A Review on the Progress in Chemo-Enzymatic Processes for CO2 Conversion and Upcycling
Abstract
:1. Introduction
2. Overview of Chemical Methods for CO2 Conversion
2.1. Electrochemical

2.2. Thermal
3. In Vitro Conversion of CO2
3.1. CO2 Conversion via Single Enzyme In Vitro
- Conversion of CO2 by oxidoreductases;
- Conversion of CO2 by lyases;
- Conversion of CO2 by synthases.
3.1.1. Conversion of CO2 by Oxidoreductases
| Product | Enzyme | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| Bicarbonate (HCO3−) | Carbonic anhydrase | Rapid cycle |
| [50,51,52] |
| Formate (HCOO−) | Formate dehydrogenase | Obtaining value-added products |
| [53,54,55] |
| Carbon dioxide reductase |
|
| [42,43,56] | |
| Methanol (CH3OH) | Multiple dehydrogenases (FDH + FaDH + ADH) |
|
| [13,57,58] |
| Methane (CH4) | Remodeled Nitrogenase | Not inhibited by H2 |
| [59,60] |
| Carbon Monoxide (CO) | Carbon monoxide dehydrogenase |
|
| [45] |

3.1.2. Conversion of CO2 by Lyases
3.2. CO2 Conversion via Multienzyme In Vitro
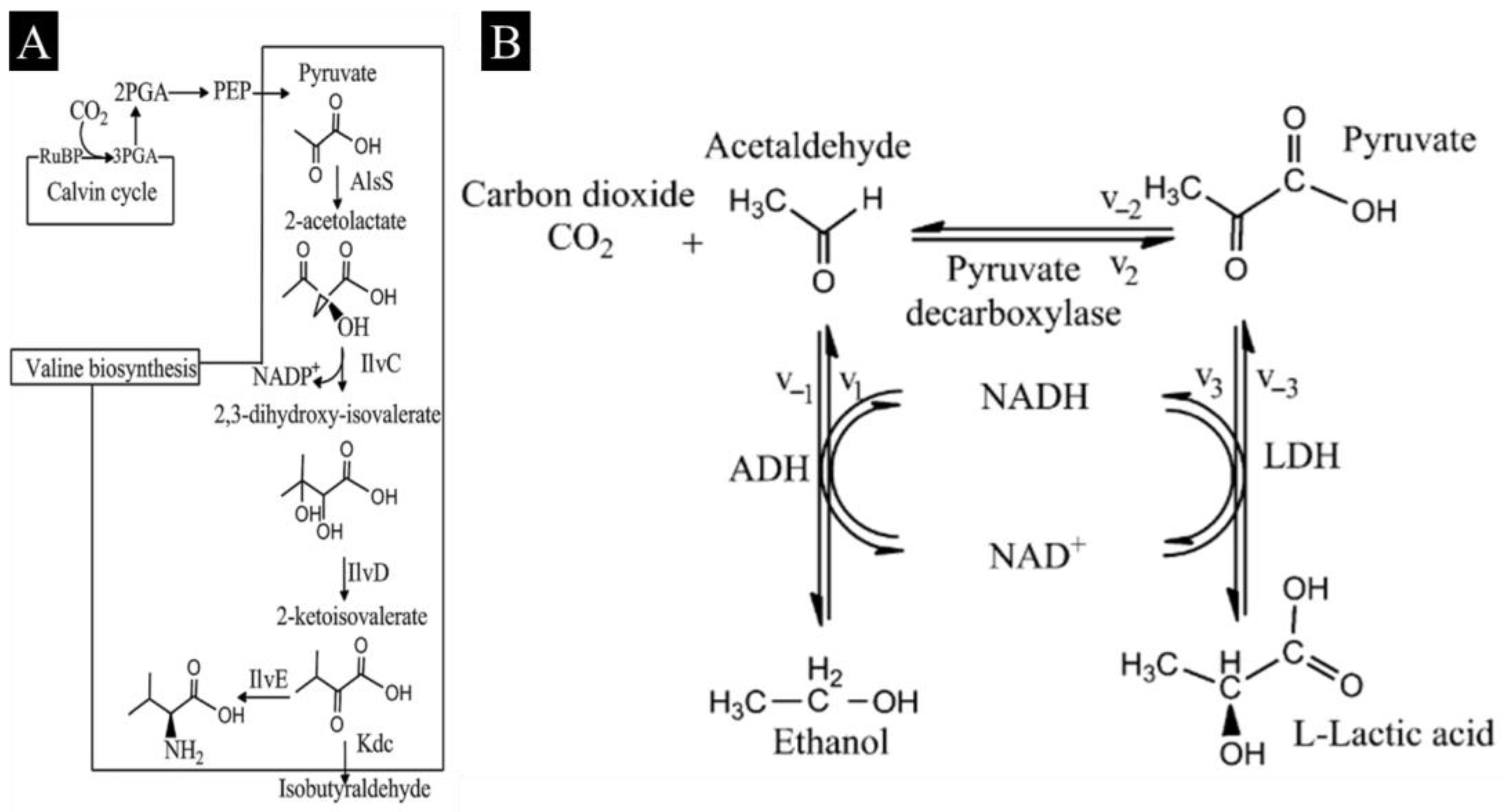
3.2.1. Conversion of CO2 to Methanol by Multiple Dehydrogenases
3.2.2. Conversion of CO2 to Other Fuels/Chemicals/Materials by Use of a Multienzyme System

4. Applications of Chemo-Enzymatic Approaches for CO2 Upcycling
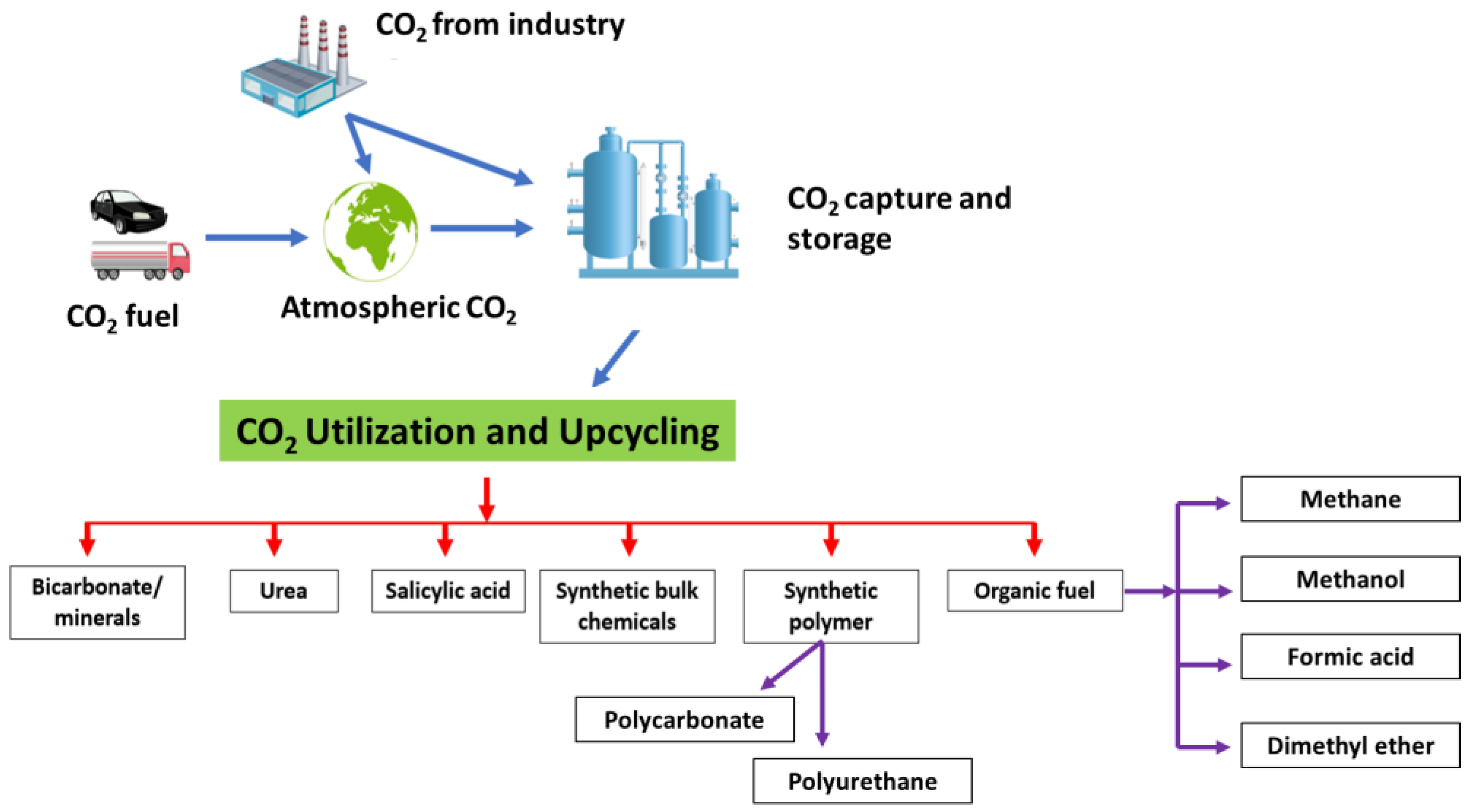
4.1. Conversion into Minerals
4.2. Conversion into Chemicals
4.3. Conversion into Organic Fuels
4.3.1. Conversion into Methane and Methanol
4.3.2. Conversion into Ethanol
4.3.3. Conversion into Formic Acid
4.3.4. Conversion into Dimethyl Ether (DME)
4.3.5. Conversion into Olefins
4.4. CO2 Utilization in Polymer Synthesis
4.4.1. Chemo-Enzymatic Synthesis of Polycarbonates
4.4.2. Chemo-Enzymatic Synthesis of Polyurethanes
5. Significance of Chemo-Enzymatic Methods in Comparison
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Najafabadi, A.T. CO2 chemical conversion to useful products: An engineering insight to the latest advances toward sustainability. Int. J. Energy Res. 2013, 37, 485–499. [Google Scholar] [CrossRef]
- Van Calster, G.; Reins, L. The Paris Agreement on Climate Change. In Paris Agreement on Climate Change: A Commentary; Edward Elgar Publishing: Cheltenham, UK, 2021; pp. 11–22. [Google Scholar] [CrossRef]
- Krzywanski, J.; Ashraf, W.M.; Czakiert, T.; Sosnowski, M.; Grabowska, K.; Zylka, A.; Kulakowska, A.; Skrobek, D.; Mistal, S.; Gao, Y. CO2 Capture by Virgin Ivy Plants Growing up on the External Covers of Houses as a Rapid Complementary Route to Achieve Global GHG Reduction Targets. Energies 2022, 15, 1683. [Google Scholar] [CrossRef]
- International Energy Agency. An Energy Sector Roadmap to Carbon Neutrality in China, An Energy Sect. Roadmap to Carbon Neutrality China; IEA: Paris, France, 2021. [Google Scholar] [CrossRef]
- Markandan, K.; Chai, W.S. Perspectives on Nanomaterials and Nanotechnology for Sustainable Bioenergy Generation. Materials 2022, 15, 7769. [Google Scholar] [CrossRef]
- Alshalif, A.F.; Irwan, J.M.; Othman, N.; Al-Gheethi, A.A.; Shamsudin, S. A systematic review on bio-sequestration of carbon dioxide in bio-concrete systems: A future direction. Eur. J. Environ. Civ. Eng. 2022, 26, 1209–1228. [Google Scholar] [CrossRef]
- Sharma, T.; Sharma, S.; Kamyab, H.; Kumar, A. Energizing the CO2 utilization by chemo-enzymatic approaches and potentiality of carbonic anhydrases: A review. J. Clean. Prod. 2020, 247, 119138. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef] [Green Version]
- Mets, L. System for the Production of Methane from CO2. European Patent EP2032709A1, 3 November 2009. [Google Scholar]
- Van Duc Long, N.; Lee, J.; Koo, K.K.; Luis, P.; Lee, M. Recent progress and novel applications in enzymatic conversion of carbon dioxide. Energies 2017, 10, 473. [Google Scholar] [CrossRef] [Green Version]
- Alpdağtaş, S.; Turunen, O.; Valjakka, J.; Binay, B. The challenges of using NAD+-dependent formate dehydrogenases for CO2 conversion. Crit. Rev. Biotechnol. 2022, 42, 953–972. [Google Scholar] [CrossRef]
- Han, G.H.; Bang, J.; Park, G.; Choe, S.; Jang, Y.J.; Jang, H.W.; Kim, S.Y.; Ahn, S.H. Recent Advances in Electrochemical, Photochemical, and Photoelectrochemical Reduction of CO2 to C2+ Products. Small 2023, 2205765. [Google Scholar] [CrossRef]
- Obert, R.; Dave, B.C. Enzymatic conversion of carbon dioxide to methanol: Enhanced methanol production in silica sol-gel matrices. J. Am. Chem. Soc. 1999, 121, 12192–12193. [Google Scholar] [CrossRef]
- Baskaya, F.S.; Zhao, X.; Flickinger, M.C.; Wang, P. Thermodynamic feasibility of enzymatic reduction of carbon dioxide to methanol. Appl. Biochem. Biotechnol. 2010, 162, 391–398. [Google Scholar] [CrossRef]
- Cazelles, R.; Drone, J.; Fajula, F.; Ersen, O.; Moldovan, S.; Galarneau, A. Reduction of CO2 to methanol by a polyenzymatic system encapsulated in phospholipids-silica nanocapsules. New J. Chem. 2013, 37, 3721–3730. [Google Scholar] [CrossRef]
- Marpani, F.; Pinelo, M.; Meyer, A.S. Enzymatic conversion of CO2 to CH3OH via reverse dehydrogenase cascade biocatalysis: Quantitative comparison of efficiencies of immobilized enzyme systems. Biochem. Eng. J. 2017, 127, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Meyer, A.S.; Mateiu, R.V.; Pinelo, M. Cascade catalysis in membranes with enzyme immobilization for multi-enzymatic conversion of CO2 to methanol. New Biotechnol. 2015, 32, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kaczur, J.J.; Sajjad, S.D.; Masel, R.I. Electrochemical conversion of CO2 to formic acid utilizing SustainionTM membranes. J. CO2 Util. 2017, 20, 208–217. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Malkhandi, S.; Yeo, B.S. Electrochemical conversion of carbon dioxide to high value chemicals using gas-diffusion electrodes. Curr. Opin. Chem. Eng. 2019, 26, 112–121. [Google Scholar] [CrossRef]
- Yuan, J.; Hao, C. Solar-driven photoelectrochemical reduction of carbon dioxide to methanol at CuInS2 thin film photocathode. Sol. Energy Mater. Sol. Cells 2013, 108, 170–174. [Google Scholar] [CrossRef]
- Yeo, J.S.; Lee, J.H.; Yoo, E.J. Electrochemical properties of environment-friendly lithium-tin liquid metal battery. Electrochim. Acta 2018, 290, 228–235. [Google Scholar] [CrossRef]
- Rosen, B.A.; Salehi-Khojin, A.; Thorson, M.R.; Zhu, W.; Whipple, D.T.; Kenis, P.J.A.; Masel, R.I. Ionic Liquid—Mediated Selective Conversion of CO2 to CO at Low Overpotentials. Science 2011, 334, 643–644. [Google Scholar] [CrossRef]
- Parkin, A.; Seravalli, J.; Vincent, K.A.; Ragsdale, S.W.; Armstrong, F.A. Rapid and efficient electrocatalytic CO2/CO interconversions by Carboxydothermus hydrogenoformans CO dehydrogenase I on an electrode. J. Am. Chem. Soc. 2007, 129, 10328–10329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, W.; Lee, S.H.; Shin, J.W.; Lee, S.P.; Kim, Y. Highly Selective Electrocatalytic Conversion of CO2 to CO at −0.57 V (NHE) by Carbon Monoxide Dehydrogenase from Moorella thermoacetica. J. Am. Chem. Soc. 2003, 125, 14688–14689. [Google Scholar] [CrossRef] [PubMed]
- Zarandi, R.F.; Rezaei, B.; Ghaziaskar, H.S.; Ensafi, A.A. Electrochemical conversion of CO2 to methanol using a glassy carbon electrode, modified by Pt@histamine-reduced graphene oxide. Int. J. Hydrogen Energy 2019, 44, 30820–30831. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Narayanan, V.A.; Yaashikaa, P.R.; Swetha, S.; Reshma, B. A comprehensive review on different approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Huang, C.; Mao, D.; Guo, X.; Yu, J. Microwave-Assisted Hydrothermal Synthesis of CuO–ZnO–ZrO2 as Catalyst for Direct Synthesis of Methanol by Carbon Dioxide Hydrogenation. Energy Technol. 2017, 5, 2100–2107. [Google Scholar] [CrossRef]
- Ud, I.; Shaharun, M.S.; Naeem, A.; Tasleem, S.; Ra, M. Carbon nano fiber-based copper/zirconia catalyst for hydrogenation of CO2 to methanol. J. CO2 Util. 2017, 21, 145–155. [Google Scholar]
- Liang, Z.; Gao, P.; Tang, Z.; Lv, M.; Sun, Y. Three dimensional porous Cu-Zn/Al foam monolithic catalyst for CO2 hydrogenation to methanol in microreactor. J. CO2 Util. 2017, 21, 191–199. [Google Scholar] [CrossRef]
- Collins, S.E.; Baltanás, M.A.; Bonivardi, A.L. An infrared study of the intermediates of methanol synthesis from carbon dioxide over Pd/β-Ga2O3. J. Catal. 2004, 226, 410–421. [Google Scholar] [CrossRef]
- Bonivardi, A.L.; Chiavassa, D.L.; Querini, C.A.; Baltanás, M.A. Enhancement of the catalytic performance to methanol synthesis from CO2/H2 by gallium addition to palladium/silica catalysts. Stud. Surf. Sci. Catal. 2000, 130, 3747–3752. [Google Scholar] [CrossRef]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Willauer, H.D. Effects of ceria-doping on a CO2 hydrogenation iron-manganese catalyst. Catal. Commun. 2010, 11, 816–819. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Catalytic thermal conversion of CO2 into fuels: Perspective and challenges. Renew. Sustain. Energy Rev. 2019, 115, 109333. [Google Scholar] [CrossRef]
- Ning, W.; Koizumi, N.; Yamada, M. Researching Fe catalyst suitable for CO2-containing syngas for Fischer-Tropsch synthesis. Energy Fuels 2009, 23, 4696–4700. [Google Scholar] [CrossRef]
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Fratalocchi, L.; Lietti, L. CO2 hydrogenation to hydrocarbons over Co and Fe-based Fischer-Tropsch catalysts. Catal. Today 2016, 277, 161–170. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Evans, J.; Feria, L.; Vidal, A.B.; Liu, P.; Nakamura, K. CO2 hydrogenation on Au/TiC, Cu/TiC, and Ni/TiC catalysts: Production of CO, methanol, and methane. J. Catal. 2013, 307, 162–169. [Google Scholar] [CrossRef]
- Asara, G.G.; Ricart, J.M.; Rodriguez, J.A.; Illas, F. Exploring the activity of a novel Au/TiC(001) model catalyst towards CO and CO2 hydrogenation. Surf. Sci. 2015, 640, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, G.; Kong, W.; Zhang, S.; Bao, Y.; Zhao, H.; Wang, L.; Zhou, L.; Jiang, Y. Electrocatalytic NAD(P)H regeneration for biosynthesis. Green Chem. Eng. 2023. [Google Scholar] [CrossRef]
- Yan, L.; Liu, G.; Liu, J.; Bai, J.; Li, Y.; Chen, H.; Zhou, L.; Gao, J.; Jiang, Y. Hierarchically porous metal organic framework immobilized formate dehydrogenase for enzyme electrocatalytic CO2 reduction. Chem. Eng. J. 2022, 450, 138164. [Google Scholar] [CrossRef]
- Reda, T.; Plugge, C.M.; Abram, N.J.; Hirst, J. Reversible interconversion of carbon dioxide and formate by an electroactive enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 10654–10658. [Google Scholar] [CrossRef] [Green Version]
- Beller, M.; Bornscheuer, U.T. CO2 Fixation through Hydrogenation by Chemical or Enzymatic Methods. Angew. Chem. Int. Ed. 2014, 53, 4527–4528. [Google Scholar] [CrossRef] [PubMed]
- Schuchmann, K.; Müller, V. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Science 2013, 342, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.F.; Himeda, Y.; Wang, W.H.; Hashiguchi, B.; Periana, R.; Szalda, D.J.; Muckerman, J.T.; Fujita, E. Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nat. Chem. 2012, 4, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, A.; Wang, V.C.C.; Woolerton, T.W.; Bell, S.; Fontecilla-Camps, J.C.; Can, M.; Ragsdale, S.W.; Chaudhary, Y.S.; Armstrong, F.A. How Light-Harvesting Semiconductors Can Alter the Bias of Reversible Electrocatalysts in Favor of H2 Production and CO2 Reduction. J. Am. Chem. Soc. 2013, 135, 15026–15032. [Google Scholar] [CrossRef] [PubMed]
- Woolerton, T.W.; Sheard, S.; Reisner, E.; Pierce, E.; Ragsdale, S.W.; Armstrong, F.A. Efficient and Clean Photoreduction of CO2 to CO by Enzyme-Modified TiO2 Nanoparticles Using Visible Light. J. Am. Chem. Soc. 2010, 132, 2132–2133. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Sheng, W.; Tao, W.; Li, Z. Direct methanol fuel cells system—A review of dual-role electrocatalysts for oxygen reduction and methanol oxidation. J. Mater. Sci. Technol. 2022, 114, 29–41. [Google Scholar] [CrossRef]
- Ünlü, A.; Duman-Özdamar, Z.E.; Çaloğlu, B.; Binay, B. Enzymes for Efficient CO2 Conversion. Protein J. 2021, 40, 489–503. [Google Scholar] [CrossRef]
- Yang, Z.; Moure, V.R.; Dean, D.R.; Seefeldt, L.C. Carbon dioxide reduction to methane and coupling with acetylene to form propylene catalyzed by remodeled nitrogenase. Proc. Natl. Acad. Sci. USA 2012, 109, 19644–19648. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.T.; Zhang, L.; Chen, H.L.; Zhang, H.M. Selective separation of low concentration CO2 using hydrogel immobilized CA enzyme based hollow fiber membrane reactors. Chem. Eng. Sci. 2010, 65, 3199–3207. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Grace, A.N.; Chu, D.H.; Nam, S.C.; Yoon, Y.; Yoon, S.H.; Jeong, S.K. CO2 absorption and sequestration as various polymorphs of CaCO3 using sterically hindered amine. Langmuir 2013, 29, 15655–15663. [Google Scholar] [CrossRef]
- Annunziato, G.; Angeli, A.; D’Alba, F.; Bruno, A.; Pieroni, M.; Vullo, D.; De Luca, V.; Capasso, C.; Supuran, C.T.; Costantino, G. Discovery of New Potential Anti-Infective Compounds Based on Carbonic Anhydrase Inhibitors by Rational Target-Focused Repurposing Approaches. ChemMedChem 2016, 11, 1904–1914. [Google Scholar] [CrossRef]
- Thauer, R.K. A fifth pathway of carbon fixation. Science 2007, 318, 1732–1733. [Google Scholar] [CrossRef]
- Castillo, R.; Oliva, M.; Martí, S.; Moliner, V. A theoretical study of the catalytic mechanism of formate dehydrogenase. J. Phys. Chem. B 2008, 112, 10013–10022. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.K.; Baeg, J.O.; Oh, G.H.; Park, N.J.; Kong, K.J.; Kim, J.; Hwang, D.W.; Biswas, S.K. A photocatalyst-enzyme coupled artificial photosynthesis system for solar energy in production of formic acid from CO2. J. Am. Chem. Soc. 2012, 134, 11455–11461. [Google Scholar] [CrossRef]
- Ceccaldi, P.; Schuchmann, K.; Müller, V.; Elliott, S.J. The Hydrogen Dependent CO2 reductase: The first completely co tolerant fefe-hydrogenase. Energy Environ. Sci. 2017, 10, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Z.; Shi, J.; Wu, H.; Jiang, Z.; Zhang, W.; Song, X.; Ai, Q. Bioinspired approach to multienzyme cascade system construction for efficient carbon dioxide reduction. ACS Catal. 2014, 4, 962–972. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Stufano, P.; MacYk, W.; Baran, T.; Fragale, C.; Costa, M.; Aresta, M. Hybrid technologies for an enhanced carbon recycling based on the enzymatic reduction of CO2 to methanol in water: Chemical and photochemical NADH regeneration. ChemSusChem 2012, 5, 373–378. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Ensign, S.A.; Rasche, M.E. Carbonyl Sulfide and Carbon Dioxide as new Substrates, and Carbon Disulfide as a new Inhibitor, of Nitrogenase. Biochemistry 1995, 34, 5382–5389. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Yang, Z.Y.; Lukoyanov, D.A.; Harris, D.F.; Dean, D.R.; Raugei, S.; Hoffman, B.M. Reduction of Substrates by Nitrogenases. Chem. Rev. 2020, 120, 5082–5106. [Google Scholar] [CrossRef]
- Wu, Z.; Nan, Y.; Zhao, Y.; Wang, X.; Huang, S.; Shi, J. Immobilization of carbonic anhydrase for facilitated CO2 capture and separation. Chin. J. Chem. Eng. 2020, 28, 2817–2831. [Google Scholar] [CrossRef]
- Jensen, E.L.; Clement, R.; Kosta, A.; Maberly, S.C.; Gontero, B. A new widespread subclass of carbonic anhydrase in marine phytoplankton. ISME J. 2019, 13, 2094–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuffaro, D.; Nuti, E.; Rossello, A. An overview of carbohydrate-based carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 1906–1922. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, Y.; Jiang, Z.; Wang, X.; Wang, X.; Zhang, S.; Han, P.; Yang, C. Enzymatic conversion of carbon dioxide. Chem. Soc. Rev. 2015, 44, 5981–6000. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, H.; Lu, Y. Enhanced stability and chemical resistance of a new nanoscale biocatalyst for accelerating CO2 absorption into a carbonate solution. Environ. Sci. Technol. 2013, 47, 13882–13888. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Gang, H.; Chung, J.; Gu, M.B. Carbonic anhydrase assisted calcium carbonate crystalline composites as a biocatalyst. Green Chem. 2012, 14, 2216–2220. [Google Scholar] [CrossRef]
- Forsyth, C.; Yip, T.W.S.; Patwardhan, S.V. CO2 sequestration by enzyme immobilized onto bioinspired silica. Chem. Commun. 2013, 49, 3191–3193. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, S.; Van Hest, J.C.M. Multienzyme systems: Bringing enzymes together in vitro. Soft Matter 2012, 8, 1736–1746. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Chen, Y.; Tan, T.; Nielsen, J. Third-generation biorefineries as the means to produce fuels and chemicals from CO2. Nat. Catal. 2020, 3, 274–288. [Google Scholar] [CrossRef]
- Alissandratos, A.; Easton, C.J. Biocatalysis for the application of CO2 as a chemical feedstock. Beilstein J. Org. Chem. 2015, 11, 2370–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, E.T.; Lee, S. Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Dunn, M.F.; Niks, D.; Ngo, H.; Barends, T.R.M.; Schlichting, I. Tryptophan synthase: The workings of a channeling nanomachine. Trends Biochem. Sci. 2008, 33, 254–264. [Google Scholar] [CrossRef]
- Conrado, R.J.; Varner, J.D.; DeLisa, M.P. Engineering the spatial organization of metabolic enzymes: Mimicking nature’s synergy. Curr. Opin. Biotechnol. 2008, 19, 492–499. [Google Scholar] [CrossRef]
- El-Zahab, B.; Donnelly, D.; Wang, P. Particle-Tethered NADH for Production of Methanol from CO2 Catalyzed by Coimmobilized Enzymes. Biotechnol. Bioeng. 2008, 99, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Schlager, S.; Dumitru, L.M.; Haberbauer, M.; Fuchsbauer, A.; Neugebauer, H.; Hiemetsberger, D.; Wagner, A.; Portenkirchner, E.; Sariciftci, N.S. Electrochemical Reduction of Carbon Dioxide to Methanol by Direct Injection of Electrons into Immobilized Enzymes on a Modified Electrode. ChemSusChem 2016, 9, 631–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuk, S.K.; Singh, R.K.; Nam, D.H.; Singh, R.; Lee, J.-K.; Park, C.B. Photoelectrochemical Reduction of Carbon Dioxide to Methanol through a Highly Efficient Enzyme Cascade. Angew. Chemie Int. Ed. 2017, 56, 3827–3832. [Google Scholar] [CrossRef] [PubMed]
- Netto, C.G.C.M.; Andrade, L.H.; Toma, H.E. Carbon dioxide/methanol conversion cycle based on cascade enzymatic reactions supported on superparamagnetic nanoparticles. An. Acad. Bras. Cienc. 2018, 90, 593–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, X.; El-Zahab, B.; Zhao, X.; Liu, Y.; Wang, P. Enzymatic synthesis of L-lactic acid from carbon dioxide and ethanol with an inherent cofactor regeneration cycle. Biotechnol. Bioeng. 2011, 108, 465–469. [Google Scholar] [CrossRef]
- Atsumi, S.; Higashide, W.; Liao, J.C. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 2009, 27, 1177–1180. [Google Scholar] [CrossRef]
- Damiani, D.; Litynski, J.T.; McIlvried, H.G.; Vikara, D.M.; Srivastava, R.D. The US department of Energy’s R&D program to reduce greenhouse gas emissions through beneficial uses of carbon dioxide. Greenh. Gases Sci. Technol. 2012, 2, 9–16. [Google Scholar] [CrossRef]
- Biermann, M.; Gruß, H.; Hummel, W.; Gröger, H. Guerbet Alcohols: From Processes under Harsh Conditions to Synthesis at Room Temperature under Ambient Pressure. ChemCatChem 2016, 8, 895–899. [Google Scholar] [CrossRef]
- Alper, E.; Orhan, O.Y. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Xu, S.; Lu, Y.; Li, J.; Jiang, Z.; Wu, H. Efficient Conversion of CO2 to Methanol Catalyzed by Three Dehydrogenases Co-Encapsulated in an Alginate-Silica (ALG-SiO2) Hybrid Gel. Ind. Eng. Chem. Res. 2006, 45, 4567–4573. [Google Scholar] [CrossRef]
- Yang, S.; Peng, S.; Xu, J.; Luo, Y.; Li, D. Methane and nitrous oxide emissions from paddy field as affected by water-saving irrigation. Phys. Chem. Earth Parts A/B/C 2012, 53–54, 30–37. [Google Scholar] [CrossRef]
- Yong, J.K.J.; Stevens, G.W.; Caruso, F.; Kentish, S.E. The use of carbonic anhydrase to accelerate carbon dioxide capture processes. J. Chem. Technol. Biotechnol. 2015, 90, 3–10. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef] [Green Version]
- Bahruji, H.; Armstrong, R.D.; Esquius, J.R.; Jones, W.; Bowker, M.; Hutchings, G.J. Hydrogenation of CO2 to Dimethyl Ether over Brønsted Acidic PdZn Catalysts. Ind. Eng. Chem. Res. 2018, 57, 6821–6829. [Google Scholar] [CrossRef]
- Cai, T.; Sun, H.; Qiao, J.; Zhu, L.; Zhang, F.; Zhang, J.; Tang, Z.; Wei, X.; Yang, J.; Yuan, Q.; et al. Cell-free chemo-enzymatic starch synthesis from carbon dioxide. Science 2021, 373, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Desmons, S.; Grayson-Steel, K.; Nuñez-Dallos, N.; Vendier, L.; Hurtado, J.; Clapés, P.; Fauré, R.; Dumon, C.; Bontemps, S. Enantioselective Reductive Oligomerization of Carbon Dioxide into l-Erythrulose via a Chemo-enzymatic Catalysis. J. Am. Chem. Soc. 2021, 143, 16274–16283. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castañera, F.; Álvarez-Rodríguez, J.; Candela, N.; Maroto-Valiente, Á. First Phenol Carboxylation with CO2 on Carbon Nanostructured C@Fe-Al2O3 Hybrids in Aqueous Media under Mild Conditions. Nanomaterials 2021, 11, 190. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, X.; Wen, X.; Zhou, Y.; Zhou, L.; Li, H.; Tao, L.; Li, Q.; Du, S.; Liu, T.; et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 2020, 12, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Mazari, S.A.; Hossain, N.; Basirun, W.J.; Mubarak, N.M.; Abro, R.; Sabzoi, N.; Shah, A. An overview of catalytic conversion of CO2 into fuels and chemicals using metal organic frameworks. Process Saf. Environ. Prot. 2021, 149, 67–92. [Google Scholar] [CrossRef]
- He, Z.; Qian, Q.; Ma, J.; Meng, Q.; Zhou, H.; Song, J.; Liu, Z.; Han, B. Water-Enhanced Synthesis of Higher Alcohols from CO2 Hydrogenation over a Pt/Co3O4 Catalyst under Milder Conditions. Angew. Chem. Int. Ed. 2016, 55, 737–741. [Google Scholar] [CrossRef]
- Zhu, Q. Developments on CO2-utilization technologies. Clean Energy 2019, 3, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Guild, C.; Suib, S.L. Thermal, electrochemical, and photochemical conversion of CO2 to fuels and value-added products. J. CO2 Util. 2013, 2, 64. [Google Scholar] [CrossRef]
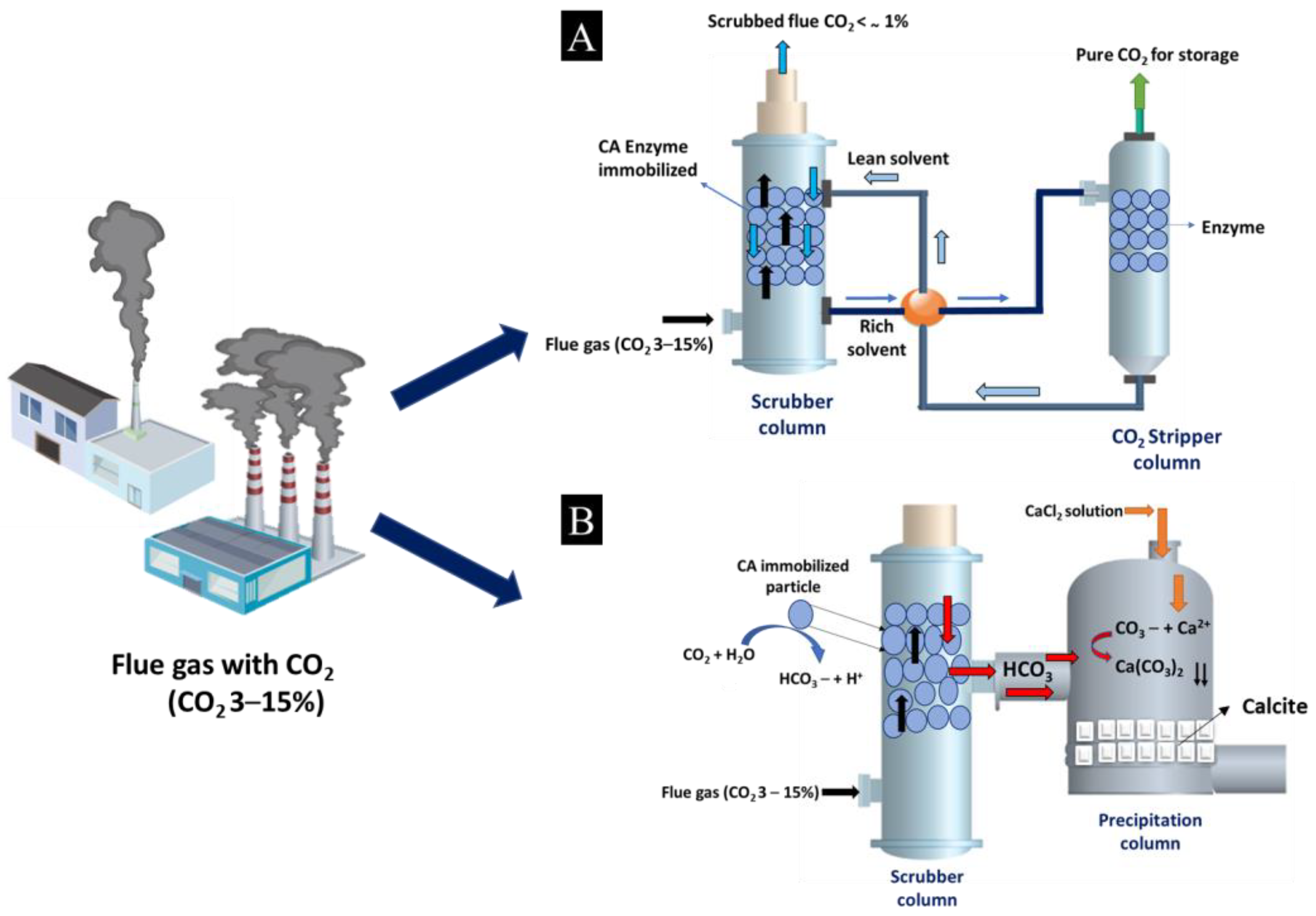
- Gundersen, M.T.; Von Solms, N.; Woodley, J.M. Enzymatically assisted CO2 removal from flue-gas. Energy Procedia 2014, 63, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Kanth, B.K.; Lee, J.; Pack, S.P. Carbonic anhydrase: Its biocatalytic mechanisms and functional properties for efficient CO2 capture process development. Eng. Life Sci. 2013, 13, 422–431. [Google Scholar] [CrossRef]
- Hargis, C.W.; Chen, I.A.; Devenney, M.; Fernandez, M.J.; Gilliam, R.J.; Thatcher, R.P. Calcium Carbonate Cement: A Carbon Capture, Utilization, and Storage (CCUS) Technique. Materials 2021, 14, 2709. [Google Scholar] [CrossRef] [PubMed]
- Chafik, A.; El Hassani, K.; Essamadi, A.; Çelik, S.Y.; Mavi, A. Efficient sequestration of carbon dioxide into calcium carbonate using a novel carbonic anhydrase purified from liver of camel (Camelus dromedarius). J. CO2 Util. 2020, 42, 101310. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Schlossmacher, U.; Schröder, H.C.; Lieberwirth, I.; Glasser, G.; Korzhev, M.; Neufurth, M.; Wang, X. Enzyme-accelerated and structure-guided crystallization of calcium carbonate: Role of the carbonic anhydrase in the homologous system. Acta Biomater. 2014, 10, 450–462. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzym. Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef]
- Kim, H.S.; Hong, S.-G.; Woo, K.M.; Seijas, V.T.; Kim, S.; Lee, J.; Kim, J. Precipitation-Based Nanoscale Enzyme Reactor with Improved Loading, Stability, and Mass Transfer for Enzymatic CO2 Conversion and Utilization. ACS Catal. 2018, 8, 6526–6536. [Google Scholar] [CrossRef]
- Effendi, S.S.W.; Chiu, C.-Y.; Chang, Y.-K.; Ng, I.-S. Crosslinked on novel nanofibers with thermophilic carbonic anhydrase for carbon dioxide sequestration. Int. J. Biol. Macromol. 2020, 152, 930–938. [Google Scholar] [CrossRef]
- Lim, H.K.; Kim, D.R.; Hwang, I.T. Sequestration of CO2 into CaCO3 using Carbonic Anhydrase Immobilization on Functionalized Aluminum Oxide. Appl. Biochem. Microbiol. 2019, 55, 375–379. [Google Scholar] [CrossRef]
- Bednár, A.; Nemestóthy, N.; Bakonyi, P.; Fülöp, L.; Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K.; Bélafi-Bakó, K. Enzymatically-boosted ionic liquid gas separation membranes using carbonic anhydrase of biomass origin. Chem. Eng. J. 2016, 303, 621–626. [Google Scholar] [CrossRef]
- Duan, L.; Li, H.; Zhang, Y. Synthesis of Hybrid Nanoflower-Based Carbonic Anhydrase for Enhanced Biocatalytic Activity and Stability. ACS Omega 2018, 3, 18234–18241. [Google Scholar] [CrossRef] [Green Version]
- Heuer, J.; Kraus, Y.; Vučak, M.; Zeng, A.P. Enhanced sequestration of carbon dioxide into calcium carbonate using pressure and a carbonic anhydrase from alkaliphilic Coleofasciculus chthonoplastes. Eng. Life Sci. 2022, 22, 178–191. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Abu-Reesh, I.M.; Mohanakrishna, G.; Bulut, M.; Pant, D. Advanced Routes of Biological and Bio-Electrocatalytic Carbon Dioxide (CO2) Mitigation toward Carbon Neutrality. Front. Energy Res. 2020, 8, 94. [Google Scholar] [CrossRef]
- Kim, H.S.; Hong, S.-G.; Yang, J.; Ju, Y.; Ok, J.; Kwon, S.-J.; Yeon, K.-M.; Dordick, J.S.; Kim, J. 3D-Printed interfacial devices for biocatalytic CO2 conversion at gas-liquid interface. J. CO2 Util. 2020, 38, 291–298. [Google Scholar] [CrossRef]
- Gao, H. A study on the spatio-temporal evolution of Tibetan inbound tourism flow network based on social networks. Proceedings of 2021 the International Conference on Environmental Remote Sensing and Big Data (ERSBD 2021), Wuhan, China, 29–31 October 2021; He, Y., Weng, C.-H., Eds.; SPIE: Bellingham, WA, USA, 2021; p. 42. [Google Scholar] [CrossRef]
- Iijima, T.; Yamaguchi, T. Efficient regioselective carboxylation of phenol to salicylic acid with supercritical CO2 in the presence of aluminium bromide. J. Mol. Catal. A Chem. 2008, 295, 52–56. [Google Scholar] [CrossRef]
- Iijima, T.; Yamaguchi, T. K2CO3-catalyzed direct synthesis of salicylic acid from phenol and supercritical CO2. Appl. Catal. A Gen. 2008, 345, 12–17. [Google Scholar] [CrossRef]
- Longtai, L.; Biao, G.; Xuebin, L.; Gang, W.; Limin, G. Research progress on hydrogenation of carbon dioxide. Ind. Catal. 2021, 29, 1–10. [Google Scholar] [CrossRef]
- Martins, J.A.; Miguel, C.V.; Rodrigues, A.E.; Madeira, L.M. Novel Adsorption-Reaction Process for Biomethane Purification/Production and Renewable Energy Storage. ACS Sustain. Chem. Eng. 2022, 10, 7833–7851. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research progress on CO2 capture and utilization technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.G. CO2 Hydrogenation to Methanol over ZrO2-Containing Catalysts: Insights into ZrO2 Induced Synergy. ACS Catal. 2019, 9, 7840–7861. [Google Scholar] [CrossRef]
- Lau, N.-S.; Matsui, M.; Abdullah, A.A.-A. Cyanobacteria: Photoautotrophic Microbial Factories for the Sustainable Synthesis of Industrial Products. BioMed Res. Int. 2015, 2015, 754934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dexter, J.; Fu, P. Metabolic engineering of cyanobacteria for ethanol production. Energy Environ. Sci. 2009, 2, 857. [Google Scholar] [CrossRef]
- Moret, S.; Dyson, P.J.; Laurenczy, G. Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. Nat. Commun. 2014, 5, 4017. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Joó, F. Free formic acid by hydrogenation of carbon dioxide in sodium formate solutions. Catal. Commun. 2011, 14, 74–76. [Google Scholar] [CrossRef]
- Chai, M.; Bazaz, S.R.; Daiyan, R.; Razmjou, A.; Warkiani, M.E.; Amal, R.; Chen, V. Biocatalytic micromixer coated with enzyme-MOF thin film for CO2 conversion to formic acid. Chem. Eng. J. 2021, 426, 130856. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 Recycling to Dimethyl Ether: State-of-the-Art and Perspectives. Molecules 2017, 23, 31. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Lu, W.; Wang, M.; Xu, X. Low-temperature synthesis of DME from CO2/H2 over Pd-modified CuO–ZnO–Al2O3–ZrO2/HZSM-5 catalysts. Catal. Commun. 2004, 5, 367–370. [Google Scholar] [CrossRef]
- Fang, X.; Jia, H.; Zhang, B.; Li, Y.; Wang, Y.; Song, Y.; Du, T.; Liu, L. A novel in situ grown Cu-ZnO-ZrO2/HZSM-5 hybrid catalyst for CO2 hydrogenation to liquid fuels of methanol and DME. J. Environ. Chem. Eng. 2021, 9, 105299. [Google Scholar] [CrossRef]
- Ren, S.; Fan, X.; Shang, Z.; Shoemaker, W.R.; Ma, L.; Wu, T.; Li, S.; Klinghoffer, N.B.; Yu, M.; Liang, X. Enhanced catalytic performance of Zr modified CuO/ZnO/Al2O3 catalyst for methanol and DME synthesis via CO2 hydrogenation. J. CO2 Util. 2020, 36, 82–95. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Mota, N.; Ordoñez, E.M.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Direct Synthesis of Dimethyl Ether from CO2: Recent Advances in Bifunctional/Hybrid Catalytic Systems. Catalysts 2021, 11, 411. [Google Scholar] [CrossRef]
- Pawelec, B.; Guil-López, R.; Mota, N.; Fierro, J.; Yerga, R.N. Catalysts for the Conversion of CO2 to Low Molecular Weight Olefins—A Review. Materials 2021, 14, 6952. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Porosoff, M.D. Development of Tandem Catalysts for CO2 Hydrogenation to Olefins. ACS Catal. 2019, 9, 2639–2656. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Ji, X.; Wei, J.; Wen, Z.; Yao, R.; Xu, H.; Ge, Q. Directly converting carbon dioxide to linear α-olefins on bio-promoted catalysts. Commun. Chem. 2018, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Wang, X.; Wang, F. Synthesis and properties of carbon dioxide based copolymers. Sci. Sin. Chim. 2018, 48, 883–893. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.-L.; Wu, H.-W.; Lin, Z.-I.; Liao, M.-G.; Su, Y.-C.; Chen, C.-K.; Ko, B.-T. Synthesis of functional CO2-based polycarbonates via dinuclear nickel nitrophenolate-based catalysis for degradable surfactant and drug-loaded nanoparticle applications. Polym. Chem. 2021, 12, 1244–1259. [Google Scholar] [CrossRef]
- Shuai, L.; Xingyuan, M. Research Progress of Blocked Solvent-Free Polyurethane. Mater. Sustain. Dev. Green Manuf. Process. Mater. 2019, 33, 3892–3899. [Google Scholar]
- Ying, Z.; Zhang, C.; Jiang, S.; Wu, Q.; Zhang, B.; Yu, Y.; Lan, M.; Cheng, H.; Zhao, F. Synthesis of a novel hydrophobic polyurea gel from CO2 and amino-modified polysiloxane. J. CO2 Util. 2016, 15, 131–135. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, H.-Y.; Shi, R.-H.; Wu, P.-X.; Lin, W.-W.; Zhang, C.; Arai, M.; Zhao, F.-Y. Direct Synthesis of Polyurea Thermoplastics from CO2 and Diamines. ACS Appl. Mater. Interfaces 2019, 11, 47413–47421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sha, F.; Li, Y.; Zhang, J. Synthesis of polyurethane based on indirect utilization strategy of CO2. J. Environ. Chem. Eng. 2021, 9, 105309. [Google Scholar] [CrossRef]
- Spinner, N.S.; Vega, J.A.; Mustain, W.E. Recent progress in the electrochemical conversion and utilization of CO2. Catal. Sci. Technol. 2012, 2, 19–28. [Google Scholar] [CrossRef]
- Hong, S.G.; Jeon, H.; Kim, H.S.; Jun, S.H.; Jin, E.; Kim, J. One-pot enzymatic conversion of carbon dioxide and utilization for improved microbial growth. Environ. Sci. Technol. 2015, 49, 4466–4472. [Google Scholar] [CrossRef] [PubMed]



| Catalyst | Products | Ref. |
|---|---|---|
| Carboxydothermus hydrogenoformans CO Dehydrogenase I [Ch Ni-CODH I] | Carbon monoxide (CO) | [24] |
| Dehydrogenases in an ALG−SiO2 | Methanol | [83] |
| Formate Dehydrogenase | Formate | [41] |
| Nitrogenase | Methane | [84] |
| Carbonic anhydrase | Bicarbonate (HCO3−) | [85] |
| Nanocatalyst (Na–Fe3O4/HZSM-5) | Gasoline | [86] |
| Nanoparticles (PdZn/TiO2) | Dimethyl Ether | [87] |
| Inorganic catalyst ZnO-ZrO | Starch | [88] |
| Formolase(FLS) and D-fructose-6-phosphate aldolase (FSA) | Carbohydrate (l-Erythrulose) | [89] |
| Hybrid nanocatalyst (C@Fe–Al2O3) | Salicylic acid | [90] |
| PdCu alloy nanoparticles | Urea | [91] |
| Metallic heterogenous catalyst | Cyclo carbonates | [92] |
| Cu–Ru–Metallic Organic Framework (MOF) | Ethanol | [92] |
| Platinum/cobalt catalysts | Butanol-based fuel | [93] |
| Approaches | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Electrochemical |
|
| [94] |
| Thermal |
|
| [34,95] |
| Chemo-enzymatic |
|
| [27,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markandan, K.; Sankaran, R.; Wei Tiong, Y.; Siddiqui, H.; Khalid, M.; Malik, S.; Rustagi, S. A Review on the Progress in Chemo-Enzymatic Processes for CO2 Conversion and Upcycling. Catalysts 2023, 13, 611. https://doi.org/10.3390/catal13030611
Markandan K, Sankaran R, Wei Tiong Y, Siddiqui H, Khalid M, Malik S, Rustagi S. A Review on the Progress in Chemo-Enzymatic Processes for CO2 Conversion and Upcycling. Catalysts. 2023; 13(3):611. https://doi.org/10.3390/catal13030611
Chicago/Turabian StyleMarkandan, Kalaimani, Revathy Sankaran, Yong Wei Tiong, Humaira Siddiqui, Mohammad Khalid, Sumira Malik, and Sarvesh Rustagi. 2023. "A Review on the Progress in Chemo-Enzymatic Processes for CO2 Conversion and Upcycling" Catalysts 13, no. 3: 611. https://doi.org/10.3390/catal13030611






