Plasma-Enhanced Chemical Looping Oxidative Coupling of Methane through Synergy between Metal-Loaded Dielectric Particles and Non-Thermal Plasma
Abstract
:1. Introduction
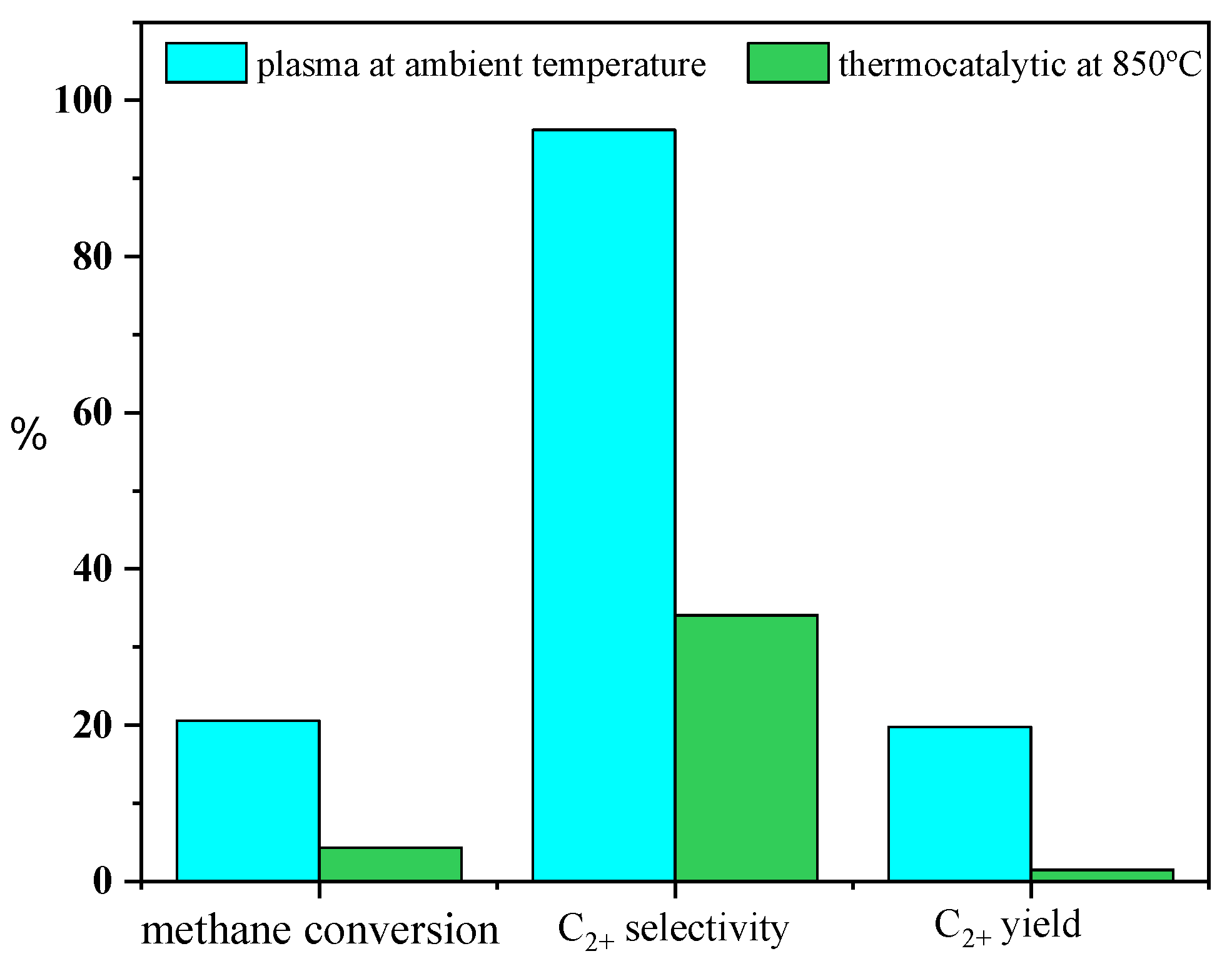
2. Results and Discussion
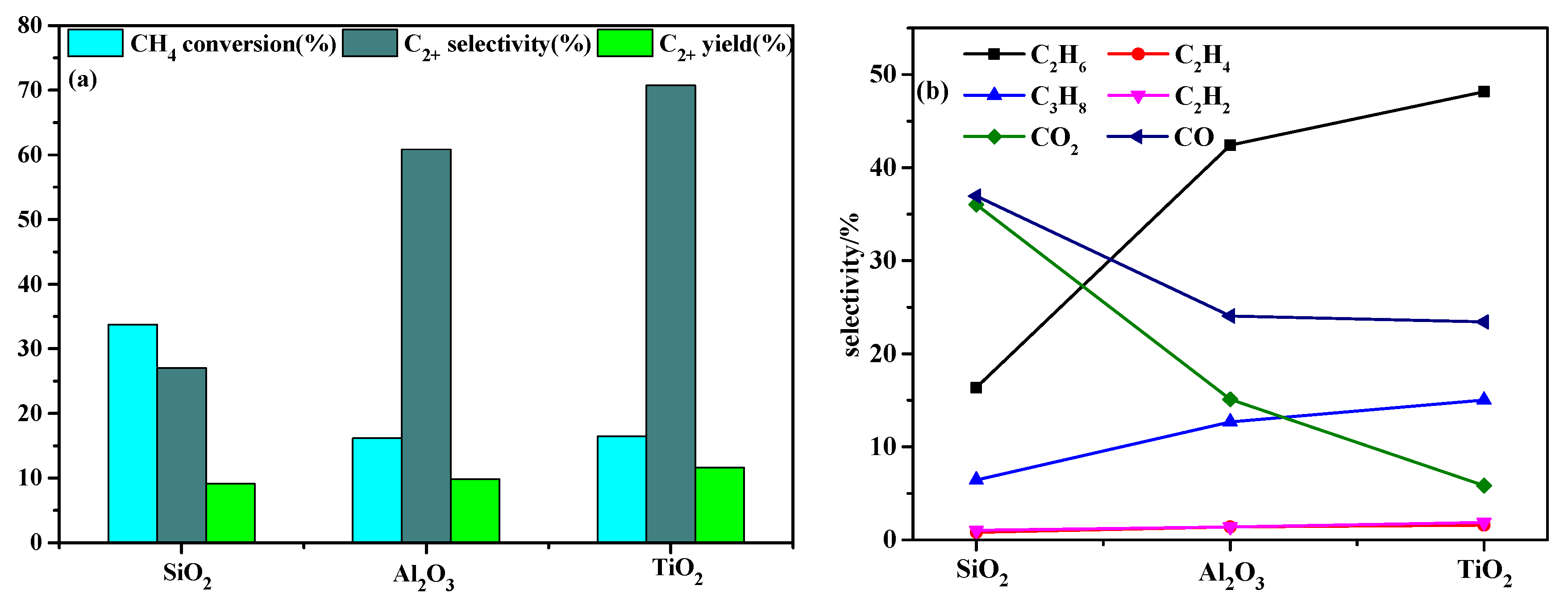
2.1. Effect of Dielectric Materials
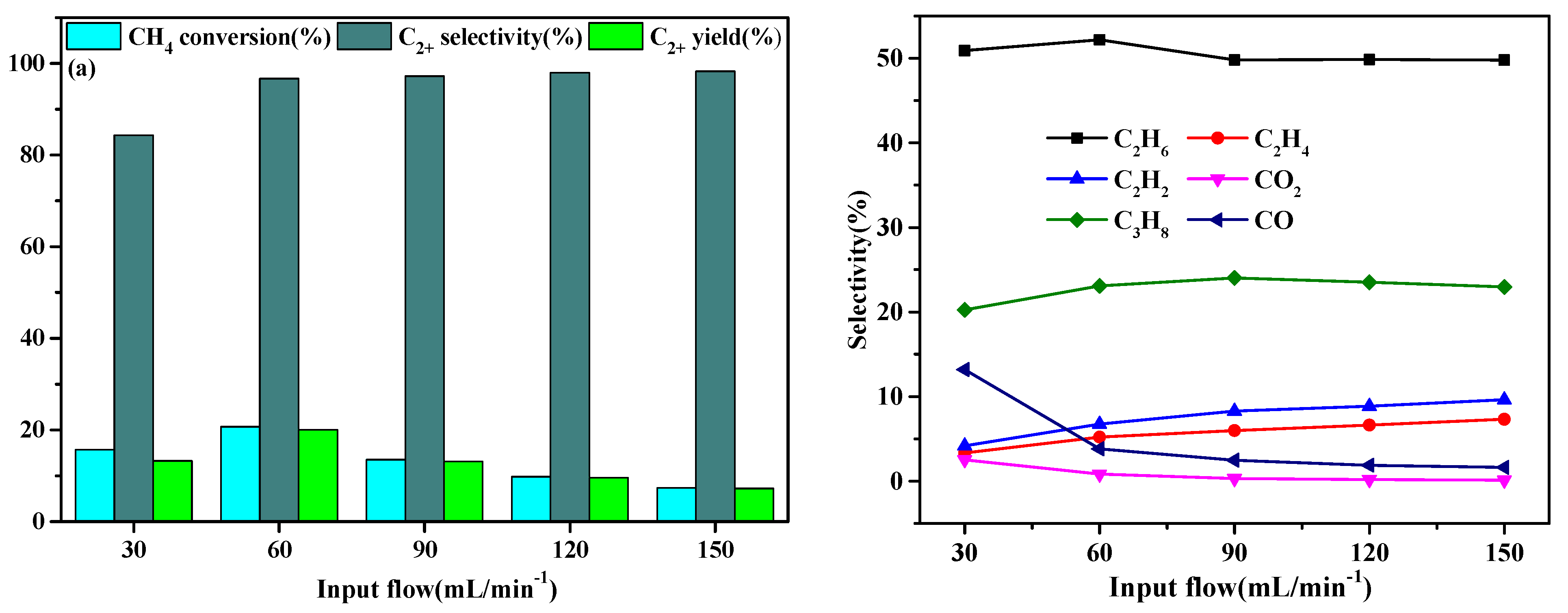
2.2. Effect of Flow Rate
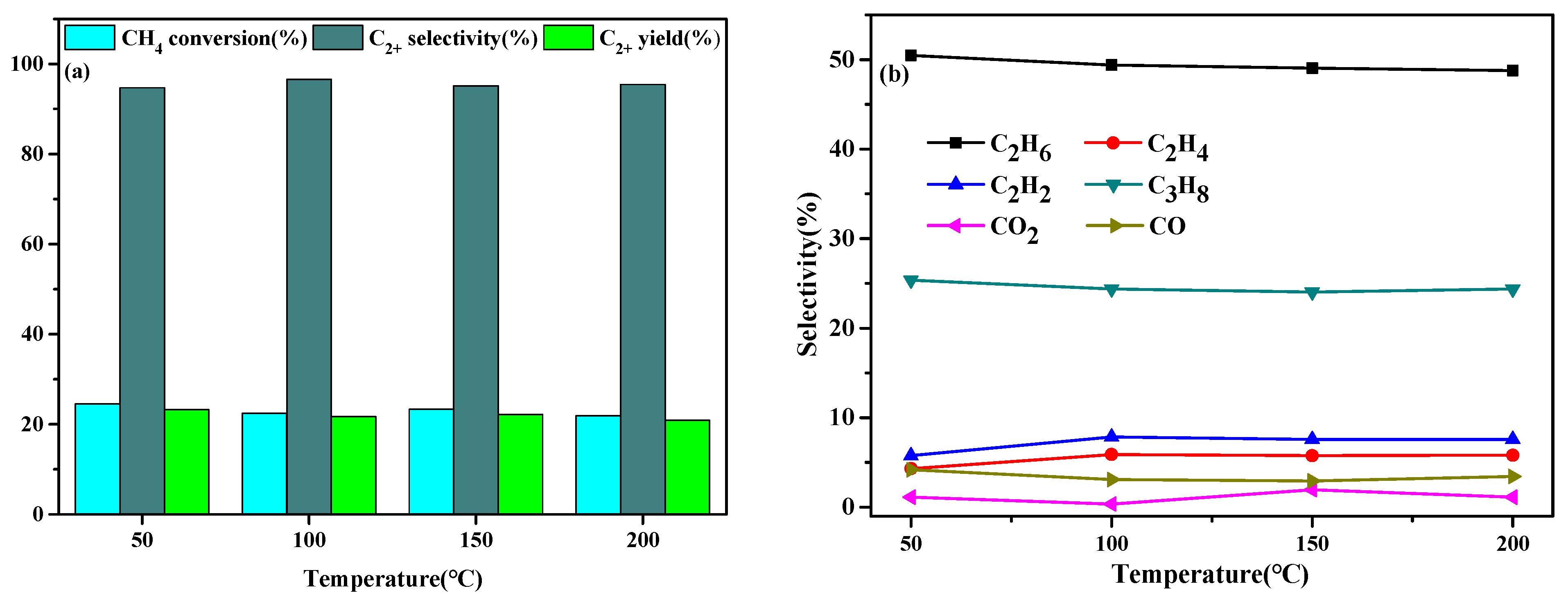
2.3. Effect of Temperature
2.4. Effect of Applied Voltage
3. Characterization
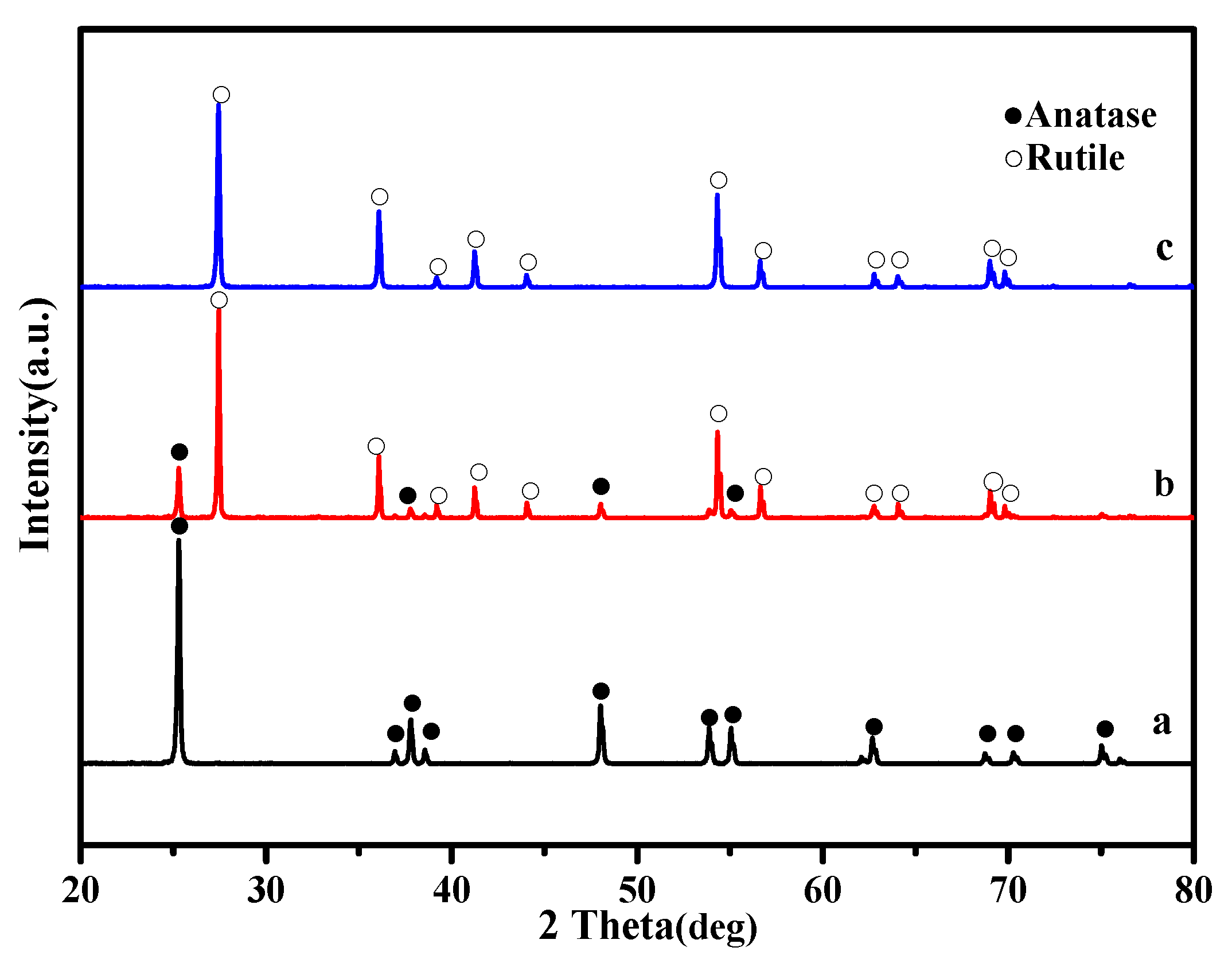
3.1. XRD
3.2. XPS
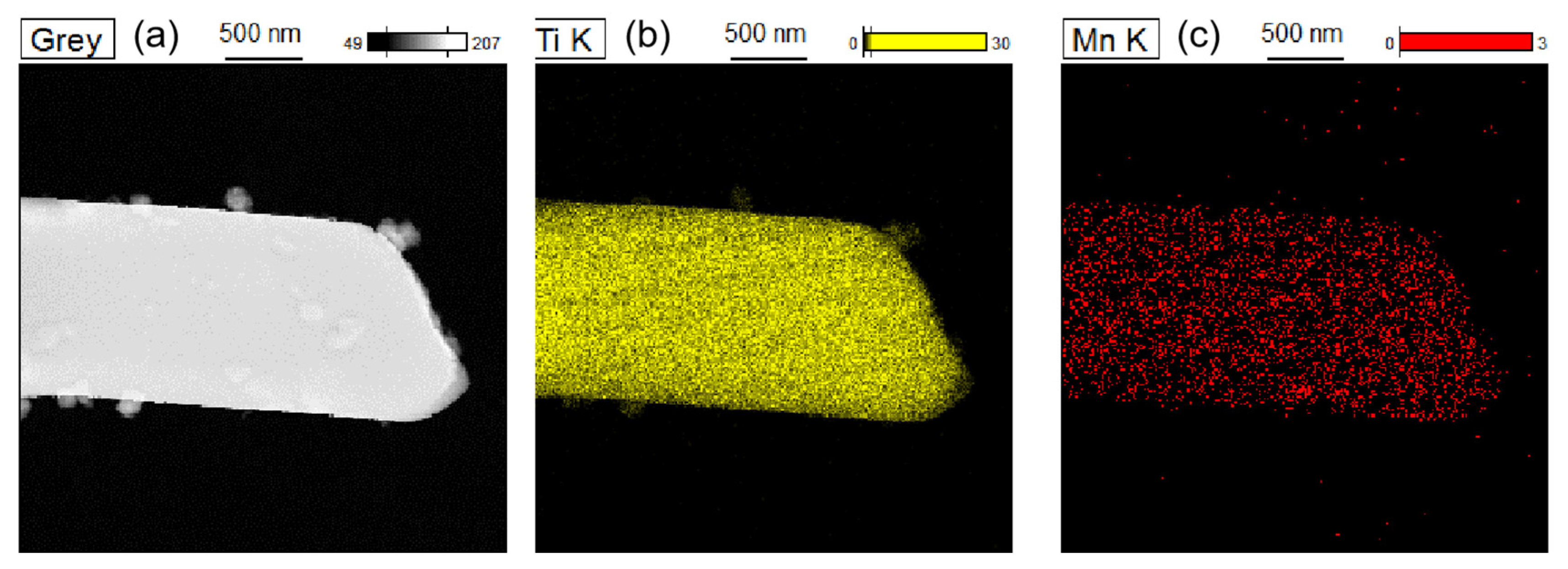
3.3. TEM–EDS
3.4. H2-TPR
3.5. Chemical Looping Redox Experiments
4. Experimental
4.1. Preparation of the Catalyst
4.2. Material Characterization
4.3. Catalytic Test
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boswell, R. Is gas hydrate energy within reach? Science 2009, 325, 957–958. [Google Scholar] [CrossRef] [PubMed]
- McFarland, E. Unconventional chemistry for unconventional natural gas. Science 2012, 338, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Gao, Y.; Iftikhar, S.; Li, F. Ce stabilized Ni–SrO as a catalytic phase transition sorbent for integrated CO2 capture and CH4 reforming. J. Mater. Chem. A 2022, 10, 3077–3085. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, Y.; Li, K.; Zhu, X.; Wang, H.; Jiang, L.; Wei, Y.; Shan, S.; Zheng, Y. LaFe0.8Co0.15Cu0.05O3 Supported on Silicalite-1 as a Durable Oxygen Carrier for Chemical Looping Reforming of CH4 Coupled with CO2 Reduction. ACS Appl. Mater. Interfaces 2022, 14, 39004–39013. [Google Scholar] [CrossRef]
- Li, Z.; Feng, X.; Gu, Z.; Lu, C.; Li, D.; Zhu, X.; Jiang, L.; Deng, G.; Li, K. Enhanced performance of the CeO2MgO oxygen carrier by NiO for chemical looping CO2 splitting. Fuel Process. Technol. 2022, 225, 107045. [Google Scholar] [CrossRef]
- Holmen, A. Direct conversion of methane to fuels and chemicals. Catal. Today 2009, 142, 2–8. [Google Scholar] [CrossRef]
- Hou, Y.; Han, W.; Xia, W.; Wan, H. Structure Sensitivity of La2O2CO3 Catalysts in the Oxidative Coupling of Methane. ACS Catal. 2015, 5, 1663–1674. [Google Scholar] [CrossRef]
- Wang, H.; Schmack, R.; Paul, B.; Albrecht, M.; Sokolov, S.; Rümmler, S.; Kondratenko, E.V.; Kraehnert, R. Porous silicon carbide as a support for Mn/Na/W/SiC catalyst in the oxidative coupling of methane. Appl. Catal. A-Gen. 2017, 537, 33–39. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, S.F. Numerical simulation of particle/monolithic two-stage catalyst bed reactor with beds-interspace distributed dioxygen feeding for oxidative coupling of methane. Comput. Chem. Eng. 2016, 90, 247–259. [Google Scholar] [CrossRef]
- Guo, X.; Fang, G.; Li, G.; Ma, H. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 2014, 344, 616–619. [Google Scholar] [CrossRef]
- Goujard, V.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. Carbon Dioxide Reforming of Methane Using a Dielectric Barrier Discharge Reactor: Effect of Helium Dilution and Kinetic Model. Plasma Chem. Plasma Process. 2011, 31, 315–325. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.; Dou, L.; Zhang, C.; Zhang, S.; Gao, Y.; Tu, X.; Shao, T. Plasma enhanced anti-coking performance of Pd/CeO2 catalysts for the conversion of methane. Sustain. Energy Fuels 2022, 6, 98–109. [Google Scholar] [CrossRef]
- Kim, J.; Go, D.B.; Hicks, J.C. Synergistic effects of plasma-catalyst interactions for CH4 activation. Phys. Chem. Chem. Phys. 2017, 19, 13010–13021. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yi, Y.; Wang, L.; Yan, J.; Guo, H. Pt/TS-1 Catalyst Promoted C–N Coupling Reaction in CH4–NH3 Plasma for HCN Synthesis at Low Temperature. ACS Catal. 2018, 8, 10219–10224. [Google Scholar] [CrossRef]
- Bogaerts, A.; Berthelot, A.; Heijkers, S.; Kolev, S.; Snoeckx, R.; Sun, S.; Trenchev, G.; Van Laer, K.; Wang, W. CO2 conversion by plasma technology: Insights from modeling the plasma chemistry and plasma reactor design. Plasma Sources Sci. Technol. 2017, 26, 063001. [Google Scholar] [CrossRef]
- Debek, R.; Azzolina-Jury, F.; Travert, A.; Mauge, F. A review on plasma-catalytic methanation of carbon dioxide-Looking for an efficient catalyst. Renew. Sustain. Energy Rev. 2019, 116, 109427. [Google Scholar] [CrossRef]
- Liu, J.L.; Wang, X.; Li, X.S.; Likozar, B.; Zhu, A.M. CO2 conversion, utilisation and valorisation in gliding arc plasma reactors. J. Phys. D Appl. Phys. 2020, 53, 253001. [Google Scholar] [CrossRef]
- Qin, Y.; Niu, G.H.; Wang, X.; Luo, D.B.; Duan, Y.X. Status of CO2 conversion using microwave plasma. J. CO2 Util. 2018, 28, 283–291. [Google Scholar] [CrossRef]
- Barboun, M.; Mehta, P.; Herrera, F.; Go, D.; Schneider, W. Distinguishing Plasma Contributions to Catalyst Performance in Plasma-Assisted Ammonia Synthesis. ACS Sustain. Chem. Eng. 2019, 7, 32. [Google Scholar] [CrossRef]
- Zhu, X.B.; Liu, J.; Hu, X.L.; Zhou, Z.J.; Li, X.B.; Wang, W.T.; Wu, R.B.; Tu, X. Plasma-catalytic synthesis of ammonia over Ru-based catalysts: Insights into the support effect. J. Energy Inst. 2022, 102, 240–246. [Google Scholar] [CrossRef]
- Xu, C.; Tu, X. Plasma-assisted methane conversion in an atmospheric pressure dielectric barrier discharge reactor. J. Energy Chem. 2013, 22, 420–425. [Google Scholar] [CrossRef]
- Thanyachotpaiboon, K.; Chavadej, S.; Caldwell, T.A. Conversion of Methane to Higher Hydrocarbons in AC Nonequilibrium Plasmas. AIChE J. 2010, 44, 2252–2257. [Google Scholar] [CrossRef]
- Bouchoul, N.; Fourré, E.; Duarte, A.; Tanchoux, N.; Louste, C.; Batiot-Dupeyrat, C. Plasma-metal oxides coupling for CH4-CO2 transformation into syngas and/or hydrocarbons, oxygenates. Catal. Today 2021, 369, 62–68. [Google Scholar] [CrossRef]
- Kim, J.; Jeoung, J.; Jeon, J.; Kim, J.; Mok, Y.S.; Ha, K.S. Effects of dielectric particles on non-oxidative coupling of methane in a dielectric barrier discharge plasma reactor. Chem. Eng. J. 2019, 377, 119896. [Google Scholar] [CrossRef]
- Bouchoul, N.; Touati, H.; Fourré, E.; Clacens, J.-M.; Batonneau-Gener, I.; Batiot-Dupeyrat, C. Plasma-catalysis coupling for CH4 and CO2 conversion over mesoporous macroporous Al2O3: Influence of the physico-chemical properties. Appl. Catal. B-Environ. 2021, 295, 120262. [Google Scholar] [CrossRef]
- Han, Q.; Tanaka, A.; Matsumoto, M.; Endo, A.; Kubota, Y.; Inagaki, S. Conversion of methane to C2 and C3 hydrocarbons over TiO2/ZSM-5 core-shell particles in an electric field. RSC Adv. 2019, 9, 34793–34803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krawczyk, K.; Młotek, M.; Ulejczyk, B.; Schmidt-Szałowski, K. Methane conversion with carbon dioxide in plasma-catalytic system. Fuel 2014, 117, 608–617. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhang, M.; Yu, T.; Chen, B.; Xu, Y.; Crocker, M.; Zhu, X.; Zhu, Y.; Wang, R.; et al. Synergy between β-Mo2C Nanorods and Non-thermal Plasma for Selective CO2 Reduction to CO. Chem 2020, 6, 3312–3328. [Google Scholar] [CrossRef]
- Liu, L.; Das, S.; Zhang, Z.; Kawi, S. Nonoxidative Coupling of Methane over Ceria-Supported Single-Atom Pt Catalysts in DBD Plasma. ACS Appl. Mater. Interfaces 2022, 14, 5363–5375. [Google Scholar] [CrossRef]
- Takanabe, K.; Iglesia, E. Rate and selectivity enhancements mediated by OH radicals in the oxidative coupling of methane catalyzed by Mn/Na2WO4/SiO2. Angew. Chem. Int. Ed. Engl. 2008, 47, 7689–7693. [Google Scholar] [CrossRef]
- Kasinathan, P.; Park, S.; Choi, W.; Hwang, Y.K.; Chang, J.S.; Park, Y.K. Plasma-Enhanced Methane Direct Conversion over Particle-Size Adjusted MOx/Al2O3 (M = Ti and Mg) Catalysts. Plasma Chem. Plasma Process. 2014, 34, 1317–1330. [Google Scholar] [CrossRef]
- Kim, S.S.; Kwon, B.; Kim, J. Plasma catalytic methane conversion over sol–gel derived Ru/TiO2 catalyst in a dielectric-barrier discharge reactor. Catal. Commun. 2007, 8, 2204–2207. [Google Scholar] [CrossRef]
- Liu, C.; Marafee, A.; Mallinson, R.; Lobban, L. Methane conversion to higher hydrocarbons in a corona discharge over metal oxide catalysts with OH groups. Appl. Catal. A-Gen. 1997, 164, 21–33. [Google Scholar] [CrossRef]
- Keller, G.E.; Bhasin, M.M. Synthesis of ethylene via oxidative coupling of methane: I. Determination of active catalysts. J. Catal. 1982, 73, 9–19. [Google Scholar] [CrossRef]
- Cheng, Z.; Baser, D.S.; Nadgouda, S.G.; Qin, L.; Fan, J.A.; Fan, L.-S. C2 Selectivity Enhancement in Chemical Looping Oxidative Coupling of Methane over a Mg–Mn Composite Oxygen Carrier by Li-Doping-Induced Oxygen Vacancies. ACS Energy Lett. 2018, 3, 1730–1736. [Google Scholar] [CrossRef]
- Jiang, S.C.; Ding, W.X.; Zhao, K.; Huang, Z.; Wei, G.Q.; Feng, Y.Y.; Lv, Y.J.; He, F. Enhanced Chemical looping oxidative coupling of methane by Na-doped LaMnO3 redox catalysts. Fuel 2021, 299, 120932. [Google Scholar] [CrossRef]
- Zeng, D.; Wang, C.; Liu, T.; Ou, W.; Xiao, R. Enhanced performance of chemical looping methane oxidative coupling by the synergistic effect of TiO2 doped Na2WO4/Mn2O3/SiO2 oxygen carriers. Fuel Process. Technol. 2022, 234, 107320. [Google Scholar] [CrossRef]
- Lunsford, J.H. The catalytic oxidative coupling of methane. Angew. Chem. Int. Ed. Engl. 1995, 34, 970–980. [Google Scholar] [CrossRef]
- Kiani, D.; Sourav, S.; Wachs, I.E.; Baltrusaitis, J. Synthesis and molecular structure of model silica-supported tungsten oxide catalysts for oxidative coupling of methane (OCM). Catal. Sci. Technol. 2020, 10, 3334–3345. [Google Scholar] [CrossRef]
- Elkins, T.; Hagelin-Weaver, H. Characterization of Mn–Na2WO4/SiO2 and Mn–Na2WO4/MgO catalysts for the oxidative coupling of methane. Appl. Catal. A-Gen. 2015, 497, 96–106. [Google Scholar] [CrossRef]
- Arndt, S.; Otremba, T.; Simon, U.; Yildiz, M.; Schubert, H.; Schomäcker, R. Mn–Na2WO4/SiO2 as catalyst for the oxidative coupling of methane. What is really known? Appl. Catal. A-Gen. 2012, 425, 53–61. [Google Scholar] [CrossRef]
- Kim, G.J.; Ausenbaugh, J.T.; Hwang, H.T. Effect of TiO2 on the Performance of Mn/Na2WO4 Catalysts in Oxidative Coupling of Methane. Ind. Eng. Chem. Res. 2021, 60, 3914–3921. [Google Scholar] [CrossRef]
- Chen, H.; Lee, H.; Chen, S.; Chao, Y.; Chang, M. Review of plasma catalysis on hydrocarbon reforming for hydrogen production—Interaction, integration, and prospects. Appl. Catal. B-Environ. 2008, 85, 1–9. [Google Scholar] [CrossRef]
- Hou, S.; Cao, Y.; Xiong, W.; Liu, H. Site requirements for the oxidative coupling of methane on SiO2-supported Mn catalysts. Ind. Eng. Chem. Res. 2006, 45, 7077–7083. [Google Scholar] [CrossRef]
- Chawdhury, P.; Bhargavi, K.; Subrahmanyam, C. A single-stage partial oxidation of methane to methanol: A step forward in the synthesis of oxygenates. Sustain. Energy Fuels 2021, 5, 3351–3362. [Google Scholar] [CrossRef]
- Seyed, M.N.; Savadkoohi, H.A.; Feizabadi, S.Y. Methane Conversion to C2 Hydrocarbons Using Dielectric-barrier Discharge Reactor: Effects of System Variables. Plasma Chem. Plasma Process. 2008, 28, 189–202. [Google Scholar] [CrossRef]
- Miao, Y.; Kreider, P.; Reddick, I.; Pommerenck, J.; Collin, R.; AuYeung, N.; von Jouanne, A.; Jovanovic, G.; Yokochi, A. Methane Coupling to Ethylene and Longer-Chain Hydrocarbons by Low-Energy Electrical Discharge in Microstructured Reactors. Ind. Eng. Chem. Res. 2021, 60, 6950–6958. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.H.; Ha, J.M.; Kim, D.H. Plasma assisted oxidative coupling of methane (OCM) over Ag/SiO2 and subsequent regeneration at low temperature. Appl. Catal. A-Gen. 2018, 557, 39–45. [Google Scholar] [CrossRef]
- Huu, P.; Gil, S.; Patrick, D.; Anne, G.-F.; Khacef, A. Plasma-catalytic hybrid reactor: Application to methane removal. Catal. Today 2015, 257, 86–92. [Google Scholar]
- Gunawidjaja, R.; Anderson, B.; Eilers, H. Structural and spectroscopic characterization of irreversible phase changes in rapidly heated precursors of europium-doped titania nanoparticles. J. Solid State Chem. 2018, 258, 15–23. [Google Scholar] [CrossRef]
- Zhang, P.; Xiong, J.; Wei, Y.C.; Li, Y.F.; Zhang, Y.L.; Tang, J.J.; Song, W.Y.; Zhao, Z.; Liu, J. Exposed {0 0 1} facet of anatase TiO2 nanocrystals in Ag/TiO2 catalysts for boosting catalytic soot combustion: The facet-dependent activity. J. Catal. 2021, 398, 109–122. [Google Scholar] [CrossRef]
- Zhao, K.; Li, L.W.; Zheng, A.Q.; Huang, Z.; He, F.; Shen, Y.; Wei, G.Q.; Li, H.B.; Zhao, Z.L. Synergistic improvements in stability and performance of the double perovskite-type oxides La2−xSrxFeCoO6 for chemical looping steam methane reforming. Appl. Energy 2017, 197, 393–404. [Google Scholar] [CrossRef]
- Xie, P.; Pu, T.; Nie, A.; Hwang, S.; Purdy, S.C.; Yu, W.; Su, D.; Miller, J.T.; Wang, C. Nanoceria-Supported Single-Atom Platinum Catalysts for Direct Methane Conversion. ACS Catal. 2018, 8, 4044–4048. [Google Scholar] [CrossRef]
- Yang, N.Z.; Guo, R.T.; Pan, W.G.; Chen, Q.L.; Wang, Q.S.; Lu, C.Z. The promotion effect of Sb on the Na resistance of Mn/TiO2 catalyst for selective catalytic reduction of NO with NH3. Fuel 2016, 169, 87–92. [Google Scholar] [CrossRef]










| Sample | Oxygen Distribution/% | |||
|---|---|---|---|---|
| O2− | O22−/O− | OH− | H2O | |
| TiO2 | 21.94 | 37.96 | 36.05 | 4.06 |
| Fresh 5%Mn(NO3)2-TiO2 | 17.02 | 28.41 | 45.33 | 8.63 |
| Reduced 5%Mn(NO3)2-TiO2 | 17.82 | 22.73 | 49.26 | 10.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Deng, J.; Wang, X.; Zhao, K.; Zheng, M.; Song, D.; Huang, Z.; Lin, Y.; Liu, A.; Zheng, A.; et al. Plasma-Enhanced Chemical Looping Oxidative Coupling of Methane through Synergy between Metal-Loaded Dielectric Particles and Non-Thermal Plasma. Catalysts 2023, 13, 557. https://doi.org/10.3390/catal13030557
Kang S, Deng J, Wang X, Zhao K, Zheng M, Song D, Huang Z, Lin Y, Liu A, Zheng A, et al. Plasma-Enhanced Chemical Looping Oxidative Coupling of Methane through Synergy between Metal-Loaded Dielectric Particles and Non-Thermal Plasma. Catalysts. 2023; 13(3):557. https://doi.org/10.3390/catal13030557
Chicago/Turabian StyleKang, Shunshun, Jinlin Deng, Xiaobo Wang, Kun Zhao, Min Zheng, Da Song, Zhen Huang, Yan Lin, Anqi Liu, Anqing Zheng, and et al. 2023. "Plasma-Enhanced Chemical Looping Oxidative Coupling of Methane through Synergy between Metal-Loaded Dielectric Particles and Non-Thermal Plasma" Catalysts 13, no. 3: 557. https://doi.org/10.3390/catal13030557





