Highly Efficient Self-Assembled Activated Carbon Cloth-Templated Photocatalyst for NADH Regeneration and Photocatalytic Reduction of 4-Nitro Benzyl Alcohol
Abstract
:1. Introduction
2. Results and Discussion
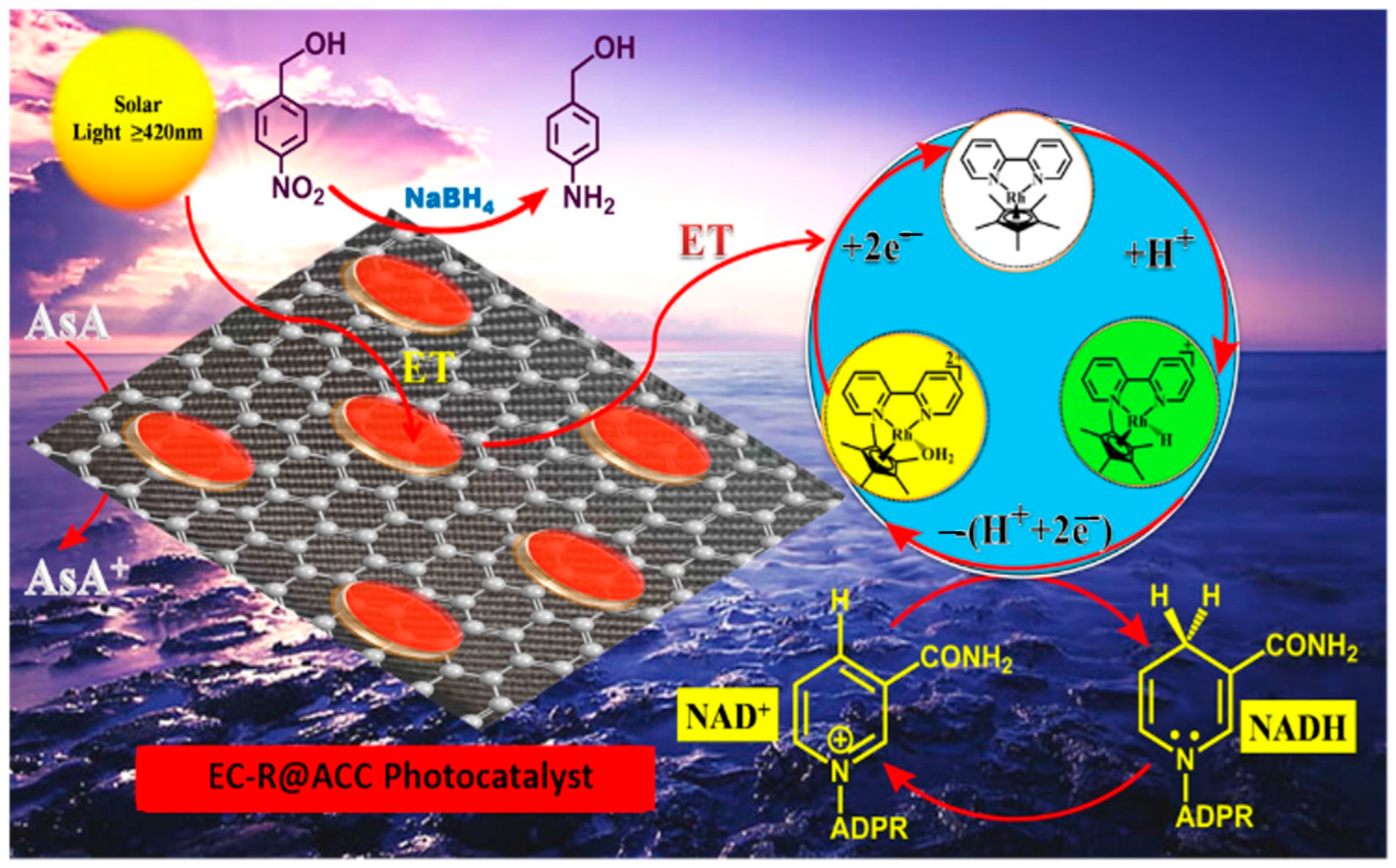
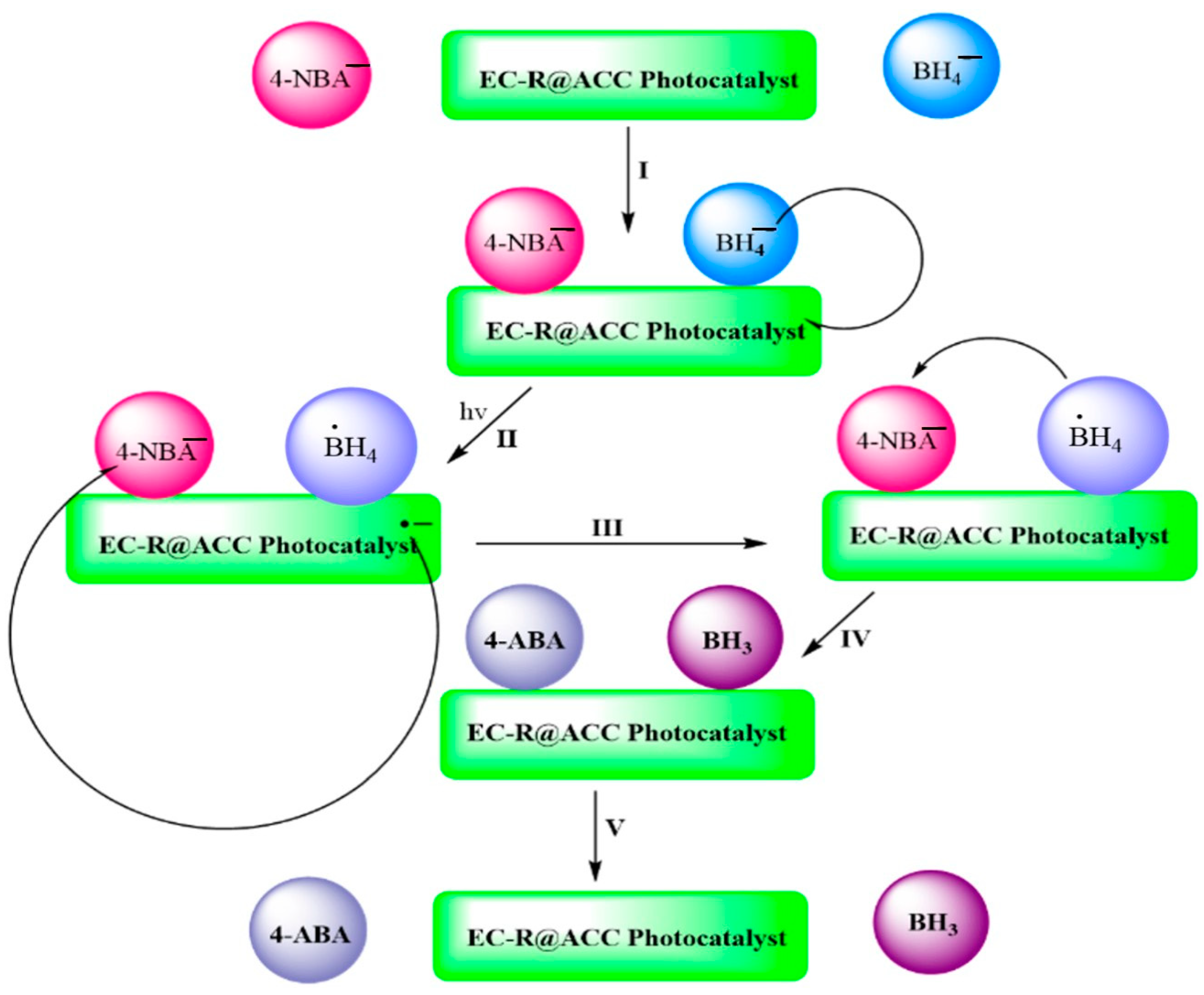
2.1. Mechanistic Pathway for the Photoreduction of 4-NBA
2.2. Presence of Conformational Isomers during 1,4-NADH Synthesis
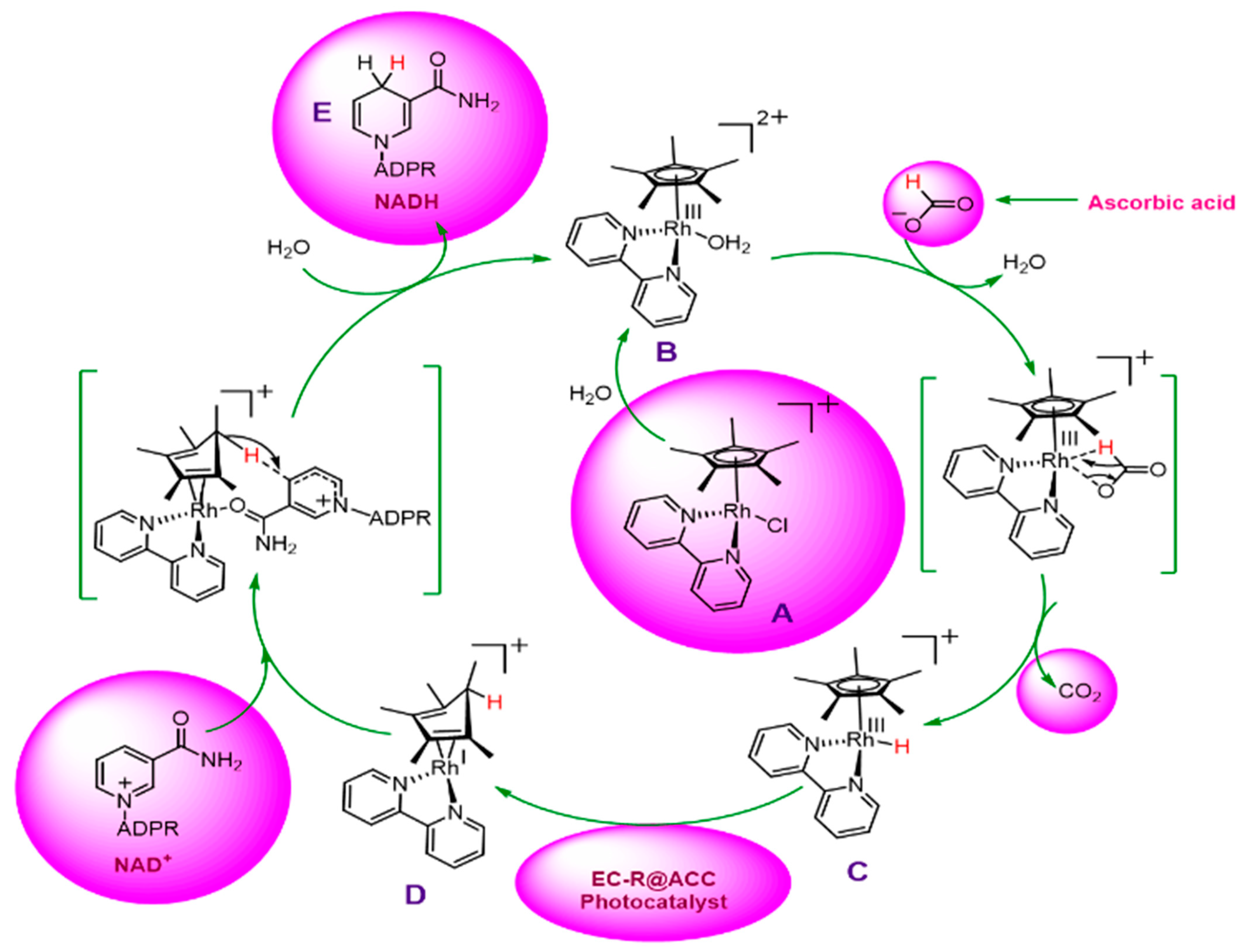
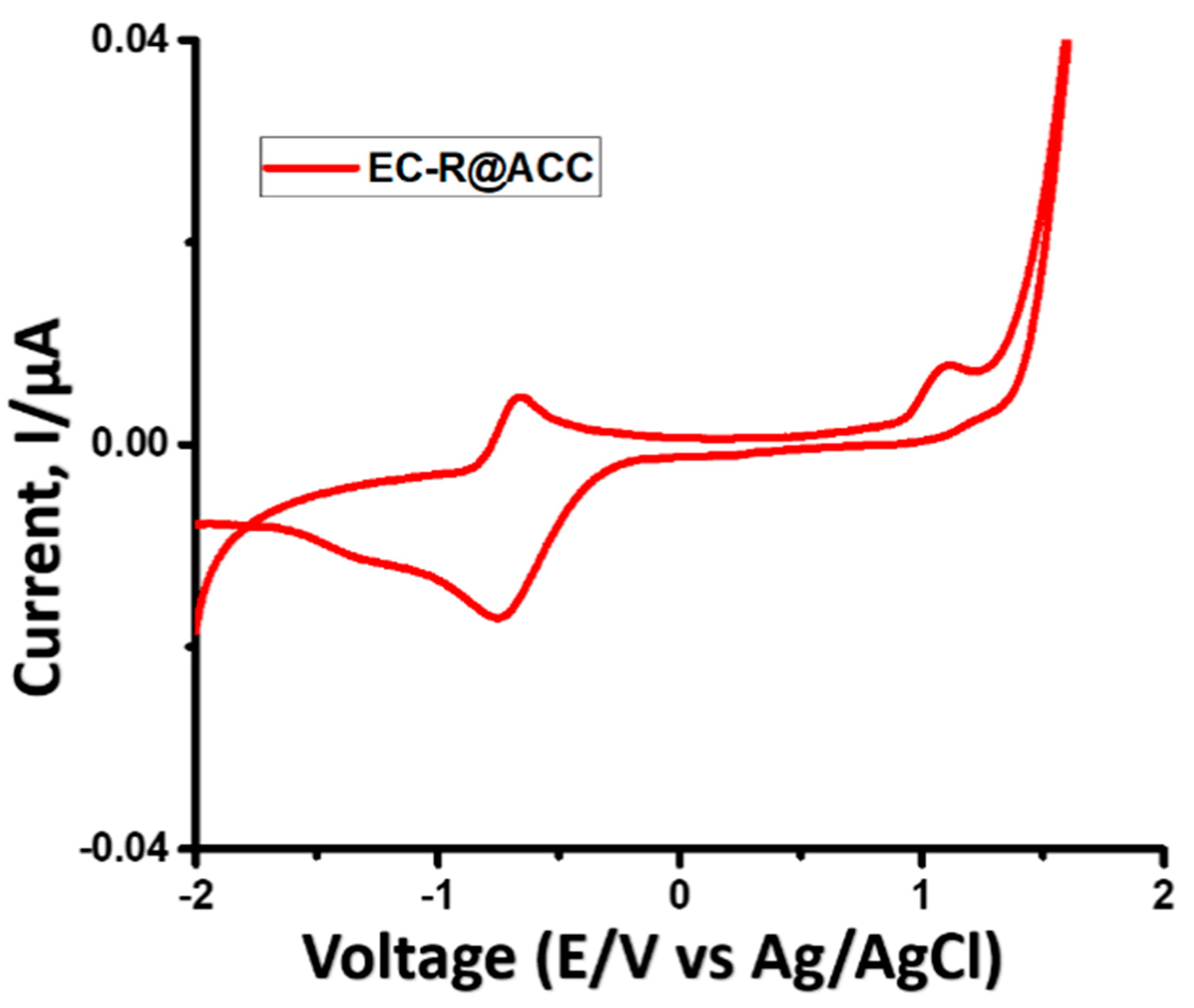
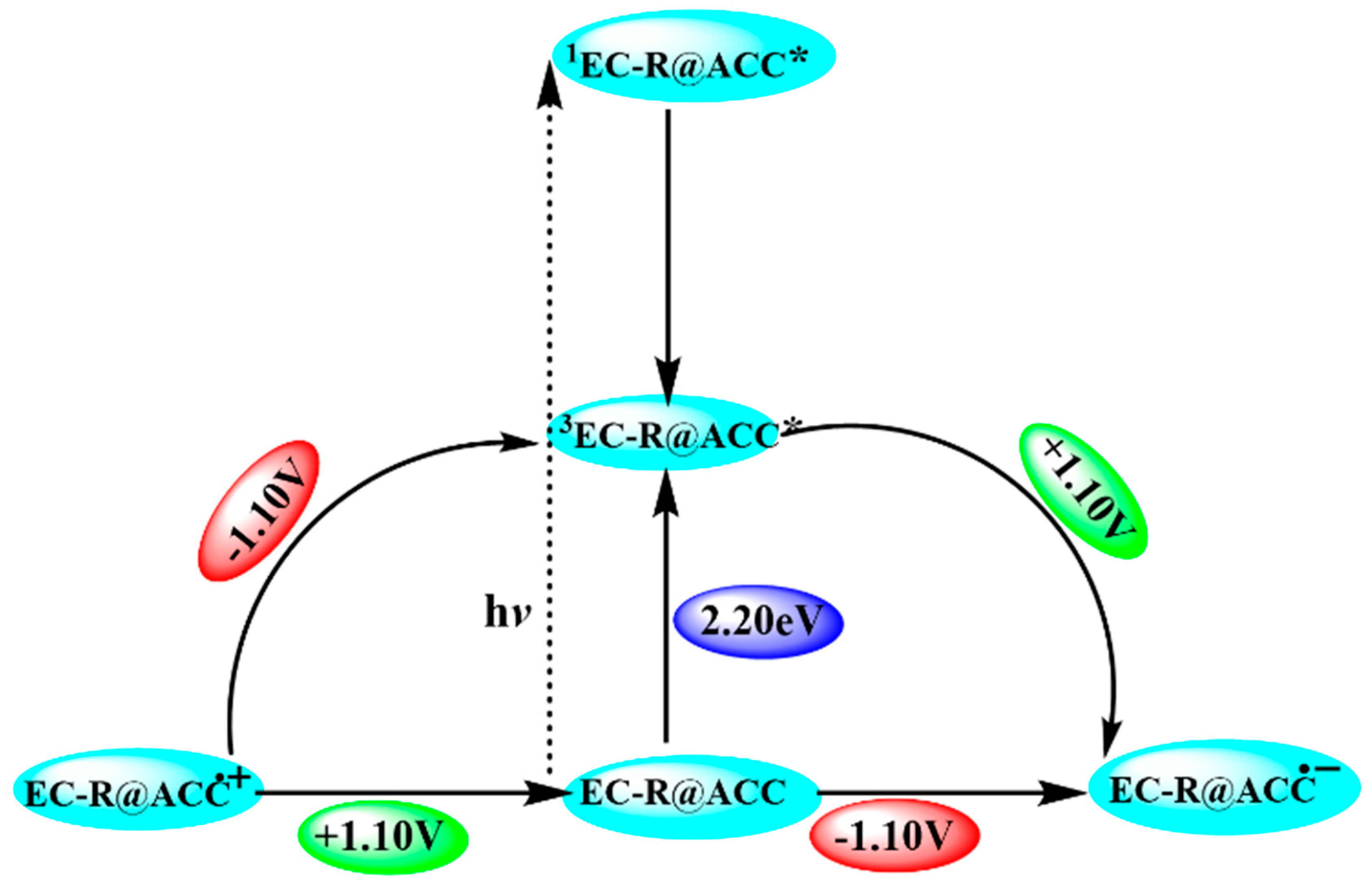
2.3. Reaction Mechanism of Photocatalytic NADH Regeneration
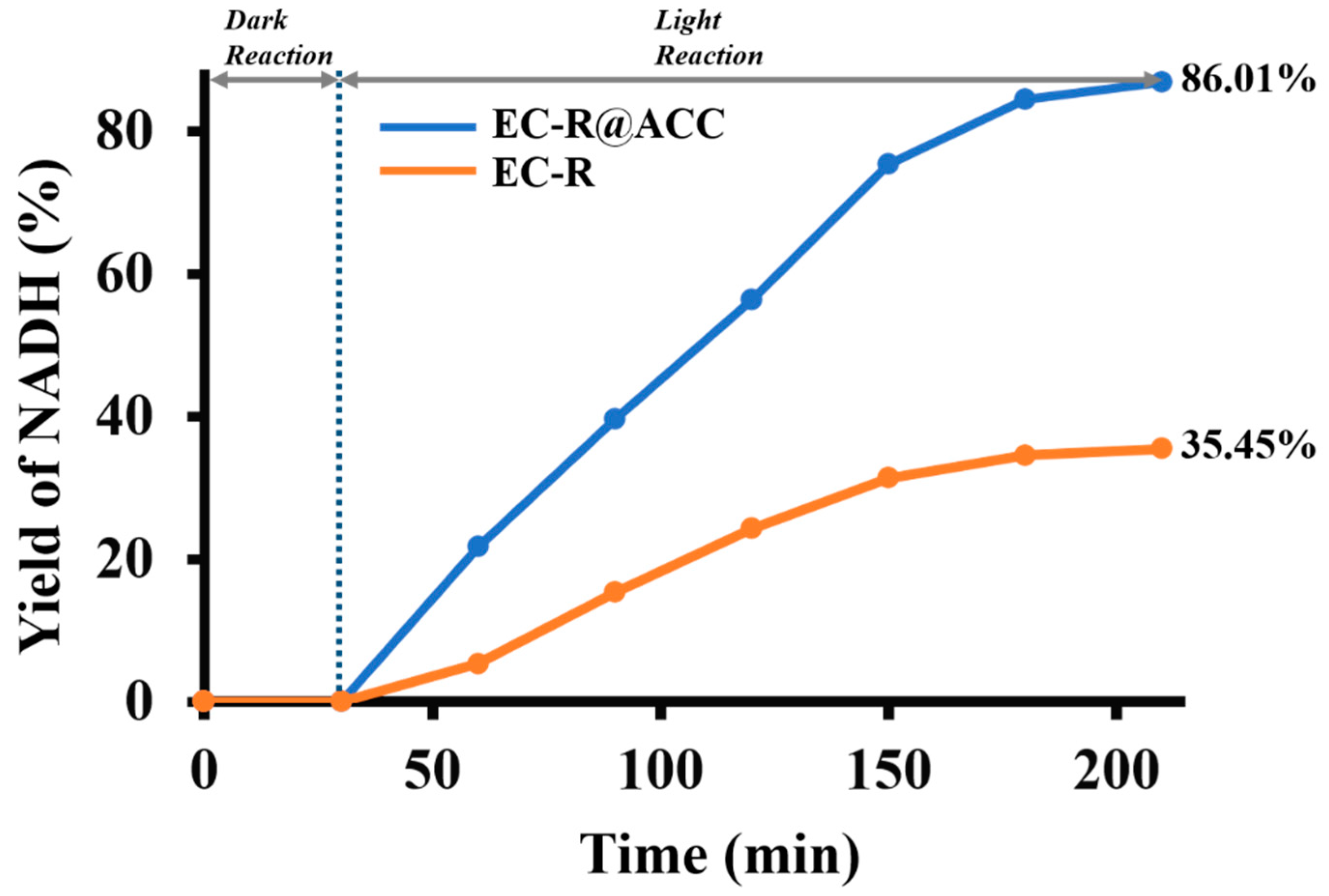
2.4. Solar Light-Induced Catalytic 1,4 NADH Regeneration
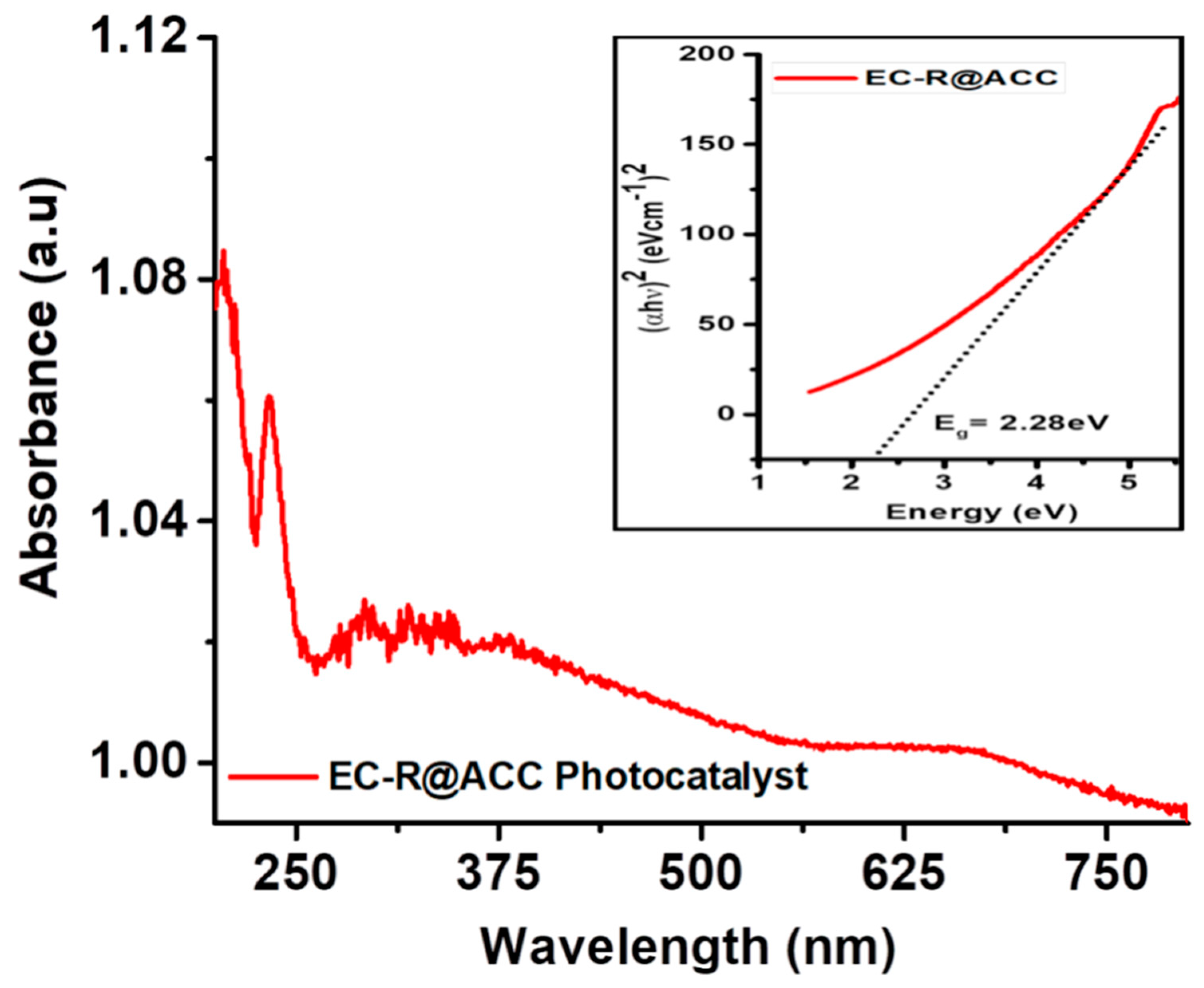
2.5. Study of UV-Visible Spectra of Newly Designed Solar Light Spectrum Responsive EC-R@ACC Photocatalyst
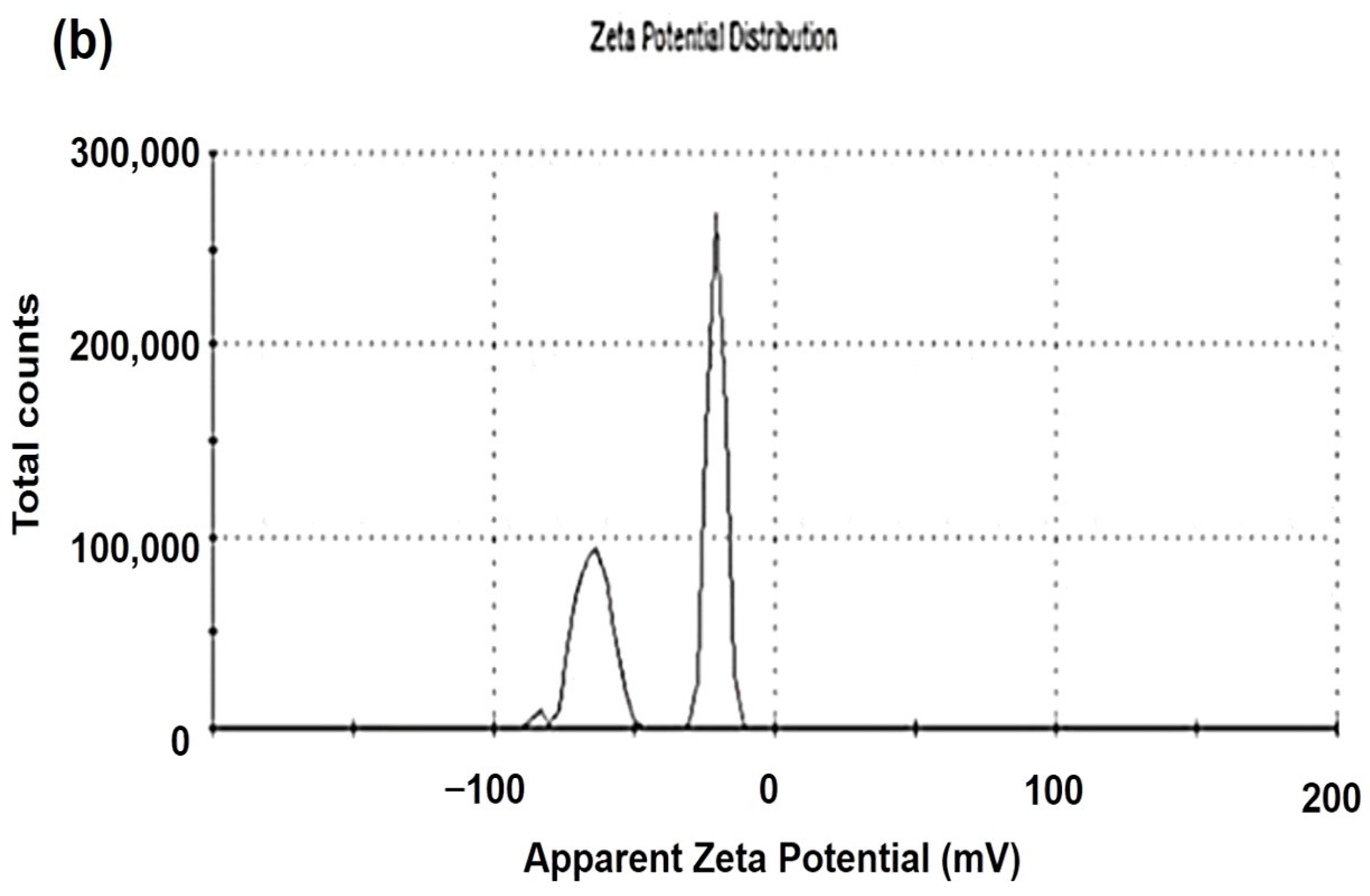
2.6. Study of Zeta Potential of ACC and EC-R@ACC Photocatalyst
3. Experimental Details
3.1. Materials and Chemicals
3.2. Synthesis of ACC
3.3. Synthesis of EC-R@ACC Photocatalyst
3.4. Synthesis of Rh-Complex
3.5. Synthesis of 4-ABA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horvath, I.T.; Anastus, P.T. Innovations and Green Chemistry. Chem. Rev. 2007, 107, 2169–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamali, A.A.; Solangi, A.R.; Memon, N.; Nizamani, S.M.; Khaskheli, A.A.; Hussain, M.; Mahmoud, M.H.; Fouad, H.; Akhtar, M.S. Abiotic Degradation of Imidacloprid Pesticide with L-Threonine Capped Nickel Nanoparticles. Sci. Adv. Mater. 2021, 13, 2043–2048. [Google Scholar] [CrossRef]
- Izzudin, N.M.; Jalil, A.A.; Aziz, F.F.; Azami, A.; Ali, M.S.; Hassan, M.W.; Rahman, A.F.A.; Fauzi, A.A.; Vo, N.S. Simultaneous remediation of hexavalent chromium and organic pollutants in wastewater using period 4 transition metal oxide-based photocatalysts: A review. Environ. Chem. Lett. 2021, 19, 4489–4517. [Google Scholar] [CrossRef]
- Lv, Y.; Zhan, Q.; Yu, X. Microbial-Induced Mineralization of Zinc Ions Based on the Degradation of Toluene and Its Characterization. Sci. Adv. Mater. 2021, 13, 656–661. [Google Scholar] [CrossRef]
- Lang, X.; Chen, X.; Zhao, J. Rational Design of Hybrid Nanostructures for Advanced Photocatalysis. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zheng, Z.; Peng, B.; Wu, X.; Huang, X.; Chen, X. Simultaneous Degradation of p-Nitrophenol and Recovery of Copper from Wastewater in Electrochemical Reactor under High Salinity. Sci. Adv. Mater. 2021, 13, 2450–2459. [Google Scholar] [CrossRef]
- Dalle, K.E.; Julien, W.; Jane, J.L.; Bertrand, R.; Isabell, S.K.; Erwin, R. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752. [Google Scholar] [CrossRef]
- Yadav, R.K.; Oh, G.; Park, N.-J.; Kumar, A.; Kong, K.-J.; Baeg, J.-O. Highly selective solar-driven methanol from CO2 by a photocatalyst/biocatalyst integrated system. J. Am. Chem. Soc. 2014, 136, 16728. [Google Scholar] [CrossRef]
- Yoon, S.K.; Choban, E.R.; Kane, C.; Tzedakis, T.; Kenis, P.J.A. Active control of the depletion boundary layers in microfluidic electrochemical reactors. J. Am. Chem. Soc. 2005, 127, 10466. [Google Scholar] [CrossRef]
- Liu, J.; Antoniettia, M. Bio-inspired NADH regeneration by carbon nitride photocatalysis using diatom templates. Energy Environ. Sci. 2013, 6, 1486–1493. [Google Scholar] [CrossRef] [Green Version]
- Maenaka, Y.; Suenobu, T.; Fukuzumi, S. Efficient Catalytic Interconversion between NADH and NAD+ Accompanied by Generation and Consumption of Hydrogen with a Water-Soluble Iridium Complex at Ambient Pressure and Temperature. J. Am. Chem. Soc. 2012, 134, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kohler, V.; Paul, C.E.; Hollmann, F.; Ward, T.R. Efficient In Situ Regeneration of NADH Mimics by an Artificial Metalloenzyme. ACS Catal. 2016, 6, 3553. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.C.; Mohlmann, L.; Antonietti, M.; Wang, X.; Blechert, S. Aerobic oxidative coupling of amines by carbon nitride photocatalysis with visible light. Angew. Chem. Int. Ed. 2011, 50, 657–660. [Google Scholar]
- Su, F.; Mathew, S.C.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-Catalyzed Selective Oxidation of Alcohols Using O2 and Visible Light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef]
- Singh, S.; Yadav, R.; Kim, T.; Singh, C.; Singh, P.; Chaubey, S.; Singh, A.; Beag, J.; Gupta, S.; Tiwary, D. Generation and Regeneration of the C(sp3)–F Bond and 1,4-NADH/NADPH via Newly Designed S-gC3N4@Fe2O3/LC Photocatalysts under Solar Light. Energy Fuels 2022, 36, 8402–8412. [Google Scholar] [CrossRef]
- Sawunyama, P.; Fujishima, A.; Hashimoto, K. Titanium dioxide photocatalysis. Langmuir 1999, 15, 3551–3556. [Google Scholar] [CrossRef]
- Ischay, M.A.; Anzovino, M.E.; Du, J.; Yoon, T.P. Efficient Visible Light Photocatalysis of [2+2] Enone Cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. [Google Scholar] [CrossRef]
- Zeitler, K. Photoredox Catalysis with Visible Light. Angew. Chem. Int. Ed. 2009, 48, 9785–9789. [Google Scholar] [CrossRef]
- Narayanam, J.M.R.; Tucker, J.W.; Stephenson, C.R.J. Electron-Transfer Photoredox Catalysis: Development of a Tin-Free Reductive Dehalogenation Reaction. J. Am. Chem. Soc. 2009, 131, 8756–8757. [Google Scholar] [CrossRef]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Shih, H.W.; Vander, M.N.; Wal, R.L.; MacMillan, D.W.C.; Grange, R.L. Enantioselective α-Benzylation of Aldehydes via Photoredox Organocatalysis. J. Am. Chem. Soc. 2010, 132, 13600–13603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Wu, L.Z.; Zhou, L.; Han, X.; Yang, Q.Z.; Zhang, L.P.; Tung, C.H. Photoresponsive hydrogen-bonded supramolecular polymers based on a stiff stilbene. Unit. J. Am. Chem. Soc. 2004, 126, 3440–3441. [Google Scholar]
- Haag, B.A.; Mosrin, M.; Ila, H.; Malakhov, V.; Knochel, P. Regio- and Chemoselective Metalation of Arenes and Heteroarenes Using Hindered Metal Amide Bases. Angew. Chem. Int. Ed. 2011, 50, 9511–9954. [Google Scholar] [CrossRef] [PubMed]
- Schmermund, L.; Jurkas, V.; Özgen, F.F.; Barone, G.D.; Grimm, H.C.; Winkler, C.K.; Schmidt, S.; Kourist, R.; Krouti, W. Photo-Biocatalysis: Biotransformations in the Presence of Light. ACS Catal. 2019, 9, 4115–4144. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Wu, C.; Zhang, Z.; Pan, Q.; Hu, F.; Wang, R.; Li, P.; Huang, X.; Li, Z. Fully Conjugated Two-Dimensional sp2-Carbon Covalent Organic Frameworks as Artificial Photosystem I with High Efficiency. Angew. Chem. 2019, 58, 5376–5381. [Google Scholar] [CrossRef]
- Rajeshwar, K. Solar Energy Conversion and Environmental Remediation Using Inorganic Semiconductor–Liquid Interfaces: The Road Traveled and the Way Forward. J. Phys. Chem. Lett. 2011, 2, 1301–1309. [Google Scholar] [CrossRef]
- Yohannes, A.; Su, Y.; Yao, S. Emerging Applications of Metal−Organic Frameworks for Environmental Remediation. ACS Symp. Ser. 2021, 1395, 1–23. [Google Scholar]
- Roy, A.; Sharma, A.; Yadav, S.; Jule, L.T.; Krishnaraj, R. Nanomaterials for Remediation of Environmental Pollutants. Bioinorg Chem. Appl. 2021, 28, 1764647. [Google Scholar] [CrossRef]
- Gopinath, K.; Panchamoorthy, M.; Nagarajan, V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-Light Photocatalysts and Their Perspectives for Building Photocatalytic Membrane Reactors for Various Liquid Phase Chemical Conversions. Catalyst 2020, 10, 1334. [Google Scholar] [CrossRef]
- Mohsin, M.; Ishaq, T.; Bhatti, I.A.; Maryam; Jilani, A.; Melaibari, A.A.; Abu-Hamdeh, N.H. Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel. Nanomaterials 2023, 13, 546. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, H.; Cui, W.; Lee, S.C.; Wang, L.; Dong, F. Bi-based photocatalysts for light-driven environmental and energy applications: Structural tuning, reaction mechanisms, and challenges. EcoMat 2020, 2, e12047. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Films 2016, 605, 2–19. [Google Scholar] [CrossRef] [Green Version]
- Long, X.; Feng, C.; Yang, S.; Ding, D.; Feng, J.; Liu, M.; Chen, Y.; Tan, J.; Peng, X.; Shi, J.; et al. Oxygen doped graphitic carbon nitride with regulatable local electron density and band structure for improved photocatalytic degradation of bisphenol A. J. Chem. Eng. 2022, 435, 1385–8947. [Google Scholar] [CrossRef]
- Choudhury, S.; Baeg, J.-O.; Park, N.-J.; Yadav, R.K. A Photocatalyst/Enzyme Couple That Uses Solar Energy in the Asymmetric Reduction of Acetophenones. Angew. Chem. 2012, 51, 11624–11628. [Google Scholar] [CrossRef] [PubMed]
- Sakhare, P.A.; Pawar, S.S.; Bhat, T.S.; Yadav, S.D.; Patil, G.R.; Patil, P.S.; Sheikh, A.D. Magnetically Recoverable BiVO4/NiFe2O4 Nanocomposite Photocatalyst For Efficient Detoxification of Polluted Water Under Collected Sunlight. Mater. Res. Bull. 2020, 129, 110908. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Fang, B.; Xing, Z.; Sun, D.; Li, Z.; Zhou, W. Hollow semiconductor photocatalysts for solar energy conversion. APM 2022, 1, 100021. [Google Scholar] [CrossRef]
- Yadav, R.K.; Baeg, J.-O.; Oh, G.; Park, N.-J.; Kong, K.; Kim, J.; Hwang, D.W.; Biswas, S.K. A Photocatalyst–Enzyme Coupled Artificial Photosynthesis System for Solar Energy in Production of Formic Acid from CO2. J. Am. Chem. Soc. 2012, 134, 11455–11461. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Gao, S.; Lin, Y.; Li, H. A facile route to n-type TiO2-nanotube/p-type boron-doped-diamond heterojunction for highly efficient photocatalysts. Chem. Comm. 2010, 46, 3119. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen-Doped Titanium Dioxide (N-TiO2): Synopsis of Synthesis Methodologies, Doping Mechanisms, Property Evaluation and Visible Light Photocatalytic Applications. Photochemical 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Li, K.; Zeng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.Y.C.; Huang, H. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef] [PubMed]
- Plakas, K.V.; Taxintari, A.; Karabelas, A.J. Enhanced Photo-Catalytic Performance of Activated Carbon Fibers for Water Treatment. Water 2019, 11, 1794. [Google Scholar] [CrossRef] [Green Version]
- Lawtae, P.; Tangsathitkulchai, C. The Use of High Surface Area Mesoporous-Activated Carbon from Longan Seed Biomass for Increasing Capacity and Kinetics of Methylene Blue Adsorption from Aqueous Solution. Molecules 2021, 26, 6521. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Yadav, R.K.; Kim, T.W.; Upare, P.P.; Gupta, A.K.; Singh, A.P.; Yadav, B.C.; Dwivedi, D.K. In Situ Prepared Solar Light-Driven Flexible Actuated Carbon Cloth-Based Nanorod Photocatalyst for Selective Radical-Radical Coupling to Vinyl Sulfides. Photochem. Photobiol. 2021, 97, 955–962. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, Z.; Wang, J.; Chen, Z.; Yin, J.; Zhang, Z.; Huang, J.; Qian, J.; Wang, X. Harvesting Solar Energy by Flowerlike Carbon Cloth Nanocomposites for Simultaneous Generation of Clean Water and Electricity. ACS Appl. Mater. 2021, 13, 27129–27139. [Google Scholar] [CrossRef]
- Ndlwana, L.; Raleie, N.; Dimpe, K.M.; Ogutu, H.F.; Oseghe, E.O.; Motsa, M.M.; Msagati, T.A.M.; Mamba, B.B. Sustainable Hydrothermal and Solvothermal Synthesis of Advanced Carbon Materials in Multidimensional Applications: A Review. Materials 2021, 14, 5094. [Google Scholar] [CrossRef]
- Carmen, Z.; Daniela, S. Rijeka: Textile Organic Dyes–Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents–A Critical Overview. IntechOpen 2012, 3, 55–86. [Google Scholar]
- Sahiner, N.; Ozay, H.; Ozay, O.; Aktas, N. A soft hydrogel reactor for cobalt nanoparticle preparation and use in the reduction of nitrophenols. Appl. Catal. B Environ. 2010, 101, 137–143. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Hu, Z. Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environ. Sci. Process. Impacts 2013, 15, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gazi, S.; Ananthakrishnan, R. Metal-free-photocatalytic reduction of 4-nitrophenol by resin-supported dye under the visible irradiation. Appl. Catal. B Environ. 2011, 105, 317–325. [Google Scholar] [CrossRef]
- Krishna, M.B.M.; Venkatramaiah, N.; Venkatesan, R.; Rao, D.N. Synthesis and structural, spectroscopic and nonlinear optical measurements of graphene oxide and its composites with metal and metal free porphyrins. J. Mater. Chem. 2012, 22, 3059–3068. [Google Scholar] [CrossRef]
- Chaubey, S.; Singh, P.; Singh, C.; Singh, S.; Shreya, S.; Yadav, R.K.; Mishra, S.; Jeong, Y.J.; Biswas, B.K.; Kim, T.W. Ultra-efficient synthesis of bamboo-shape porphyrin framework for photocatalytic CO2 reduction and consecutive C-S/C-N bonds formation. J. CO2 Util. 2022, 59, 101968. [Google Scholar] [CrossRef]
- Yadav, S.N.; Kumar, B.; Yadav, R.K.; Singh, P.; Gupta, S.K.; Singh, S.; Singh, A.P. Synthesis of highly efficient selenium oxide hybridized g-C3N4 photocatalyst for NADH/NADPH regeneration to facilitate solar-to-chemical reaction. Main Group Chem. 2022, 21, 1077–1089. [Google Scholar] [CrossRef]
- Kumar, K.S.; Huerta, G.V.; Castellanos, A.R.; Varaldo, H.M.P.; Feria, O.S. Microwave Assisted Synthesis and Characterizations of Decorated Activated Carbon. Int. J. Electrochem. Sci. 2012, 7, 5484–5494. [Google Scholar]
- Chaubey, S.; Yadav, R.K.; Kim, T.W.; Singh, A.P.; Kumar, K.; Yadav, B.C. Ultrahigh sun-light-responsive/not responsive integrated catalyst for C-S arylation/humidity sensing. Vietnam J. Chem. 2021, 59, 500–510. [Google Scholar]
- Singh, C.; Kim, T.W.; Yadav, R.K.; Baeg, J.O.; Gole, V.; Singh, A.P. Flexible Covalent Porphyrin Framework Film: An Emerged Platform for Photocatalytic C-H Bond Activation. App. Surface Sci. 2021, 544, 148938. [Google Scholar] [CrossRef]
- Singh, P.; Yadav, R.K.; Kim, T.W.; Kumar, A.; Dwivedi, D.K. Chitosan-based fluorescein isothiocyanate film as a highly efficient metal-free photocatalyst for solar-light-mediated direct C-H arylation. Int. J. Energy Res. 2021, 45, 5964–5973. [Google Scholar] [CrossRef]
- Lacroix, M.R.; Gao, X.Y.; Liu, S.H. Strauss, Unusually sharp FTIR ν(OH) bands and very weak O single bondH⋯F hydrogen bonds in M2(H2O)1,2B12F12 hydrates (Mdouble bond Nasingle bond Cs). J. Fluorine Chem. 2019, 217, 105–108. [Google Scholar] [CrossRef]
- Yen, C.Y.; Lee, C.H.; Lin, Y.F.; Lin, H.L.; Hsiao, Y.H.; Liao, S.H.; Chuang, C.Y.; Ma, C.C.M. Proton conductivity of Nafion/ex situ Stöber silica nanocomposite membranes as a function of silica particle size and temperature. J. Power Sources 2007, 173, 36–44. [Google Scholar] [CrossRef]
- Thompson, W.R.; Cai, M.; Ho, M.; Pemberton, J.E. Hydrolysis and Condensation of Self-Assembled Monolayers of (3-Mercaptopropyl) trimethoxysilane on Ag and Au Surfaces. Langmuir 1997, 13, 2291–2302. [Google Scholar] [CrossRef]
- Bano, M.; Ahirwar, D.; Thomas, M.; Naikoo, G.A.; Sheikh, M.U.-D.; Khan, F. Hierarchical synthesis of silver monoliths and their efficient catalytic activity for the reduction of 4-nitrophenol to 4-aminophenol. New J. Chem. 2016, 40, 6787–6795. [Google Scholar] [CrossRef]
- Singh, S.; Yadav, R.; Kim, T.; Singh, C.; Singh, P.; Singh, A.; Singh, A.; Singh, A.; Beag, J.; Gupta, S. Rational design of a graphitic carbon nitride catalytic–biocatalytic system as a photocatalytic platform for solar fine chemical production from CO2. React. Chem. Eng. 2022, 7, 1566–1572. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, R.K.; Park, N.J.; Baeg, J.O. Facile One-Pot Two-Step Synthesis of Novel in Situ Selenium-Doped Carbon Nitride Nanosheet Photocatalysts for Highly Enhanced Solar Fuel Production from CO2. ACS Appl. Nano Mater. 2017, 1, 47–54. [Google Scholar] [CrossRef]



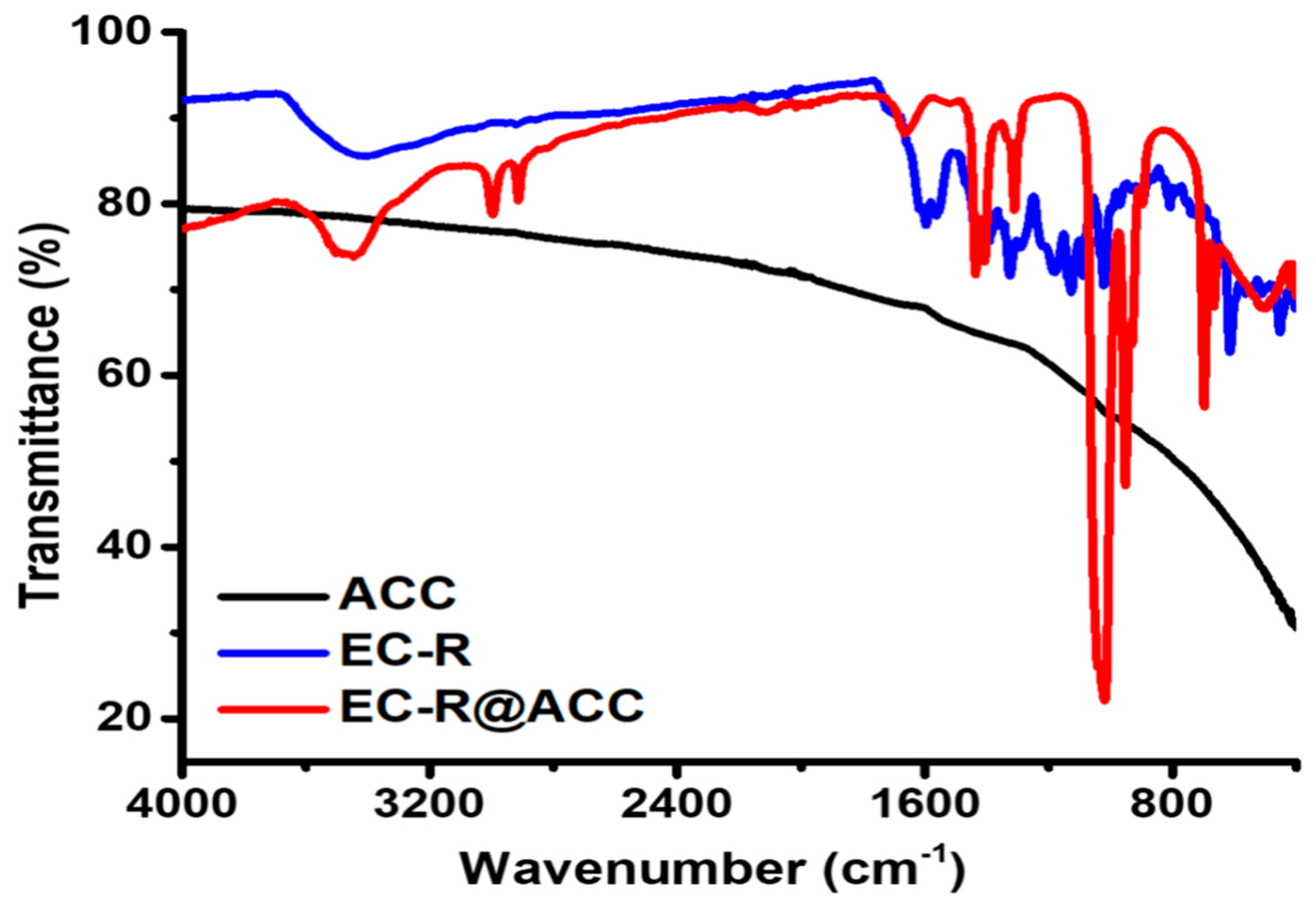


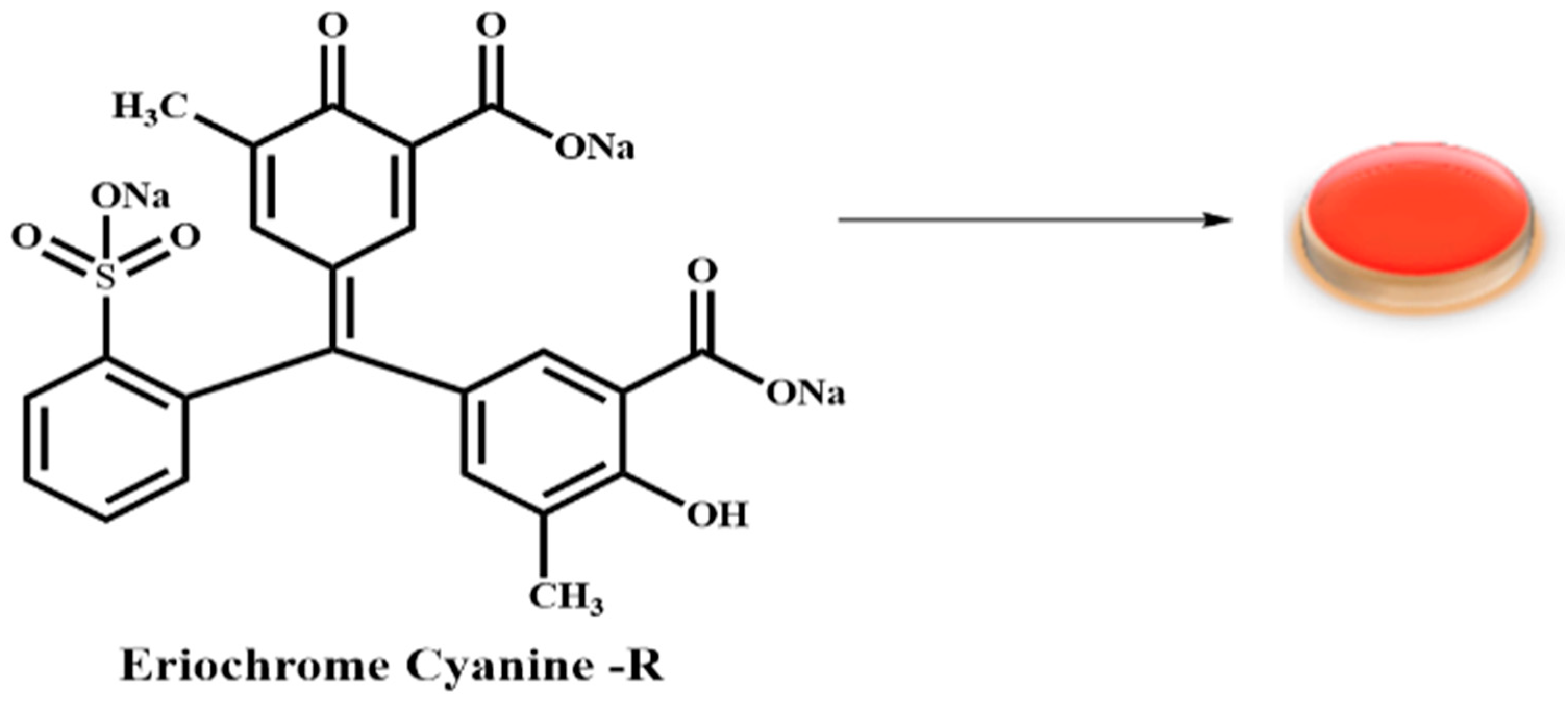
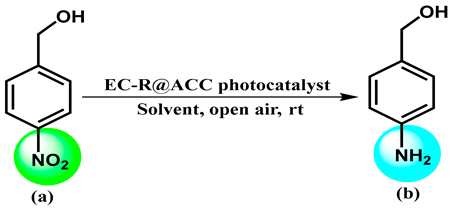 | ||||||
|---|---|---|---|---|---|---|
| Entry | Solar Light | Photocatalyst | Solvent | Time | Conversion (%) | Yield (%) |
| 1. | Yes | EC-R@ACC | C2H5OH | 12 | 50 | 49 |
| 2. | Yes | EC-R@ACC | C2H5OH | 6 | 47 | 48 |
| 3. | Yes | EC-R@ACC | PEG | 12 | 55 | 56 |
| 4. | Yes | EC-R@ACC | PEG | 6 | 51 | 49 |
| 5. | Yes | EC-R@ACC | CH2Cl2 | 12 | 60 | 58 |
| 6. | Yes | EC-R@ACC | CH2Cl2 | 6 | 58 | 57 |
| 7. | Yes | EC-R@ACC | DMF | 12 | 97 | 96 |
| 8. | Yes | EC-R@ACC | DMF | 6 | 79 | 78 |
| 9. | Yes | EC-R | DMF | 6 | 46 | 45 |
| 10. | No | EC-R@ACC | DMF | 12 | 05 | 05 |
| 11. | Yes | EC-R@ACC | Absent | 12 | 10 | 10 |
| 12. | Yes | Absent | DMF | 12 | 05 | 05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, V.; Yadav, R.K.; Umar, A.; Ibrahim, A.A.; Singh, S.; Shahin, R.; Shukla, R.K.; Tiwary, D.; Dwivedi, D.K.; Singh, A.K.; et al. Highly Efficient Self-Assembled Activated Carbon Cloth-Templated Photocatalyst for NADH Regeneration and Photocatalytic Reduction of 4-Nitro Benzyl Alcohol. Catalysts 2023, 13, 666. https://doi.org/10.3390/catal13040666
Gupta V, Yadav RK, Umar A, Ibrahim AA, Singh S, Shahin R, Shukla RK, Tiwary D, Dwivedi DK, Singh AK, et al. Highly Efficient Self-Assembled Activated Carbon Cloth-Templated Photocatalyst for NADH Regeneration and Photocatalytic Reduction of 4-Nitro Benzyl Alcohol. Catalysts. 2023; 13(4):666. https://doi.org/10.3390/catal13040666
Chicago/Turabian StyleGupta, Vaibhav, Rajesh K. Yadav, Ahmad Umar, Ahmed A. Ibrahim, Satyam Singh, Rehana Shahin, Ravindra K. Shukla, Dhanesh Tiwary, Dilip Kumar Dwivedi, Alok Kumar Singh, and et al. 2023. "Highly Efficient Self-Assembled Activated Carbon Cloth-Templated Photocatalyst for NADH Regeneration and Photocatalytic Reduction of 4-Nitro Benzyl Alcohol" Catalysts 13, no. 4: 666. https://doi.org/10.3390/catal13040666
APA StyleGupta, V., Yadav, R. K., Umar, A., Ibrahim, A. A., Singh, S., Shahin, R., Shukla, R. K., Tiwary, D., Dwivedi, D. K., Singh, A. K., Singh, A. K., & Baskoutas, S. (2023). Highly Efficient Self-Assembled Activated Carbon Cloth-Templated Photocatalyst for NADH Regeneration and Photocatalytic Reduction of 4-Nitro Benzyl Alcohol. Catalysts, 13(4), 666. https://doi.org/10.3390/catal13040666











