Abstract
Pure water, i.e., a sign of life, continuously circulates and is contaminated by different discharges. This emerging environmental problem has been attracting the attention of scientists searching for methods for the treatment of wastewater contaminated by multiple recalcitrant compounds. Various physical and chemical methods are used to degrade contaminants from water bodies. Traditional methods have certain limitations and complexities for bioenergy production, which motivates the search for new ways of sustainable bioenergy production and wastewater treatment. Biological strategies have opened new avenues to the treatment of wastewater using oxidoreductase enzymes for the degradation of pollutants. Fungal-based fuel cells (FFCs), with their catalysts, have gained considerable attention among scientists worldwide. They are a new, ecofriendly, and alternative approach to nonchemical methods due to easy handling. FFCs are efficiently used in wastewater treatment and the production of electricity for power generation. This article also highlights the construction of fungal catalytic cells and the enzymatic performance of different fungal species in energy production and the treatment of wastewater.
1. Introduction
The rapid industrial and global population growth has polluted water and depleted the resources of fossil fuels to fulfill the excessive demand for energy production. The quality of water is deteriorating due to the continuous mixing of undesirable chemicals [1]. The need for water quality improvement and preservation is continuously growing day by day due to agricultural, civilization, and industrial activities, leading to environmental and global changes. Wastewater is defined as a combination of liquid, water with wastes from residential areas, commercial sites, institutions, and industrial establishments together with ground, surface, and storm water [2].
Nonpoint sources contaminate valuable water resources. Organic pollutants are hazardous and toxic; hence, chemical processes are most suitable to remediate and eliminate the inorganic matter, dyes, and recalcitrant matter. Various techniques (biological, physical, and chemical) are used to treat organic-compounds polluted the wastewater. Traditional methods have certain limitations for bioenergy production, e.g., large spaces, high capital cost, and complexities linked with the production process. The demands for sustainable bioenergy production have been increasing in the world as an alternative to nonchemical methods for power generation. Biological degradation involves the use of microorganisms (fungi, algae, bacteria, and enzymes), which utilize the maximum land area, exhibit very high sensitivity toward toxic agents, and require a long consumption time [3].
The exploration of novel and efficient approaches have attracted the attention of environmental scientists to cleanup and remediation of the contaminated water bodies. The fungal potential to generate bioelectricity from biodegradable wastewater reduces the cost of conversion [4]. Biotic sources exploit different species of fungi for bioenergy generation. However, very little data are available on the use of “fungal-mediated electrochemical system” for energy production. Minimal resources, higher prices of fossil fuels, and increasing global warming issues have motivated the scientists to design alternative “renewable” energy sources, e.g., fungal cell factories.
The fungal fuel cell is a device that uses fungi as catalysts to generate electricity by oxidizing the inorganic compounds of biomass [5]. A few researchers believe that this technology is not only used for the production of electricity. It also depends on the ability of the electrode associated with the fungi to degrade the toxics and waste materials [6]. Biomass/organic material is a sustainable alternative approach to address this issue. Fungal fuel cells (FFCs) provide electricity directly through the “biodegradation” of raw materials by fungal cells [7].
It is proposed that fungal species are used for energy generation, taking advantage of their potential as “novel cell factories”. Saccharomyces or Pichia fungi are used in these cells [8]. Fungal cells have nine times higher potential to generate energy accumulated in sewage sludge than conventional methods [9]. Fungal species have a strong potential to generate power using the presence of complex enzymatic systems. These species can rapidly grow on waste materials and degrade these materials within a shorter time for the production of “bioenergy”. The use of fungi in “bioremediation” is a promising technique [10].
This approach is also called an “Oxidative Biocatalyst” approach. The efficiency of this strategy can be maximized by using different fungal growth and environmental parameters with redox mediator systems [11]. The fungal approach is the best method for energy production and wastewater treatment in a cost-effective manner. Integrated physical, chemical, and biological wastewater treatment are discussed in detail with current challenges in terms of achieving good treatment efficiencies to meet the discharge standards during wastewater treatment and bioelectricity generation.
Objectives
- To construct bioelectrochemical devices, where the fungus (catalyst) is used for the oxidation of inorganic matter for electricity generation.
- To generate power/energy from wastewater (substrate) using fungal electrochemical technology (FET) in an ecofriendly manner.
- Compared to conventional methods, biodegradation, using cost-effective and economical technologies, is greatly preferred with improved outcomes. The goal of this review provides a design for a much more cost-effective system with a principle of wastes removal using fungal fuel cells. Studies on wastewater treatment in FFCs with electricity generation are also presented. Thus, biodegradation using FFCs is considered to be a highly economical, ecofriendly, and more prominent way to solve these problems.
1.1. Oleaginous Fungi
Oleaginous microorganisms have potential for biodiesel formulation and production. These are used as an alternative renewable energy sources. Oleaginous fungi have numerous advantages, e.g., lower land requirements, short cultivation time, and maximum production of fatty acids (oils) [12]. A few oleaginous species metabolize xylose and assist in lipid production from “lignocellulosic hydrolysates” [13]. These fungal species become more oleaginous, when different organic substrates (glucose and sucrose) are added to their growth medium. Each species has particular abilities to utilize organic substrates and enhance the lipid yield. It is noticed that in a fungal consortium, less productive species always follow a more productive species during co-metabolism. This way of combination is yielding more biomass than single cultures.
The genera Mucor and Aspergillus have been recognized to store up to 80% of oils (in cells) in specific conditions [14]. Strains that have high lipid contents and metabolize TAG (triacylglycerides) usually preferred to formulate and generate biofuels efficiently. Zygomycetes are a class of excellent oleaginous fungal species, providing palmitic and oleic acids that are used for biodiesel formation. Additionally, anaerobic fungi are an arsenal of extracellular multienzyme complexes. These fungi are involved in the breakdown of various biomasses for biogas generation. Zygomycetes, such as Mortierella isabelline has reported to have a 60–70% lipid content [15].
Oleaginous yeast (Rhodotorula mucilaginosa SML) has been using for the treatment of food industry effluents. The overall yeast lipid content for the effluent treatment was 67.95 w/w% of dry cell biomass. The extracted yeast oil was used for transesterification and showed a 98% conversion of oil to methanol. The fatty acid composition was compatible with petroleum diesel, making it applicable for alternative biofuel production. Thus, this strategy proved efficient in the removal of contaminants of industrial wastes suggested as a new sustainable source for biodiesel production [16].
The biofilm of Wickerhamomyces anomalus (yeast) on the anodic electrode of a single-chamber fuel cell fed with zinc and copper electrodes and pineapple waste (substrate) is used for fuel production. Current (4.95667 ± 0.54 mA) and voltage peaks (0.99 ± 0.03 V) were generated for 16 and 20 days, respectively. The maximum power density of 513.99 ± 6.54 mW/m2 at a current density of 6.123 A/m2 was generated [17].
1.2. Hydrolytic and Lignolytic Fungi
Hydrolytic and ligninolytic fungi are suitable candidates for the production of biofuels or bioethanol. A few basidiomycetes have been reporting to secrete extracellular enzymes that degrade the waste materials [18]. Fungal peroxidases (manganese-dependent peroxidase and lignin peroxidase) degrade the lignin, hemicellulose, and polyaromatic phenols [19].
Fungal cells are known for the generation of bioelectricity, good-quality biofuel production, and wastewater treatment. The best-known biofuel-producing fungal species are Rhodosporidium toruloides, Cryptococcus sp., Yarrowia lipolytica, Penicillium sp., Aspergillus sp., and Trichoderma reesei. Species that have the potential to produce biodiesel or electricity generation transfer the e− via cytochrome C. These include Candida sp., Colletotrichum sp., Saccharomyces cerevisiae, Penicillium sp., Alternaria sp., Rhizopus sp., and Aspergillus sp. Cells constructed from these species are called “Fungal-based FCs” [20].
Energy-generating fungal biocatalysts increase the electron transmission rate through extensive networking of fungal hyphae and produce stable electricity, which contributes to “external electrochemical operations”. Due to this unique property, fungi, and yeasts are preferred over bacterial cells for wastewater treatment and electricity generation [21].
1.3. Effects of Environmental Factors on Fungal Growth and Metabolism
- pH
A few fungal species grow in a broad pH range, while some species grow in a narrower pH range. The optimum growth of fungal species appears to correspond to a specific pH value [22]. The fungal ability to grow at a pH >7 is required during industrial production. The substrate with a pH below 7.00 inhibits the growth of contaminants (bacteria) without affecting the yield. A slight increase in pH of FFCs, inhibits fungal growth and metabolism. Fungal catalyst formation (oxidoreductase) and catalytic action are highly stable at an acidic pH (3–6). A low pH induces mobility and unfolding of the enzyme proteins.
- Temperature
Temperature plays an important role in fungal growth, metabolism, and electricity generation using fungal fuel cells. The temperature of system facilitates the cells metabolism and their enzymatic reactions. In wood-rotting fungi, oxidoreductase is produced in an optimum temperature range (25–30 °C), which depends on mesophilic and thermophilic fungal species [23]. The enzyme system of mesophilic basidiomycetes is thermostable at elevated temperatures. Optimum temperature is also favorable for the efficient maintenance of fungal systems in fuel cells during their metabolic mechanisms. A slight decrease or increase in temperature leads to denaturation and inactivation of the cell components, which consequently stops the work of fuel cells with no power generation.
- Ionic strength
Higher ionic conductivity also influences the work of fungal fuel cell. Ionic conductivity is directly proportional to power generation due to minimum internal resistance. High ionic conductivity increases the power output of FCs. Protons and electrons can easily move from one compartment to another for the completion of a circuit.
- Salinity
About 90% fungal species can tolerate at 3 to 6% salt stress. Halotolerant fungal species are better adapted to the salty environment [24]. Marine fungi with a dark cell wall can tolerate higher salinity than moniliaceous fungi [25]. The habitats of marine fungal species have a strong influence on their adaptation to salt and metabolic functioning.
Hyperosmotic stress in fungi is linked with the inhibition of cell wall extension and cellular expansion, resulting a reduction in their growth [26]. Excess in everything is bad. Maximum ions can alter protein, membrane integrity, and nucleic metabolism, which may change the enzymatic activity and catalytic performance during fungal growth and functioning of fuel cells [27]. Organic osmotica (compounds) are called compatible solutes, as these solutes can store high concentrations of salts without interfering with cell metabolism. Polyols (mannitol, arabitol, and glycerol) and non-reducing saccharides (trehalose) are soluble carbohydrates found in basidiomycetes and ascomycetes. These solutes help the fungi to grow efficiently in a salt-stress environment. A maximum salt range destroys the fungal product yield as well.
1.4. Enzymatic Treatment by Biocatalytic Fungal Species
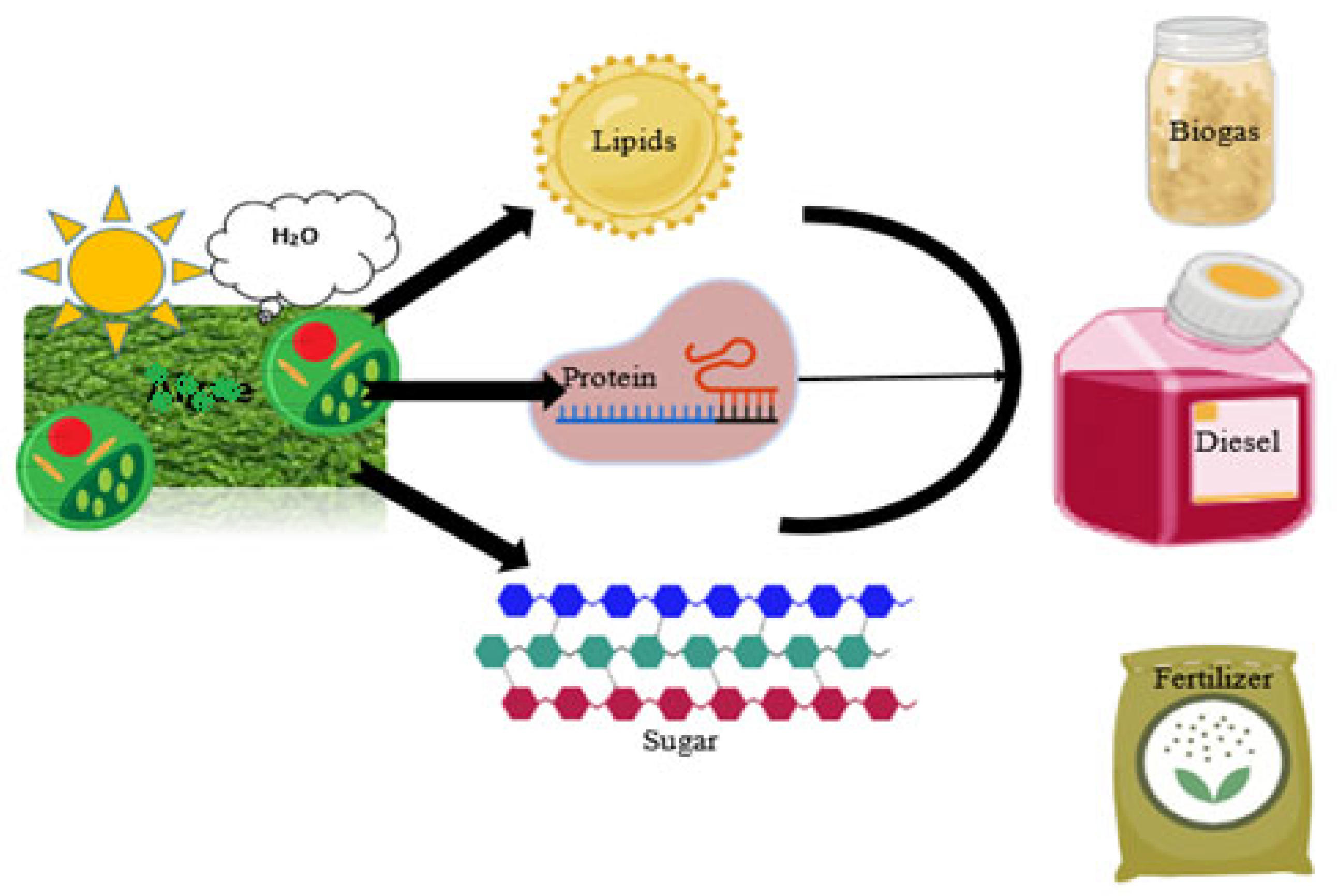
Catalysts are needed to accelerate the maximum biodiesel production. Biofuels are categorized into bioethanol, biohydrogen, and biodiesel (Figure 1). During the enzymatic treatment of wastewater (substrate), low-chain carbon compounds are produced, which utilized during microbial oxidation. The basic oxidoreductases, such as laccases, lignin peroxidases, and manganese peroxidases, are extracellular enzymes. In contrast, glucose oxidase and cellobiose dehydrogenase are auxiliary enzymes isolated from white-rot fungi.
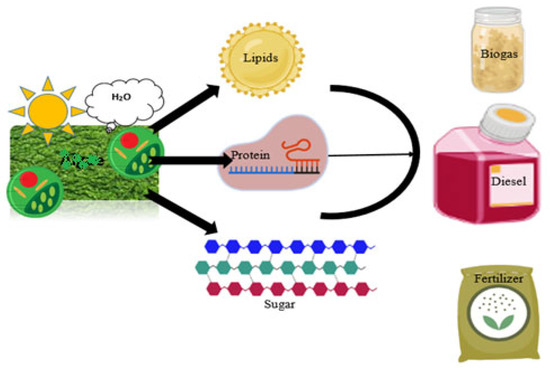
Figure 1.
Fungal species utilize sunlight and water for the metabolic production of lipids, protein, and sugar used for the manufacturing of biodiesel, biogas, and biofertilizers.
Biocatalysts are exoelectrogens that oxidize organic material and deliver electrons from anode to cathode to generate the electricity. Exoelectrogens are now investigated for the development of FFCs, which potentially convert the diverse organic substances (activated sludge of waste water) into electricity, ethanol, and H2 [28]. Sporotrichum pruinosum (white-rot fungus) efficiently degrades pollutants, organic biomass, and chemical substances with the use of an extracellular enzyme [29].
Biocatalyst is deposited onto the carbon anode as floating biomass in yeast-based FFCs [30]. Yeast-based FFCs have the following advantages: (i) degradation of very complex substrates (starch and cellulose-based substrates) into simple organic molecules; (ii) survival in an anaerobic environment [31]; and (iii) simple and easy production, rapid development, and sensitivity of the strains. Except for yeast, other fungal species are also exploited as biocatalysts for both wastewater treatment and electrochemical approaches.
Scientists are motivated toward the development of such mediator systems as fungal-based FCs [21]. Pure Saccharomyces cerevisiae (yeast) is a model organisms used as biocatalysts in FFCs. Christwardana et al. [30] indicated the significance of yeast cells in MFCs due to their unique features and sustainability. S. cerevisiae has been extensively studied and characterized as a biocatalyst in biological fuel cells [32,33,34]. This species is nonpathogenic to non-target organisms (humans), has a high growth rate, easy to culture in anaerobic environments, and grows very well at room temperature [35]; hence, it can be used for the effective treatment of wastewater [36,37]. In addition to the low cost and rapid multiplication, the species remains active and survives even in a dried environment for a longer period [38]. Carbon-neutral fuel, referred to as bioethanol, is produced from plant waste and bacterial/algal biomass [39]. It is also produced from yeast and fungi in anaerobic conditions [40], especially S. cerevisiae, which is considered to have great potential in the production of bioethanol.
Candida melibiosica, Kluyveromyces marxianus, Blastobotrys adeninivorans, Pichia anomala, P. polymorpha, and Saccharomyces cerevisiae yeasts are used as biocatalysts in FFCs with/without an external mediator. Kluyveromyces marxianus is a promising yeast species producing maximum power at higher temperatures, when grown in natural (organic) substrates. Other fungal species, e.g., Saccharomyces cerevisiae [33], Candida melibiosica [36], Blastobotrys adeninivorans [37], Hansenula polymorpha [41], and Pichia anomala [42] all are a potential source for catalysts in FFCs.
Exogenous mediators, such as methylene blue (MB) and neutral red (NR), are used to increase the transport of electrons between anodes and microbes. The yeast cell surface-displayed dehydrogenases include cellobiose dehydrogenase (CDH) and pyranose dehydrogenase (PDH) [43]. Both CDH- and PDH-based biocatalysts are used in the anodic compartment of FFCs.
1.5. Structure of Fungal-Mediated Fuel Cells
Protons and electrons are generated through the oxidation of organic matter in the aqueous solution of anode compartment, when fungi used as catalyst. The external circuit is used to transmit the electrons toward the cathode, while the proton exchange membrane (PEM) facilitates proton diffusion [44,45,46]. At the cathode, e− and protons are used for the reduction reaction and eventually change oxygen to water [47]. Potter [48] was the first person, who liberated electrical energy from yeast cells in 1911. Fungi can transfer electrons to the anode electrode in three possible ways: (1) direct contact; (2) pili/conductive wires; and (3) redox mediators/electron shuttle [49].
The advantages of FFCs include sustainable nonchemical character, minimum sludge generation, optimum temperature, wide range of substrates, low power consumption, and good performance [50]. This is a promising alternative technique used to explore the fungal potential in the conversion of organic substrates into electricity. The performance of FFCs depends on multiple factors, e.g., configuration of the cell, choice of the substrate, anodic material, biocatalyst, electro-catalyst (at the cathode), and environmental conditions. Prasad et al. [42] observed that fungi are more active than bacteria in MFCs.
Fungal fuel cells (FFCs) are operated in a closed-system mode on the principle of oxidation–reduction reaction through a series of electrochemical and microbial pathways. The anaerobic environment is maintained in the anodic compartment [51]. The e− and protons in the anode chamber are produced through oxidation of the substrate by fungi, and oxygen reduced by a terminal electron acceptor in the cathodic chamber.
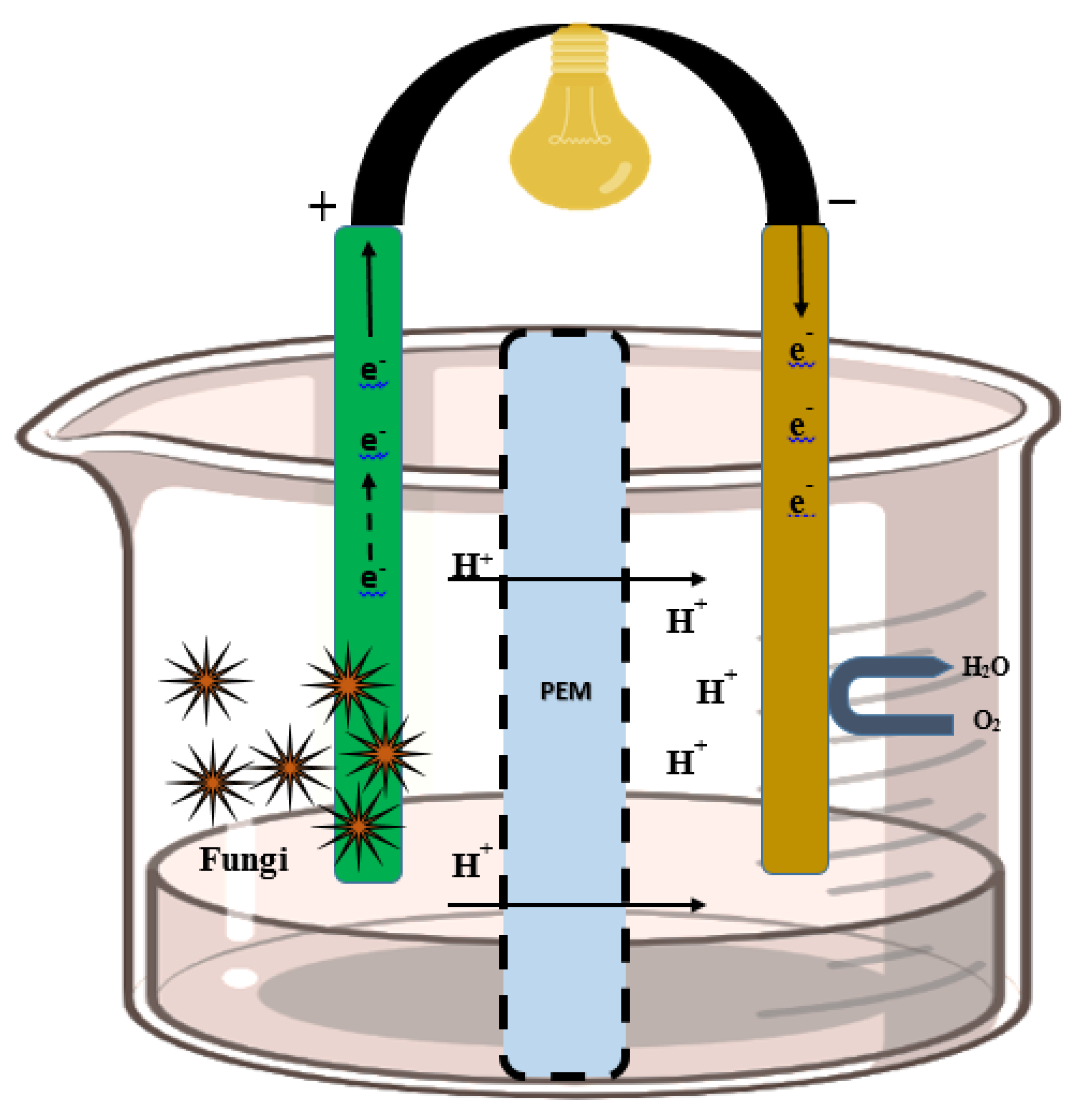
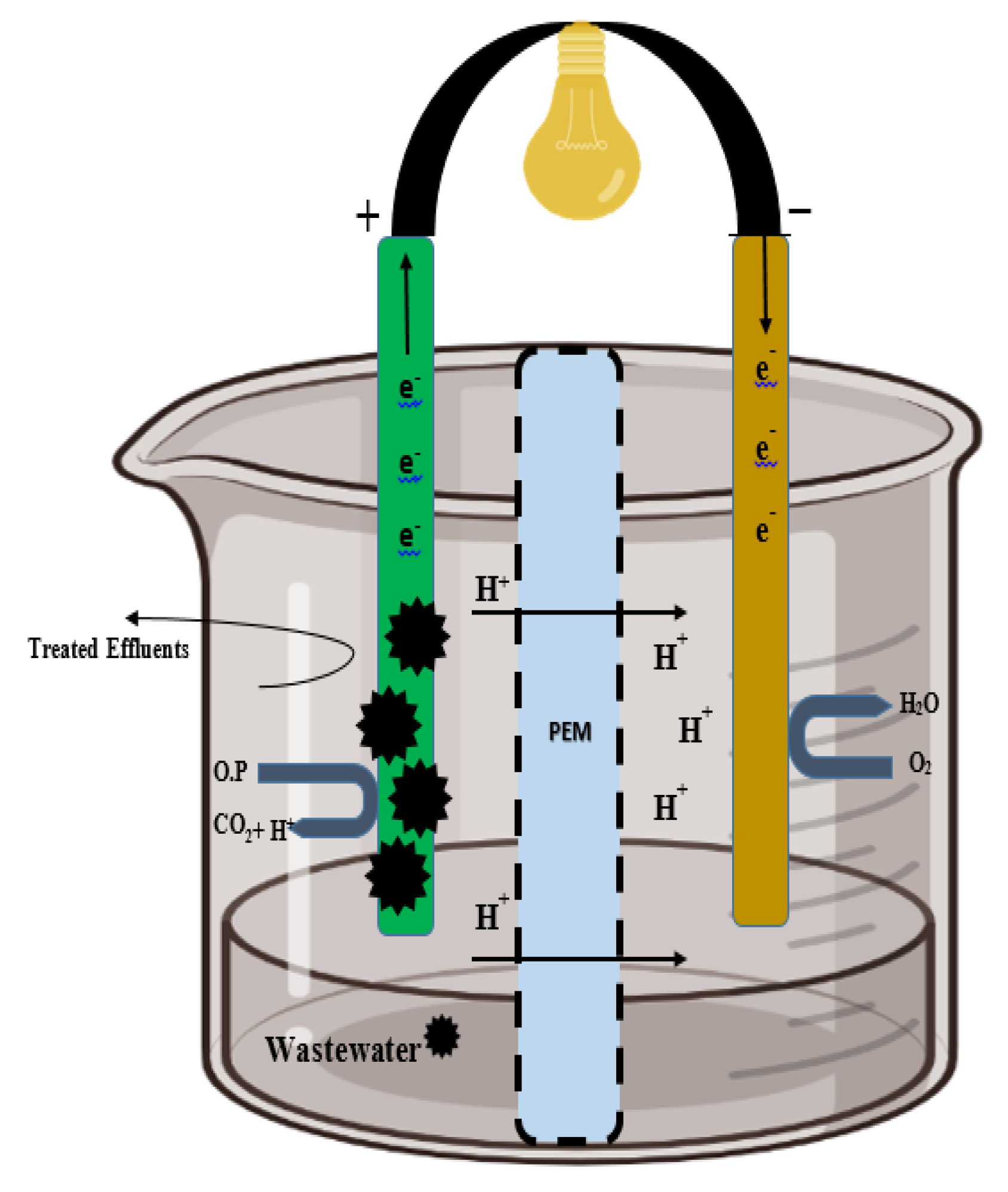
Fungi used at the anode to transport e− via redox-active fungal protein and synthetic mediators. Anodic fungal cells oxidize the substrates and produce electrons and protons. The e− is absorbed by the electrode, and protons flow toward the cathode via a PEM. The protons are transferred through the PEM to the cathode. Subsequently, the electron and proton combination produce a molecule of water (at the cathode) for the completion of the bioelectrochemical reaction. At the cathode, the fungal enzyme catalyzes the reaction and the final electron receiver is O2 and H2O produced during this reaction. Additionally, airtight compartments, sufficient space within the chamber, an outlet, and inlets are certain prerequisites in the arrangement of the PEM and electrodes into a system (Figure 2).
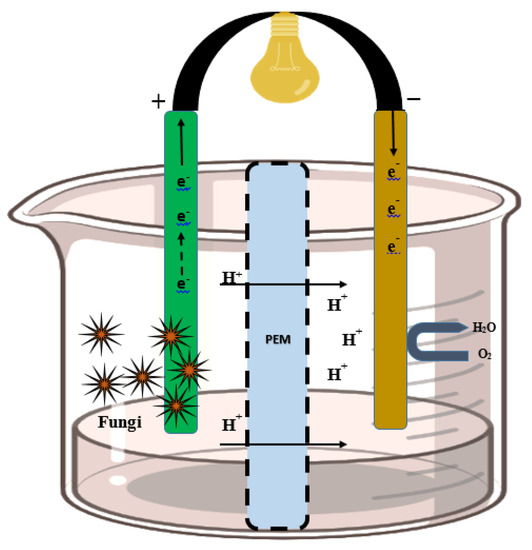
Figure 2.
Structure of the fungal fuel cell. The left side of the cell is the fungal catalyst electrode (anodic chamber), and protons are transferred from the proton exchange membrane to the cathodic chamber (where catalytic oxidation takes place).
A membrane separator called a proton exchange membrane (PEM), divides the cell into two distinct cathodic and anodic chambers. An extracellular microorganism with the ability to transfer electrons is called an exoelectrogen (Biocatalyst) [52]. Membrane separators (PEM, salt bridge, anion and cation exchange membrane, microfiltration membrane, glass fiber) have such features as low permeability and high conductivity for optimum FFC performance [53].
The membrane (Nafion), cathode (Platinum), and anode (carbon cloth and carbon paper) materials are expensive and fragile. Fungal FCs with a low-cost electrode, high power output, and membrane materials with good scalability should develop to treat different effluents (de-sizing, bleaching, dyeing, and printing effluents).
Potent microbes improve the electron transfer and facilitate the degradation of biological substrates, e.g., Shewanella oneidensis or the hyphal networks of T. versicolor facilitate electron transfer onto the anode efficiently. Nearly 30 days are required for the formation of homogeneous bacterial and fungal biofilms on the electrode [54].
1.6. Electron Transfer (ET) Mechanism
There are two types of e− transfer mechanisms. Fungal consortia demonstrated a better ET mechanism than single species.
- Direct ET: two types via outer cytochrome and nanowire.
- Indirect ET: (mediated/mediator electron transfer) reactive diffusible redox mediators (RMs) enhance the reaction rate and increase the range of degraded substrates.
1.7. Types of Electrodes
The electrode material influences the performance of FFC, which has a direct impact on the kinetics of electrodes [55].
Anode: A carbon-based anode is cost-effective and noncorrosive (modifier carbon paper, carbon felt, carbon cloth, carbon nanotubes, graphene, stainless steel, titanium, and gold) [56]. These materials improve the characteristics of anodic surface material and provide an appropriate platform for fungal biofilm formation with an active catalyst. The anode quality enhances the high surface area, chemical/electrical stability, and biocompatibility [57]. The anodic electrode increases the efficiency of FFCs. This serves as a driving feature for power generation. Thus, the anodic material seems to be a suitable strategy to enhance performance.
Reaction in the anodic chamber:
C6H12O6 + 6H2O → 6CO2 + 24H+ + 24e−
Iron and iron oxide nanoparticles, graphite, carbon cloth, and carbon felt are effective anode catalysts improving the efficiency of a fungal fuel cell (FFC) for industrial wastewater treatment. Wastewater is used nowadays as an energy source. This is a promising approach to meet the increasing energy needs in place of fossil fuels [58]. Biocatalysts provide clean, sustainable, and renewable energy sources by utilizing exoelectrogenic organisms [59].
There are several advantages of FFCs; however, their utilization is still limited due to the high cost of their components and their low power output [60]. This limitation can overcome by using an appropriate anode surface morphology (large surface area, superhydrophilicity, high electrical conductivity, excellent chemical stability, high porosity, biocompatibility, and chemistry) with improved electron transfer process [6,61]. The hydrophobic nature of anode, negatively affects the microbial adhesion and enhances the interfacial resistance for e− transfer due to insufficient adhesion on anode surface. This minimize the power and current density [62], which can be overcome with the use of a carbonaceous anode and modification methods (chemical function group treatment, physical treatment, acid heat treatment, and transition metal coating techniques) [63].
Cathode and Cathodic compartment: Types of substrate act as a cathode in FFCs (biodegradable waste such as brewery and sewage wastewater, rich in organics such as glucose, sucrose, lignocellulose, acetate, biomass materials, etc). The reduction of oxygen takes place in this compartment. This is a key interaction in energy conversion and biological respiration [64]. Electrons from the anode are received by cathode through an external circuit and protons are transported via the PEM. This is essential in the reduction reaction between electrons and protons resulting in H2O formation. The cathode affects the total cell voltage output and has a high redox potential.
Reaction in the cathodic chamber
6O2 + 24H+ + 24e− → 12H2O
The biocathode is an alternative low-cost, sustainable, stable, and nonchemical option used currently in FFCs. Due to certain biological components in the cathode, the term ’biocathode’ is used. The fungus is embedded in an oxygenated cathodic chamber and establishes a mutual configuration of a dual compartment-based yeast fuel cell.
1.8. Reactor Configuration
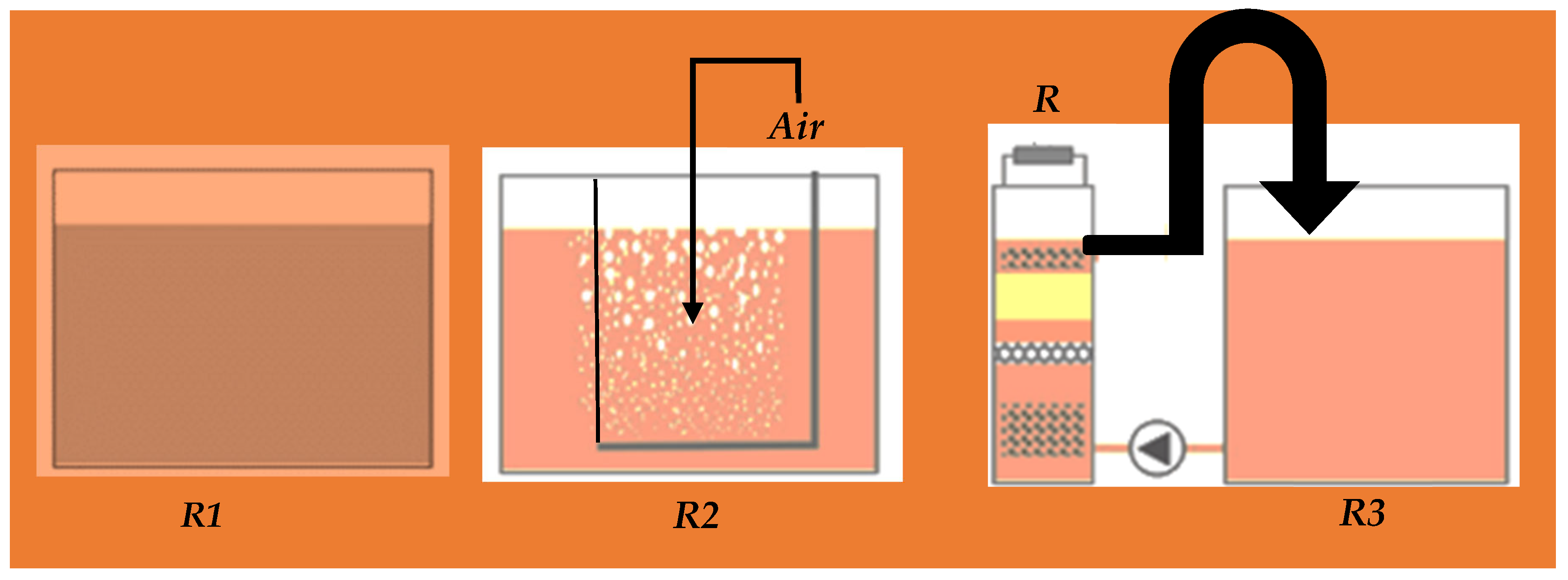
The reactor configuration influences the performance of biological fuel cells. There are two construction designs: (1) single [65] and (2) dual-chambers [66] to evaluate the in vitro performance. According to the mode of aeration, FFCs are classified into different configurations (Figure 3):
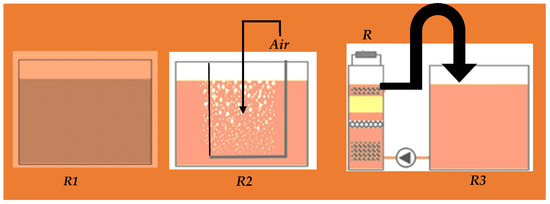
Figure 3.
Reactors for the measurement of wastewater parameters; without-air reactor (R1)—the reactor 15 L and the air is interface in wastewater sewage; with-air reactor (R2)—15 L with constantly aerated wastewater; and membrane less FFC (R3)—reduces COD to 90%.
(1) Aqueous cathode: in this cell, water is bubbled with air for the supply of dissolved O2 to the electrode [67]; (2) air cathode: to minimize the cost and maximize the energy output, the air cathode is designed. Carbon electrodes generate energy in the absence of a PEM. The power density (494 mW/m2) is much better in this type of cell than in the aqueous cathode [68]; (3) downflow: this membrane less fuel cell is constructed with downflow feeding to generate electricity from wastewater. Water is fed directly onto the cathode, which is horizontally installed in the upper part of the FFC. Oxygen is utilized readily from the air and concentration of dissolved oxygen in the wastewater has little effect on the power generation. The maximum power density of 37.4 mW/m2 is generated by this type of cell and mostly used in brewery wastewater treatment [69]; (4) upflow: upflow reactors have advantages in retaining the maximum cell density and mass transfer efficiency. In this type of cell, the recirculation rate can improve the upflow rate. At a recirculation rate of 4.8 RV/h, a power density of 356 ± 24 mW/m2 is produced from this cell [70]; (5) miniature: a low-cost mini tubular fuel cell is developed for the treatment of groundwater contaminated with benzene and for the monitoring of wells. An increase in the length and density and a decrease in size of char particles at the anode effectively reduce the internal resistance. This type of cell removes 95% of benzene and generates a power density of 38 mW/m2 [71]; (6) stacked: this easy-to-operate FFC in septic tanks comprises a common base and multiple pluggable units, which are connected in series or parallel for electricity generation during waste treatment. Three parallel-connected units produce a power density of 142 ± 6.71 mW/m2 [72]; (7) large scale: in this cell, multiple operational conditions can be tested (different flow rates, application of external resistors, and poised anodic potentials). This results in the highest COD removal efficiency (94.6 ± 1.0%) at an applied resistance of 10 Ω across each circuit. Results of eight stages of operation (325 days total) indicate that this fuel cell can sustain treatment rates over a long-term period and are robust enough to sustain performance even after system perturbations [73]; (8) tubular: two ceramic stacks, mullite (m-stack) and terracotta (t-stack) are developed to produce energy. Each stack contains 12 identical fuel cells, which are arranged in cascades and tested under different electrical configurations. The m-stack and the t-stack are found to produce a maximum power of 800 μW and 520 μW, respectively [74]; and (9) salt bridge: a salt bridge is used instead of membrane system. The low power output (2.2 mW/m2) is directly attributed to the higher internal resistance of the salt bridge (19920 ± 50 Ω) compared to the membrane system (1286 ± 1 Ω). Oxygen diffusion from the cathode to the anode chamber is a factor in power generation [75].
2. Methods for Degradation
2.1. Physical Methods
Physical methods for wastewater treatment remove substances with the use of naturally occurring forces like gravity, electrical attraction, and van der Waal forces. In the mechanism of physical treatment, no change is found in chemical structure of the target substances, while in some cases, the physical state will be changed, as in vaporization, and isolated or scattered substances often caused to agglomerate.
The following methods are easy to use and cost-effective/inexpensive, but have numerous disadvantages.
- Adsorption: The method is easy to use and cost-effective, and ensures the regeneration of adsorbents and disposal of generated sludge. Activated lignin and coal are applied as surfaces for adsorption used for degradation [76].
- Coagulation, flocculation, and sedimentation: Coagulation, flocculation, and sedimentation techniques are efficient approaches to remove pollutants; however, both tend to be selective toward specific types of contaminants [77].
- Reverse osmosis and filtration: These are effective but expensive methods for wastewater treatment. They generate secondary waste during their performances (drawback). Filtration is an integral component of drinking water and wastewater treatment applications, which include ultrafiltration, microfiltration, nanofiltration, and reverse osmosis. These techniques remove the color from wastewater. Each membrane process is best suited for a particular water treatment function [78].
2.2. Chemical Methods
The conversion or removal of contaminants is achieved by the addition of chemicals or chemical reactions. Chemical treatments include precipitation, adsorption, and disinfection. These processes are activated by adding aluminum, calcium, and ferric ions, etc.
The following techniques are promising and effective in the degradation of organic compounds [79].
- Advanced oxidation processes (AOPs): These degrade various organic compounds. This approach generates reactive OH− radicals for subsequent reactions with organic pollutants resulting in the degradation of pollutants into smaller intermediates. This is a costly process and demands a continuous input of expensive, reactive, and corrosive chemicals with large amounts of energy.
- Electrochemical destruction: Direct electroreduction has lost its popularity as a means of destruction of dyes in an aqueous solution because it offers very poor decontamination of wastewater compared to other electrochemical treatments.
- NaOCl: Wastewater is treated with sodium hypochlorite, allowed to stand for 1 d in the dark, and then neutralized with sodium thiosulfate [80]. The neutralized sample is used for the determination of hypochlorite treatment effects during the wastewater cleanliness.
2.3. Biological Methods
A modern society without the utilization of chemicals in pulp, leather, pharmaceutical, and paper industries is not possible. However, the consumption of chemicals contaminates the environment and causes harmful effects [81]. Chemical and physical methods include electrochemical methods applicable for wastewater decolorization [82]. These methods are quite expensive, have low removal efficiencies, produce toxic intermediates, and exhibit high specificity for dyes [83]. There are environmental friendlier and potentially less expensive methods for the removal of Ops (organic pollutants) from contaminated water. Numerous microorganisms, e.g., bacteria, yeasts, and fungi, have the potential to decolorize different types of organic compounds [84]. The modification of living cells of yeast by polypyrrole (PPy) was evaluated. A microbial fuel cell using yeast modified by a solution containing 0.05 M pyrrole generated maximal power of 47.12 mW/m2, which is 8.32 mW/m2 higher than the system, which not based on yeast [85]. The yeast-based FC technology showed great potential to harness energy (bioelectricity and biohydrogen) from xylose. Herein, the yeast strain (Cystobasidium slooffiae JSUX1) facilitated the reduction and assembly of graphene oxide (GO) nanosheets reduced to 3D rGO hydrogels on the carbon felt (CF) anode surface. This fuel cell enhanced, by two times, the bioelectricity and biohydrogen production from xylose [86].
The performance of a fuel cell is estimated in terms of pollutant removal and electricity generation. Pollutant removal can measure in terms of organic removal, also called a change in equivalent COD (chemical oxygen demand) between the effluent and the influent. Wastewater pollution from numerous sources is removed by using FFCs, in which organisms decompose organic compounds and convert this chemical energy into electrical energy [2]. The most important advantages of this method are the low concentrations of reagents needed for mild conditions and the degradation of a wide range of substrates. A disadvantage of this method is the high cost of enzymes, which ameliorated by the use of recombinant DNA technology [87].
The biodegradable organic matter ranges from pure compounds (acetate, cysteine, glucose, and ethanol) to mixtures comprising organic compounds (liquid municipal waste, leachate landfills, animal waste, liquid waste of industrial and agricultural origin) [88]. Biological treatment removes these biodegradable organic contaminants. Biological processes degrade dyes contained in wastewater through decolorization carried out by fungal strains [89]. These processes are slow; however, their efficiency is satisfactory. Enzymatic decolorization is now used for the decolorization of dye effluents. There are several problems, e.g., the cost, stability, and product inhibition of enzymes [89]. Anaerobic treatments degrade a wide variety of synthetic dyes [90]. However, successfully aerobic conditions are applied to decolorize the dyes. A few industries use biological treatment to dispose of biodegradable materials, e.g., food processing dyes, dairy wastes, paper, plastics, brewery wastes, and petrochemicals [91,92]. The fungal method is more economical in decolorization via adsorption (living or dead), microbial biomass formation, and bioremediation systems. Fungal organisms degrade and accumulate different pollutants [93].
3. Metabolism of Fungi
Fungi, which are ubiquitous organisms, play a vital role in ecosystems. They can grow on different substrates and function for an indefinite time period. A pivotal parameter determining the cell potential is a metabolic pathway of the microorganism. The performance of FFCs is influenced by fungal metabolism and growth. Fungi have a complex cellular organization and use two pathways for electron transfer. The substrate (glucose) oxidation results in the production of two molecules of NADH/glucose (glycolysis), while mediators interact with the component of ETC (keeps ETC functioning and produces electrons from TCA) [94]. Both metabolic pathways are essential for the removal of waste from the substrates by providing electrons. Any disturbance in these pathways disturb the system, resulting in lower power generation.
The consumption of non-renewable energy creates many problems such as the “availability of fossil fuel stocks for future releases of a huge quantity of toxic gases or particles”, which have influence at the global level and stimulate changes in the climate. Fungal biofuel cells (bioelectrochemical system) utilize the living cell for the production of bioelectricity. This cell can drive electricity or other energy generation currents by the use of living cell interaction. Fungal fuel cells and enzymatic biofuel cells can improve sustainable energy production with an efficient conversion system compared to chemical fuels [95].
The diverse group of yeast, molds, and filamentous fungi can remediate various industrial wastewaters. Mycodegradation destroys wood, paper, textile, plastic, and leather materials. The mycelia of several species facilitate degradation. Fungi degrade pesticides, dyes, polychlorinated biphenyls, hydrocarbons, and phenolic and chlorinated compounds with the use of different enzymes (laccases, manganese peroxidase, and lignin peroxidases) [96]. Irpex lacteus and Pleurotus ostreatus degrade PAH from contaminated industrial soil [97]. Many fungi (Fusarium oxysporum, Mucor alternans, Tricoderma viride, and Phanerochaete chrysosporium) can degrade DDT.
Numerous white-rot fungi (Phanerochaete chrysosporium, Pleurotus ostreatus, Trametes versicolor, Irpex lacteus, and Lentinula edodes) can degrade various toxic compounds through their numerous reductive and oxidative mechanisms. Endosulfan is oxidized to endosulfan sulfate through the catalyze-based mechanism of Tricoderma harzianum. Fungi are suitable for biotreating oil-based sediments and PAH-contaminated cuttings [98]. Phanerochaete chrysosporium fungi oxidize pyrene, benzo[a]pyrene, anthracene, and fluorine into quinines using MnP and LiP [99].
Fungal Chitosan: Fungal chitin is an economically attractive pollutant-adsorbing material next to cellulose [100]. Chitosan or glucosamine is a derivative of chitin found in the cell wall of a few fungi (Mucorales). Reactive Red 2 contaminated water is decolorized by macro fungi [101]. Chitosan beads are used for the treatment of aqueous solutions containing perfluorooctane sulfonate (PFOS) [102]. The biosorption efficiency of fungal biomass can be increased by modification processes. Chitosan poly vermiculite hydrogel adsorbents remove methylene blue from aqueous solutions [103].
4. Role of Fungal Enzymes and Modifications in FFCs
Extracellular ligninolytic oxidative enzymes, e.g., valuable extracellular oxidoreductases (laccases, lignin peroxide, and manganese peroxide) help the fungi to degrade dyes and xenobiotic compounds [104]. Intracellular enzymes are recovered from the cell wall of fungal mycelia [105]. Multiple enzyme systems are successfully used for the efficient breakdown of diverse types of organic pollutants by oxidation or degradation into smaller intermediates.
Oxidoreductase is renowned for its ability to degrade numerous types of organic pollutants [106]. Reactive diffusible redox mediators (RMs) based on oxidoreductase dramatically increase the reaction rate and a broad range of substrates are degraded by these enzymes [107]. The advantages of enzymatic degradation include the low concentration of reagents in mild conditions, with their ability to break down a wide range of substrates. The disadvantage of high cost of enzymes can be improved by using recombinant DNA technology.
Lactate dehydrogenase and ferricyanide reductase are redox enzymes [42]. Fungi exhibit a similar mechanism of electron transfer to that in bacteria. Mediator-less FFCs and fungal electrogenic efficiency are examined by Sayed and Abdelkareem [21], and studies on fungal-mediated electron transport have received attention due to the presence of fungal redox proteins (lactic acid dehydrogenase or ferricyanide reductase).
Wood degraders (white-rot fungi) secrete many extracellular enzymes such as laccase a multi copper oxidative enzyme. Fungal laccase is a 4Cu-containing oxidoreductase biocatalyst that can transfer electrons and has a higher capacity for redox reactions (for organic and aromatic compounds) using an enzyme-mediated system. Laccase catalyzes the oxidation reactions and increases the chances of smooth biological degradation mechanisms. Laccase accepts e− from atm. O2 (electron acceptor) catalyzes the 1e− oxidation reaction of phenolic compounds, which facilitates the catabolism of organic compounds [108]. Consequently, at the same time, laccase act as a cathode catalyst in a fungal fuel cell (FFC). White-rot fungi metabolize the laccase to accumulate the nutrients in the soil by lignin degeneration or organic components of the ecosystem. They are suitable and cost-effective for the sustainable development of power generation from FFCs through the in situ elimination of laccase.
Fungi play a dual role in FFCs [109], e.g., at the anode, fungi facilitate e− transfer via their respiratory proteins or chemical mediators. At the cathode, they reduce the terminal electron acceptors (oxygen). Recent investigations have shown the direct electron transfer through cytochrome C [110]. The in situ laccase of white-rot fungi shown to increase the efficiency of FFCs. This involved the oxidization of aromatic amines and phenolic mixtures using atmospheric oxygen (terminal electron acceptor) [111]. Laccase is extensively manipulated to degrade the organic pollutants, phenolic compounds, triclosan, bisphenol A [112], synthetic and natural hormones such as estrone (E1), 17b-estradiol (E2), estriol (E3), and aromatic dyes [113]. Lignin is degraded as laccase is generated. In the treatment of rubber-processing wastewater, sludge is used at the anode (as a substrate), while laccase is deposited on the cathode under optimal systems to generate electricity. Ganoderma lucidum (strain BCRC 36123), Pleurotus ostreatus, and T. versicolor are well-known laccase-producing fungal species with high efficiency in the production of energy because of an incapacitated layer of fungal enzymes (at the cathode) [114]. Laccase also returns the nutrients by degradation of plant lignin in soil and produces large amount of power in dual-chamber rather than single-chamber FFCs [115]. Laccase used on the biocathode to minimizes the cost of FFC manufacturing.
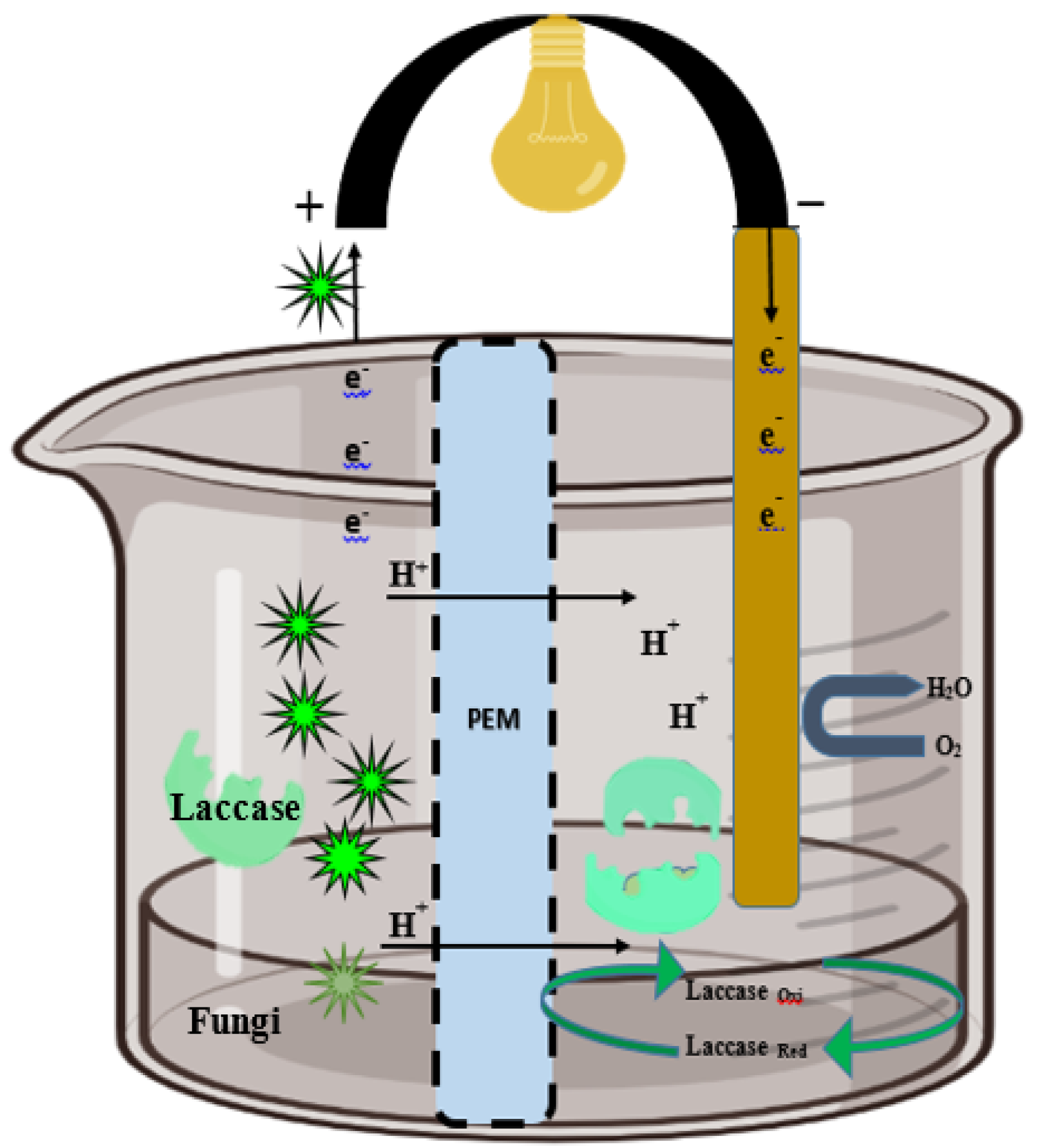
Laccase-producing Ganoderma lucidum BCRC 36123 was planted on the cathode surface of a single-chamber FFC to degrade a dye synergistically with a community of anaerobic microbes in the anode chamber (Figure 4). The laccase activity (1063 ± 26 U/L) of white-rot fungi (Ceriporiopsis subvermispora, Pycnoporus cinnabarinus, Phanerochaete chrysosporium, and Trametes pubescens) efficiently removed 71–77% of COD and 87–92% of phenolic compounds at a pH of 5.0 [116] (Strong 2010). Fungal laccase hydrolyzed winery wastewater and effectively removed the phenolic compounds, COD, and colors [117].
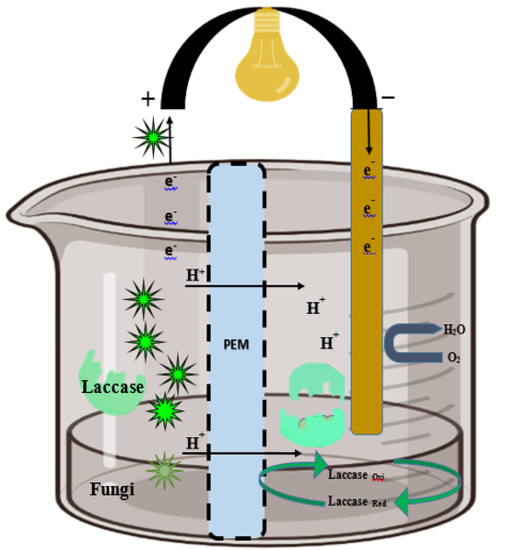
Figure 4.
Extracellular fungal laccase catalyst in the anodic chamber, which enzymatically oxidizes wastes during wastewater treatment in the cathodic chamber with reduction of O2 into water. The movement of e− from the anode to cathode lit the bulb during the completion of circuit.
Peroxidases are other oxidoreductase enzymes found in fungi. They efficiently oxidize a wide range of substrates [118]. These are effective and extensively used in dye degradation [113]. Only a few studies have reported the use of peroxidases for PPCP degradation [119]. Pollutant-degrading peroxidases are heme peroxidases and non-heme peroxidases. The heme-based peroxidases are further classified into four superfamilies: peroxidase cyclooxygenase, peroxidase–catalase, peroxidase–peroxygenase, and peroxidase–chlorite dismutase. The peroxidase–catalase superfamily is the most abundant in fungi and further subdivided into three additional families, e.g., F1 (intracellular bacterial catalase), F2 (secretory fungal peroxidases such as lignin and manganese), and F3 (secreted plant peroxidases) [120].
4.1. Modification in Yeast-Based Cells
The yeast-based fuel cell (YBFC) is a novel technique used to purify different types of wastewater and convert chemical energy into electrical energy at the cost of active biocatalysts [121]. The performance of YBFCs is influenced by the performance of the electrodes. The electrode performance of yeast-based cells are investigated by coating carbon paper with a thin layer of gold or cobalt (of thickness 5 or 30 nm). The electrode performance is assessed by measuring the electrode half-cell potential efficiency during degradation of chemical substances, pollutants, and organic biomass on the budget of extracellular enzymes [29].
4.2. Factors Affecting FFC Performance
Certain biological (external resistance, substrate type with concentration, and choice of inoculum) and physical factors (reactor configuration, electrode material, and separator) efficiently increase the cell functioning [122], e.g.:
- Using a fungal biocatalyst.
- The type of fuel for the FFC.
- The chemical energy of the substrate (converted into electrical energy).
- The use of mediators (ABTS 2,2′-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid)) that are effective in e− transfer from the electrode to laccase.
- An airtight anodic chamber in a dual-chamber.
- Cathode chamber is filled with laccase secreted by white-rot fungi and sufficient nutrient growth medium for the optimum growth of fungal cells.
5. Mechanism of FFCs in Wastewater Treatment
Different types of FCs are operated and reported in wastewater treatment with the generation of biohydrogen, biogas, and other biofuels/energy. Later on, biogas is converted into electric power. Recent developments and researches on designing of fungal fuel cell and its application emphasize the bioenergy generation for future.
Environmental scientists try to devise the methods of degradation or removal of pollutants from the water supply. Water bodies are rich in organic pollutants. These pollutants have diverse chemical structures, e.g., dyes present in textile waste streams are classified into anthraquinone, basic, acidic, and azo dyes [123]. Organic aromatic compounds are carcinogenic in nature and pose serious health risks for humans and aquatic organisms [124]. Organic pollutants are suitable substrates for the growth of bacteria that reduce the oxygen level in water bodies and increase their turbidity with color to decrease the photosynthetic growth of water biota [125]. Human consumption and improper disposal of personal care products contribute to their release into ground and surface water [126]. Efficient degradation depends on the proper binding and orientation of organic compounds in the active sites of peroxidase enzymes. Hence, the substrate structure is a very important parameter in the degradation of pollutants.
Biological approaches are environmentally friendlier, less expensive, and widely used for the handling and removal of harmful substances from wastewater [127,128]. Fungal enzymes (peroxidases and laccases) metabolize and degrade pollutants into less-toxic forms [129]. Diverse fungal enzymes (exocellular and endocellular) in fungus-based FCs are used for waste biodegradation activity. Peroxidases and laccases degrade many types of organic pollutants. Mostly, studies are focused on the treatment of “pure (neat) pollutants” or “simulated wastewater”. This presents a major challenge and weakness in the field of enzyme-based remediation of organic pollutants. Only a few studies use enzyme-based systems for real wastewater samples (complex and complicated) rather than neat solutions.
In FFCs, the anaerobic anode and aerobic cathode chambers are separated by a proton exchanger membrane. The anode chamber comprises both respiring and fermenting microbial communities, which enhances the versatility of FCs in the degradation of pollutants [130]. In the fungal fuel cell, electrochemically active fungal species can oxidize different organic compounds present in wastewater in the anode chamber and produce electrons and protons, which are transported toward the cathodic chamber for reduction of O2 into water (Figure 5). A membrane separates both the anode and cathode compartments. An external resistor set between the anode and the cathode harvests the electricity easily.
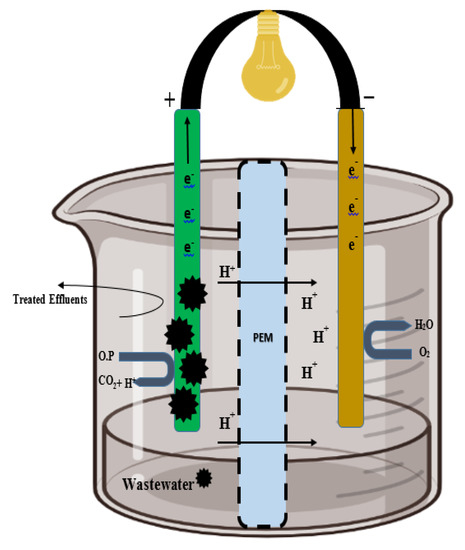
Figure 5.
Wastewater with effluents and organic pollutants is placed in anodic regions, where fungal oxidoreductase enzymes are deposited on the electrode. Carbon, electrons, and protons are generated from the effluents by oxidoreductase action and transferred to the cathode where the reduction of O2 into water takes place. The external resistor between the anode and the cathode harvests the electricity easily.
First, wastewater enters the anode chamber, where fermenting microbes convert the large organic molecules into smaller fermented products (lactate), which are further oxidized by anaerobic (respiring) bacteria to release CO2, protons, and e−. When e− does not find a suitable acceptor in the anolyte, electrons pass to the anode interface and transfer through an external wire to the cathode. Here, they bind O2 and protons and convert them into water molecules. This is a final step of the circuit [92]. Electrons are released during organic matter degradation and travel toward anodes, eventually being oxidized by electron acceptors (cathode) [131]. The electron donating substrates are used in the anode chamber. A few electron acceptors are characterized by fast kinetics, high redox potential, low cost, and by their importance in environmental sustainability [132]. Oxygen is one of the most convenient electron acceptor utilize commonly in FFCs. The oxygen availability, with its high oxidation potential, produces a clean product, i.e., water [133]. These fuel cells not only generate electricity, also recover and eliminate the compounds from wastewater [134].
The concentration of cations is higher than that of protons, and these cations accumulate in the cathode chamber, resulting an increase in pH of chamber and a decrease in pH of the anodic chamber. This reduces the efficiency of fungal activity and thermodynamics of the fuel cell [135]. Hence, modification of the pore size of membranes can ensure the transfer of only protons and no other cations. Different approaches, e.g., cation and anion exchange membranes (EM), have been suggested to solve the problem of the pH gradient on both sides of the cell membrane [136]. Ionic liquid membranes can improve the fuel cells and ensure the selective transport of only protons and no cations via the membrane. This improvement enhances the efficiency of fungal activity in the anode chamber (not affected) due to absence of transport of cations present in textile wastewater across the membrane. The energy obtained in this process (wastewater treatment) is five times greater than energy consumed during the treatment [137].
The conventional biological process is not sufficient in wastewater treatment due to the inhibition of “biocatalytic activity”. The deposition of catalysts on electrodes has gained huge interest, as it accelerate the reaction kinetics (at electrodes). Cathode catalysts enhance the rates of the reduction reaction, while anode catalysts enhance the oxidation rate.
Enzymes secreted by fungi and algae, either individually or in symbiotic association, catalyze the toxic pollutants into a less harmful form. Valuable products are generated in wastewater contaminated by fungal mycelia and fungal interventions such as different enzymes and lipids during the treatment of distillery wastewater to minimize contaminants [138].
COD removal takes place at the cathode and anode, which is a major concern in the treatment of wastewater. A fungal-based study on the treatment of water and sewage wastewater for energy generation was conducted by Fernandez de Dios et al. [139]. The biodegradation involved efficient species remediating harmful toxicants for energy production. Agro-industries release organic pollutants dissolved in wastewater [115], as do fermentation, pulp, and paper industries. Many countries have implemented stringent regulations and norms for the disposal of agro-based industrial wastewater. Such norms are quite challenging, as they insist on the steady adoption of cost-effective and innovative technologies.
The catalytic platinum and gold increase the price of electrodes. To reduce electricity production and wastewater treatment costs, catalysts should not comprise valuable metals [140]. Therefore, fungi play a role as anode catalysts to adjust the appropriate cathode catalysts.
5.1. Role of the Cathode in Wastewater Treatment
The cathode must be good, robust, mechanically strong, and highly conductive with the best catalytic properties [55]. Cathode materials require high power generation with Coulombic efficiency. Therefore, the main challenge to minimize the expenses is the target aspect of fuel cell technology. Such electrode is successfully applied for the treatment of wastewater [141]. Cathodes are abiotic and biocathodic. Biotic types can be aerobic or anaerobic [142]. Mediators/or catalysts are required in abiotic cathodes for oxygen reduction reactions. Maximum expense and poor stability causes the catalyst poisoning (Pt-based), which vanquish by engaging the biotic cathodes (where microorganisms assist in the cathodic reactions) [143]. Biocathodes have an advantage over abiotic cathodes, a few interesting benefits are cost reduction and the omission of costly mediators or expensive metal catalysts (platinum) [57].
Role of the Anode in Wastewater Treatment
The selection of an anode is also critical in the performance of FFCs. It plays an important role in e− transfer from microbes to the electron acceptor. Conventional cells comprise bioanodes and abiotic cathodes.
5.2. Integrated Treatment Processes
Water for dilution is required in bioelectrochemical processes to reduce the organic matter in fungal pretreated effluents, which is a major disadvantage of FCs. Effluent dilution is possible by using two repeated fungal cocultivation processes followed by treatment in FFCs. Hence, ecofriendly treatment processes with lesser environmental and economical footprints should focus on the achievement of highest treatment efficiency. Different enzymes and other derivatives of fermentation broth make the fungal fermentation technology profitable for industrial implementation. Catalytic thermolysis is an effective process for the destruction of color and organic compounds in complex wastewater [144].
Coupling processes can improve the efficiency of distillery wastewater treatment with improved COD and color removal. Single-stage anaerobic digestion is insufficient for the higher removal of organic matter. Therefore, combined single- or multistage aerobic and chemical oxidation treatment is required [145]. Sequential anaerobic–aerobic treatment of fungal fuel cell (FFC) is a better modification [146]. The treatment of malt whiskey distillery wastewater removed 52% of COD and BOD in a UASB reactor (first stage), 70% COD and BOD in batch aerobic degradation (second stage), and >99% of COD and BOD, when combined with thermophilic fungi (third stage) [147].
Trametes pubescens pretreatment followed by anaerobic digestion was helpful in the removal of COD (53.3%) and polyphenolic compounds (72.5%) from wine distillery wastewater. Similarly, winery effluent treated with fungi supplemented with C and nutrient media followed by anaerobic digestion for 2 days; this procedure reduced the COD level (99.5%) [148]. A study consisting of a combination of fungal pretreatment, submerged MBR, and the secondary digestion of wine distillery wastewater showed 86% decrease in COD concentration [149]. Fungal biodegradation carried out with exo-enzymes released by Aspergillus sp. combined with grain-based distillery stillage removed 94% of total COD (first time), while higher efficiency (99%) was achieved by support of fungal degradation processes (second time) [105].
5.3. Role of Yeast Cells in Wastewater Treatment
Fungal degraders are widely used in the treatment of wastewater due to their consumption of substrates. The yeast wastewater is a dual-stage (anaerobic) biological wastewater [150]. Yeast cells used in FFCs represent two types: oxidizing and fermenting [151]. Fermenting yeasts utilize 6C sugar and ferment into CO2 and alcohol. Yeast cells metabolize inorganic compounds and hazardous materials. The yeast cell wall is very thick and cell membrane can separate from the cell wall, which is difficult to obtain. Therefore, yeasts are processed in a transplasma membrane ET system also called “plasma membrane (PM) oxidoreductase”. The PM–NADH–oxidoreductase system located throughout the membrane is also involved in transportation of e− using NADH and NADPH to the external electron acceptor/anode. Yeast fuel cells play an active role in e− transfer during ATP synthesis along with NAD+ into NADH reduction. The oxidation of glucose is the main source of energy production by yeast cell factories [33].
Yeasts efficiently treat wastewater. Yeasts and fungi shown decolorization of various organic compounds efficiently [152]. Yeasts are used to remove dyes and heavy metals from wastewater [153]. Yeast cells are well-known producers of different lipids, enzymes, and glycolipids suitable for wastewater treatment, as wastewater contains maximum concentrations of heavy metals, ion, organic matter, and domestic sewages also. The yeast consortium quickly degrades high concentrations of organic matter [154,155]. Biodegradable organic wastewater, ferricyanide potassium, acid orange, and acetate are also used as anolytes or anolyte feed.
Algae, in combination with fungi, are effective in the generation of electricity in MFCs (microbial fuel cells) using molasses substrate. The transport of protons from yeasts to algae proceeds with the help of microporous tubes rooted in activated bleaching earth, called an ion exchange medium, for the production of electricity. A strain of Galactomyces reessii reported to utilize synthetic wastewater and rubber industry sludge for the generation of electricity [156]. The unique ability of yeast species to transfer electrons extracellularly helps to advance the fuel cell technologies and to purify wastewater as a biocatalyst. Natural and genetically modified yeasts with improved enzyme action are useful for the degradation of toxic substances and exhibit the capacity for the generation of electricity. Candida melibiosica has shown the high phytase activity to remediate phytates and a maximum potential for electricity generation. This opens up novel avenues for nonchemical and more sustainable ecofriendly approaches in the purification of phosphate-polluted wastewater as well as many other xenobiotic contaminants [157].
A double-chambered fuel cell has generated electricity due to the ability of Pichia fermentans to utilize wheat straw hydrolyzate. This hydrolyzate was prepared by degrading the wheat straw in the presence of Phlebia brevispora, Phanerochaete chrysosporium, and Phlebia floridensis (white-rot fungi). The maximum electrochemical response of 20.13 ± 0.052 mWm−2 and 20.42 ± 0.071 mWm−2 was recorded in hydrolysate using P. floridensis and P. chrysosporium, respectively [158].
The electricity was produced from yeast wastewater in membraneless (ML) fuel cells with a Cu–Ag cathode and a power of 6.38 mW and a cell voltage of 1.09 V were obtained. This research proved a feasible way to obtain the maximum bioelectricity from fuel cells (fed with yeast wastewater) [159].
5.4. Pharmaceutical Wastewater Treatment
Pharmaceutical industries release pharmaceutical products in water on daily basis [160], the treatment of which is a challenging task. Wastewater treatment plants are the point sources of pharmaceutically active compounds; however, these are not designed for the elimination of active compounds [160]. Long-term exposure to compounds in the environment may result in chronic and acute damage, behavioral changes, reproductive impairment, inhibition of cell proliferation, and accumulation in tissues [161]. Unchanged antibiotics from pharmaceutical wastewater reaching the sewage system may result in the emergence of antibiotic resistance in fungi and aquatic organisms. They may also change the microbial community structure. Different treatment techniques for pharmaceutical wastewater require a large area, costly chemicals, treatment methods, and high energy input. In turn, the low biodegradability [162] and generation of toxic byproducts have stimulated scientists to construct FFCs as an efficient alternative not requiring costly chemicals and capable of generating energy without toxic byproducts.
5.5. Heavy Metal-Loaded Wastewater
Water, which faces various types of pollution and degradation, is a precious commodity. It only seems inexhaustible, whereas the whole world in the short or long term will face the problem of its scarcity. This urge makes wastewater one of the most valuable resource for energy and water. Conventional remediation treatments are neither environmentally friendly nor economical. A new process is required to overcome these issues, where the conservation and recovery of energy will become possible. Fungal fuel cells are emerging as a promising technique for the mitigation of pollution.
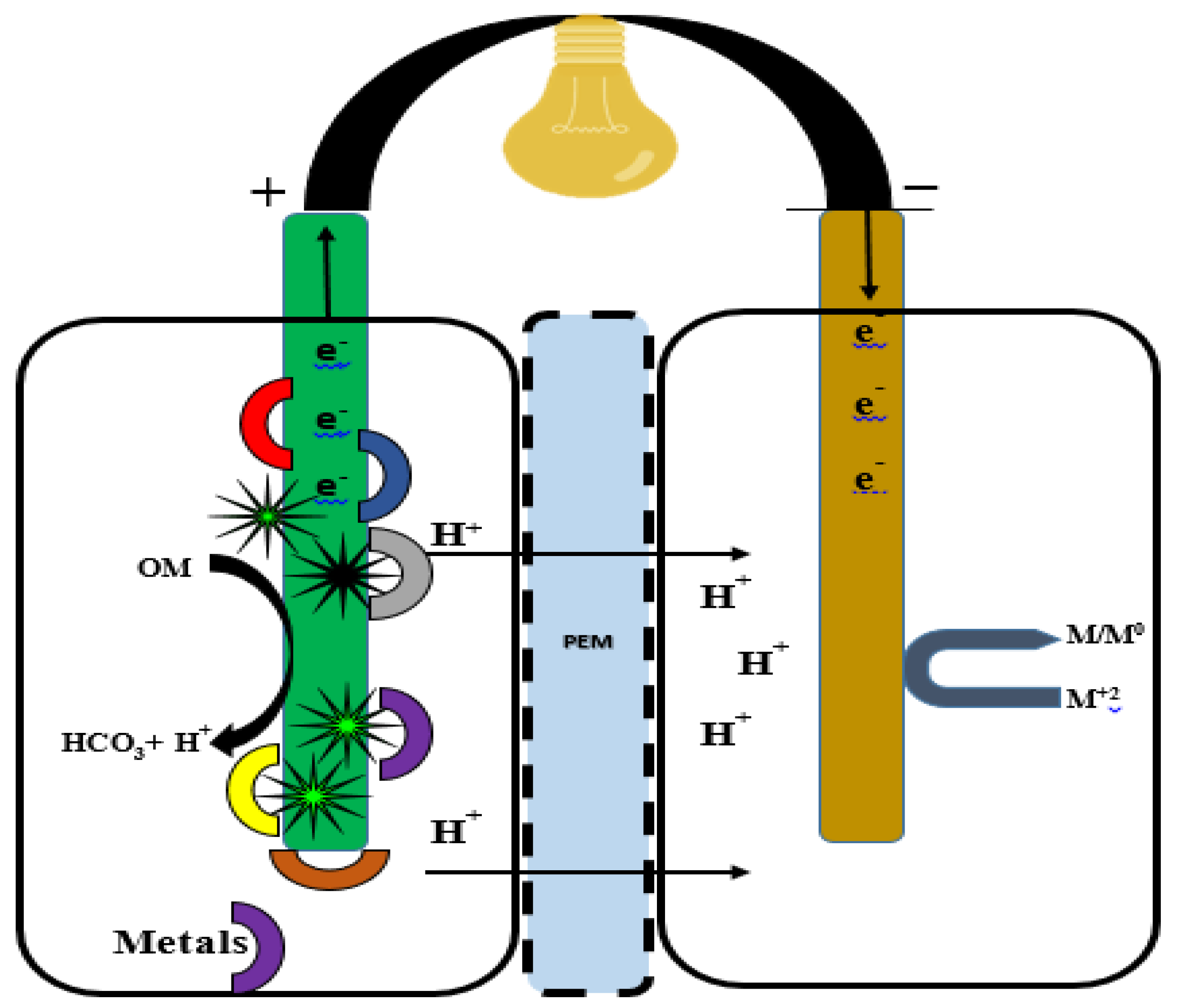
FFCs provide a strategy to treat wastewater and remove or recover heavy metals. These bioelectrochemical systems utilize fungal catalytic activity in the form of biofilms to oxidize inorganic compounds with the production of electric current. It is a sustainable bioremediation method for energy production (Figure 6).
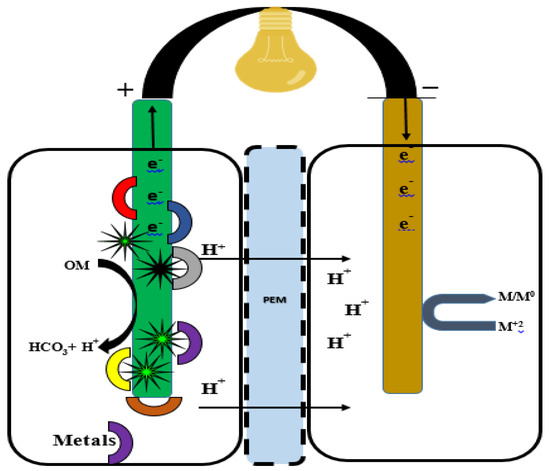
Figure 6.
A double-chambered fungal fuel cell for the treatment of heavy metals. Heavy metal-contaminated water is placed in the anodic cell (aerobic compartment), where fungi enzymatically release carbon, e− and H+ transfer via PEM and external wire to the cathodic cell (anaerobic compartment), where the reduction of the metal from M+2 to M/M0 takes places.
5.6. Agro–Industrial Wastewater Management
Agro-industries demand large volume of water during manufacturing processes and generate wastewater. This agricultural wastewater has a high content of N, P, and organic compounds can also be treated using FFC technology [163]. The wastewater is loaded with organic matter, proteins, oils, grease, and sugars, which increase the COD of ground and surface water. This causes severe environmental pollution, because such wastewater not biodegradable easily due to the addition of bacterial end products and high chemical stability. Industrial wastewater is especially useful in FFCs because of its constant composition.
5.7. Biodegradation of Distillery Wastewater
The bioremediation of distillery wastewater using biological and natural pathways leads to the degradation of organic pollutants. Via aerobic degradation, the white-rot fungus Phanerochaete chrysosporium can remove organic matter (COD and BOD) effectively [164]. Fungal biodegradation is an inexpensive technique where wastewater dilution is not required. A pseudo-second-order rate is used for the kinetic model of fungal biodegradation of distillery wastewater [165]. Aspergillus awamori removed 39.3% of COD from grain-based distillation [166]. A mixed consortium of six fungal species, i.e., Penicillium pinophilum, Aspergillus flavus, Alternaria gaisen, Fusarium verticillioides, Pleurotus florida, and Aspergillus niger are used to remove 65.4% of COD in distillery wastewater treatment [167].
Batch aerobic treatment with the use of yeast and fungal species is very effective in the remediation of distillery wastewater. This treatment remove >85% of COD in a single phase, while a minimum cultivation time of 7–10 days is required for a higher removal of organics [164].
Distillery fermentation produces a large volume of wastewater called stillage. This wastewater has a lower pH, unconvertible organic fractions, and a maximum % of dissolved inorganic matter. Dark brown molasses stillage comprises higher chemical oxygen demand, biochemical oxygen demand, and inorganic impurities. Grain stillage is characterized by a lower chemical oxygen demand, acidic pH (3.4–4.1), and exhibits significant potential for pollution upon discharge. Physical, chemical, and biological integrated treatments of distillery stillage are discussed in detail with current challenges. Distillery stillage is used as a substrate in fungal fuel cells, encouraging observations in terms of achieving high treatment efficiency to meet the discharge standards, bioelectricity generation, and value-added products recovery.
Xylanase (17.3 U/mL) production from the phyllosphere yeast (Pseudozyma antarctica) removed 63% of DOC (dissolved organic compound) from wastewater by utilizing bioethanol distillery wastewater (substrate) [168]. This is an expensive source for the production of biomaterials. Oleaginous yeasts (Rhodosporidium toruloides and Chlorella pyrenoidosa) successfully degraded distillery wastewater effluent with a 43.65 ± 1.74% lipid content and a 3.54 ± 0.04 g/L lipid yield associated with an 86.11 ± 0.41% COD reduction [169]. Maximum mycelial biomass of Calvatia gigantea (2.75 g/100 mL/4.5 days) was obtained in optimized conditions when the fungus was cultivated on raw distillery wastewater [170]. Candida utilis biomass utilized shochu wastewater and removed 62.9% of DOC [171].
5.8. Degradation of Ethanol Distillery Wastewater
Pollutants from ethanol production vary significantly in wastewater. Winery wastewater comprises low concentration of organic matters and high amount of polyphenolics and nutrients [172]. The fermentation of beet molasses and sugarcane releases aldehydes and ketones, which impart flavor. The wastewater from this process was characterized by a high level of biodegradable organic matter [173], maximum nutrient contents (sulfate, chloride, calcium, magnesium, and potassium), and a low pH [174]. This type of wastewater constitutes severe water and land pollution.
COD and phenol is removed from winery wastewater using yeast-mediated fuel cells. In this study, electrochemical properties are monitored. The results indicated the laccase activity of yeast strain ET-KK. A maximal current and power density of 139.17 ± 1.44 mA/m2 and 38.74 ± 0.80 mW/m2 is generated. The COD and phenol removal from winery wastewater was 79.14 ± 0.92% and 85.04 ± 0.07%, respectively [175].
5.9. Degradation of Dye Wastewater
Trametes versicolor is a well-known white-rot fungus used for the treatment of dye wastewater [83]. Fungal culture adjusts its metabolism through its modification in environmental conditions. Intra- and extracellular enzymes are helpful in fungal metabolic activities and the degradation of different dyes from textile wastewater. The best examples are lignin, laccase, peroxidase, and manganese [176] from white-rot fungi (Coriolopsis sp., Pleurotus eryngii, and Penicillium simplicissimum) [177].
In bioanode–biocathode FFCs, the dye wastewater acts as anolyte and catholyte. The maximum COD removal and decolorization are observed in the first 12 h, because a higher substrate consumption at cathode and anode occurs during the initial hours. The maximal cell potential are recorded to be 706 mV in a fungal FC, with power densities of 276.9 mWm−2. The reactor was also tested for the biodegradation of RR 195 dye from wastewater along with bioelectricity production. The overall COD and color removal efficiency was 72%, and 95% [178].
Fungal fuel cells are an emerging technique that effectively treats wastewater with simultaneous electricity production. In a study on the decolorization and degradation of azo dyes (remazol brilliant blue, mordant blue 9, acid red1, and orange G), wheat straw hydrolyzate substrate are used in FFC. The hydrolysate was prepared by the degradation of wheat straw by P. floridensis, P. chrysosporium, and P. brevispora, while Pichia fermentans (yeast) used as a biocatalyst. Dye decolorization was carried out in a fungus yeast-mediated single-chambered FC. The maximum power density was recorded (34.99 mW m−2) on the 21st day. The best response to dye decolorization was observed in MB9 (96%) with P. floridensis followed by RBB (90–95%), AR1 (38%), and OG (76%) [179].
Mechanism: FCs transform azo dyes into less colorful compounds but fail to degrade and mineralize them completely. The decolorization of wastewater containing azo dyes and other types of dye takes place through the movement of anode and cathode electrons via external circuit. Azo dyes in the catholyte act as e− acceptors and decolorize dyes via reductive cathode reactions. The dye-reducing reactions progress better in anaerobic conditions, as O2 competes for e− from the cathode, and reaction rate heavily depends on the pH of the catholyte [90].
Two-in-one systems: First stage (reductive transformation): Azo dye wastewater is placed as an anolyte (anode chamber), where dye molecules are reduced by bacteria (anaerobic) to form aromatic amines. Second Stage: This water is then shifted to an aerobic bioreactor, where it is further degraded into smaller compounds [180]. This two-in-one system is very effective; however, it is structurally complex and expensive to operate and construct. The proton exchanger membrane prevents the movement of pollutants and keeps transformed products in their respective chambers.
White-rot fungi are the only group of organisms that completely degrade azo dyes. Laccase produce, when fungi degrade lignin (a highly heterogeneous aromatic polymer) that is abundant in the natural habitats of white-rot fungi [181]. The laccase catalyst has replaced the noble metals in catalyzing the reduction of O2 [182]. Single-chamber FCs have no cathode chamber and their cathodes are directly exposed to air for maximum oxygen availability [183]. A feasible test in growing a laccase-producing white-rot fungus on the cathode surface of a single-chamber FC is the possibility of proton exchanger membrane, which, when replaced by a layer of polyvinyl alcohol-hydrogel, allows the pollutant to diffuse from the anode to cathode chamber [184].
5.10. Applications of FFCs
- The management of the environment
- The generation of bioelectricity
6. Future Prospective
In future, fungal fuel cell technique may become the part of energy system at the expense of waste products for electricity generation from wastewater, where organic contents are usually in a much smaller range of 260–450 mWm−2 [185]. The absolute goal in near future is to design a cell that capable of producing the highest amount of energy on the expenditure of lowest possible energy input.
7. Conclusions
Conventional methods can treat loaded wastewater, while biological methods such as FFCs represent a sustainable technique in lowering the wastewater impact on the environment. This remediation system is profitable, ecofriendly, and cheaper than other conventional methods. FFCs offer an enduring solution for the degradation of wastewater contaminants into non/less-toxic forms with simultaneous generation of electricity by using fungal oxidoreductase. The fungal enzymes clean up the contaminated wastewater with maintaining of their regular metabolism. The “Industrial Revolution” forces the valorization of chemical wastes. Fungal cells are suitable candidates for the valorization of wastes in water and the simultaneous production of electrical energy.
Author Contributions
Conceptualization and methodology, A.U.; software, M.G.; validation, A.U. and M.G.; formal analysis, A.U.; investigation, A.U.; resources, A.U. and M.G.; data curation, A.U.; writing—original draft preparation, A.U. and M.G.; writing—review and editing, A.U., Ł.S. and M.G.; visualization, A.U.; supervision, M.G.; project administration, A.U.; funding acquisition, A.U. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lvovich, M.I. Water Resources and Their Future; Litho Crafters Inc.: Eastpointe, MI, USA, 1979. [Google Scholar]
- Adeel, S.; Abrar, S.; Kiran, S.; Farooq, T.; Gulzar, T.; Jamal, M. Sustainable Application of Natural Dyes in Cosmetic Industry. Handbook of Renewable Materials for Coloration and Finishing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 189–211. [Google Scholar]
- Hanafi, M.F.; Sapawe, N. A review on the current techniques and technologies of organic pollutants removal from water/wastewater. Mater. Today Proc. 2022, 31, A158–A165. [Google Scholar] [CrossRef]
- Narita, J.; Okano, K.; Tateno, T.; Tanino, T.; Sewaki, T.; Sung, M.H.; Fukuda, H.; Kondo, A. Display of active enzymes on the cell surface of Escherichia coli using PgsA anchor protein and their application to bioconversion. Appl. Microbiol. Biotechnol. 2006, 70, 564–572. [Google Scholar] [CrossRef]
- Lovley, D.R. Microbial fuel cells: Novel microbial physiologies and engineering approaches. Curr. Opin. Biotechnol. 2006, 17, 327–332. [Google Scholar] [CrossRef]
- Franks, A.E.; Nevin, K.P. Microbial fuel cells. A currentreview. Energies 2010, 3, 899–919. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Hill, G.A. Continuous microbial fuel cell using a photoautotrophic cathode and a fermentative anode. Can. J. Chem. Eng. 2012, 90, 1006–1010. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Overview of recent advancements in the microbial fuel cell from fundamentals to applications: Design, major elements, and scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Zhang, G.; Yang, Q.; Lu, J.; Xia, T.; Peng, L.; Wang, Y. Cascading of engineered bioenergy plants and fungi sustainable for low-cost bioethanol and high-value biomaterials under green-like biomass processing. Renew. Sustain. Energy Rev. 2021, 137, 110586. [Google Scholar] [CrossRef]
- Vernet, G.; Hobisch, M.; Kara, S. Process intensification in oxidative biocatalysis. Curr. Opin. Green Sustain. Chem. 2022, 38, 100692. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Kurosawa, K.; Wewetze, S.J.; Sinskey, A.J. Engineering xylose metabolism in triacylglycerol producing Rhodococcus opacus for lignocellulosic fuel production. Biotechnol. Biofuels 2013, 6, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.; Sundaresan, M.; Suresh, A.; Thajuddin, N.; Thangaraj, R.; Vinothini, G. Oleaginous microorganisms for biofuel development. Environ. Sci. Eng. 2017, 12, 243–263. [Google Scholar]
- Fakas, S.; Papanikolaou, S.; Batsos, A.; Galiotou-Panayotou, M.; Mallouchos, A.; Aggelis, G. Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Bio. Bioenergy 2009, 33, 573–580. [Google Scholar] [CrossRef]
- Sundaramahalingam, M.A.; Sivashanmugam, P. Concomitant strategy of wastewater treatment and biodiesel production using innate yeast cell (Rhodotorula mucilaginosa) from food industry sewerage and its energy system analysis. Renew Energ. 2023, 208, 52–62. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; Nazario-Naveda, R.; Benites, S.M.; Gallozzo-Cardenas, M.; Delfín-Narciso, D.; Díaz, F. Use of Pineapple Waste as Fuel in Microbial Fuel Cell for the Generation of Bioelectricity. Molecules 2022, 27, 7389. [Google Scholar] [CrossRef]
- Beopoulos, A.; Nicaud, J.M. Yeast: A new oil producer? Ol. Corps Gras. Lipides 2012, 19, 22–28. [Google Scholar] [CrossRef]
- Hofrichter, M. Review: Lignin conversion by manganese peroxidase (MnP). Enzym. Microb. Technol. Recent Adv. Lignin Biodegrad. 2002, 30, 454–466. [Google Scholar] [CrossRef]
- Sekrecka-Belniak, A.; Toczyłowska-Mamińska, R. Fungi-based microbial fuel cells. Energies 2018, 19, 2827. [Google Scholar] [CrossRef]
- Sayed, T.; Abdelkareem, M.A. Yeast as a Biocatalyst in Microbial Fuel Cell. Old Yeasts-New Quest. 2017, 317, 41–65. [Google Scholar]
- Hallsworth, J.E.; Magan, N. Culture age, temperature, and pH affect the polyol and trehalose contents of fungal propagules. Appl. Environ. Microbiol. 1996, 62, 2435–2442. [Google Scholar] [CrossRef]
- Deska, M.; Kończak, B. Immobilized fungal laccase as” green catalyst” for the decolourization process–State of the art. Process Biochem. 2019, 84, 112–123. [Google Scholar] [CrossRef]
- Huang, J.; Lu, C.; Qian, X.; Huang, Y.; Zheng, Z.; Shen, Y. Effect of salinity on the growth, biological activity and secondary metabolites of some marine fungi. Acta Oceanol. Sin. 2011, 30, 118. [Google Scholar] [CrossRef]
- Cantrell, S.A.; Casillas-Martínez, L.; Molina, M. Characterization of fungi from hypersaline environments of solar salterns using morphological and molecular techniques. Mycol. Res. 2006, 110, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Salama, K.H. Cellular basis of salinity tolerance in plants. Environ. Exp. Bot. 2004, 52, 113–122. [Google Scholar] [CrossRef]
- Patil, S.A.; Hagerhall, C.; Gorton, L. Electron transfer mechanisms between microorganisms and electrodes in bio electrochemical systems. Bioanal. Rev. 2012, 4, 159–192. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011, 28, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Effects of methylene blue and methyl red mediators on performance of yeast based microbial fuel cells adopting polyethylenimine coated carbon felt as anode. J. Power Sources 2018, 396, 1–11. [Google Scholar] [CrossRef]
- Mao, L.; Verwoerd, W.S. Selection of organisms for systems biology study of microbial electricity generation: A review. Int. J. Energy Environ. Eng. 2013, 4, 17. [Google Scholar] [CrossRef]
- Ganguli, R.; Dunn, B.S. Kinetics of anode reactions for a yeast-catalysed microbial fuel cell. Fuel Cells 2009, 9, 44–52. [Google Scholar] [CrossRef]
- Gunawardena, A.; Fernando, S.; To, F. Performance of a yeast-mediated biological fuel cell. Int. J. Mol. Sci. 2008, 9, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Raghavulu, S.V.; Goud, R.K.; Sarma, P.N.; Mohan, S.V. Saccharomyces cerevisiae as anodic biocatalyst for power generation in biofuel cell: Influence of redox condition and substrate load. Bioresour. Technol. 2011, 102, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Schaetzle, O.; Barriere, F.; Baronian, K. Bacteria and yeasts as catalysts in microbial fuel cells: Electron transfer from micro-organisms to electrodes for green electricity. Energy Environ. Sci. 2008, 1, 607–620. [Google Scholar] [CrossRef]
- Hubenova, Y.; Mitov, M. Extracellular electron transfer in yeast-based biofuel cells: A review. Bioelectrochemistry 2015, 106, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Haslett, N.D.; Rawson, F.J.; Barriere, F.; Kunze, G.; Pasco, N.; Gooneratne, R.; Baronian, K.H.R. Characterisation of yeast microbial fuel cell with the yeast Arxula adeninivorans as the biocatalyst. Biosens. Bioelectron. 2011, 26, 3742–3747. [Google Scholar] [CrossRef]
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- Hanaki, K.; Portugal-Pereira, J. The Effect of Biofuel Production on Greenhouse Gas Emission Reductions. In Biofuels and Sustainability. Science for Sustainable Societies; Takeuchi, K., Shiroyama, H., Saito, O., Matsuura, M., Eds.; Springer: Tokyo, Japan, 2018; pp. 53–71. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of second generation bioethanol production from residual biomass. Food Technol. Biotech. 2018, 56, 174. [Google Scholar] [CrossRef]
- Shkil, H.; Schulte, A.; Guschin, D.A.; Schuhmann, W. Electron transfer between genetically modified hansenula polymorpha yeast cells and electrode surfaces via complex modified redox polymers. Chem. Phys. Chem. 2011, 12, 806–813. [Google Scholar] [CrossRef]
- Prasad, D.; Arun, S.; Murugesan, M.; Padmanaban, S.; Satyanarayanan, R.S.; Berchmans, S.; Yegnaraman, V. Direct electron transfer with yeast cells and construction of a mediatorless microbial fuel cell. Biosens. Bioelectron. 2007, 22, 2604–2610. [Google Scholar] [CrossRef]
- Gal, I.; Schlesinger, O.; Amir, L.; Alfonta, L. Yeast surface display of dehydrogenases in microbial fuel-cells. Bioelectrochemistry. 2016, 112, 53–60. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Yakhmi, J.V. Microbial fuel cells to recover heavy metals. Environ. Chem. Lett. 2014, 12, 483–494. [Google Scholar] [CrossRef]
- Miskan, M.; Ismail, M.; Ghasemi, M.; Md Jahim, J.; Nordin, D.; Abu Bakar, M.H. Characterization of membrane biofouling and its effect on the performance of microbial fuel cell. Int. J. Hydrog. Energy 2016, 41, 543–552. [Google Scholar] [CrossRef]
- Darvishi, Y.; Hassan-Beygi, S.R.; Zarafshan, P.; Hooshyari, K.; Malaga-Toboła, U.; Gancarz, M. Numerical Modeling and Evaluation of PEM Used for Fuel Cell Vehicles. Materials 2021, 14, 7907. [Google Scholar] [CrossRef]
- Woo, S.; Lee, S.; Taning, A.Z.; Yang, T.H.; Park, S.H.; Yim, S.D. Current understanding of catalyst/ionomer interfacial structure and phenomena affecting the oxygen reduction reaction in cathode catalyst layers of proton exchange membrane fuel cells. Curr. Opin. Electrochem. 2020, 21, 289–296. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. B Biol. Sci. 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336. [Google Scholar] [CrossRef]
- Sayed, E.T.; Tsujiguchi, T.; Nakagawa, N. Catalytic activity of baker’s yeast in a mediatorless microbial fuel cell. Bioelectrochemistry 2012, 86, 97–101. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Ho, C.H.; Yi, J.; Wang, X. Biocatalytic continuous manufacturing of diabetes drug: Plantwide process modeling, optimization, and environmental and economic analysis. ACS Sustain. Chem. Engineer. 2018, 7, 1038–1051. [Google Scholar] [CrossRef]
- Kim, I.S.; Chae, K.J.; Choi, M.J.; Verstraete, W.; Kim, I.S.; Chae, K.J.; Choi, M.J.; Verstraete, W. Microbial fuel cells: Recent advances, bacterial communities and application beyond electricity generation. Environ. Eng. Res. 2008, 13, 51–65. [Google Scholar] [CrossRef]
- Nimje, V.R.; Chen, C.Y.; Chen, H.R.; Chen, C.C.; Huang, Y.M.; Tseng, M.J.; Cheng, K.C.; Chang, Y.F. Comparative bioelectricity production from various wastewaters in microbial fuel cells using mixed cultures and a pure strain of Shewanella oneidensis. Bioresour. Technol. 2012, 104, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Mustakeem, M. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4, 1–11. [Google Scholar] [CrossRef]
- Richter, H.; McCarthy, K.; Nevin, K.P.; Johnson, J.P.; Rotello, V.M.; Lovley, D.R. Electricity generation by geobacter sulfurreducens attached to gold electrodes. Langmuir 2008, 24, 4376–4379. [Google Scholar] [CrossRef]
- Watanabe, K. Recent developments in microbial fuel cell technologies for sustainable bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. [Google Scholar] [CrossRef]
- Wu, D.; Yi, X.; Tang, R.; Feng, C.; Wei, C. Single microbial fuel cell reactor for coking wastewater treatment: Simultaneous carbon and nitrogen removal with zero alkaline consumption. Sci. Total Environ. 2018, 621, 497–506. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.J.; Lee, J.W.; Kim, K.Y.; Kim, I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Qu, Y.; Feng, Y. Enhanced Shewanella oneidensis MR-1 anode performance by adding fumarate in microbial fuel cell. Chem. Engineer. J. 2017, 328, 697–702. [Google Scholar] [CrossRef]
- Santoro, C.; Babanova, S.; Artyushkova, K.; Cornejo, J.A.; Ista, L.; Bretschger, O.; Marsili, E.; Atanassov, P.; Schuler, A.J. Influence of anode surface chemistry on microbial fuel cell operation. Bioelectrochemistry 2015, 106, 141–149. [Google Scholar] [CrossRef]
- Xu, H.; Quan, X.; Xiao, Z.; Chen, L. Effect of anodes decoration with metal and metal oxides nanoparticles on pharmaceutically active compounds removal and power generation in microbial fuel cells. Chem. Engineer. J. 2018, 335, 539–547. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Abu-Reesh, I.M.; Kondaveeti, S.; Al-Raoush, R.I.; He, Z. Enhanced treatment of petroleum refinery wastewater by short-term applied voltage in single chamber microbial fuel cell. Bioresour. Technol. 2018, 253, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.A.; Ghadge, A.N.; Ghangrekar, M.M. Simultaneous organic matter removal and disinfection of wastewater with enhanced power generation in microbial fuel cell. Bioresour. Technol. 2014, 163, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of photobioreactors for mass cultivation of photosynthetic organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Erable, B.; Bergel, A. First air-tolerant effective stainless steel microbial anode obtained from a natural marine biofilm. Bioresour. Technol. 2009, 100, 3302–3307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Utomo, H.D.; Yu, L.S.; Yi, D.C.Z.; Jun, O.J. Recycling solid waste and bioenergy generation in MFC dual-chamber model. Energy Procedia 2017, 143, 424429. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 40404046. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, W.; Zhang, X.; Tao, G. Electricity generation in a membrane-less microbial fuel cell with down-flow feeding onto the cathode. Bioresour. Technol. 2011, 102, 73247328. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.H.; Kokko, M.E.; Puhakka, J.A. Power generation in fed-batch and continuous up-flow microbial fuel cell from synthetic wastewater. Energy 2015, 91, 235241. [Google Scholar] [CrossRef]
- Chang, S.H.; Wu, C.H.; Wang, R.C.; Lin, C.W. Electricity production and benzene removal from groundwater using low-cost mini tubular microbial fuel cells in a monitoring well. J. Environ. Manag. 2017, 193, 551557. [Google Scholar] [CrossRef]
- Yazdi, H.; Alzate-Gaviria, L.; Ren, Z.J. Pluggable microbial fuel cell stacks for septic wastewater treatment and electricity production. Bioresour. Technol. 2015, 180, 258263. [Google Scholar] [CrossRef]
- Lu, M.; Chen, S.; Babanova, S.; Phadke, S.; Salvacion, M.; Mirhosseini, A.; Chan, S.; Carpenter, K.; Cortese, R.; Bretschger, O. Long-term performance of a 20-L continuous flow microbial fuel cell for treatment of brewery wastewater. J. Power Sources 2017, 356, 274287. [Google Scholar] [CrossRef]
- Tremouli, A.; Greenman, J.; Ieropoulos, I. Investigation of ceramic MFC stacks for urine energy extraction. Bioelectrochemistry 2018, 123, 1925. [Google Scholar] [CrossRef]
- Min, B.; Cheng, S.; Logan, B.E. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 2005, 39, 16751686. [Google Scholar] [CrossRef] [PubMed]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Sudoh, R.; Islam, M.S.; Sazawa, K.; Okazaki, T.; Hata, N.; Taguchi, S.; Kuramitz, H. Removal of dissolved humic acid from water by coagulation method using polyaluminum chloride (PAC) with calcium carbonate as neutralizer and coagulant aid. J. Environ. Chem. Eng. 2015, 3, 770–774. [Google Scholar] [CrossRef]
- Gautami, G.; Khanam, S. Selection of optimum configuration for multiple effect evaporator system. Desalination 2012, 288, 16–23. [Google Scholar] [CrossRef]
- Serpone, N.; Artemev, Y.M.; Ryabchuk, V.K.; Emeline, A.V.; Horikoshi, S. Lightdriven advanced oxidation processes in the disposal of emerging pharmaceutical contaminants in aqueous media: A brief review. Curr. Opin. Green Sustain. Chem. 2017, 6, 18–33. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166, 603–643. [Google Scholar] [CrossRef]
- Harrison, R.M. (Ed.) Pollution: Causes, Effects and Control, 3rd ed.; Royal Society of Chemistry: Cambridge, UK, 2001. [Google Scholar]
- Robinson, T.; Chandran, B.; Nigam, P. Studies on the production of enzymes by white rot-fungi for the decolorisation of textile dyes. Enzym. Microb. Technol. 2001, 29, 575–579. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; Fernandes, D.L.A.; Tavares, A.P.M.; Xavier, A.B.M.R.; Cammarota, M.C.; Coutinho, J.A.P.; Coelho, M.A.Z. Decolorization of dyes from textile wastewater by Trametes versicolor. Environ. Technol. 2004, 25, 1313–1320. [Google Scholar] [CrossRef]
- Qu, J.; Xu, Y.; Ai, G.M.; Liu, Y.; Liu, Z.P. Novel Chryseobacterium sp. PYR2 degrades various organochlorine pesticides (OCPs) and achieves enhancing removal and complete degradation of DDT in highly contaminated soil. J. Environ. Manag. 2015, 161, 350–357. [Google Scholar] [CrossRef]
- Zinovicius, A.; Rozene, J.; Merkelis, T.; Bruzaite, I.; Ramanavicius, A.; Morkvenaite-Vilkonciene, I. Evaluation of a yeast–polypyrrole biocomposite used in microbial fuel cells. Sensors 2022, 22, 327. [Google Scholar] [CrossRef] [PubMed]
- Moradian, J.M.; Mi, J.L.; Dai, X.; Sun, G.F.; Du, J.; Ye, X.M.; Yong, Y.C. Yeast-induced formation of graphene hydrogels anode for efficient xylose-fueled microbial fuel cells. Chemosphere 2022, 291, 132963. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sehgal, R.; Gupta, R. Microbes and wastewater treatment. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 239–255. [Google Scholar]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q. Peroxidase mediated decolorization and remediation of wastewater containing industrial dyes: A review. Rev. Environ. Sci. Biotechnol. 2010, 9, 117–140. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.A.; Ramli, S.; Mamat, N.H.; Khan, S.; Gomes, C. Chemical and biosensor technologies for wastewater quality management. Int. J. Adv. Res. Publ. 2017, 1, 1–10. [Google Scholar]
- Hamza, R.A.; Iorhemen, O.T.; Tay, J.H. Advances in biological systems for the treatment of high-strength wastewater. J. Water Process Engineer. 2016, 10, 128–142. [Google Scholar] [CrossRef]
- Arfin, T.; Sonawane, K.; Saidankar, P.; Sharma, S. Role of microbes in the bioremediation of toxic dyes. Integ. Green Chem. Sustain. Eng. 2019, 26, 443–472. [Google Scholar]
- Behera, B.C. Citric acid from Aspergillus niger: A comprehensive overview. Crit. Rev. Microbiol. 2020, 46, 727–749. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; Cabanillas-Chirinos, L.; Nazario-Naveda, R.; Gallozzo-Cardenas, M.; Diaz, F.; Delfin-Narciso, D.; Rojas-Villacorta, W. Use of Tangerine Waste as Fuel for the Generation of Electric Current. Sustainability 2023, 15, 3559. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Angayarkanni, J.; Das, A.; Palaniswamy, M. Mycoremediation of Benzo [a] pyrene by Pleurotus ostreatus isolated from Wayanad district in Kerala, India. Int. J. Pharm. Bio. Sci. 2012, 2, 84–93. [Google Scholar]
- Bhatt, M.; Cajthaml, T.; Šašek, V. Mycoremediation of PAH-contaminated soil. Folia Microbiol. 2002, 47, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Okparanma, R.N.; Ayotamuno, J.M.; Davis, D.D.; Allagoa, M. Mycoremediation of polycyclic aromatic hydrocarbons (PAH)-contaminated oil-based drill-cuttings. Afr. J. Biotech. 2013, 10, 5149–5156. [Google Scholar]
- Peng, R.H.; Xiong, A.S.; Xue, Y.; Fu, X.Y.; Gao, F.; Zhao, W.; Tian, Y.S.; Yao, Q.H. Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microb. Rev. 2008, 32, 927–955. [Google Scholar] [CrossRef] [PubMed]
- Roorer, G.L.; Hsien, T.Y.; Way, J.D. Synthesis of porous-magnetic chitosan beads for removal of cadmium ions from wastewater. Ind. Eng. Chem. Res. 1993, 32, 2170–2178. [Google Scholar] [CrossRef]
- Akar, T.; Divriklioglu, M. Biosorption applications of modified fungal biomass for decolorization of reactive red 2 contaminated solutions, batch and dynamic flow mode studies. Bioresour. Technol. 2010, 101, 7271–7277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Deng, S.; Yu, G.; Huang, J. Removal of perfluorooctane sulfonate from aqueous solution by crosslinked chitosan beads, sorption kinetics and uptake mechanism. Bioresour. Technol. 2011, 102, 2265–2271. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Wang, A. Enhanced adsorption of methylene blue from aqueous solution by chitosan-g-poly, (acrylic acid)/vermiculite hydrogel composites. J. Environ. Sci. 2010, 22, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Wesenberg, D.; Kyriakides, I.; Agathos, S.N. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol. Adv. VI Int. Symp. Environ. Biotechnol. 2003, 22, 161–187. [Google Scholar] [CrossRef]
- Ghosh Ray, S.; Ghangrekar, M.M. Comprehensive review on treatment of high-strength distillery wastewater in advanced physico-chemical and biological degradation pathways. Int. J. Environ. Sci. Tech. 2019, 16, 527–546. [Google Scholar] [CrossRef]
- Rauf, M.A.; Salman Ashraf, S. Survey of recent trends in biochemically assisted degradation of dyes. Chem. Eng. J. 2012, 209, 520–530. [Google Scholar] [CrossRef]
- Adelaja, O.; Keshavarz, T.; Kyazze, G. The effect of salinity, redox mediators and temperature on anaerobic biodegradation of petroleum hydrocarbons in microbial fuel cells. J. Hazard Mater. 2015, 283, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Shleev, S.; Tkac, J.; Christenson, A.; Ruzgas, T.; Yaropolov, A.I.; Whittaker, J.W.; Gorton, L. Direct electron transfer between copper-containing proteins and electrodes. 20th Anniversary of Biosensors and Bioelectronics. Biosens. Bioelectron. 2005, 20, 2517–2554. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Pontié, M.; Younesi, H.; Nacef, M.; Rahimpour, A.; Rahimnejad, M.; Bouchenak Khelladi, R.M. Biodegradation of acetaminophen and its main by-product 4-aminophenol by Trichoderma harzianum versus mixed biofilm of Trichoderma harzianum/Pseudomonas fluorescens in a fungal microbial fuel cell. J. Appl. Electrochem. 2021, 51, 581–596. [Google Scholar] [CrossRef]
- Wilkinson, S.; Klar, J.; Applegarth, S. Optimizing biofuel cell performance using a targeted mixed mediator combination. Electroanalysis 2006, 18, 2001–2007. [Google Scholar] [CrossRef]
- Leonowicz, A.; Cho, N.; Luterek, J.; Wilkolazka, A.; Wojtas-Wasilewska, M.; Matuszewska, A.; Hofrichter, M.; Wesenberg, D.; Rogalski, J. Fungal laccase: Properties and activity on lignin. J. Basic Microbiol. 2001, 41, 185–227. [Google Scholar] [CrossRef]
- Daâssi, D.; Prieto, A.; Zouari-Mechichi, H.; Martínez, M.J.; Nasri, M.; Mechichi, T. Degradation of bisphenol A by different fungal laccases and identification of its degradation products. Int. Biodeterior. Biodegr. 2016, 110, 181–188. [Google Scholar] [CrossRef]
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic decolorization and degradation of azo dyes—A review. Int. Biodeterio. Biodegr. 2015, 104, 21–31. [Google Scholar] [CrossRef]
- Mani, P.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. Decolourisation of Acid orange 7 in a microbial fuel cell with a laccase-based biocathode: Influence of mitigating pH changes in the cathode chamber. Enzym. Microb. Technol. 2017, 96, 170–176. [Google Scholar] [CrossRef]
- Wu, C.; Liu, X.W.; Li, W.W.; Sheng, G.P.; Zang, G.L.; Cheng, Y.Y.; Shen, N.; Yang, Y.P.; Yu, H.Q. A white rot fungus is used as a biocathode to improve electricity production of a microbial fuel cell. Appl. Energy 2012, 98, 594–596. [Google Scholar] [CrossRef]
- Strong, P.J. Fungal remediation of Amarula distillery wastewater. World J. Microbiol. Biotechnol. 2010, 26, 133–144. [Google Scholar] [CrossRef]
- Strong, P.J.; Burgess, J.E. Fungal and enzymatic remediation of a wine lees and five wine-related distillery wastewaters. Bioresour. Technol. 2008, 99, 6134–6142. [Google Scholar] [CrossRef] [PubMed]
- Dunford, H.B.; Stillman, J.S. On the function and mechanism of action of peroxidases. Coord. Chem. Rev. 1976, 19, 187–251. [Google Scholar] [CrossRef]
- Wang, J.; Majima, N.; Hirai, H.; Kawagishi, H. Effective removal of endocrine-disrupting compounds by lignin peroxidase from the white-rot fungus Phanerochaete sordida YK-624. Curr. Microbiol. 2012, 64, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Zamocky, M.; Jakopitsch, C.; Furtmüller, P.G.; Dunand, C.; Obinger, C. The peroxidase–cyclooxygenase superfamily: Reconstructed evolution of critical enzymes of the innate immune system. Proteins 2008, 72, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Abubackar, H.N.; Biryol, I.; Ayol, A. Yeast industry wastewater treatment with microbial fuel cells: Effect of electrode materials and reactor configurations. Int. J. Hydrog. Energy 2023, 48, 12424–12432. [Google Scholar] [CrossRef]
- Bhagchandanii, D.D.; Babu, R.P.; Khanna, N.; Pandit, S.; Jadhav, D.A.; Khilari, S.; Prasad, R. A Comprehensive Understanding of Electro-Fermentation. Fermentation 2020, 6, 92. [Google Scholar] [CrossRef]
- Langhals, H. Color chemistry. In Synthesis, Properties and Applications of Organic Dyes and Pigments, 3rd ed.; Heinrich, Z., Ed.; VCH: Weinheim, Germany, 2004; pp. 5291–5292. [Google Scholar]
- Martins, M.; Santos, J.M.; Diniz, M.S.; Ferreira, A.M.; Costa, M.H.; Costa, P.M. Effects of carcinogenic versus non-carcinogenic AHR-active PAHs and their mixtures: Lessons from ecological relevance. Environ. Res. 2015, 138, 101–111. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Sridharan, P.V. (Eds.) Looking Back to Think Ahead: GREEN India 2047 (Growth with Resource Enhancement of Environment and Nature); TERI: New Delhi, India, 1998. [Google Scholar]
- Burkina, V.; Zlabek, V.; Zamaratskaia, G. Effects of pharmaceuticals present in aquatic environment on Phase I metabolism in fish. Environ. Toxicol. Pharmacol. 2015, 40, 430–444. [Google Scholar] [CrossRef]
- Chaalal, O.; Zekri, A.Y.; Soliman, A.M. A novel technique for the removal of strontium from water using thermophilic bacteria in a membrane reactor. J. Ind. Eng. Chem. 2015, 21, 822–827. [Google Scholar] [CrossRef]
- Liu, H.; Guo, S.; Jiao, K.; Hou, J.; Xie, H.; Xu, H. Bioremediation of soils contaminated with heavy metals and 2,4,5-trichlorophenol by fruiting body of Clitocybe maxima. J. Hazard Mater. 2015, 294, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Alcala, I.; Guillén-Navarro, J.M.; Fernández-López, C. Pharmaceutical biological degradation, sorption and mass balance determination in a conventional activated-sludge wastewater treatment plant from Murcia, Spain. Chem. Eng. J. 2017, 316, 332–340. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Peng, J.; Wu, X.; Gao, S.; Mao, L. The effect of dissolved organic matter on soybean peroxidase-mediated removal of triclosan in water. Chemosphere. 2017, 172, 399–407. [Google Scholar] [CrossRef]
- Momoh, O.L.Y. A novel electron acceptor for microbial fuel cells: Nature of circuit connection on internal resistance. J. Biochem. Technol. 2011, 2, 216–220. [Google Scholar]
- Lu, M.; Li, F.Y. Cathode reactions and applications in microbial fuel cells: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2504–2525. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Microbial fuel cells-challenges and applications. Environ. Sci. Technol. 2006, 40, 5172–5180. [Google Scholar] [CrossRef] [PubMed]
- He, C.S.; Mu, Z.X.; Yang, H.Y.; Wang, Y.Z.; Mu, Y.; Yu, H.Q. Electron acceptors for energy generation in microbial fuel cells fed with wastewaters: A mini-review. Chemosphere 2015, 140, 12–17. [Google Scholar] [CrossRef]
- Hernandez-Fernandez, F.J.; Perez de los Ríos, A.; Mateo-Ramírez, F.; Juarez, M.D.; Lozano-Blanco, L.J.; Godínez, C. New application of supported ionic liquids membranes as proton exchange membranes in microbial fuel cell for wastewater treatment. Chem. Eng. J. 2015, 279, 115–119. [Google Scholar] [CrossRef]
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Liew, K.B.; Ismail, M. Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: A comprehensive review. Renew. Sustain. Energy Rev. 2013, 28, 575–587. [Google Scholar] [CrossRef]
- Xie, S.; Lawlor, P.G.; Frost, J.P.; Hu, Z.; Zhan, X. Effect of pig manure to grass silage ratio on methane production in batch anaerobic co-digestion of concentrated pig manure and grass silage. Bioresour. Technol. 2011, 102, 5728–5733. [Google Scholar] [CrossRef]
- Mohana, S.; Acharya, B.K.; Madamwar, D. Bioremediation concepts for treatment of distillery effluent. In Biotechnology for Environmental Management and Resource Recovery; Springer: New Delhi, India, 2013; pp. 261–278. [Google Scholar]
- Fernandez de Dios, M.A.; del Campo, A.G.; Fernandez, F.J.; Rodrigo, M.; Pazos, M.; Sanroman, M.A. Bacterial–fungal interactions enhance power generation in microbial fuel cells and drive dye de colorization by an ex situ and in situ electroFenton process. Bioresour. Technol. 2013, 148, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Liew, K.B.; Daud, W.R.W.; Ghasemia, M.; Leong, J.X.; Lim, S.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrog. Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.E. Microbial fuel cell as new technology for bioelectricity generation: A review. Alexandr. Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef]
- Modestra, J.A.; Chiranjeevi, P.; Mohan, S.V. Cathodic material effect on electron acceptance towards bioelectricity generation and wastewater treatment. Renew. Energy 2016, 98, 178–187. [Google Scholar] [CrossRef]
- Kalathil, S.; Patil, S.A.; Pant, D. Microbial fuel cells: Electrode materials. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Vadgama, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 309–318. [Google Scholar] [CrossRef]
- Kazemi, N.; Tavakoli, O.; Seif, S.; Nahangi, M. High-strength distillery wastewater treatment using catalytic sub-and supercritical water. J. Supercrit. Fluids 2015, 97, 74–80. [Google Scholar] [CrossRef]
- Latif, M.A.; Ghufran, R.; Wahid, Z.A.; Ahmad, A. Integrated application of upfow anaerobic sludge blanket reactor for the treatment of wastewaters. Water Res. 2011, 45, 4683–4699. [Google Scholar] [CrossRef] [PubMed]
- Anupama, S.; Pradeep, N.V.; Hampannavar, U.S. Anaerobic followed by aerobic treatment approaches for Spentwash using MFC and RBC. Sugar Tech. 2013, 13, 197–202. [Google Scholar] [CrossRef]
- Apollo, S.; Onyango, M.S.; Ochieng, A. An integrated anaerobic digestion and UV photocatalytic treatment of distillery wastewater. J. Hazard Mater. 2013, 261, 435–442. [Google Scholar] [CrossRef]
- Melamane, X.; Tandlich, R.; Burgess, J. Anaerobic digestion of fungally pre-treated wine distillery wastewater. Afr. J. Biotechnol. 2007, 6, 1990–1993. [Google Scholar]
- Melamane, X.L.; Tandlich, R.; Burgess, J.E. Submerged membrane bioreactor and secondary digestion in the treatment of wine distillery wastewater. Part II: The effect of fungal pre-treatment on wine distillery wastewater digestion. Fresenius Environ. Bull. 2007, 16, 162–167. [Google Scholar]
- Włodarczyk, B.; Włodarczyk, P.P. Analysis of the potential of an increase in yeast output resulting from the application of additional process wastewater in the evaporator station. Appl. Sci. 2019, 9, 2282. [Google Scholar] [CrossRef]
- Rozene, J.; Morkvenaite-Vilkonciene, I.; Bruzaite, I.; Dzedzickis, A.; Ramanavicius, A. Yeast-based microbial biofuel cell-mediated by 9,10- phenantrenequinone. Electrochim. Acta 2021, 373, 137918. [Google Scholar] [CrossRef]
- Que, Y.; Xie, Y.; Li, T.; Yu, C.; Tu, S.; Yao, C. An N-heterocyclic carbene-catalyzed oxidative γ-aminoalkylation of saturated carboxylic acids through in situ activation strategy: Access to δ-lactam. Org. Lett. 2015, 17, 6234–6237.143. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, S.; Moghadas, B.K.; Esmaeili, H.; Khoo, F.S.; Gholami, G.; Ghasemi, M. Improving the surface properties of adsorbents by surfactants and their role in the removal of toxic metals from wastewater: A review study. Process Saf. Environ. Prot. 2021, 148, 775–795. [Google Scholar] [CrossRef]
- Chigusa, K.; Hasegawa, T.; Yamamoto, N.; Watanabe, Y. Treatment of wastewater from oil manufacturing plant by yeasts. Water Sci. Technol. 1996, 34, 51–58. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, M.; Lv, W.; Liu, F. Study on sludge expansion during treatment of salad oil manufacturing wastewater by yeast. Environ. Technol. 2001, 22, 533–542. [Google Scholar] [CrossRef]
- Chaijak, P.; Lertworapreecha, M.; Sukkasem, C. Phenol removal from palm oil mill effluent using Galactomyces reessii termite-associated yeast. Pol. J. Environ. Stud. 2018, 27, 39–44. [Google Scholar] [CrossRef]
- Hubenova, Y.; Georgiev, D.; Mitov, M. Enhanced phytate dephosphorylation by using Candida melibiosica yeast-based biofuel cell. Biotechnol. Lett. 2014, 36, 1993–1997. [Google Scholar] [CrossRef]
- Pal, M.; Shrivastava, A.; Sharma, R.K. Electroactive biofilm development on carbon fiber anode by Pichia fermentans in a wheat straw hydrolysate based microbial fuel cell. Biomass Bioenerg. 2023, 168, 106682. [Google Scholar] [CrossRef]
- Włodarczyk, B.; Włodarczyk, P.P. Electricity Production from Yeast Wastewater in Membrane-Less Microbial Fuel Cell with Cu-Ag Cathode. Energies 2023, 16, 2734. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Biological combination processes for efficient removal of pharmaceutically active compounds from wastewater: A review and future perspectives. J. Environ. Chem. Eng. 2017, 5, 35903603. [Google Scholar] [CrossRef]
- Patneedi, C.B.; Prasadu, K.D. Impact of pharmaceutical wastes on human life and environment. Rasayan J. Chem. 2015, 8, 6770. [Google Scholar]
- Ismail, Z.Z.; Habeeb, A.A. Experimental and modeling study of simultaneous power generation and pharmaceutical wastewater treatment in microbial fuel cell based on mobilized biofilm bearers. Renew. Energy 2017, 101, 12561265. [Google Scholar] [CrossRef]
- Zub, S.; Kurisso, T.; Menert, A.; Blonskaja, V. Combined biological treatment of high-sulphate wastewater from yeast production. Water Environ. J. 2008, 22, 274–286. [Google Scholar] [CrossRef]
- Hossain, S.M. Aerobic treatment of distillery wastewater using Phanerochaete chrysosporium. Indian J. Environ. Prot. 2007, 27, 362–366. [Google Scholar]
- Mullai, P.; Sathian, S.; Sabarathinam, P.L. Kinetic modelling of distillery wastewater biodegradation using Paecilomyces variotii. Chem. Eng. World 2007, 42, 98–106. [Google Scholar]
- Ray, S.G.; Ghangrekar, M.M. Enhancing organic matter removal, biopolymer recovery and electricity generation from distillery wastewater by combining fungal fermentation and microbial fuel cell. Bioresour. Technol. 2015, 176, 8–14. [Google Scholar]
- Ravikumar, R.; Vasanthi, N.S.; Saravanan, K. Single factorial experimental design for decolorizing anaerobically treated distillery spent wash using Cladosporium Cladosporioides. Int. J. Environ. Sci. Technol. 2011, 8, 97–106. [Google Scholar] [CrossRef]
- Watanabe, T.; Suzuki, K.; Sato, I.; Morita, T.; Koike, H.; Shinozaki, Y.; Ueda, H.; Koitabashi, M.; Kitamoto, H.K. Simultaneous bioethanol distillery wastewater treatment and xylanase production by the phyllosphere yeast Pseudozyma antarctica GB-4(0). AMB Express 2015, 5, 121. [Google Scholar] [CrossRef]
- Ling, J.; Tian, Y.; de Toledo, R.A.; Shim, H. Cost reduction for the lipid production from distillery and domestic mixed wastewater by Rhodosporidium toruloides via the reutilization of spent seed culture medium. Energy 2016, 136, 135–141. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, C.; Luo, F.; Zhang, C.; Wang, T.; Wei, Q. Optimization of Calvatia gigantea mycelia production from distillery wastewater. J. Inst. Brew. 2015, 121, 78–86. [Google Scholar] [CrossRef]
- Watanabe, T.; Iefuji, H.; Kitamoto, H.K. Treatment of Candida utilis biomass production from shochu wastewater; the effects of maintaining a low pH on DOC removal and feeding cultivation on biomass production. SpringerPlus 2013, 2, 514. [Google Scholar] [CrossRef] [PubMed]
- Melamane, X.L.; Strong, P.J.; Burgess, J.E. Treatment of wine distillery wastewater: A review with emphasis on anaerobic membrane reactors. S. Afr. J. Enol. Vitic. 2007, 28, 25. [Google Scholar] [CrossRef]
- Sheehan, G.J.; Greenfield, P.F. Utilisation, treatment and disposal of distillery wastewater. Water Res. 1980, 14, 257–277. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Fujimura, Y.; Shigematsu, T.; Morimura, S.; Kida, K. Anaerobic treatment performance and microbial population of thermophilic upflow anaerobic filter reactor treating awamori distillery wastewater. J. Biosci. Bioeng. 2007, 104, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kongthale, G.; Sotha, S.; Michu, P.; Madloh, A.; Wetchapan, P.; Chaijak, P. Electricity Production and Phenol Removal of Winery Wastewater by Constructed Wetland—Microbial Fuel Cell Integrated with Ethanol Tolerant Yeast. Biointerface Res. Appl. Chem. 2023, 13, 157. [Google Scholar]
- Chen, S.H.; Yien Ting, A.S. Biodecolorization and biodegradation potential of recalcitrant triphenylmethane dyes by Coriolopsis sp. isolated from compost. J. Environ. Manag. 2015, 150, 274–280. [Google Scholar] [CrossRef]
- Chen, S.H.; Yien Ting, A.S. Biosorption and biodegradation potential of triphenylmethane dyes by newly discovered Penicillium simplicissimum isolated from indoor wastewater sample. Int. Biodeterior. Biodegr. 2015, 103, 1–7. [Google Scholar] [CrossRef]
- Raqba, R.; Rafaqat, S.; Ali, N.; Munis, M.F.H. Biodegradation of Reactive Red 195 azo dye and Chlorpyrifos organophosphate along with simultaneous bioelectricity generation through bacterial and fungal based biocathode in microbial fuel cell. J. Water Process Eng. 2022, 50, 103177. [Google Scholar] [CrossRef]
- Pal, M.; Shrivastava, A.; Sharma, R.K. Wheat straw-based microbial electrochemical reactor for azo dye decolorization and simultaneous bioenergy generation. J. Environ. Manag. 2022, 323, 116253. [Google Scholar] [CrossRef]
- Rawat, D.; Sharma, R.S.; Karmakar, S.; Arora, L.S.; Mishra, V. Ecotoxic potential of a presumably non-toxic azo dye. Ecotoxicol. Environ. Saf. 2018, 148, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Kadir, W.N.A.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T. Harvesting and pre-treatment of microalgae cultivated in wastewater for biodiesel production: A review. Energy Conv. Manag. 2018, 171, 1416–1429. [Google Scholar] [CrossRef]
- Mani, A.; Hameed, S.A.S. Improved bacterial-fungal consortium as an alternative approach for enhanced decolourization and degradation of azo dyes: A review. Nat. Environ. Pollut. Technol. 2019, 18, 49–64. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Boddula, R.; Pothu, R. Microbial fuel cells: Technologically advanced devices and approach for sustainable/renewable energy development. Energ. Conver. Manag. X. 2022, 13, 100160. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kim, Y.; Logan, B.E. Energy capture from thermolytic solutions in microbial reverse-electrodialysis cells. Science 2012, 335, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).