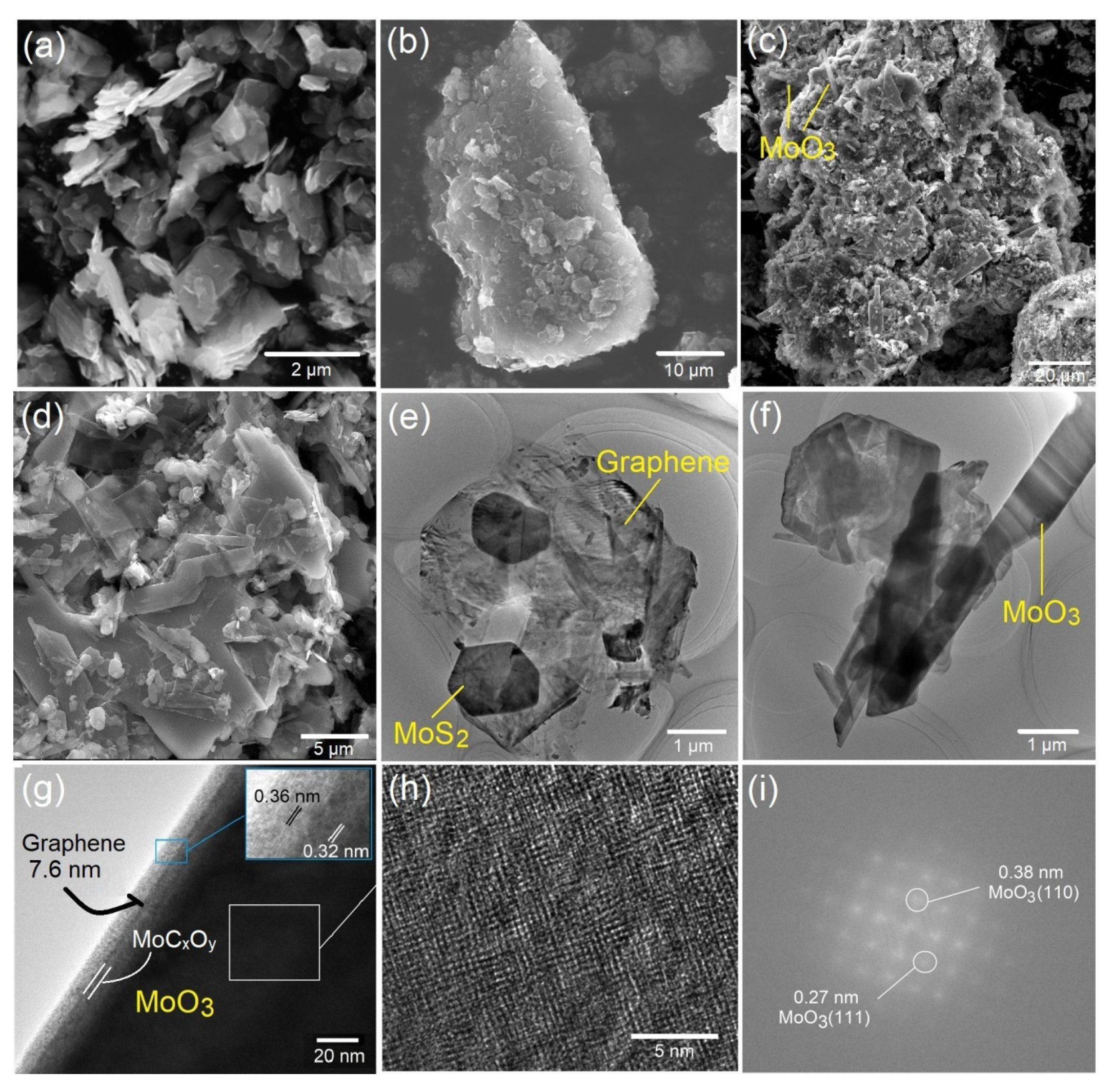
2.4. SEM Characterization
2.5. TEM Characterization
2.6. Surface Characterization
2.7. Catalytic Formation of Hexagonal MoS2 Nanocrystals and α-MoO3
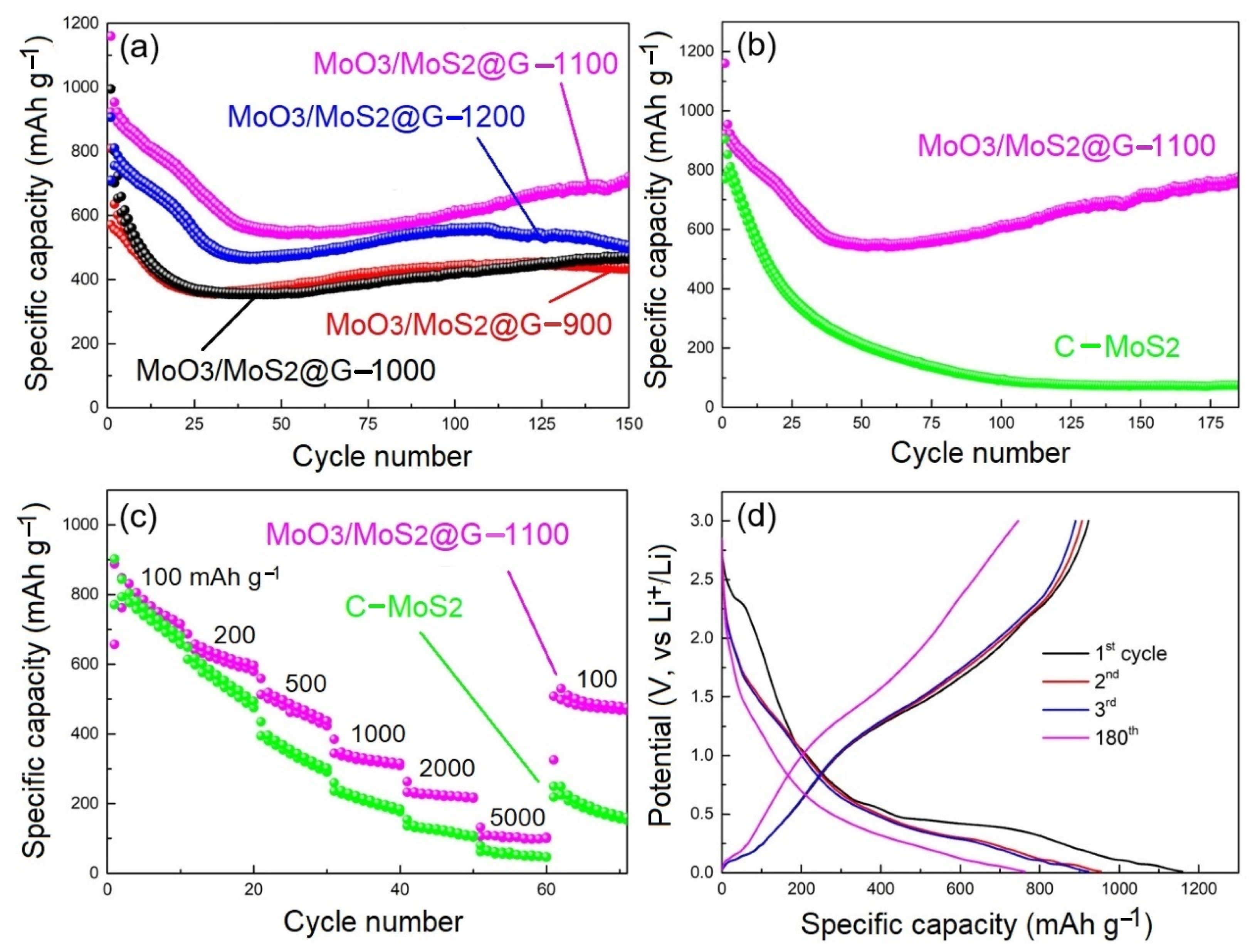
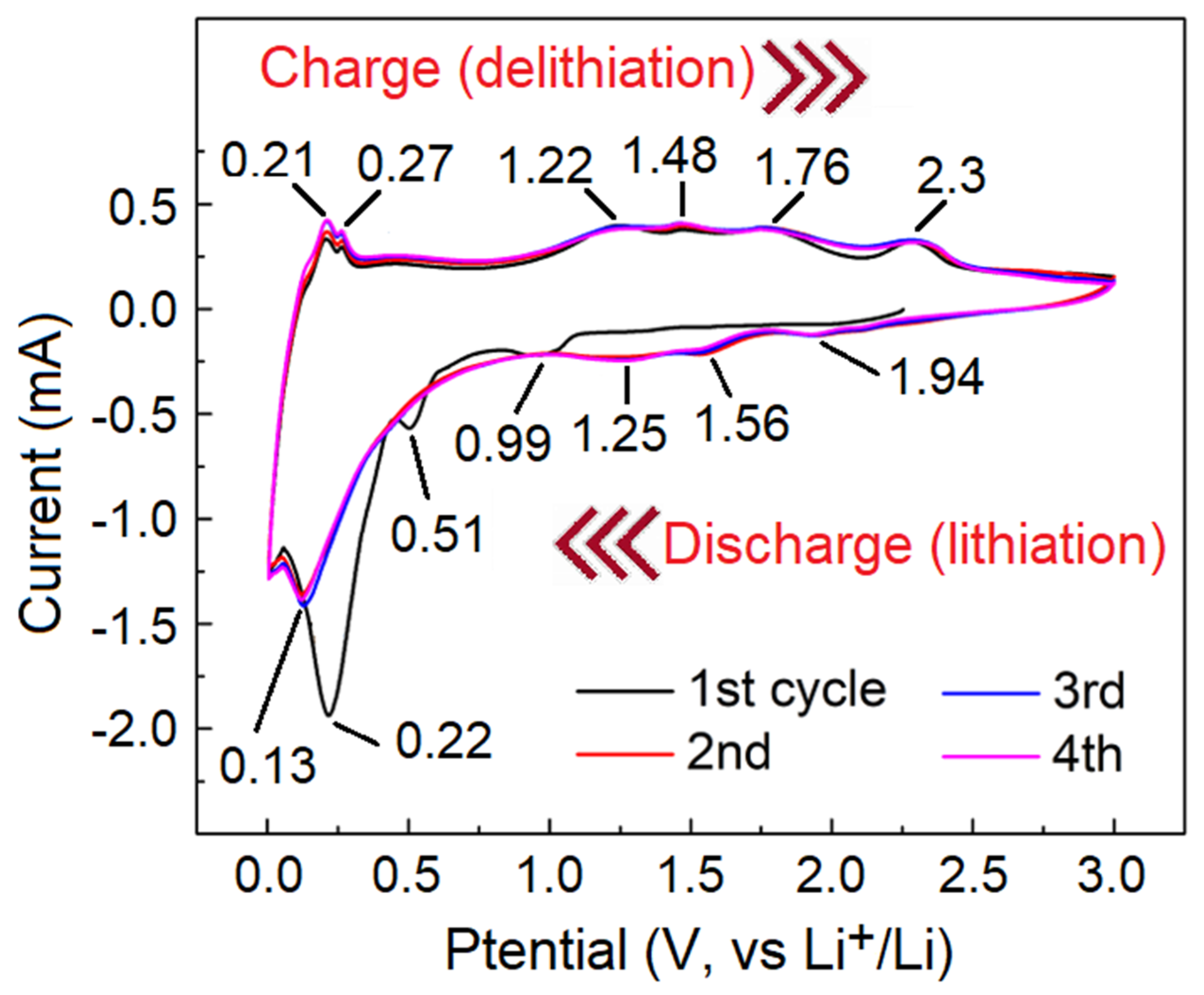
2.8. Electrochemical Characterization of MoO3/MoS2@G Samples
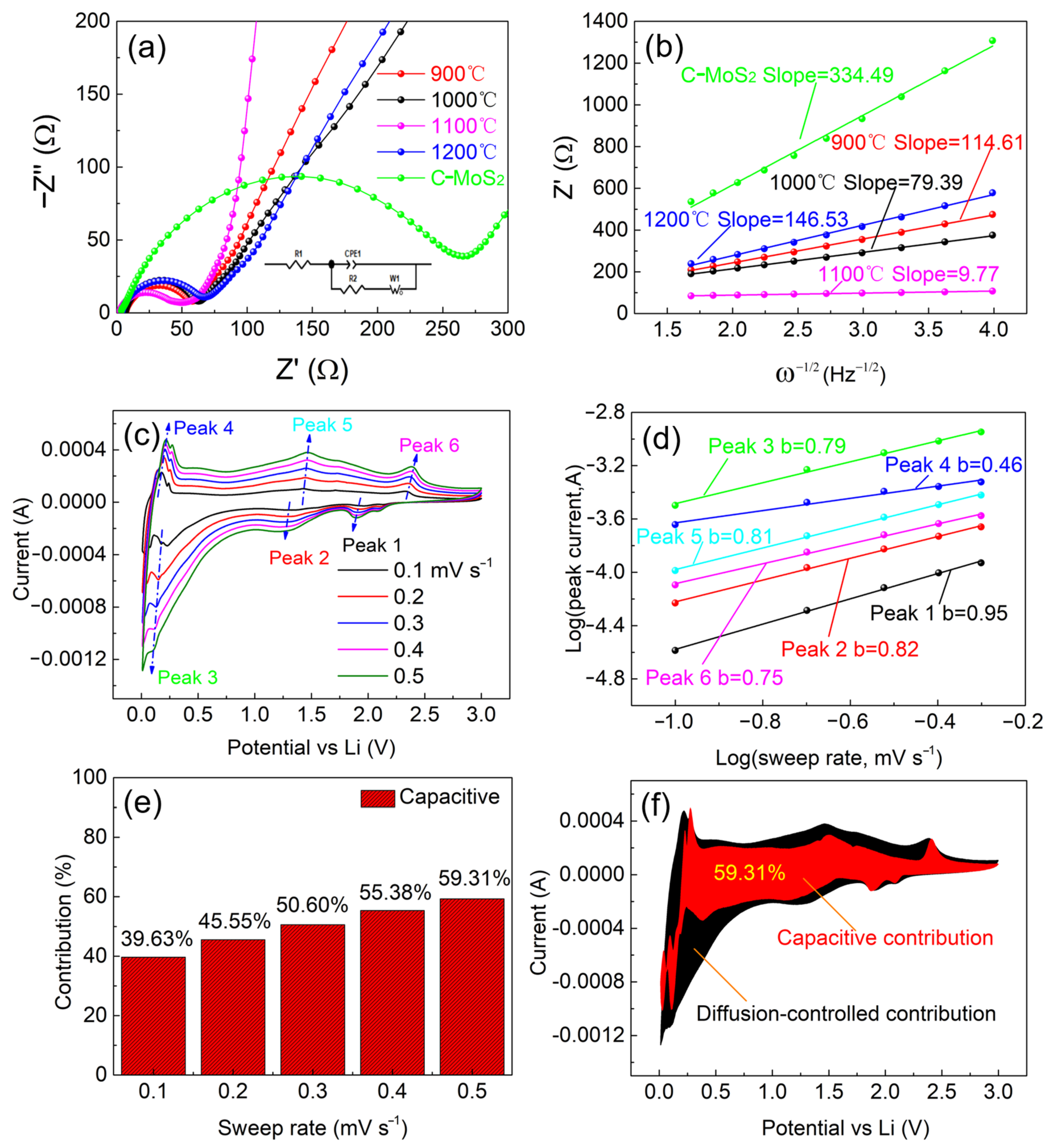
2.9. Impedance Spectroscopy and Electrode Kinetics
2.10. Contribution of Diffusion- and Surface-Phenomena
3. Discussion
4. Materials and Methods
4.1. Synthesis of MoS2/MoO3@Graphene Nanocomposites
4.2. Morphological, Structural and Surface Characterizations
4.3. Electrochemical Characterizations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292. [Google Scholar] [CrossRef]
- Chen, W.D.; Liang, J.; Yang, Z.H.; Li, G. A Review of lithium-ion battery for electric vehicle applications and beyond. Energy Procedia 2019, 158, 4363. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and challenges of next-generation “beyond Li-ion” batteries for electric vehicles and grid decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kamali, A.R. Clean preparation of Fe2SiO4 coated Fe2O3 integrated with graphene for Li-ion storage application. Colloids Surf. A 2023, 656, 130275. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Bondarev, D.; Amatucci, G.G.; Yushin, G. Battery materials for low-cost electric transportation. Mater. Today 2021, 42, 57–72. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition metal oxide anodes for electrochemical energy storage in lithium- and sodium-ion batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.S.; Wang, L.; He, X.M. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Lee, S.; Youn, S.; Yong, C.; Chung, S.; Chui, Y.; Lee, S. High electrical and thermal conductivities of a PAN-based carbon fiber via boron-assisted catalytic graphitization. Carbon 2022, 199, 70–79. [Google Scholar] [CrossRef]
- Bonijoly, M.; Oberlin, M.; Oberlin, A. A possible mechanism for natural graphite formation. Int. J. Coal Geol. 1982, 1, 283–312. [Google Scholar] [CrossRef]
- Zhao, H.; Rezaei, A.; Kamali, A.R. Electrolytic conversion of natural graphite into carbon nanostructures with enhanced electrical conductivity and Na-ion storage performance. J. Electrochem. Soc. 2022, 169, 054512. [Google Scholar] [CrossRef]
- Zhao, L.; Ding, B.C.; Qin, X.Y.; Wang, Z.J.; Lv, W.; He, Y.B.; Yang, Q.H.; Kang, F.Y. Revisiting the roles of natural graphite in ongoing lithium-ion batteries. Adv. Mater. 2022, 32, 2106704. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geological Survey. Mineral Commodity Summaries, Graphite (Natural), January 2022. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022.pdf (accessed on 1 January 2022).
- Zaghib, K.; Song, X.; Guerfi, A.; Rioux, R.; Kinoshit, K. Purification process of natural graphite as anode for Li-ion batteries: Chemical versus thermal. J. Power Source 2003, 119–121, 8–15. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Ma, C.; Han, G. One-step fabrication of fluorine-doped graphite derived from a low-grade microcrystalline graphite ore for potassium-ion batteries. Energy Fuels 2020, 34, 8993–9001. [Google Scholar] [CrossRef]
- Ani, T.A.; Leinonen, S.; Ahtola, T.; Salvador, D. High-grade flake graphite deposits in metamorphic schist belt, central Finland—Mineralogy and beneficiation of graphite for lithium-ion nattery applications. Minerals 2020, 10, 680. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsena, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209. [Google Scholar] [CrossRef]
- Wang, W.; Qin, J.W.; Yin, Z.G.; Cao, M.H. Achieving fully reversible conversion in MoO3 for lithium ion batteries by rational introduction of CoMoO4. ACS Nano 2016, 10, 10106–10116. [Google Scholar] [CrossRef]
- Li, F.Y.; Cabrera, C.R.; Chen, Z.F. Theoretical design of MoO3-based high-rate lithium ion battery electrodes: The effect of dimensionality reduction. J. Mater. Chem. A 2014, 2, 19180. [Google Scholar] [CrossRef]
- Sahu, S.R.; Rikka, V.R.; Haridoss, P.; Chatterjee, A.; Gopalan, R.; Prakash, R. A novel α-MoO3/single-walled carbon nanohorns composite as high-performance anode material for fast-charging lithium-ion battery. Adv. Energy Mater. 2020, 10, 2001627. [Google Scholar] [CrossRef]
- Wang, T.X.; Li, J.; Zhao, G.L. Synthesis of MoS2 and MoO3 hierarchical nanostructures using a single-source molecular precursor. Powder Technol. 2014, 253, 347–351. [Google Scholar] [CrossRef]
- Yang, J.; Qu, Y.D.; Lin, X.H.; Wang, L.Y.; Zheng, Z.H.; Zhuang, J.; Duan, L.F. MoO3/MoS2 flexible paper as sulfur cathode with synergistic suppress shuttle effect for lithium-sulfur batteries. Electrochim. Acta 2022, 418, 140378. [Google Scholar] [CrossRef]
- Zhao, S.P.; Zha, Z.L.; Liu, X.; Tian, H.X.; Wu, Z.Y.; Li, W.F.; Sun, L.B.; Liu, B.; Chen, Z.G. Core−sheath structured MoO3@MoS2 composite for high performance lithium-ion battery anodes. Energy Fuels 2020, 34, 11498–11507. [Google Scholar] [CrossRef]
- Du, J.L.; Wu, H.D.; Wang, X.R.; Qi, C.Y.; Mao, W.; Ren, T.Q.; Qiao, Q.D.; Yang, Z.X. Ternary MoS2/MoO3/C nanosheets as high-performance anode materials for lithium-ion batteries. J. Electron. Mater. 2018, 47, 6767. [Google Scholar] [CrossRef]
- Vieira, L.; Neto, J.D.R.M.; Ferreira, O.P.; Torresi, R.M.; Torresi, S.I.C.D.; Alves, O.L. Template conversion of MoO3 to MoS2 nanoribbons: Synthesis and electrochemical properties. RSC Adv. 2018, 8, 30346–30353. [Google Scholar] [CrossRef]
- Pareek, A.; Kim, H.G.; Paik, P.; Borse, P.H. Ultrathin MoS2-MoO3 nanosheets functionalized CdS photoanodes for effective charge transfer in photoelectrochemical (PEC) cells. J. Mater. Chem. A 2017, 5, 1541. [Google Scholar] [CrossRef]
- Villevieille, C.; Wang, X.J.; Krumeich, F.; Nesper, R.; Novák, P. MoS2 coating on MoO3 nanobelts: A novel approach for a high specific charge electrode for rechargeable Li-ion batteries. J. Power Source 2015, 279, 636–644. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.J.; Deng, L.; Ding, M.; Li, J.Q.; He, X.M. Perpendicular growth of few-layered MoS2 nanosheets on MoO3 nanowires fabricated by direct anion exchange reactions for high performance lithium-ion batteries. J. Mater. Chem. A 2016, 4, 17764. [Google Scholar] [CrossRef]
- Li, B.; Yang, S.X.; Huo, N.J.; Li, Y.T.; Yang, J.H.; Li, R.X.; Fan, C.; Lu, F.Y. Growth of large area few-layer or monolayer MoS2 from controllable MoO3 nanowire nuclei. RSC Adv. 2014, 4, 26407. [Google Scholar] [CrossRef]
- Zhu, W.; Kamali, A.R. Green preparation of nanostructured β-MoO3/hexagonal-shaped MoS2/graphene with enhanced lithium-ion storage performance. J. Alloys Compd. 2023, 932, 167724. [Google Scholar] [CrossRef]
- Carlsson, A.; Brorson, M.; Topsoe, H. Supported metal sulphide nanoclusters studied by HAADF-STEM. J. Microsc. 2006, 223, 179. [Google Scholar] [CrossRef]
- Cai, G.M.; Jian, J.K.; Chen, X.L.; Lei, M.; Wang, W.Y. Regular hexagonal MoS2 microflakes grown from MoO3 precursor. Appl. Phys. 2007, 89, 783. [Google Scholar] [CrossRef]
- Li, P.G.; Lei, M.; Wang, X.F.; Tang, H.L.; Tang, W.H. Thermal conversion of tungsten oxide nanorods to tungsten disulfide nanoflakes. J. Alloys Compd. 2009, 474, 463. [Google Scholar] [CrossRef]
- Leong, S.X.; Mayorga-Martinez, C.C.; Chia, X.Y.; Luxa, J.; Sofer, Z.; Pumera, M. 2H → 1T phase change in direct synthesis of WS2 nanosheets via solution-based electrochemical exfoliation and their catalytic properties. ACS Appl. Mater. Interfaces 2017, 9, 26350. [Google Scholar] [CrossRef] [PubMed]
- Ai, R.Q.; Guan, X.; Li, J.; Yao, K.K.; Chen, P.; Zhang, Z.W.; Duan, X.D.; Duan, X.F. Growth of single-crystalline cadmium iodide nanoplates, CdI2/MoS2 (WS2, WSe2) van der waals heterostructures, and patterned arrays. ACS Nano 2017, 11, 3413. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, T.; Wang, X.Q.; Ma, L.B.; Chen, R.P.; Zhu, H.F.; Yuan, X.; Yan, C.Z.; Zhu, G.Y.; Lv, H.L.; et al. Controlled growth and photoconductive properties of hexagonal SnS2 nanoflakes with mesa-shaped atomic steps. Nano Res. 2017, 10, 1434. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, W.B.; Huang, K.; Chen, H.M. Electronic structure, optical properties and band edges of layered MoO3: A first-principles investigation. Comput. Mater. Sci. 2017, 130, 242–248. [Google Scholar] [CrossRef]
- Kamali, A.R.; Fray, D.J. Solid phase growth of tin oxide nanostructures. Mater. Sci. Eng. B 2012, 177, 819–825. [Google Scholar] [CrossRef]
- He, Z.K.; Sun, Q.; Shi, Z.; Xie, K.; Kamali, A.R. Molten salt synthesis of oxygen-deficient SnO2 crystals with enhanced electrical conductivity. Appl. Surf. Sci. 2019, 465, 397–404. [Google Scholar] [CrossRef]
- Deng, X.Q.; Zhu, M.H.; Ke, J.; Feng, Y.F.; Li, W.R.; Xiong, D.P.; He, M. Synthesis and electrochemical performances of ternary nanocomposite SnO2@MoO3@graphene as high-performance anode material for lithium-ion batteries. Chem. Phys. Lett. 2021, 770, 138408. [Google Scholar] [CrossRef]
- Liu, X.X.; Wu, Y.; Wang, H.W.; Wang, Y.F.; Huang, C.F.; Liu, L.M.; Wang, Z.J. Two-dimensional β-MoO3@C nanosheets as high-performance negative materials for supercapacitors with excellent cycling stability. RSC Adv. 2020, 10, 17497–17505. [Google Scholar] [CrossRef]
- Zhu, J.; Uslamin, E.A.; Kosinov, N.; Hensen, E.J.M. Tuning the reactivity of molybdenum (oxy)carbide catalysts by the carburization degree: CO2 reduction and anisole hydrodeoxygenation. Catal. Sci. Technol. 2020, 10, 3635–3645. [Google Scholar] [CrossRef]
- Zheng, Y.; Tang, Y.; Gallagher, J.R.; Gao, J.; Miller, J.T.; Wachs, I.E.; Podkolzin, S.G. Molybdenum oxide, oxycarbide, and carbide: Controlling the dynamic composition, size, and catalytic activity of zeolite supported nanostructures. J. Phys. Chem. C 2019, 123, 22281–22292. [Google Scholar] [CrossRef]
- Kado, T. Epitaxial molybdenum oxycarbide thin films synthesized by inductively coupled radio-frequency plasma assisted magnetron sputtering. Thin Solid Film. 2006, 515, 2481–2484. [Google Scholar] [CrossRef]
- Shahsank, M.; Naik, H.S.B.; Sumedha, H.N.; Nagaraju, G. Implementing an in-situ carbon formation of MoO3 nanoparticles for high performance lithium-ion battery. Ceram. Int. 2021, 47, 10261–10267. [Google Scholar] [CrossRef]
- Yoon, A.; Kim, J.H.; Yoon, J.C.; Lee, Y.D.; Lee, Z. Van der Waals Epitaxial Formation of Atomic Layered α-MoO3 on MoS2 by Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.R.; Fray, D.J. Molten salt corrosion of graphite as a possible way to make carbon nanostructures. Carbon 2013, 56, 121–131. [Google Scholar] [CrossRef]
- Kamali, A.R.; Yang, J. Effect of molten salts on the structure, morphology and electrical conductivity of PET-derived carbon nanostructures. Polym. Degrad. Stab. 2020, 177, 109184. [Google Scholar] [CrossRef]
- Kamali, A.R.; Schwandt, C.; Fray, D.J. On the oxidation of electrolytic carbon nanomaterials. Corros. Sci. 2012, 54, 307–313. [Google Scholar] [CrossRef]
- Kamali, A.R.; Divitini, G.; Schwandt, C.; Fray, D.J. Correlation between microstructure and thermokinetic characteristics of electrolytic carbon nanomaterials. Corros. Sci. 2012, 64, 90–97. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Yin, Z.; Li, H.; Liu, J.; Cao, X.; Zhang, Q.; Zhang, H. Layer thinning and etching of mechanically exfoliated MoS2 nanosheets by thermal annealing in air. Small 2013, 9, 3314–3319. [Google Scholar]
- Zhou, H.; Yu, F.; Liu, Y.; Zou, X.; Cong, C.; Qiu, C.; Yu, T.; Yan, Z.; Shen, X.; Sun, L.; et al. Thickness-dependent patterning of MoS2 sheets with well-oriented triangular pits by heating in air. Nano Res. 2013, 6, 703–711. [Google Scholar] [CrossRef]
- Rao, R.; Islam, A.E.; Campbell, P.M.; Vogel, E.M.; Maruyama, B. In situ thermal oxidation kinetics in few layer MoS2. 2D Mater. 2017, 4, 025058. [Google Scholar] [CrossRef]
- Afanasiev, P.; Lorentz, C. Oxidation of nanodispersed MoS2 in ambient air: The products and the mechanistic steps. J. Phys. Chem. C 2019, 123, 7486–7494. [Google Scholar] [CrossRef]
- Spychalski, W.L.; Pisarek, M.; Szoszkiewicz, R. Microscale insight into oxidation of single MoS2 crystals in air. J. Phys. Chem. C 2017, 121, 26027–26033. [Google Scholar] [CrossRef]
- Szoszkiewicz, R.; Rogala, M.; Dąbrowski, P. Surface-bound and volatile Mo oxides produced during oxidation of single MoS2 crystals in air and high relative humidity. Materials 2020, 13, 3067. [Google Scholar] [CrossRef]
- Kamali, A.R. Green Production of Carbon Nanomaterials in Molten Salts and Applications; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Hu, X.L.; Zhang, W.; Liu, X.X.; Mei, Y.N.; Huang, Y.H. Nanostructured Mo-based electrode materials for electrochemical energy storage. Chem. Soc. Rev. 2015, 44, 2376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, J.X.; Xu, J.S.; Liu, S.L.; Li, L.; Zhang, C.; Liu, T.X. High-temperature solvent-free sulfidation of MoO3 confined in a polypyrrole shell: MoS2 nanosheets encapsulated in a nitrogen, sulfur dual-doped carbon nanoprism for efficient lithium storage. Nanoscale 2018, 10, 7536–7543. [Google Scholar] [CrossRef]
- Li, J.; Hou, S.; Liu, T.Z.; Wang, L.K.; Mei, C.; Guo, Y.Y.; Zhao, L.Z. Hierarchical hollow-nanocube Ni−Co skeleton@MoO3/MoS2 hybrids for improved-performance lithium-ion batteries. Chem. Eur. J. 2020, 26, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xia, X.; Lv, P.; Zhang, J.; Hou, X.; Zhao, M.; Ao, K.; Wang, D.; Lu, K.; Qiao, H.; et al. C@TiO2/MoO3 composite nanofibers with 1T-phase MoS2 nanograin dopant and stabilized interfaces as anodes for Li- and Na-ion batteries. ChemSusChem 2018, 11, 4060–4070. [Google Scholar] [CrossRef]
- Liu, H.W.; Wang, J. One-pot synthesis of ZnCo2O4 nanorod anodes for high power Lithium ions batteries. Electrochim. Acta 2013, 92, 371. [Google Scholar] [CrossRef]
- Zhang, J.J.; He, P.; Xia, Y.Y. Electrochemical kinetics study of Li-ion in Cu6Sn5 electrode of lithium batteries by PITT and EIS. J. Electroanal. Chem. 2008, 624, 161. [Google Scholar] [CrossRef]
- Lei, D.; Shang, W.Z.; Zhang, X.; Li, Y.P.; Qiao, S.M.; Zhong, Y.P.; Deng, X.Y.; Shi, X.S.; Zhang, Q.; Hao, C.; et al. Facile synthesis of heterostructured MoS2-MoO3 nanosheets with active electrocatalytic sites for high-performance lithium−sulfur batteries. ACS Nano 2021, 15, 20478–20488. [Google Scholar] [CrossRef]
- Zhao, B.; Song, D.Y.; Ding, Y.W.; Li, W.R.; Wang, Z.X.; Jiang, Y.; Zhang, J.J. Size-tunable SnS2 nanoparticles assembled on graphene as anodes for high performance lithium/sodium-ion batteries. Electrochim. Acta 2020, 354, 13673. [Google Scholar] [CrossRef]
- Zhang, P.C.; Cai, B.; Feng, Y.; Pan, H.; Yao, J.F. Constructing MoO3@MoO2 heterojunction on g-C3N4 nanosheets with advanced Li-ion storage ability. J. Alloys Compd. 2021, 875, 160077. [Google Scholar] [CrossRef]
- Kamali, A.R.; Li, S. Molten salt-assisted valorization of waste PET plastics into nanostructured SnO2@terephthalic acid with excellent Li-ion storage performance. Appl. Energy 2023, 334, 120692. [Google Scholar] [CrossRef]
- Zhu, W.; Kamali, A.R. Green molten salt synthesis and Li-ion storage performance of sodium dimolybdate. J. Alloys Compd. 2020, 831, 154781. [Google Scholar] [CrossRef]
- Kamali, A.R.; Ye, J. Reactive molten salt modification of ilmenite as a green approach for the preparation of inexpensive Li ion battery anode materials. Miner. Eng. 2021, 172, 107175. [Google Scholar] [CrossRef]
- Zhuan, Y.; Ma, J.L.; Feng, W.J. Highlighting the implantation of metal particles into hollow cavity yeast-based carbon for improved electrochemical performance of lithium–sulfur batteries. Catalysts 2022, 12, 951. [Google Scholar] [CrossRef]
- Zeng, G.; Wang, J.; Dai, M.; Meng, Y.; Luo, H.; Lin, L.; Zang, K.; Meng, Z.; Pan, X. Natural iron minerals in an electrocatalytic oxidation system and in situ pollutant removal in groundwater: Applications, mechanisms, and challenges. Sci. Total Environ. 2023, 871, 161826. [Google Scholar] [CrossRef]
- Enneffatia, M.; Rasheed, M.; Louatia, B.; Guidaraa, K.; Shihab, S.; Barillé, R. Investigation of structural, morphology, optical properties and electrical transport conduction of Li0.25Na0.75CdVO4 compound. J. Phys. Conf. Ser. 2021, 1795, 012050. [Google Scholar] [CrossRef]
- Rasheed, M.; Shihab, S.; Sabah, O.W. An investigation of the Structural, Electrical and Optical Properties of Graphene-Oxide Thin Films Using Different Solvents. J. Phys. Conf. Ser. 2021, 1795, 012052. [Google Scholar] [CrossRef]
- Nazarian-Samani, M.; Kamali, A.R.; Mobarra, R.; Nazarian-Samani, M. Phase transformations of Ni-15 wt. % B powders during mechanical alloying and annealing. Mater. Lett. 2010, 64, 309–312. [Google Scholar] [CrossRef]
- Seifi, T.; Kamali, A.R. Enhanced dispersion and antibacterial activity of mechanically exfoliated graphite flakes in the presence of n-hexane and NaCl. Mater. Lett. 2021, 304, 130730. [Google Scholar] [CrossRef]
- Seifi, T.; Kamali, A.R. The influence of mechanochemical treatment in hexane on dispersibility and floatability of graphite flakes with enhanced water evaporation performance. Colloids Surf. A 2022, 638, 128326. [Google Scholar] [CrossRef]
- Wang, Z.G.; Lia, Q.; Xu, H.X.; Dahl-Petersen, C.; Yang, Q.; Cheng, D.J.; Cao, D.P.; Besenbacher, F.; Lauritsen, J.V.; Helveg, S.; et al. Controllable etching of MoS2 basal planes for enhanced hydrogen evolution through the formation of active edge sites. Nano Energy 2018, 49, 634–643. [Google Scholar] [CrossRef]
- Yorulmaz, B.; Özden, A.; Şar, H.; Ay, F.; Sevik, C.; Perkgöz, N.K. CVD growth of monolayer WS2 through controlled seed formation and vapor density. Mater. Sci. Semicond. Process. 2019, 93, 158. [Google Scholar] [CrossRef]
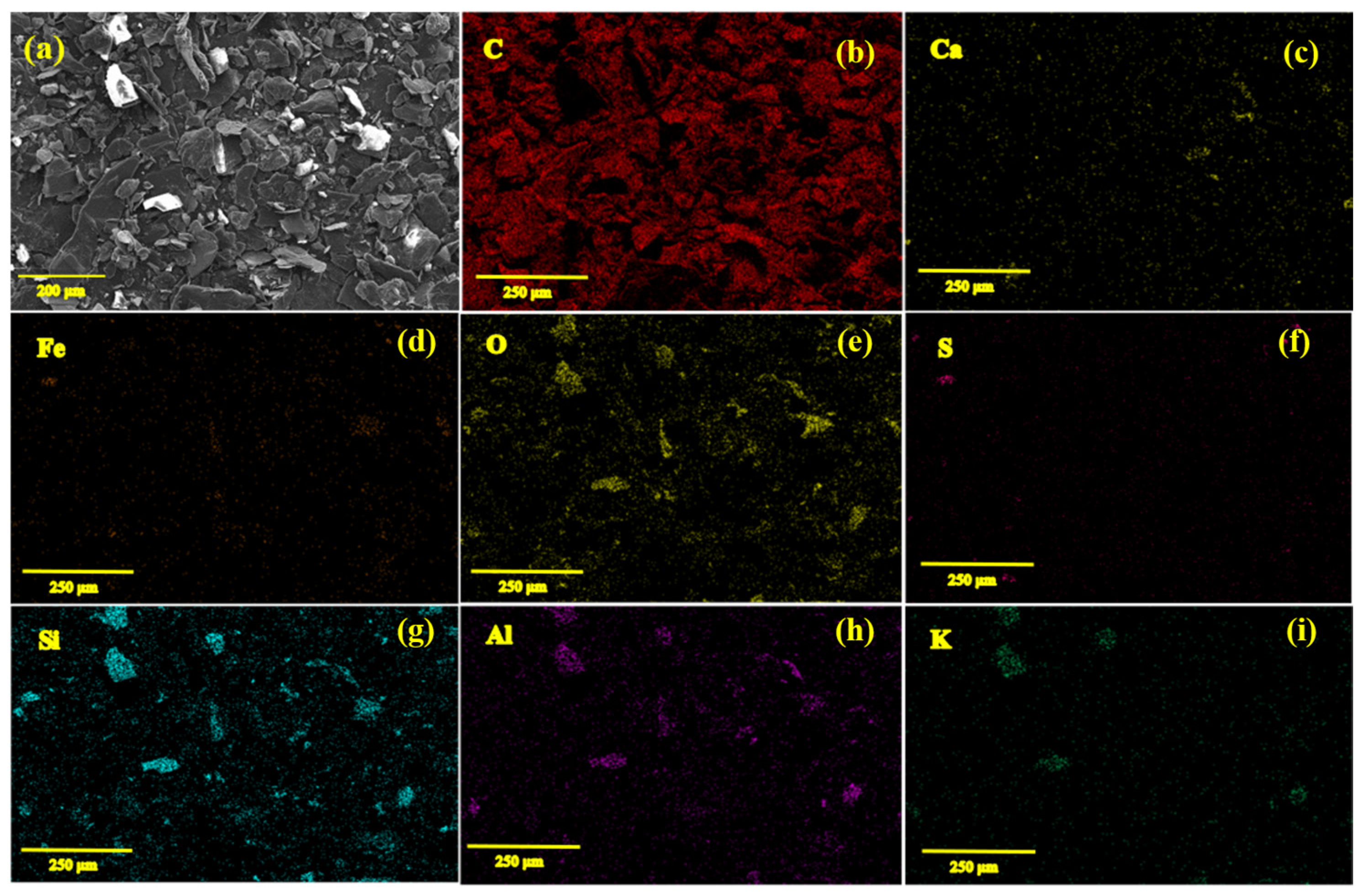



| C | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | S |
|---|---|---|---|---|---|---|
| 74.3 | 9.27 | 6.92 | 2.97 | 2.29 | 2.17 | 2.08 |
| Sample | S | Mo | C | Si | Al | Fe | Ca | O |
|---|---|---|---|---|---|---|---|---|
| MoO3/MoS2@G-900 | 5.78 | 44.33 | 22.6 | 5.15 | 1.92 | 1.65 | 1.69 | 16.88 |
| MoO3/MoS2@G-1000 | 5.59 | 45.18 | 23.3 | 6.36 | 2.42 | 1.15 | 1.74 | 14.26 |
| MoO3/MoS2@G-1100 | 2.72 | 46.01 | 17.8 | 6.78 | 2.48 | 1.56 | 1.65 | 21.00 |
| MoO3/MoS2@G-1200 | 10.2 | 48.48 | 15.6 | 5.96 | 2.07 | 1.82 | 1.18 | 14.69 |
| Electrode | Initial Coulombic Efficiency (%) | Specific Capacity after 100 Cycles (mAh/g) | Specific Capacity after 150 Cycles (mAh/g) |
|---|---|---|---|
| C-MoS2 | 85.13 | 94.8 | 73.5 |
| MoO3/MoS2@G-900 | 70.64 | 444.1 | 442.8 |
| MoO3/MoS2@G-1000 | 71.13 | 422.2 | 472.7 |
| MoO3/MoS2@G-1100 | 79.58 | 616.3 | 721.2 |
| MoO3/MoS2@G-1200 | 78.36 | 560.1 | 505.9 |
| Sample | Re (Ω) | Rct (Ω) | σ (Ω s−1/2) | DLi (cm2 s−1) |
|---|---|---|---|---|
| MoO3/MoS2@G-900 | 6.90 | 63.65 | 114.61 | 8.7 × 10−21 |
| MoO3/MoS2@G-1000 | 6.56 | 53.56 | 79.39 | 1.8 × 10−20 |
| MoO3/MoS2@G-1100 | 2.21 | 40.71 | 9.77 | 1.2 × 10−18 |
| MoO3/MoS2@G-1200 | 3.92 | 62.9 | 146.53 | 5.3 × 10−21 |
| C-MoS2 | 3.56 | 263.8 | 334.49 | 1.0 × 10−21 |
| Mo-Oxide Based Material | Precursor Materials | Preparation Process | Performance |
|---|---|---|---|
| Capacity (mAh/g)/Cycle/Current Density (mA/g) | |||
| Core−sheath structured MoO3@MoS2 composite [23] | Rhododendron petals, (NH4)6Mo7O24·4H2O, CH4N2S | In situ growth at 550 °C (2 h) + 180 °C (24 h) | 400/30th/200 |
| Ternary MoS2/MoO3/C nanosheets [24] | Organic amine, MoO3 | CVD at 800 °C (3 h) under N2 | 961.4/120th/430 |
| MoO3/MoS2 nanoribbons [25] | Molybdic acid, glacial acetic acid | Hydrothermal treatment at 800 °C (30 min) | - |
| MoS2/MoO3 nanosheets [26] | CdCl2, (NH)2CS, (NH4)2MoO4, NH2OH.HCl | Hydrothermal treatment at 180 °C (30 h) | - |
| MoS2 coated MoO3 nanobelts [27] | Mo metal powder 10 mL H2O2 | Hydrothermal treatment at 180 °C (12 h) | 1400/50th/100 |
| MoS2 nanosheets on MoO3 nanowires [28] | (NH4)6Mo7O24·4H2O, HNO3,thiourea | Hydrothermal treatment at 180 °C (24 h) | 800/100th/100 |
| Core-shell MoO3/MoS2 nanowires [29] | MoO3 powders, sulfur, N2 | Thermal treatment at 700 °C | |
| MoS2/MoO3@graphene [This work] | MoS2, natural graphite, NaCl | Heating at 1100 °C (20 min) | 316/40th/1000 |
| 774/180th/100 |
| Raw Material | MoS2 (10 g)-NaCl (100 g)-Graphite (5 g) | |||
|---|---|---|---|---|
| Ball-milled sample | 112.00 g | |||
| Heat-treatment Temperature | 900 °C | 1000 °C | 1100 °C | 1200 °C |
| Sample | MoO3/MoS2@G-900 | MoO3/MoS2@G-1000 | MoO3/MoS2@G-1100 | MoO3/MoS2@G-1200 |
| Before heat-treatment | 28.00 g | 28.00 g | 28.00 g | 28.00 g |
| After washing | 3.31 | 2.60 | 2.29 | 2.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Kamali, A.R. Molten Salt-Assisted Catalytic Preparation of MoS2/α-MoO3/Graphene as High-Performance Anode of Li-Ion Battery. Catalysts 2023, 13, 499. https://doi.org/10.3390/catal13030499
Zhu W, Kamali AR. Molten Salt-Assisted Catalytic Preparation of MoS2/α-MoO3/Graphene as High-Performance Anode of Li-Ion Battery. Catalysts. 2023; 13(3):499. https://doi.org/10.3390/catal13030499
Chicago/Turabian StyleZhu, Wenhui, and Ali Reza Kamali. 2023. "Molten Salt-Assisted Catalytic Preparation of MoS2/α-MoO3/Graphene as High-Performance Anode of Li-Ion Battery" Catalysts 13, no. 3: 499. https://doi.org/10.3390/catal13030499
APA StyleZhu, W., & Kamali, A. R. (2023). Molten Salt-Assisted Catalytic Preparation of MoS2/α-MoO3/Graphene as High-Performance Anode of Li-Ion Battery. Catalysts, 13(3), 499. https://doi.org/10.3390/catal13030499









