Abstract
Recent advances in atmospheric plasmas have led to the formation of nonthermal plasma (NTP). In recent decades, a number of novel plasma diagnostic approaches have been implemented and reported in order to better understand the physics of NTP. The use of NTP is a novel approach to producing reactive oxygen and nitrogen species. Plasma technology has many applications, including electrical device microfabrication, biomedicine, dentistry, agriculture, ozone generation, chemical synthesis, surface treatment, coating, and disease therapy. Furthermore, NTP is thought to be a successful strategy for the degradation of hazardous pollutants in the environment, making it a future hope. Recent studies showed that various operating parameters affect the yield of NTP-based technology. Especially, the presence of a catalyst, properly placed in an NTP reactor, leads to a significant increase in process performance as compared to NTP alone. Scientists have looked at using NTP in conjunction with catalysts to remove various sorts of pollutants from the environment. In this context, review articles are crucial due to the prevalence of NTP-based applications and ongoing developments. This review will describe recent advancements in NTP-based biomedical applications, bacterial inactivation, food preservation and storage, and environmental catalytic formulations. This review could be useful in providing a platform for advancements in biological applications and environmental protection through the use of NTP technology.
1. Introduction
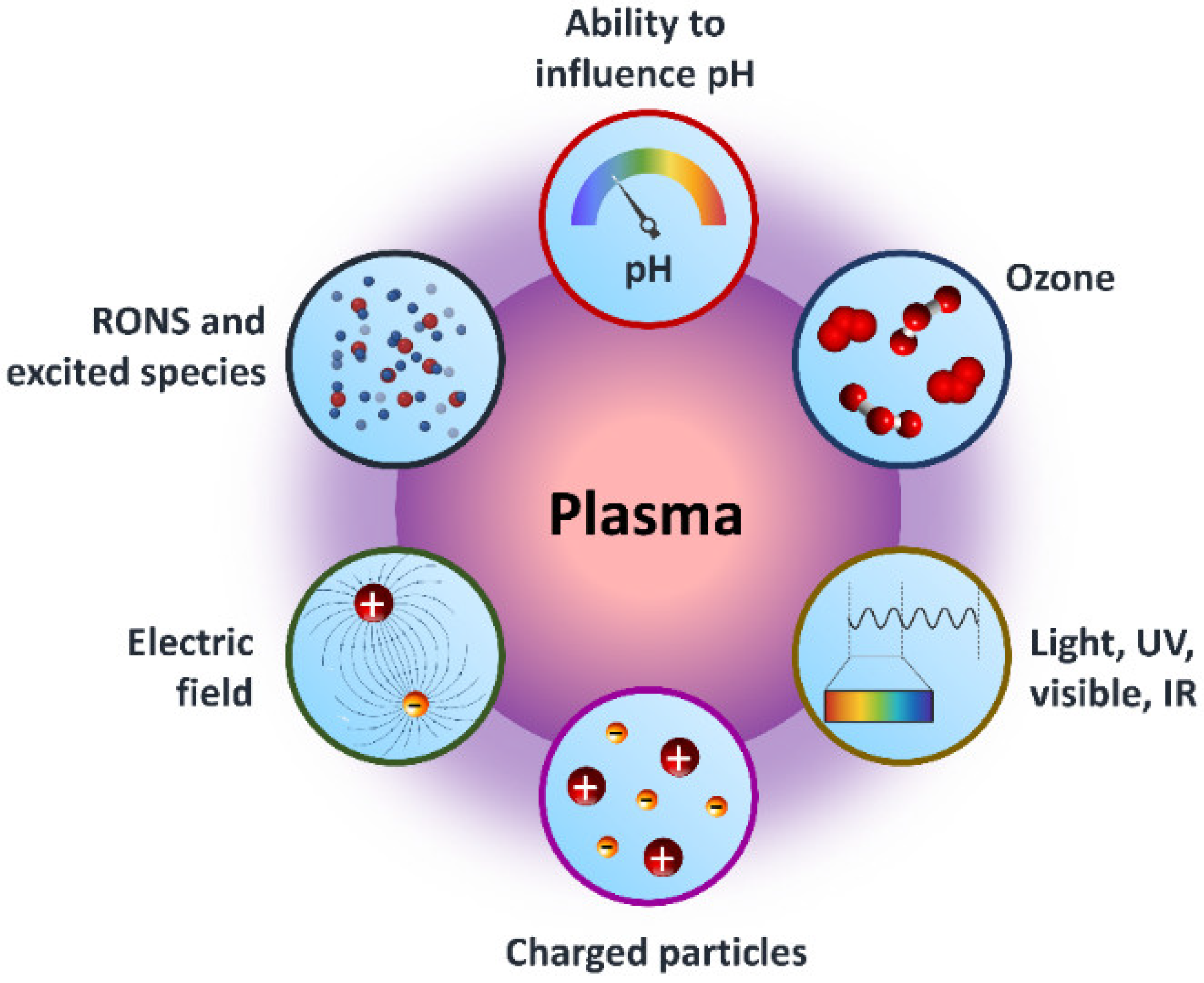
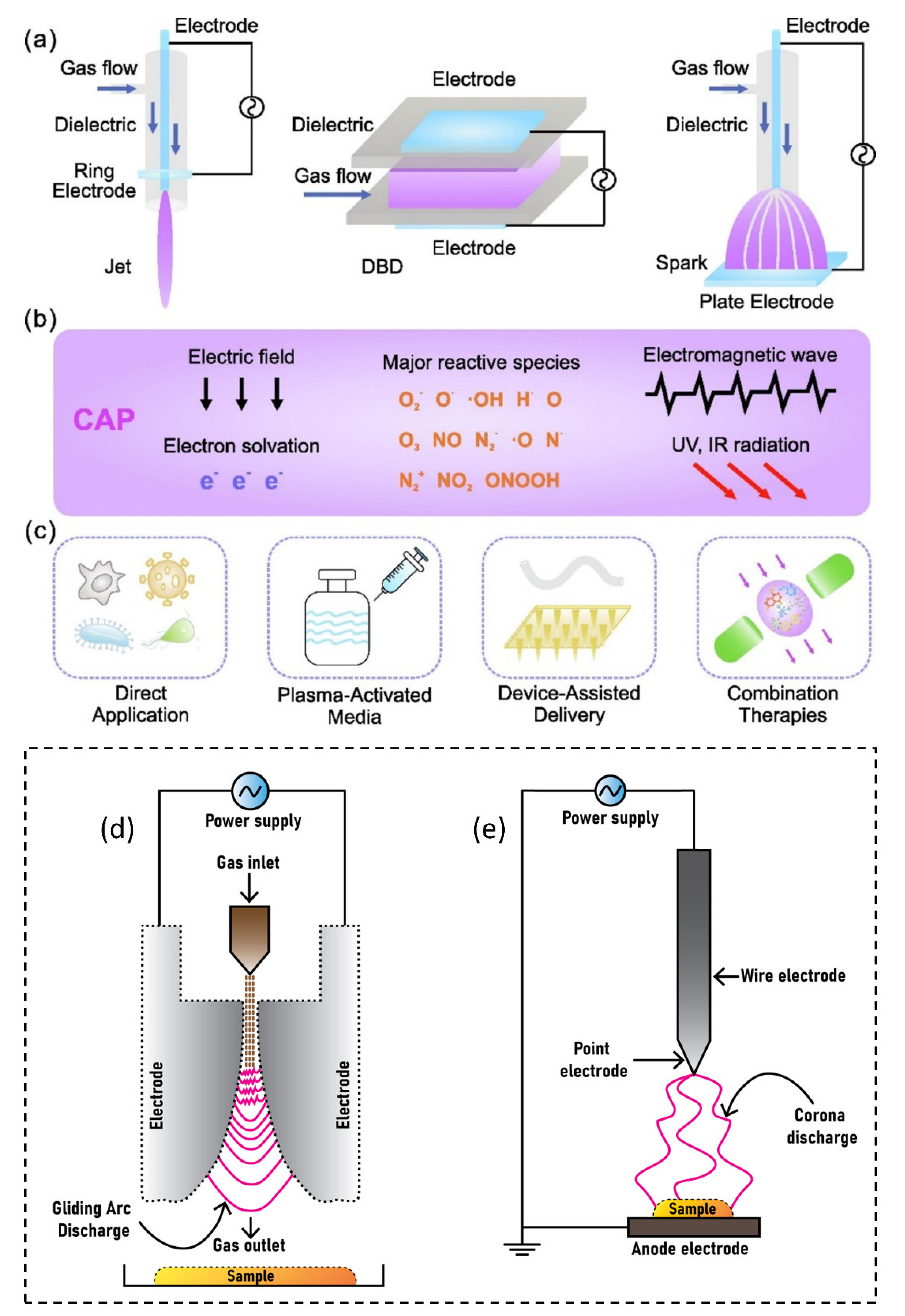
The American physicist Irving Langmuir identified plasma as a fourth state of matter in 1922 [1]. Plasmas are gases that have been totally or partially ionized. In order to remove electrons from atoms, enough energy is given to the gas, resulting in a combination of free electrons, free radicals, neutrals, and positively charged species [2,3,4]. Nonthermal plasma (NTP) or cold atmospheric plasma differs from thermal plasma because of the properties of electrons, ions, and neutrality. The charge species, reactive oxygen species (ROS), reactive nitrogen species (RNS), electromagnetic field, electric field, ability to influence pH, visible light, charged particles, neutral species, ozone, and ultraviolet (UV) radiations were all found in NTP as shown in Figure 1, making it a feasible tool for a variety of tasks [5,6,7,8,9]. The temperature of plasma electrons might reach tens of thousands of kelvin, significantly exceeding the neutral gas’ temperature of roughly room temperature. In bulk, most plasmas are electrically neutral, which adds to plasma’s unique properties and categorization as the fourth state of matter. Plasma parameters can vary dramatically depending on the energy source and amount delivered. Furthermore, NTP devices such as jet plasma, dielectric barrier discharge (DBD) plasma, and spark plasma depicted in Figure 2a often operate in a room environment, making them ideal for life science research [9,10,11,12] and a wide range of biomedical applications [13,14,15,16,17,18,19,20,21,22]. Sterilization, skin disinfection, oral/dental disease treatment, blood coagulation, wound healing, cancer therapy, and immunotherapy have all seen new possibilities in medicine by using NTP [23]. Gliding arc discharge (Figure 2d) and corona discharge (Figure 2e) devices are commonly used for environmental applications such as the removal of gaseous pollutants.
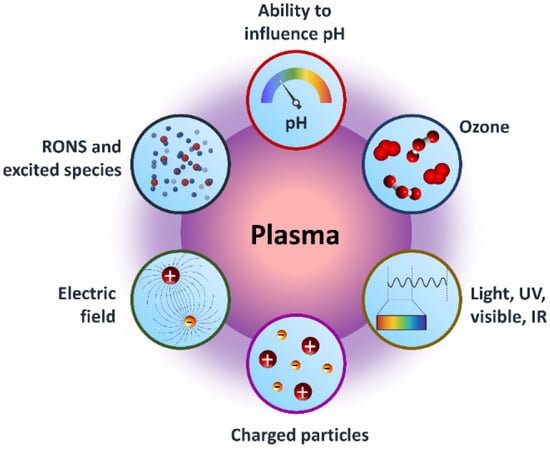
Figure 1.
Graphical representation of core components of NTP for dealing with the surroundings in a variety of applications.
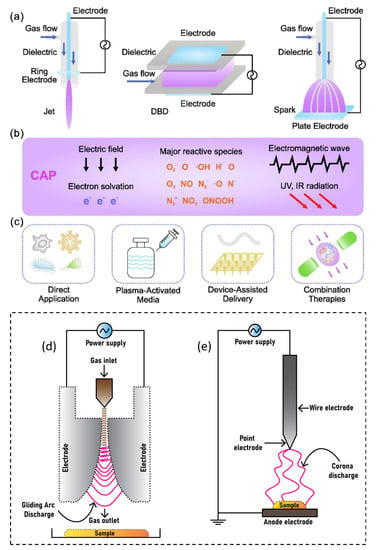
Figure 2.
NTP delivery schematic for biomedical applications: (a) typical NTP devices, including plasma jet, DBD, and spark discharge; (b) plasma environment containing reactive species, electrons, other ions, emissions, waves, and physical forces; and (c) plasma delivery strategies, including direct application, plasma-activated media, device-assisted delivery, and therapeutic approaches. Reprinted with permission from Ref. [1]. Copyright 2022, Elsevier. The most common devices used for environmental applications are (d) gliding arc discharge plasma and (e) corona discharge plasma.
1.1. Plasma
As plasma makes up 99% of the universe, it is the most prevalent kind of matter. The Sun and other stars, galaxies, solar winds, lightning, and the aurora borealis are all examples of the plasma state. Plasma televisions, neon and fluorescent lights, and plasma displays are well-known applications for man-made plasma. From Figure 1 and Figure 2c, it can be seen that plasma is made up of neutral, ionized, and/or excited charged particles, ions, and molecules, and ozone with the ability to influence the pH of solutions, as well as the existence of different ROS and RNS. A significant source of UV and vacuum UV radiation is plasma made up of several gases [24]. Plasma is a unique material treatment technology since it can employ a single component or a mix of components.
1.2. Thermal and Nonthermal Plasmas
All of the particles in a thermal plasma are at about the same temperature and completely ionized. Hot, completely ionized, and equilibrium plasma are other names for thermal plasma. Depending on the demands, equilibrium plasma is used in a variety of applications [25]. Plasma is a (partially) ionized gas made up of neutral species (molecules, radicals, excited species), ions, photons, and electrons. As the electron temperature is much higher than the temperature of heavy species (ions and neutrals) in nonthermal plasma (NTP) or non-equilibrium plasma, radicals and excited species are formed at temperatures that are closer to room temperature. This nonthermal energy distribution provides a potential route to getting around both the kinetic and thermodynamic constraints on the chemical conversion of reactants into desired products. Cold, partially ionized, atmospheric pressure, low temperature, and non-equilibrium plasma are other names for NTP. The NTP technology is suitable for treating a range of biological materials, including solids, liquids, and aerosols because it is at a low temperature when applied. There are several uses for two different types of NTP, low pressure and atmospheric pressure [26,27,28,29]. Although atmospheric-pressure plasma is only permitted in areas with strong electric fields, low-pressure plasma can spread across a considerable area [30]. When compared to low-pressure plasma, atmospheric pressure may offer more appealing applications. Plasma sustainability is frequently achieved via electric discharges. Despite the fact that the presence of reactive species affects the temperature of gas, it has a much higher chemical reactivity than the source gas. Atmospheric pressure plasma has been used in biological systems for practical reasons [28].
2. Generation of Reactive Species in NTP Discharge
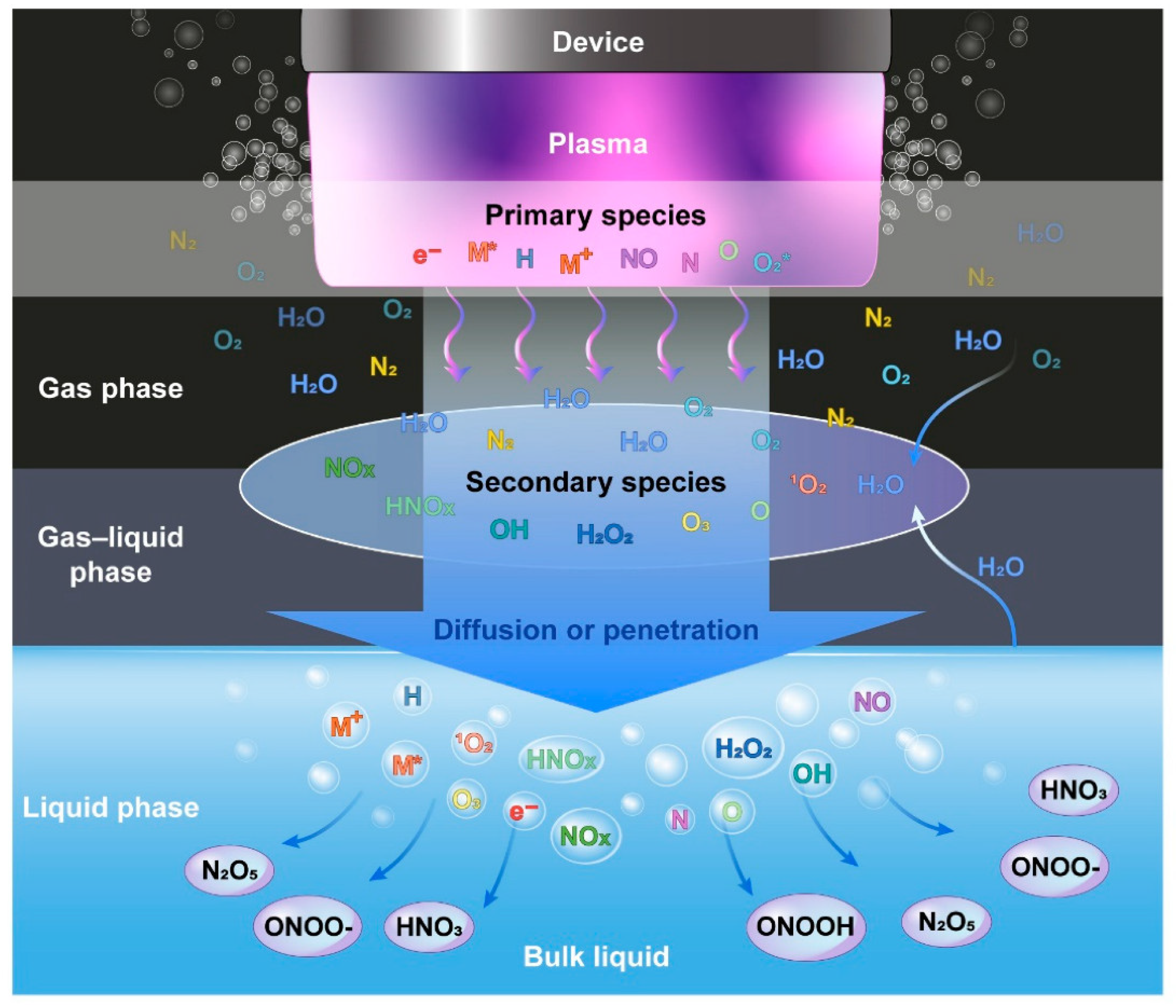
The NTP is well recognized for generating exceptionally high concentrations of reactive species. NTP produces a variety of reactive oxygen species (ROS), including OH radicals, O2, H2O2, O, O3, and 1O2, as well as reactive nitrogen species (RNS), such as NO, NO2, N2O, N2O5, and atomic N [5]. Some of them, such as OH radicals, O, NO, N, and 1O2, have a short lifespan [19]. Helium plasma discharge was also applied by a number of studies [31,32,33,34]. The mechanisms by which ROS are produced in helium plasma jets have been well reported [35]. A number of species, including N2+, atomic (He, O) radicals, and molecular OH, were discovered in helium discharge [32,34]. The RONS, also referred to as primary reactive species, are generated by the energy released during collisions between accelerating electrons and neutrals. In the gas phase right after the collision, electrons (e−), ionized neutrals and gas (M+), excited neutrals and gas (M*), N, O, atomic H, NO, and O2*- are produced [13]. They are categorized as primary reactive species [36], and the intensities of these species are very high in the plasma region. The lifetimes of primary reactive species are relatively short; for example, the lifespan of OH radicals, NO, and O2*−, is 2.7 μs, 1.2 μs, 1.4 μs, and 1.3 μs, respectively [37]. Some of these reactive species immediately experience radiative decay, while others combine with neutrals, water molecules, and other reactive species. The main reactive species change into secondary reactive species such as H2O2, NO2, NO3, and O3 [38] in an ambient environment, as shown in Figure 3. The liquid phase (or another target) is where the RONS produced in the gas phase dissolve and form tertiary reactive species. The tertiary reactive species are formed when the RONS produced in the gas phase dissolve in the liquid phase [36]. Long-living reactive species include O3, H2O4, NO3, and NO2 because of their lifespans of a few milliseconds over several days [39]. Water can dissolve H2O2, NO2, and NO3; NO2 and NO3 are immediately converted into NO2− and NO3−, respectively [13,40]. Depending on the plasma source, working gas, power source, treatment time, and sample volume, these reactive species can reduce the pH of the target liquid by up to 2 [40]. However, whether a target is dry or aqueous affects the chemistry of ROS and RNS formation in a target [40]. The target, gas region, plasma/target interface, and discharge region of RONS formation are schematically depicted in Figure 3.
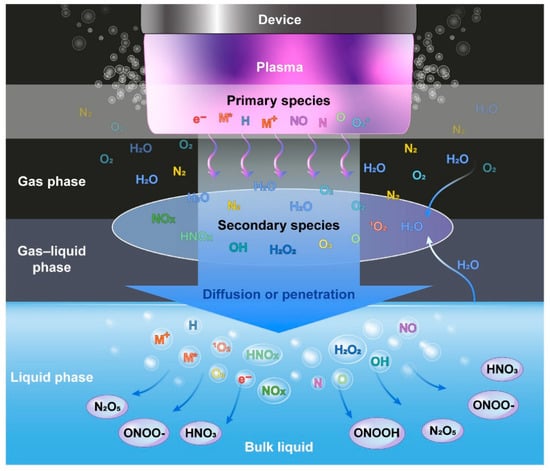
Figure 3.
Graphical representation of formation of reactive species in plasma discharge, gas phase, gas–liquid phase, and liquid phase [38].
DBD plasma and jet plasma have been shown to produce high levels of ROS [26,41,42]. Indirect plasmas are produced between two electrodes of certain devices and transferred to the application region through a gas flow. ROS are often produced at the border of jets with the surrounding air by a variety of causes. Several authors claim that ROS generated by plasma can induce morphological alterations, membrane depolarization, lipid peroxidation, and DNA damage in cells [43,44,45,46]. The transport of reactive oxygen/nitrogen species (RONS) is the major mechanism of NTP anti-neoplastic action [47]. The quantity of reactive species created in plasma-treated liquids is extremely important in plasma therapy. Several lines of study now focus on utilizing plasma to treat cancer using the ROS generation feature [48,49,50]. In human cells, plasma treatment induces the mitochondrial membrane potential to depolarize, resulting in the generation of ROS [51]. The therapeutic effects of air plasma have been linked to the generation of RONS such as H2O2, Ox, OH, •O2, and NOx as a result of mitochondrial membrane potential depolarization and ROS accumulation [49]. Previous reports provide a more thorough explanation of plasma–liquid interactions and the plasma roadmap [23,52,53].
3. NTP Application for Cancer Treatment
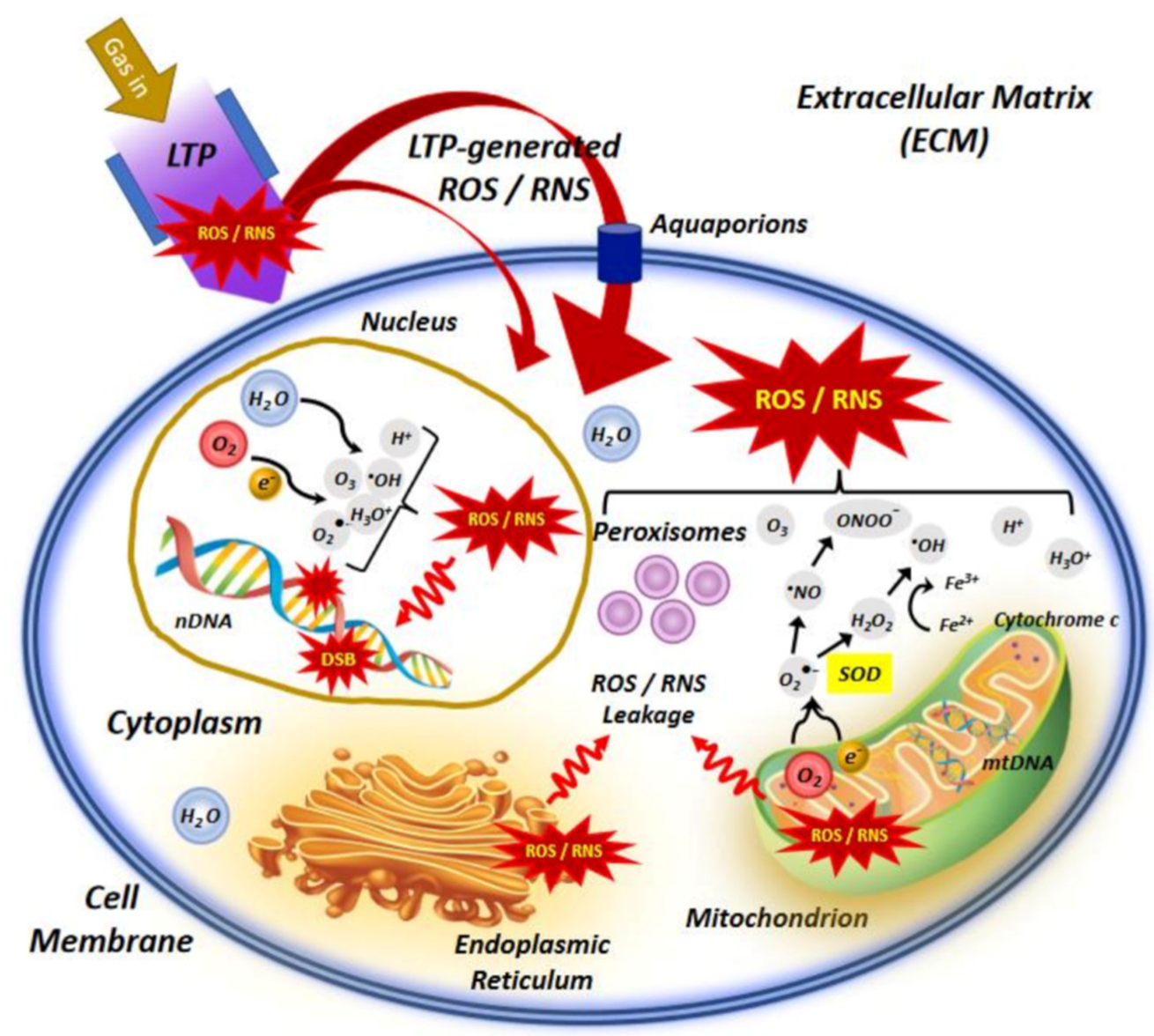
The NTP technology has advanced dramatically in the previous two decades, from theoretical and experimental study to real-world implementations. The generation of plasma at atmospheric pressure and room temperatures to deal with biological systems has given rise to a new multidisciplinary field known as “plasma medicine” [2]. NTP has a variety of potentials in biomedical engineering nowadays [54]. Figure 4 depicts the interaction of NTP with the biological system, indicating the main molecular mechanisms involved in the use of LTP in cancer treatment. NTP technology has the potential to provide a less intrusive surgical procedure for removing particular cells without causing injury to the surrounding tissue. Traditional laser surgery relies on heat contact, which can result in unintentional cell death (necrosis) and lasting tissue damage. NTP contact with tissue, on the other hand, may allow selective cell elimination without necrosis [55]. Cell detachment without influencing cell viability, regulated cell death, and other interactions are examples of these interactions. It can also be employed for cosmetic approaches to dermis reticular architecture regeneration.
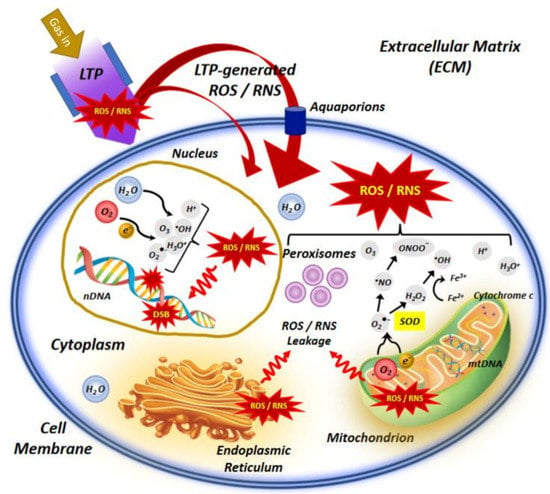
Figure 4.
An illustration of the interaction of NTP or low−temperature plasma (LTP) with the cell, indicating the main molecular mechanisms involved in the use of LTP in cancer treatment [56].
The goal of plasma contact with tissue is to operate below the temperature damage threshold and cause chemically specific reactions or alterations, rather than to denature the tissue. The presence of plasma, in particular, can enhance chemical processes that have the desired effect. Pressure, gas composition, and power may all be adjusted to enhance chemical reactions. As a result, finding plasma conditions that generate a positive influence on tissue without having heat treatment is crucial. NTP produces a variety of reactive species that can be employed to increase cancer cells’ oxidative stress and ultimately kill them [41,57,58]. Different ROS can be produced in plasma, and some of them can cause oxidative stress in cells [47]. As a result, it can alter any pathway that is regulated by or connected to ROS, either directly or indirectly [41,59]. Recent research has found that cancer cells generate more ROS [60] and are thus prone to oxidative stress higher than normal cells, making them more appropriate for targeting by ROS in combination with plasma technology [61,62]. Because NTP-killing activity is greater in cancer cells than in normal cells, the outcome of an NTP cancer treatment is more optimistic [62]. NTP medicinal applications have achieved this incredible breakthrough from initial discovery through fundamental scientific research to clinical applications [63].
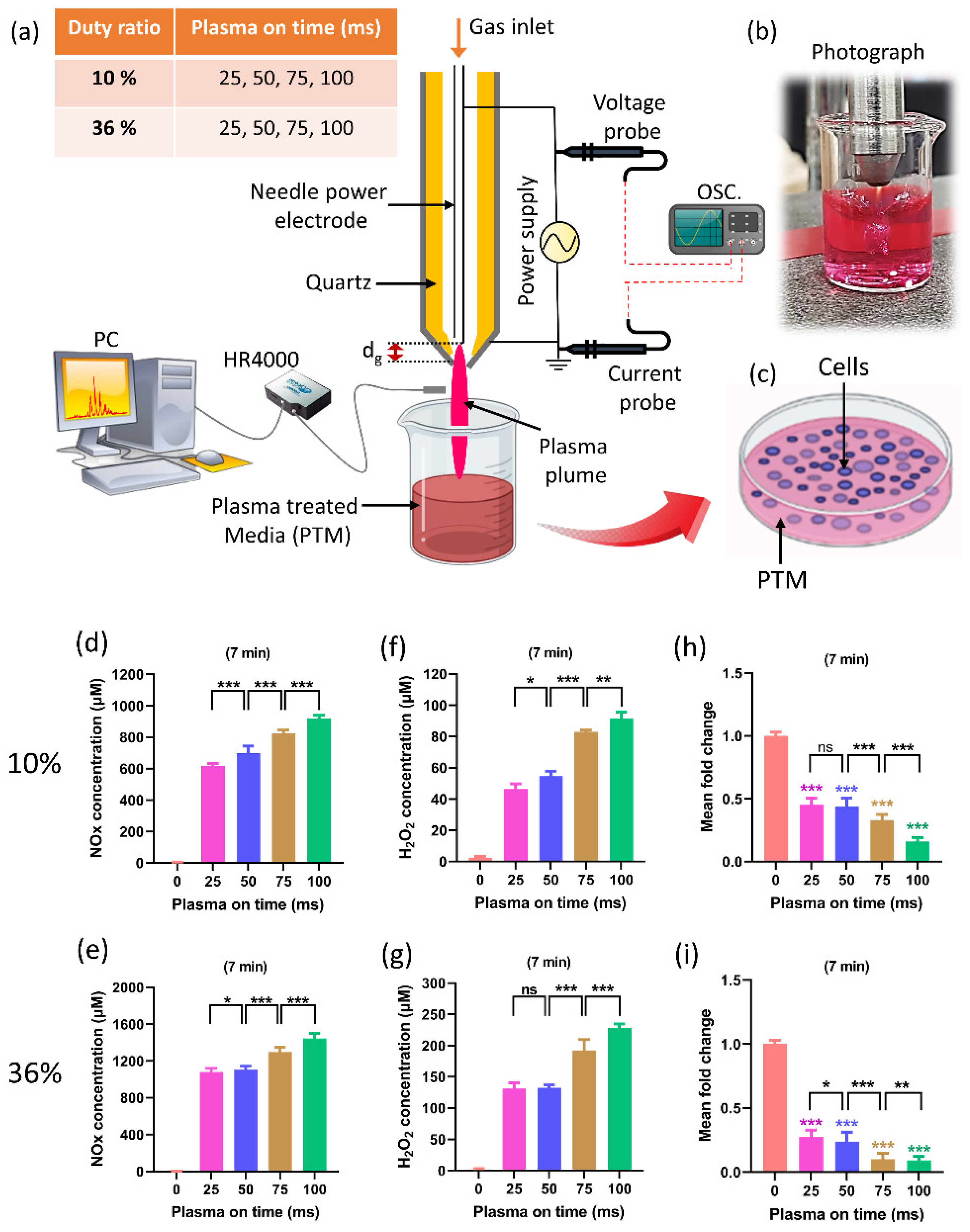
Previous studies dealt with the optimal duration of treatment to maximize plasma’s anticancer effects [33,64,65,66]. The improvement of plasma on-time during treatment is a critical issue that needs to be addressed. The biomedical effects are directly impacted by RONS levels, which are directly impacted by plasma on-time. Recently, research on the effects of plasma on-times was conducted while keeping the duty ratio and treatment time fixed (Figure 5) [67]. The selected plasma on-times are 25, 50, 75, and 100 ms for 10% and 36% duty ratios, respectively. Figure 5a–c depict, respectively, the experimental setups, a photo taken during the preparation of PTM using a soft plasma jet, and the application of PTM to cells. Figure 5d,e show that the NOx concentration in PTM corresponds to the plasma on-time, in 10% and 36% duty ratios. Similarly, Figure 5f,g show the concentrations of H2O2. Figure 5h,i indicate the viability of the U87-MG cell line when PTM was applied. It is interesting to note that by increasing the plasma on-time, the levels of ROS/RNS dramatically increased in PTM and significantly impacted the viability and ATP levels of the U87-MG cell line. The findings of this study offer a significant indication of progress by introducing the optimization of plasma on-time to enhance the effectiveness of the soft plasma jet for biomedical applications [67]. It is interesting to observe from this research that the ROS/RNS levels can be altered to suit needs by adjusting the plasma on-time within the fixed duty ratio and treatment time.
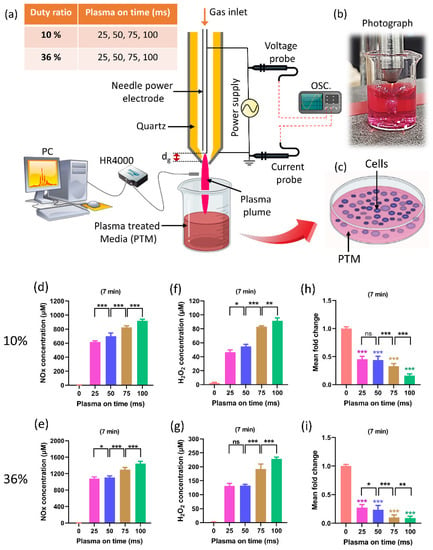
Figure 5.
(a) The schematic of soft plasma jet and experimental setup. When preparing PTM, two fixed duty ratios of 10% and 36% were kept, as well as a fixed treatment time of 7 min; only the plasma on-time was changed (25, 50, 75, and 100 ms). (b) Photograph of soft jet during treatment, and (c) the application of PTM to the U87-MG cell line. (d,e) The NOx content in PTM by changing the plasma on-time when duty ratio (10% and 36%) and treatment time are fixed. (f,g) H2O2 content corresponding to plasma on-time in 10% and 36% duty ratio. (h,i) The cell viability by changing the plasma on-time. The NOx and H2O2 content significantly increased when only plasma on-time increased to 25, 50, 75, and 100 ms which shows further decline in the viability of brain cancer cells [67]. The significance of treatment groups indicated by * p < 0.05, ** p < 0.01, *** p < 0.001, ns—not significant.
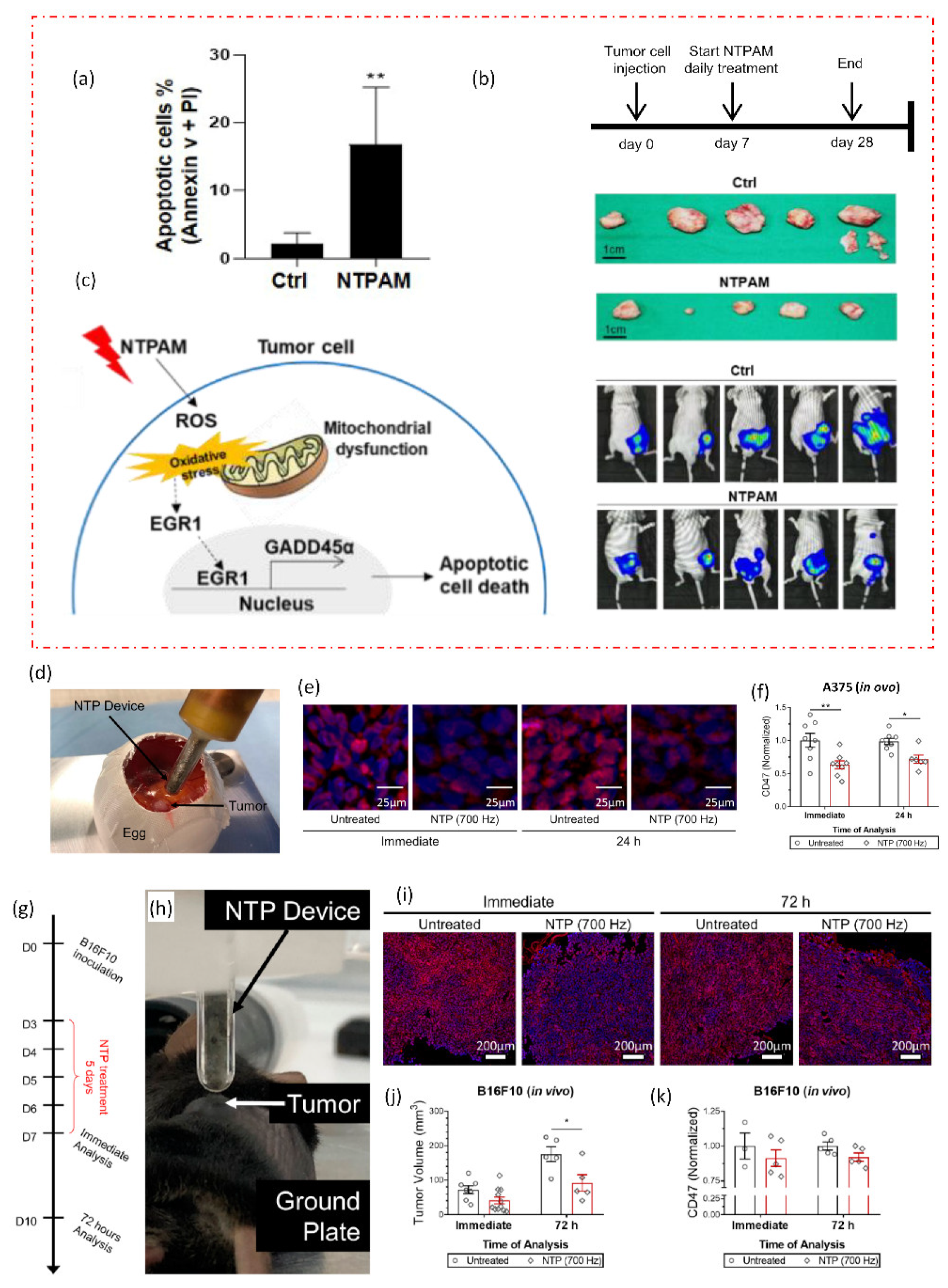
A new cancer treatment modality is being developed by the multidisciplinary field of plasma medicine, which combines plasma physics, chemistry, biology, and clinical medicine. In order to specifically target malignant cells for the prevention of cell proliferation and tumor progression, it primarily relies on the use of low-temperature plasmas in atmospheric pressure. A comprehensive discussion is given of intracellular mechanisms of action, important pathways, and the selectivity of NTP against cancer cells as shown in Figure 4. The in vivo, in vitro, and in ovo experiments are all studied using the NTP technology. Figure 6 depicts two recent in vivo and in ovo experiments using NTP for anticancer purposes. According to reports and widespread knowledge, the NTP raises the rate of apoptosis in cancer cells when compared to the control group (Figure 6a) [68].
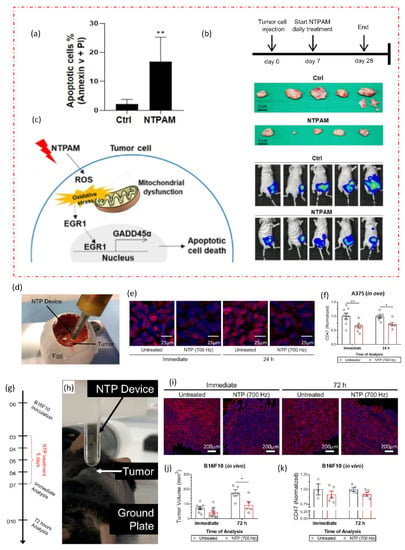
Figure 6.
NTP applications in in vivo and in ovo studies. (a) The effects of nonthermal plasma-activated medium (NTPAM) on apoptosis in thyroid cancer (THCA) cells, which exhibit an increased percentage of apoptosis following NTPAM treatment. NTPAM has anticancer activity in an in vivo xenograft model. (b) Diagrammatic representation of the in vivo experimental plan. Following the injection of mice with FRO-Luc cells, final tumor images of cancer cells tracked with the IVIS imaging system, and last tumor images. (c) potential mechanism according to study, the THCA ROS/EGR1/GADD45α axis is induced by NTPAM [68]. In an in ovo model, NTP treatment reduced CD47 expression in the A375 melanoma tumors. (d) Illustration of fertilized eggs that have been treated directly with NTP. Tumors were removed immediately after treatment or 24 h later, sectioned, and (e) stained with CD47 (red) and counterstained with DAPI (blue). (f) CD47 quantification was compared to untreated. In vivo, NTP treatment decreased tumor volume and marginally decreased CD47 expression. (g,h) Experimental design and NTP treatment directly for 5 days after developing B16F10 melanoma tumors in mice. (i) Tumors were removed either immediately after treatment or 72 h after treatment. (j) Following treatment, tumor volumes were also decreased. (k) CD47 quantification [69]. The significance of treatment groups indicated by * p < 0.05, ** p < 0.01, ns—not significant.
There is still a lack of understanding of the molecular mechanisms underlying NTP’s therapeutic effect on thyroid cancer. Understanding the anticancer effects of NTP-activated medium (NTPAM) on thyroid cancer cells and clarifying the signaling mechanisms causing NTPAM-induced thyroid cancer cell death was explored in a recent study [68]. The in vivo analysis demonstrates that, when compared to control groups, NTPAM-treated groups had significantly lower tumor weights (Figure 6b). The findings of the reported study showed that NTPAM inhibited THCA cell growth more potently than the control did and that ROS controlled EGR1/GADD45α to mediate NTPAM-induced apoptotic cell death (Figure 6c). Recent research offers a fresh understanding of the basic chemical mechanisms underlying NTP–cancer cell interactions as well as a previously unrecognized benefit of current NTP cancer therapy: lowering immunosuppressive signals on the surface of cancer cells (Figure 6g–k) [69]. Researchers can better explain many disagreements by having a thorough understanding of the underlying mechanisms. This includes choosing the best plasma parameters to regulate the combination and concentration of reactive species, delivering plasma to deep-lying tumors, and figuring out the best plasma dose to achieve particular clinical translational outcomes. Designing low-temperature plasma sources that satisfy medical device technical standards is a unique approach for cancer therapy in clinical trials, but it still has to be safer and more effective. Table 1 provides a summary of some recent NTP developments made by researchers for anti-cancer applications.

Table 1.
An overview of recent NTP developments for anti-cancer applications.
4. Role of Plasma Technology in Food Decontamination and Storage
4.1. Microbial Inactivation
Plasma technology’s source of ROS and RNS is mainly concerned with increasing the yields of cultivars by stimulating seedling growth and inactivating microorganisms to increase the shelf life of food storage and fulfill the scarcity of food from all over the world [90,91]. Several treatment methods increased the concentration of intrabacterial reactive species such as H2O2, NO3, NO2, and O3. These reactive species are caused by oxidative stress, which prevented the ability of biofilms to regenerate [92]. The inactivation of E. coli bacteria was reported due to the UV radiation produced during the PAW [93]. The PAW has bactericidal properties due to the RONS. Conidium is an asexually generated fungal spore, a notorious plant disease inhibited by the reactive species in the PAW [94]. Most crops are destroyed by serious diseases by wall-less bacteria called phytoplasmas. Yellow grapevines can easily become infected with phytoplasma [95]. Phytoplasma caused disease in yellow grapevines controlled by the plasma-treated liquids which stimulate the defensive enzymes stilbene synthase and phenylalanine ammonia. The presence of RONS in PAW solution results in oxidative stress, which activates the stilbene metabolic pathway and increases the antioxidant properties. Resultantly, the research shows that PAW treatment can be used to enhance plants’ resistance to diseases [96].
4.2. Effect of NTP on Biofilms
Biofilms are multicellular communities of microbial cells, connected to a surface indefinitely and enveloped by a matrix predominantly consisting of polysaccharides, extracellular nucleic acid, and protein. In nature, more than 95% of the bacteria exist as a biofilm which favors their growth on solid surfaces [97]. Bacterial biofilm formation involves many steps. Quorum sensing is a special type of signaling required which occurs between microbial cells, and the transcription of a different set of genes is also a basic requirement for biofilm formation. Bacterial inactivation or bio-decontamination, as well as the sterilization of surfaces by NTP, have gained a lot of interest in recent years [98]. It is well-known that NTP has antibacterial effectivity and can inactivate planktonic bacteria, yeast, and spores. The effects of plasma application on biofilms seem to be a promising new direction in biofilm removal technology [99]. Since NTP, particularly atmospheric-pressure plasma jets (APPJs), are frequently operated at temperatures close to room temperature, they can be used directly on heat-sensitive materials such as human tissues. Due to the APPJs’ transitory character and accompanying thermal non-equilibrium, the plasma chemistry is improved, and charged species are transported to the targets quickly and efficiently [100]. The reactive species formed by plasmas are expected to comprise a combination of charged particles and chemically active species producing ultraviolet (UV) light (e.g., O3, O2, NO, H2O2, and OH) which contribute to the anti-microbial effects by inflicting damage on DNA, lipids, proteins, etc., leading to cellular structure degradation [101]. As we know, bacterial biofilms consist of DNA, proteins, and polysaccharides which are affected or damaged by plasma-generated reactive species which are made up of charged particles and chemically active species and the generation of UV radiation.
4.3. Sustaining Food Freshness and Storage
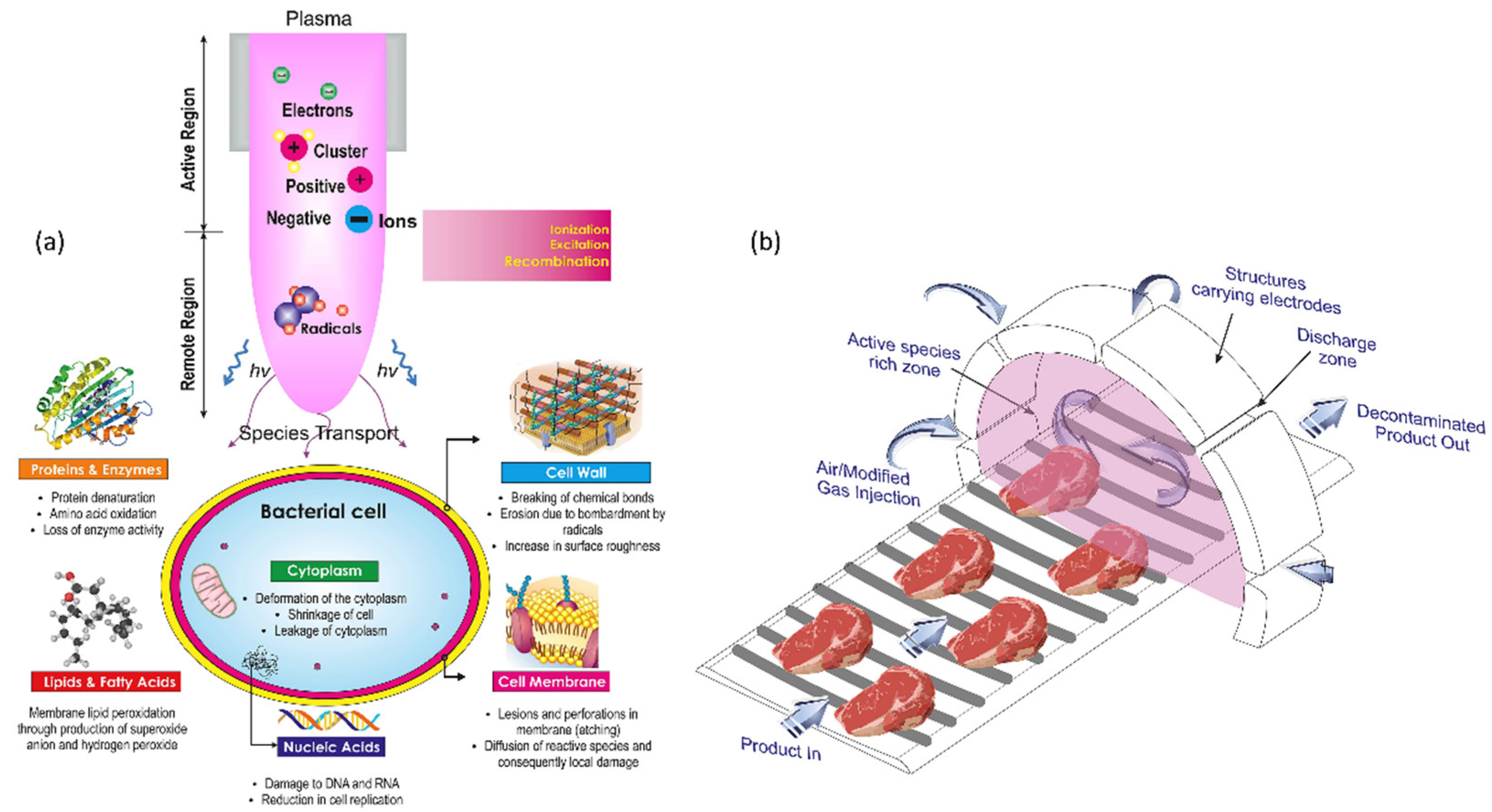
NTP technology has been suggested as an advanced method to increase food freshness and food storage for longer use. The freshness of freshly cut lettuce was reported to be maintained for a long time after washing with PAW. The lettuce tissue organelles were sustained as fresh properties such as color, texture, and taste after washing with PAW treatment [102,103]. PAW treatment has the ability to maintain the freshness of fresh-cut kiwifruit. Kiwifruits were sprayed with PAW solution or deionized water and submerged in S. aureus suspension. The reactive species in PAW generated oxidative stress, which in turn damaged the membrane of bacterial cells and ultimately lead to cell death. Furthermore, PAW treatment had no detrimental effects on the kiwifruits’ quality. They were less affected by reactive species oxidative species damage due to the antioxidant enzymes in PAW [104]. Plasma therapy effectively suppressed B. cinerea spore germination and mycelial development in vitro, as well as gray mold decomposition in blueberries infected with B. cinerea during postharvest storage [105]. Figure 7 shows the action of NTP on bacterial cell structures, resulting in functional loss and sterilization which helps in food preservation.
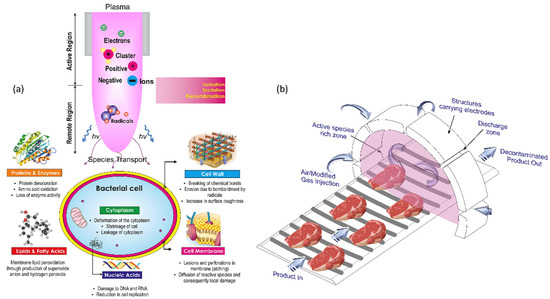
Figure 7.
(a) A diagram depicting the action of NTP on bacterial cell structures, resulting in functional loss and sterilization. (b) Conceptual model for an industrial-level continuous NTP disinfecting unit. Reprinted with permission from Ref. [106]. Copyright 2017, Elsevier.
The microbial inactivation in a liquid substrate is mostly caused by oxidative stressors brought on by the reactive species, and NTP is regarded as an improved oxidation approach. Typically, seed treatment is conducted to guard against infections that could harm seeds during the early stages of seedling development. If this phase is skipped, pathogens, insects, and plant diseases will only attack, disrupting the germination process and plant growth. However, some seed treatments that use chemicals can be extremely detrimental since they expose people to chemicals when treating large amounts of seeds, which can lead to inadvertent poisoning, damage to the seeds, and risk for farmers or workers. The presence of microorganisms (bacteria and fungus) that will impact crops is the industry’s top concern right now. Recently, the agriculture sector has implemented a number of methods to limit the growth of bacteria and fungi on crops. The creation of bacterially resistant strains, the use of chemical fumigants, the drainage of the soil, and particular plowing techniques are a few of the methods used to manage bacteria in the agriculture sector. The denaturation of proteins, lipids, and other micronutrients during some of these reactions, however, could result in unfavorable alterations to the nutritional quality of food. Furthermore, when applying this technology for food applications, it is important to thoroughly research the genotoxicity of reactive species. The application of cold plasma for microbial eradication can be used across a variety of food substrates, including cheese, fruits, and meat products. In addition, it is utilized to modify the rate at which seeds germinate. It is an environmentally friendly method that is utilized as an alternative to conventional methods for food preservation and other possible uses. The Summary of recent NTP advancements for applications in food preservation and bacterial inactivation are provided in Table 2.

Table 2.
Summary of recent NTP advancements for applications in food preservation and bacterial inactivation.
5. NTP Technology to Combat COVID-19
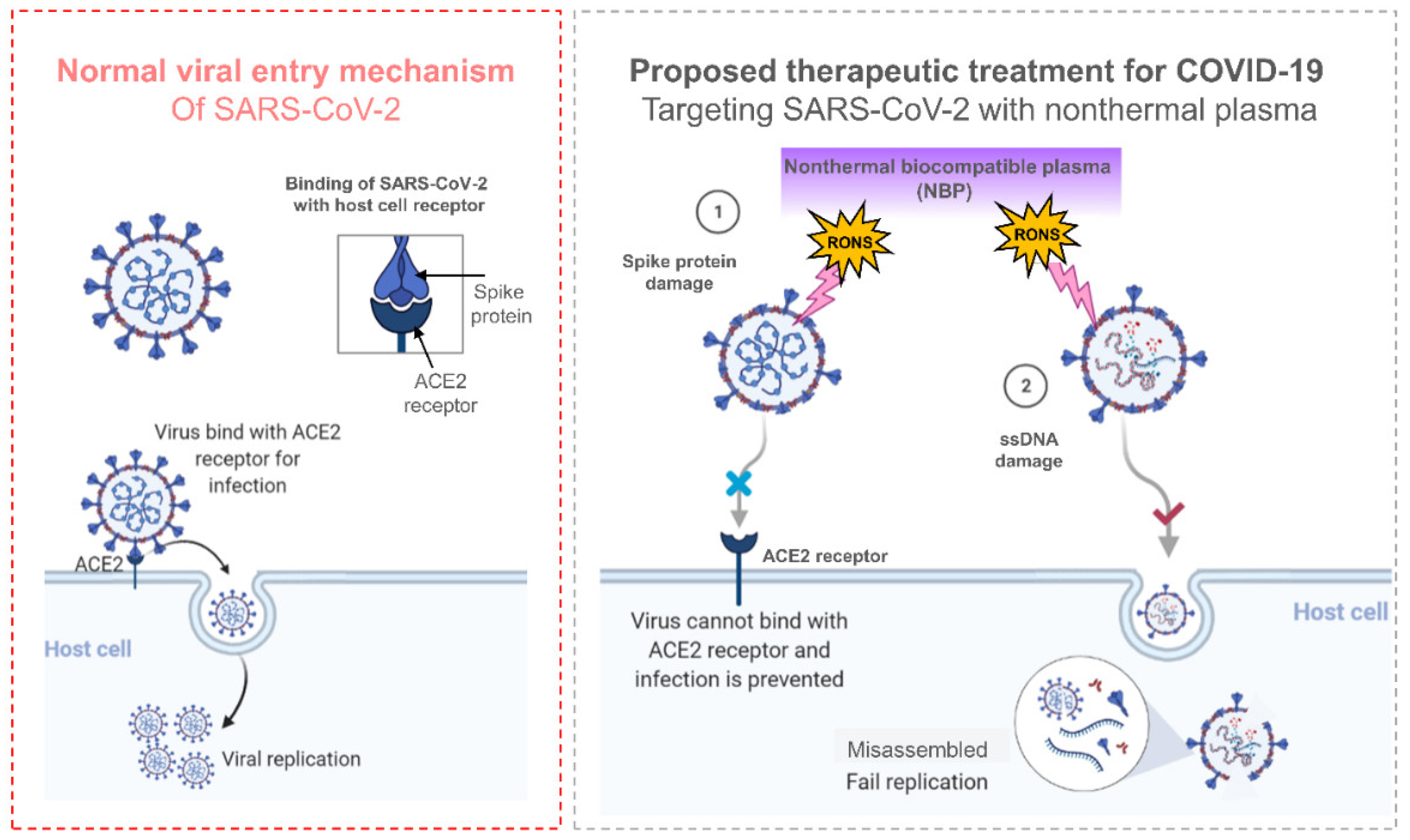
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic known as COVID-19 has become a new global health crisis. It has halted economies in every nation it has impacted, with 200 million infections and more than 4.3 million deaths reported [23]. Plasmas are used in many different fields, primarily to modify the surfaces of solids, but they can also be used to eliminate microbes such as viruses. A biological method to measure the in vivo DNA damage brought on by bacteriophage lambda viruses exposed to air plasma was proposed by Yashuda et. al. ten years ago [138]. NTP has the benefit of being easily produced from air, water, and electricity, providing a sterilization solution at a lower cost without the cost and logistics of maintaining expensive and robust supply chains required for conventional methods that depend on necessities such as alcohol and hydrogen peroxide [139,140,141]. In order to effectively deactivate MS2 bacteriophages in aerosols while utilizing a minimal pressure drop across the reactor for air sterilization against airborne bacteriophages, Xia et. al. designed and constructed a packed-bed DBD plasma reactor. This procedure is effective at halting the airborne transmission of viral infections [142]. As several other researchers have discovered, NTP significantly increases the amount of protein oxidation that takes place in the viral capsid or coat. By producing ROS, NTP treatment puts bacteriophages into a dormant state. NTP operating parameters may favor viral DNA damage or protein inactivation, but further research is needed to confirm this. The mechanism for inactivating SARS-CoV-2 by using NTP is shown in Figure 8. The SARS-CoV-2 spike proteins bind to the ACE2 receptor during typical viral entry, allowing the virus to enter the host cell and replicate there. NTP caused damage to the spike proteins in SARS-CoV-2, which prevented the virus from attaching to the ACE2 receptor and preventing infection. However, NTP also causes DNA/RNA damage, which prevents SARS-CoV-2 from replicating. Additionally, plasma has been shown to alter exterior virus components necessary for attachment, such as the spike protein, and degrade pure SARS-CoV-2 RNA [28,29,143,144]. The NTP technology for viruses is still in its infancy and requires more research. More precise approaches are desperately needed to clarify which NTP particles are most important and how they affect viruses.
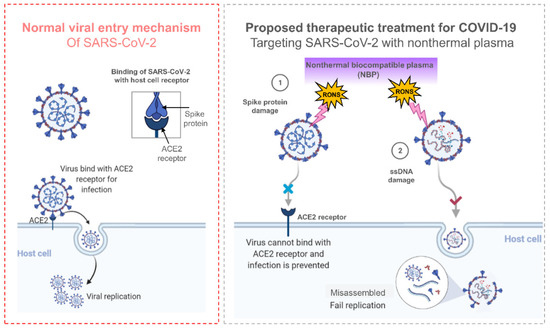
Figure 8.
The mechanism by which SARS-CoV-2 normally enters a host cell and the idea behind NTP-based inactivation. NTP-produced RONS bind directly to the surface of virus particles. Due to membrane-bound spike proteins’ improper binding to ACE-2 receptors, they prevent the virus from entering lung cells. Additionally, even though the virus passes through the alveolar plasma membrane, contact viral components are not synthesized in lung cells as a result of genetic information damage. The capsid proteins that protect RNA are decomposed and the molecular binding of RNA is broken [143].
6. Applications of NTP for Environmental Protections
Controlling the dissipation of volatile organic compounds (VOCs) and hazardous contaminants from water is one of the most demanding environmental issues [145,146,147]. Long-term VOC exposure may result in a variety of illnesses, notably cancer, cardiovascular disease, and numerous other possible disorders [148]. Similarly, the presence of industrial effluents such as dyes and pharmaceutical waste in the aquatic system is a concern (threat) for the environment because of the fact of their nonbiodegradability, toxicity, and potential carcinogenic nature [149]. Therefore, controlling this environmental pollution has become an important issue and a hot research topic worldwide. Different technologies have been used for controlling these pollutants, including thermal and catalytic incineration [150], adsorption [151], condensation [152], coagulation [153], biofiltration [154], and membrane separation [155]. However, these kinds of treatments are often inefficient at removing pollutants such as pesticides, dyes, pharmaceutical compounds, and VOCs, which are increasingly present in the environment. Owing to this, advanced oxidation processes (AOPs) have been considered possible alternatives thanks to their extreme oxidizing capacity and their efficiency in the removal of bio-recalcitrant compounds [156]. Among these, NTP represents an innovative AOP for water treatment [157]. With respect to “thermal” plasma, the NTP system maintains at relatively low temperatures and is able to generate charge carriers. As reported in the literature, NTP technology is widely used for different applications and especially for the removal of pollutants from the environment [158]. It is well known that the NTP process could produce active species such as O3, H2O2, •OH, and O• which play a key role in the oxidative degradation of pollutants [159]. The start of the process is an important phase that remains poorly understood in the destruction of VOCs with low-temperature plasmas. A pioneering modeling work indicated that the radicals appear to be the dominant destruction species at low ethylene concentrations and low specific energy deposition (SED) values, whereas the metastable ones are found to be the dominant destruction species at high ethylene concentrations and high SED values. In addition to the reactions with radicals, the simulations study revealed that quenching reactions with metastable nitrogen N2 (equation image) appear to be an important process for the destruction of ethylene [160]. Atomic oxygen is the main destruction species in dry air at low SED and low ethylene concentrations, which are generally used for industrial uses [160]. For various ethylene concentrations, the effect of the SED on removal effectiveness and selectivity toward CO and CO2 was investigated. The presented model by a research group led by Aerts et. al. enables the identification of the pathway for destruction in both dry and humid air. The latter is found to be primarily initiated by metastable N2 molecules, but O atoms and OH radicals dominate the subsequent stages of destruction [161]. However, some disadvantages of NTP “alone” in the degradation of organic contaminants are mostly associated with the creation of undesirable by-products, which might limit its industrial applicability. To circumvent these constraints, an intriguing technique combining NTP and heterogeneous catalysis has been proposed to improve pollutant degradation efficiency while simultaneously reducing the generation of reaction by-products [162]. The effect of NTP combined with catalysts enhances the generation of different types of active species (such as hydroxyls) and the activity and stability of catalytic materials [163].
6.1. Plasma Catalysis
Plasma catalysis is increasingly being used in a wide range of gas conversion processes, such as the conversion of CO2 into high-value chemicals and fuels, the activation of CH4 to form hydrogen, the generation of higher hydrocarbons or oxygenates, and the synthesis of NH3 [164,165]. Other uses, such as those for reducing air pollution, such as the removal of VOCs, particulate matter, and NOx, are already well-established [166]. Because the electron temperature is substantially higher than the temperature of the heavy species, radicals, and excited species are generated at temperatures closer to ambient in an NTP or non-equilibrium plasma (ions and neutrals). This nonthermal energy distribution has the ability to bypass the kinetic and thermodynamic constraints on the chemical conversion of reactants into desired products. If directed correctly, this energy can drive endothermic, equilibrium-limited processes under conditions when equilibrium conversions are negligible. Similarly, energy can accelerate reaction pathways that are kinetically slow under the current conditions [167,168,169]. The very energetic electrons in an NTP create (rotationally, vibrationally, and electrically) excited species, ions, and radicals through inelastic collisions with feedstock molecules, resulting in a multitude of novel species and states that are unreachable at bulk thermal temperatures. Because NTPs can contain a wide range of highly reactive species, it is difficult to operate them in a fashion that results in the generation of single products with high yield and selectivity. The integration of plasma and catalysts provides the possibility of combining the benefits of the two and permitting conversions that are now difficult [170,171]. The use of NTP in conjunction with heterogeneous catalysts is classified into two types based on the position of the catalyst: in-plasma catalysis (IPC) and post-plasma catalysis (PPC). The former is a two-stage method, where the catalyst is situated downstream of the plasma reactor, whereas the latter is a one-stage technique in which the catalyst is subjected to active plasma. The activation of the catalyst by NTP is linked to synergetic effects in plasma catalysis. All activation pathways include ozone, local heating, UV, the alteration of work function, lattice oxygen stimulation, adsorption/desorption, and electron–hole pair creation, as well as direct interactions among gas-phase radicals and adsorbed contaminants [166,170,172,173]. In a previous report, a thorough and in-depth explanation of plasma catalysts was presented [166].
For example, Yang et al. investigated NOx degradation in a coaxial dual-dielectric barrier reactor using a mixed catalyst. It was discovered that the synthesized TiO2x could accomplish significant degradation effects (84.84%, SIE = 401.27 J L1) in an oxygen-rich plasma catalytic system, outperforming TiO2 (73.99%) and a single plasma degradation process (26.00%). The presence of Ti3+ and oxygen vacancies in TiO2x caused a very narrow band gap, which helped to catalyze deeply the oxidation of NOx to NO2 and NO3 during the plasma-induced "pseudo-photocatalysis" process. Meanwhile, TiO2x increased the discharge current and efficiency, indicating its significant activating effect in the reaction. Reduced TiO2x demonstrated a remarkable degrading effect in a long-term plasma-catalysis procedure while retaining its intrinsic crystal structure and morphology [174]. Capp et al. conducted a thorough investigation into the effects of combining titanium di-oxide (TiO2) photocatalysts with nonthermal atmospheric pressure nitrogen–oxygen plasmas, which increased the production of ozone and dinitrogen pentoxide (N2O5) while limiting the formation of harmful nitrogen dioxide (NO2) and nitrous oxide (N2O) by-products. Magnetron sputtering was used to produce TiO2 coatings atop barium titanate (BaTiO3) particles for use in a packed-bed dielectric barrier discharge reactor (DBD). Titanium dioxide can influence plasma chemistry in the DBD by acting as a sink for atomic oxygen, catalyzing the generation of superoxide anion radicals (O2−), and changing the dielectric constant of the BaTiO3 particles. This study discusses the complicated interaction of these influences on the chemistry of oxygen and nitrogen plasmas. The effect of photocatalyst surface characteristics, gas composition, and residence duration on the reaction pathways for ozone and nitrogen oxide (NxOy) generation was studied. The photocatalytic activity of titanium dioxide was enhanced by annealing the coated surface, and it was later discovered to allow the synthesis of ozone, boost the formation of N2O5, and greatly decrease the formation of hazardous NO2 and N2O with a residence time of 0.011 s [175]. Plasma also has promising potential for catalyst synthesis and treatment. However, fuller knowledge of the underlying physical and chemical processes is required. Diagnostic experiments, studies into the physicochemical mechanisms underpinning plasma–catalyst interactions, and chemical activities occurring at the catalyst surface can all help to uncover this. The key problem is creating catalysts that are inexpensive, highly active, and stable while also being suitable for the plasma environment. It is impossible to overestimate the importance of understanding thermal, electrical, and photocatalysis.
6.2. Influence of NTP on the Catalytic Processes
6.2.1. The Properties of Catalyst
For catalyst preparation, NTP technology was employed. Plasma treatment improves the dispersal of active catalytic elements and alters the stability and catalytic activity of exposed catalyst material [176]. NTP can also change the oxidation state of the catalyst. In particular, when a Mn2O3 catalyst is exposed to a DBD plasma for an extended period of time, the occurrence of Mn3O4, a lower-valent manganese oxide with a strong oxidizing potential, is demonstrated by X-ray diffraction spectra [177]. After many hours of discharge operation, fewer parent Ti-O bonds are detected on TiO2 surfaces owing to plasma–catalyst interactions [178]. Even new sorts of active sites with distinctive characteristics are being developed, such as persistent Al-O-O* with a lifespan of more than two weeks, as found in Al2O3 pores in IPC experiments, may emerge [179]. Plasma bombardment can cause a change in catalyst composition as well as an augmentation or reduction in specific surface area.
6.2.2. Adsorption
Adsorption processes are crucial in plasma–catalytic reaction systems [180]. If the catalyst has a high capacity for pollutant adsorption, the pollutant persistence duration in the reactor is extended. In the instance of IPC, the contaminant level in the discharge zone increases. The increased likelihood of collision between polluting molecules and active species improves the removal rate.
6.2.3. Plasma-Mediated Activation of Photocatalysts
Contaminants bind to the surface of porous semiconductors, which are subjected to UV light in photocatalytic activity. UV photons form electron–hole pairs, causing the adsorbed contaminant to be oxidized by valence band holes. The oxidation products are finally desorbed. TiO2 is one of the most effective photocatalysts (together with ZnO, ZnS, CdS, Fe2O3, and WO3) for the breakdown of a wide spectrum of organic pollutants. The surfactant-assisted sol–gel technique was combined with microwave plasma calcination to produce TiO2 nanopowders. To reduce calcination time and ensure energy-efficient photocatalyst production, plasma calcination was performed for only 20–30 min. Under these conditions, mixed anatase-rutile phased TiO2 nanoparticles were produced. Microwave plasma calcination reduced the photocatalyst’s band gap energy by 40%. Surfactants were discovered to be ineffective in phase transitions of synthetic TiO2. The absorption of the O-Ti-O band spanning between 415 and 420 cm−1 was confirmed by FTIR analysis. After plasma calcination, the hydroxyl bands (OH) were found to be less stretched. The band gap energies of the normally calcined HA-Ti, NA-Ti, and MS-Ti samples were 5.09 eV, 4.88 eV, and 5.06 eV, respectively. The band gap energies of plasma-calcined MTHA-Ti, MTNNA-Ti, and MTMS-Ti samples were calculated to be approximately 4.92 eV, 3.11 eV, and 4.96 eV, respectively. The combined effect of photocatalyst, plasma reactive species, and UV radiation increased the efficiency of methylene blue dye degradation. After 30 min of DC plasma jet exposure, the maximum degradation efficiency of 95% was attained. After four dye degradation cycles, the catalyst preserved approximately 93–95% degradation efficiency [181].
A DBD reactor with BaTiO3 and TiO2 photocatalysts was used to examine plasma-photocatalytic CO2 conversion into CO and O2. The combination of plasma with the BaTiO3 and TiO2 photocatalysts in the CO2 DBD slightly increases the gas temperature of the plasma by 6–11 °C compared to the CO2 discharge in the absence of a catalyst at an SED of 28 kJ/L, while the plasma gas temperature in the gas phase is almost the same as the temperature on the surface of the photocatalysts (BaTiO3 and TiO2) in the plasma-catalytic DBD reactor. The combination of plasma with BaTiO3 and TiO2 catalysts has a synergistic impact that increases CO2 conversion and energy efficiency by a factor of 2.5 when compared to the plasma reaction without a catalyst. The presence of catalyst pellets in the plasma gap is found to play a dominant role in inducing plasma’s physical effects such as the enhancement of the electric field and the production of more energetic electrons and reactive species, which leads to chemical effects and contributes to CO2 conversion. We discovered that the intensity of UV emissions produced by the CO2 DBD is much lower than that emitted by external UV sources (e.g., UV lamps), which are routinely utilized to activate photocatalysts in conventional photocatalytic reactions. This phenomenon implies that the UV emissions produced by the CO2 DBD play only a minor role in the activation of the BaTiO3 and TiO2 catalysts in the plasma-photocatalytic conversion of CO2 and that their contribution to the exceptional performance of the plasma-photocatalytic reaction and the synergy of plasma photocatalysis could be very weak or negligible [182]. When a TiO2-based photocatalyst is combined with a nonthermal plasma, various synergistic effects occur that are not all related to photocatalytic mechanisms. To gain a comprehensive knowledge of plasma/TiO2 synergy, at least four effects must be distinguished: (1) TiO2 catalytic material enhances injected energy for the same applied voltage due to quicker filament breaking, most likely due to a larger amount of adsorbed charges and a bigger local electric field due to TiO2’s high permittivity. (2) TiO2 may alter the breakdown mechanisms of filaments with the same supplied energy. The porosity of the catalytic material boosts the effectiveness of reactions triggered by plasma species, most likely due to improved contact between reagents when they are adsorbed. If TiO2 is sufficiently activated, increased photocatalytic reactivity involving electron–hole pairs of TiO2 may occur in interaction with the plasma phase [183].
6.2.4. Thermal Activation
Despite gas heating raising catalyst surface temperatures, the heating impact is often insufficient to compensate for the thermal activation of the catalyst. Hot spots can emerge in packed-bed reactors; however, adapted heating is driven by powerful microdischarges running between the sharp edges and corners of neighboring pellets. Catalytic VOC elimination can be aided by increasing catalyst temperatures [184]. A low-temperature plasma reactor is used for the plasma-catalytic conversion of CO2. Combining plasma plus a catalyst is an active technique to obtain high conversion rates while consuming little energy. TiO2 is a common catalyst used in dielectric barrier discharges. According to Guaitella et al., the plasma–photocatalyst synergy provides enhanced conversions and energy efficiency. This research distinguishes three key synergistic effects: (a) the influence of catalysts on power input; (b) the effect of porosity on C2H2 oxidation; and (c) the photocatalytic degradation of C2H2 on TiO2 during plasma exposure. According to reports, the presence of glass fibers enhances the injected power at a constant voltage substantially. Glass fiber macroporosity and nanoparticles both play small roles in C2H2 oxidation enhancement. The generation of O atoms near the surface is most likely responsible for the increased removal efficiency of C2H2 from porous material [183]. Mei et al. developed a coaxial DBD reactor for the atmospheric pressure and low-temperature plasma-catalytic conversion of pure CO2 into CO and O2. According to the findings, the synergy between the plasma and the photocatalyst improves energy efficiency and conversion rates. A plasma-driven photocatalytic process aids in CO2 conversion [182].
7. NTP for Catalytic VOCs Abatement
Controlling VOC emissions from anthropogenic sources has emerged as a critical issue in environmental protection as a result of growing global awareness. One of the most promising methods for the handling of VOCs is nonthermal plasma catalysis, which has high efficiency, good by-product selectivity, and superior carbon balancing (VOCs) [185]. For example, Hoseini et al. synthesized a Mn2O3 catalyst from a spent alkaline battery using a hydrometallurgical technique. Manganese oxide was impregnated at various percentages on alumina and utilized as a catalyst for the oxidation of benzene, toluene, and xylene (BTX) in a hybrid plasma–catalytic process. Whereas MnAl catalysts produced a comparable benzene and toluene oxidation percentage (97–98%), xylene oxidation was problematic and dependent on the Mn percent, so the catalyst with 10% wt. of manganese oxide indicated 74% of xylene oxidation while higher or lower Mn contents exhibited lower oxidation percent. The effect of the plasma input voltage, the catalyst position in the plasma reactor, the BTX flow rate, and the catalyst loadings on the experimental parameters was examined. The results revealed that benzene and toluene were practically totally oxidized regardless of the input plasma voltage, whereas the percentage of xylene oxidation increased as the voltage was increased. Furthermore, the position of the catalyst in the reactor had no effect on BTX conversion; however, increasing the flow rate resulted in a decrease in BTX removal efficiency [177]. We discussed this section in detail and divided them into the three most investigated target chemicals, namely, trichloroethylene, benzene, and toluene.
7.1. Trichloroethylene
Chlorinated VOCs (CVOCs) are often present in industrial waste gases because they are extensively used as chemicals and solvents in industry. Because of its diverse range of solvent uses and degreaser in industry, trichloroethylene is perhaps the most prevalent CVOC discharged into the atmosphere. However, the International Agency of Cancer (IARC) reports that TCE is carcinogenic to humans, suggesting that its atmospheric emission must be prohibited to protect public health [186]. The performance of TCE decomposition for TiO2 catalysts has been examined by Oda et al. in relation to the initial TCE concentration, pellet size, and sintering temperature. The breakdown voltage to produce NTP is significantly lower when the barrier-type reactor is filled with TiO2 sintered at 673 K as compared to when the reactor is empty and when the reactor is filled with TiO2 sintered at 1373 K. According to their hypothesis, the disk-shaped dielectric pellets sintered at 673 K had a non-uniform geometrical distribution that disrupted the electric field and produced an electric field concentration at the pellets’ contacting region. This result suggests that contacting point discharges or surface discharges occur on the surfaces of the pellets, lowering the breakdown voltage and increasing the decomposition energy efficiency. Additionally, they show that too-fine TiO2 particles disrupt the gas flow and result in insufficient plasma filling of the discharge area [187].
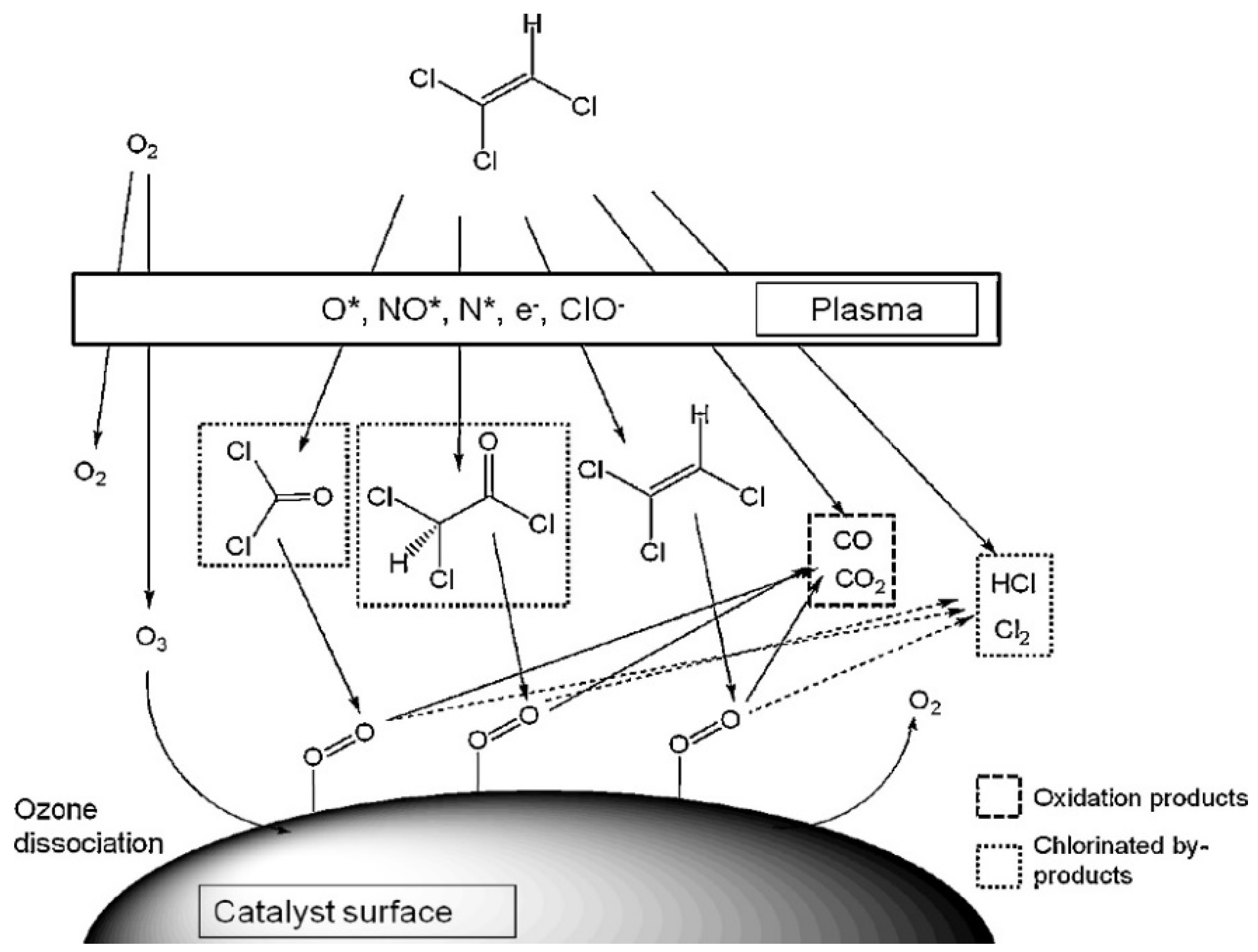
Han et al. have further investigated the direct and indirect processes’ effects of the manganese dioxide post-plasma catalyst. For the direct process, oxygen species mostly convert TCE into DCAC when excited species (or electrons) collide with O2. The improved decomposition efficiency for the direct procedure is attributed to the oxygen species formed during ozone breakdown at the surface of MnO2 oxidizing the continuing TCE into trichloroacetaldehyde (CCl3-CHO, TCAA). At an energy density of 120 J/L, the COx production increases from 15% to 35% when MnO2 is present. Increasing the energy density to 400 J/L results in a COx yield of 98%. Similar results are found for the indirect approach, even if the COx yield is not as good [188]. The degradation of trichloroethylene (TCE) in dry air was examined using a multi-pin-to-plate mixed negative DC corona/glow discharge with the MnO2 catalyst positioned downstream of the plasma reactor [189]. The activation energy for the plasma-catalytic system was much lower (1.5 kcal/mol) than it was for the pure catalytic system (8.7 kcal/mol), indicating that the oxygenated intermediates created by the plasma are more favorable to catalytic oxidation than TCE (Figure 9). Compared to the separate systems, the combined application of plasma and catalysis enhanced TCE abatement. To begin, electron–molecule collisions transform N2 and O2 molecules into a complex mixture of ionized, excited, radical, and metastable species capable of decomposing TCE into oxygenated intermediates and ultimate oxidation products. Second, in the gas phase, ozone dissociates on the catalyst surface to generate peroxide groups and molecular oxygen. These surface species accelerate TCE oxidation to CO, CO2, HCl, and Cl2. This oxidation is more efficient if oxygenated molecules, such as phosgene and DCAC, reach the catalyst’s surface. A summary of the plasma-catalytic TCE abatement provided in Table 3.
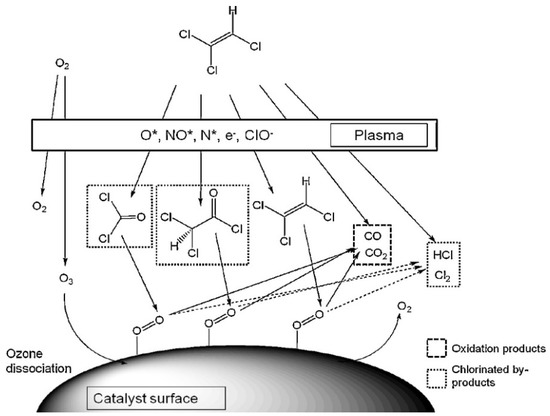
Figure 9.
Plausible reaction pathway for the plasma-catalytic TCE abatement. Reprinted with permission from Ref. [189]. Copyright 2014, Elsevier.

Table 3.
Summary of the plasma-catalytic TCE abatement.
7.2. Benzene
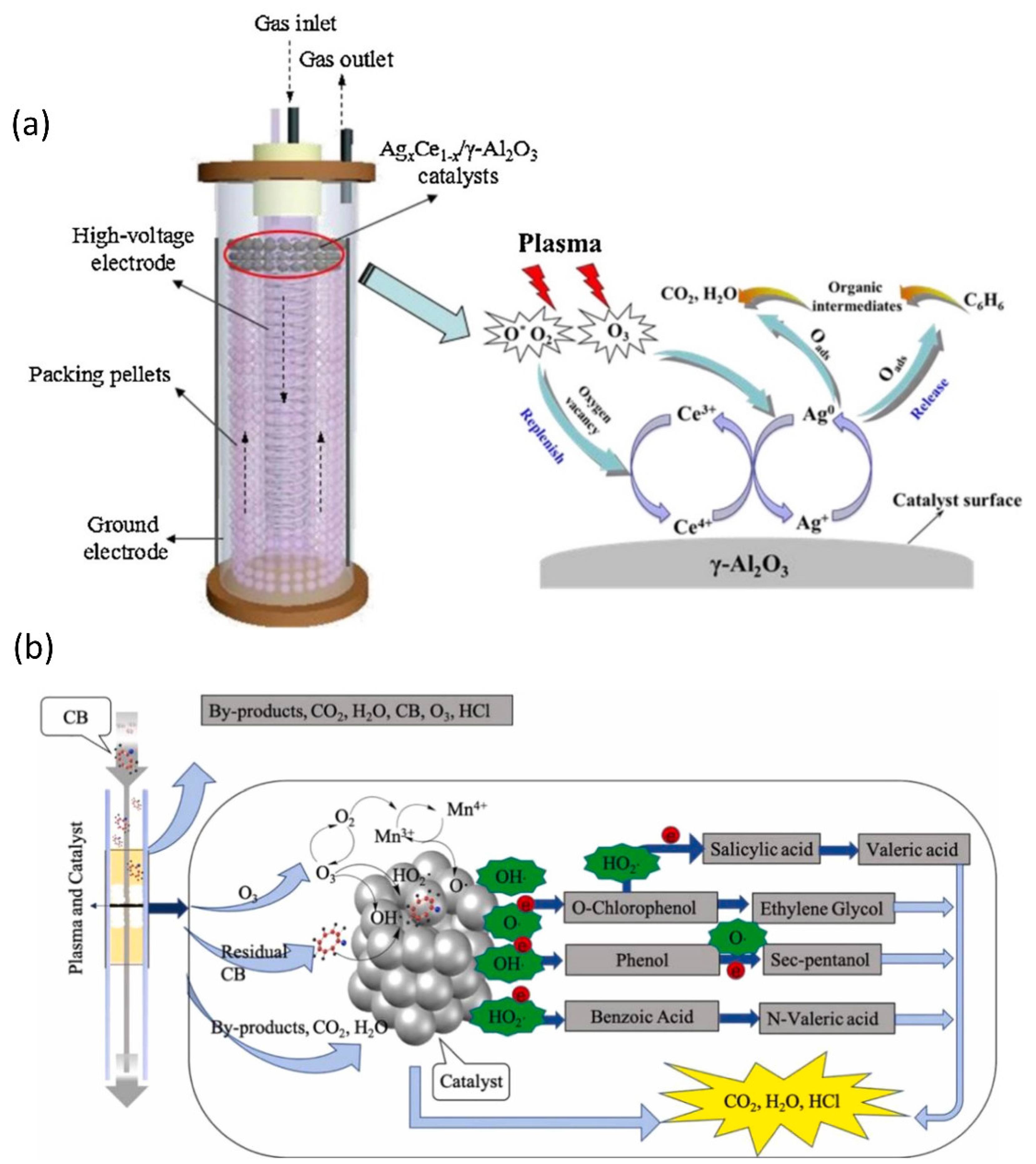
Various catalyst formulations and reactor designs have been reported by various research groups to improve the degradation of benzene with NTP. Jiang et al., for instance, used an optimized surface/packed-bed hybrid discharge (SPBHD) plasma in combination with MOx (M = Ag, Mn, Cu, or Fe) catalysts in a post-plasma-catalysis (PPC) system to accomplish the degradation of benzene [196]. When plasma was combined with all MOx/Al2O3 catalysts, the benzene degrading was strengthened, and the mineralization phase was considerably accelerated toward total oxidation. The same group has used in-plasma catalysis (IPC) and post-plasma catalysis (PPC) configurations of plasma-assisted catalysis to degrade benzene via hybrid surface/packed-bed discharge (HSPBD) plasmas over a sequence of Agx Ce1-x/-Al2O3 catalysts (Figure 10a). The plasma and Agx Ce1-x/-Al2O3 catalyst combination considerably increased the reaction performance when compared to the plasma-only process, and the combined degradation efficiency is a synergistic impact rather than primarily an additive effect. Both benzene molecules and intermediates formed by direct plasma reactions can be adsorbed on the catalyst surface in a PPC arrangement where the Ag0.9Ce0.1/-Al2O3 catalyst is placed downstream of area II. Following that, active oxygen species created on the catalyst surface via surface reactions can primarily oxidize benzene and intermediate molecules into CO2 and H2O, while the resulting oxygen vacancies can be replaced by gas phase oxygen and active oxygen species generated in the NTP process. As a rate-determining step, the alternate oxidation and reduction processes of metal active sites on the catalyst surface provide a continual supply of active oxygen species [197]. LaMnO3 and OMS-2 catalysts were used in a plasma-catalytic reactor for the study of chlorobenzene removal (Figure 10b). The combination of NTP and manganese-based catalyst significantly improved CB removal efficiency, CO2 selectivity, and energy efficiency when compared to NTP alone. Meanwhile, the use of a catalyst can considerably lower O3 and NOx production while also preventing the development of byproducts. The OMS-2 catalyst outperformed the LaMnO3 catalyst in terms of activity, with a maximum CB removal efficiency of 89.5% and a CO2 selectivity of 57.6%. More than 15 byproducts were discovered in the NTP reactors, with nitrogenous organic molecules (intermediate A) being detected in the NTP reactor without a catalyst. This was mostly owing to manganese-based catalysts boosting partial particle energy above the nitrogen-to-nitrogen triple bond dissociation energy (9.8 eV).
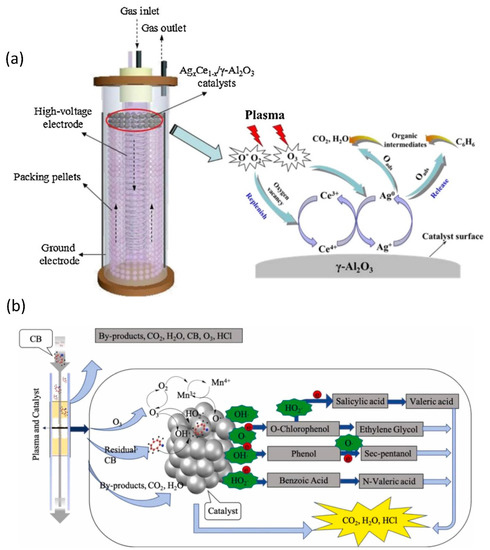
Figure 10.
(a) Proposed synthetic catalytic mechanism for organic compound removal in the NTP + Agx Ce1−x/γ-Al2O3 system. Reprinted with permission from Ref. [197]. Copyright 2016, Elsevier. (b) The most likely chlorobenzene elimination reaction pathways in the plasma-catalytic method. Reprinted with permission from Ref. [198]. Copyright 2021, Elsevier.
Many different types of small molecules were found in the NTP reactor with OMS-2, including oxalic acid (intermediate C), 2-pentanone (intermediate D), sec-pentanol (intermediate E), glycerol (intermediate F), valeric acid (intermediate G), and ethylene glycol (intermediate H). As a result, the inclusion of a manganese-based catalyst to the deep oxidation reaction intermediate reduced the development of hazardous byproducts. Overall, the OMS-2 catalyst was more favorable for CB mineralization than the typical NTP reactor and the NTP with LaMnO3 reactor, which promoted deep oxidation and reduced by-product generation. Based on the results of the preceding investigation, the OMS-2 catalyst with the best catalytic effect was chosen, and three different reaction routes were proposed. In the first route, a series of active species (such as O•, HO•, and HO2•) and high-energetic electrons in the plasma-catalytic system could attack CB, and subsequently, dechlorination occurred through the substitution and ionization reactions, and benzene (intermediate I) and phenol(intermediate J) was generated. The benzene ring was then opened by high-energy molecules, resulting in a sequence of alkanes (intermediate E), organic acids (intermediate C, G), and alcohols (intermediate F, H) that were eventually degraded to CO2, H2O, and HCl. For the second reaction pathway, high-energy electrons bombarded the C-H bond to cause the de-H reaction, and then a reaction with HO• occurred to form O-chlorophenol (intermediate B). Under the action of free radicals and electrons, O-chlorophenol is replaced by dechlorinated carboxyl groups to generate salicylic acid (intermediate O), or the benzene ring is opened and substituted to form an alcohol (intermediate H) to form salicylic acid, eventually creating CO2, H2O, and HCl. The third method involved converting CB to benzoic acid (intermediate K). Benzoic acid performed a sequence of complex chemical reactions to generate a series of alkanes and organic acids before decomposing into CO2, H2O, and HCl [198].
The breakdown of gas-phase benzene was studied by Kim et al. utilizing plasma-driven catalyst (PDC) reactors filled with TiO2, Pt/TiO2, or Ag/TiO2. For a comparison study, a standard BaTiO3 packed-bed plasma reactor was also used. Compared to those using the traditional BaTiO3 packed-bed plasma reactor, the PDC reactors were shown to be more effective in terms of energy efficiency and the elimination of undesired reaction products. The test materials’ catalytic activity for the breakdown of benzene went in the following order: Ag/TiO2, TiO2, and Pt/TiO2. Additionally, it was discovered that a silver (Ag) catalyst improved the CO2 [199]. Lee et al. used hybrid systems of discharge plasma and photocatalysts to carry out the breakdown of gas-phase benzene. By suppressing secondary products, a hybrid plasma–photocatalyst system improved CO2 selectivity. It was crucial to use a high-porosity material for the hybrid system in order to maximize CO2 selectivity. For this, benzene conversion and selectivity to CO2 were significantly improved when utilizing Al2O3 as a catalytic support [200]. Table 4 provides a brief overview of the plasma-catalytic benzene abatement.

Table 4.
Summary of the plasma-catalytic benzene abatement.
7.3. Toluene
The catalytic process based on NTP has been widely researched for toluene reduction. Some of the important papers are explained briefly. By oxidizing toluene in a dielectric barrier discharge (DBD) reactor, X. Xu et al. assessed the catalysts’ NTP catalytic performance. The findings revealed that Co-MCM-41 and Co-MCM-41 imp have different levels of catalytic activity and stability. The well-dispersed cobalt species, strong ozone decomposition activities, and high capacity to break down organic intermediates on catalyst surfaces were identified as the key causes of the improved catalytic performance and stability [210]. In an attempt to apply Ag-Mn/-Al2O3 materials for the removal of toluene through an adsorption plasma-catalytic process, Qin et al. investigated the effects of changing the sequence in which Ag and Mn were impregnated. The results demonstrate that related to the co-impregnated catalyst Ag-Mn-C/-Al2O3, the catalyst with Ag first impregnated Ag(F)-Mn/-Al2O3, and bare -Al2O3, Mn was attached first (Ag-Mn(F)/-Al2O3), and the catalyst had a longer breakthrough time, lower emission of toluene, and improved carbon balance [211]. W. Xu et al. used a series of Ni-SBA catalysts in conjunction with an adsorption–discharge plasma system to catalyze the removal of low concentrations of toluene. The outcomes revealed that after 60 min of plasma catalysis, the 5% Ni-SBA sample had a greater toluene mineralization rate (71.8%) than the other samples [212]. Next, a single-stage coaxial DBD reactor was implemented to compare the simultaneous removal of toluene and styrene under NTP and NTP catalysis (Figure 11a,b). To better understand the mechanism of degradation and the consequences of coexistence, the effects of VOC mixture, humidity, materials filling in the discharge zoon, removal efficiency, COx selectivity, and by-product types and emission levels were thoroughly explored. According to experimental findings, when treated with styrene during plasma treatment, toluene elimination was severely impeded. However, under the same circumstances, styrene was little impacted. It was discovered that the conversion of toluene was constrained by benzaldehyde, which served as the main organic waste from styrene and consumed the oxidizing particles (O and OH) [213]. The decomposition of toluene in a dielectric barrier discharge (DBD) reactor with a number of CeO2-MnOx catalysts was studied by Wang et al. (Figure 11c). The plasma-catalytic system greatly enhances the removal of toluene when compared to the NTP alone approach, and CeO2-MnOx catalysts outperform pure CeO2 and MnOx catalysts in terms of catalytic performance. With an input power of 24 W, the Ce1Mn1 catalyst achieves the maximum CO2 selectivity and removal efficiency, which are 95.94% and 90.73%, respectively. C-H in the methyl group has the lowest dissociation energy (3.7 eV), followed by C-H in the benzene ring (4.3 eV), C-C in the coupled methyl group with the benzene ring (4.4 eV), C-C in the benzene ring (5.0–5.3 eV), and C = C in the benzene ring (5.0–5.3 eV) (5.5 eV).
Thus, the easiest process in toluene decomposition is the destruction of C-H outside the benzene ring, which results in the creation of the benzyl radical. Benzaldehyde and benzyl alcohol can be formed via the reaction between the benzyl radical and O. Furthermore, phenyl produced by breaking a C–C bond can react with H•, CH3•, and OH• to form benzene, o-xylene, and phenol separately. Meanwhile, the ring-containing intermediate products can couple to form a polymer compound. A series of active species (O•, OH•, H•) and high energetic electrons (>5.5 eV) can attack the benzene ring to generate ring-opening products. These chain intermediates are oxidized to CO2 and H2O and then to toluene. Intermediates and reactive species in the gas phase are adsorbed onto the surface of catalysts, where they are oxidized further by active surface oxygen species. As a result, oxygen atom species are critical in the breakdown of toluene on the surface of catalysts. Interactions between MnOx and CeO2 can increase oxygen mobility on the catalysts and speed up the formation of active oxygen species. This active atomic oxygen generated by the catalyst, plasma, and O3 may significantly improve toluene removal efficiency and CO2 selectivity [214]. Additionally, using a simple in situ Ce doping technique, a series of Ba1xCexTiO3 perovskite catalysts with high specific surface areas (68.6–85.6 m2 g−1) were developed and evaluated for their ability to catalytically degrade toluene (Figure 11d). The catalysts and nonthermal plasma worked in cooperation to generate a powerful result. When BC4T (Ce/Ti molar ratio = 4:100) was packed in a coaxial dielectric barrier discharge reactor at a specific input energy of 508 J L−1, the results showed the maximum decomposition efficiency (100%), COx selectivity (98.1%), and CO2 selectivity (63.9%), and the lowest O3 generation (0 ppm). The C-H in the methyl group was destroyed by high-energy electrons to generate a benzyl group because it had the lowest dissociation energy in toluene. The benzyl group was structurally unstable, and it reacted with oxygen species (such as •O and •OH) to produce benzaldehyde and phenylmethanol. Because benzaldehyde and phenylmethanol polymerized with methyl formate, respectively, methyl benzoylformate and -hydroxy-methyl ester-benzeneacetic acid were identified. In a DBD-catalytic reactor, the plasma catalytic performance of toluene decomposition over various transition metal-supported catalysts was assessed recently [215]. At room temperature, the toluene decomposition and energy efficiency were highest for γ-Al2O3 supported with 1 wt% MnO2. The ability of catalysts to decompose O3 was correlated with the efficiency of toluene decomposition. According to the results of the study, 1 wt% Mn/γ-Al2O3 was better at catalyzing the conversion of toluene to carbonate and bicarbonate via the breaking of C-C bonds from benzoic acid [215]. Recent studies have looked at the plasma-catalyzed CO2 hydrogenation over ZnO and Pd/ZnO using a tabular DBD reactor at low temperatures. With the help of online mass spectroscopy (MS), optical emission spectroscopy (OES) diagnostics, and a newly developed integrated DBD/FTIR gas cell reactor, it may be possible to better understand the mechanisms and pathways of complex plasma-catalyzed chemical reactions, particularly plasma-assisted surface reactions [216]. In a recent study, the effects of surface temperature and plasma irradiation on catalyst surfaces were also assessed. The concentration of adsorbed species decreased as the catalyst surface temperature increased, promoting desorption and gas-phase ammonia production [217]. The experimental confirmation of surface reactions involving plasma-activated intermediates should serve as a guide for establishing more precise descriptions of the steps in plasma-activated catalytic ammonia synthesis [217].
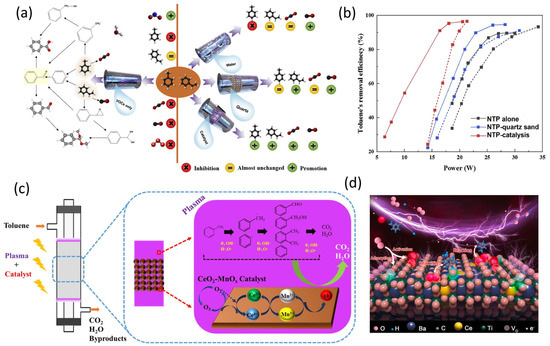
Figure 11.
(a) Summary of suggested degradation pathways and NTP system effect on the simultaneous removal of binary VOCs mixture. (b) Comparison of quartz sand and catalyst filling on VOCs’ removal efficiency of toluene. Reaction conditions: toluene (styrene): 50 ppm, air as carrier gas, and GHSV of 60,000 mL·(g·h)−1. Reused with permission from [213]. Copyright 2021, Elsevier. (c) Plausible reaction pathway for the plasma-catalytic Ttoluene abatement. Reused with permission from [214]. Copyright 2016, Elsevier. (d) Schematic diagram of the plasma-catalytic decomposition of toluene. Reprinted with permission from Ref. [218]. Copyright 2021, Elsevier.
Figure 11.
(a) Summary of suggested degradation pathways and NTP system effect on the simultaneous removal of binary VOCs mixture. (b) Comparison of quartz sand and catalyst filling on VOCs’ removal efficiency of toluene. Reaction conditions: toluene (styrene): 50 ppm, air as carrier gas, and GHSV of 60,000 mL·(g·h)−1. Reused with permission from [213]. Copyright 2021, Elsevier. (c) Plausible reaction pathway for the plasma-catalytic Ttoluene abatement. Reused with permission from [214]. Copyright 2016, Elsevier. (d) Schematic diagram of the plasma-catalytic decomposition of toluene. Reprinted with permission from Ref. [218]. Copyright 2021, Elsevier.
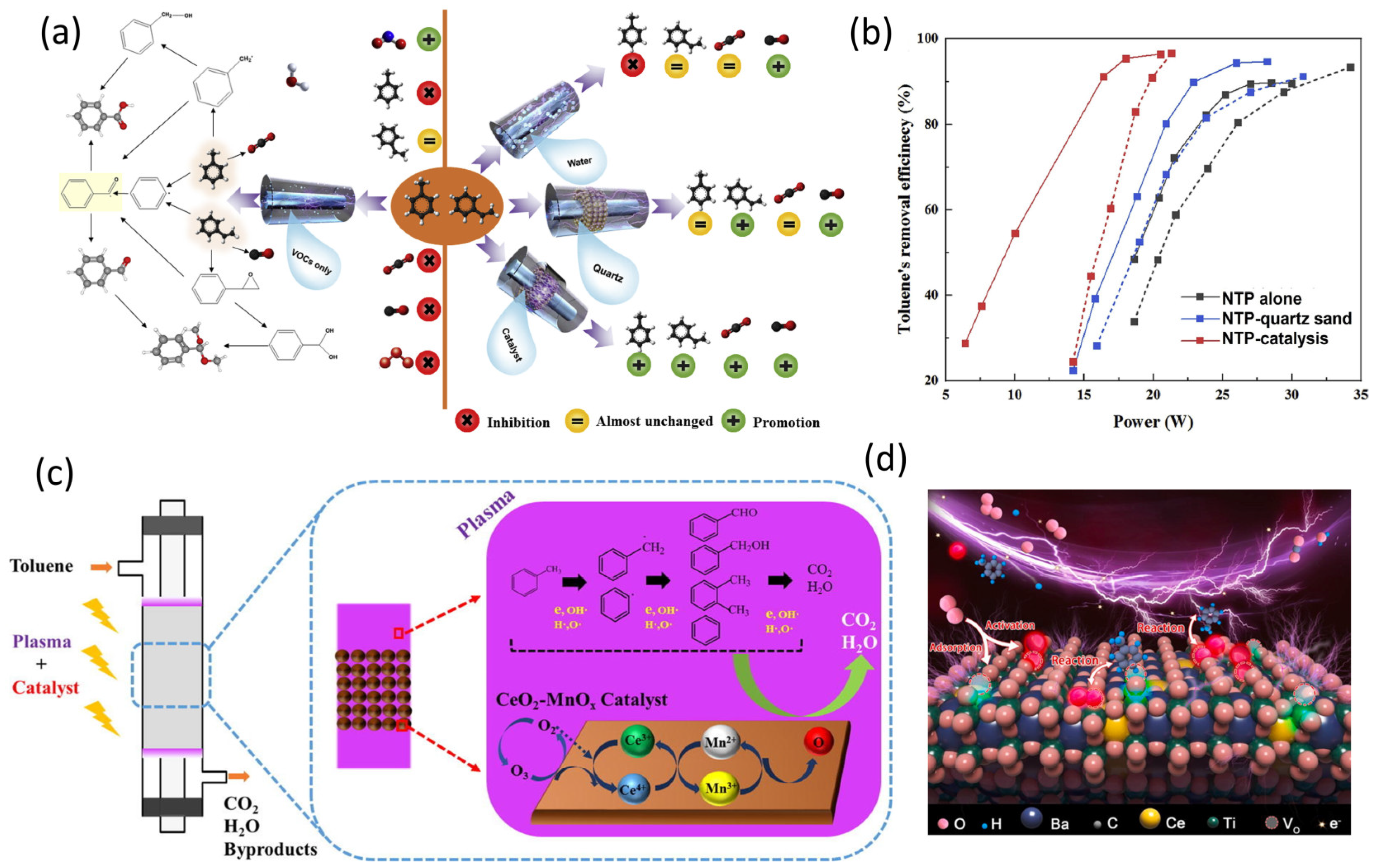
Furthermore, the plasma-generated species easily dissolved the C-C link connecting the methyl group and the benzene ring to form a phenyl group, which reacted with •N2O and •CH3 to form nitrobenzene and mesitylene, respectively. A considerable quantity of ROS were created on the catalyst surface in our plasma-catalytic hybrid system, which successfully promoted intermediate degradation. The majority of the intermediates were oxidized to CO2 and H2O [218]. A brief summary of the plasma-catalytic toluene abatement is provided in Table 5.

Table 5.
Summary of the plasma-catalytic toluene abatement.
8. Nonthermal Plasma Coupled with Catalyst for the Degradation of Water Pollutants
One of the most promising technologies for degrading dangerous contaminants in wastewater is nonthermal plasma. Recent research has shown that different operating parameters have an impact on the yield of processes based on nonthermal plasma (NTP). In particular, a catalyst that is properly positioned in the NTP reactor causes a considerable improvement in process performance compared to NTP alone. Several studies have been carried out to evaluate the potential of NTP in conjunction with catalysts for the removal of various types of pollutants in an aqueous solution. It is obvious that defining an ideal situation that can work for all forms of pollutants and the many kinds of catalysts utilized in this situation is still difficult. However, it was clearly explained that the operational factors are crucial. However, it is often challenging to understand how plasma can affect the catalyst and the generation of the oxidizing species most in charge of degrading pollutants. The purpose of this review is to offer an insight into catalytic compositions used in conjunction with NTP technology to remove water contaminants. Specifically, the reactor architecture to be used when NTP was coupled with a catalyst, the placement of the catalyst in the reactor, and the function of the primary oxidizing species were provided.
8.1. Decontamination of Pharmaceutical Compounds
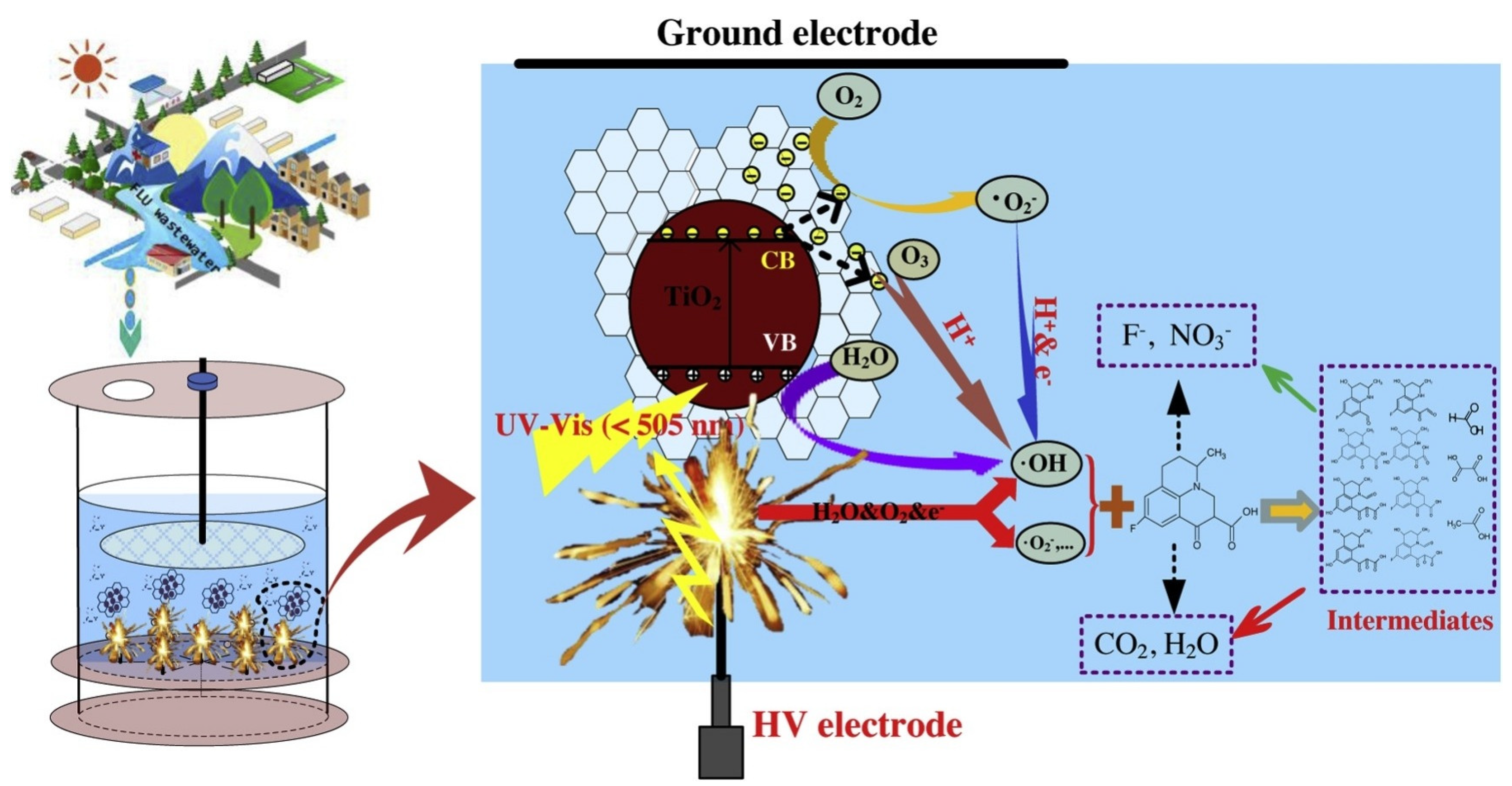
Drug usage has recently increased the variety of toxins entering the environment. Different pharmaceutical chemicals do not completely degrade during wastewater treatment operations due to their low biodegradability and strong chemical stability. According to reports, the combination of NTP and a catalyst is a viable technique for removing pharmaceutical pollutants from wastewater. For instance, Guo et al. created a hybrid rGO-TiO2 nanocomposite for the PDP system’s synergetic degradation of fluoroquinolones (Figure 12). According to the degrading performance testing, the composite has the best catalytic performance, with a 99.4% elimination efficiency in the PDP system, which is better than the 64.8% in the solitary PDP system [227].
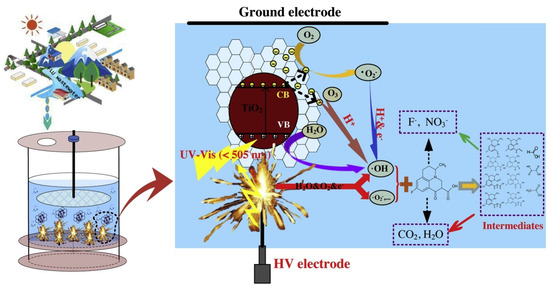
Figure 12.
Proposed synthetic catalytic mechanism for pharmaceutical compound degradation in the NTP + graphene/TiO2 system. Reprinted with permission from Ref. [227]. Copyright 2019, Elsevier.
In order to degrade aqueous amoxicillin in plasma, Ansari et al. produced ZnO and -Fe2O3 as a nanocatalyst (ZnO/-Fe2O3) and paired it with DBD (AMX). According to the results, the AMX degradation was greatly boosted by the ZnO/-Fe2O3 composite catalyst, going from 75.0% (when employing a single plasma reactor) to 99.3% under ideal conditions [228]. Another recent study examined how DBD in conjunction with Bi2WO6-rMoS2 could degrade sulfamethoxazole (SMZ) in wastewater. The combined effect of the DBD plasma reactor and Bi2WO6-rMoS2 nanocomposites significantly increased the degradation efficiency of the antibacterial medication (97.6% vs. 72.5%). Ozone and hydrogen peroxide were utilized as indirect indicators of the generation of the •OH radicals by the authors to analyze the impact of oxidizing species in this work [229]. Triclocarban (TCC) was reported to degrade in water using a dielectric barrier discharge plasma in conjunction with TiO2-activated carbon fibers by Wang et al. (ACFs). The degradation of triclosan (TCS) from pharmaceuticals and personal care products followed a very similar procedure. In this case, a DBD reactor and activated carbon fibers are provided using this method. In this study, the electric discharge creates plasma that can be used to synthesize ACFs in situ and reuse them for additional treatment cycles in addition to degrading TCS [230]. Table 6 summarizes the primary operating conditions and catalysts employed for the degradation of pharmaceutical compounds in aqueous phase by NTP coupled with catalysts.

Table 6.
Summary of the main operating conditions and catalysts used for the degradation of pharmaceutical compounds in aqueous phase by NTP coupled with catalysts.
8.2. Removal of Dyes
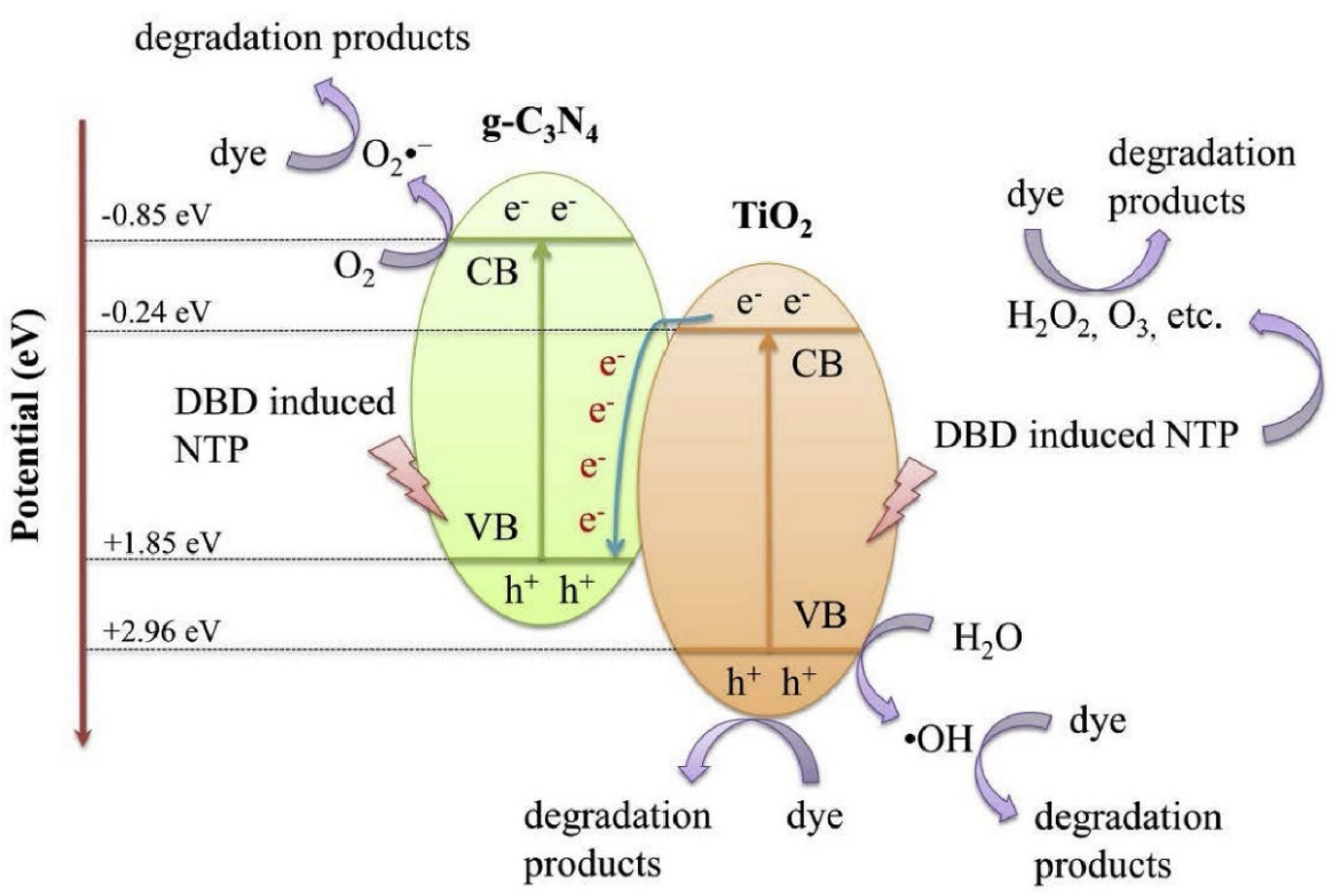
One of the most prominent pollutant groups in wastewater from textile and other industrial processes is composed of organic dyes. Because the dyes may be poisonous and are visible in surface waterways, the removal of this type of pollutant from wastewater is a significant issue from an environmental perspective. A method that looks promising for the degradation of this kind of pollutant is the combination of NTP and a catalyst. Numerous studies proving the effectiveness of the procedure are described in the literature. The effectiveness of NTP (DBD reactor) in conjunction with g-C3N4/TiO2 for the degradation of the acid orange 7 dye in an aqueous solution was reported by Liu’s research group (Figure 13) [239].
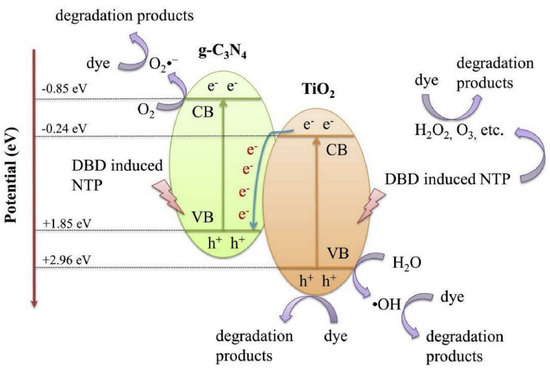
Figure 13.
Proposed synthetic catalytic mechanism for dye degradation in the NTP + g−C3N4/TiO2 system. Reprinted with permission from Ref. [239]. Copyright 2020, Elsevier.
Guo et al. also report on the AO7 degradation, demonstrating the use of activated carbon and pulsed discharge plasma (PDP) (AC). Due to the AC’s adsorbent and catalytic properties, its presence in the PDP/AC combination helps to provide a synergistic effect. The creation of the OH• and O• radicals was investigated in particular as it relates to the impact of AC addition on the production of activated species [240]. Zhang et al. suggested using activated carbon fibers along with TiO2 for the application. In particular, they describe how to employ a PDP reactor with activated carbon fiber loaded with TiO2 to degrade methyl orange (MO) in an aqueous solution [241]. Iervolino et al. reported degrading acid orange 7 dye (AO7) in an aqueous solution utilizing immobilized Fe2O3 on glass spheres as a catalyst in a DBD reactor. The authors demonstrate that using catalytic packed material enables the total degradation of AO7 pollutant to be achieved in a relatively short amount of time (~10 min) with the supplied voltage of 12 kV, which is below that typically employed for this technique [242]. Table 7 provides a concise overview of the operating conditions and catalysts utilized for the degradation of dyes in aqueous phase by NTP.

Table 7.
A summary of the key operating conditions and catalyst utilized for the degradation of dyes in aqueous phase by NTP combined with a catalyst.
Additionally, DBD combined with BiPO4 has been found to degrade the crystal violet dye in an aqueous solution. The CV dye was not shown to be adsorbed by this catalyst, but it does participate in the photogeneration of reactive species. In fact, its inclusion in the DBD reactor enabled the dye’s degradation to increase by up to 28% after 12 min of exposure [243]. Furthermore, it has been noticed in this circumstance that an increase in input power improves dye degradation up to a particular power value, after which, a reduction in the dye’s degradation efficiency was observed.
9. Concluding Remarks
NTP application in medicine has grown from a concept, inspired by plasma physicists, to an interdisciplinary research area with increasing acceptance in the medical industry. The use of NTP for therapeutic purposes can be used to achieve a variety of medical objectives, including the decontamination of wounds and skin, the promotion of wound healing, the suppression of cancer, the management of multi-drug resistant bacteria that reside in wounds, and dental and aesthetic procedures. However, it is crucial to recognize and take advantage of the special benefits of plasma therapies in comparison to other forms of treatment [23]. A cutting-edge strategy is the formation of reactive species using NTP sources. These reactive species played a vital role in anticancer and various medical applications. To date, many studies (in vivo and in vitro) have shown that NTP has the potential to suppress cancer/tumors without harming healthy cells (Table 1) [23]. Preclinical and clinical trials should be carried out and assessed so that the interactions between plasma and biological cells and tissues can be fully understood.
Additionally, NTP technology has been used extensively in the food industry for the past few decades to sterilize food to keep it fresh and increase its storage life. Table 2 provides a summary of recent developments on this research topic along with some important findings. It is clear that food intrinsic factors must be taken into consideration when developing a plasma treatment because they affect the cell inactivation behavior and effectiveness of NTP [116]. To lessen toxicity and fungal contamination, this technique can be used as a substitute for the thermal process [119]. NTP may be able to reduce the number of bacteria on surfaces, but longer treatment times are required [122].
Recently, it is reported that NTP technology has the potential to combat COVID-19 [28,143]. As NTP is produced with less energy and has an electron at a temperature much higher than bulk gas molecules, it can inactivate airborne viruses [251]. When using NBP for virus inactivation, it is essential to set the correct parameters and choose treatment times that allow particles to interact with the contaminated material. We believe that one of the areas of viral inactivation where plasma may be a more significant advancement is water decontamination. NTP may render troublesome enteric viruses and hardy plant viruses inactive for use in humans and/or for agricultural purposes. In any case, it is important to first evaluate any potential genotoxic and cytotoxic effects of plasma-activated water on people and plants. As several other researchers have discovered, NTP significantly increases the amount of protein oxidation that takes place in the viral capsid or coat. By producing ROS, NTP treatment puts bacteriophages into a dormant state. NTP operating parameters may favor viral DNA damage or protein inactivation, but further research is needed to confirm this. Almost every study on NTP inactivation of viruses is distinct from the others because they either deal with the treatment of various liquid volumes, various viruses, or a particular plasma source with unique properties. It is challenging to directly compare these studies and define any common inactivation parameters or mechanism of action conclusions as a result of their excessive diversity. NTP technology for viruses is still in its infancy, so more research is needed. In order to better understand which plasma particles are crucial and how they affect viruses, more precise methods are clearly needed.
Among the best techniques for removing harmful chemicals from the environment is NTP. The yield of NTP-based technology is affected by a variety of operational factors. Catalysts can, from a physical standpoint, serve as a medium for enhancing the discharge intensity or lengthening the time that reactants are retained in the plasma zone [252,253]. When a catalyst is properly added to the NTP reactor, process performance is greatly improved compared to NTP alone. As a result, several scientists have investigated using NTP in combination with catalysts to remove numerous types of environmental pollutants. With a focus on different types of environmental pollutants removal in NTP alone and a plasma-catalytic system, this review might be useful to give a description of the significant advancements in the application of the plasma-catalytic system for environmental pollutants removal mainly in the past several years. Due to its particular synergistic effect, plasma catalysis is recommended for the effective removal of environmental contaminants. Based on the characteristics of the target pollutant and the reaction conditions, it is better to select an appropriate catalyst and plasma-catalysis system configuration. Particularly, plasma-catalytic systems have effectively created and used transition metal oxide-based catalysts with increased activity. The main factors affecting the performance of plasma-catalytic systems are catalyst synthesis parameters (such as the calcination temperature, aging duration, precursor type, and supports) and operating parameters [166]. In the plasma-catalytic system, the surface characteristics of the catalyst are crucial for catalytic activity. Although significant efforts have been made to investigate the activation and characterization of catalysts in plasma, advancing our knowledge of the synergetic mechanism of plasma and catalysts, there are still many significant obstacles that must be overcome through future research. There are limitations to the advancements in the formation of plasma-catalytic systems that are closer to practical industrial applications, demanding additional research in the future.
Author Contributions
Conceptualization, S.M., R.K. and I.H.; methodology, S.M., R.K. and I.H.; software, S.M.; validation, S.M., R.K., J.N.R., R.J. and I.H.; formal analysis, S.M., R.K., J.N.R., R.J., M.I. and I.H.; investigation, S.M. and R.K.; resources, S.M., R.K. and I.H.; data curation, S.M. and R.K.; writing—original draft preparation, S.M., R.K., J.N.R., R.J., M.I. and I.H.; writing—review and editing, S.M., R.K., J.N.R., R.J., M.I. and I.H.; visualization, S.M., R.K. and I.H.; supervision, E.H.C. and I.H.; project administration, I.H.; funding acquisition, I.H. and E.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the National Research Foundation of Korea (NRF) Grant through the Korean Government under Grant NRF-2022R1A2C1004257 and Grant NRF-2021R1A6A1A03038785, the Basic Science Research Program through funded by the Ministry of Education (2020R1I1A1A01073071), and by ITRC (Information Technology Research Center) support program (IITP-2020-0-01846), also by the Excellent researcher support project of Kwangwoon University in 2022.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold atmospheric plasma delivery for biomedical applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015, 24, 33001. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef]
- Ghimire, B.; Lee, G.J.; Mumtaz, S.; Choi, E.H. Scavenging effects of ascorbic acid and mannitol on hydroxyl radicals generated inside water by an atmospheric pressure plasma jet. AIP Adv. 2018, 8, 75021. [Google Scholar] [CrossRef]
- Lamichhane, P.; Veerana, M.; Lim, J.S.; Mumtaz, S.; Shrestha, B.; Kaushik, N.K.; Park, G.; Choi, E.H. Low-Temperature Plasma-Assisted Nitrogen Fixation for Corn Plant Growth and Development. Int. J. Mol. Sci. 2021, 22, 5360. [Google Scholar] [CrossRef]
- Lamichhane, P.; Adhikari, B.C.; Nguyen, L.N.; Paneru, R.; Ghimire, B.; Mumtaz, S.; Lim, J.S.; Hong, Y.J.; Choi, E.H. Sustainable nitrogen fixation from synergistic effect of photo-electrochemical water splitting and atmospheric pressure N2 plasma. Plasma Sources Sci. Technol. 2020, 29, 45026. [Google Scholar] [CrossRef]
- Han, I.; Rana, J.N.; Kim, J.-H.; Choi, E.H.; Kim, Y. A Non-thermal Biocompatible Plasma-Modified Chitosan Scaffold Enhances Osteogenic Differentiation in Bone Marrow Stem Cells. Pharmaceutics 2022, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Hong, Y.J.; Ghimire, B.; Choi, J.; Mumtaz, S.; Choi, E.H. Measurement of electron density in transient spark discharge by simple interferometry. Results Phys. 2021, 20, 103693. [Google Scholar] [CrossRef]
- Lu, X.; Laroussi, M.; Puech, V. On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sources Sci. Technol. 2012, 21, 34005. [Google Scholar] [CrossRef]
- Yoon, S.; Jeong, K.; Mumtaz, S.; Choi, E.H. Electromagnetic pulse shielding effectiveness of circular multi-waveguides for fluids. Results Phys. 2020, 16, 102946. [Google Scholar] [CrossRef]
- Kuchenbecker, M.; Bibinov, N.; Kaemlimg, A.; Wandke, D.; Awakowicz, P.; Viöl, W. Characterization of DBD plasma source for biomedical applications. J. Phys. D Appl. Phys. 2009, 42, 45212. [Google Scholar] [CrossRef]
- Lamichhane, P.; Paneru, R.; Nguyen, L.N.; Lim, J.S.; Bhartiya, P.; Adhikari, B.C.; Mumtaz, S.; Choi, E.H. Plasma-assisted nitrogen fixation in water with various metals. React. Chem. Eng. 2020, 5, 2053–2057. [Google Scholar] [CrossRef]
- Mumtaz, S.; Uhm, H.; Lim, J.S.; Choi, E.H. Output-Power Enhancement of Vircator Based on Second Virtual Cathode Formed by Wall Charge on a Dielectric Reflector. IEEE Trans. Electron Devices 2022, 69, 2043–2050. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Mumtaz, S.; Lim, J.S.; Jang, J.H.; Kim, D.; Sahu, B.D.; Bogaerts, A.; Choi, E.H. Evaluation of non-thermal effect of microwave radiation and its mode of action in bacterial cell inactivation. Sci. Rep. 2021, 11, 14003. [Google Scholar] [CrossRef]
- Tornin, J.; Labay, C.; Tampieri, F.; Ginebra, M.-P.; Canal, C. Evaluation of the effects of cold atmospheric plasma and plasma-treated liquids in cancer cell cultures. Nat. Protoc. 2021, 16, 2826–2850. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Bhartiya, P.; Kaushik, N.; Adhikari, M.; Lamichhane, P.; Lee, S.-J.; Kaushik, N.K.; Choi, E.H. Pulsed high-power microwaves do not impair the functions of skin normal and cancer cells in vitro: A short-term biological evaluation. J. Adv. Res. 2020, 22, 47–55. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, S.; Zhang, J.; Wang, Z.; Liu, D.; Guo, L.; Cheng, C.; Cheng, Y.; Xu, D.; Kong, M.G.; et al. Plasma-activated thermosensitive biogel as an exogenous ROS carrier for post-surgical treatment of cancer. Biomaterials 2021, 276, 121057. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, P.; Ghimire, B.; Mumtaz, S.; Paneru, R.; Ki, S.H.; Choi, E.H. Control of hydrogen peroxide production in plasma activated water by utilizing nitrification. J. Phys. D Appl. Phys. 2019, 52, 265206. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Kaushik, N.; Lamichhane, P.; Mumtaz, S.; Paneru, R.; Bhartiya, P.; Kwon, J.S.; Mishra, Y.K.; Nguyen, L.Q.; Kaushik, N.K.; et al. In situ plasma-assisted synthesis of polydopamine-functionalized gold nanoparticles for biomedical applications. Green Chem. 2020, 22, 6588–6599. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, D.; Ki, S.; Mumtaz, S.; Shaik, A.M.; Han, I.; Hong, Y.J.; Park, G.; Choi, E.H. Characteristics of a Rollable Dielectric Barrier Discharge Plasma and Its Effects on Spinach-Seed Germination. Int. J. Mol. Sci. 2023, 24, 4638. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, I.; Agarwal, S.; Ahedo, E.; Alves, L.L.; Baalrud, S.; Babaeva, N.; Bogaerts, A.; Bourdon, A.; Bruggeman, P.J.; Canal, C.; et al. The 2022 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2022, 55, 373001. [Google Scholar] [CrossRef]
- Machala, Z.; Pavlovich, M.J. A New Phase in Applied Biology. Trends Biotechnol. 2018, 36, 577–578. [Google Scholar] [CrossRef]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Li, Y.; Ho Kang, M.; Sup Uhm, H.; Joon Lee, G.; Ha Choi, E.; Han, I. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma activated nitric oxide water on cervical cancer cells. Sci. Rep. 2017, 7, 45781. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Yepez, X.; Xu, L.; Keener, K. In-package cold plasma technologies. J. Food Eng. 2019, 244, 21–31. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef]
- Jenns, K.; Sassi, H.P.; Zhou, R.; Cullen, P.J.; Carter, D.; Mai-Prochnow, A. Inactivation of foodborne viruses: Opportunities for cold atmospheric plasma. Trends Food Sci. Technol. 2022, 124, 323–333. [Google Scholar] [CrossRef]
- Mozetič, M.; Vesel, A.; Primc, G.; Zaplotnik, R. Chapter 2—Introduction to Plasma and Plasma Diagnostics. In Non-Thermal Plasma Technology for Polymeric Materials; Thomas, S., Mozetič, M., Cvelbar, U., Špatenka, P., Praveen, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–65. ISBN 978-0-12-813152-7. [Google Scholar]
- Yan, W.; Xia, Y.; Bi, Z.; Song, Y.; Wang, D.; Liu, D. Numerical investigation of underwater discharge generated in a single helium bubble at atmospheric pressure. Phys. Plasmas 2019, 26, 23504. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Chánová, E.; Syková, E.; Dejneka, A.; Kubinová, Š. Cell death induced by ozone and various non-thermal plasmas: Therapeutic perspectives and limitations. Sci. Rep. 2014, 4, 7129. [Google Scholar] [CrossRef]
- Cheng, X.; Sherman, J.; Murphy, W.; Ratovitski, E.; Canady, J.; Keidar, M. The Effect of Tuning Cold Plasma Composition on Glioblastoma Cell Viability. PLoS ONE 2014, 9, e98652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Z.; Shen, J.; Li, X.; Ding, L.; Ma, J.; Lan, Y.; Xia, W.; Cheng, C.; Sun, Q.; et al. Effects and Mechanism of Atmospheric-Pressure Dielectric Barrier Discharge Cold Plasmaon Lactate Dehydrogenase (LDH) Enzyme. Sci. Rep. 2015, 5, 10031. [Google Scholar] [CrossRef]
- Yonemori, S.; Nakagawa, Y.; Ono, R.; Oda, T. Measurement of OH density and air–helium mixture ratio in an atmospheric-pressure helium plasma jet. J. Phys. D Appl. Phys. 2012, 45, 225202. [Google Scholar] [CrossRef]
- Lu, X.; Keidar, M.; Laroussi, M.; Choi, E.; Szili, E.J.; Ostrikov, K. Transcutaneous plasma stress: From soft-matter models to living tissues. Mater. Sci. Eng. R Rep. 2019, 138, 36–59. [Google Scholar] [CrossRef]
- Attri, P.; Kim, Y.H.; Park, D.H.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.N.; Fridman, A.; Choi, E.H. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci. Rep. 2015, 5, 9332. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, C.-H. Applications of Plasma-Activated Liquid in the Medical Field. Biomedicines 2021, 9, 1700. [Google Scholar] [CrossRef]
- Kondeti, V.S.S.K.; Phan, C.Q.; Wende, K.; Jablonowski, H.; Gangal, U.; Granick, J.L.; Hunter, R.C.; Bruggeman, P.J. Long-lived and short-lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free Radic. Biol. Med. 2018, 124, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef]
- Ja Kim, S.; Min Joh, H.; Chung, T.H. Production of intracellular reactive oxygen species and change of cell viability induced by atmospheric pressure plasma in normal and cancer cells. Appl. Phys. Lett. 2013, 103, 153705. [Google Scholar] [CrossRef]
- Akter, M.; Lim, J.S.; Choi, E.H.; Han, I. Non-Thermal Biocompatible Plasma Jet Induction of Apoptosis in Brain Cancer Cells. Cells 2021, 10, 236. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.J.; Wang, S.B.; Choi, E.H.; Han, I. Non-Thermal Bio-Compatible Plasma Induces Osteogenic Differentiation of Human Mesenchymal Stem/Stromal Cells With ROS-Induced Activation of MAPK. IEEE Access 2020, 8, 36652–36663. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Reactive oxygen species in seminal plasma as a cause of male infertility. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.N.; Mumtaz, S.; Choi, E.H.; Han, I. ROS production in response to high-power microwave pulses induces p53 activation and DNA damage in brain cells: Radiosensitivity and biological dosimetry evaluation. Front. Cell Dev. Biol. 2023, 11, 1067861. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of Non-Thermal Plasma on Mammalian Cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.-S. Targeting Cancer Cells with Reactive Oxygen and Nitrogen Species Generated by Atmospheric-Pressure Air Plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.-S. Atmospheric-Pressure Plasma Jet Induces Apoptosis Involving Mitochondria via Generation of Free Radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef]
- Samukawa, S.; Hori, M.; Rauf, S.; Tachibana, K.; Bruggeman, P.; Kroesen, G.; Whitehead, J.C.; Murphy, A.B.; Gutsol, A.F.; Starikovskaia, S.; et al. The 2012 Plasma Roadmap. J. Phys. D Appl. Phys. 2012, 45, 253001. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Laroussi, M.; Kong, M.; Morfill, G.; Stolz, W. Plasma Medicine: Applications of Low-Temperature Gas Plasmas in Medicine and Biology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Stoffels, E.; Kieft, I.E.; Sladek, R.E.J.; van den Bedem, L.J.M.; van der Laan, E.P.; Steinbuch, M. Plasma needle for in vivo medical treatment: Recent developments and perspectives. Plasma Sources Sci. Technol. 2006, 15, S169–S180. [Google Scholar] [CrossRef]
- Alizadeh, E.; Ptasińska, S. Recent Advances in Plasma-Based Cancer Treatments: Approaching Clinical Translation through an Intracellular View. Biophysica 2021, 1, 48–72. [Google Scholar] [CrossRef]
- Ratovitski, E.A.; Cheng, X.; Yan, D.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. Anti-Cancer Therapies of 21st Century: Novel Approach to Treat Human Cancers Using Cold Atmospheric Plasma. Plasma Process. Polym. 2014, 11, 1128–1137. [Google Scholar] [CrossRef]
- Pai, K.; Timmons, C.; Roehm, K.D.; Ngo, A.; Narayanan, S.S.; Ramachandran, A.; Jacob, J.D.; Ma, L.M.; Madihally, S.V. Investigation of the Roles of Plasma Species Generated by Surface Dielectric Barrier Discharge. Sci. Rep. 2018, 8, 16674. [Google Scholar] [CrossRef]
- Kim, C.-H.; Bahn, J.H.; Lee, S.-H.; Kim, G.-Y.; Jun, S.-I.; Lee, K.; Baek, S.J. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J. Biotechnol. 2010, 150, 530–538. [Google Scholar] [CrossRef]
- Chien, P.-C.; Chen, C.-Y.; Cheng, Y.-C.; Sato, T.; Zhang, R.-Z. Selective inhibition of melanoma and basal cell carcinoma cells by short-lived species, long-lived species, and electric fields generated from cold plasma. J. Appl. Phys. 2021, 129, 163302. [Google Scholar] [CrossRef]
- Golpour, M.; Alimohammadi, M.; Mohseni, A.; Zaboli, E.; Sohbatzadeh, F.; Bekeschus, S.; Rafiei, A. Lack of Adverse Effects of Cold Physical Plasma-Treated Blood from Leukemia Patients: A Proof-of-Concept Study. Appl. Sci. 2022, 12, 128. [Google Scholar] [CrossRef]
- Choi, E.H.; Kaushik, N.K.; Hong, Y.J.; Lim, J.S.; Choi, J.S.; Han, I. Plasma bioscience for medicine, agriculture and hygiene applications. J. Korean Phys. Soc. 2022, 80, 817–851. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Choi, E.H. Cold Atmospheric Plasma Sources for Cancer Applications and Their Diagnostics. In Plasma Cancer Therapy; Keidar, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 53–73. ISBN 978-3-030-49966-2. [Google Scholar]
- Sato, T.; Yokoyama, M.; Johkura, K. A key inactivation factor of HeLa cell viability by a plasma flow. J. Phys. D Appl. Phys. 2011, 44, 372001. [Google Scholar] [CrossRef]
- Bekeschus, S.; Masur, K.; Kolata, J.; Wende, K.; Schmidt, A.; Bundscherer, L.; Barton, A.; Kramer, A.; Bröker, B.; Weltmann, K.-D. Human Mononuclear Cell Survival and Proliferation is Modulated by Cold Atmospheric Plasma Jet. Plasma Process. Polym. 2013, 10, 706–713. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Lim, J.S.; Javed, R.; Choi, E.H.; Han, I. Effect of Plasma On-Time with a Fixed Duty Ratio on Reactive Species in Plasma-Treated Medium and Its Significance in Biological Applications. Int. J. Mol. Sci. 2023, 24, 5289. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-N.; Oh, C.; Chang, J.W.; Liu, L.; Lim, M.A.; Jin, Y.L.; Piao, Y.; Kim, H.J.; Won, H.-R.; Lee, S.E.; et al. EGR1/GADD45α Activation by ROS of Non-Thermal Plasma Mediates Cell Death in Thyroid Carcinoma. Cancers 2021, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Razzokov, J.; Verswyvel, H.; Privat-Maldonado, A.; De Backer, J.; Yusupov, M.; Cardenas De La Hoz, E.; Ponsaerts, P.; Smits, E.; Bogaerts, A. Oxidation of Innate Immune Checkpoint CD47 on Cancer Cells with Non-Thermal Plasma. Cancers 2021, 13, 579. [Google Scholar] [CrossRef]
- Almeida-Ferreira, C.; Silva-Teixeira, R.; Gonçalves, A.C.; Marto, C.M.; Sarmento-Ribeiro, A.B.; Caramelo, F.; Botelho, M.F.; Laranjo, M. Cold Atmospheric Plasma Apoptotic and Oxidative Effects on MCF7 and HCC1806 Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 1698. [Google Scholar] [CrossRef]
- Jo, A.; Bae, J.H.; Yoon, Y.J.; Chung, T.H.; Lee, E.-W.; Kim, Y.-H.; Joh, H.M.; Chung, J.W. Plasma-activated medium induces ferroptosis by depleting FSP1 in human lung cancer cells. Cell Death Dis. 2022, 13, 212. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Christodoulou, A.-M.; Tachliabouri, M.; Meropoulis, S.; Christopoulou, M.-E.; Karalis, T.T.; Chatzopoulos, A.; Skandalis, S.S. Cold Atmospheric Plasma Attenuates Breast Cancer Cell Growth Through Regulation of Cell Microenvironment Effectors. Front. Oncol. 2022, 11, 826865. [Google Scholar] [CrossRef]
- Jinno, R.; Komuro, A.; Yanai, H.; Ono, R. Antitumor abscopal effects in mice induced by normal tissue irradiation using pulsed streamer discharge plasma. J. Phys. D Appl. Phys. 2022, 55, 17LT01. [Google Scholar] [CrossRef]
- Schweigert, I.; Zakrevsky, D.; Milakhina, E.; Gugin, P.; Biryukov, M.; Patrakova, E.; Koval, O. A grounded electrode beneath dielectric targets, including cancer cells, enhances the impact of cold atmospheric plasma jet. Plasma Phys. Control. Fusion 2022, 64, 44015. [Google Scholar] [CrossRef]
- Yu, H.; Song, X.; Yang, F.; Wang, J.; Sun, M.; Liu, G.; Ahmad, N.; Zhou, Y.; Zhang, Y.; Shi, G.; et al. Combined effects of vitamin C and cold atmospheric plasma-conditioned media against glioblastoma via hydrogen peroxide. Free Radic. Biol. Med. 2023, 194, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, A.; Sander, C.; Eggers, B.; Weiss, M.; Egger, E.; Kramer, F.-J.; Erb, H.H.H.; Mustea, A.; Stope, M.B. Pleiotropic Devitalization of Renal Cancer Cells by Non-Invasive Physical Plasma: Characterization of Molecular and Cellular Efficacy. Cancers 2023, 15, 481. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Bien-Möller, S.; Marx, S.; Bekeschus, S.; Schroeder, H.W.S.; Mustea, A.; Stope, M.B. Devitalization of Glioblastoma Cancer Cells by Non-invasive Physical Plasma: Modulation of Proliferative Signalling Cascades. Anticancer Res. 2023, 43, 7–18. [Google Scholar] [CrossRef]
- Golpour, M.; Alimohammadi, M.; Sohbatzadeh, F.; Fattahi, S.; Bekeschus, S.; Rafiei, A. Cold atmospheric pressure plasma treatment combined with starvation increases autophagy and apoptosis in melanoma in vitro and in vivo. Exp. Dermatol. 2022, 31, 1016–1028. [Google Scholar] [CrossRef]
- Patrakova, E.; Biryukov, M.; Troitskaya, O.; Gugin, P.; Milakhina, E.; Semenov, D.; Poletaeva, J.; Ryabchikova, E.; Novak, D.; Kryachkova, N.; et al. Chloroquine Enhances Death in Lung Adenocarcinoma A549 Cells Exposed to Cold Atmospheric Plasma Jet. Cells 2023, 12, 290. [Google Scholar] [CrossRef]
- Choi, J.-H.; Gu, H.-J.; Park, K.-H.; Hwang, D.-S.; Kim, G.-C. Anti-Cancer Activity of the Combinational Treatment of Noozone Cold Plasma with p-FAK Antibody-Conjugated Gold Nanoparticles in OSCC Xenograft Mice. Biomedicines 2022, 10, 2259. [Google Scholar] [CrossRef]
- Nitsch, A.; Strakeljahn, S.; Jacoby, J.M.; Sieb, K.F.; Mustea, A.; Bekeschus, S.; Ekkernkamp, A.; Stope, M.B.; Haralambiev, L. New Approach against Chondrosoma Cells—Cold Plasma Treatment Inhibits Cell Motility and Metabolism, and Leads to Apoptosis. Biomedicines 2022, 10, 688. [Google Scholar] [CrossRef]
- Mihai, C.-T.; Mihaila, I.; Pasare, M.A.; Pintilie, R.M.; Ciorpac, M.; Topala, I. Cold Atmospheric Plasma-Activated Media Improve Paclitaxel Efficacy on Breast Cancer Cells in a Combined Treatment Model. Curr. Issues Mol. Biol. 2022, 44, 1995–2014. [Google Scholar] [CrossRef]
- Qi, M.; Xu, D.; Wang, S.; Li, B.; Peng, S.; Li, Q.; Zhang, H.; Fan, R.; Chen, H.; Kong, M.G. In Vivo Metabolic Analysis of the Anticancer Effects of Plasma-Activated Saline in Three Tumor Animal Models. Biomedicines 2022, 10, 528. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Ginebra, M.-P.; Tornín, J.; Canal, C. Cold atmospheric plasma enhances doxorubicin selectivity in metastasic bone cancer. Free Radic. Biol. Med. 2022, 189, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; Berman, R.; Connors, J.; Haddad, E.K.; Miller, V.; Nonnemacher, M.R.; Dampier, W.; Wigdahl, B.; Krebs, F.C. Immunomodulatory Effects of Non-Thermal Plasma in a Model for Latent HIV-1 Infection: Implications for an HIV-1-Specific Immunotherapy. Biomedicines 2023, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nakatsu, Y.; Tanaka, H.; Koga, K.; Ishikawa, K.; Shiratani, M.; Hori, M. Effects of plasma-activated Ringer’s lactate solution on cancer cells: Evaluation of genotoxicity. Genes Environ. 2023, 45, 3. [Google Scholar] [CrossRef]
- Shojaei, E.; Zare, S.; Shirkavand, A.; Eslami, E.; Fathollah, S.; Mansouri, P. Biophysical evaluation of treating adipose tissue-derived stem cells using non-thermal atmospheric pressure plasma. Sci. Rep. 2022, 12, 11127. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Sahun, M.; Biscop, E.; Verswyvel, H.; De Waele, J.; De Backer, J.; Theys, C.; Cuypers, B.; Laukens, K.; Berghe, W.V.; et al. Acquired non-thermal plasma resistance mediates a shift towards aerobic glycolysis and ferroptotic cell death in melanoma. Drug Resist. Updat. 2023, 67, 100914. [Google Scholar] [CrossRef]
- Mohamed, H.; Gebski, E.; Reyes, R.; Beane, S.; Wigdahl, B.; Krebs, F.C.; Stapelmann, K.; Miller, V. Differential Effect of Non-Thermal Plasma RONS on Two Human Leukemic Cell Populations. Cancers 2021, 13, 2437. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, X.; Yang, W.; Zhang, Y.; Ye, Z.; Hu, S.; Ye, C.; Li, Y.; Lan, Y.; Shen, J. In vitro antimicrobial effects and mechanism of air plasma-activated water on Staphylococcus aureus biofilm. Plasma Process. Polym. 2020, 17, 1900270. [Google Scholar] [CrossRef]
- Lukes, P.; Clupek, M.; Babicky, V.; Sunka, P. Ultraviolet radiation from the pulsed corona discharge in water. Plasma Sources Sci. Technol. 2008, 17, 24012. [Google Scholar] [CrossRef]
- Šimečková, J.; Krčma, F.; Klofáč, D.; Dostál, L.; Kozáková, Z. Influence of Plasma-Activated Water on Physical and Physical–Chemical Soil Properties. Water 2020, 12, 2357. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Jones, P. Phytoplasmas: Genomes, Plant Hosts and Vectors; CABI: Wallingford, UK, 2009; ISBN 1845935314. [Google Scholar]
- Liu, X.; Li, Y.; Wang, S.; Huangfu, L.; Zhang, M.; Xiang, Q. Synergistic antimicrobial activity of plasma-activated water and propylparaben: Mechanism and applications for fresh produce sanitation. LWT 2021, 146, 111447. [Google Scholar] [CrossRef]
- Chen, T.-P.; Su, T.-L.; Liang, J. Plasma-Activated Solutions for Bacteria and Biofilm Inactivation. Curr. Bioact. Compd. 2017, 13, 59–65. [Google Scholar] [CrossRef]
- Foest, R.; Schmidt, M.; Becker, K. Microplasmas, an emerging field of low-temperature plasma science and technology. Int. J. Mass Spectrom. 2006, 248, 87–102. [Google Scholar] [CrossRef]
- Koban, I.; Holtfreter, B.; Hübner, N.-O.; Matthes, R.; Sietmann, R.; Kindel, E.; Weltmann, K.-D.; Welk, A.; Kramer, A.; Kocher, T. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro—Proof of principle experiment. J. Clin. Periodontol. 2011, 38, 956–965. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, J.; Zhang, Z.; Ma, J.; Ma, R.; Zhao, Y.; Sun, Q.; Qian, S.; Zhang, H.; Ding, L.; et al. Inactivation Effects of Non-Thermal Atmospheric-Pressure Helium Plasma Jet on Staphylococcus aureus Biofilms. Plasma Process. Polym. 2015, 12, 827–835. [Google Scholar] [CrossRef]
- Ikawa, S.; Kitano, K.; Hamaguchi, S. Effects of pH on Bacterial Inactivation in Aqueous Solutions due to Low-Temperature Atmospheric Pressure Plasma Application. Plasma Process. Polym. 2010, 7, 33–42. [Google Scholar] [CrossRef]
- Schnabel, U.; Handorf, O.; Stachowiak, J.; Boehm, D.; Weit, C.; Weihe, T.; Schäfer, J.; Below, H.; Bourke, P.; Ehlbeck, J. Plasma-functionalized water: From bench to prototype for fresh-cut lettuce. Food Eng. Rev. 2021, 13, 115–135. [Google Scholar] [CrossRef]
- Yu, N.-N.; Ketya, W.; Choi, E.-H.; Park, G. Plasma Promotes Fungal Cellulase Production by Regulating the Levels of Intracellular NO and Ca2+. Int. J. Mol. Sci. 2022, 23, 6668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, R.; Liu, D.; Wang, W.; Niu, J.; Xia, Y.; Qi, Z.; Zhao, Z.; Song, Y. Effect of nonthermal plasma-activated water on quality and antioxidant activity of fresh-cut kiwifruit. IEEE Trans. Plasma Sci. 2019, 47, 4811–4817. [Google Scholar] [CrossRef]
- Hu, X.; Sun, H.; Yang, X.; Cui, D.; Wang, Y.; Zhuang, J.; Wang, X.; Ma, R.; Jiao, Z. Potential use of atmospheric cold plasma for postharvest preservation of blueberries. Postharvest Biol. Technol. 2021, 179, 111564. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Han, J.-Y.; Park, S.-H.; Kang, D.-H. Effects of plasma bubble-activated water on the inactivation against foodborne pathogens on tomatoes and its wash water. Food Control 2023, 144, 109381. [Google Scholar] [CrossRef]
- Katsaros, G.; Giannoglou, M.; Chanioti, S.; Roufou, S.; Javaheri, A.; de Oliveira Mallia, J.; Gatt, R.; Agalou, A.; Beis, D.; Valdramidis, V. Production, characterization, microbial inhibition, and in vivo toxicity of cold atmospheric plasma activated water. Innov. Food Sci. Emerg. Technol. 2023, 84, 103265. [Google Scholar] [CrossRef]
- Charoux, C.M.G.; Free, L.; Hinds, L.M.; Vijayaraghavan, R.K.; Daniels, S.; O’Donnell, C.P.; Tiwari, B.K. Effect of non-thermal plasma technology on microbial inactivation and total phenolic content of a model liquid food system and black pepper grains. LWT 2020, 118, 108716. [Google Scholar] [CrossRef]
- Dasan, B.G.; Yildirim, T.; Boyaci, I.H. Surface decontamination of eggshells by using non-thermal atmospheric plasma. Int. J. Food Microbiol. 2018, 266, 267–273. [Google Scholar] [CrossRef]
- Hadinoto, K.; Rao, N.R.H.; Astorga, J.B.; Zhou, R.; Biazik, J.; Zhang, T.; Masood, H.; Cullen, P.J.; Prescott, S.; Henderson, R.K.; et al. Hybrid plasma discharges for energy-efficient production of plasma-activated water. Chem. Eng. J. 2023, 451, 138643. [Google Scholar] [CrossRef]
- Guo, L.; Yao, Z.; Yang, L.; Zhang, H.; Qi, Y.; Gou, L.; Xi, W.; Liu, D.; Zhang, L.; Cheng, Y.; et al. Plasma-activated water: An alternative disinfectant for S protein inactivation to prevent SARS-CoV-2 infection. Chem. Eng. J. 2021, 421, 127742. [Google Scholar] [CrossRef]
- Shan, C.; Wu, H.; Zhu, Y.; Zhou, J.; Yan, W.; Jianhao, Z.; Liu, X. Preservative effects of a novel bacteriocin from Lactobacillus panis C-M2 combined with dielectric barrier discharged cold plasma (DBD-CP) on acquatic foods. Food Sci. Technol. Int. 2022, 28, 10820132221094720. [Google Scholar] [CrossRef]
- Wang, J.; Han, R.; Liao, X.; Ding, T. Application of plasma-activated water (PAW) for mitigating methicillin-resistant Staphylococcus aureus (MRSA) on cooked chicken surface. LWT 2021, 137, 110465. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Li, X.; Xiao, J.; Sun, J.; Guo, L. Inactivation of Escherichia coli on broccoli sprouts via plasma activated water and its effects on quality attributes. LWT 2022, 154, 112761. [Google Scholar] [CrossRef]
- Smet, C.; Noriega, E.; Rosier, F.; Walsh, J.L.; Valdramidis, V.P.; Van Impe, J.F. Impact of food model (micro)structure on the microbial inactivation efficacy of cold atmospheric plasma. Int. J. Food Microbiol. 2017, 240, 47–56. [Google Scholar] [CrossRef]
- Patange, A.D.; Simpson, J.C.; Curtin, J.F.; Burgess, C.M.; Cullen, P.J.; Tiwari, B.K. Inactivation efficacy of atmospheric air plasma and airborne acoustic ultrasound against bacterial biofilms. Sci. Rep. 2021, 11, 2346. [Google Scholar] [CrossRef]
- Lim, J.; Byeon, Y.-S.; Hong, E.J.; Ryu, S.; Kim, S.B. Effect of post-discharge time of plasma-treated water (PTW) on microbial inactivation and quality of fresh-cut potatoes. J. Food Process. Preserv. 2021, 45, e15387. [Google Scholar] [CrossRef]
- Esmaeili, Z.; Hosseinzadeh Samani, B.; Nazari, F.; Rostami, S.; Nemati, A. The green technology of cold plasma jet on the inactivation of Aspergillus flavus and the total aflatoxin level in pistachio and its quality properties. J. Food Process. Eng. 2023, 46, e14189. [Google Scholar] [CrossRef]
- Lin, C.-M.; Patel, A.K.; Chiu, Y.-C.; Hou, C.-Y.; Kuo, C.-H.; Dong, C.-D.; Chen, H.-L. The application of novel rotary plasma jets to inhibit the aflatoxin-producing Aspergillus flavus and the spoilage fungus, Aspergillus niger on peanuts. Innov. Food Sci. Emerg. Technol. 2022, 78, 102994. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Ma, T. Properties of plasma-activated water with different activation time and its effects on the quality of button mushrooms (Agaricus bisporus). LWT 2021, 147, 111633. [Google Scholar] [CrossRef]
- Xiang, Q.; Kang, C.; Zhao, D.; Niu, L.; Liu, X.; Bai, Y. Influence of organic matters on the inactivation efficacy of plasma-activated water against E. coli O157:H7 and S. aureus. Food Control 2019, 99, 28–33. [Google Scholar] [CrossRef]
- Thomas-Popo, E.; Mendonça, A.; Misra, N.N.; Little, A.; Wan, Z.; Moutiq, R.; Coleman, S.; Keener, K. Inactivation of Shiga-toxin-producing Escherichia coli, Salmonella enterica and natural microflora on tempered wheat grains by atmospheric cold plasma. Food Control 2019, 104, 231–239. [Google Scholar] [CrossRef]
- Yadav, B.; Spinelli, A.C.; Misra, N.N.; Tsui, Y.Y.; McMullen, L.M.; Roopesh, M.S. Effect of in-package atmospheric cold plasma discharge on microbial safety and quality of ready-to-eat ham in modified atmospheric packaging during storage. J. Food Sci. 2020, 85, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Benjakul, S. The combined effect of squid pen chitooligosaccharides and high voltage cold atmospheric plasma on the shelf-life extension of Asian sea bass slices stored at 4 °C. Innov. Food Sci. Emerg. Technol. 2020, 64, 102339. [Google Scholar] [CrossRef]
- Roh, S.H.; Oh, Y.J.; Lee, S.Y.; Kang, J.H.; Min, S.C. Inactivation of Escherichia coli O157:H7, Salmonella, Listeria monocytogenes, and Tulane virus in processed chicken breast via atmospheric in-package cold plasma treatment. LWT 2020, 127, 109429. [Google Scholar] [CrossRef]
- Xia, T.; Yang, M.; Marabella, I.; Lee, E.M.; Olson, B.; Zarling, D.; Torremorell, M.; Clack, H.L. Inactivation of airborne porcine reproductive and respiratory syndrome virus (PRRSv) by a packed bed dielectric barrier discharge non-thermal plasma. J. Hazard. Mater. 2020, 393, 122266. [Google Scholar] [CrossRef]
- Moutiq, R.; Misra, N.N.; Mendonça, A.; Keener, K. In-package decontamination of chicken breast using cold plasma technology: Microbial, quality and storage studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef]
- Kim, Y.E.; Min, S.C. Inactivation of Salmonella in ready-to-eat cabbage slices packaged in a plastic container using an integrated in-package treatment of hydrogen peroxide and cold plasma. Food Control 2021, 130, 108392. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Chantakun, K.; Benjakul, S. Microbial, chemical qualities and shelf-life of blue swimming crab (Portunus armatus) lump meat as influenced by in-package high voltage cold plasma treatment. Food Biosci. 2021, 43, 101274. [Google Scholar] [CrossRef]
- Ramezan, Y.; Hematabadi, H.; Ramezan, M.; Khani, M.R.; Kamkari, A.; Najafi Tabrizi, A. Effect of cold atmospheric plasma torch distance on the microbial inactivation and sensorial properties of ready-to-eat olivier salad. Food Sci. Technol. Int. 2022, 28, 10820132221108708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pal, R.K.; Yen, H.-W.; Naik, S.P.; Orzeszko, M.K.; Mazzeo, A.; Salvi, D. Cold plasma from flexible and conformable paper-based electrodes for fresh produce sanitation: Evaluation of microbial inactivation and quality changes. Food Control 2022, 137, 108915. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhang, Y.; Lü, X.; Zhao, L.; Song, Y.; Zhang, L.; Jiang, H.; Zhang, J.; Ge, W. Processing sheep milk by cold plasma technology: Impacts on the microbial inactivation, physicochemical characteristics, and protein structure. LWT 2022, 153, 112573. [Google Scholar] [CrossRef]
- De Baerdemaeker, K.; Van der Linden, I.; Nikiforov, A.; Zuber, S.; De Geyter, N.; Devlieghere, F. Non-thermal plasma inactivation of Salmonella Typhimurium on different matrices and the effect of selected food components on its bactericidal efficacy. Food Res. Int. 2022, 151, 110866. [Google Scholar] [CrossRef]
- Zorzi, V.; Berardinelli, A.; Gozzi, G.; Ragni, L.; Vannini, L.; Ceccato, R.; Parrino, F. Combined effect of atmospheric gas plasma and UVA light: A sustainable and green alternative for chemical decontamination and microbial inactivation of fish processing water. Chemosphere 2023, 317, 137792. [Google Scholar] [CrossRef]
- Man, C.; Zhang, C.; Fang, H.; Zhou, R.; Huang, B.; Xu, Y.; Zhang, X.; Shao, T. Nanosecond-pulsed microbubble plasma reactor for plasma-activated water generation and bacterial inactivation. Plasma Process. Polym. 2022, 19, 2200004. [Google Scholar] [CrossRef]
- Lee, S.H.I.; Fröhling, A.; Schlüter, O.; Corassin, C.H.; De Martinis, E.C.P.; Alves, V.F.; Pimentel, T.C.; Oliveira, C.A.F. Cold atmospheric pressure plasma inactivation of dairy associated planktonic cells of Listeria monocytogenes and Staphylococcus aureus. LWT 2021, 146, 111452. [Google Scholar] [CrossRef]
- Yasuda, H.; Miura, T.; Kurita, H.; Takashima, K.; Mizuno, A. Biological Evaluation of DNA Damage in Bacteriophages Inactivated by Atmospheric Pressure Cold Plasma. Plasma Process. Polym. 2010, 7, 301–308. [Google Scholar] [CrossRef]
- Hongzhuan, Z.; Ying, T.; Xia, S.; Jinsong, G.; Zhenhua, Z.; Beiyu, J.; Yanyan, C.; Lulu, L.; Jue, Z.; Bing, Y.; et al. Preparation of the inactivated Newcastle disease vaccine by plasma activated water and evaluation of its protection efficacy. Appl. Microbiol. Biotechnol. 2020, 104, 107–117. [Google Scholar] [CrossRef]
- Khalili, M.; Daniels, L.; Lin, A.; Krebs, F.C.; Snook, A.E.; Bekeschus, S.; Bowne, W.B.; Miller, V. Non-thermal plasma-induced immunogenic cell death in cancer. J. Phys. D Appl. Phys. 2019, 52, 423001. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, R.; Yang, L.; Wang, K.; Zhang, Q.; Su, X.; Yang, B.; Zhang, J.; Fang, J. Non-thermal plasma for inactivated-vaccine preparation. Vaccine 2016, 34, 1126–1132. [Google Scholar] [CrossRef]
- Xia, T.; Kleinheksel, A.; Lee, E.M.; Qiao, Z.; Wigginton, K.R.; Clack, H.L. Inactivation of airborne viruses using a packed bed non-thermal plasma reactor. J. Phys. D Appl. Phys. 2019, 52, 255201. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Mumtaz, S.; Choi, E.H. Nonthermal Biocompatible Plasma Inactivation of Coronavirus SARS-CoV-2: Prospects for Future Antiviral Applications. Viruses 2022, 14, 2685. [Google Scholar] [CrossRef]
- Peterhans, E. Reactive oxygen species and nitric oxide in viral diseases. Biol. Trace Elem. Res. 1997, 56, 107–116. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Adebayo, B.; Gelles, T.; Rownaghi, A.; Rezaei, F. Abatement of gaseous volatile organic compounds: A process perspective. Catal. Today 2020, 350, 100–119. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Shuai, J.; Kim, S.; Ryu, H.; Park, J.; Lee, C.K.; Kim, G.-B.; Ultra, V.U.; Yang, W. Health risk assessment of volatile organic compounds exposure near Daegu dyeing industrial complex in South Korea. BMC Public Health 2018, 18, 528. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.Y.L.; Lo, C.K.Y.; Kan, C. Textile dyes and human health: A systematic and citation network analysis review. Color. Technol. 2018, 134, 245–257. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, L.; Deák, G.; Jóver, B.; Kalló, D. Thermal and thermo-catalytic degradation of high-density polyethylene waste. J. Anal. Appl. Pyrolysis 2004, 72, 235–242. [Google Scholar] [CrossRef]
- Khader, E.H.; Mohammed, T.J.; Mirghaffari, N.; Salman, A.D.; Juzsakova, T.; Abdullah, T.A. Removal of organic pollutants from produced water by batch adsorption treatment. Clean Technol. Environ. Policy 2022, 24, 713–720. [Google Scholar] [CrossRef]
- Li, K.; Wang, E.; Wang, Q.; Husnain, N.; Li, D.; Fareed, S. Improving the removal of inhalable particles by combining flue gas condensation and acoustic agglomeration. J. Clean. Prod. 2020, 261, 121270. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Cornejo-Ponce, L.; Rajendran, R.; Manavalan, K.; Femilaa Rajan, V.; Awad, F. A review on biofiltration techniques: Recent advancements in the removal of volatile organic compounds and heavy metals in the treatment of polluted water. Bioengineered 2022, 13, 8432–8477. [Google Scholar] [CrossRef]
- Gan, G.; Fan, S.; Li, X.; Zhang, Z.; Hao, Z. Adsorption and membrane separation for removal and recovery of volatile organic compounds. J. Environ. Sci. 2022, 123, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Mustafa, M.F.; Fu, X.; Liu, Y.; Abbas, Y.; Wang, H.; Lu, W. Volatile organic compounds (VOCs) removal in non-thermal plasma double dielectric barrier discharge reactor. J. Hazard. Mater. 2018, 347, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, Y.; Li, S.; Xu, N.; Fu, Y. Environment pollutants removal with non-thermal plasma technology. Int. J. Low-Carbon Technol. 2022, 17, 446–455. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, D.; Qiu, R.; Tang, Y.; Du, C. Non-thermal plasma technology for organic contaminated soil remediation: A review. Chem. Eng. J. 2017, 313, 157–170. [Google Scholar] [CrossRef]
- Aerts, R.; Tu, X.; De Bie, C.; Whitehead, J.C.; Bogaerts, A. An Investigation into the Dominant Reactions for Ethylene Destruction in Non-Thermal Atmospheric Plasmas. Plasma Process. Polym. 2012, 9, 994–1000. [Google Scholar] [CrossRef]
- Aerts, R.; Tu, X.; Van Gaens, W.; Whitehead, J.C.; Bogaerts, A. Gas Purification by Nonthermal Plasma: A Case Study of Ethylene. Environ. Sci. Technol. 2013, 47, 6478–6485. [Google Scholar] [CrossRef]
- Nam, S.-N.; Choong, C.E.; Hoque, S.; Farouk, T.I.; Cho, J.; Jang, M.; Snyder, S.A.; Meadows, M.E.; Yoon, Y. Catalytic non-thermal plasma treatment of endocrine disrupting compounds, pharmaceuticals, and personal care products in aqueous solution: A review. Chemosphere 2022, 290, 133395. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Cheng, Z.; Sun, Z.; Chen, D.; Yu, J.; Chen, J. Non-thermal plasma coupled with catalysis for VOCs abatement: A review. Process Saf. Environ. Prot. 2021, 153, 139–158. [Google Scholar] [CrossRef]
- Ong, M.Y.; Nomanbhay, S.; Kusumo, F.; Show, P.L. Application of microwave plasma technology to convert carbon dioxide (CO2) into high value products: A review. J. Clean. Prod. 2022, 336, 130447. [Google Scholar] [CrossRef]
- Ray, D.; Ye, P.; Yu, J.C.; Song, C. Recent progress in plasma-catalytic conversion of CO2 to chemicals and fuels. Catal. Today 2022, in press. [Google Scholar] [CrossRef]
- Bogaerts, A.; Tu, X.; Whitehead, J.C.; Centi, G.; Lefferts, L.; Guaitella, O.; Azzolina-Jury, F.; Kim, H.-H.; Murphy, A.B.; Schneider, W.F.; et al. The 2020 plasma catalysis roadmap. J. Phys. D Appl. Phys. 2020, 53, 443001. [Google Scholar] [CrossRef]
- Whitehead, J.C. Plasma catalysis: A solution for environmental problems. Pure Appl. Chem. 2010, 82, 1329–1336. [Google Scholar] [CrossRef]
- Bogaerts, A.; Zhang, Q.-Z.; Zhang, Y.-R.; Van Laer, K.; Wang, W. Burning questions of plasma catalysis: Answers by modeling. Catal. Today 2019, 337, 3–14. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Engelmann, Y.; van ‘t Veer, K.; Postma, R.S.; Bogaerts, A.; Lefferts, L. Plasma-driven catalysis: Green ammonia synthesis with intermittent electricity. Green Chem. 2020, 22, 6258–6287. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef]
- Mehta, P.; Barboun, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Catalysis Enabled by Plasma Activation of Strong Chemical Bonds: A Review. ACS Energy Lett. 2019, 4, 1115–1133. [Google Scholar] [CrossRef]
- Chung, W.-C.; Mei, D.-H.; Tu, X.; Chang, M.-B. Removal of VOCs from gas streams via plasma and catalysis. Catal. Rev. 2019, 61, 270–331. [Google Scholar] [CrossRef]
- Whitehead, J.C. Plasma–catalysis: The known knowns, the known unknowns and the unknown unknowns. J. Phys. D Appl. Phys. 2016, 49, 243001. [Google Scholar] [CrossRef]
- Yang, X.; Qu, J.; Wang, L.; Luo, J. In-plasma-catalysis for NOx degradation by Ti3+ self-doped TiO2−x/γ-Al2O3 catalyst and nonthermal plasma. RSC Adv. 2021, 11, 24144–24155. [Google Scholar] [CrossRef]
- Capp, S.C.; Sawtell, D.A.G.; Banks, C.E.; Kelly, P.J.; Abd-Allah, Z. The effect of TiO2 coatings on the formation of ozone and nitrogen oxides in non-thermal atmospheric pressure plasma. J. Environ. Chem. Eng. 2021, 9, 106046. [Google Scholar] [CrossRef]
- Yan, C.; Waitt, C.; Akintola, I.; Lee, G.; Easa, J.; Clarke, R.; Geng, F.; Poirier, D.; Otor, H.O.; Rivera-Castro, G.; et al. Recent Advances in Plasma Catalysis. J. Phys. Chem. C 2022, 126, 9611–9614. [Google Scholar] [CrossRef]
- Hoseini, S.; Rahemi, N.; Allahyari, S.; Tasbihi, M. Application of plasma technology in the removal of volatile organic compounds (BTX) using manganese oxide nano-catalysts synthesized from spent batteries. J. Clean. Prod. 2019, 232, 1134–1147. [Google Scholar] [CrossRef]
- Abedi, K.; Ghorbani-Shahna, F.; Jaleh, B.; Bahrami, A.; Yarahmadi, R. Enhanced performance of non-thermal plasma coupled with TiO2/GAC for decomposition of chlorinated organic compounds: Influence of a hydrogen-rich substance. J. Environ. Health Sci. Eng. 2014, 12, 119. [Google Scholar] [CrossRef]
- Roland, U.; Holzer, F.; Kopinke, F.-D. Combination of non-thermal plasma and heterogeneous catalysis for oxidation of volatile organic compounds: Part 2. Ozone decomposition and deactivation of γ-Al2O3. Appl. Catal. B Environ. 2005, 58, 217–226. [Google Scholar] [CrossRef]
- Parastaev, A.; Hoeben, W.F.L.M.; van Heesch, B.E.J.M.; Kosinov, N.; Hensen, E.J.M. Temperature-programmed plasma surface reaction: An approach to determine plasma-catalytic performance. Appl. Catal. B Environ. 2018, 239, 168–177. [Google Scholar] [CrossRef]
- Shukrullah, S.; Ayyaz, M.; Naz, M.Y.; Ibrahim, K.A.; AbdEl-Salam, N.M.; Mohamed, H.F. Post-synthesis plasma processing and activation of TiO2 photocatalyst for the removal of synthetic dyes from industrial wastewater. Appl. Phys. A 2021, 127, 307. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; Wu, C.; Ashford, B.; Williams, P.T.; Tu, X. Plasma-photocatalytic conversion of CO2 at low temperatures: Understanding the synergistic effect of plasma-catalysis. Appl. Catal. B Environ. 2016, 182, 525–532. [Google Scholar] [CrossRef]
- Guaitella, O.; Thevenet, F.; Puzenat, E.; Guillard, C.; Rousseau, A. C2H2 oxidation by plasma/TiO2 combination: Influence of the porosity, and photocatalytic mechanisms under plasma exposure. Appl. Catal. B Environ. 2008, 80, 296–305. [Google Scholar] [CrossRef]
- Pan, K.L.; Pan, G.T.; Chong, S.; Chang, M.B. Removal of VOCs from gas streams with double perovskite-type catalysts. J. Environ. Sci. 2018, 69, 205–216. [Google Scholar] [CrossRef]
- Feng, X.; Liu, H.; He, C.; Shen, Z.; Wang, T. Synergistic effects and mechanism of a non-thermal plasma catalysis system in volatile organic compound removal: A review. Catal. Sci. Technol. 2018, 8, 936–954. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef]
- Oda, T.; Yamaji, K.; Takahashi, T. Decomposition of dilute trichloroethylene by nonthermal plasma processing-gas flow rate, catalyst, and ozone effect. IEEE Trans. Ind. Appl. 2004, 40, 430–436. [Google Scholar] [CrossRef]
- Han, S.-B.; Oda, T. Decomposition mechanism of trichloroethylene based on by-product distribution in the hybrid barrier discharge plasma process. Plasma Sources Sci. Technol. 2007, 16, 413–421. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Mora, M.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J.; De Geyter, N.; Leys, C.; Morent, R. TCE abatement with a plasma-catalytic combined system using MnO2 as catalyst. Appl. Catal. B Environ. 2014, 156–157, 94–100. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Nguyen Dinh, M.T.; Nuns, N.; Giraudon, J.-M.; De Geyter, N.; Leys, C.; Lamonier, J.-F.; Morent, R. Combination of non-thermal plasma and Pd/LaMnO3 for dilute trichloroethylene abatement. Chem. Eng. J. 2016, 283, 668–675. [Google Scholar] [CrossRef]
- Veerapandian, S.K.P.; De Geyter, N.; Giraudon, J.-M.; Lamonier, J.-F.; Morent, R. The Use of Zeolites for VOCs Abatement by Combining Non-Thermal Plasma, Adsorption, and/or Catalysis: A Review. Catalysts 2019, 9, 98. [Google Scholar] [CrossRef]
- Dinh, M.T.N.; Giraudon, J.-M.; Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Lamonier, J.-F. Post plasma-catalysis for total oxidation of trichloroethylene over Ce–Mn based oxides synthesized by a modified “redox-precipitation route”. Appl. Catal. B Environ. 2015, 172–173, 65–72. [Google Scholar] [CrossRef]
- Yu, X.; Dang, X.; Li, S.; Li, Y.; Wang, H.; Jing, K.; Dong, H.; Liu, X. Enhanced activity of plasma catalysis for trichloroethylene decomposition via metal-support interaction of SiOCo/Mn bonds over CoMnOX/ZSM-5. Sep. Purif. Technol. 2023, 305, 122553. [Google Scholar] [CrossRef]
- Jiang, N.; Kong, X.; Lu, X.; Peng, B.; Liu, Z.; Li, J.; Shang, K.; Lu, N.; Wu, Y. Promoting streamer propagation, active species generation and trichloroethylene degradation using a three-electrode nanosecond pulsed sliding DBD nanosecond plasma. J. Clean. Prod. 2022, 332, 129998. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, Z.; Leus, K.; Shen, Z.; Huang, Y.; Wang, C.; De Geyter, N.; Morent, R. The remarkable oxidation of trichloroethylene in a post-plasma-catalytic system over Ag-Mn-Ce/HZSM-5 catalysts. Fuel 2023, 334, 126746. [Google Scholar] [CrossRef]
- Jiang, N.; Qiu, C.; Guo, L.; Shang, K.; Lu, N.; Li, J.; Wu, Y. Post Plasma-Catalysis of Low Concentration VOC Over Alumina-Supported Silver Catalysts in a Surface/Packed-Bed Hybrid Discharge Reactor. Water Air Soil Pollut. 2017, 228, 113. [Google Scholar] [CrossRef]
- Jiang, N.; Hu, J.; Li, J.; Shang, K.; Lu, N.; Wu, Y. Plasma-catalytic degradation of benzene over Ag–Ce bimetallic oxide catalysts using hybrid surface/packed-bed discharge plasmas. Appl. Catal. B Environ. 2016, 184, 355–363. [Google Scholar] [CrossRef]
- Jiang, L.; Yao, Z.; Wang, P.; Chen, J.; Zhang, Y.; Huang, W.; Cao, X.; Xu, Y. Removal of chlorobenzene over LaMnO3 and OMS-2 catalysts in a dielectric barrier discharge reactor. J. Environ. Chem. Eng. 2021, 9, 105898. [Google Scholar] [CrossRef]
- Kim, H.-H.; Lee, Y.-H.; Ogata, A.; Futamura, S. Plasma-driven catalyst processing packed with photocatalyst for gas-phase benzene decomposition. Catal. Commun. 2003, 4, 347–351. [Google Scholar] [CrossRef]
- Lee, B.-Y.; Park, S.-H.; Lee, S.-C.; Kang, M.; Choung, S.-J. Decomposition of benzene by using a discharge plasma–photocatalyst hybrid system. Catal. Today 2004, 93–95, 769–776. [Google Scholar] [CrossRef]
- Chae, J.O.; Demidiouk, V.; Yeulash, M.; Choi, I.C.; Jung, T.G. Experimental study for indoor air control by plasma-catalyst hybrid system. IEEE Trans. Plasma Sci. 2004, 32, 493–497. [Google Scholar] [CrossRef]
- Kim, H.-H.; Ogata, A.; Futamura, S. Atmospheric plasma-driven catalysis for the low temperature decomposition of dilute aromatic compounds. J. Phys. D Appl. Phys. 2005, 38, 1292–1300. [Google Scholar] [CrossRef]
- Malik, M.A.; Minamitani, Y.; Schoenbach, K.H. Comparison of catalytic activity of aluminum oxide and silica gel for decomposition of volatile organic compounds (VOCs) in a plasmacatalytic Reactor. IEEE Trans. Plasma Sci. 2005, 33, 50–56. [Google Scholar] [CrossRef]
- Kim, H.-H.; Ogata, A.; Futamura, S. Oxygen partial pressure-dependent behavior of various catalysts for the total oxidation of VOCs using cycled system of adsorption and oxygen plasma. Appl. Catal. B Environ. 2008, 79, 356–367. [Google Scholar] [CrossRef]
- Pan, W.; Meng, J.; Gu, T.; Zhang, Q.; Zhang, J.; Wang, X.; Bu, C.; Liu, C.; Xie, H.; Piao, G. Plasma-catalytic steam reforming of benzene as a tar model compound over Ni-HAP and Ni-γAl2O3 catalysts: Insights into the importance of steam and catalyst support. Fuel 2023, 339, 127327. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Z.; Zhu, Y.; Tao, S.; Chen, M.; Zhang, Z.; Jiang, Z.; Shangguan, W. RE-NiOx (RE = Ce, Y, La) composite oxides coupled plasma catalysis for benzene oxidation and by-product ozone removal. J. Rare Earths 2023, in press. [Google Scholar] [CrossRef]
- Jiang, Z.; Fang, D.; Liang, Y.; He, Y.; Einaga, H.; Shangguan, W. Catalytic degradation of benzene over non-thermal plasma coupled Co-Ni binary metal oxide nanosheet catalysts. J. Environ. Sci. 2023, 132, 1–11. [Google Scholar] [CrossRef]
- Dahiru, U.H.; Saleem, F.; Al-Sudani, F.T.; Zhang, K.; Harvey, A.P. Decomposition of benzene vapour using non-thermal plasmas: The effect of moisture content on eliminating solid residue. J. Environ. Chem. Eng. 2022, 10, 107767. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; Zhou, X.; Liang, J.; Liu, Y.; Chang, D.; Yang, D. Degradation of Benzene Using Dielectric Barrier Discharge Plasma Combined with Transition Metal Oxide Catalyst in Air. Catalysts 2022, 12, 203. [Google Scholar] [CrossRef]
- Xu, X.; Wu, J.; Xu, W.; He, M.; Fu, M.; Chen, L.; Zhu, A.; Ye, D. High-efficiency non-thermal plasma-catalysis of cobalt incorporated mesoporous MCM-41 for toluene removal. Catal. Today 2017, 281, 527–533. [Google Scholar] [CrossRef]
- Qin, C.; Huang, X.; Dang, X.; Huang, J.; Teng, J.; Kang, Z. Toluene removal by sequential adsorption-plasma catalytic process: Effects of Ag and Mn impregnation sequence on Ag-Mn/γ-Al2O3. Chemosphere 2016, 162, 125–130. [Google Scholar] [CrossRef]
- Xu, W.; Jiang, X.; Chen, H.; Chen, X.; Chen, L.; Wu, J.; Fu, M.; Ye, D. Adsorption-discharge plasma system for toluene decomposition over Ni-SBA catalyst: In situ observation and humidity influence study. Chem. Eng. J. 2020, 382, 122950. [Google Scholar] [CrossRef]
- Liu, R.; Song, H.; Li, B.; Li, X.; Zhu, T. Simultaneous removal of toluene and styrene by non-thermal plasma-catalysis: Effect of VOCs interaction and system configuration. Chemosphere 2021, 263, 127893. [Google Scholar] [CrossRef]
- Wang, B.; Chi, C.; Xu, M.; Wang, C.; Meng, D. Plasma-catalytic removal of toluene over CeO2-MnOx catalysts in an atmosphere dielectric barrier discharge. Chem. Eng. J. 2017, 322, 679–692. [Google Scholar] [CrossRef]
- Jiang, B.; Xu, K.; Li, J.; Lu, H.; Fei, X.; Yao, X.; Yao, S.; Wu, Z. Effect of supports on plasma catalytic decomposition of toluene using in situ plasma DRIFTS. J. Hazard. Mater. 2021, 405, 124203. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, J.; Wang, Y.; Li, J.; Wang, N.; Harding, J.; Mo, S.; Chen, L.; Chen, P.; Fu, M.; et al. Plasma-Catalytic CO2 Hydrogenation over a Pd/ZnO Catalyst: In Situ Probing of Gas-Phase and Surface Reactions. JACS Au 2022, 2, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.R.; Ashford, B.; Hong, J.; Murphy, A.B.; Chen, J.G. Identifying Surface Reaction Intermediates in Plasma Catalytic Ammonia Synthesis. ACS Catal. 2020, 10, 14763–14774. [Google Scholar] [CrossRef]
- Wu, K.; Sun, Y.; Liu, J.; Xiong, J.; Wu, J.; Zhang, J.; Fu, M.; Chen, L.; Huang, H.; Ye, D. Nonthermal plasma catalysis for toluene decomposition over BaTiO3-based catalysts by Ce doping at A-sites: The role of surface-reactive oxygen species. J. Hazard. Mater. 2021, 405, 124156. [Google Scholar] [CrossRef]
- Karuppiah, J.; Reddy, E.L.; Reddy, P.M.K.; Ramaraju, B.; Karvembu, R.; Subrahmanyam, C. Abatement of mixture of volatile organic compounds (VOCs) in a catalytic non-thermal plasma reactor. J. Hazard. Mater. 2012, 237–238, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, C.; Renken, A.; Kiwi-Minsker, L. Catalytic non-thermal plasma reactor for abatement of toluene. Chem. Eng. J. 2010, 160, 677–682. [Google Scholar] [CrossRef]
- Xu, W.; Chen, B.; Jiang, X.; Xu, F.; Chen, X.; Chen, L.; Wu, J.; Fu, M.; Ye, D. Effect of calcium addition in plasma catalysis for toluene removal by Ni/ZSM-5: Acidity/basicity, catalytic activity and reaction mechanism. J. Hazard. Mater. 2020, 387, 122004. [Google Scholar] [CrossRef]
- Bo, Z.; Yang, S.; Kong, J.; Zhu, J.; Wang, Y.; Yang, H.; Li, X.; Yan, J.; Cen, K.; Tu, X. Solar-Enhanced Plasma-Catalytic Oxidation of Toluene over a Bifunctional Graphene Fin Foam Decorated with Nanofin-like MnO2. ACS Catal. 2020, 10, 4420–4432. [Google Scholar] [CrossRef]
- Yang, S.; Yang, H.; Yang, J.; Qi, H.; Kong, J.; Bo, Z.; Li, X.; Yan, J.; Cen, K.; Tu, X. Three-dimensional hollow urchin α-MnO2 for enhanced catalytic activity towards toluene decomposition in post-plasma catalysis. Chem. Eng. J. 2020, 402, 126154. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, C.; Chen, D.; Chen, J.; Zhang, S.; Ye, J.; Yu, J.; Dionysiou, D.D. A novel array of double dielectric barrier discharge combined with TiCo catalyst to remove high-flow-rate toluene: Performance evaluation and mechanism analysis. Sci. Total Environ. 2019, 692, 940–951. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Jiang, S.; Liu, S.; Cao, J.; Ai, Y. Enhanced catalytic performance and reduced by-products emission on plasma catalytic oxidation of high-concentration toluene using Mn-Fe/rGO catalysts. J. Environ. Chem. Eng. 2022, 10, 108770. [Google Scholar] [CrossRef]
- Zhou, A.; Liu, J.-L.; Zhu, B.; Li, X.-S.; Zhu, A.-M. Plasma catalytic removal of VOCs using cycled storage-discharge (CSD) mode: An assessment methodology based on toluene for reaction kinetics and intermediates. Chem. Eng. J. 2022, 433, 134338. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, N.; Wang, H.; Shang, K.; Lu, N.; Li, J.; Wu, Y. Enhanced catalytic performance of graphene-TiO2 nanocomposites for synergetic degradation of fluoroquinolone antibiotic in pulsed discharge plasma system. Appl. Catal. B Environ. 2019, 248, 552–566. [Google Scholar] [CrossRef]
- Ansari, M.; Hossein Mahvi, A.; Hossein Salmani, M.; Sharifian, M.; Fallahzadeh, H.; Hassan Ehrampoush, M. Dielectric barrier discharge plasma combined with nano catalyst for aqueous amoxicillin removal: Performance modeling, kinetics and optimization study, energy yield, degradation pathway, and toxicity. Sep. Purif. Technol. 2020, 251, 117270. [Google Scholar] [CrossRef]
- Zheng, K.; Sun, Y.; Gong, S.; Jiang, G.; Zheng, X.; Yu, Z. Degradation of sulfamethoxazole in aqueous solution by dielectric barrier discharge plasma combined with Bi2WO6-rMoS2 nanocomposite: Mechanism and degradation pathway. Chemosphere 2019, 222, 872–883. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Feng, J.; Xin, L.; Ma, J. Degradation of triclocarban in water by dielectric barrier discharge plasma combined with TiO2/activated carbon fibers: Effect of operating parameters and byproducts identification. Chem. Eng. J. 2016, 300, 36–46. [Google Scholar] [CrossRef]
- Xin, L.; Sun, Y.; Feng, J.; Wang, J.; He, D. Degradation of triclosan in aqueous solution by dielectric barrier discharge plasma combined with activated carbon fibers. Chemosphere 2016, 144, 855–863. [Google Scholar] [CrossRef]
- Gong, S.; Sun, Y.; Zheng, K.; Jiang, G.; Li, L.; Feng, J. Degradation of levofloxacin in aqueous solution by non-thermal plasma combined with Ag3PO4/activated carbon fibers: Mechanism and degradation pathways. Sep. Purif. Technol. 2020, 250, 117264. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, N.; Wang, H.; Lu, N.; Shang, K.; Li, J.; Wu, Y. Degradation of antibiotic chloramphenicol in water by pulsed discharge plasma combined with TiO2/WO3 composites: Mechanism and degradation pathway. J. Hazard. Mater. 2019, 371, 666–676. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Y.; Zhang, C.; Yu, Z. Decomposition of acetaminophen in water by a gas phase dielectric barrier discharge plasma combined with TiO2-rGO nanocomposite: Mechanism and degradation pathway. J. Hazard. Mater. 2017, 323, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Jankunaite, D.; Tichonovas, M.; Buivydiene, D.; Radziuniene, I.; Racys, V.; Krugly, E. Removal of Diclofenac, Ketoprofen, and Carbamazepine from Simulated Drinking Water by Advanced Oxidation in a Model Reactor. Water Air Soil Pollut. 2017, 228, 353. [Google Scholar] [CrossRef]
- Rong, S.-P.; Sun, Y.-B.; Zhao, Z.-H. Degradation of sulfadiazine antibiotics by water falling film dielectric barrier discharge. Chin. Chem. Lett. 2014, 25, 187–192. [Google Scholar] [CrossRef]
- Giardina, A.; Tampieri, F.; Marotta, E.; Paradisi, C. Air non-thermal plasma treatment of Irgarol 1051 deposited on TiO2. Chemosphere 2018, 210, 653–661. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Yao, S.; Peng, Y.; Xu, Y. Plasma-catalytic degradation of tetracycline hydrochloride over Mn/γ-Al2O3 catalysts in a dielectric barrier discharge reactor. Plasma Sci. Technol. 2019, 21, 65503. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Hu, R.; Wei, Y.; Yun, W.; Nian, P.; Feng, J.; Zhang, A. Synergistic degradation of acid orange 7 dye by using non-thermal plasma and g-C3N4/TiO2: Performance, degradation pathways and catalytic mechanism. Chemosphere 2020, 249, 126093. [Google Scholar] [CrossRef]
- Guo, H.; Wang, H.; Wu, Q.; Zhou, G.; Yi, C. Kinetic analysis of acid orange 7 degradation by pulsed discharge plasma combined with activated carbon and the synergistic mechanism exploration. Chemosphere 2016, 159, 221–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, X.; Han, Y.; Yuan, H.; Deng, S.; Xiao, H.; Shen, F.; Wu, X. Application of titanium dioxide-loaded activated carbon fiber in a pulsed discharge reactor for degradation of methyl orange. Chem. Eng. J. 2010, 162, 1045–1049. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Pepe, G.; Campiglia, P.; Palma, V. Degradation of Acid Orange 7 Azo Dye in Aqueous Solution by a Catalytic-Assisted, Non-Thermal Plasma Process. Catalysts 2020, 10, 888. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Shen, Z.; Lu, S.; Su, K.; Yuan, S.; Hu, Z.; Zhang, A.; Feng, J. Non-thermal plasma and BiPO4 induced degradation of aqueous crystal violet. Sep. Purif. Technol. 2017, 179, 135–144. [Google Scholar] [CrossRef]
- Shen, Y.; Han, S.; Xu, Q.; Wang, Y.; Xu, Z.; Zhao, B.; Zhang, R. Optimizing degradation of Reactive Yellow 176 by dielectric barrier discharge plasma combined with TiO2 nano-particles prepared using response surface methodology. J. Taiwan Inst. Chem. Eng. 2016, 60, 302–312. [Google Scholar] [CrossRef]
- Tarkwa, J.-B.; Acayanka, E.; Jiang, B.; Oturan, N.; Kamgang, G.Y.; Laminsi, S.; Oturan, M.A. Highly efficient degradation of azo dye Orange G using laterite soil as catalyst under irradiation of non-thermal plasma. Appl. Catal. B Environ. 2019, 246, 211–220. [Google Scholar] [CrossRef]
- Vaiano, V.; Miranda, L.N.; Pepe, G.; Basilicata, M.G.; Campiglia, P.; Iervolino, G. Catalytic non-thermal plasma process for the degradation of organic pollutants in aqueous solution. J. Environ. Chem. Eng. 2022, 10, 107841. [Google Scholar] [CrossRef]
- Hua, W.; Kang, Y. Synergistic degradation of Orange G in water via water surface plasma assisted with β-Bi2O3/CaFe2O4. Korean J. Chem. Eng. 2023, 40, 1–11. [Google Scholar] [CrossRef]
- Mohamed, W.A.A.; Fahmy, A.; Helal, A.; Ahmed, E.A.E.; Elsayed, B.A.; Kamoun, E.A.; Gad, E.A.M. Degradation of local Brilliant Blue R dye in presence of polyvinylidene fluoride/MWCNTs/TiO2 as photocatalysts and plasma discharge. J. Environ. Chem. Eng. 2022, 10, 106854. [Google Scholar] [CrossRef]
- Alarcón-Hernández, F.B.; Montiel-Palacios, E.; Fuentes-Albarrán, M.C.; de León, A.T.; Gadea-Pacheco, J.L.; Tlatelpa-Becerro, A. Behavior of the AB52 dye degradation in liquid medium by different electrical power non-thermal plasma at atmospheric pressure. Rev. Mex. Ing. Química 2022, 21, IA2793. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, X.; Li, J.; Zhang, J. Study on degradation of alizarine reds in simulated dye wastewater by gas-liquid two-phase discharge plasma. Chem. Eng. Process—Process Intensif. 2022, 181, 109114. [Google Scholar] [CrossRef]
- Assadi, I.; Guesmi, A.; Baaloudj, O.; Zeghioud, H.; Elfalleh, W.; Benhammadi, N.; Khezami, L.; Assadi, A.A. Review on inactivation of airborne viruses using non-thermal plasma technologies: From MS2 to coronavirus. Environ. Sci. Pollut. Res. 2022, 29, 4880–4892. [Google Scholar] [CrossRef] [PubMed]
- Neyts, E.C.; Bogaerts, A. Understanding plasma catalysis through modelling and simulation—A review. J. Phys. D Appl. Phys. 2014, 47, 224010. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Dong, F. Activation and characterization of environmental catalysts in plasma-catalysis: Status and challenges. J. Hazard. Mater. 2022, 427, 128150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).