Heterogeneous Chitosan@copper Catalyzed Selective C(sp3)–H Sulfonylation of Ketone Hydrazones with Sodium Sulfinates: Direct Access to β-Ketosulfones
Abstract
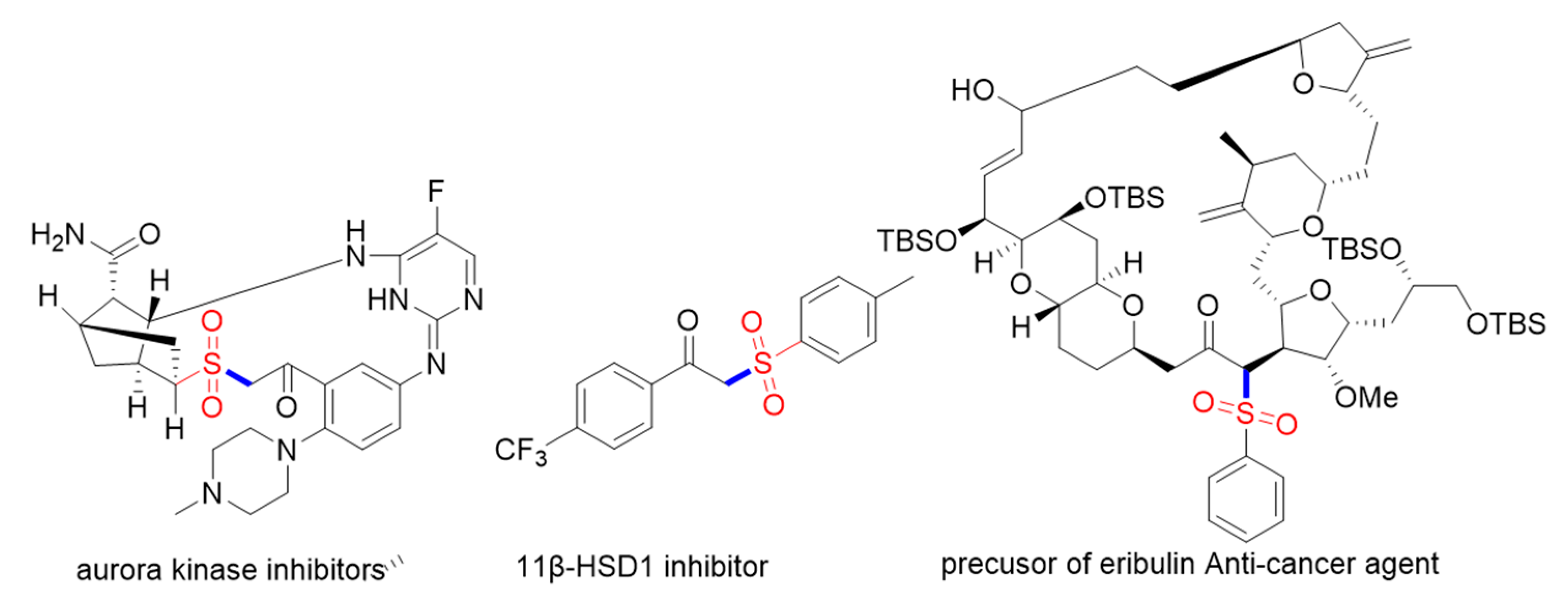
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Synthesis of Biomass-Derived Cu Catalysts
3.3. Preparation of Ketone Hydrazones
3.4. Heterogeneous Cu-Catalyzed Sulfonylation of Ketone Hydrazone with Sodium Sulfinates
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T.; Liu, X. Recent advances in C-S bond formation via C–H bond functionalization and decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.; del Pozo, C.; Sorochinsky, A.; Fustero, S.; Soloshonok, V.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2015, 114, 2432–2506. [Google Scholar] [CrossRef]
- Shen, C.; Xia, H.; Yan, H.; Chen, X.; Ranjit, S.; Xie, X.; Tan, D.; Lee, R.; Yang, Y.; Xing, B.; et al. A concise, efficient synthesis of sugar-based benzothiazoles through chemoselective intramolecular C-S coupling. Chem. Sci. 2012, 3, 2388–2393. [Google Scholar] [CrossRef]
- Xu, J.; Shen, C.; Zhu, X.; Zhang, P.; Ajitha, M.; Huang, K.; An, Z.; Liu, X. Remote C-H Activation of Quinolines through Copper-Catalyzed Radical Cross-Coupling. Chem. Asian J. 2016, 11, 882–892. [Google Scholar] [CrossRef]
- Sarver, P.; Bissonnette, N.; MacMillan, D. Decatungstate-Catalyzed C(sp3)-H Sulfinylation: Rapid Access to Diverse Organosulfur Functionality. J. Am. Chem. Soc. 2021, 143, 9737–9747. [Google Scholar] [CrossRef] [PubMed]
- Mellah, M.; Voituriez, A.; Schulz, E. Electropolymerized Cr-salen complexes for the heterogeneous asymmetric hetero Diels-Alder reaction. Chem. Rev. 2007, 107, 5133–5209. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chen, H.; Tsai, Y. NH2OH-HCl-Mediated Umpolung α-Methylsulfonylation of α-Sulfonyl Ketones with Methylsulfoxides: Synthesis of α, β-Bis-sulfonyl Arylketones. Org. Lett. 2019, 21, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P.; Green, F.; Rose, E. Synthesis of acetylenes from carboxylic acid derivatives via β-keto sulfones. J. Am. Chem. Soc. 1978, 100, 4852–4858. [Google Scholar] [CrossRef]
- Swenson, R.; Sowin, T.; Zhang, H. Synthesis of substituted quinolines using the dianion addition of N-Boc-anilines and alpha-tolylsulfonyl-alpha, beta-unsaturated ketones. J. Org. Chem. 2002, 67, 9182–9185. [Google Scholar] [CrossRef]
- Li, F.; Su, J.; Xu, Y.; Liu, J.; Yu, Y.; Wang, C.; Li, Z.; Li, C.; Wang, L. A glucose oxidase-hemoglobin system for efficient oxysulfonylation of alkenes/alkynes in water. Mol. Catal. 2021, 500, 111336–111341. [Google Scholar] [CrossRef]
- Yang, H.; Carter, R.; Zakharov, L. Enantioselective Total Synthesis of Lycopodine. J. Am. Chem. Soc. 2008, 130, 9238–9239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curti, C.; Laget, M.; Carle, A.; Gellis, A.; Vanelle, P. Rapid synthesis of sulfone derivatives as potential anti-infectious agents. Eur. J. Med. Chem. 2007, 42, 880–884. [Google Scholar] [CrossRef]
- Kumar, A.; Muthyala, M. 1-Butyl-3-methylimidazolium p-toluenesulfinate: A novel reagent for synthesis of sulfones and β-ketosulfones in ionic liquid. Tetrahedron Lett. 2011, 52, 5368–5370. [Google Scholar] [CrossRef]
- Chang, M.; Cheng, Y.; Lu, Y. Bi(OTf)3-Mediated Cycloisomerization of γ-Alkynyl Arylketones: Application to the Synthesis of Substituted Furans. Org. Lett. 2015, 17, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Pampana, V.; Charpe, V.; Saqadevan, D.; Lin, C.; Hwu, J.; Hwang, K. Oxy-sulfonylation of terminal alkynes via C-S coupling enabled by copper photoredox catalysis. Green Chem. 2021, 23, 3569–3574. [Google Scholar] [CrossRef]
- Wen, J.; Yang, X.; Sun, Z.; Yang, J.; Han, P.; Liu, Q.; Dong, H.; Gu, M.; Huang, L.; Wang, H. Biomimetic photocatalytic sulfonation of alkenes to access β-ketosulfones with single-atom iron site. Green Chem. 2020, 22, 230–237. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, J.; Zhao, G.; Qi, Y.; Wang, H.; Lei, A. Dioxygen-Triggered Oxidative Radical Reaction: Direct Aerobic Difunctionalization of Terminal Alkynes toward β-Keto Sulfones. J. Am. Chem. Soc. 2013, 135, 11481–11487. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, J.; Peng, P.; Zhang, G.; Huang, Z.; Yi, H.; Miller, J.; Lei, A. Operando X-ray absorption and EPR evidence for a single electron redox process in copper catalysis. Chem. Sci. 2015, 6, 4851–4854. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Kumar, A. Amino Acid-Catalyzed Direct Synthesis of β-Keto Sulfones via Aerobic Difunctionalization of Terminal Alkynes in an Aqueous Medium. ACS Sustain. Chem. Eng. 2019, 7, 9182–9188. [Google Scholar] [CrossRef]
- Vennstra, G.; Zwaneburg, B. An Improved Synthesis of Sulfones using Tetrabutyl-ammonium Sulfinates. Synthesis 1975, 8, 519–520. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Z. Hypervalent iodine in synthesis 66: One pot preparation of b-keto sulfones by reaction of ketones, [hydroxy(tosyloxy)iodo]benzene, and sodium sulfinates. Synth. Commun. 2001, 31, 3145–3149. [Google Scholar] [CrossRef]
- Lin, B.; Kuang, J.; Chen, J.; Hua, Z.; Khakyzadeh, V.; Xia, Y. A one-pot protocol for the synthesis of beta-ketosulfones from alpha, alpha-dibromoketones. Org. Chem. Front. 2019, 6, 2647–2653. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, Y.; Chen, J.; Yu, X.; Xiao, W.; Chen, J. Copper-Catalyzed Radical Cross-Coupling of Oxime Esters and Sulfinates for Synthesis of Cyanoalkylated Sulfones. ChemCatChem 2019, 11, 5300–5306. [Google Scholar] [CrossRef]
- He, J.; Chen, G.; Zhang, B.; Xiao, W.J.; Liu, F.; Li, C. Catalytic Decarboxylative Radical Sulfonylation. Chem 2020, 6, 1149–1159. [Google Scholar] [CrossRef]
- Chen, G.; He, S.; Cheng, L.; Liu, F. Copper-Catalyzed N-Directed Distal C(sp3)–H Sulfonylation and Thiolation with Sulfinate Salts. Org. Lett. 2021, 23, 8338–8342. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Kumari, A.; Kumar, J. Recent advances in the synthesis and applications of β-keto sulfones: New prospects for the synthesis of β-keto thiosulfones. Org. Biomol. Chem. 2021, 19, 3087–3118. [Google Scholar] [CrossRef]
- Ghosh, S.; Samanta, S.; Ghosh, A.; Neogi, S.; Hajra, A. Advances in Oxosulfonylation Reaction. Adv. Synth. Catal. 2020, 362, 4552–4581. [Google Scholar] [CrossRef]
- Chawla, R.; Singh, A.; Yadav, L. K2S2O8-Mediated Aerobic Oxysulfonylation of Olefins into β-Keto Sulfones in Aqueous Media. Eur. J. Org. Chem. 2014, 2014, 2032–2036. [Google Scholar] [CrossRef]
- Handa, S.; Fennewald, J.; Lipshutz, B. Aerobic Oxidation in Nanomicelles of Aryl Alkynes, in Water at Room Temperature. Angew. Chem. Int. Ed. 2014, 53, 3432–3435. [Google Scholar] [CrossRef]
- Singh, A.; Chawla, R.; Yadav, L. An organocatalytic synthesis of N-sulfonyl imines using chloramine-T in aqueous medium. Tetrahedron Lett. 2014, 55, 2845–2848. [Google Scholar] [CrossRef]
- Reddy, R.; Kumar, J.; Kumari, A. Unprecedented Reactivity of β-Iodovinyl Sulfones: An Efficient Synthesis of β-Keto Sulfones and β-Keto Thiosulfones. Eur. J. Org. Chem. 2019, 2019, 3771–3775. [Google Scholar] [CrossRef]
- Reddy, R.; Kumari, A. Synthesis and applications of sodium sulfinates (RSO2Na): A powerful building block for the synthesis of organosulfur compounds. RSC Adv. 2021, 11, 9130–9221. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.; Hamze, A. An update on the use of sulfinate derivatives as versatile coupling partners in organic chemistry. Org. Biomol. Chem. 2020, 45, 9136–9161. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hofman, K.; Friedrich, M.; Manolikakes, G. Recent Advances in the Synthesis and Direct Application of Sulfinate Salts. Eur. J. Org. Chem. 2020, 2020, 4664–4676. [Google Scholar] [CrossRef]
- Smith, J.; Dixon, J.; deGruyter, J.; Baran, P. Alkyl Sulfinates: Radical Precursors Enabling Drug Discovery. J. Med. Chem. 2019, 62, 2256–2280. [Google Scholar] [CrossRef]
- Wei, W.; Liu, C.; Yang, D.; Wen, J.; You, J.; Suo, Y.; Wang, H. Copper-catalyzed direct oxysulfonylation of alkenes with dioxygen and sulfonylhydrazides leading to β-ketosulfones. Chem. Commun. 2013, 49, 10239–10241. [Google Scholar] [CrossRef]
- Tang, X.; Huang, L.; Xu, Y.; Yang, J.; Wu, W.; Jiang, H. Copper-Catalyzed Coupling of Oxime Acetates with Sodium Sulfinates: An Efficient Synthesis of Sulfone Derivatives. Angew. Chem. Int. Ed. 2014, 53, 4205–4208. [Google Scholar] [CrossRef]
- To, T.; Tran, C.; Nguyen, N.; Nguyen, H.; Nguyen, A.; Phan, A.; Phan, N. An efficient access to β-ketosulfones via β-sulfonylvinylamines: Metal-organic framework catalysis for the direct C-S coupling of sodium sulfinates with oxime acetates. RSC Adv. 2018, 8, 17477–17485. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Huang, X.; You, S.; Cai, M. Heterogeneous copper-catalyzed oxidative coupling of oxime acetates with sodium sulfinates: An efficient and practical synthesis of β-keto sulfones. Appl. Organometal. Chem. 2019, 33, 5001–5011. [Google Scholar] [CrossRef]
- Xu, J.; Shen, C.; Qin, X.; Wu, J.; Zhang, P.; Liu, X. Oxidative Sulfonylation of Hydrazones Enabled by Synergistic Copper/Silver Catalysis. J. Org. Chem. 2021, 86, 3706–3720. [Google Scholar] [CrossRef]
- Lan, X.; Wang, N.; Bai, C.; Zhang, W.; Xing, Y.; Wen, J.; Wang, Y.; Li, Y. Ligand-Mediated and Copper-Catalyzed C(sp3)-H Bond Functionalization of Aryl Ketones with Sodium Sulfinates under Mild Conditions. Sci. Rep. 2015, 5, 18391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavari, I.; Shaabanzadeh, S. Electrochemical Synthesis of β-Ketosulfones from Switchable Starting Materials. Org. Lett. 2020, 22, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Huang, B.; Wei, W.; Li, J.; Lin, G.; Liu, Y.; Ding, J.; Sun, P.; Wang, H. Visible-light initiated direct oxysulfonylation of alkenes with sulfinic acids leading to β-ketosulfones. Green Chem. 2016, 18, 5630–5634. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Z.; Zhang, Y.; Xu, J.; Gang, X.; Shen, C.; Zhang, P. Selective Mono- and Diamination of Ketones in a Combined Copper-Organocatalyst System. Org. Lett. 2022, 24, 3614–3619. [Google Scholar] [CrossRef]
- Molnár, Á. The use of chitosan-based metal catalysts in organic transformations. Coord. Chem. Rev. 2019, 388, 126–171. [Google Scholar] [CrossRef]
- Hammi, N.; Chen, S.; Michon, C.; Royer, S.; El Kadib, A. Cu nanoparticles embedded on reticular chitosan-derived N-doped carbon: Application to the catalytic hydrogenation of alkenes, alkynes and N-heteroarenes. Mol. Catal. 2022, 519, 112104–112112. [Google Scholar] [CrossRef]
- Shen, C.; Xu, J.; Yu, W.; Zhang, P. A highly active and easily recoverable chitosan@copper catalyst for the C-S coupling and its application in the synthesis of zolimidine. Green Chem. 2014, 16, 3007–3012. [Google Scholar] [CrossRef]
- Hertrich, M.; Scharnagl, F.; Pews-Davtyan, A.; Kreyenschulte, C.; Lund, H.; Bartling, S.; Jackstell, R.; Beller, M. Supported Cobalt Nanoparticles for Hydroformylation Reactions. Chem. Eur. J. 2019, 25, 5534–5538. [Google Scholar] [CrossRef]
- Sahoo, B.; Surkus, A.; Pohl, M.; Radnik, J.; Schneider, M.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. A Biomass-Derived Non-Noble Cobalt Catalyst for Selective Hydrodehalogenation of Alkyl and (Hetero)Aryl Halides. Angew. Chem. Int. Ed. 2017, 56, 11242–11247. [Google Scholar] [CrossRef]
- Sahoo, B.; Formenti, D.; Topf, C.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. Biomass-Derived Catalysts for Selective Hydrogenation of Nitroarenes. ChemSusChem 2017, 10, 3035–3039. [Google Scholar] [CrossRef]
- Shen, C.; Xu, J.; Ying, B.; Zhang, P. Heterogeneous Chitosan@Copper(II)-Catalyzed Remote Trifluoromethylation of Aminoquinolines with the Langlois Reagent by Radical Cross-Coupling. ChemCatChem 2016, 8, 3560–3564. [Google Scholar] [CrossRef]
- Shen, H.; Shen, C.; Chen, C.; Wang, A.; Zhang, P. Novel glycosyl pyridyl-triazole@palladium nanoparticles: Efficient and recoverable catalysts for C-C cross-coupling reactions. Catal. Sci. Technol. 2015, 5, 2065–2071. [Google Scholar] [CrossRef]
- Ying, B.; Xu, J.; Zhu, X.; Shen, C.; Zhang, P. Catalyst-Controlled Selectivity in the Synthesis of C2- and C3-Sulfonate Esters from Quinoline N-Oxides and Aryl Sulfonyl Chlorides. ChemCatChem 2016, 8, 2604–2608. [Google Scholar] [CrossRef]
- Guo, L.; An, Q.; Xiao, Z.; Zhai, S.; Cui, L. Inherent N-doped Honeycomb-like Carbon/Fe3O4 Composites with Versatility for Efficient Microwave Absorption and Wastewater Treatment. ACS Sustain. Chem. Eng. 2019, 7, 9237–9248. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, F.; Zhang, F.; Dong, Z. Renewable chitosan-derived Cobalt@N-doped porous carbon for efficient aerobic esterification of alcohols under air. Nanoscale 2019, 11, 17736–17745. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Tang, X.; Wang, P.; Zhao, Z.; Ba, X.; Jiang, Y.; Zhang, X. Metal-Organic Frameworks Decorated Cu2O Heterogeneous Catalysts for Selective Oxidation of Styrene. Catalysts 2022, 5, 487. [Google Scholar] [CrossRef]
- Zhan, G.; Fan, L.; Zhao, F.; Huang, Z.; Chen, B.; Yang, X.; Zhou, S. Fabrication of Ultrathin 2D Cu-BDC Nanosheets and the Derived Integrated MOF Nanocomposites. Adv. Funct. Mater. 2019, 29, 1806720. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of Polycrystalline Copper Thin Films at Ambient Conditions. J. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the Activity Origin of Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2020, 132, 5388–5392. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; Chen, J.; Wang, X.; Mai, Y. Efficient and Stable Cu-Cu2O@NC Catalysts for Selective Catalytic Conversion of Glycerol to Lactic Acid. ChemCatChem 2023, 15, e202201139. [Google Scholar] [CrossRef]
- Kar, A.; Srivastava, R. Selective Synthesis of Cu-Cu2O/C and CuO-Cu2O/C Catalysts for Pd Free C-C, C-N Coupling and Oxidation Reactions. Inorg. Chem. Front. 2019, 6, 576–589. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, L.; Zhao, B.; Huang, L.; Yu, S.; Ragauskas, A. Highly selective hydrogenation of phenol to cyclohexanone over a Pd-loaded N-doped carbon catalyst derived from chitosan. J. Colloid Interface Sci. 2022, 605, 82–90. [Google Scholar] [CrossRef]
- Li, Z.; Wen, C.; Wang, R.; Zheng, H.; Xie, K. Chloride-free Cu2O/AC catalyst prepared by pyrolysis of copper acetate and catalytic oxycarbonylation. Chem. J. Chin. Univ. 2009, 10, 2024–2031. [Google Scholar]
- Chang, M.-Y.; Cheng, Y.-C.; Lu, Y.-J. Synthesis of substituted benzenes via Bi(OTf)3-mediated intramolecular carbonyl allylation of α-prenyl or α-geranyl β-arylketosulfones. Org. Lett. 2015, 17, 3142–3145. [Google Scholar] [CrossRef]
- Wang, N.; Liu, L.; Xu, W.; Zhang, M.; Huang, Z.; Shi, D.; Zhao, Y. Rhodium(III)-Catalyzed Oxidative Annulation of Ketoximes with Sulfonamide: A Direct Approach to Indazoles. Org. Lett. 2019, 21, 365–368. [Google Scholar] [CrossRef]
- Wei, Y.; Yoshikai, N. Modular pyridine synthesis from oximes and enals through synergistic copper/iminium catalysis. J. Am.Chem. Soc. 2013, 135, 3756–3759. [Google Scholar] [CrossRef]
- Tsui, G.C.; Glenadel, Q.; Lau, C.; Lautens, M. Rhodium(I)-catalyzed addition of arylboronic acids to (benzyl-/arylsulfonyl)acetonitriles: Efficient synthesis of (Z)-β-sulfonylvinylamines and β-keto sulfones. Org. Lett. 2011, 13, 208–211. [Google Scholar] [CrossRef]
- Deng, S.; Liang, E.; Wu, Y.; Tang, X. Efficient sulfonylation of ketones with sodium sulfinates for the synthesis of β-keto sulfones. Tetrahedron Lett. 2018, 59, 3955–3957. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, Y.; Zhang, Y.; Yu, S. Sulfonation and trifluoromethylation of enol acetates with sulfonyl chlorides using visible-light photoredox catalysis. Eur. J. Org. Chem. 2013, 2013, 5485–5492. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, F.; Yue, H.; Zhao, X.; Li, J.; Wei, W. Photocatalyst-free visible light-induced synthesis of β-oxo sulfones via oxysulfonylation of alkenes with arylazo sulfones and dioxygen in air. Adv. Synth. Catal. 2019, 361, 5277–5282. [Google Scholar] [CrossRef]
- Xiong, Y.-S.; Weng, J.; Lu, G. Manganese(III)-mediated and -catalyzed decarboxylative hydroxysulfonylation of arylpropiolic acids with sodium sulfinates in water. Adv. Synth. Catal. 2018, 360, 1611–1616. [Google Scholar] [CrossRef]
- Singh, A.K.; Chawla, R.; Keshari, T.; Yadav, V.K.; Yadav, L.D.S. Aerobic oxysulfonylation of alkenes using thiophenols: An efficient one-pot route to β-ketosulfones. Org. Biomol. Chem. 2014, 12, 8550–8554. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-Y.; Cheng, Y.-C.; Lu, Y.-J. One-pot access to sulfonylmethyl arylpyrroles via the domino aerobic wacker-type aminocyclization/1,4-sulfonyl migration. Org. Lett. 2014, 16, 6252–6255. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Ji, Q.; Liao, P.; Anderson, E.A.; Bi, X. Silver-catalyzed stereoselective aminosulfonylation of alkynes. Angew. Chem. Int. Ed. 2017, 56, 13805–13808. [Google Scholar] [CrossRef] [PubMed] [Green Version]









 | ||||
|---|---|---|---|---|
| Entry | Catalyst (mol %) | Oxidant | Solvent | Yield (%) b |
| 1 | CuxOy@CS-300 | Na2S2O8 | acetone | 21 |
| 2 | CuxOy@CS-400 | Na2S2O8 | acetone | 56 |
| 3 | CuxOy@CS-500 | Na2S2O8 | acetone | trace |
| 4 | Cu | Na2S2O8 | acetone | trace |
| 5 | Cu2O | Na2S2O8 | acetone | 55 |
| 6 | CuO | Na2S2O8 | acetone | trace |
| 7 | - | Na2S2O8 | acetone | 0 |
| 8 | CuxOy@CS-400 | K2S2O8 | acetone | 81 |
| 9 | CuxOy@CS-400 | (NH4)2S2O8 | acetone | 41 |
| 10 | CuxOy@CS-400 | K2S2O8 | MeCN | 23 |
| 11 | CuxOy@CS-400 | K2S2O8 | H2O | 0 |
| 12 | CuxOy@CS-400 | K2S2O8 | toluene | 35 |
| 13 | CuxOy@CS-400 | K2S2O8 | acetone | 82 c |
| 14 | CuxOy@CS-400 | K2S2O8 | acetone | 57 d |
| 15 | CuxOy@CS-400 | K2S2O8 | acetone | 80 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, J.; Zheng, K.; Lin, Z.; Jin, H.; Yu, W.; Shen, C.; Jia, A.; Zhang, Q. Heterogeneous Chitosan@copper Catalyzed Selective C(sp3)–H Sulfonylation of Ketone Hydrazones with Sodium Sulfinates: Direct Access to β-Ketosulfones. Catalysts 2023, 13, 726. https://doi.org/10.3390/catal13040726
Qiao J, Zheng K, Lin Z, Jin H, Yu W, Shen C, Jia A, Zhang Q. Heterogeneous Chitosan@copper Catalyzed Selective C(sp3)–H Sulfonylation of Ketone Hydrazones with Sodium Sulfinates: Direct Access to β-Ketosulfones. Catalysts. 2023; 13(4):726. https://doi.org/10.3390/catal13040726
Chicago/Turabian StyleQiao, Jun, Kai Zheng, Zhiwei Lin, Huiye Jin, Wenbo Yu, Chao Shen, Aiquan Jia, and Qianfeng Zhang. 2023. "Heterogeneous Chitosan@copper Catalyzed Selective C(sp3)–H Sulfonylation of Ketone Hydrazones with Sodium Sulfinates: Direct Access to β-Ketosulfones" Catalysts 13, no. 4: 726. https://doi.org/10.3390/catal13040726
APA StyleQiao, J., Zheng, K., Lin, Z., Jin, H., Yu, W., Shen, C., Jia, A., & Zhang, Q. (2023). Heterogeneous Chitosan@copper Catalyzed Selective C(sp3)–H Sulfonylation of Ketone Hydrazones with Sodium Sulfinates: Direct Access to β-Ketosulfones. Catalysts, 13(4), 726. https://doi.org/10.3390/catal13040726






