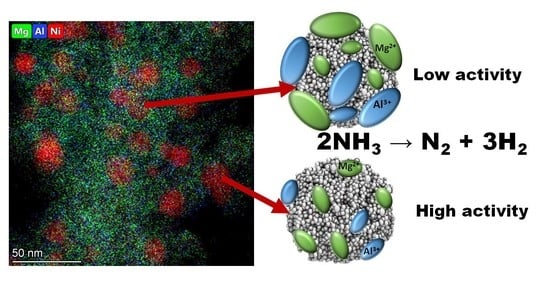
Layered Double Hydroxide-Derived Ni-Mg-Al Catalysts for Ammonia Decomposition Process: Synthesis and Characterization
Abstract
1. Introduction
2. Results and Discussion
3. Experimental
3.1. Synthesis of Nickel Catalysts
3.2. Physicochemical Methods for the Catalysts Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okura, K.; Miyazaki, K.; Muroyama, H.; Matsui, T.; Eguchi, K. Ammonia decomposition over Ni catalysts supported on perovskite-type oxides for the on-site generation of hydrogen. RSC Adv. 2018, 8, 32102–32110. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; He, M.; Hong, X.; Chen, X.; Feng, G.; Lu, Z.-H. Hydrogen production via selective dehydrogenation of hydrazine borane and hydrous hydrazine over MoOx-promoted Rh catalyst. Int. J. Hydrogen Energy 2019, 44, 28430–28440. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Ammonia as a green fuel and hydrogen source for vehicular applications. Fuel Process. Technol. 2009, 90, 729–737. [Google Scholar] [CrossRef]
- Guo, J.; Chen, P. Catalyst: NH3 as an Energy Carrier. Chem 2017, 3, 709–712. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Yin, S.-F.; Zhang, Q.-H.; Xu, B.-Q.; Zhu, W.-X.; Ng, C.-F.; Au, C.-T. Investigation on the catalysis of COx-free hydrogen generation from ammonia. J. Catal. 2004, 224, 384–396. [Google Scholar] [CrossRef]
- Im, Y.; Muroyama, H.; Matsui, T.; Eguchi, K. Investigation on catalytic performance and desorption behaviors of ruthenium catalysts supported on rare-earth oxides for NH3 decomposition. Int. J. Hydrogen Energy 2022, 47, 32543–32551. [Google Scholar] [CrossRef]
- Ju, X.; Liu, L.; Zhang, X.; Feng, J.; He, T.; Chen, P. Highly Efficient Ru/MgO Catalyst with Surface-Enriched Basic Sites for Production of Hydrogen from Ammonia Decomposition. ChemCatChem 2019, 11, 4161–4170. [Google Scholar] [CrossRef]
- Ju, X.; Liu, L.; Yu, P.; Guo, J.; Zhang, X.; He, T.; Wu, G.; Chen, P. Mesoporous Ru/MgO prepared by a deposition-precipitation method as highly active catalyst for producing COx-free hydrogen from ammonia decomposition. Appl. Catal. B Environ. 2017, 211, 167–175. [Google Scholar] [CrossRef]
- Do, Q.C.; Kim, Y.; Le, T.A.; Kim, G.J.; Kim, J.-R.; Kim, T.-W.; Lee, Y.-J.; Chae, H.-J. Facile one-pot synthesis of Ni-based catalysts by cation-anion double hydrolysis method as highly active Ru-free catalysts for green H2 production via NH3 decomposition. Appl. Catal. B Environ. 2022, 307, 121167. [Google Scholar] [CrossRef]
- Okura, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Promotion effect of rare-earth elements on the catalytic decomposition of ammonia over Ni/Al2O3 catalyst. Appl. Catal. A Gen. 2015, 505, 77–85. [Google Scholar] [CrossRef]
- Kurtoğlu, S.F.; Sarp, S.; Yılmaz Akkaya, C.; Yağcı, B.; Motallebzadeh, A.; Soyer-Uzun, S.; Uzun, A. COx-free hydrogen production from ammonia decomposition over sepiolite-supported nickel catalysts. Int. J. Hydrogen Energy 2018, 43, 9954–9968. [Google Scholar] [CrossRef]
- Lucentini, I.; Casanovas, A.; Llorca, J. Catalytic ammonia decomposition for hydrogen production on Ni, Ru and Ni Ru supported on CeO2. Int. J. Hydrogen Energy 2019, 44, 12693–12707. [Google Scholar] [CrossRef]
- Muroyama, H.; Saburi, C.; Matsui, T.; Eguchi, K. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Appl. Catal. A Gen. 2012, 443–444, 119–124. [Google Scholar] [CrossRef]
- Okura, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Ammonia Decomposition over Nickel Catalysts Supported on Rare-Earth Oxides for the On-Site Generation of Hydrogen. ChemCatChem 2016, 8, 2988–2995. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Chiodo, V.; Donato, S.; Bonura, G.; Cavallaro, S. Steam and auto-thermal reforming of bio-ethanol over MgO and CeO2CeO2 Ni supported catalysts. Int. J. Hydrogen Energy 2006, 31, 2193–2199. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakamori, T. Steam reforming of methane over nickel catalysts at low reaction temperature. Appl. Catal. A Gen. 2004, 258–266, 107–114. [Google Scholar] [CrossRef]
- Yan, H.; Xu, Y.-J.; Gu, Y.-Q.; Li, H.; Wang, X.; Jin, Z.; Shi, S.; Si, R.; Jia, C.-J.; Yan, C.-H. Promoted Multimetal Oxide Catalysts for the Generation of Hydrogen via Ammonia Decomposition. J. Phys. Chem. C 2016, 120, 7685–7696. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Stepinska, N.; Chalupka, K.; Nowosielska, M.; Maniukiewicz, W.; Rogowski, J.; Goswami, N.; Vasilev, K.; Szynkowska, M. I Effect of the support composition on catalytic and physicochemical properties of Ni catalysts in oxy-steam reforming of methane. Catal. Today 2021, 364, 46–60. [Google Scholar] [CrossRef]
- Kim, H.-M.; Jang, W.-J.; Yoo, S.-Y.; Shim, J.-O.; Jeon, K.-W.; Na, H.-S.; Lee, Y.-L.; Jeon, B.-H.; Bae, J.W.; Roh, H.-S. Low temperature steam reforming of methane using metal oxide promoted Ni-Ce0.8 Zr0.2 O2 catalysts in a compact reformer. Int. J. Hydrogen Energy 2018, 43, 262–270. [Google Scholar] [CrossRef]
- Roh, H.-S.; Eum, I.-H.; Jeong, D.-W. Low temperature steam reforming of methane over Ni–Ce(1−x) Zr(x) O2 catalysts under severe conditions. Renew. Energy 2012, 42, 212–216. [Google Scholar] [CrossRef]
- Huang, C.; Li, H.; Yang, J.; Wang, C.; Hu, F.; Wang, X.; Lu, Z.-H.; Feng, G.; Zhang, R.; Ce0. 6Zr0.3Y0.1O2 solid solutions-supported Ni Co bimetal nanocatalysts for NH3 decomposition. Appl. Surf. Sci. 2019, 478, 708–716. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, J.; Ge, Q.; Xu, H.; Li, W. Effects of CeO2 addition on Ni/Al2O3 catalysts for the reaction of ammonia decomposition to hydrogen. Appl. Catal. B Environ. 2008, 80, 98–105. [Google Scholar] [CrossRef]
- Arene, F. Magnesia-supported nickel catalysts, I. Factors affecting the structure and morphological properties. J. Catal. 1991, 132, 58–67. [Google Scholar] [CrossRef]
- Yu, Y.; Gan, Y.-M.; Huang, C.; Lu, Z.-H.; Wang, X.; Zhang, R.; Feng, G. Ni/La2O3 and Ni/MgO–La2O3 catalysts for the decomposition of NH3 into hydrogen. Int. J. Hydrogen Energy 2020, 45, 16528–16539. [Google Scholar] [CrossRef]
- Yao, L.; Shi, T.; Li, Y.; Zhao, J.; Ji, W.; Au, C.-T. Core–shell structured nickel and ruthenium nanoparticles: Very active and stable catalysts for the generation of COx-free hydrogen via ammonia decomposition. Catal. Today 2011, 164, 112–118. [Google Scholar] [CrossRef]
- Takehira, K. Recent development of layered double hydroxide-derived catalysts − Rehydration, reconstitution, and supporting, aiming at commercial application. Appl. Clay Sci. 2017, 136, 112–141. [Google Scholar] [CrossRef]
- Chen, S.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D.; Centi, G. Electrocatalytic Synthesis of Ammonia at Room Temperature and Atmospheric Pressure from Water and Nitrogen on a Carbon-Nanotube-Based Electrocatalyst. Angew. Chem. Int. Ed. 2017, 56, 2699–2703. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Wang, P.; Kosaka, T.; Takaki, K. Steam reforming of CH4 over supported Ni catalysts prepared from a Mg–Al hydrotalcite-like anionic clay. Phys. Chem. Chem. Phys. 2003, 5, 3801–3810. [Google Scholar] [CrossRef]
- Lin, X.; Li, R.; Lu, M.; Chen, C.; Li, D.; Zhan, Y.; Jiang, L. Carbon dioxide reforming of methane over Ni catalysts prepared from Ni–Mg–Al layered double hydroxides: Influence of Ni loadings. Fuel 2015, 162, 271–280. [Google Scholar] [CrossRef]
- Balsamo, N.; Mendieta, S.; Oliva, M.; Eimer, G.; Crivello, M. Synthesis and Characterization of Metal Mixed Oxides from Layered Double Hydroxides. Proc. Mater. Sci. 2012, 1, 506–513. [Google Scholar] [CrossRef][Green Version]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.C.; Henriques, C.A.; Monteiro, J.L.F. Influence of Ni content on physico-chemical characteristics of Ni, Mg, Al-Hydrotalcite like compounds. Mater. Res. 2003, 6, 563–568. [Google Scholar] [CrossRef]
- Cherepanova, S.V.; Leont’eva, N.N.; Arbuzov, A.B.; Drozdov, V.A.; Belskaya, O.B.; Antonicheva, N.V. Structure of oxides prepared by decomposition of layered double Mg–Al and Ni–Al hydroxides. J. Solid State Chem. 2015, 225, 417–426. [Google Scholar] [CrossRef]
- Seeman, V.; Feldbach, E.; Kärner, T.; Maaroos, A.; Mironova-Ulmane, N.; Popov, A.I.; Shablonin, E.; Vasil’chenko, E.; Lushchik, A. Fast-neutron-induced and as-grown structural defects in magnesium aluminate spinel crystals with different stoichiometry. Opt. Mater. 2019, 91, 42–49. [Google Scholar] [CrossRef]
- Romero, A.; Jobbágy, M.; Laborde, M.; Baronetti, G.; Amadeo, N. Ni(II)–Mg(II)–Al(III) catalysts for hydrogen production from ethanol steam reforming: Influence of the activation treatments. Catal. Today 2010, 149, 407–412. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Duraczyska, D.; Launay, F.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Methane dry reforming over hydrotalcite-derived Ni–Mg–Al mixed oxides: The influence of Ni content on catalytic activity, selectivity and stability. Catal. Sci. Technol. 2016, 6, 6705–6715. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Yang, W.; Kim, Y.; Liu, P.K.T.; Sahimi, M.; Tsotsis, T.T. A study by in situ techniques of the thermal evolution of the structure of a Mg–Al–CO3 layered double hydroxide. Chem. Eng. Sci. 2002, 57, 2945–2953. [Google Scholar] [CrossRef]
- Hibino, T. Decarbonation Behavior of Mg-Al-CO3 Hydrotalcite-like Compounds during Heat Treatment. Clays Clay Miner. 1995, 43, 427–432. [Google Scholar] [CrossRef]
- Su, Q.; Gu, L.; Yao, Y.; Zhao, J.; Ji, W.; Ding, W.; Au, C.-T. Layered double hydroxides derived Nix(MgyAlzOn) catalysts: Enhanced ammonia decomposition by hydrogen spillover effect. Appl. Catal. B Environ. 2017, 201, 451–460. [Google Scholar] [CrossRef]
- Robertson, S. Determination of reducibility and identification of alloying in copper-nickel-on-silica catalysts by temperature-programmed reduction. J. Catal. 1975, 37, 424–431. [Google Scholar] [CrossRef]
- González, A.R.; Asencios, Y.J.O.; Assaf, E.M.; Assaf, J.M. Dry reforming of methane on Ni–Mg–Al nano-spheroid oxide catalysts prepared by the sol–gel method from hydrotalcite-like precursors. Appl. Surf. Sci. 2013, 280, 876–887. [Google Scholar] [CrossRef]
- Perez-Lopez, O.W.; Senger, A.; Marcilio, N.R.; Lansarin, M.A. Effect of composition and thermal pretreatment on properties of Ni–Mg–Al catalysts for CO2 reforming of methane. Appl. Catal. A Gen. 2006, 303, 234–244. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhou, X.P.; Au, C.T. A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl. Catal. A Gen. 2004, 277, 1–9. [Google Scholar] [CrossRef]
- Hu, X.-C.; Wang, W.-W.; Jin, Z.; Wang, X.; Si, R.; Jia, C.-J. Transition metal nanoparticles supported La-promoted MgO as catalysts for hydrogen production via catalytic decomposition of ammonia. J. Energy Chem. 2019, 38, 41–49. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, L.; Zheng, W.; Xu, S.; Yu, Q.; Qiu, X. Nickel-induced structure transformation in hydrocalumite for enhanced ammonia decomposition. Int. J. Hydrogen Energy 2020, 45, 12244–12245. [Google Scholar] [CrossRef]
- Sato, K.; Abe, N.; Kawagoe, T.; Miyahara, S.; Honda, K.; Nagaoka, K. Supported Ni catalysts prepared from hydrotalcite-like compounds for the production of hydrogen by ammonia decomposition. Int. J. Hydrogen Energy 2017, 42, 6610–6617. [Google Scholar] [CrossRef]
- Debye, P. Zerstreuung von Röntgenstrahlen. Ann. Phys. 1915, 351, 809–823. [Google Scholar] [CrossRef]
- Guinier, A. X-ray Diffraction in Crystals, Imperfect Crystals and Amorphous Bodies; Dover: New York, NY, USA, 1994. [Google Scholar]
- Hall, B.D. Debye function analysis of structure in diffraction from nanometer-sized particles. J. Appl. Phys. 2000, 87, 1666–1675. [Google Scholar] [CrossRef]
- Tsybulya, S.V.; Yatsenko, D.A. X-ray diffraction analysis of ultradisperse systems: The Debye formula. J. Struct. Chem. 2012, 53, 150–165. [Google Scholar] [CrossRef]
- Beyerlein, K.R. A review of Debye Function Analysis. Powder Diffr. 2013, 28, S2–S10. [Google Scholar] [CrossRef][Green Version]
- Yatsenko, D.A.; Tsybulya, S.V. DIANNA (Diffraction Analysis of Nanopowders): Software for structural analysis of ultradisperse systems by X-ray methods. Bull. Russ. Acad. Sci. Phys. 2012, 76, 382–384. [Google Scholar] [CrossRef]
- Yatsenko, D.; Tsybulya, S. DIANNA (diffraction analysis of nanopowders)—A software for structural analysis of nanosized powders. Cryst. Mater. 2018, 233, 61–66. [Google Scholar] [CrossRef]
- Li, Y.; Wen, J.; Ali, A.M.; Duan, M.; Zhu, W.; Zhang, H.; Chen, C.; Li, Y. Size structure–catalytic performance correlation of supported Ni/MCF-17 catalysts for COx-free hydrogen production. Chem. Commun. 2018, 54, 6364–6367. [Google Scholar] [CrossRef]
- Henpraserttae, S.; Charojrochkul, S.; Klysubun, W.; Lawtrakul, L.; Toochinda, P. Reduced Temperature Ammonia Decomposition Using Ni/Zr-Doped Al2O3 Catalyst. Catal. Lett. 2018, 148, 1775–1783. [Google Scholar] [CrossRef]
- Henpraserttae, S.; Charojrochkul, S.; Lawtrakul, L.; Toochinda, P. Ni-based Catalysts for Hydrogen Production from Ammonia Decomposition: Effect of Dopants and Urine Application. ChemistrySelect 2018, 3, 11842–11850. [Google Scholar] [CrossRef]
- Sima, D.; Wu, H.; Tian, K.; Xie, S.; Foo, J.J.; Li, S.; Wang, D.; Ye, Y.; Zheng, Z.; Liu, Y.-Q. Enhanced low temperature catalytic activity of Ni/Al–Ce0.8Zr0.2O2 for hydrogen production from ammonia decomposition. Int. J. Hydrogen Energy 2020, 45, 9342–9352. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Weng, C.-C.; Chen, C.; Yuan, Z.-Y. Catalytic decomposition of ammonia to COx-free hydrogen over Ni/ZSM-5 catalysts: A comparative study of the preparation methods. Appl. Catal. A Gen. 2018, 562, 49–57. [Google Scholar] [CrossRef]

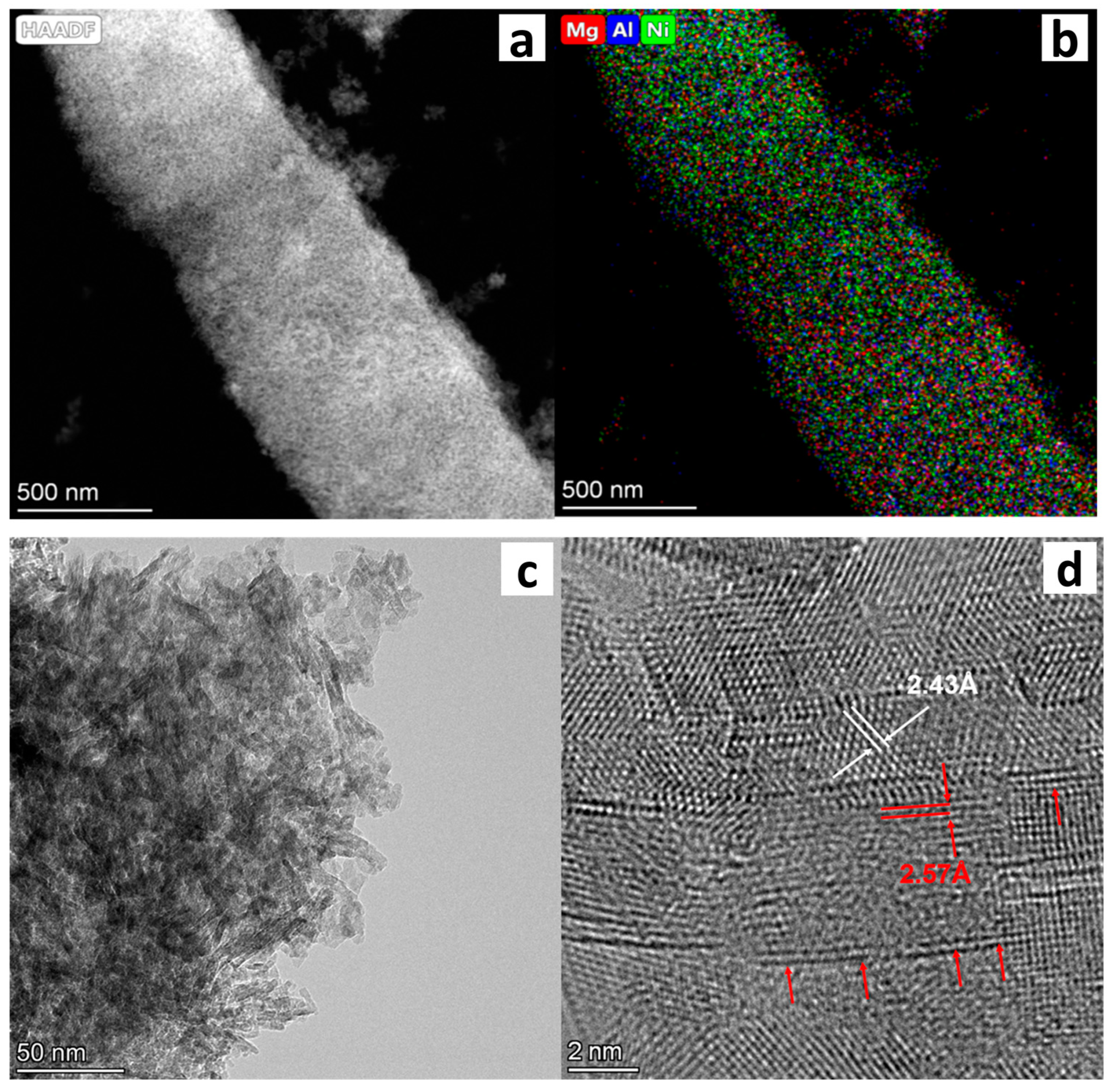
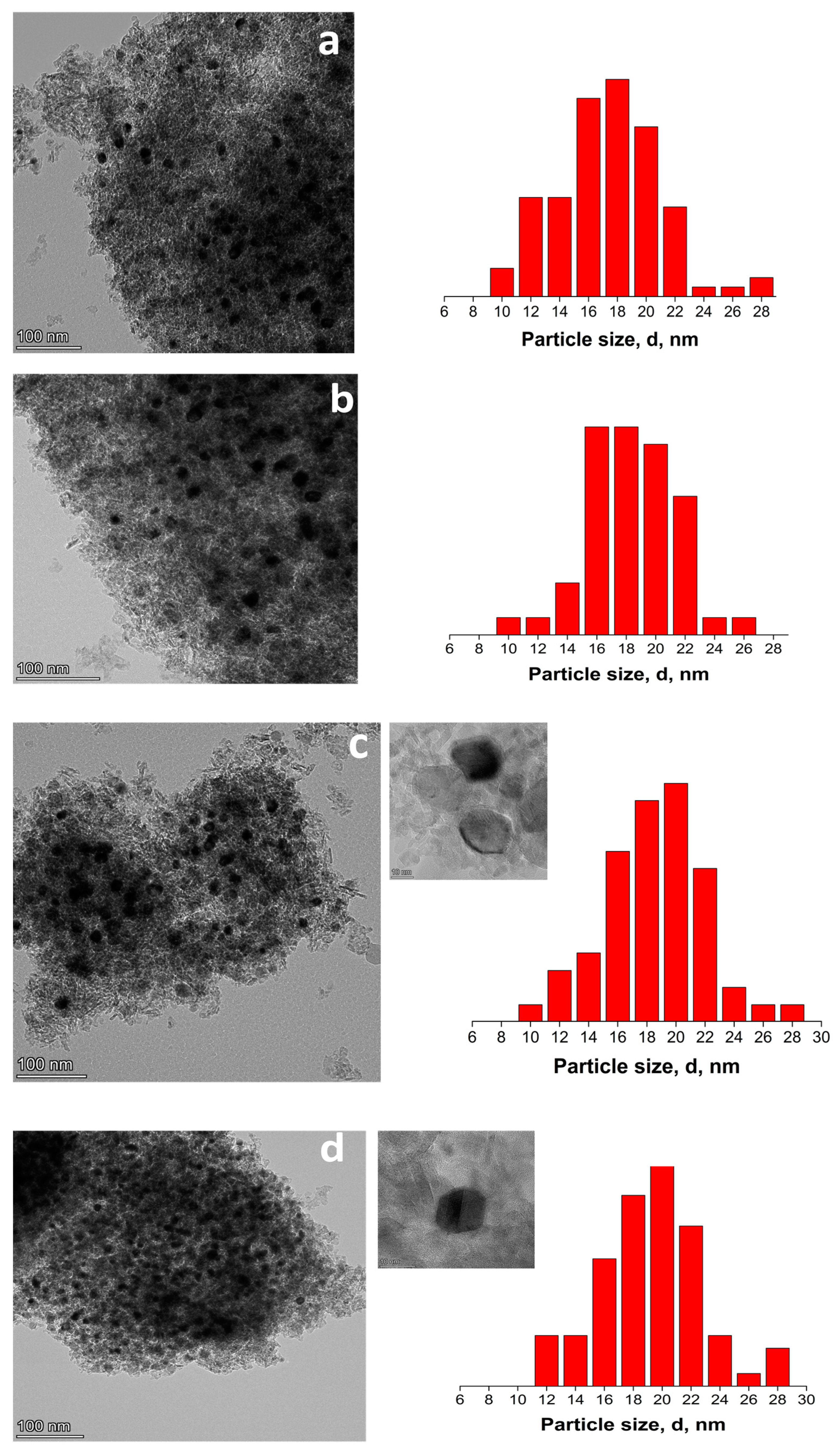

| Sample | NiO Content, wt.% | 110 °C | 600 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBET, m2/g | Unit Cell Parameters | D001, nm | D110, nm | SBET, m2/g | a, nm | D, nm | ||||
| Exp. | Calc. | a, Å | c, Å | |||||||
| NiMg4Al2-HT | 22.5 | 22.1 | 52.7 | 3.048 | 23.036 | 8.5 | 13.0 | 209.1 | 4.181 | 4.0 |
| Ni1.3Mg3.7Al2-HT | 28.4 | 27.9 | 46.9 | 3.047 | 23.045 | 8.0 | 12.0 | 189.5 | 4.179 | 4.0 |
| Ni1.6Mg3.4Al2-HT | 33.8 | 33.2 | 52.1 | 3.047 | 23.089 | 8.0 | 11.0 | 179.8 | 4.174 | 4.0 |
| Ni2Mg3Al2-HT | 43.4 | 41.1 | 35.1 | 3.047 | 23.125 | 7.5 | 11.0 | 156.1 | 4.174 | 4.0 |
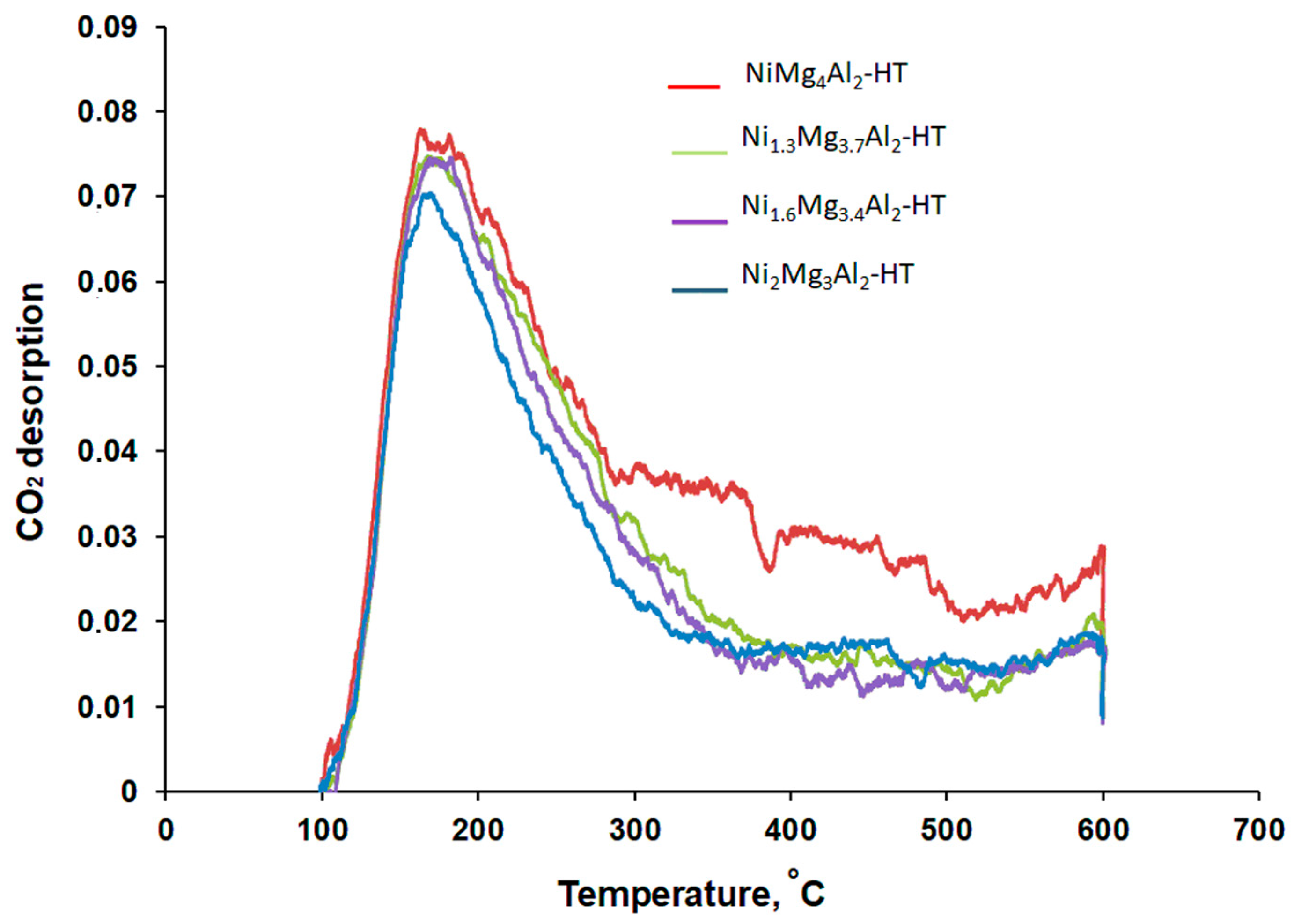
| Catalyst | Tмax, °C | The Amount of CO2 Desorbed | |
|---|---|---|---|
| µmol/g | µmol/m2 | ||
| Ni1Mg4Al2-HT | ~163, with the shoulder at more high T | 183 | 0.9 |
| Ni1.3Mg3.7Al2-HT | 169 | 143 | 0.8 |
| Ni1.6Mg3.4Al2-HT | 169 | 148 | 0.8 |
| Ni2Mg4Al2-HT | 166 | 133 | 0.9 |
| Catalyst | Ni Content wt.% | GHSV, mL/(gcat·h) | T, °C | Conversion, % | H2 Rate, mmol/(gcat·min) | T50, °C | Ref. |
|---|---|---|---|---|---|---|---|
| NiMg4Al2-HT | 16.9 | 72,000 | 500 | 20.7 | 16.6 | 608 | This study |
| Ni1.3Mg3.7Al2-HT | 21.5 | 72,000 | 500 | 25.9 | 20.8 | 570 | This study |
| Ni1.6Mg3.4Al2-HT | 25.3 | 72,000 | 500 | 26.2 | 21.1 | 600 | This study |
| Ni2Mg3Al2-HT | 30.7 | 72,000 | 500 | 29.6 | 23.8 | 590 | This study |
| NIAP-13-02 | 22.5 | 72,000 | 500 | 15.8 | 12.7 | 616 | This study |
| Ni0.6Al0.9On | 40.5 | 30,000 | 500 | 31.0 | 10.4 | 530 | [42] |
| Ni0.6Mg0.3Al0.6On | 40.1 | 30,000 | 500 | 42.0 | 14.1 | 508 | [42] |
| 20Ni/La-MgO | 20 | 22,000 | 500 | 84.0 | 21.0 | 449 | [47] |
| Ni/C-LDHs-ST | 23.6 | 30,000 | 500 | 20.4 | 6.8 | 549 | [48] |
| Ni_MgAl | 15.0 | 60,000 | 500 | 17.0 | 11.4 | 582 | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorova, Z.A.; Borisov, V.A.; Pakharukova, V.P.; Gerasimov, E.Y.; Belyaev, V.D.; Gulyaeva, T.I.; Shlyapin, D.A.; Snytnikov, P.V. Layered Double Hydroxide-Derived Ni-Mg-Al Catalysts for Ammonia Decomposition Process: Synthesis and Characterization. Catalysts 2023, 13, 678. https://doi.org/10.3390/catal13040678
Fedorova ZA, Borisov VA, Pakharukova VP, Gerasimov EY, Belyaev VD, Gulyaeva TI, Shlyapin DA, Snytnikov PV. Layered Double Hydroxide-Derived Ni-Mg-Al Catalysts for Ammonia Decomposition Process: Synthesis and Characterization. Catalysts. 2023; 13(4):678. https://doi.org/10.3390/catal13040678
Chicago/Turabian StyleFedorova, Zaliya A., Vadim A. Borisov, Vera P. Pakharukova, Evgeniy Y. Gerasimov, Vladimir D. Belyaev, Tatyana I. Gulyaeva, Dmitriy A. Shlyapin, and Pavel V. Snytnikov. 2023. "Layered Double Hydroxide-Derived Ni-Mg-Al Catalysts for Ammonia Decomposition Process: Synthesis and Characterization" Catalysts 13, no. 4: 678. https://doi.org/10.3390/catal13040678
APA StyleFedorova, Z. A., Borisov, V. A., Pakharukova, V. P., Gerasimov, E. Y., Belyaev, V. D., Gulyaeva, T. I., Shlyapin, D. A., & Snytnikov, P. V. (2023). Layered Double Hydroxide-Derived Ni-Mg-Al Catalysts for Ammonia Decomposition Process: Synthesis and Characterization. Catalysts, 13(4), 678. https://doi.org/10.3390/catal13040678