Abstract
In this study, Co-modified alkalinized g-C3N4 (named Co-CNK-OH) was prepared for the Fenton-like photocatalytic degradation of tetracycline (TC) via a simple yet effective calcination–impregnation method. In all samples of CNK-OH with different Co2+ loadings, Co-CNK-OH catalyst with the optimal content (9%) exhibited the highest catalytic activity, with 87.1% tetracycline removal and 50% removal efficiency of the total organic carbon (TOC). Mechanism studies revealed that the 9%Co-CNK-OH catalyst had the lower electrical resistance after alkalization treatment and Co2+ modification, leading to a significantly accelerated interfacial charge transfer to the electron acceptor as well as effectively separating electrons and holes. The intermediates generated during the TC degradation in the photo-Fenton process were detected by HPLC-MS, which proved that the holes, superoxide radicals, and singlet oxygen are the key reactive species in the Fenton-like photocatalysis. This study provides a new option for the treatment of TC in wastewater.
1. Introduction
In recent years, an endless variety of antibiotics has been synthesized and manufactured as therapeutic drugs to treat infectious diseases in humans, animals, and even plants around the world [1]. Antibiotic-induced sewage pollution is highly concerned as a large quantity of antibiotics has entered water bodies with the widespread application of antibiotics. Tetracycline (TC), which is widely used to treat diseases in livestock animals, is difficult to degrade in nature, resulting in the continuous release and accumulation of TC in the natural water environment [2,3]. Therefore, it is urgent to develop more effective methods for degrading tetracycline in wastewater treatment technologies.
Common methods used to degrade TC fall into several categories, such as biological treatment, adsorption and desorption, membrane treatment, and advanced oxidation technologies [4,5,6,7]. Among the advanced oxidation technologies, the Fenton process is currently a popular method of water-pollution treatment. However, the classical Fenton process has many limitations on its practical applications, such as a relatively narrow pH range and heavy iron deposition, leading to increased treatment costs and low efficiency in the utilization of H2O2 [8,9]. Thus, Fenton-like technologies have increasingly become alternative choices [10,11,12].
Graphite-like carbon nitride (g-C3N4), a common two-dimensional (2D) material, has attracted extensive research attention in the field of photocatalysis because of its non-toxicity, low cost, good stability, and visible-light response [13,14,15]. Moreover, g-C3N4 can stimulate the generation of hydroxyl radicals (∙OH) from H2O2 and is often used as a Fenton-like catalyst [16]. However, the rapid recombination of electron–hole pairs in g-C3N4 limits its application on the degradation of organic pollutants [17]. To solve this problem, Thi Quyen et al. [18] improved the light absorption behavior by doping S in g-C3N4, which can effectively reduce the recombination rate of electron–hole pairs in g-C3N4. Bao et al. [19] prepared Cu-doped g-C3N4 by a simple template-mediated supramolecular-self-assembly method, in which the specific surface area of the g-C3N4 was considerably increased while achieving enhanced light absorption and higher separation and transfer efficiency of the photogenerated carriers. The energy bandgap in g-C3N4 can be adjusted by doping with metallic or nonmetallic elements to prevent the recombination of electrons and holes [14,18,19,20,21,22]. Alkalinization treatment is an important surface/interface modulation tool to reduce the energy bandgap by grafting hydroxyl groups, which can inhibit the recombination of photogenerated electrons and holes and improve the Fenton-like catalytic activity, with limited improvement in the photocatalytic performance [23,24,25]. The combination of alkalization treatment and metal-ion modification can effectively enhance the performance of photocatalysis. For example, Cong et al. [26] improved the specific surface area and electrochemical activity by embedding Co ions in g-C3N4, and the produced Co-g-C3N4 showed excellent catalytic performance in the PMS-activated degradation of TC.
To the best of our knowledge, a Co-doped g-C3N4 alkalized catalyst has never been reported. In this work, a novel Co2+-doped g-C3N4 alkalized catalyst (Co-CNK-OH) was prepared by a simple calcination–impregnation approach. On the one hand, the basic treatment can transplant numerous hydroxyl groups on the surface of the g-C3N4 to generate active hydroxyl radicals and decrease the energy bandgap of the g-C3N4. On the other hand, the doping of metal ions can significantly improve the Fenton-like catalytic activity of the g-C3N4 through the variable valence state of the metal ions. Compared with similar products, the Co-CNK-OH catalyst exhibits superior degradation performance for TC under visible-light irradiation. This study provides a new option for the treatment of tetracycline in wastewater.
2. Results
2.1. Characterization of As-Prepared Samples
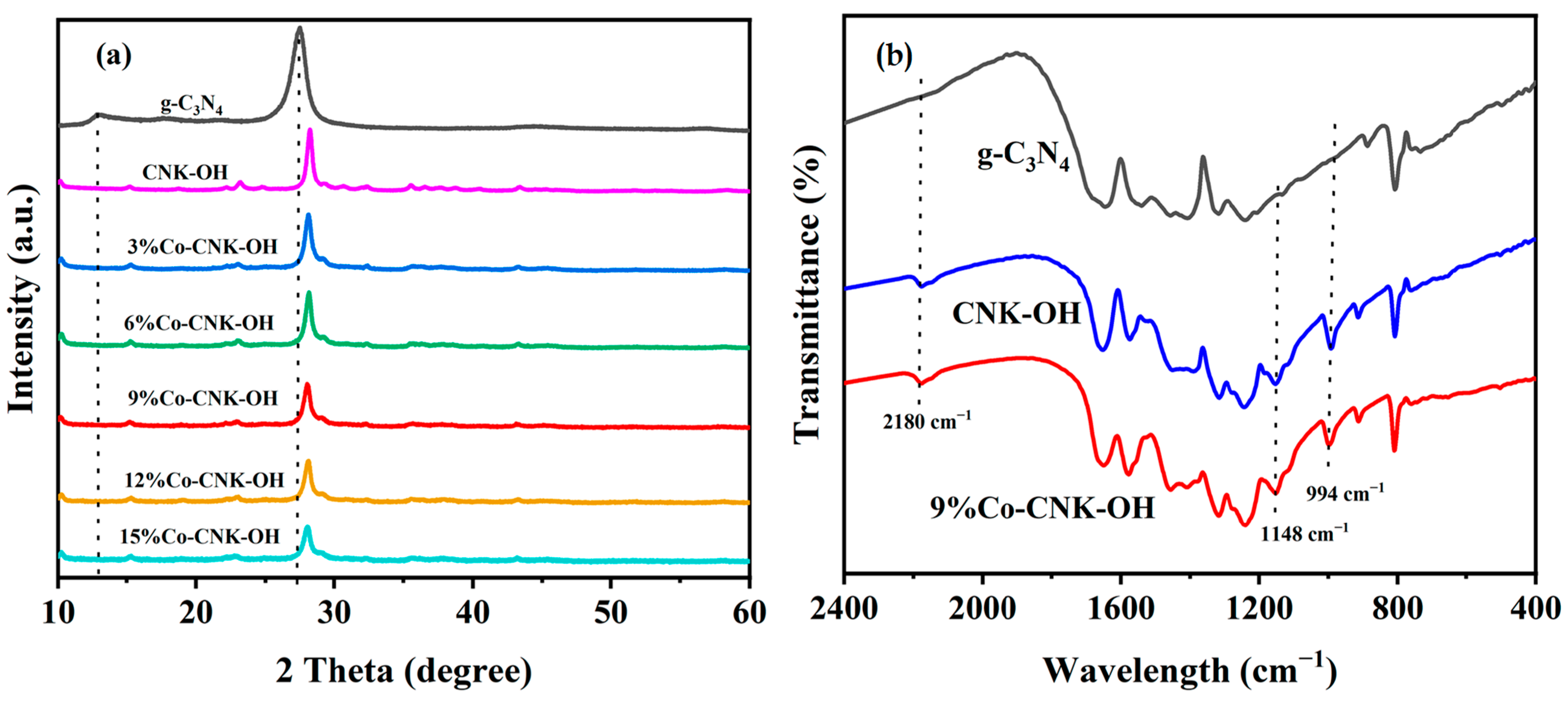
Figure 1a shows the XRD spectra of the as-prepared g-C3N4, CNK-OH, and Co-CNK-OH heterojunctions with different Co contents. Two typical characteristic peaks for g-C3N4 were found at 13.0° and 27.4°, corresponding to the (100) and (200) crystal planes of the g-C3N4, respectively [27]. The (100) plane at 13.0° disappeared compared with that of the pure g-C3N4 when the g-C3N4 was alkalized with KCl and NH4Cl, implying that K+ ions were embedded into the g-C3N4 plane and an attraction force between K and N atoms. This was consistent with the results of the K 2p XPS spectra [28]. As for the (200) plane, a slight shift in the peak from 27.4° to 28.1° for the CNK-OH indicated a smaller interlayer spacing of the (200) plane in the CNK-OH [16]. The intensity of the diffraction peak gradually decreased with increasing Co2+ content, indicating that Co2+ was successfully doped in the framework of the g-C3N4 and that the Co-doping partly damaged the in-plane structure of the g-C3N4 [29]. The XRD diffraction peak of the CNK-OH was basically unchanged, even with Co2+ ions loading, indicating that Co2+ ions might have been embedded in the center of the triazine ring unit [30,31].
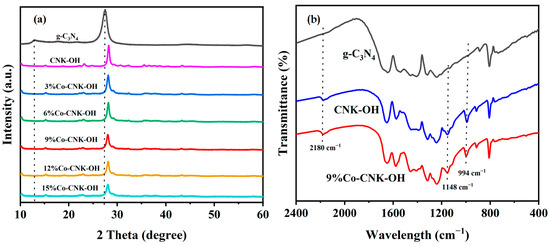
Figure 1.
The XRD patterns of g-C3N4, CNK-OH and Co-CNK-OH (a) and FT-IR spectra of g-C3N4, CNK-OH, and 9%Co-CNK-OH (b).
FT-IR was used to investigate the chemical structures and compositions of the g-C3N4, CNK-OH, and 9%Co-CNK-OH materials (Figure 1b), and characteristic peaks at 800 cm−1 and between 1200 and 1700 cm−1 were observed for all the samples. The peak at 800 cm−1 was attributed to the typical vibration of the repeating triazine unit. The absorption band at 1200-1700 cm−1 could be assigned to the stretching vibrations of the aromatic CN heterocycles in the g-C3N4 [32]. Compared with the peaks for the g-C3N4, three additional peaks appeared at ~994 cm−1, 1148 cm−1, and 2180 cm−1 for the CNK-OH and 9%Co-CNK-OH by the introduction of K+ ions. The peaks at 994 cm−1 and 1148 cm−1 corresponded to the vibrational modes of the O-H and C-O or C=O bonds, respectively, verifying that hydroxyl groups were effectively grafted on the surface of the g-C3N4 by this alkalinization modification method [27]. The band at 2180 cm−1 belonged to the cyano group (C≡N), which suggested that the introduction of K+ ions ruptured the C-N-C bonds in the g-C3N4 to form new C≡N bonds [16]. The FT-IR spectrum of the 9%Co-CNK-OH sample was similar to that of the CNK-OH sample, and no evident change was found because of the metal modification.
2.2. Morphology and Structural Properties
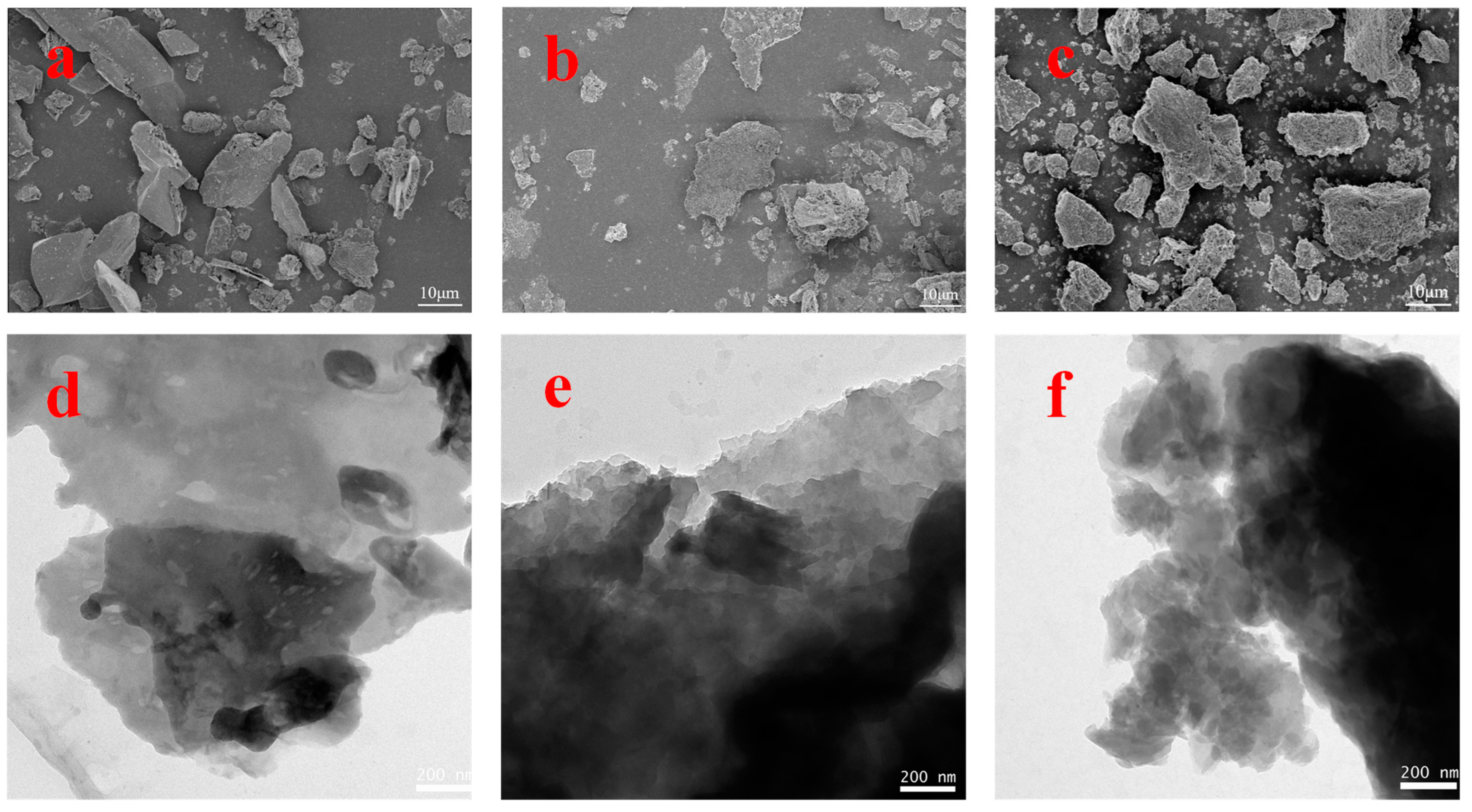
To investigate the microstructures of the g-C3N4, CNK-OH, and 9%Co-CNK-OH materials, SEM and TEM images of the as-prepared samples are shown in Figure 2a–f.
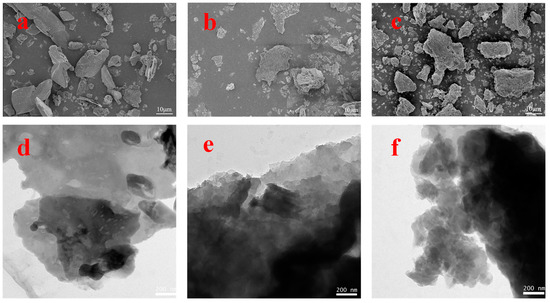
Figure 2.
SEM images of g-C3N4 (a), CNK-OH (b), Co-CNK-OH (c) and TEM images of g-C3N4 (d), CNK-OH (e), and 9%Co-CNK-OH (f).
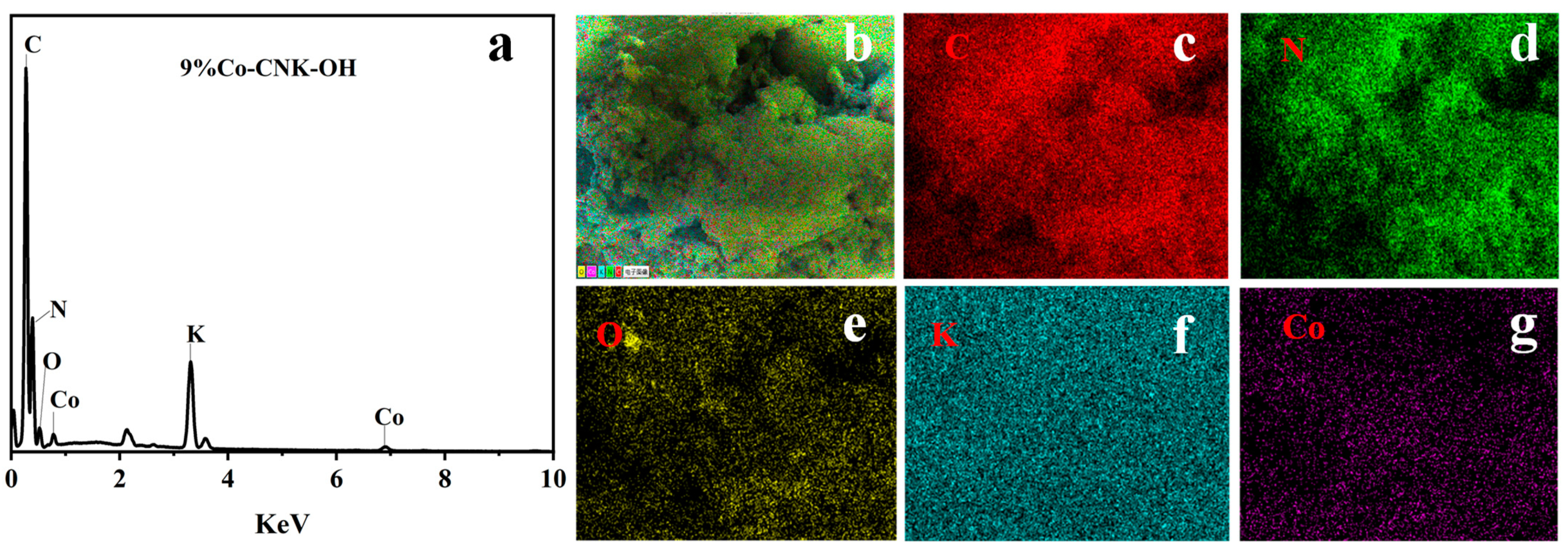
To confirm the elemental distribution, the EDS and elemental mapping images of the Co-CNK-OH are shown (Figure 3a–g). It was obvious that the surface of the g-C3N4 was smooth while the surface of the CNK-OH became rougher with dispersed particles after the alkalization treatment (Figure 2a,b), which may have been due to the influence of the grafted hydroxyl group on the surface of the g-C3N4 [33]. Compared with the CNK-OH and g-C3N4 surfaces, smaller solid particles were observed, and the surface of the Co-CNK-OH was much rougher after the Co2+ modification (Figure 2c). From the microstructure of the CNK-OH, numerous nanosheets on the surface could be clearly seen (Figure 2e). However, the modification with Co2+ resulted in a more fragmented and uniformly dispersed nanosheet structure, in agreement with the SEM analysis results (Figure 2e,f). As for Co-CNK-OH, it was clear that C, N, O, K, and Co were uniformly distributed on the surface of the Co-CNK-OH material in Figure 3c–g, and the previously mentioned evidence implied the formation of Co-CNK-OH heterojunctions at the microscopic level.
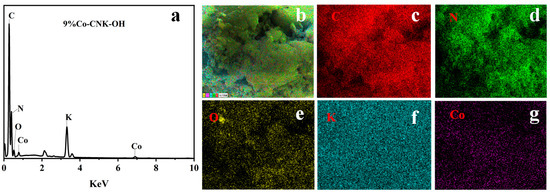
Figure 3.
EDS of 9%Co-CNK-OH (a) and the elemental mapping images of 9%Co-CNK-OH (b–g).
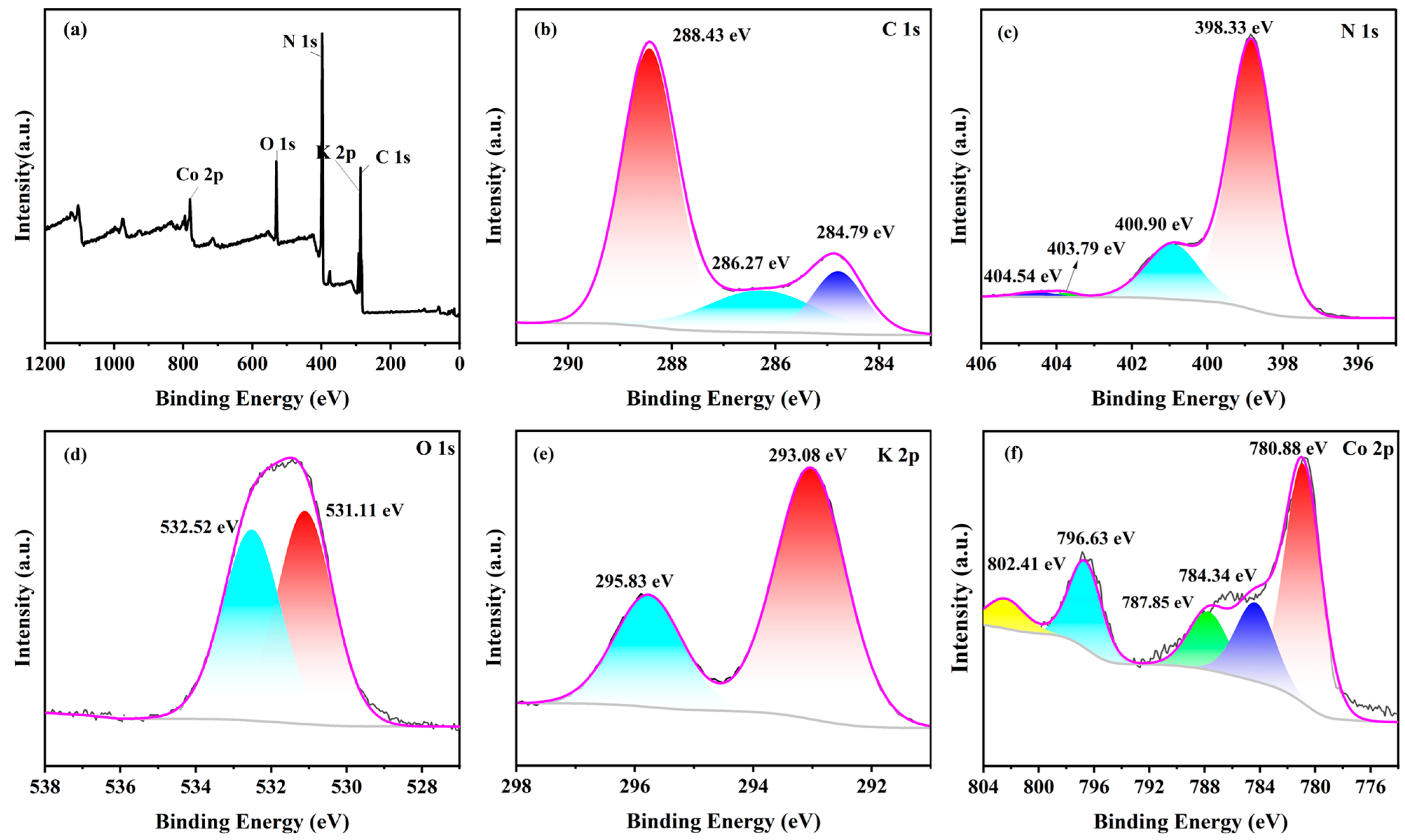
To clarify the detailed chemical bonding and valence states of the constituent elements in Co-CNK-OH, the XPS spectra of the 9%Co-CNK-OH show the peaks of C 1s, N 1s, O 1s, K 2p, and Co 2p (Figure 4a). In the spectrum of C 1s (Figure 4b), the characteristic peaks at binding energy (BE) = 288.43 and 284.79 eV correspond to the N=C=N group and C=C bond of the g-C3N4, respectively [34]. The weak peak at 286.27 eV was due to the C-OH group [31], confirming the grafting of the hydroxyl groups on the surface of the g-C3N4. Four characteristics peaks at 398.33, 400.90, 403.79, and 404.54 eV were found in the N 1s spectrum (Figure 4c). The characteristic peaks at 398.33, 400.90, and 403.79 eV could be ascribed to the C-N=C, N-(C)3, and C-N-H groups in the g-C3N4, respectively [14,35], while the peak at 404.54 eV was caused by the delocalization of the π-electrons in the g-C3N4 [31]. In the O 1s spectrum (Figure 4d), two peaks were fitted, in which the peak at 532.52 eV was assigned to the binding of water molecules, while the peak at 531.11 eV belonged to the OH bond [31,36], which further proved the implantation of the hydroxyl group in the g-C3N4. The K 2p spectrum depicts two characteristic peaks. The peak at 293.08 eV could be attributed to the N-K bond (Figure 4e), such as that in KN3, indicating that K+ ions can break the C-N bond in g-C3N4 and insert into the plane of g-C3N4 by the mutual attraction between the K and N atoms. This is crucial to the subsequent grafting of the surface hydroxyl groups, in accordance with the results of the FTIR [16]. As for the spectrum of Co 2p (Figure 4f), the characteristic peaks at 780.88 and 796.63 eV were the main peaks of Co 2p3/2 and Co 2p1/2, respectively; BE of Co 2p1/2 (796.63 eV) was lower than that of metallic cobalt (799 eV) while BE of Co 2p3/2 (780.88 eV) was higher than that of cobalt oxide (779–780 eV), suggesting that the doped Co2+ ions combined with the N atoms in the g-C3N4 to form Co-N bonds [37,38,39]; the peaks at 784.34, 787.85, and 802.41 eV are satellite peaks, representing the vibrations of the Co2+ ions [26,40,41]. From the combined results of the XRD, FT-IR, SEM, TEM, EDS, and XPS, it was concluded that Co2+ ions were doped in CNK-OH.
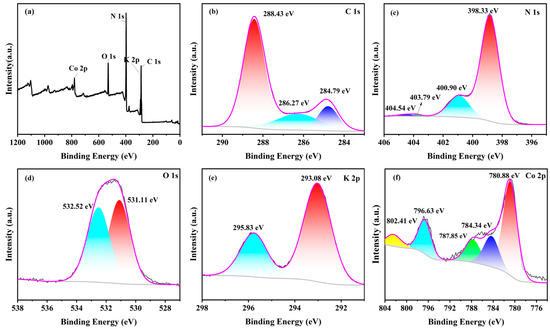
Figure 4.
The XPS spectra of 9%Co-CNK-OH: (a) full spectrum, (b) C 1s, (c) N 1s, (d) O 1s, (e) K 2p, and (f) Co 2p.
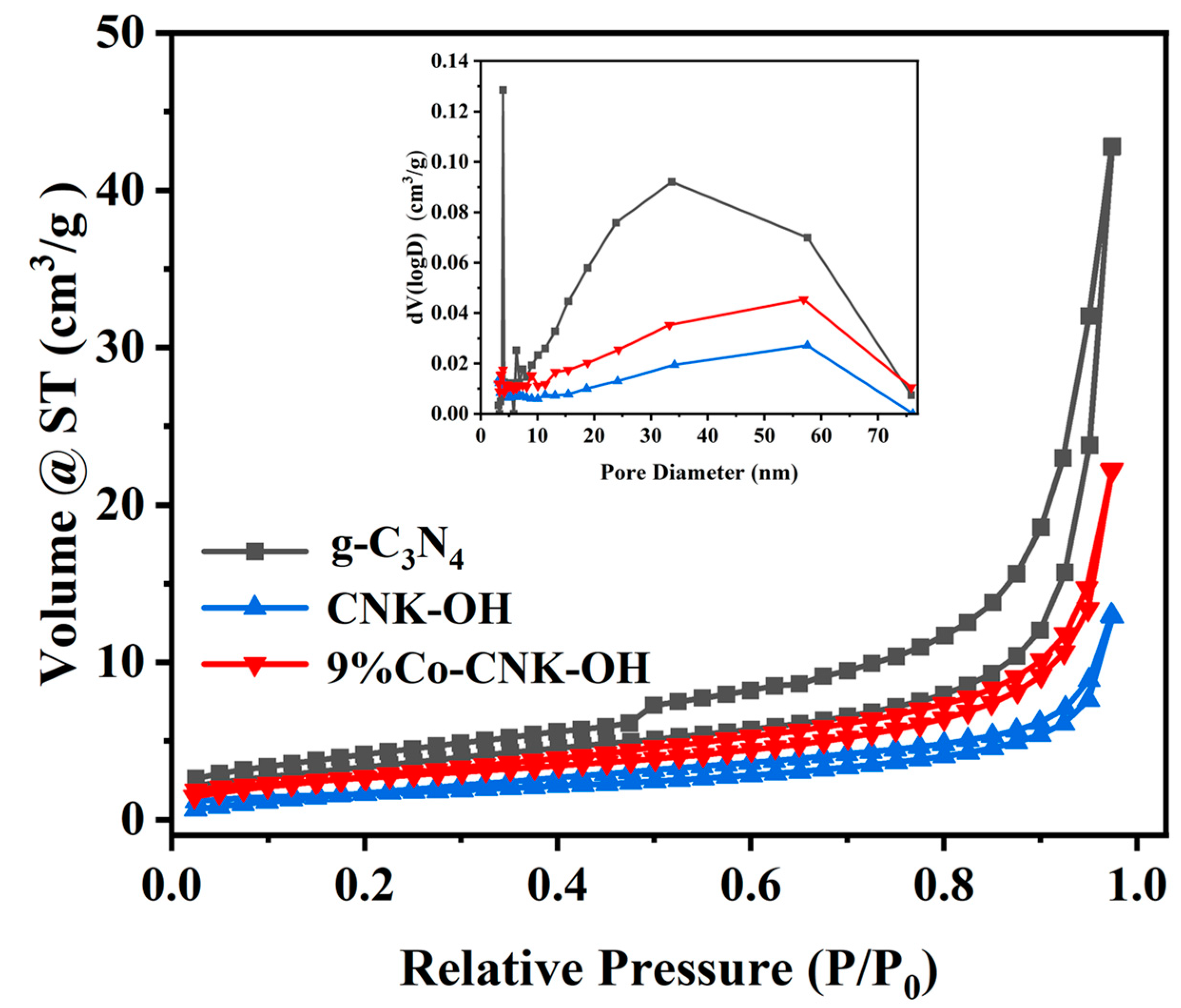
The N2 adsorption–desorption isotherms and corresponding pore-size-distribution curves of the g-C3N4, CNK-OH, and 9%Co-CNK-OH were further analyzed (Figure 5), and all the samples belonged to type IV [42].
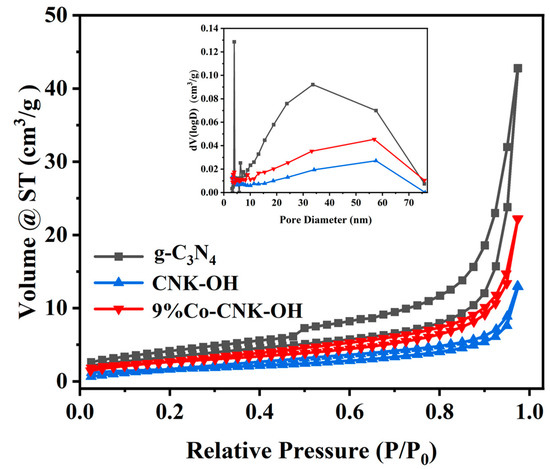
Figure 5.
Nitrogen adsorption-desorption isotherms (outer figure) and corresponding pore-size distribution curves (sub-figure) for g-C3N4, CNK-OH and 9%Co-CNK-OH.
From Table 1, g-C3N4, CNK-OH, and 9%Co-CNK-OH had specific surface areas of 15.279, 5.146, and 8.243 m2/g, respectively. The specific surface area of CNK-OH was smaller than that of g-C3N4, which could be attributed to the mixing of KCl with the g-C3N4 samples, whereby K+ ions entered into g-C3N4 to inhibit N2 adsorption and partially block the mesopores [24]; however, a slightly increased specific surface area was obtained after Co2+ loading.

Table 1.
Specific surface areas and pore-size parameters of g-C3N4, CNK-OH and 9%Co-CNK-OH.
2.3. Optical and Photoelectric Properties
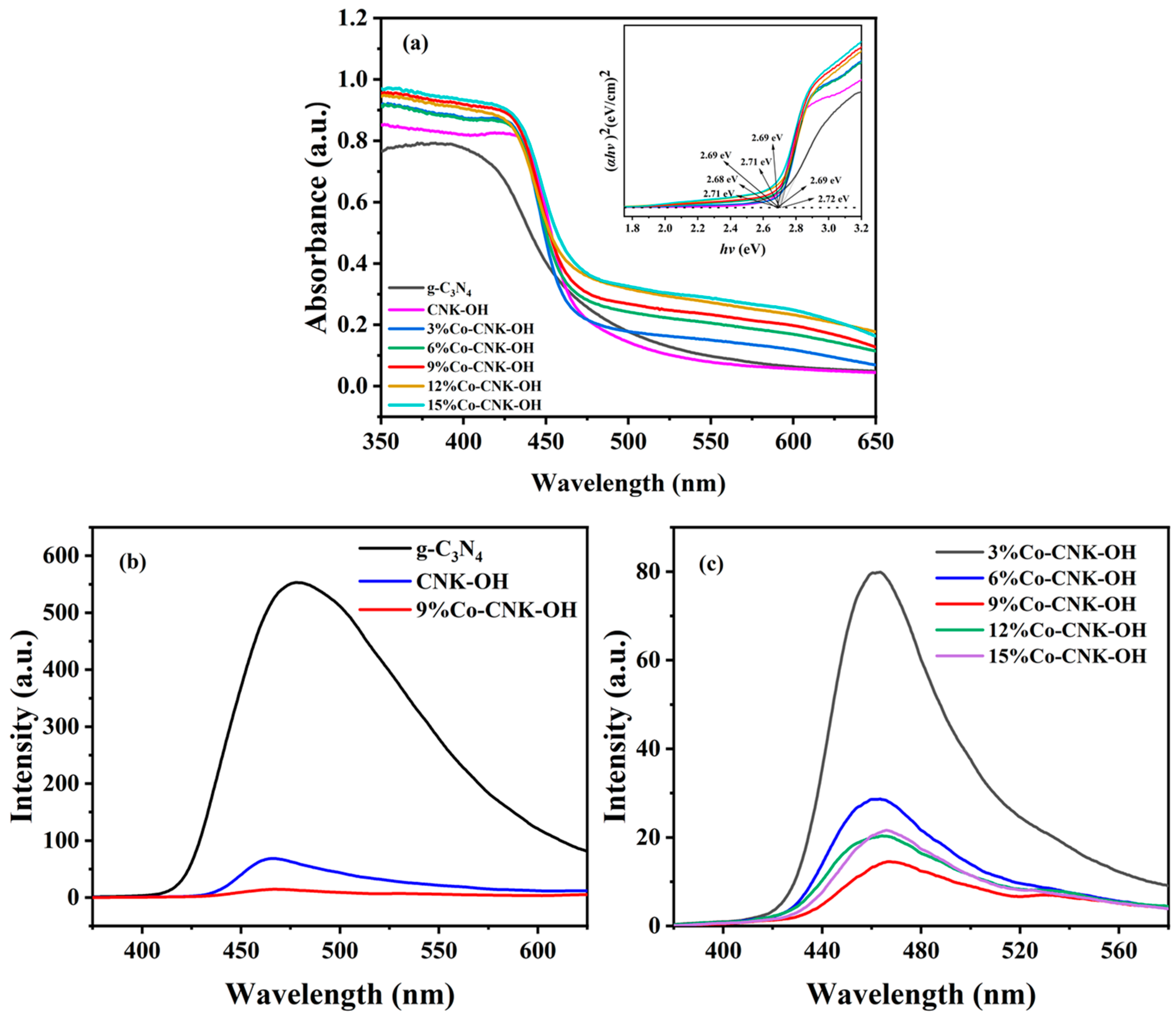
The optical properties of the catalysts were measured by the UV–Vis diffuse reflectance spectra (Figure 6a).
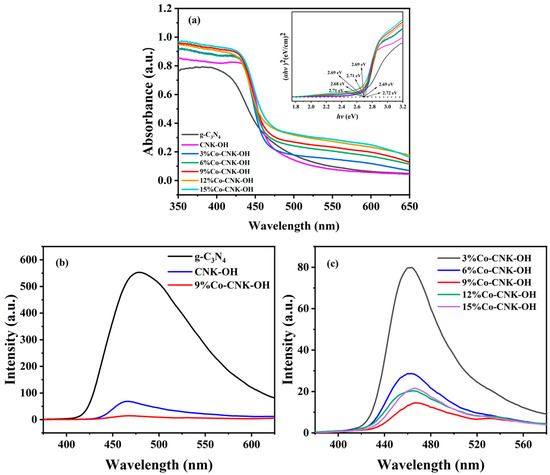
Figure 6.
UV-vis diffuse reflectance patterns of g-C3N4, CNK-OH and Co-CNK-OH (outer figure), plots of (αhv)2 versus hv of g-C3N4, CNK-OH and Co-CNK-OH (sub-figure) (a); PL emission spectra of g-C3N4, CNK-OH, and 9%Co-CNK-OH (b); and PL emission spectra of X%Co-CNK-OH (c).
The absorption edge of g-C3N4 was located at about 460 nm, with a large bandgap energy of about 2.7 eV. Compared with the g-C3N4 and CNK-OH absorption edges, those of all the Co-CNK-OH catalysts with different Co contents shifted slightly toward the long-wave range, and the intensities of the visible-light absorbance were increased by the Co2+ modification. The Eg values of the g-C3N4, CNK-OH, and Co-CNK-OH were deduced from the linear extension of the (ahν)2 versus hv curve to the X-axis. The Eg values of g-C3N4, CNK-OH, 3%Co-CNK-OH, 6%Co-CNK-OH, 9%Co-CNK-OH, 12%Co-CNK-OH, and 15%Co-CNK-OH were 2.71, 2.69, 2.72, 2.71, 2.68, 2.69, and 2.69 eV, respectively (Table 2).

Table 2.
Eg, EFB, ECB, and EVB of g-C3N4, CNK-OH and Co-CNK-OH.
PL emission spectroscopy is an effective method to detect the combination of photogenerated electrons and holes. The fluorescence intensity of g-C3N4 was very strong, while the fluorescence intensity of the CNK-OH modified by alkalization was sharply reduced to about 1/8 of that of g-C3N4 (Figure 6b, c). The PL intensity of Co-CNK-OH further decreased compared with that of CNK-OH, indicating that the introduction of Co2+ ions could effectively promote the separation of electrons and holes. The PL intensity of 9%Co-CNK-OH reached the minimum value and then PL intensity increased instead while further increasing the Co content, suggesting that the optimum dopant was 9%Co-CNK-OH for the most efficient separation of electron–hole pairs.
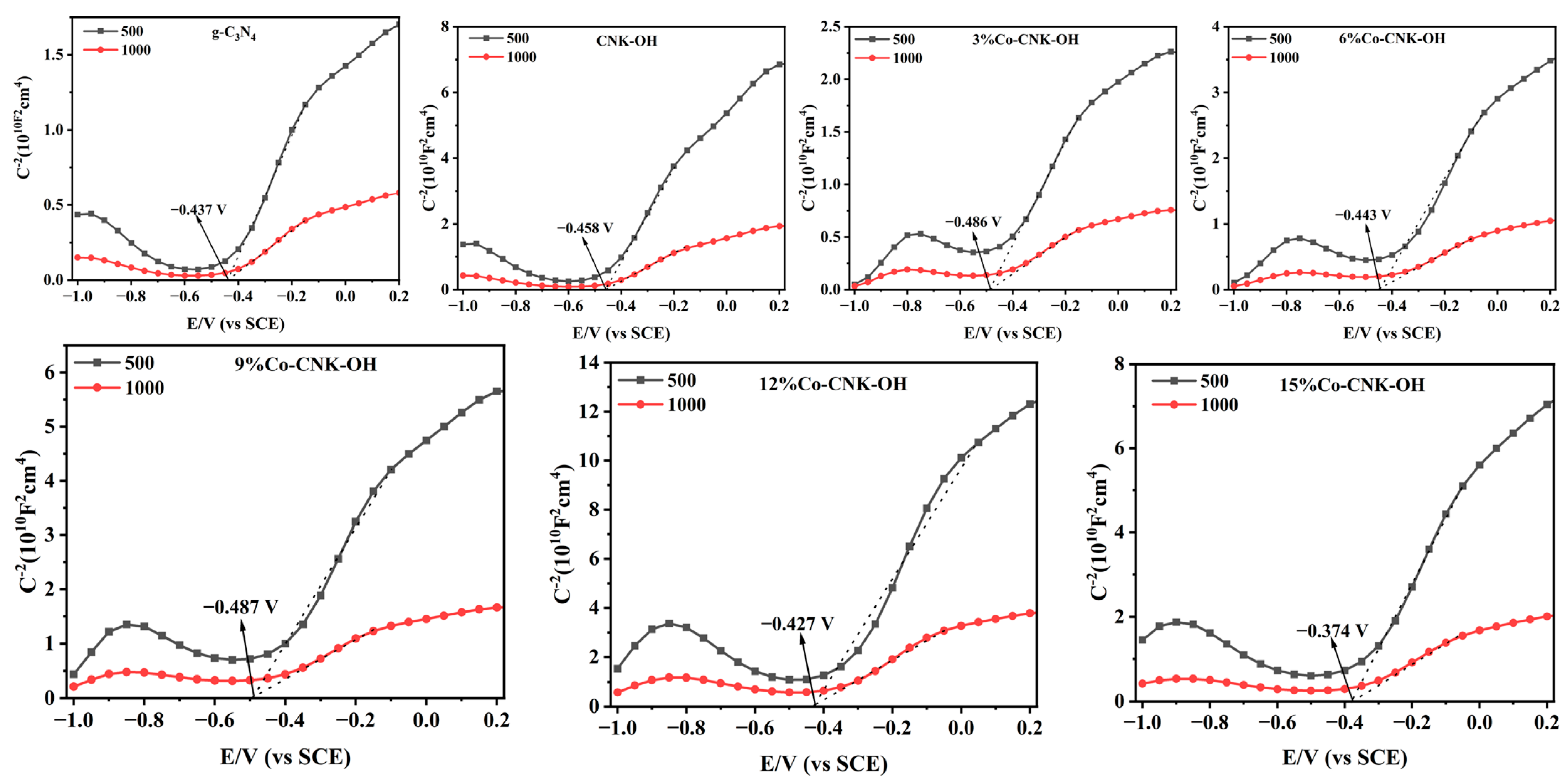
Mott–Schottky curves were applied to detect the type of semiconductor. As shown in Figure 7, the observed curves of all the samples show significantly positive slopes, indicating that g-C3N4, CNK-OH, and Co-CNK-OH were all categorized as n-type semiconductors. As listed in Table 2, the EFB and EVB of g-C3N4, CNK-OH, and Co-CNK-OH were calculated by extending the linear part of C−2 = 0 to obtain EFB, ECB = EFB + 0.197 V (the standard electrode potential of Ag/AgCl), and EVB = Eg + ECB [16].
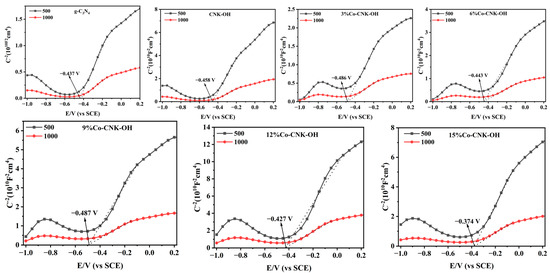
Figure 7.
Mott–Schottky curves of g-C3N4, CNK-OH, and Co-CNK-OH.
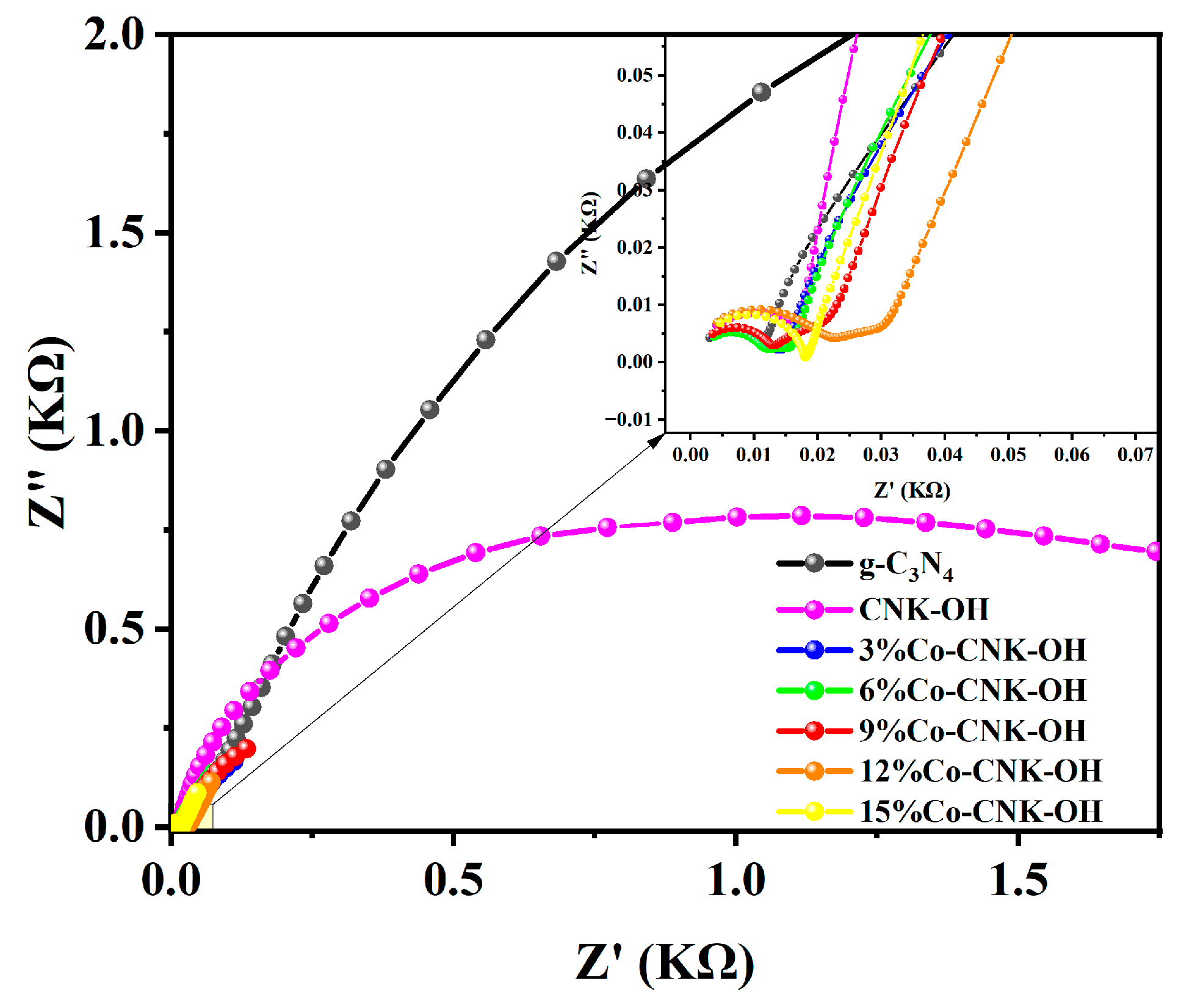
Electrochemical impedance spectroscopy (EIS) is commonly used to probe the separation efficiency of electrons and resistance to interfacial charge transfer of semiconductor materials. The EIS curves of g-C3N4, CNK-OH, and Co-CNK-OH are shown in Figure 8. The arc radius of 9%Co-CNK-OH was significantly smaller than those of the other samples, implying that the electrical resistance of 9%Co-CNK-OH was the lowest, in accordance with the best electron-transfer performance and suggesting that Co2+ modification significantly promote the interfacial charge to the electron acceptor and separate electrons and holes effectively [43].
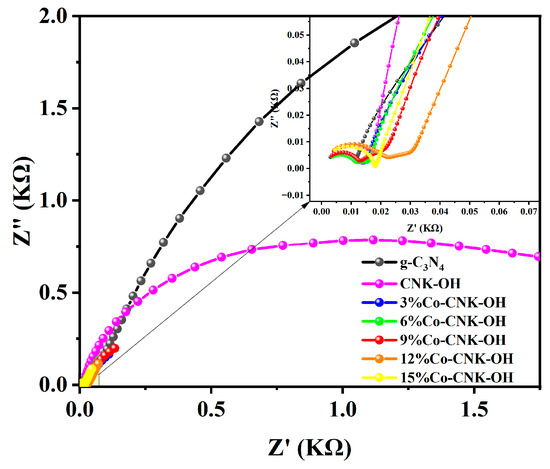
Figure 8.
EIS of g-C3N4, CNK-OH, and Co-CNK-OH (outer figure) and enlarged view of 0.00-0.07 KΩ (sub-figure).
2.4. Catalytic Performance and Mechanism of As-Prepared Samples
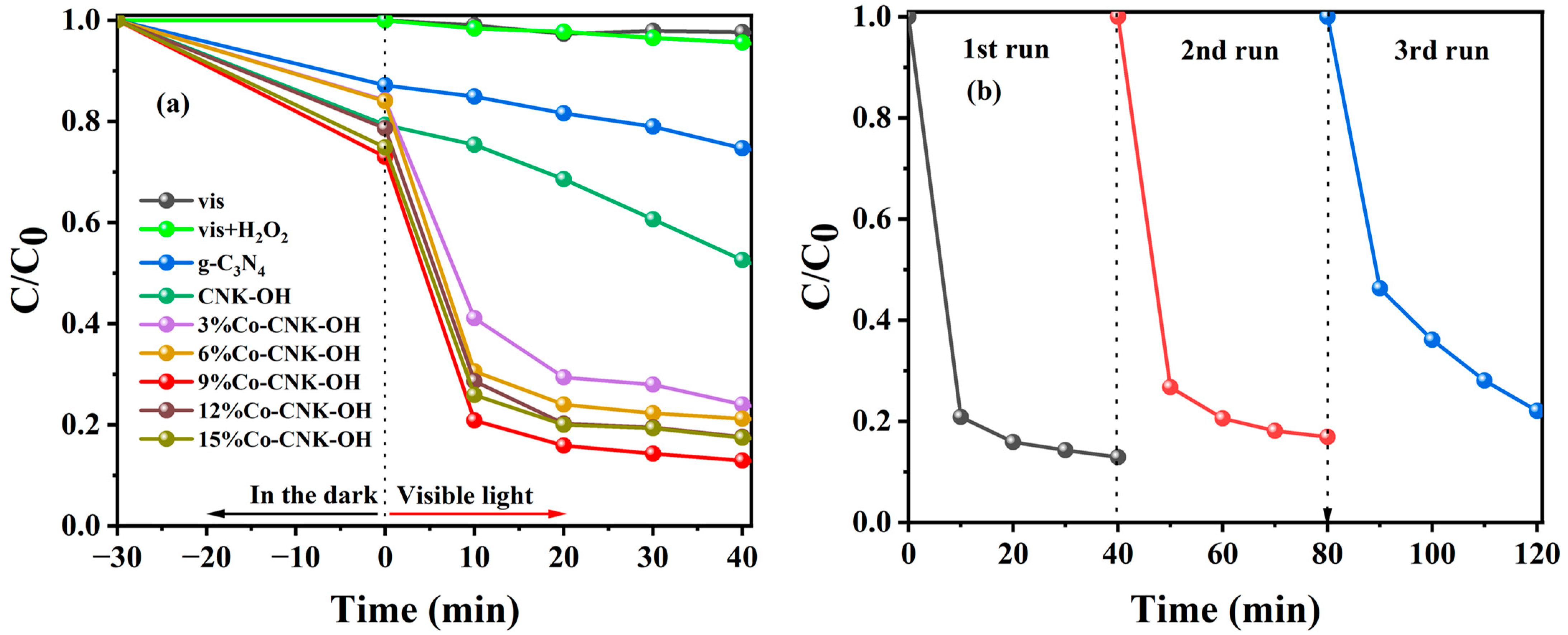
In order to evaluate the catalytic performance of Co-CNK-OH, the optimal experiments were designed, and the results are shown in Figure S1. From Figure S1, the optimal reaction conditions for the TC degradation were as follows: the amount of catalyst was 50 mg L−1, the concentration of H2O2 was 30 mM, pH = 7, and the initial concentration of the TC was 20 mg L−1. Different reaction systems were applied to TC degradation. As shown in Figure 9a, with visible light alone or with visible light and H2O2, only a slight self-degradation of the TC occurred in 40 min. Adding g-C3N4 and CNK-OH led to the degradation efficiency of TC reaching 25.30 and 47.45%, respectively, indicating that the alkalized g-C3N4 promoted the degradation of TC because of the introduction of hydroxyl groups. It was noteworthy that the Co modification enhanced the degradation efficiency of TC by Co-CNK-OH. In detail, the degradation efficiency of TC increased with increasing Co content (3–9%) and then decreased with further increases in the Co content, which could be explained by the introduction of defects that act as a complex center for the charge carriers [44]. The highest-efficiency TC degradation with 9%Co-CNK-OH was 87.10% in 40 min. The leaching of Co2+ ions after catalysis was also investigated by inductively coupled plasma mass spectrometry (ICP-MS). The result of the ICP-MS indicated that the concentration of the free Co2+ in the solution after the reaction was 0.21 mg/L, which complies with China’s emission standards for copper, cobalt, and nickel industrial sources (GB25467-2010, 1 mg/L).
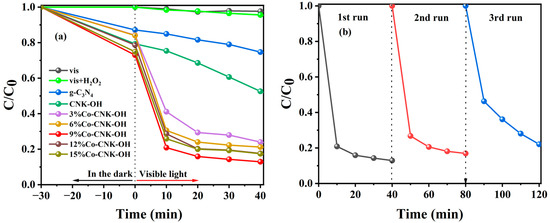
Figure 9.
Degradation curves of TC in different reaction systems (a), cycle test of 9%Co-CNK-OH for TC degradation (b). Reaction conditions: catalyst dosage: 50 mg L−1, H2O2: 30 mM, pH: 7, and TC: 20 mg L−1.
The reusability and stability of the catalyst are critical for practical applications. In order to evaluate the durability of the catalysts, three cycles of repeated experiments were carried out with 9%Co-CNK-OH under similar conditions (Figure 9b). It could be observed that the degradation efficiency of TC decreased from 87.1% to 77.95%, suggesting that the Co-CNK-OH sample had excellent stability.
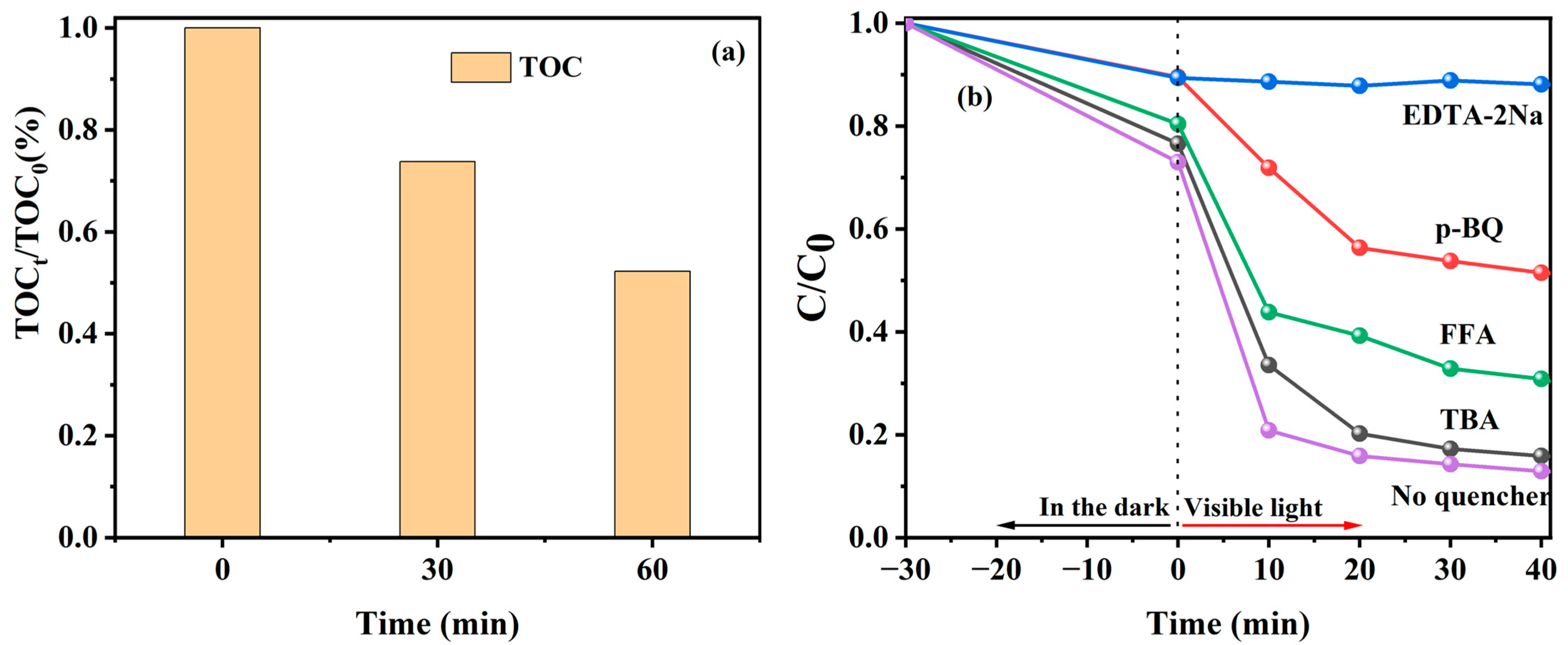
To monitor the effect of the mineralization property of the photocatalyst on organic pollutants, the TOC removal efficiency of TC by the 9%Co-CNK-OH catalyst under visible-light irradiation was investigated (Figure 10a). The TOC removal efficiency of the TC by the 9%CO-CNK-OH catalyst was 50% after 60 min. The mineralization property of the 9%Co-CNK-OH catalyst is better than some previous reports (41%) [45].
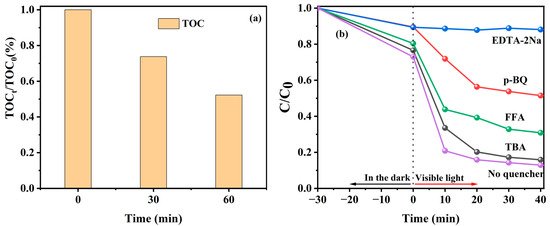
Figure 10.
TOC changes in TC by 9%Co-CNK-OH under visible light (a); photo-Fenton degradation efficiencies of TC by 9%Co-CNK-OH with different scavengers under visible light (b).
To figure out the mechanism of TC degradation by 9%Co-CNK-OH, TBA, EDTA-2Na, p-BQ, and FFA were used to capture ∙OH, h+, ∙O2− and 1O2, respectively (Figure 10b). Compared with the efficiency of TC removal (87.10%) by 9%Co-CNK-OH without any trapping-agent addition, the efficiency of TC degradation was reduced by 2.95%, 75.25%, 38.60%, and 17.98% with the addition of TBA, EDTA-2Na, p-BQ, and FFA, respectively, suggesting that h+, ∙O2−, and 1O2 are the main active species for the photocatalytically assisted Fenton-like degradation of TC by Co-CNK-OH.
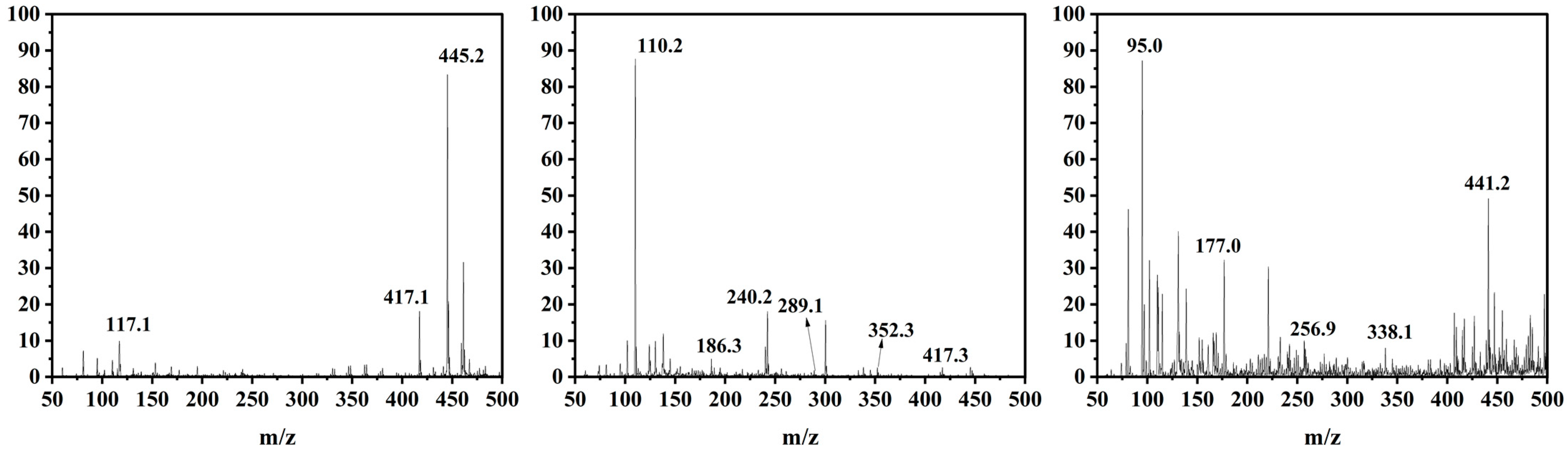
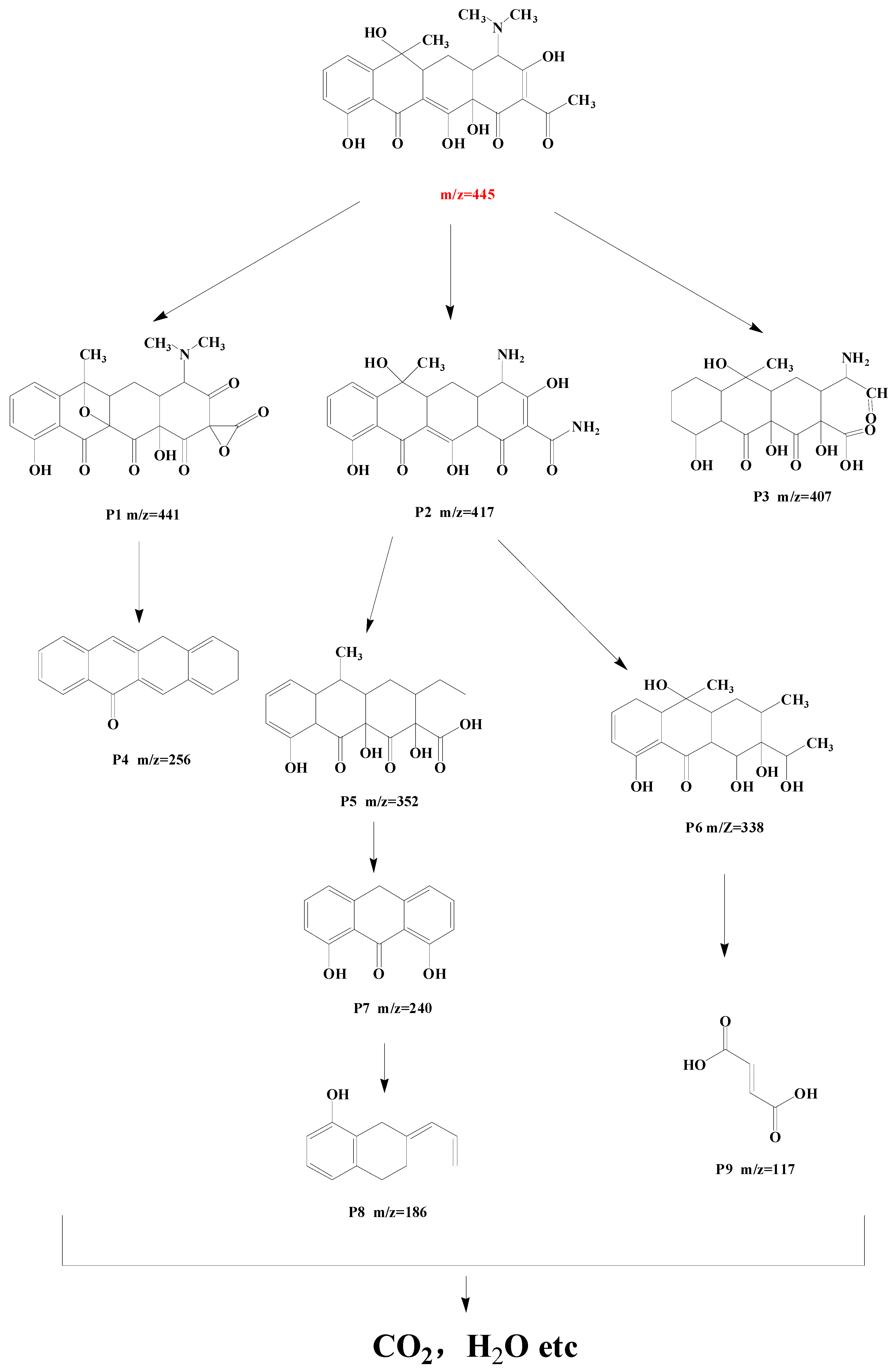
The possible intermediates generated during TC degradation in the photo-Fenton process were investigated by HPLC-MS, as well as the possible TC-degradation pathway by 9%Co-CNK-OH (Figure 11 and Figure 12). The main intermediates were named P1 (m/z = 441), P2 (m/z = 417), P3 (m/z = 407), P4 (m/z = 256), P5 (m/z = 352), P6 (m/z = 338), P7 (m/z = 240), P8 (m/z = 186), P9 (m/z = 117), etc. First, the TC molecules were attacked by reactive substances (h+, ·O2−, 1O2 or ·OH) to form P1 (m/z = 441), P2 (m/z = 417), and P3 (m/z = 407) [46]. P1 (m/z = 441) was produced by demethylation and then reacted to form P4 (m/z=256) by dehydroxylation, deamidation, and ring-opening reactions. P2 (m/z = 417) lost amine and carbonyl groups by the deamidation reaction to produce P5 (m/z = 352) [47]. Then, P5 was further attacked by free radicals to form P6 (m/z = 338) by the rupture of the cyclic hydrocarbon [48], followed by the ringing-open reaction due to the action of ∙O2− to produce P7 (m/z = 240) with the loss of methanol [49]. P8 (m/z = 186) was obtained by the ringing-open reaction and dihydroxylation of P7 (m/z = 240) [22]. Another possibility is that P6 (m/z = 338) underwent demethylation and hydroxylation to produce P9 (m/z = 117). Finally, these smaller molecules can be completely mineralized to produce H2O and CO2 or other molecules.
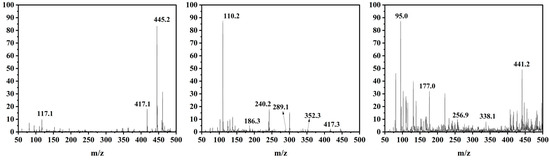
Figure 11.
Mass spectra of tetracycline and its corresponding intermediates during photo-Fenton degradation of TC.
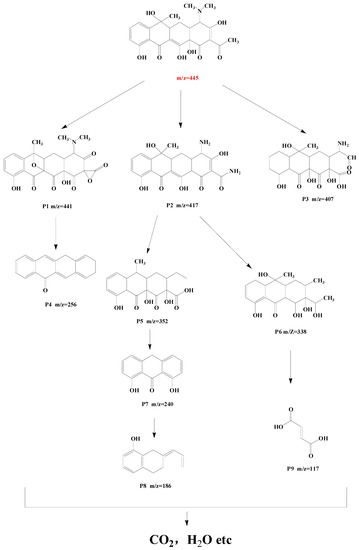
Figure 12.
Possible TC degradation pathway by 9%Co-CNK-OH.
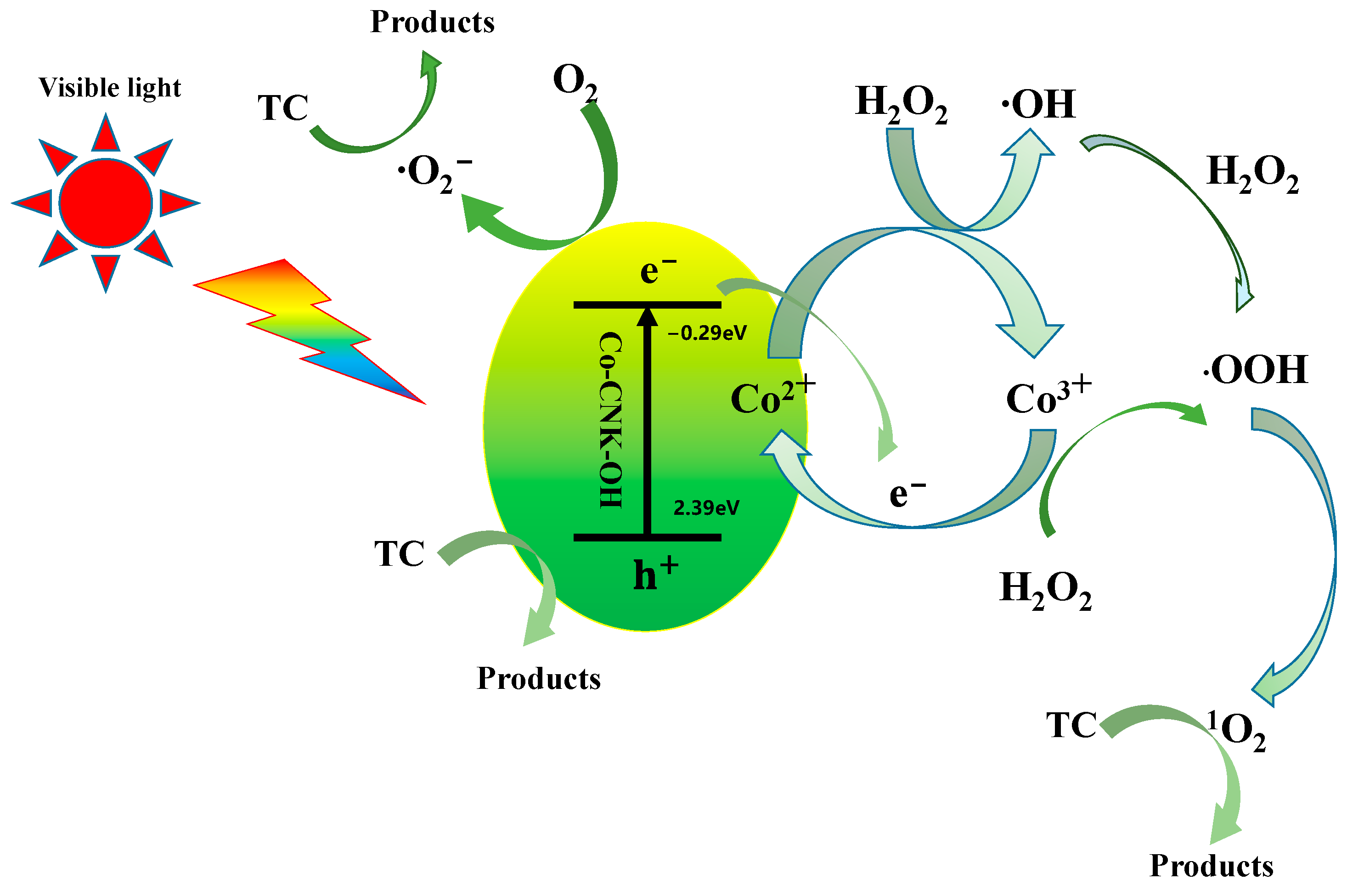
In view of this analysis, a possible mechanism for TC degradation by 9%Co-CNK-OH catalyst is proposed (Figure 13). First, numerous photogenerated electrons and holes in the conduction and valence bands were generated under visible-light irradiation and an internal electric field for the 9%Co-CNK-OH samples and ∙O2− was formed. The addition of H2O2 promoted the formation of both 1O2 and ∙O2−. The Co (III)/Co (II) redox cycle accelerated the consumption of the photogenerated electrons and then promoted the separation of the photogenerated electrons and holes while keeping the continuous generation of 1O2, ∙O2−, and h+ in the system [50,51]. Therefore, TC degradation by 9%Co-CNK-OH was attributed to the synergistic effect of photocatalysis and Fenton reactions. The possible reactions are listed as follows:
9%Co-CNK-OH + hv → 9%Co-CNK-OH (e− + h+)
e− + O2 → ∙O2−
Co2+ + H2O2 → Co3+ + HO∙ + OH−
Co3+ + H2O2 → Co2+ + HOO∙ + H+
Co3+ + e− → Co2+
HO∙ + H2O2 → HOO∙ + H2O
HOO∙ + HOO∙ → 1O2 + H2O2
(h+, 1O2, ∙O2−) + TC → CO2 + H2O
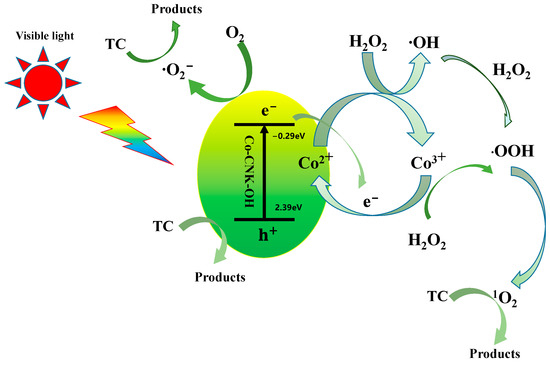
Figure 13.
Possible photocatalytic mechanism of TC degradation by Co-CNK-OH in Fenton-like photocatalytic systems.
3. Materials and Methods
3.1. Chemicals
Melamine (C3H6N6, 99%) and furfuryl alcohol (C5H6O2, 98%) were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China). Potassium chloride (KCl, 99.5%), ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), and cobalt chloride hexahydrate (CoCl2·6H2O, 99%) were purchased from Comeo Chemical Reagent Co., Ltd. (Tianjin, China). Ammonium chloride (NH4Cl, 99.5%) was purchased from Fengfan chemical reagent technology Co., Ltd. (Tianjin, China). Tetracycline (TC) was purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). Hydrochloric acid (HCl, 36–38%) was provided by Haohua chemical reagent Co., Ltd. (Luoyang, China), and p-benzoquinone (p-BQ) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Tert-butanol (t-BuOH, 99.8%) was provided by Yong chemical reagent Co., Ltd. (Tianjin, China). Hydrogen peroxide (H2O2, 30%) was obtained from Xilong Chemical Co., Ltd. (Shantou, China). All chemical reagents were used without any purification.
3.2. Materials Synthesis
3.2.1. Preparation of CNK-OH
The steps for preparing alkalinized g-C3N4 (CNK-OH) were the same as those followed by Li. First, 9 g of melamine, 45 g of KCl, and 0.6 g of NH4Cl were mixed and ground thoroughly in a mortar for 30 min. Then, the mixture was transferred to a ceramic crucible, heated to 550 °C at 2.3 °C/min in a muffle furnace, kept there for 4 h, and cooled naturally in the air. The obtained solid was ground into powder, dissolved in 200 mL of deionized water, stirred for 6 h, washed by vacuum filtration, dried in an oven at 80 °C for 12 h, and ground again to obtain the as-prepared CNK-OH. Under the same conditions, pure g-C3N4 was prepared with 4.5 g of melamine but without KCl or NH4Cl.
3.2.2. Fabrication of Co-CNK-OH Composites
Co-CNK-OH composites were prepared by an impregnation method [16]. First, 0.48 g of CNK-OH was dispersed in 100 mL of deionized water (denoted as solution A); a certain amount of CoCl2∙6H2O was dissolved in 100 mL of deionized water (denoted as solution B). Then, solution B was added dropwise to solution A while stirred slowly, then stirred for 36 h at room temperature. The obtained suspension was sonicated for 3 h, washed three times each with deionized water and ethanol, and dried in an oven at 80 °C for 12 h to obtain the as-prepared Co-CNK-OH sample. The mass ratios of CoCl2∙6H2O and CNK-OH were designed between 3 and 15%, and the Co-CNK-OH samples loaded with different Co contents are expressed as weight percentages of Co-CNK-OH for simplicity. Accordingly, 3%Co-CNK-OH, 6%Co-CNK-OH, 9%Co-CNK-OH, 12%Co-CNK-OH, and 15%Co-CNK-OH samples were prepared.
3.3. Degradation Experiments
First, a certain amount of the as-prepared catalyst was dispersed in 100 mL of a 20 mg/L TC solution and stirred magnetically for 30 min under dark conditions to establish adsorption–desorption equilibrium. A certain amount of H2O2 was added, while a 300 W Xe lamp (λ ≥ 420 nm) was turned on at the same time, and about 3 mL of the suspension was taken out every 10 min, filtered through a 0.22 μm filter tip to remove the catalyst, and the absorbance was measured at 357 nm by spectrophotometry to evaluate the efficiencies of the TC degradation by the different catalysts. Every run was measured 3 times, and the relative error was less than 5%.
3.4. TC Mineralization Experiment
The sampling procedure for TC mineralization was based on that of the catalytic experiment. A total organic carbon analyzer was used to monitor the filtrate, and the TOC removal efficiency of TC was calculated with the following equation:
where TOC0 is the initial TOC value of the TC solution, and TOCt indicates the TOC value measured at any time t.
3.5. Trapping Test of Active Species
The active species in the degradation process were investigated using tert-butyl alcohol (TBA, 150 mM), p-benzoquinone (p-BQ, 10 mM), disodium ethylenediaminetetraacetate (EDTA-2Na, 10 mM), and furfuryl alcohol (FFA, 10 mM) to capture hydroxyl radicals (∙OH), superoxide radicals (∙O2−), photogenerated holes (h+), and singlet oxygen (1O2), respectively, and the experimental procedure was consistent with that used for the photocatalytically assisted Fenton-like degradation of TC by 9%Co-CNK-OH.
4. Conclusions
In this study, Co-CNK-OH photocatalysts were prepared by a calcination–impregnation method, and the 9%Co-CNK-OH showed the best degradation efficiency (87.1% TC degradation in 40 min and 50% TOC removal in an hour). The doping of Co2+ expanded the absorption range of visible light and accelerated the transfer of the photogenerated electrons at the interface of the catalysts. The Co (III)/Co (II) redox cycle not only accelerated the consumption of the photogenerated electrons and then promoted the separation of the photogenerated electrons and holes but also accelerated the continuous generation of 1O2, ∙O2−, and h+ in the system. The addition of H2O2 and the Co2+/Co3+ redox cycle promoted the production of 1O2 and ∙O2− and, thus, improved the TC degradation efficiency. The mechanism study of Co-CNK-OH for the Fenton-like photocatalytic degradation of TC was further proposed and confirmed that h+, ∙O2−, and 1O2 are the main reactive species in the TC degradation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13040715/s1, Figure S1: The influence of initial TC concentration(a), H2O2 concentration(b), pH value(c) and Catalyst addition (d)on degradation efficiency of TC.
Author Contributions
Conceptualization, J.W. and D.H.; methodology, D.H. and J.L.; experiments, D.H. and Q.S.; data curation, D.H., Q.S. and M.Z.; writing—original draft preparation, D.H.; guidance, review, and editing, J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 22178326).
Data Availability Statement
The data generated during and/or analysed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Zhou, Y.; Li, W.B.; Kumar, V.; Necibi, M.C.; Mu, Y.J.; Shi, C.Z.; Chaurasia, D.; Chauhan, S.; Chaturvedi, P.; Sillanpaa, M.; et al. Synthetic organic antibiotics residues as emerging contaminants waste-to-resources processing for a circular economy in China: Challenges and perspective. Environ. Res. 2022, 211, 113075. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tian, Q.; Zhang, Y.; Li, Y.; Yang, X.; Zheng, H.; Chen, L.; Li, F. Sequential combination of photocatalysis and microalgae technology for promoting the degradation and detoxification of typical antibiotics. Water Res. 2022, 210, 117985. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Y.; Huo, P.W.; Luo, Y.Y.; Liu, X.L.; Wu, D.; Gao, X.; Li, C.X.; Yan, Y.S. Performance of molecularly imprinted photocatalysts based on fly-ash cenospheres for selective photodegradation of single and ternary antibiotics solution. J. Mol. Catal. A-Chem. 2013, 378, 91–98. [Google Scholar] [CrossRef]
- Sun, S.; Geng, J.; Ma, L.; Sun, X.; Qi, H.; Wu, Y.; Zhang, R. Changes in antibiotic resistance genotypes and phenotypes after two typical sewage disposal processes. Chemosphere 2022, 291 Pt 2, 132833. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernandez-Calvino, D.; Nunez-Delgado, A.; Fernandez-Sanjurjo, M.J.; Arias-Estevez, M.; Alvarez-Rodriguez, E. Estimation of adsorption/desorption Freundlich's affinity coefficients for oxytetracycline and chlortetracycline from soil properties: Experimental data and pedotransfer functions. Ecotoxicol. Environ. Saf. 2020, 196, 110584. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.X.; Zhang, H.M.; Feng, Y.J.; Yang, F.L.; Zhang, J.P. Removal of trace antibiotics from wastewater: A systematic study of nanofiltration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 2014, 240, 211–220. [Google Scholar] [CrossRef]
- Liu, M.; Xia, H.; Yang, W.; Liu, X.; Xiang, J.; Wang, X.; Hu, L.; Lu, F. Novel Cu-Fe bi-metal oxide quantum dots coupled g-C3N4 nanosheets with H2O2 adsorption-activation trade-off for efficient photo-Fenton catalysis. Appl. Catal. B Environ. 2022, 301, 120765. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Qian, X.; Wu, Y.; Kan, M.; Fang, M.; Yue, D.; Zeng, J.; Zhao, Y. FeOOH quantum dots coupled g-C3N4 for visible light driving photo- Fenton degradation of organic pollutants. Appl. Catal. B Environ. 2018, 237, 513–520. [Google Scholar] [CrossRef]
- Li, Y.; Luo, N.; Tian, Z.; Li, H.; Yang, M.; Shang, W.; Shen, Y.; Qu, M.; Zhou, A. H2O2-free photo-Fenton degradation of organic pollutants on thermally exfoliated g-C3N4. Colloids Surf. A-Physicochem. Eng. Asp. 2020, 586, 124190. [Google Scholar] [CrossRef]
- Cui, K.P.; Yang, T.T.; Chen, Y.H.; Weerasooriya, R.; Li, G.H.; Zhou, K.; Chen, X. Magnetic recyclable heterogeneous catalyst Fe3O4/g-C3N4 for tetracycline hydrochloride degradation via photo-Fenton process under visible light. Environ. Technol 2022, 43, 3341–3354. [Google Scholar] [CrossRef]
- Lu, N.; Liu, N.; Zhang, C.; Su, Y.; Shang, K.; Jiang, N.; Li, J.; Wu, Y. CO2 conversion promoted by potassium intercalated g-C3N4 catalyst in DBD plasma system. Chem. Eng. J. 2021, 417, 129283. [Google Scholar] [CrossRef]
- Lu, Z.-Z.; Li, S.-Q.; Xiao, J.-Y. Synergetic effect of Na-Ca for enhanced photocatalytic performance in NOx degradation by g-C3N4. Catal. Lett. 2021, 151, 370–381. [Google Scholar] [CrossRef]
- Zhou, M.; Jing, L.; Dong, M.; Lan, Y.; Xu, Y.; Wei, W.; Wang, D.; Xue, Z.; Jiang, D.; Xie, J. Novel broad-spectrum-driven g-C3N4 with oxygen-linked band and porous defect for photodegradation of bisphenol A, 2-mercaptophenthiazole and ciprofloxacin. Chemosphere 2021, 268, 128839. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, Y.; Deng, F.; Wang, M.; Chen, D. Porous Z-scheme MnO2/Mn-modified alkalinized g-C3N4 heterojunction with excellent Fenton-like photocatalytic activity for efficient degradation of pharmaceutical pollutants. Sep. Purif. Technol. 2020, 246, 116890. [Google Scholar] [CrossRef]
- Lu, X.; Jin, Y.; Zhang, X.; Xu, G.; Wang, D.; Lv, J.; Zheng, Z.; Wu, Y. Controllable synthesis of graphitic C3N4/ultrathin MoS2 nanosheet hybrid nanostructures with enhanced photocatalytic performance. Dalton. Trans. 2016, 45, 15406–15414. [Google Scholar] [CrossRef]
- Thi Quyen, V.; Jae Kim, H.; Kim, J.; Thi Thu Ha, L.; Thi Huong, P.; My Thanh, D.; Minh Viet, N.; Quang Thang, P. Synthesizing S-doped graphitic carbon nitride for improvement photodegradation of tetracycline under solar light. Sol. Energy 2021, 214, 288–293. [Google Scholar] [CrossRef]
- Bao, J.; Bai, W.; Wu, M.; Gong, W.; Yu, Y.; Zheng, K.; Liu, L. Template-mediated copper doped porous g-C3N4 for efficient photodegradation of antibiotic contaminants. Chemosphere 2022, 293, 133607. [Google Scholar] [CrossRef]
- Zhou, M.; Dong, G.; Yu, F.; Huang, Y. The deep oxidation of NO was realized by Sr multi-site doped g-C3N4 via photocatalytic method. Appl. Catal. B Environ. 2019, 256, 117825. [Google Scholar] [CrossRef]
- Wu, X.; Chen, F.; Wang, X.; Yu, H. In situ one-step hydrothermal synthesis of oxygen-containing groups-modified g-C3N4 for the improved photocatalytic H2-evolution performance. Appl. Surf. Sci. 2018, 427, 645–653. [Google Scholar] [CrossRef]
- Shi, Y.X.; Li, L.L.; Xu, Z.; Sun, H.R.; Guo, F.; Shi, W.L. One-step simple green method to prepare carbon-doped graphitic carbon nitride nanosheets for boosting visible-light photocatalytic degradation of tetracycline. J. Chem. Technol. Biotechnol. 2021, 96, 3122–3133. [Google Scholar] [CrossRef]
- Wu, X.; Gao, D.; Wang, P.; Yu, H.; Yu, J. NH4Cl-induced low-temperature formation of nitrogen-rich g-C3N4 nanosheets with improved photocatalytic hydrogen evolution. Carbon 2019, 153, 757–766. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Z.-T.; Andrew Lin, K.-Y.; Wei, W.-H.; Wang, K.-H. Synergistic effect of KCl mixing and melamine/urea mixture in the synthesis of g-C3N4 for photocatalytic removal of tetracycline. J. Ind. Eng. Chem. 2022, 107, 118–125. [Google Scholar] [CrossRef]
- Song, H.; Liu, L.; Wang, H.; Feng, B.; Xiao, M.; Tang, Y.; Qu, X.; Gai, H.; Huang, T. Adjustment of the band gap of Co-doped KCl/NH4Cl/g-C3N4 for enhanced photocatalytic performance under visible light. Mater. Sci. Semicond. Process. 2021, 128, 105757. [Google Scholar] [CrossRef]
- Cong, Y.; Chen, X.; Zheng, Q.; Zhang, Y.; Lv, S.W. The calcium alginate-immobilized Co-g-C3N4 composite microspheres as an efficient mediator to activate peroxymonosulfate for degrading organic pollutants. Environ. Res 2022, 215 Pt 2, 114414. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Fang, W.Q.; Wang, H.F.; Zhang, H.; Zhao, H.; Yao, Y.; Yang, H.G. Surface hydrogen bonding can enhance photocatalytic H2 evolution efficiency. J. Mater. Chem. A 2013, 1, 14089–14096. [Google Scholar] [CrossRef]
- Li, Y.; Ouyang, S.; Xu, H.; Wang, X.; Bi, Y.; Zhang, Y.; Ye, J. Constructing Solid-Gas-Interfacial Fenton Reaction over Alkalinized-C3N4 Photocatalyst to Achieve Apparent Quantum Yield of 49% at 420 nm. J. Am. Chem. Soc. 2016, 138, 13289–13297. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Azam, Q.; Mohsin, J.; Sammia, S.; Mudassar, S. Fabrication of g-C3N4/transition metal (Fe, Co, Ni, Mn and Cr)-doped ZnO ternary composites: Excellent visible light active photocatalysts for the degradation of organic pollutants from wastewater. Mater. Res. Bull. 2021, 147, 111630. [Google Scholar]
- Chen, P.-W.; Li, K.; Yu, Y.-X.; Zhang, W.-D. Cobalt-doped graphitic carbon nitride photocatalysts with high activity for hydrogen evolution. Appl. Surf. Sci. 2017, 392, 608–615. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, Y.; Wang, L.; Ai, S.; Ding, H. Cu-modified alkalinized g-C3N4 as photocatalytically assisted heterogeneous Fenton-like catalyst. Appl. Surf. Sci. 2017, 426, 1133–1140. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Huang, S.; Li, Y.; Zhang, X.; Zeng, G.; Niu, L.; Ling, Y.; Zhang, Y. Facile construction of 2D g-C3N4 supported nanoflower-like NaBiO3 with direct Z-scheme heterojunctions and insight into its photocatalytic degradation of tetracycline. J. Hazard. Mater. 2021, 414, 125547. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Ou, M.; Zhong, Q.; Zhang, S.; Yu, L. Efficient visible-light photocatalytic oxidation of gaseous NO with graphitic carbon nitride (g-C3N4) activated by the alkaline hydrothermal treatment and mechanism analysis. J. Hazard. Mater. 2015, 300, 598–606. [Google Scholar] [CrossRef]
- Gao, Y.H.; Zhao, W.L.; Chen, Y. g-C3N4 modified by hydroxyl group on the surface prepared by double salt enhanced the visible light photocatalytic activity. Diam. Relat. Mater. 2021, 116, 108425. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, Z.; Deng, S.; Wang, H.; Wu, Z. Decorating g-C3N4 with alkalinized Ti3C2 Mxene for promoted photocatalytic CO2 reduction performance. J. Colloid. Interface Sci. 2020, 564, 406–417. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs thermal exfoliation of g-C3N4 for NOx removal under visible light irradiation. Appl. Catal. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Lu, G. Highly Efficient Hydrogen Evolution over Co(OH)2 Nanoparticles Modified g-C3N4 Co-sensitized by Eosin Y and Rose Bengal under Visible Light Irradiation. Appl. Catal. B Environ. 2016, 188, 56–64. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J. Graphene supported Co-g-C3N4 as a novel metal-macrocyclic electrocatalyst for the oxygen reduction reaction in fuel cells. Langmuir 2013, 29, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Kong, L.; Dong, Y.; Wang, G.; Wu, X.; Jiang, P. Insight into the crucial factors for photochemical deposition of cobalt cocatalysts on g-C3N4 Photocatalysts. ACS Appl. Mater. Interfaces 2018, 10, 9522–9531. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Qin, F.; Jiang, Y.; An, H.; Fang, J.; Zhang, K.; Lai, Y. Mesoporous Co–N–C composite as a sulfur host for high-capacity and long-life lithium–sulfur batteries. J. Mater. Sci. 2018, 53, 13143–13155. [Google Scholar] [CrossRef]
- Yu, J.; Chen, G.; Sunarso, J.; Zhu, Y.; Ran, R.; Zhu, Z.; Zhou, W.; Shao, Z. Cobalt oxide and cobalt-graphitic carbon core-shell based catalysts with remarkably high oxygen reduction reaction activity. Adv. Sci. 2016, 3, 1600060. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, D.; Zhou, Y.; Qin, F.; Wang, H.; Wang, W.; Yang, Y.; Zeng, G. Dual optimization approach to Mo single atom dispersed g-C3N4 photocatalyst: Morphology and defect evolution. Appl. Catal. B Environ. 2022, 303, 120904. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Li, J.; Gao, G.; Zhi, J. Onion-liked carbon-embedded graphitic carbon nitride for enhanced photocatalytic hydrogen evolution and dye degradation. Appl. Catal. B: Environ. 2022, 308, 121216. [Google Scholar] [CrossRef]
- Chen, T.; Yin, D.; Zhao, F.; Kyu, K.K.; Liu, B.; Chen, D.; Huang, K.; Deng, L.; Li, L. Fabrication of 2D heterojunction photocatalyst Co-g-C3N4/MoS2 with enhanced solar-light-driven photocatalytic activity. New J. Chem. 2019, 43, 463–473. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, Z.; Huang, D.; Qin, H.; He, Y.; Chen, M.; Zeng, G.; Xu, P. Ferrocene modified g-C3N4 as a heterogeneous catalyst for photo-assisted activation of persulfate for the degradation of tetracycline. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127024. [Google Scholar] [CrossRef]
- Yang, G.; Liang, Y.J.; Xiong, Z.R.; Yang, J.; Wang, K.; Zeng, Z.K. Molten salt-assisted synthesis of Ce4O7/Bi4MoO9 heterojunction photocatalysts for photo-Fenton degradation of tetracycline: Enhanced mechanism, degradation pathway and products toxicity assessment. Chem. Eng. J. 2021, 425, 130689. [Google Scholar] [CrossRef]
- Shi, C.D.; Yu, S.Y.; Li, C.J. Fabrication of aligned carbon nanofiber doped with SnO2-Sb for efficient electrochemical removal of tetracycline. Chem. Eng. J. 2022, 441, 136052. [Google Scholar] [CrossRef]
- Duan, R.; Ma, S.; Xu, S.; Wang, B.; He, M.; Li, G.; Fu, H.; Zhao, P. Soybean straw biochar activating peroxydisulfate to simultaneously eliminate tetracycline and tetracycline resistance bacteria: Insights on the mechanism. Water Res. 2022, 218, 118489. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Guo, F.; Shi, Y.; Li, L.; Xu, Z.; Yan, X.; Shi, W. Fe-doped g-C3N4 derived from biowaste material with Fe-N bonds for enhanced synergistic effect between photocatalysis and fenton degradation activity in a broad pH range. J. Alloy. Compd. 2022, 900, 163410. [Google Scholar] [CrossRef]
- Bai, X.; Wang, X.; Lu, X.; Liang, Y.; Li, J.; Wu, L.; Li, H.; Hao, Q.; Ni, B.-J.; Wang, C. Surface defective g-C3N4-xClx with unique spongy structure by polarization effect for enhanced photocatalytic removal of organic pollutants. J. Hazard. Mater. 2020, 398, 122897. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. Photo-accelerated Co3+/Co2+ transformation on cobalt and phosphorus Co-doped g-C3N4 for Fenton-like reaction. J. Mater. Chem. A 2021, 9, 22399–22409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).