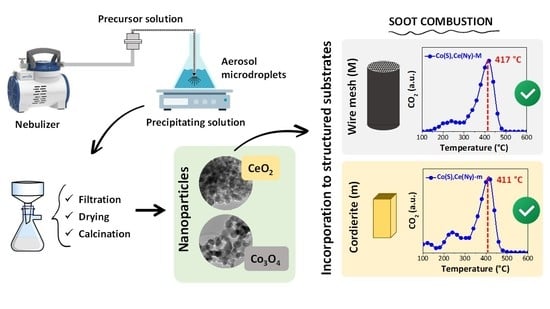
Synthesis of Co,Ce Oxide Nanoparticles Using an Aerosol Method and Their Deposition on Different Structured Substrates for Catalytic Removal of Diesel Particulate Matter
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesized Particles
2.1.1. Morphology of the Synthesized Particles
2.1.2. Catalytic Activity of Synthesized Particles
2.2. Structured Catalysts
2.2.1. Catalytic Coating
2.2.2. Catalytic Tests of Structured Systems
3. Materials and Methods
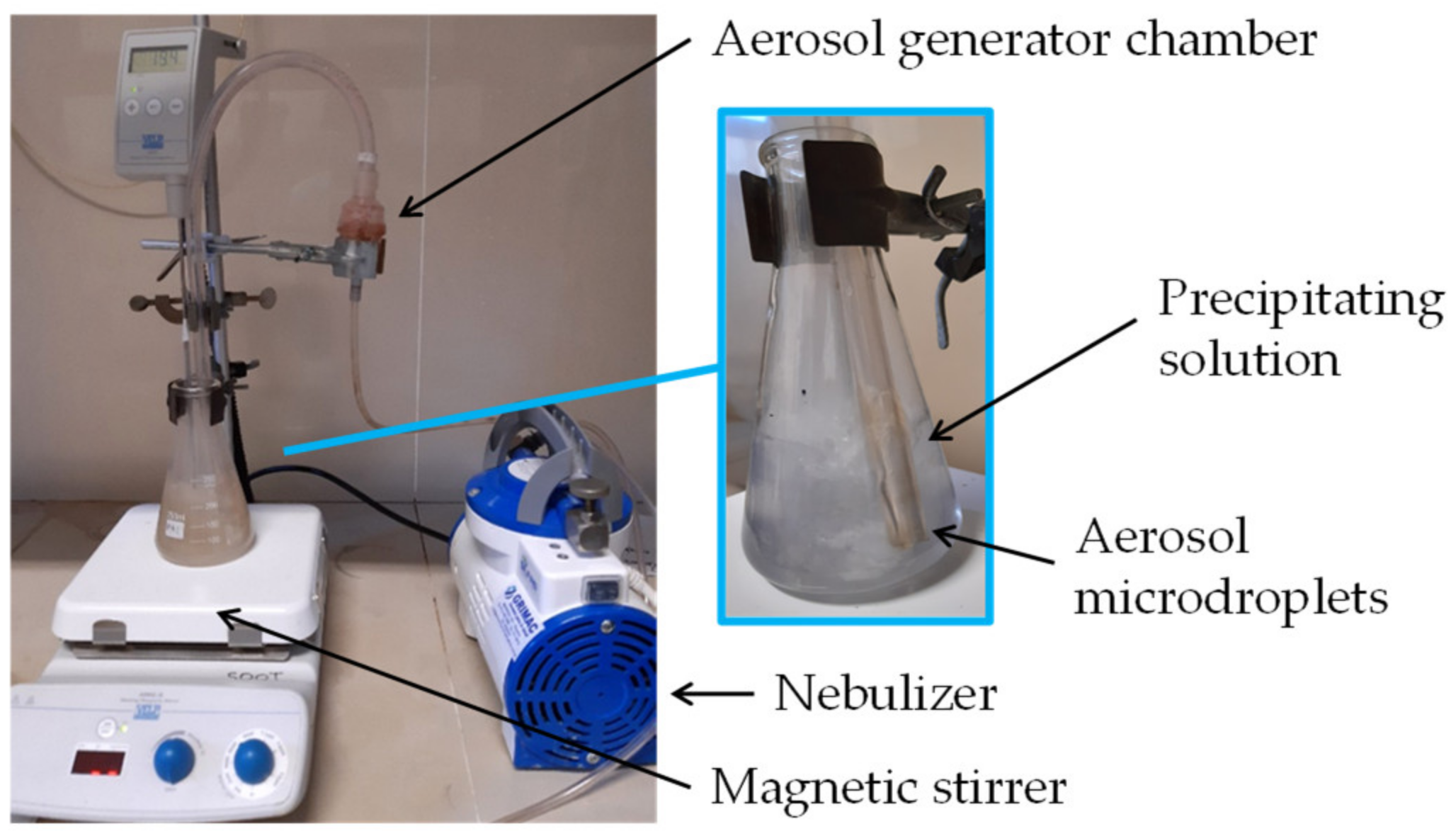
3.1. Oxide Particle Synthesis
3.2. Preparation of Structured Substrates
3.3. Deposition of Oxide Particles on Structured Substrates
3.3.1. CeO2 Suspensions
- Only commercial colloidal suspension of CeO2 nanoparticles (Ce(Ny)—Nyacol®, 20 wt./wt. %, dparticle = 10–20 nm, pH = 3);
- Synthesized CeO2 particles Ce(S) and Ce(Ny);
- Commercial CeO2 nanoparticles Ce(C) (Sigma Aldrich®, dparticle < 50 nm) and Ce(Ny).
3.3.2. Co,Ce Suspensions
- Synthesized Co3O4 particles Co(S) and Ce(Ny);
- Commercial Co3O4 nanoparticles Co(C) (Sigma Aldrich®, dparticle < 50 nm) and Ce(Ny);
- Co(S), Ce(S) and Ce(Ny);
- Co(C), Ce(C) and Ce(Ny) nanoparticles.
3.4. Characterization Techniques
3.5. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yusuf, A.A.; Dandakouta, H.; Yahuza, I.; Yusuf, D.A.; Mujtaba, M.A.; El-Shafay, A.S.; Soudagar, M.E.M. Effect of Low CeO2 Nanoparticles Dosage in Biodiesel-Blends on Combustion Parameters and Toxic Pollutants from Common-Rail Diesel Engine. Atmos. Pollut. Res. 2022, 13, 101305. [Google Scholar] [CrossRef]
- Serrano, J.R.; Novella, R.; Piqueras, P. Why the Development of Internal Combustion Engines is Still Necessary to Fight against Global Climate Change from the Perspective of Transportation. Appl. Sci. 2019, 9, 4527. [Google Scholar] [CrossRef]
- Rentería, J.; Gallego, A.; Gamboa, D.; Cacua, K.; Herrera, B. Effect of Amide-Functionalized Carbon Nanotubes as Commercial Diesel and Palm-oil Biodiesel Additives on the Ignition Delay: A Study on Droplet Scale. Fuel 2023, 338, 127202. [Google Scholar] [CrossRef]
- Ye, J.; E, J.; Peng, Q. Effects of Porosity Setting and Multilayers of Diesel Particulate Filter on the Improvement of Regeneration Performance. Energy 2022, 263, 126063. [Google Scholar] [CrossRef]
- Liu, C.; Nie, W.; Luo, C.; Hua, Y.; Yu, F.; Niu, W.; Zhang, X.; Zhang, S.; Xue, Q.; Sun, N.; et al. Numerical Study on Temporal and Spatial Distribution of Particulate Matter under Multi-Vehicle Working Conditions. SSRN Electron. J. 2022, 862, 160710. [Google Scholar] [CrossRef]
- Franklin, B.A.; Brook, R.; Arden Pope, C. Air Pollution and Cardiovascular Disease. Curr. Probl. Cardiol. 2015, 40, 207–238. [Google Scholar] [CrossRef]
- Kwon, M.; Jung, J.; Park, H.S.; Kim, N.H.; Lee, J.; Park, J.; Kim, Y.; Shin, S.; Lee, B.S.; Cheong, Y.H.; et al. Diesel Exhaust Particle Exposure Accelerates Oxidative DNA Damage and Cytotoxicity in Normal Human Bronchial Epithelial Cells through PD-L1. Environ. Pollut. 2023, 317, 120705. [Google Scholar] [CrossRef]
- Saxena, P.; Naik, V. (Eds.) Air Pollution: Sources, Impacts and Controls; CABI: London, UK, 2019. [Google Scholar] [CrossRef]
- Huang, Y.; Unger, N.; Harper, K.; Heyes, C. Global Climate and Human Health Effects of the Gasoline and Diesel Vehicle Fleets. Geohealth 2020, 4, e2019GH000240. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Sun, Z.; Chen, Y. Ambient fine particulate matter and cancer: Current evidence and future perspectives. Chem. Res. Toxicol. 2022, 36, 141–156. [Google Scholar] [CrossRef]
- Learn about Impacts of Diesel Exhaust and the Diesel Emissions Reduction Act (DERA)|US EPA. Available online: https://www.epa.gov/dera/learn-about-impacts-diesel-exhaust-and-diesel-emissions-reduction-act-dera (accessed on 25 January 2023).
- Kurien, C.; Srivastava, A.K.; Gandigudi, N.; Anand, K. Soot Deposition Effects and Microwave Regeneration Modelling of Diesel Particulate Filtration System. J. Energy Inst. 2020, 93, 463–473. [Google Scholar] [CrossRef]
- Martinovic, F.; Castoldi, L.; Deorsola, F.A. Aftertreatment Technologies for Diesel Engines: An Overview of the Combined Systems. Catalysts 2021, 11, 653. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, D.; Hu, Z.; Tan, P. Experimental Investigation into the Number, Micromorphology, Functional Group Composition, and Oxidation Activity of Particles from Diesel Engine with Biodiesel Blend. Fuel 2021, 289, 119902. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, D.; Hu, Z.; Tan, P. Particle Number, Size Distribution, Carbons, Polycyclic Aromatic Hydrocarbons and Inorganic Ions of Exhaust Particles from a Diesel Bus Fueled with Biodiesel Blends. J. Clean. Prod. 2019, 225, 627–636. [Google Scholar] [CrossRef]
- Orihuela, M.P.; Chacartegui, R.; Gómez-Martín, A.; Ramírez-Rico, J.; Becerra Villanueva, J.A. Performance Trends in Wall-Flow Diesel Particulate Filters: Comparative Analysis of Their Filtration Efficiency and Pressure Drop. J. Clean. Prod. 2020, 260, 120863. [Google Scholar] [CrossRef]
- Joshi, A. Review of Vehicle Engine Efficiency and Emissions. SAE Int. J. Adv. Curr. Pract. Mobil. 2019, 2, 734–761. [Google Scholar]
- Fayyazbakhsh, A.; Bell, M.L.; Zhu, X.; Mei, X.; Koutný, M.; Hajinajaf, N.; Zhang, Y. Engine Emissions with Air Pollutants and Greenhouse Gases and Their Control Technologies. J. Clean. Prod. 2022, 376, 134260. [Google Scholar] [CrossRef]
- Yi, C.; Fang, J.; Pu, P.; Yang, Y.; Chen, Z.; Zuo, Z.; Han, Z. Insight into Catalytic Activity of K-Ce Catalysts and K-Ce Based Mixed Catalysts on Diesel Soot Combustion. Mol. Catal. 2023, 535, 112905. [Google Scholar] [CrossRef]
- Zhao, K.; Li, J.; Wang, L.; Li, D.; Liu, B.; Li, R.; Yu, X.; Wei, Y.; Liu, J.; Zhao, Z. Preparation of Cordierite Monolith Catalysts with the Coating of K-Modified Spinel MnCo2O4 Oxide and Their Catalytic Performances for Soot Combustion. Catalysts 2022, 12, 295. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Lisi, L.; Saliva, A.; Di Benedetto, A. Catalytic Diesel Particulate Filters with Highly Dispersed Ceria: Effect of the Soot-Catalyst Contact on the Regeneration Performance. Appl. Catal. B 2016, 197, 116–124. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A Review on Oxygen Storage Capacity of CeO2-Based Materials: Influence Factors, Measurement Techniques, and Applications in Reactions Related to Catalytic Automotive Emissions Control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Araiza, D.G.; Gómez-Cortés, A.; Díaz, G. Effect of Ceria Morphology on the Carbon Deposition during Steam Reforming of Ethanol over Ni/CeO2 Catalysts. Catal. Today 2020, 349, 235–243. [Google Scholar] [CrossRef]
- Jampaiah, D.; Velisoju, V.K.; Devaiah, D.; Singh, M.; Mayes, E.L.H.; Coyle, V.E.; Reddy, B.M.; Bansal, V.; Bhargava, S.K. Flower-like Mn3O4/CeO2 Microspheres as an Efficient Catalyst for Diesel Soot and CO Oxidation: Synergistic Effects for Enhanced Catalytic Performance. Appl. Surf. Sci. 2019, 473, 209–221. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Di Benedetto, A.; Lisi, L. Synergy between Ceria and Metals (Ag or Cu) in Catalytic Diesel Particulate Filters: Effect of the Metal Content and of the Preparation Method on the Regeneration Performance. Top Catal. 2021, 64, 256–269. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Zhu, S.; Lu, J.; He, S.; Lu, H.; Song, D.; Luo, Y. Insight into the Effect of CeO2 Morphology on Catalytic Performance for Steam Reforming of Glycerol. Fuel 2023, 334, 126587. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Andana, T.; Dosa, M.; Novara, C.; Giorgis, F.; Russo, N.; Fino, D. Nanostructured Ceria-Based Materials: Effect of the Hydrothermal Synthesis Conditions on the Structural Properties and Catalytic Activity. Catalysts 2017, 7, 174. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, X.; Wang, J.; Zhao, M.; Dan, Y.; Jiang, L.; Chen, Y. Comparative Activity and Hydrothermal Stability of FeOx- And CeO2-Doped Pt-Based Catalysts for Eliminating Diesel Emissions. J. Environ. Chem. Eng. 2020, 8, 104361. [Google Scholar] [CrossRef]
- Shlapa, Y.; Solopan, S.; Sarnatskaya, V.; Siposova, K.; Garcarova, I.; Veltruska, K.; Timashkov, I.; Lykhova, O.; Kolesnik, D.; Musatov, A. Cerium Dioxide Nanoparticles Synthesized via Precipitation at Constant PH: Synthesis, Physical-Chemical and Antioxidant Properties. Colloids. Surf. B Biointerfaces 2022, 220, 112960. [Google Scholar] [CrossRef]
- Pujar, M.S.; Hunagund, S.M.; Desai, V.R.; Patil, S.; Sidarai, A.H. One-Step Synthesis and Characterizations of Cerium Oxide Nanoparticles in an Ambient Temperature via Co-Precipitation Method. AIP Conf. Proc. 2018, 1942, 050026. [Google Scholar] [CrossRef]
- Pal, J.; Chauhan, P. Study of Physical Properties of Cobalt Oxide (Co3O4) Nanocrystals. Mater. Charact. 2010, 61, 575–579. [Google Scholar] [CrossRef]
- Drasovean, R.; Monteiro, R.; Fortunato, E.; Musat, V. Optical Properties of Cobalt Oxide Films by a Dipping Sol-Gel Process. J. Non. Cryst. Solids 2006, 352, 1479–1485. [Google Scholar] [CrossRef]
- Patil, P.S.; Kadam, L.D.; Lokhande, C.D. Preparation and Characterization of Spray Pyrolysed Cobalt Oxide Thin Films. Thin Solid Film. 1996, 272, 29–32. [Google Scholar] [CrossRef]
- Brus, L.E. Electron-Electron and Electron-Hole Interactions in Small Semiconductor Crystallites: The Size Dependence of the Lowest Excited Electronic State. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Liu, S.; Xue, W.; Ji, Y.; Xu, W.; Chen, W.; Jia, L.; Zhu, T.; Zhong, Z.; Xu, G.; Mei, D.; et al. Interfacial Oxygen Vacancies at Co3O4-CeO2 Heterointerfaces Boost the Catalytic Reduction of NO by CO in the Presence of O2. Appl. Catal. B 2023, 323, 122151. [Google Scholar] [CrossRef]
- Serra, R.M.; Gómez, L.E.; Tiscornia, I.S.; de los Deharbe, M.M.; Boix, A.V. CsxCo/Na-MOR Coating on Ceramic Monoliths for Co-Adsorption of Hydrocarbons Mixture and Selective Catalytic Reduction of NOx. Catalysts 2023, 13, 106. [Google Scholar] [CrossRef]
- Gopalsamy, K.; Radhakrishnan, S.; Balachandran, S.; Azhagapillai, P. Surface Engineered Active Co3+ Species in Alkali Doped Co3O4 Spinel Catalyst with Superior O2 Activation for Efficient CO Oxidation. Surf. Interfaces 2023, 36, 102537. [Google Scholar] [CrossRef]
- Stegmayer, M.Á.; Irusta, S.; Miró, E.E.; Milt, V.G. Electrospinning Synthesis and Characterization of Nanofibers of Co, Ce and Mixed Co-Ce Oxides. Their Application to Oxidation Reactions of Diesel Soot and CO. Catal. Today 2022, 383, 266–276. [Google Scholar] [CrossRef]
- Hu, C.; Dai, P.; Chen, Z.; Zhang, H. Property and Reactivity Relationships of Co3O4 with Diverse Nanostructures for Soot Oxidation. ACS Omega 2022, 7, 44116–44123. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Xiong, J.; Wei, Y.; Jiang, N.; Li, Y.; Chi, H.; Zhao, Z.; Liu, J.; Jiao, J. Synergistic Effect of Binary Co and Ni Cations in Hydrotalcite-Derived Co2-XNixAlO Catalysts for Promoting Soot Combustion. Fuel 2022, 320, 123888. [Google Scholar] [CrossRef]
- Kupková, K.; Topka, P.; Balabánová, J.; Koštejn, M.; Jirátová, K.; Giraudon, J.-M.; Lamonier, J.-F.; Maixner, J.; Kovanda, F. Cobalt-Copper Oxide Catalysts for VOC Abatement: Effect of Co:Cu Ratio on Performance in Ethanol Oxidation. Catalysts 2023, 13, 107. [Google Scholar] [CrossRef]
- Todorova, S.; Blin, J.L.; Naydenov, A.; Lebeau, B.; Kolev, H.; Gaudin, P.; Dotzeva, A.; Velinova, R.; Filkova, D.; Ivanova, I. Co3O4-MnOx Oxides Supported on SBA-15 for CO and VOCs Oxidation. Catal. Today 2019, 357, 602–612. [Google Scholar] [CrossRef]
- Farhadi, S.; Pourzare, K.; Sadeghinejad, S. Synthesis and Characterization of Co3O4 Nanoplates by Simple Thermolysis of the [Co(NH3)6]2(C2O4)3·4H2O Complex. Polyhedron 2014, 67, 104–110. [Google Scholar] [CrossRef]
- Lavanya, S.; Kumar, T.R.; Poul Raj, I.L.; Vinoth, S.; Isaac, R.S.R.; Ganesh, V.; Yahia, I.S.; AlAbdulaal, T.H. Enhanced Structural, Optical, and Photo Sensing Properties of Ni-Doped Co3O4 Thin Films Prepared by Nebulizer Spray Pyrolysis Method. Physica. B Condens. Matter. 2022, 649, 414492. [Google Scholar] [CrossRef]
- Marcoccia, C.G.; Peluso, M.A.; Sambeth, J.E. Synthesis, Characterization and Catalytic Properties of Cobalt Oxide Recovered from Spent Lithium-Ion Batteries. Mol. Catal. 2020, 481, 110223. [Google Scholar] [CrossRef]
- Godoy, M.L.; Banús, E.D.; Miró, E.E.; Milt, V.G. Single and Double Bed Stacked Wire Mesh Cartridges for the Catalytic Treatment of Diesel Exhausts. J. Environ. Chem. Eng. 2019, 7, 103290. [Google Scholar] [CrossRef]
- Godoy, M.L.; Banús, E.D.; Sanz, O.; Montes, M.; Miró, E.; Milt, V.G. Stacked Wire Mesh Monoliths for the Simultaneous Abatement of VOCs and Diesel Soot. Catalysts 2018, 8, 16. [Google Scholar] [CrossRef]
- Godoy, M.L.; Milt, V.G.; Miró, E.E.; Banús, E.D. Scaling-up of the Catalytic Stacked Wire Mesh Filters for the Abatement of Diesel Soot. Catal. Today 2021, 394, 434–444. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Wei, S.; Zhang, Y.; Sun, R.; Zhou, T.; Bu, W.; Yao, Q.; Jiang, Z.; Chen, H. Weakly agglomerated α-Al2O3 nanopowders prepared by a novel spray precipitation method. Ceram. Int. 2018, 44, 11374–11380. [Google Scholar] [CrossRef]
- Yin, D.; Wang, J.; Liu, P.; Luo, D.; Kong, L.B.; Dong, Z.; Tang, D. Yttria nanopowders with low degree of aggregation by a spray precipitation method. Ceram. Int. 2018, 44, 20472–20477. [Google Scholar] [CrossRef]
- Peiretti, L.F.; Navascués, N.; Tiscornia, I.S.; Miró, E.E. CeO2 and Co3O4–CeO2 Nanoparticles: Effect of the Synthesis Method on the Structure and Catalytic Properties in COPrOx and Methanation Reactions. J. Mater. Sci. 2016, 51, 3989–4001. [Google Scholar] [CrossRef]
- Dzidziguri, E.L.; Sidorova, E.N.; Inkar, M.; Yudin, A.G.; Kostitsyna, E.V.; Ozherelkov, D.Y.; Slusarsky, K.V.; Nalivaiko, A.Y.; Gromov, A.A. Cobalt Nanoparticles Synthesis by Cobalt Nitrate Reduction. Mater. Res. Express. 2019, 6, 105081. [Google Scholar] [CrossRef]
- Yu, J.; Ma, B.; Shao, S.; Wang, C.; Chen, Y.; Zhang, W. Cobalt Recovery and Microspherical Cobalt Tetroxide Preparation from Ammonia Leaching Solution of Spent Lithium-Ion Batteries. Trans. Nonferrous Met. Soc. China 2022, 32, 3136–3148. [Google Scholar] [CrossRef]
- Banús, E.D.; Milt, V.G.; Miró, E.E.; Ulla, M.A. Co,Ba,K/ZrO2 Coated onto Metallic Foam (AISI 314) as a Structured Catalyst for Soot Combustion: Coating Preparation and Characterization. Appl. Catal. A Gen. 2010, 379, 95–104. [Google Scholar] [CrossRef]
- Van Setten, B.A.A.L.; Schouten, J.M.; Makkee, M.; Moulijn, J.A. Realistic Contact for Soot with an Oxidation Catalyst for Laboratory Studies. Appl. Catal. B 2000, 28, 253–257. [Google Scholar] [CrossRef]
- Banús, E.D.; Ulla, M.A.; Miró, E.E.; Milt, V.G. Co,Ba,K/ZrO2 Coated onto Metallic Foam (AISI 314) as a Structured Catalyst for Soot Combustion: Catalytic Activity and Stability. Appl. Catal. A Gen. 2011, 393, 9–16. [Google Scholar] [CrossRef]
- Leonardi, S.A.; Miró, E.E.; Milt, V.G. Activity of Catalytic Ceramic Papers to Remove Soot Particles—A Study of Different Types of Soot. Catalysts 2022, 12, 855. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mikheeva, N.N.; Mamontov, G.V.; Salaev, M.A.; Liotta, L.F. Ag/CeO2 Composites for Catalytic Abatement of CO, Soot and VOCs. Catalysts 2018, 8, 285. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, T.; Pan, C.; Shi, L.; Zhang, J. Carbon Nanotube-Assisted Synthesis and High Catalytic Activity of CeO2 Hollow Nanobeads. Mater. Chem. Phys. 2009, 113, 527–530. [Google Scholar] [CrossRef]
- Quast, T.; Aiyappa, H.B.; Saddeler, S.; Wilde, P.; Chen, Y.T.; Schulz, S.; Schuhmann, W. Single-Entity Electrocatalysis of Individual “Picked-and-Dropped” Co3O4 Nanoparticles on the Tip of a Carbon Nanoelectrode. Angew. Chem.-Int. Ed. 2021, 60, 3576–3580. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Ulla, M.A.; Miró, E.E. Zeolite Washcoating onto Cordierite Honeycomb Reactors for Environmental Applications. Chem. Eng. J. 2005, 106, 25–33. [Google Scholar] [CrossRef]
- Stegmayer, M.Á.; Godoy, M.L.; Múnera, J.F.; Miró, E.E.; Milt, V.G. In Situ Growth of Ceria Nanofibers on Cordierite Monoliths for Diesel Soot Combustion. Surf. Coat Technol. 2022, 439, 128451. [Google Scholar] [CrossRef]
- Leonardi, S.A.; Tuler, F.E.; Gaigneaux, E.M.; Debecker, D.P.; Miró, E.E.; Milt, V.G. Novel Ceramic Paper Structures for Diesel Exhaust Purification. Environ. Sci. Pollut. Res. 2018, 25, 35276–35286. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Xing, L.; Yang, Y.; Tian, Y.; Ren, W.; Zhang, J.; Zheng, L.; Li, X.; Zhao, D. Highly Efficient Catalytic Soot Combustion Performance of Hierarchically Meso-Macroporous Co3O4/CeO2 Nanosheet Monolithic Catalysts. Catal. Today 2018, 351, 83–93. [Google Scholar] [CrossRef]
- Sacco, N.A.; Banús, E.D.; Bortolozzi, J.P.; Milt, V.G.; Miró, E.E. Ultrasound-Assisted Deposition of Co-CeO2 onto Ceramic Microfibers to Conform Catalytic Papers: Their Application in Engine Exhaust Treatment. ACS Omega 2018, 3, 18334–18342. [Google Scholar] [CrossRef] [PubMed]
- Tuler, F.E.; Portela, R.; Ávila, P.; Bortolozzi, J.P.; Miró, E.E.; Milt, V.G. Development of Sepiolite/SiC Porous Catalytic Filters for Diesel Soot Abatement. Microporous Mesoporous Mater. 2016, 230, 11–19. [Google Scholar] [CrossRef]
- Brussino, P.; Bortolozzi, J.P.; Milt, V.G.; Banús, E.D.; Ulla, M.A. Alumina-Supported Nickel onto Cordierite Monoliths for Ethane Oxydehydrogenation: Coating Strategies and Their Effect on the Catalytic Behavior. Ind. Eng. Chem. Res. 2016, 55, 1503–1512. [Google Scholar] [CrossRef]
- Milt, V.G.; Banús, E.D.; Ulla, M.A.; Miró, E.E. Soot Combustion and NOx Adsorption on Co,Ba,K/ZrO2. Catal. Today 2008, 133–135, 435–440. [Google Scholar] [CrossRef]

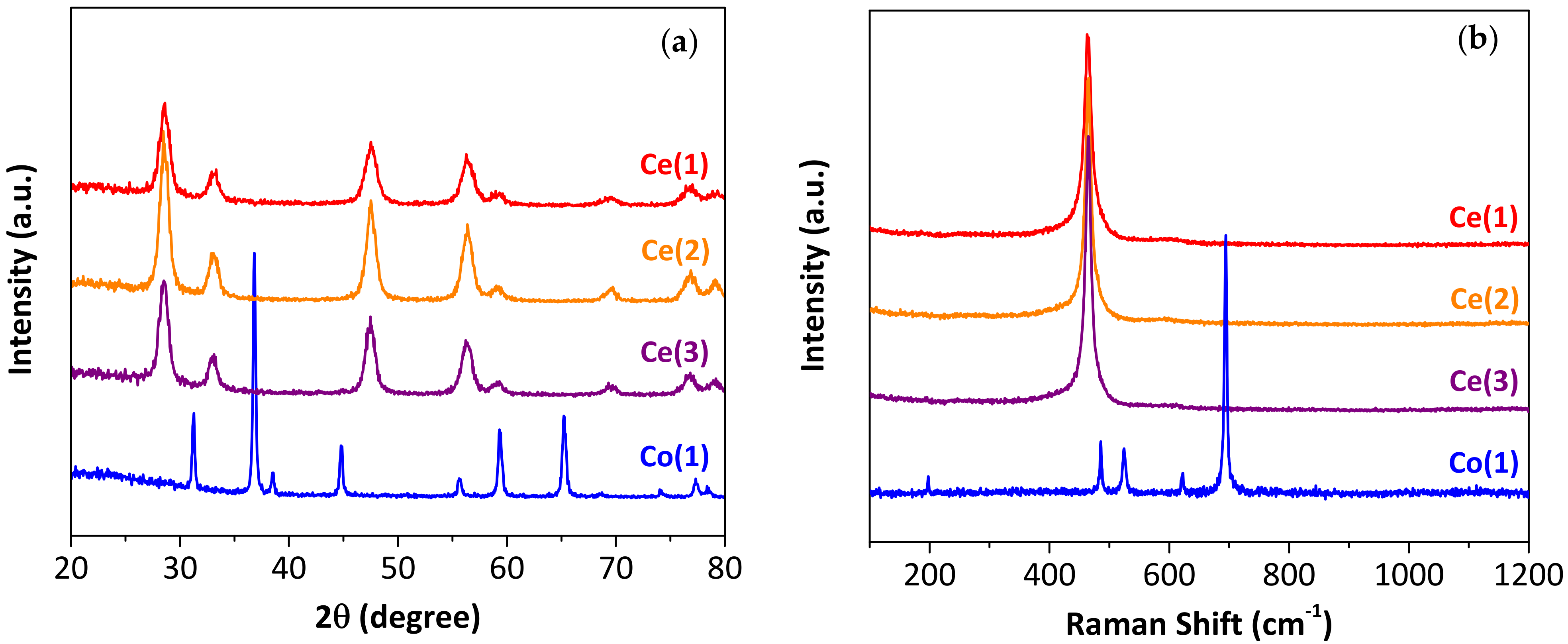
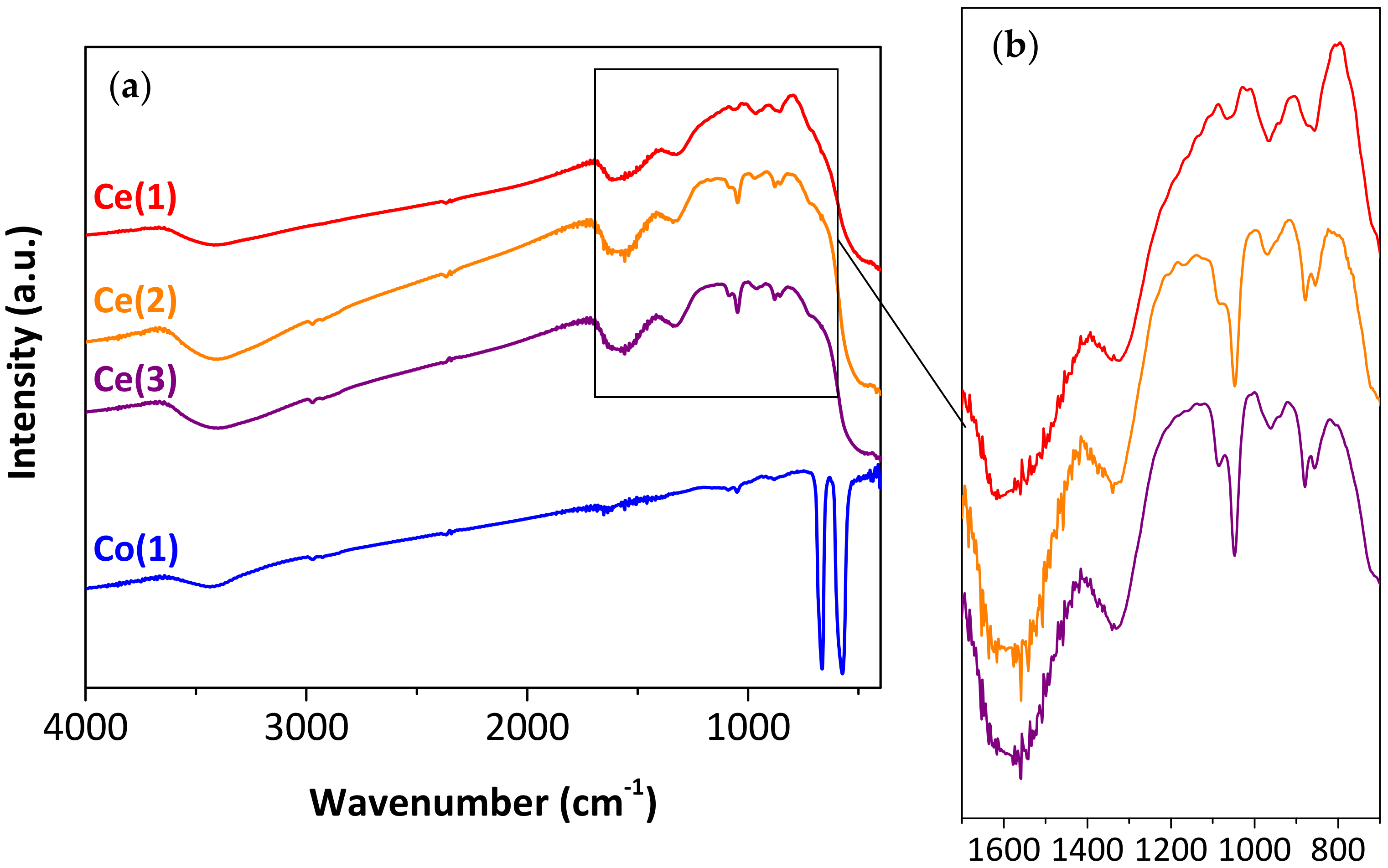
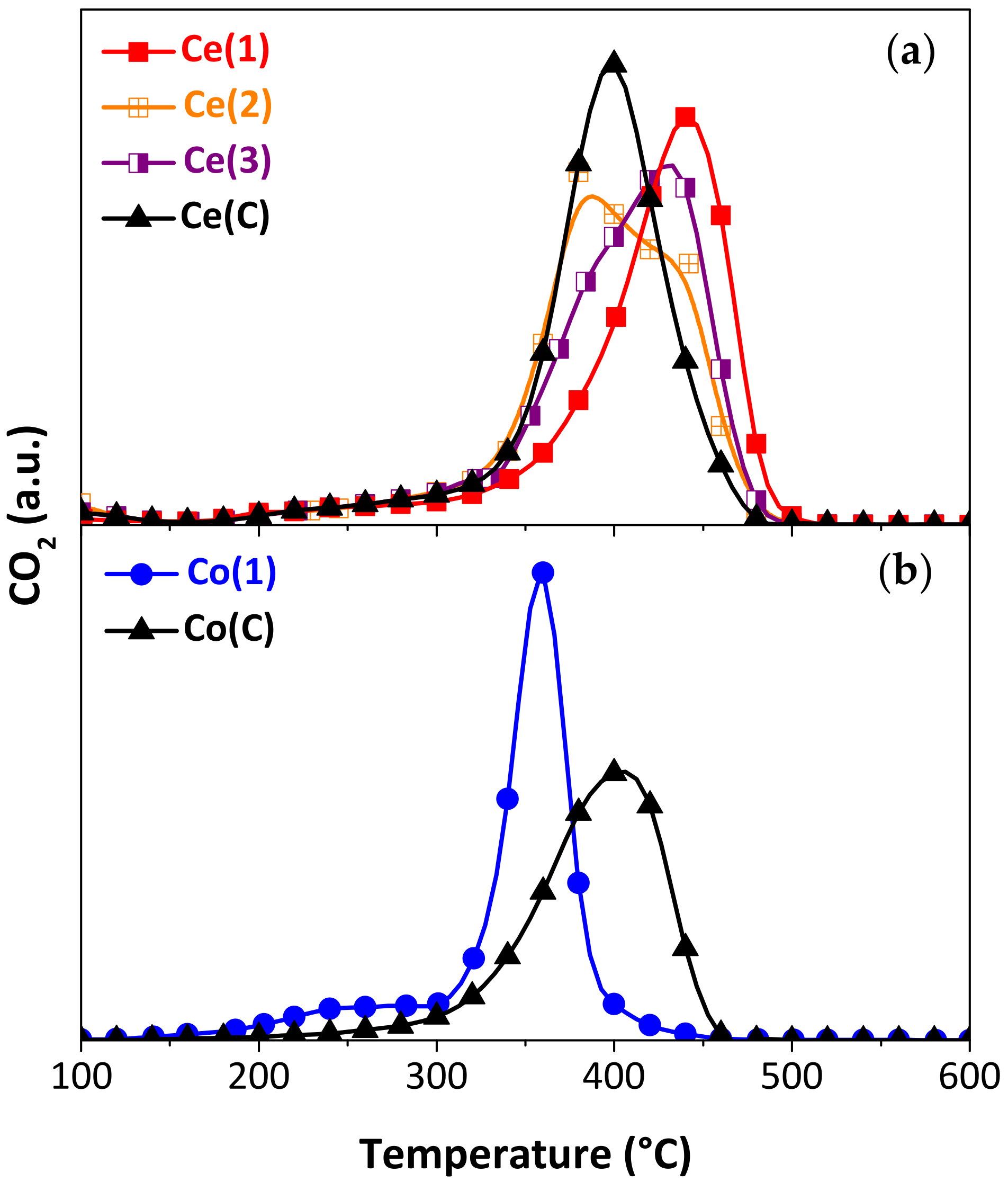


| Synthesized Particles | Sample | Precipitating Agent 1 | Drying Temp. [°C] | Particle Diameter 2 | Crystallite Size [nm] 3 |
|---|---|---|---|---|---|
| CeO2 | Ce(1) | NaOH | 130 | 1.3–1.5 µm | 7.4 |
| Ce(2) = Ce(S) 4 | NH4OH 5 | 70 | 8.4 | ||
| Ce(3) | 130 | 8.5 | |||
| Co3O4 | Co(1) = Co(S) 4 | NaOH | 130 | 50–500 nm | 33.1 |
| Samples | Adherence (%) | |
|---|---|---|
| Wire mesh monoliths (M) | Ce(Ny)-M | 71.6 |
| Ce(S),Ce(Ny)-M | 71.3 | |
| Ce(C),Ce(Ny)-M | 72.1 | |
| Co(S),Ce(Ny)-M | 84.6 | |
| Co(C),Ce(Ny)-M | 78.9 | |
| Co(S),Ce(S),Ce(Ny)-M | 90.2 | |
| Co(C),Ce(C),Ce(Ny)-M | 72.1 | |
| Cordierite monoliths (m) | Ce(Ny)-m | 94.2 |
| Ce(S),Ce(Ny)-m | 95.2 | |
| Ce(C),Ce(Ny)-m | 96.4 | |
| Co(S),Ce(Ny)-m | 94.6 | |
| Co(C),Ce(Ny)-m | 96.6 | |
| Co(S),Ce(S),Ce(Ny)-m | 93.7 | |
| Co(C),Ce(C),Ce(Ny)-m | 92.4 | |
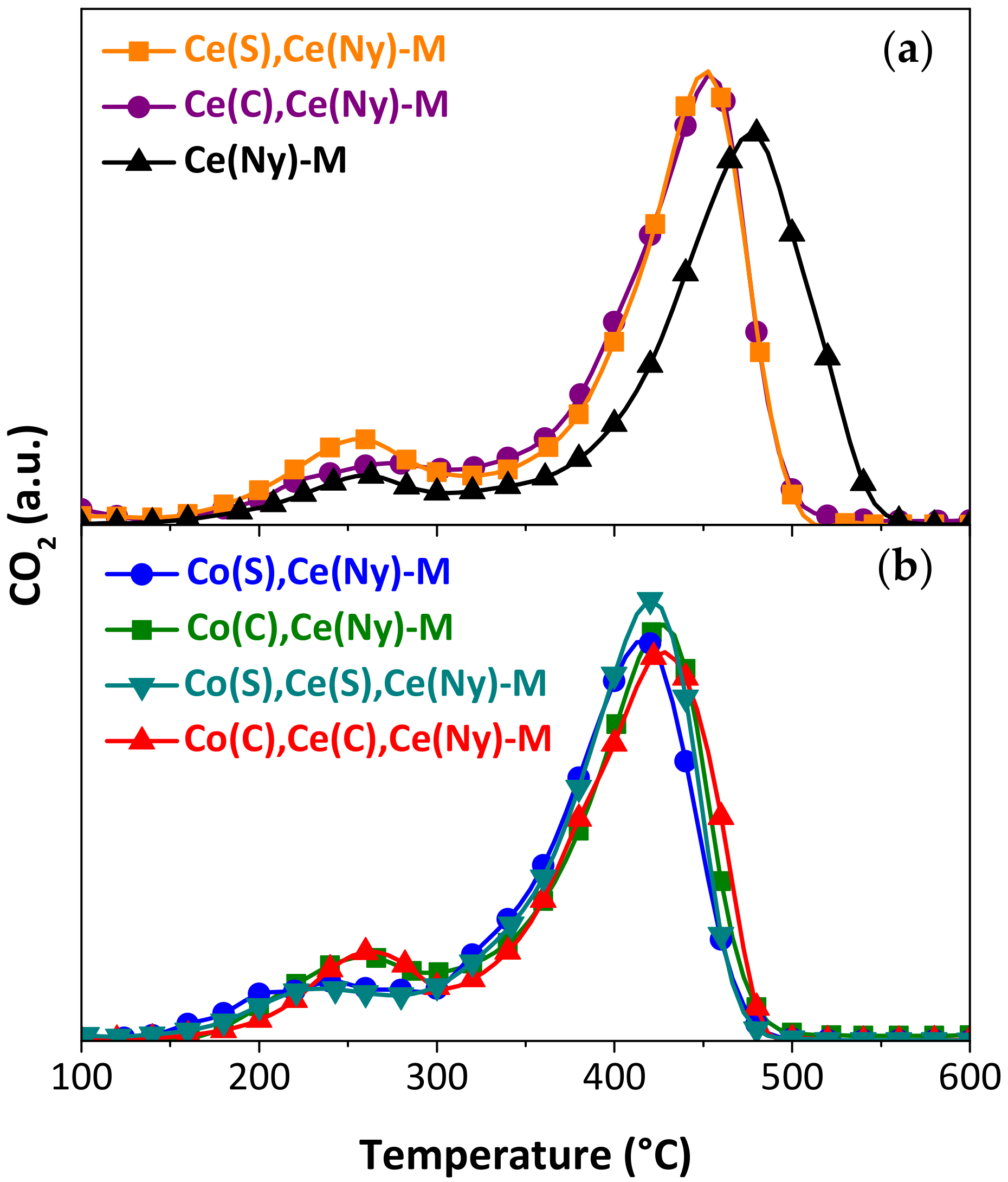
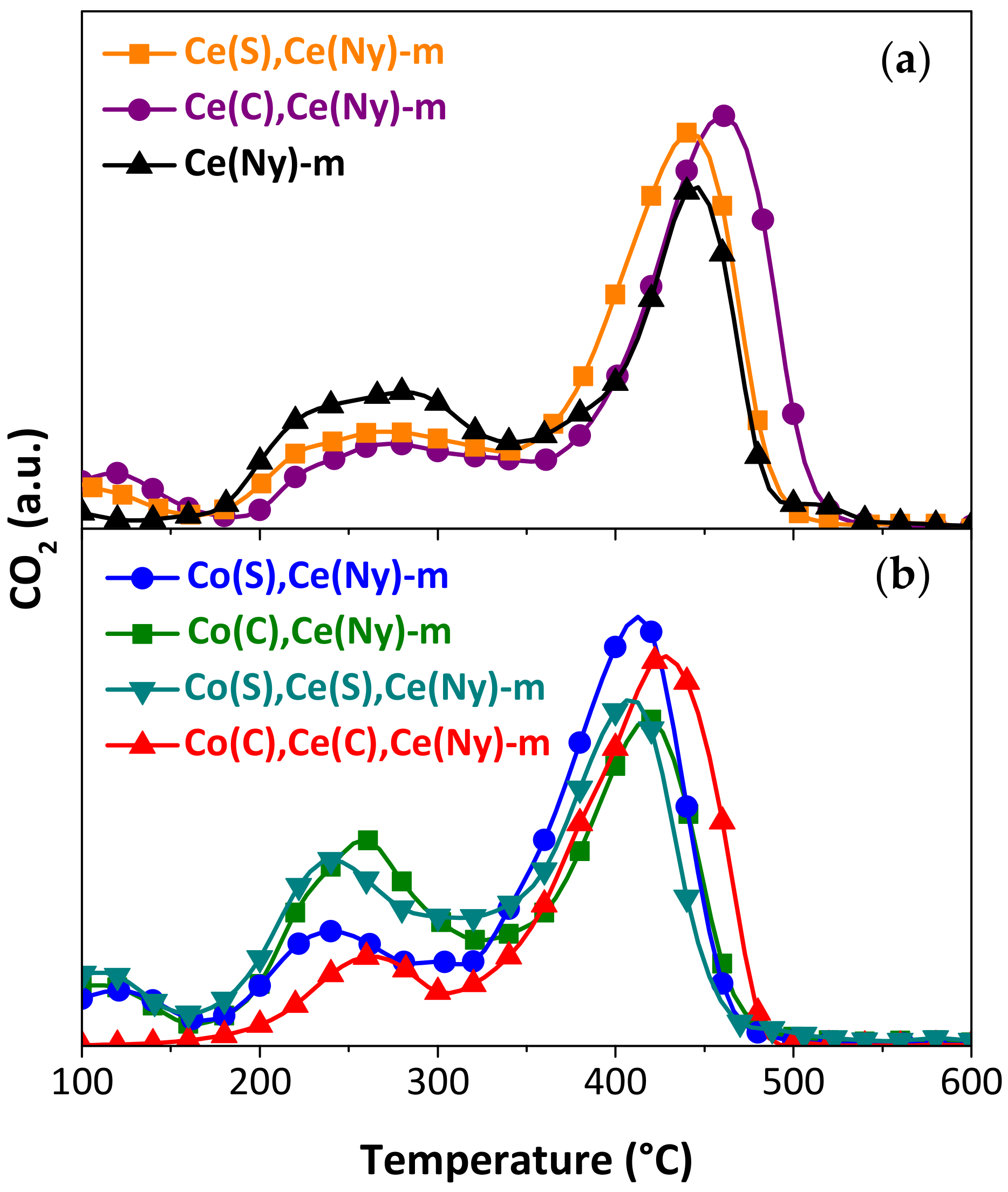
| Catalysts Incorporated to Structured Substrates | Temperature of Maximum Combustion Rate, TM (°C) | |
|---|---|---|
| Metallic Monolith Wire Mesh (M) | Ceramic Monolith Cordierite (m) | |
| Ce(Ny) | 476 | 444 |
| Ce(S),Ce(Ny) | 452 | 441 |
| Ce(C),Ce(Ny) | 452 | 461 |
| Co(S),Ce(Ny) | 417 | 411 |
| Co(C),Ce(Ny) | 427 | 418 |
| Co(S),Ce(S),Ce(Ny) | 419 | 408 |
| Co(C),Ce(C),Ce(Ny) | 429 | 429 |
| Bare substrate | 540 [44] | 524 [58] |
| Catalyst/Substrate | TM (°C) | Reference |
|---|---|---|
| Co(S),Ce(S),Ce(Ny)/wire mesh monolith | 419 | This article |
| Co(S),Ce(Ny)/wire mesh monolith | 417 | This article |
| Co(S),Ce(S),Ce(Ny)/cordierite monolith | 408 | This article |
| CeO2 fibers in-situ grown/cordierite monolith | 418 | [62] |
| Co,Ce/SiO2-Al2O3 paper with Ce(Ny) 1 | 480 | [57,63] |
| Co,Ce nanosheets/Ni foam | 390 | [64] |
| Co,Ce/SiO2-Al2O3 paper with Ce(Ny) 2 | 425 | [65] |
| Co,Ce/sepiolite-SiC monolith | 417 | [66] |
| Suspension | Suspension Composition (Mass Ratio) 1 | Structured Catalyst Name |
|---|---|---|
| CeO2 | H2O:PVA:Ce(Ny) = 5:0.1:10 | Ce(Ny)-M/m |
| H2O:PVA:Ce(S):Ce(Ny) = 5:0.1:1:5 | Ce(S),Ce(Ny)-M/m | |
| H2O:PVA:Ce(C):Ce(Ny) = 5:0.1:1:5 | Ce(C),Ce(Ny)-M/m | |
| Co,Ce | H2O:PVA:Co(S):Ce(Ny) = 5:0.1:1:10.8 | Co(S),Ce(Ny)-M/m |
| H2O:PVA:Co(C):Ce(Ny) = 5:0.1:1:10.8 | Co(C),Ce(Ny)-M/m | |
| H2O:PVA:Co(S):Ce(S):Ce(Ny) = 5:0.1:0.47:0.5:0.25 | Co(S),Ce(S),Ce(Ny)-M/m | |
| H2O:PVA:Co(C):Ce(C):Ce(Ny) = 5:0.1:0.47:0.5:0.25 | Co(C),Ce(C),Ce(Ny)-M/m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy, M.L.; Banús, E.D.; Bon, M.; Miró, E.E.; Milt, V.G. Synthesis of Co,Ce Oxide Nanoparticles Using an Aerosol Method and Their Deposition on Different Structured Substrates for Catalytic Removal of Diesel Particulate Matter. Catalysts 2023, 13, 660. https://doi.org/10.3390/catal13040660
Godoy ML, Banús ED, Bon M, Miró EE, Milt VG. Synthesis of Co,Ce Oxide Nanoparticles Using an Aerosol Method and Their Deposition on Different Structured Substrates for Catalytic Removal of Diesel Particulate Matter. Catalysts. 2023; 13(4):660. https://doi.org/10.3390/catal13040660
Chicago/Turabian StyleGodoy, María Laura, Ezequiel David Banús, Micaela Bon, Eduardo Ernesto Miró, and Viviana Guadalupe Milt. 2023. "Synthesis of Co,Ce Oxide Nanoparticles Using an Aerosol Method and Their Deposition on Different Structured Substrates for Catalytic Removal of Diesel Particulate Matter" Catalysts 13, no. 4: 660. https://doi.org/10.3390/catal13040660
APA StyleGodoy, M. L., Banús, E. D., Bon, M., Miró, E. E., & Milt, V. G. (2023). Synthesis of Co,Ce Oxide Nanoparticles Using an Aerosol Method and Their Deposition on Different Structured Substrates for Catalytic Removal of Diesel Particulate Matter. Catalysts, 13(4), 660. https://doi.org/10.3390/catal13040660