Enabling Catalysts for Biodiesel Production via Transesterification
Abstract
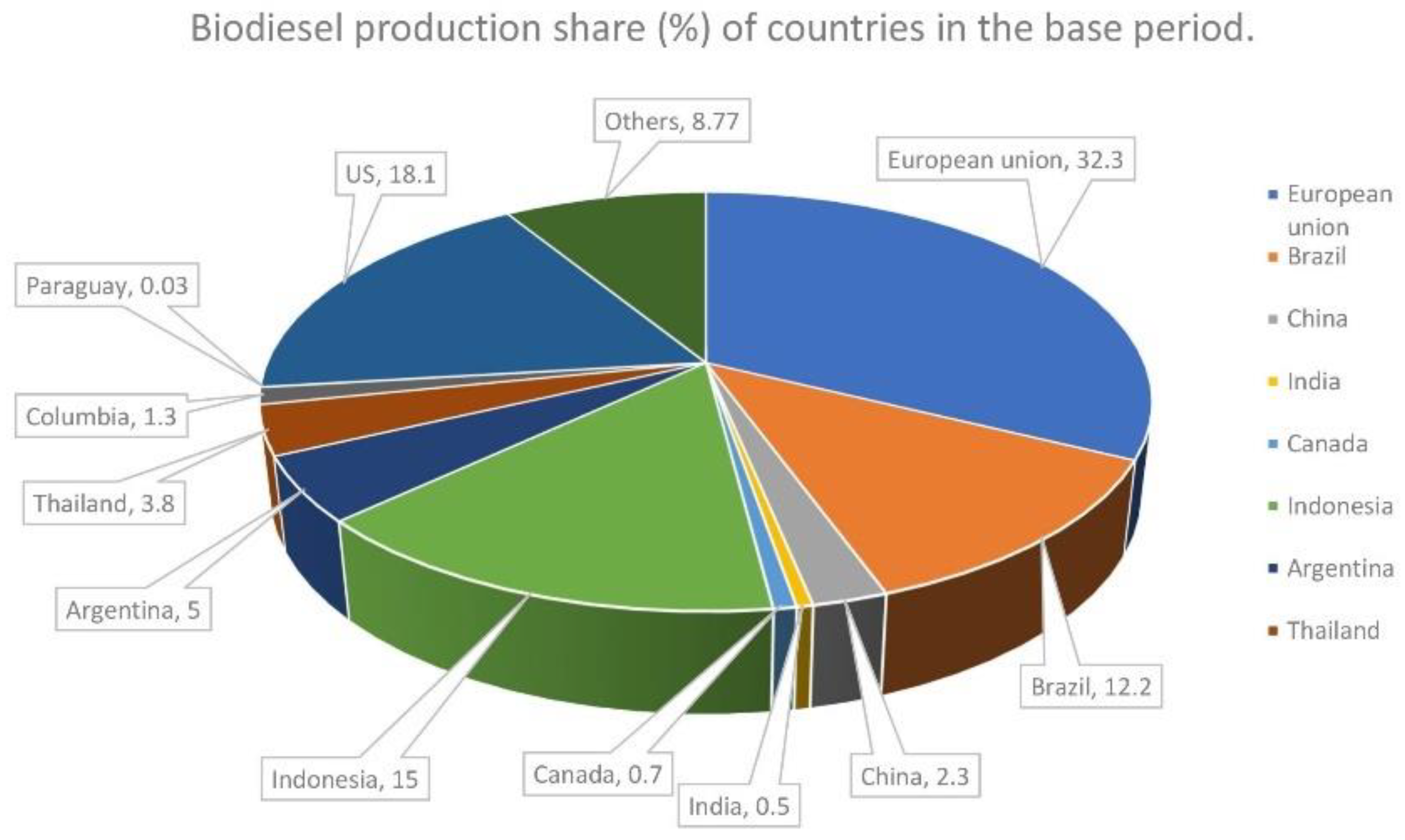
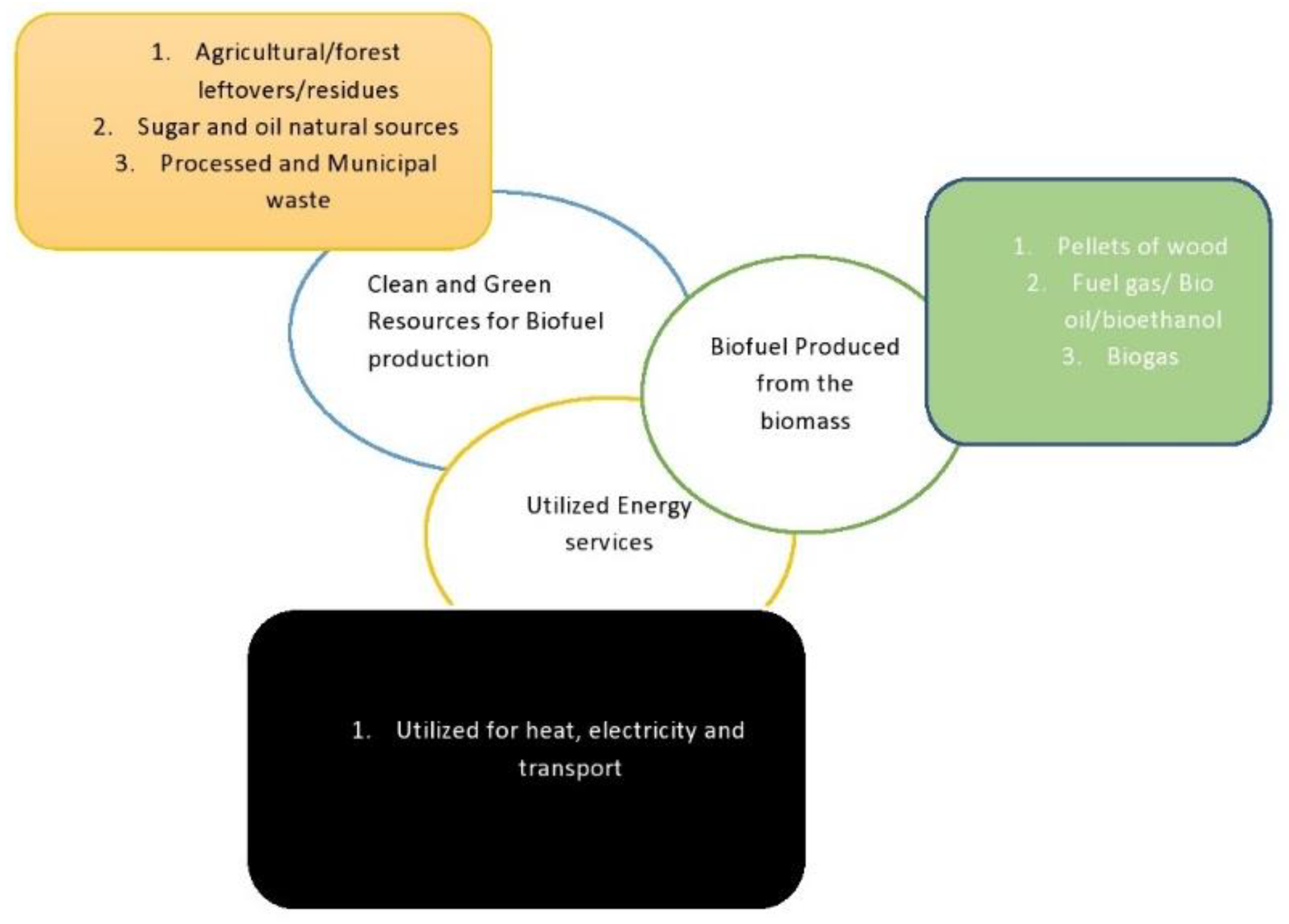
:1. Introduction
2. Homogeneous Catalysts for Biodiesel Production
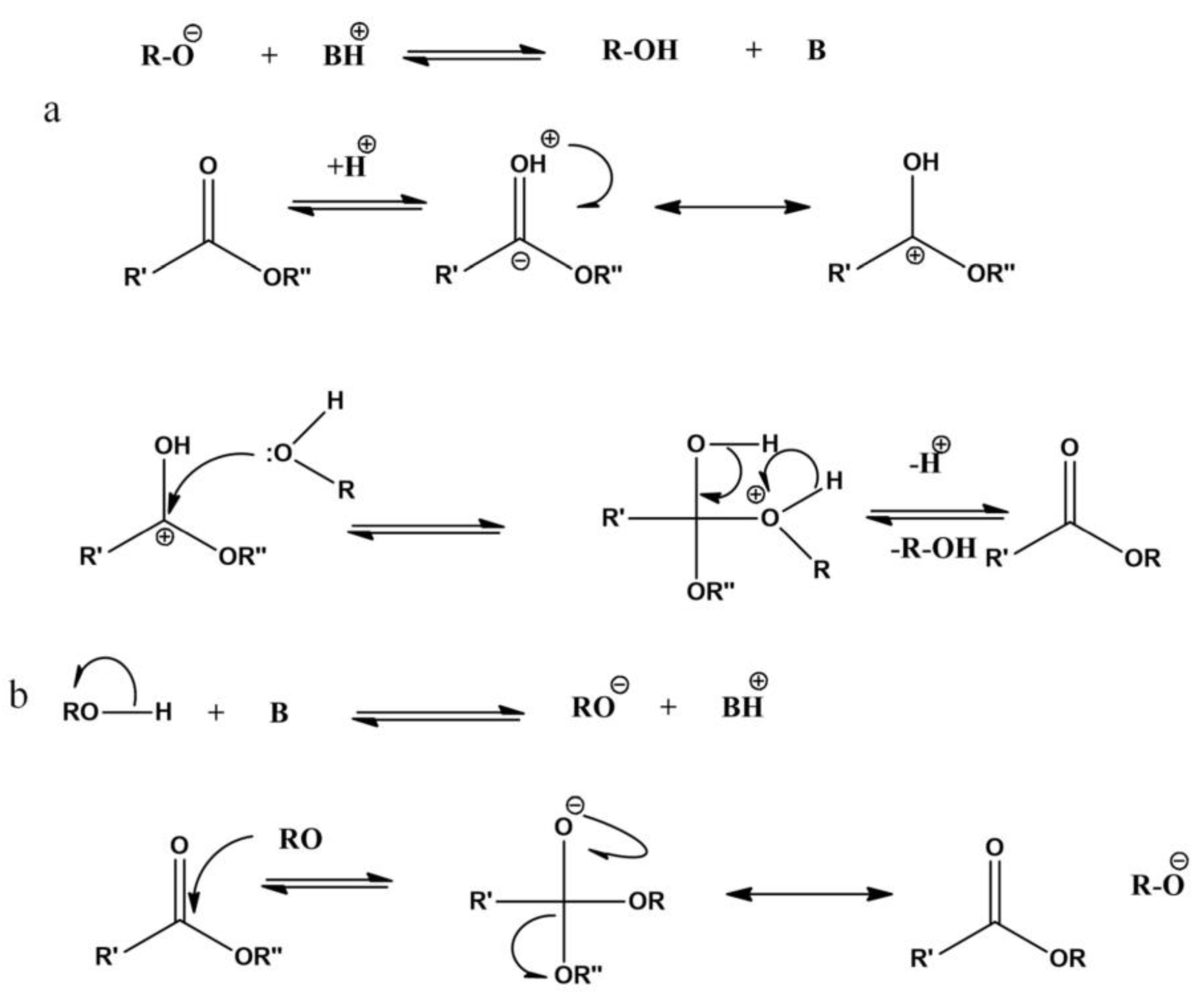
2.1. The Mechanism of Homogeneous Catalysts
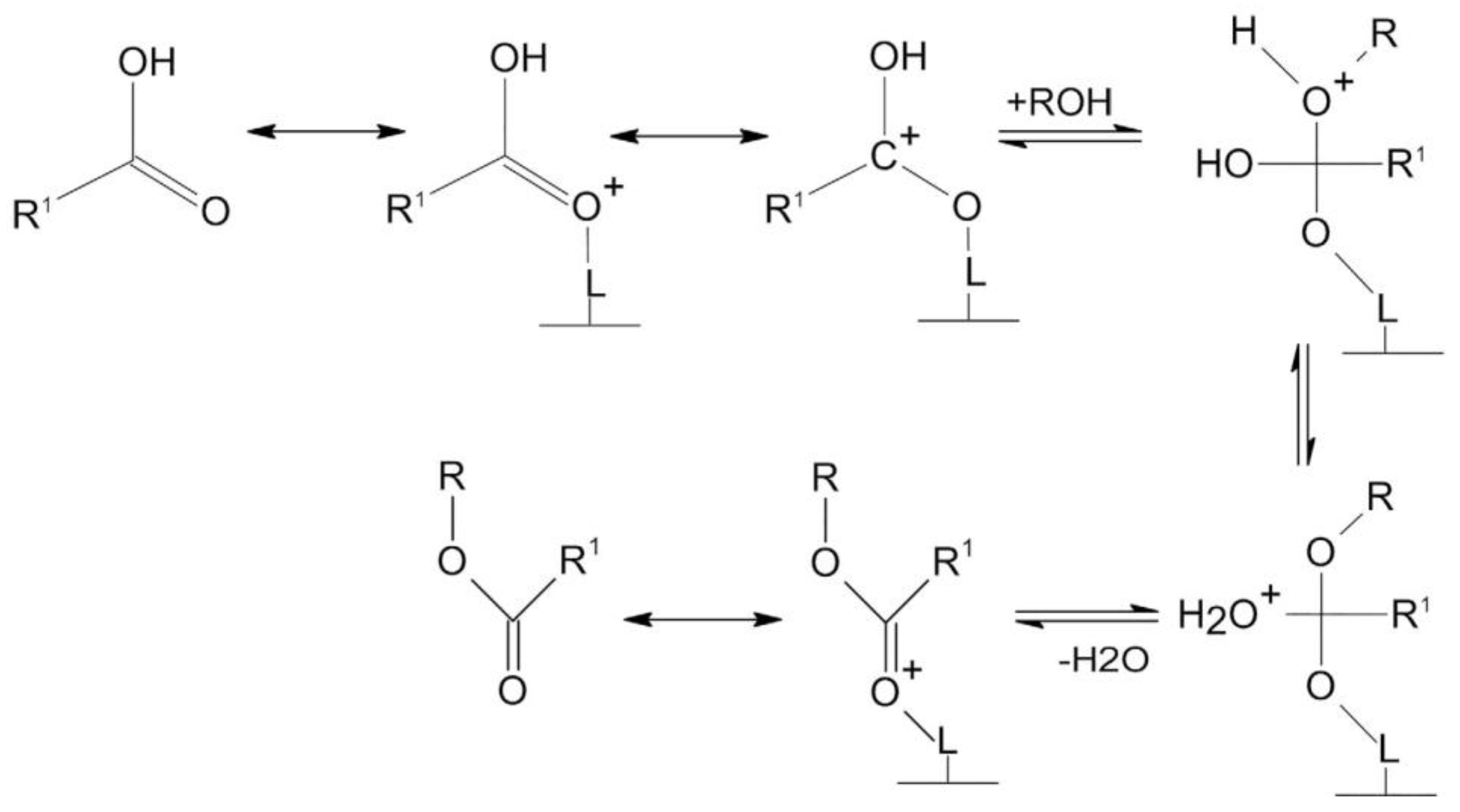
2.2. Homogeneous acid Catalysts
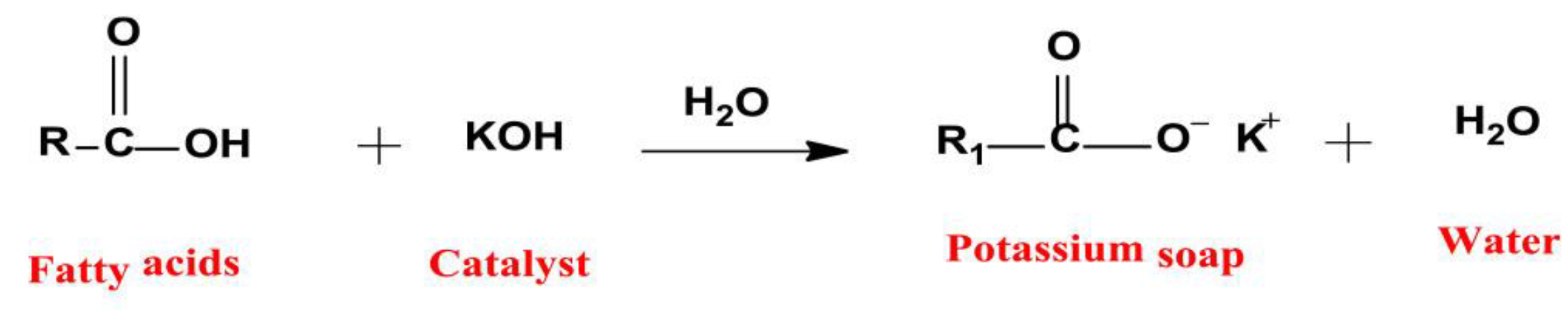
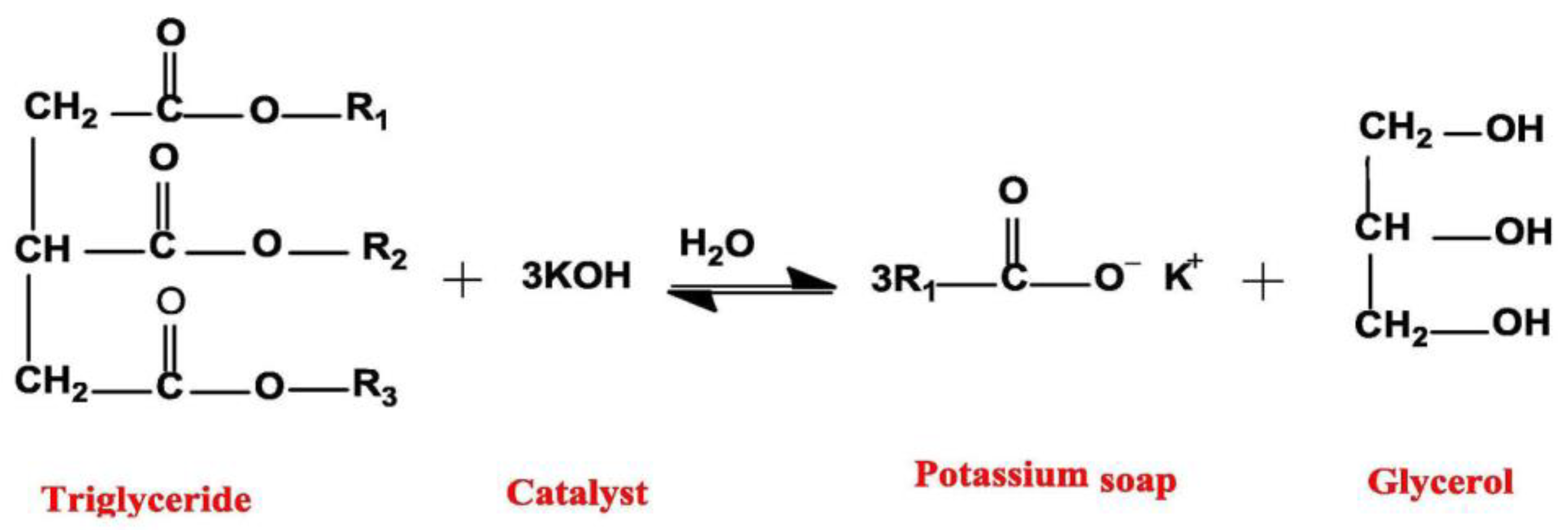
2.3. Homogeneous Base Catalysts
2.4. Derived Homogeneous Catalysts: Ionic Liquids/Deep Eutectic Solvents
3. Heterogeneous Catalysts for Biodiesel Production
3.1. The Mechanism of Heterogenous Catalysts
3.2. Heterogenous Acid Catalysts
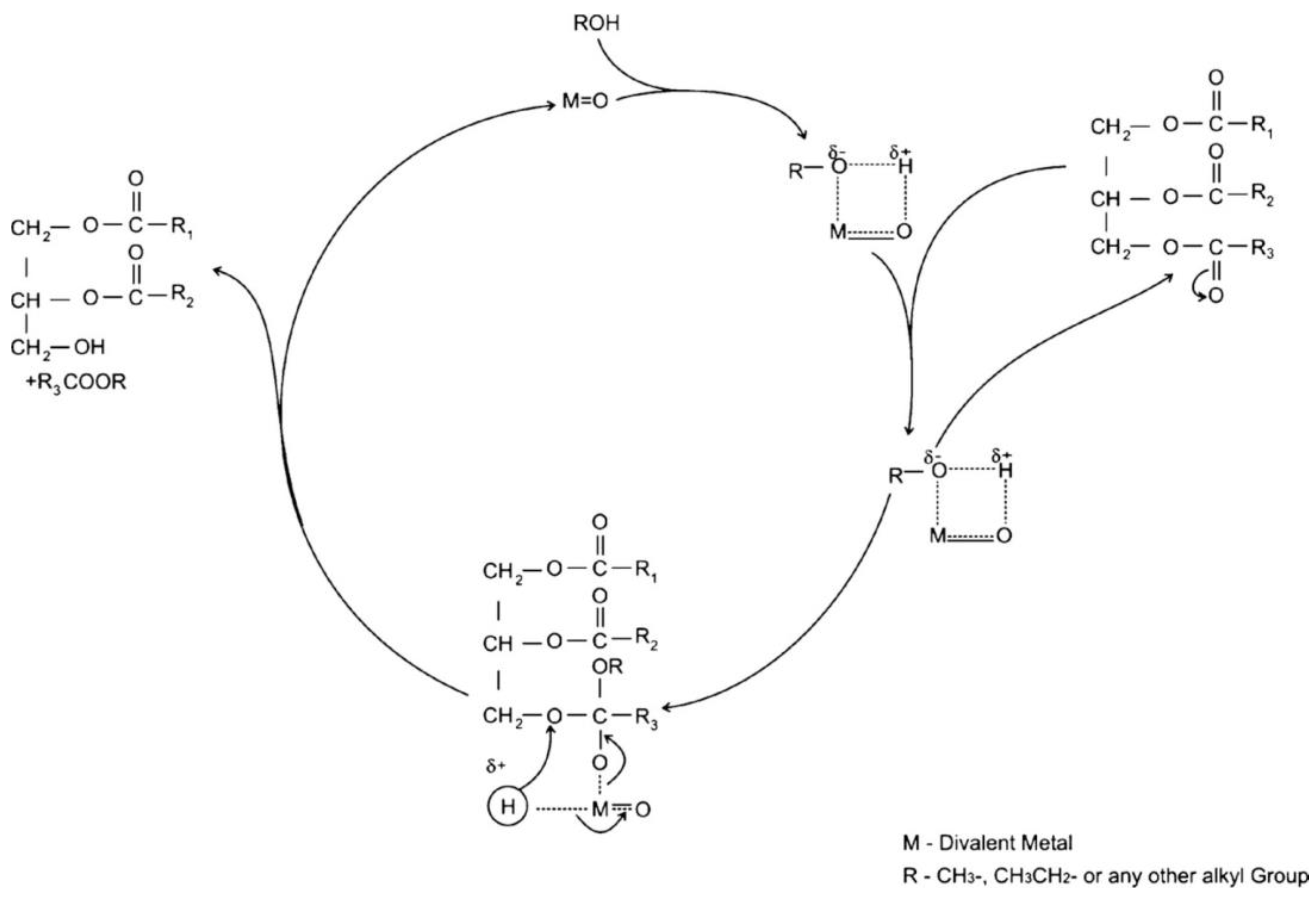
3.3. Heterogenous Base Catalysts
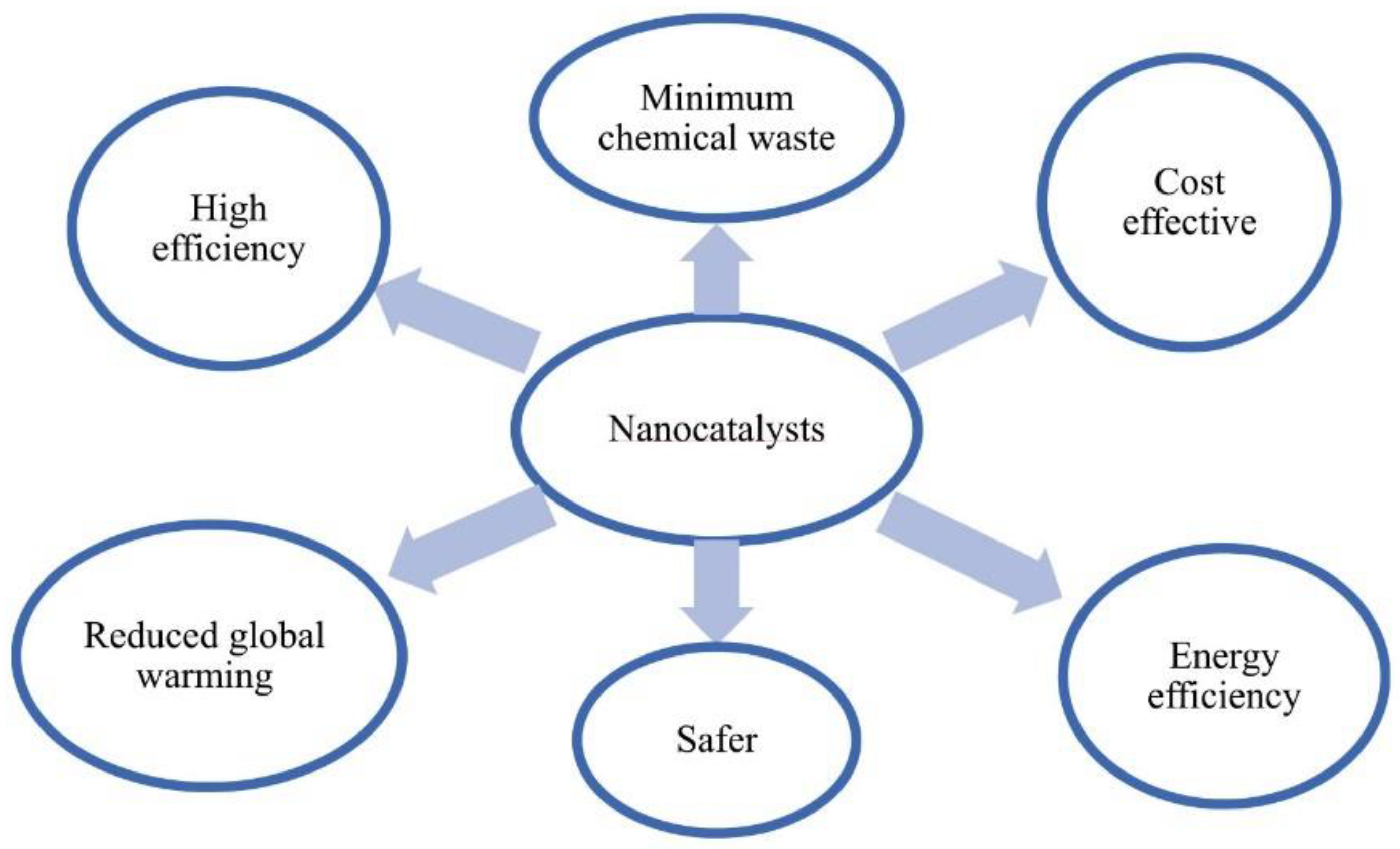
3.4. Derived Heterogenous Catalysts: Nanocatalysts/Magnetic Catalysts
4. The Advantages and Disadvantages of Different Catalysts for Biodiesel Production
5. Economic Considerations of Catalysts for Biodiesel Production
6. Challenges and Future Perspectives
- Currently, most of the catalysts used in biodiesel industrial production are homogeneous catalysts, but these catalysts are not applicable to all types of feedstocks. Moreover, homogeneous catalysts have the problem of not being reused or regenerated, which greatly increases the cost of biodiesel production.
- Homogeneous catalysts suffer from difficulties in separation. Heterogeneous solid catalysts are simple to separate, but still fall short of the expected goals for industrial use, and the residual catalyst has a large impact on biodiesel quality.
- Short catalyst lifetime, low reaction rate, and high fabrication cost are the main problems of heterogeneous catalysts.
- In the case of homogeneous catalysts, there are the problems of catalyst poisoning and contamination. In addition, active site leaching and saponification problems can lead to significant contamination generation.
- It is necessary to further accelerate the transition of heterogeneous catalysts from laboratory research to industrial applications to enrich the existing catalyst types and achieve industrial-scale applications for biodiesel. Meanwhile, the development of homogeneous catalyst-derived ionic liquid catalysts is promoted to improve the stability and reusability of homogeneous catalysts.
- Explore the recycling potential of magnetic nanocatalysts and improve the reuse performance of commercial catalysts.
- Develop efficient biomass-derived catalysts for biodiesel production to reduce the associated costs. The introduction of HPA heterogeneous catalysts and nanocatalysts with excellent catalytic properties has improved the reaction efficiency and increased the service life of the catalysts.
- Explore environmentally friendly green catalysts, such as DES, and develop efficient biomass-derived green catalysts for biodiesel production.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Quah, R.V.; Tan, Y.H.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Nolasco-Hipolito, C. An overview of biodiesel production using recyclable biomass and non-biomass derived magnetic catalysts. J. Environ. Chem. Eng. 2019, 7, 103219. [Google Scholar] [CrossRef]
- Bateni, H.; Saraeian, A.; Able, C. A comprehensive review on biodiesel purification and upgrading. Biofuel Res. J. 2017, 4, 668–690. [Google Scholar] [CrossRef]
- Esmi, F.; Borugadda, V.B.; Dalai, A.K. Heteropoly acids as supported solid acid catalysts for sustainable biodiesel production using vegetable oils: A review. Catal. Today 2022, 404, 19–34. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, T.; Cheng, C.; Wang, Q.; Lin, S.; Liu, C.; Han, X. Research Progress on Synthesis and Application of Cyclodextrin Polymers. Molecules 2021, 26, 1090. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.C.; Tiong, Y.W.; Goh, B.H.H.; Gan, Y.Y.; Mofijur, M.; Fattah, I.M.R.; Chong, C.T.; Alam, M.A.; Lee, H.V.; Silitonga, A.S.; et al. Recent advances in biodiesel production from agricultural products and microalgae using ionic liquids: Opportunities and challenges. Energy Convers. Manag. 2021, 228, 113647. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Sharma, G. Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel 2022, 311, 122543. [Google Scholar] [CrossRef]
- Jayed, M.H.; Masjuki, H.H.; Saidur, R.; Kalam, M.A.; Jahirul, M.I. Environmental aspects and challenges of oilseed produced biodiesel in Southeast Asia. Renew. Sustain. Energy Rev. 2009, 13, 2452–2462. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Kalam, M.A.; Masjuki, H.H.; Wakil, M.A. Biodiesel production, characterization, engine performance, and emission characteristics of Malaysian Alexandrian laurel oil. RSC Adv. 2014, 4, 17787–17796. [Google Scholar] [CrossRef]
- Ameen, M.; Ahmad, M.; Zafar, M.; Munir, M.; Mujtaba, M.M.; Sultana, S.; Rozina; El-Khatib, S.E.; Soudagar, M.E.M.; Kalam, M.A. Prospects of Catalysis for Process Sustainability of Eco-Green Biodiesel Synthesis via Transesterification: A State-Of-The-Art Review. Sustainability 2022, 14, 7032. [Google Scholar] [CrossRef]
- Zhu, L.; Kong, L.; Zhang, C. Numerical Study on Hysteretic Behaviour of Horizontal-Connection and Energy-Dissipation Structures Developed for Prefabricated Shear Walls. Appl. Sci. 2020, 10, 1240. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Kansedo, J.; Mubarak, N.M.; Chan, Y.S.; Nolasco-Hipolito, C. Biodiesel production from used cooking oil using green solid catalyst derived from calcined fusion waste chicken and fish bones. Renew. Energy 2019, 139, 696–706. [Google Scholar] [CrossRef]
- Hanif, M.; Bhatti, I.A.; Shahzad, K.; Hanif, M.A. Biodiesel Production from Waste Plant Oil over a Novel Nano-Catalyst of Li-TiO2/Feldspar. Catalysts 2023, 13, 310. [Google Scholar] [CrossRef]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Das, B.; Mohanty, K. A review on advances in sustainable energy production through various catalytic processes by using catalysts derived from waste red mud. Renew. Energy 2019, 143, 1791–1811. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Koranian, P.; Huang, Q.; Dalai, A.K.; Sammynaiken, R. Chemicals Production from Glycerol through Heterogeneous Catalysis: A Review. Catalysts 2022, 12, 122543. [Google Scholar] [CrossRef]
- Chandra Kishore, S.; Perumal, S.; Atchudan, R.; Sundramoorthy, A.K.; Alagan, M.; Sangaraju, S.; Lee, Y.R. A Review of Biomass-Derived Heterogeneous Catalysts for Biodiesel Production. Catalysts 2022, 12, 1501. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Costantini, A.; Califano, V. Lipase Immobilization in Mesoporous Silica Nanoparticles for Biofuel Production. Catalysts 2021, 11, 629. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Wang, F.-M.; Dinh, K.K.; Pham, T.T.; Juan, H.-Y.; Nguyen, N.P.; Ong, H.C.; Su, C.-H. Microwave-Assisted Noncatalytic Esterification of Fatty Acid for Biodiesel Production: A Kinetic Study. Energies 2020, 13, 2167. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.-H.; Li, S.-Y.; Su, C.-H.; Chien, C.-C.; Chen, Y.-J.; Huong, D.T.M. Direct transesterification of black soldier fly larvae (Hermetia illucens) for biodiesel production. J. Taiwan Inst. Chem. Eng. 2018, 85, 165–169. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.-L.; Su, C.-H.; Ong, H.C.; Juan, H.-Y.; Wu, S.-J. Bio-Derived Catalysts: A Current Trend of Catalysts Used in Biodiesel Production. Catalysts 2021, 11, 812. [Google Scholar] [CrossRef]
- de Lima, A.L.; Ronconi, C.M.; Mota, C.J.A. Heterogeneous basic catalysts for biodiesel production. Catal. Sci. Technol. 2016, 6, 2877–2891. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E.; Gumbytė, M.J.C. Rapeseed Oil Transesterification Using 1-Butanol and Eggshell as a Catalyst. Catalysts 2023, 13, 302. [Google Scholar] [CrossRef]
- Ling, J.S.J.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Saptoro, A.; Nolasco-Hipolito, C. A review of heterogeneous calcium oxide based catalyst from waste for biodiesel synthesis. SN Appl. Sci. 2019, 1, 810. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. The effects of catalysts in biodiesel production: A review. J. Ind. Eng. Chem. 2013, 19, 14–26. [Google Scholar] [CrossRef]
- Islam, A.; Taufiq-Yap, Y.H.; Chan, E.-S.; Moniruzzaman, M.; Islam, S.; Nabi, M.N. Advances in solid-catalytic and non-catalytic technologies for biodiesel production. Energy Convers. Manag. 2014, 88, 1200–1218. [Google Scholar] [CrossRef]
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Renew. Sustain. Energy Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [PubMed]
- Ruhul, A.M.; Kalam, M.A.; Masjuki, H.H.; Fattah, I.M.R.; Reham, S.S.; Rashed, M.M. State of the art of biodiesel production processes: A review of the heterogeneous catalyst. RSC Adv. 2015, 5, 101023–101044. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Đokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Shi, Y.; Yuan, H.; Shi, J. Catalysts from renewable resources for biodiesel production. Energy Convers. Manag. 2018, 178, 277–289. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.; Dinu, C. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef]
- Akubude, V.C.; Nwaigwe, K.N.; Dintwa, E. Production of biodiesel from microalgae via nanocatalyzed transesterification process: A review. Mater. Sci. Energy Technol. 2019, 2, 216–225. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Hu, Y.; Venkateswara Rao, K.T.; Xu, C.; Yang, S. Advances in production of bio-based ester fuels with heterogeneous bifunctional catalysts. Renew. Sustain. Energy Rev. 2019, 114, 109296. [Google Scholar] [CrossRef]
- Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Application of Heterogeneous Catalysts for Biodiesel Production from Microalgal Oil—A Review. Catalysts 2020, 10, 1025. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A.K. An overview on the recent advancements of sustainable heterogeneous catalysts and prominent continuous reactor for biodiesel production. J. Ind. Eng. Chem. 2020, 88, 58–77. [Google Scholar] [CrossRef]
- Hamza, M.; Ayoub, M.; Shamsuddin, R.B.; Mukhtar, A.; Saqib, S.; Zahid, I.; Ameen, M.; Ullah, S.; Al-Sehemi, A.G.; Ibrahim, M. A review on the waste biomass derived catalysts for biodiesel production. Environ. Technol. Innov. 2021, 21, 101200. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Bizuneh Gebeyehu, K.; Tessema Asfaw, B.; Chang, S.W.; Ravindran, B.; Kumar Awasthi, M. Heterogeneous base catalysts: Synthesis and application for biodiesel production—A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef]
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Ramos, M.D.N.; Milessi, T.S.; Candido, R.G.; Mendes, A.A.; Aguiar, A. Enzymatic catalysis as a tool in biofuels production in Brazil: Current status and perspectives. Energy Sustain. Dev. 2022, 68, 103–119. [Google Scholar] [CrossRef]
- Jamil, F.; Saleem, M.; Ali Qamar, O.; Khurram, M.S.; Al-Muhtaseb, A.a.H.; Inayat, A.; Akhter, P.; Hussain, M.; Rafiq, S.; Yim, H.; et al. State-of-the-art catalysts for clean fuel (methyl esters) production—A comprehensive review. J. Phys. Energy 2022, 5, 014005. [Google Scholar] [CrossRef]
- Karmee, S.K. Moving towards the Application of Biocatalysis in Food Waste Biorefinery. Fermentation 2023, 9, 73. [Google Scholar] [CrossRef]
- Xie, W.; Li, J. Magnetic solid catalysts for sustainable and cleaner biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2023, 171, 113017. [Google Scholar] [CrossRef]
- Ejikeme, P.M.; Anyaogu, I.D.; Ejikeme, C.L.; Nwafor, N.P.; Egbuonu, C.A.C.; Ukogu, K.; Ibemesi, J.A. Catalysis in Biodiesel Production by Transesterification Processes-An Insight. E-J. Chem. 2010, 7, 689051. [Google Scholar] [CrossRef]
- Granados, M.L.; Poves, M.D.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R.; Santamaría, J.; Fierro, J.L.G. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B Environ. 2007, 73, 317–326. [Google Scholar] [CrossRef]
- López, D.E.; Goodwin, J.G.; Bruce, D.A.; Lotero, E. Transesterification of triacetin with methanol on solid acid and base catalysts. Appl. Catal. A Gen. 2005, 295, 97–105. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, X.-H.; Yao, M.; Fang, Z.; Wang, Y.-T. Production of biodiesel and hydrogen from plant oil catalyzed by magnetic carbon-supported nickel and sodium silicate. Green Chem. 2016, 18, 3302–3314. [Google Scholar] [CrossRef]
- Campanelli, P.; Banchero, M.; Manna, L. Synthesis of biodiesel from edible, non-edible and waste cooking oils via supercritical methyl acetate transesterification. Fuel 2010, 89, 3675–3682. [Google Scholar] [CrossRef]
- Patil, P.; Deng, S.; Isaac Rhodes, J.; Lammers, P.J. Conversion of waste cooking oil to biodiesel using ferric sulfate and supercritical methanol processes. Fuel 2010, 89, 360–364. [Google Scholar] [CrossRef]
- Ehiri, R.; Ikelle, I.; Ozoaku, O.F. Acid-catalyzed transesterification reaction of beef tallow for biodiesel production by factor variation. Am. J. Eng. Res. 2014, 3, 174–177. [Google Scholar]
- Farag, H.A.; El-Maghraby, A.; Taha, N.A. Optimization of factors affecting esterification of mixed oil with high percentage of free fatty acid. Fuel Process. Technol. 2011, 92, 507–510. [Google Scholar] [CrossRef]
- Singh, A.; He, B.; Thompson, J.; Van Gerpen, J. Process optimization of biodiesel production using alkaline catalysts. Appl. Eng. Agric. 2006, 22, 597–600. [Google Scholar] [CrossRef]
- Abdullah; Rahmawati Sianipar, R.N.; Ariyani, D.; Nata, I.F. Conversion of palm oil sludge to biodiesel using alum and KOH as catalysts. Sustain. Environ. Res. 2017, 27, 291–295. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F. Comparison of the performance of different homogeneous alkali catalysts during transesterification of waste and virgin oils and evaluation of biodiesel quality. Fuel 2008, 87, 3572–3578. [Google Scholar] [CrossRef]
- Namwong, S.; Punsuvon, V. Biodiesel Production from Used Vegetable Oil Using Ethanol and Sodium Methoxide Catalyst. Key Eng. Mater. 2017, 723, 551–555. [Google Scholar] [CrossRef]
- Hayyan, A.; Ali Hashim, M.; Mjalli, F.S.; Hayyan, M.; AlNashef, I.M. A novel phosphonium-based deep eutectic catalyst for biodiesel production from industrial low grade crude palm oil. Chem. Eng. Sci. 2013, 92, 81–88. [Google Scholar] [CrossRef]
- Ünlü, A.E.; Arikaya, A.; Altundağ, A.; Takaç, S. Remarkable effects of deep eutectic solvents on the esterification of lactic acid with ethanol over Amberlyst-15. Korean J. Chem. Eng. 2020, 37, 46–53. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Chen, G.; Liu, S.; Ji, N.; Ding, H.; Fu, J. Preparation of phosphotungstic acid based poly(ionic liquid) and its application to esterification of palmitic acid. Renew. Energy 2019, 133, 317–324. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Pan, H.; Liu, X.; Yang, K.; Huang, S.; Yang, S. Efficient production of biodiesel with promising fuel properties from Koelreuteria integrifoliola oil using a magnetically recyclable acidic ionic liquid. Energy Convers. Manag. 2017, 138, 45–53. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, H.; Liu, J.; Dong, W.; Cao, Z.; Hu, X.; Zhou, Z. Acidic deep eutectic solvents with long carbon chains as catalysts and reaction media for biodiesel production. Renew. Energy 2020, 162, 1842–1853. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Pan, H.; Wang, A.; Souzanchi, S.; Xu, C.; Yang, S. Magnetically recyclable acidic polymeric ionic liquids decorated with hydrophobic regulators as highly efficient and stable catalysts for biodiesel production. Appl. Energy 2018, 223, 416–429. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Zhang, H.; Wang, A.; Jin, D.; Yang, S. Effective production of biodiesel from non-edible oil using facile synthesis of imidazolium salts-based Brønsted-Lewis solid acid and co-solvent. Energy Convers. Manag. 2018, 166, 534–544. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Morales, G. Heterogeneous acid catalysts for biodiesel production: Current status and future challenges. Green Chem. 2009, 11, 1285–1308. [Google Scholar] [CrossRef]
- Schuchardt, U.; Vargas, R.M.; Gelbard, G. Transesterification of soybean oil catalyzed by alkylguanidines heterogenized on different substituted polystyrenes. J. Mol. Catal. A Chem. 1996, 109, 37–44. [Google Scholar] [CrossRef]
- Ghosh, N.; Halder, G. Current progress and perspective of heterogeneous nanocatalytic transesterification towards biodiesel production from edible and inedible feedstock: A review. Energy Convers. Manag. 2022, 270, 116292. [Google Scholar] [CrossRef]
- Tajuddin, N.A.; Lee, A.F.; Wilson, K. 6—Production of biodiesel via catalytic upgrading and refining of sustainable oleagineous feedstocks. In Handbook of Biofuels Production, 2nd ed.; Luque, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing: Swaston, UK, 2016; pp. 121–164. [Google Scholar]
- Alsultan, A.G.; Asikin-Mijan, N.; Ibrahim, Z.; Yunus, R.; Razali, S.Z.; Mansir, N.; Islam, A.; Seenivasagam, S.; Taufiq-Yap, Y.H. A Short Review on Catalyst, Feedstock, Modernised Process, Current State and Challenges on Biodiesel Production. Catalysts 2021, 11, 1261. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Martínez, G.; Nogales-Delgado, S. Transesterification of Soybean Oil through Different Homogeneous Catalysts: Kinetic Study. Catalysts 2022, 12, 146. [Google Scholar] [CrossRef]
- Iram, S.; Tariq, I.; Ahmad, K.S.; Jaffri, S.B. Helianthus annuus based biodiesel production from seed oil garnered from a phytoremediated terrain. Int. J. Ambient. Energy 2020, 43, 1763–1771. [Google Scholar] [CrossRef]
- Obidike, L.I.; Yoro, K.O. Effect of zeolitic nano-catalyst on biodiesel yield and biochar formation during the pyrolysis of tallow. Biofuels 2021, 13, 683–692. [Google Scholar] [CrossRef]
- Thomas, S. Enhanced Oil Recovery—An Overview. Oil Gas Sci. Technol. 2007, 63, 9–19. [Google Scholar] [CrossRef]
- Ooi, H.K.; Koh, X.N.; Ong, H.C.; Lee, H.V.; Mastuli, M.S.; Taufiq-Yap, Y.H.; Alharthi, F.A.; Alghamdi, A.A.; Asikin Mijan, N. Progress on Modified Calcium Oxide Derived Waste-Shell Catalysts for Biodiesel Production. Catalysts 2021, 11, 194. [Google Scholar] [CrossRef]
- Chamola, R.; Khan, M.F.; Raj, A.; Verma, M.; Jain, S. Response surface methodology based optimization of in situ transesterification of dry algae with methanol, H2SO4 and NaOH. Fuel 2019, 239, 511–520. [Google Scholar] [CrossRef]
- Mekonnen, K.D.; Sendekie, Z.B. NaOH-Catalyzed Methanolysis Optimization of Biodiesel Synthesis from Desert Date Seed Kernel Oil. ACS Omega 2021, 6, 24082–24091. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Masjuki, H.H.; Kalam, M.A.; Mofijur, M.; Abedin, M.J. Effect of antioxidant on the performance and emission characteristics of a diesel engine fueled with palm biodiesel blends. Energy Convers. Manag. 2014, 79, 265–272. [Google Scholar] [CrossRef]
- Jothiramalingam, R.; Wang, M.K. Review of Recent Developments in Solid Acid, Base, and Enzyme Catalysts (Heterogeneous) for Biodiesel Production via Transesterification. Ind. Eng. Chem. Res. 2009, 48, 6162–6172. [Google Scholar] [CrossRef]
- Stojković, I.J.; Stamenković, O.S.; Povrenović, D.S.; Veljković, V.B. Purification technologies for crude biodiesel obtained by alkali-catalyzed transesterification. Renew. Sustain. Energy Rev. 2014, 32, 1–15. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Song, Z.; Zhou, T. Data-Driven Ionic Liquid Design for CO2 Capture: Molecular Structure Optimization and DFT Verification. Ind. Eng. Chem. Res. 2021, 60, 9992–10000. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Li, S.; Zhang, M.; Wang, Y.; Wang, Z.; Peng, Y.; Wang, M.; Li, X.; Pan, H. Recent advances in supported acid/base ionic liquids as catalysts for biodiesel production. Front. Chem. 2022, 10, 999607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, C.; Crittle, T.D. Choline-based deep eutectic solvents for enzymatic preparation of biodiesel from soybean oil. J. Mol. Catal. B Enzym. 2013, 85, 243–247. [Google Scholar] [CrossRef]
- Bian, Y.; Shan, Q.; Guo, C.; Liu, C.; Zhang, J. Biodiesel Production Over Esterification Catalyzed by a Novel Poly(Acidic Ionic Liquid)s. Catal. Lett. 2021, 151, 3523–3531. [Google Scholar] [CrossRef]
- Wang, X.; Yang, K.; Cai, R.; ChenYang, Y.; Huang, Z.; Han, B. Optimization and kinetics of biodiesel production from soybean oil using new tetraethylammonium ionic liquids with amino acid-based anions as catalysts. Fuel 2022, 324, 124510. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Li, L.; Ye, C.; Lin, X.; Qiu, T. Novel multi–SO3H functionalized ionic liquids as highly efficient catalyst for synthesis of biodiesel. Green Energy Environ. 2021, 6, 271–282. [Google Scholar] [CrossRef]
- Zhang, T.; Shahbaz, K.; Farid, M.M. Glycerolysis of free fatty acid in vegetable oil deodorizer distillate catalyzed by phosphonium-based deep eutectic solvent. Renew. Energy 2020, 160, 363–373. [Google Scholar] [CrossRef]
- Ranjan, A.; Dawn, S.S.; Nirmala, N.; Santhosh, A.; Arun, J. Application of deep eutectic solvent in biodiesel reaction: RSM optimization, CI engine test, cost analysis and research dynamics. Fuel 2022, 307, 121933. [Google Scholar] [CrossRef]
- Mat, R.; Adawiyah Samsudin, R.; Mohamed, M.; Johari, A. Solid Catalysts and theirs Application in Biodiesel Production. Bull. Chem. React. Eng. Catal. 2012, 7, 142–149. [Google Scholar] [CrossRef]
- Marinković, D.M.; Stanković, M.V.; Veličković, A.V.; Avramović, J.M.; Miladinović, M.R.; Stamenković, O.O.; Veljković, V.B.; Jovanović, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Refaat, A.A. Biodiesel production using solid metal oxide catalysts. Int. J. Environ. Sci. Technol. 2011, 8, 203–221. [Google Scholar] [CrossRef]
- Vásquez-Céspedes, S.; Betori, R.C.; Cismesia, M.A.; Kirsch, J.K.; Yang, Q. Heterogeneous Catalysis for Cross-Coupling Reactions: An Underutilized Powerful and Sustainable Tool in the Fine Chemical Industry? Org. Process Res. Dev. 2021, 25, 740–753. [Google Scholar] [CrossRef]
- Booramurthy, V.K.; Kasimani, R.; Pandian, S.; Ragunathan, B. Nano-sulfated zirconia catalyzed biodiesel production from tannery waste sheep fat. Environ. Sci. Pollut. Res. Int. 2020, 27, 20598–20605. [Google Scholar] [CrossRef]
- Shu, Q.; Liu, X.; Huo, Y.; Tan, Y.; Zhang, C.; Zou, L. Construction of a Brönsted-Lewis solid acid catalyst La-PW-SiO2/SWCNTs based on electron withdrawing effect of La(III) on π bond of SWCNTs for biodiesel synthesis from esterification of oleic acid and methanol. Chin. J. Chem. Eng. 2022, 44, 351–362. [Google Scholar] [CrossRef]
- Shi, W.; Zhao, J.; Yuan, X.; Wang, S.; Wang, X.; Huo, M. Effects of Brønsted and Lewis Acidities on Catalytic Activity of Heteropolyacids in Transesterification and Esterification Reactions. Chem. Eng. Technol. 2012, 35, 347–352. [Google Scholar] [CrossRef]
- Purova, R.; Narasimharao, K.; Ahmed, N.S.I.; Al-Thabaiti, S.; Al-Shehri, A.; Mokhtar, M.; Schwieger, W. Pillared HMCM-36 zeolite catalyst for biodiesel production by esterification of palmitic acid. J. Mol. Catal. A Chem. 2015, 406, 159–167. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, P.; Ansari, F.A.; Singh, B.; Bux, F. Biodiesel synthesis from microalgal lipids using tungstated zirconia as a heterogeneous acid catalyst and its comparison with homogeneous acid and enzyme catalysts. Fuel 2017, 187, 180–188. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhu, W.; Cao, F. Zn1.2H0.6PW12O40 Nanotubes with double acid sites as heterogeneous catalysts for the production of biodiesel from waste cooking oil. ChemSusChem 2009, 2, 177–183. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Calcined zirconium sulfate supported on MCM-41 silica as acid catalyst for ethanolysis of sunflower oil. Appl. Catal. B Environ. 2011, 103, 91–98. [Google Scholar] [CrossRef]
- Nath, B.; Kalita, P.; Das, B.; Basumatary, S. Highly efficient renewable heterogeneous base catalyst derived from waste Sesamum indicum plant for synthesis of biodiesel. Renew. Energy 2020, 151, 295–310. [Google Scholar] [CrossRef]
- Han, Y.-Z.; Hong, L.; Wang, X.-Q.; Liu, J.-Z.; Jiao, J.; Luo, M.; Fu, Y.-J. Biodiesel production from Pistacia chinensis seed oil via transesterification using recyclable magnetic cellulose-based catalyst. Ind. Crops Prod. 2016, 89, 332–338. [Google Scholar] [CrossRef]
- Sai, B.A.; Subramaniapillai, N.; Mohamed MS, B.K.; Narayanan, A. Optimization of continuous biodiesel production from rubber seed oil (RSO) using calcined eggshells as heterogeneous catalyst. J. Environ. Chem. Eng. 2020, 8, 103603. [Google Scholar]
- Wang, H.; Covarrubias, J.; Prock, H.; Wu, X.; Wang, D.; Bossmann, S.H. Acid-Functionalized Magnetic Nanoparticle as Heterogeneous Catalysts for Biodiesel Synthesis. J. Phys. Chem. C 2015, 119, 26020–26028. [Google Scholar] [CrossRef]
- Alaei, S.; Haghighi, M.; Toghiani, J.; Rahmani Vahid, B. Magnetic and reusable MgO/MgFe2O4 nanocatalyst for biodiesel production from sunflower oil: Influence of fuel ratio in combustion synthesis on catalytic properties and performance. Ind. Crops Prod. 2018, 117, 322–332. [Google Scholar] [CrossRef]
- Kazemifard, S.; Nayebzadeh, H.; Saghatoleslami, N.; Safakish, E. Assessment the activity of magnetic KOH/Fe3O4@Al2O3 core-shell nanocatalyst in transesterification reaction: Effect of Fe/Al ratio on structural and performance. Environ. Sci. Pollut. Res. Int. 2018, 25, 32811–32821. [Google Scholar] [CrossRef]
- Xie, W.; Gao, C.; Li, J. Sustainable biodiesel production from low-quantity oils utilizing H6PV3MoW8O40 supported on magnetic Fe3O4/ZIF-8 composites. Renew. Energy 2021, 168, 927–937. [Google Scholar] [CrossRef]
- Sahu, O. Characterisation and utilization of heterogeneous catalyst from waste rice-straw for biodiesel conversion. Fuel 2021, 287, 119543. [Google Scholar] [CrossRef]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. Nano-magnetic catalyst KF/CaO–Fe3O4 for biodiesel production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Sreeprasanth, P.S.; Srivastava, R.; Srinivas, D.; Ratnasamy, P. Hydrophobic, solid acid catalysts for production of biofuels and lubricants. Appl. Catal. A Gen. 2006, 314, 148–159. [Google Scholar] [CrossRef]
- Gardy, J.; Osatiashtiani, A.; Céspedes, O.; Hassanpour, A.; Lai, X.; Lee, A.F.; Wilson, K.; Rehan, M. A magnetically separable SO4/Fe-Al-TiO2 solid acid catalyst for biodiesel production from waste cooking oil. Appl. Catal. B Environ. 2018, 234, 268–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, S.; Lu, C.; Gong, Z.; Hu, X. Catalytic performance of NaAlO2/γ-Al2O3 as heterogeneous nanocatalyst for biodiesel production: Optimization using response surface methodology. Energy Convers. Manag. 2020, 203, 112263. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- Li, T.-F.; Wang, X.-Q.; Jiao, J.; Liu, J.-Z.; Zhang, H.-X.; Niu, L.-L.; Zhao, C.-J.; Gu, C.-B.; Efferth, T.; Fu, Y.-J. Catalytic transesterification of Pistacia chinensis seed oil using HPW immobilized on magnetic composite graphene oxide/cellulose microspheres. Renew. Energy 2018, 127, 1017–1025. [Google Scholar] [CrossRef]
- Esmaeili, H. A critical review on the economic aspects and life cycle assessment of biodiesel production using heterogeneous nanocatalysts. Fuel Process. Technol. 2022, 230, 107224. [Google Scholar] [CrossRef]
- Sharma, S.; Saxena, V.; Baranwal, A.; Chandra, P.; Pandey, L.M. Engineered nanoporous materials mediated heterogeneous catalysts and their implications in biodiesel production. Mater. Sci. Energy Technol. 2018, 1, 11–21. [Google Scholar] [CrossRef]
- Tobío-Pérez, I.; Domínguez, Y.D.; Machín, L.R.; Pohl, S.; Lapuerta, M.; Piloto-Rodríguez, R. Biomassbased heterogeneous catalysts for biodiesel production: A comprehensive review. Int. J. Energy Res. 2021, 46, 3782–3809. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Vijay Pradhap Singh, M.; Fransila, B.; Praveen Kumar, R.; Karthiga Devi, G. A review on influencing parameters of biodiesel production and purification processes. Curr. Res. Green Sustain. Chem. 2020, 1, 1–6. [Google Scholar] [CrossRef]
- Gaurav, A.; Dumas, S.; Mai, C.T.Q.; Ng, F.T.T. A kinetic model for a single step biodiesel production from a high free fatty acid (FFA) biodiesel feedstock over a solid heteropolyacid catalyst. Green Energy Environ. 2019, 4, 328–341. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, D.; Luo, Q.; Wang, J.; Deng, T.; Zhang, Y.; Ma, P. Efficient biodiesel production from oleic acid using metal-organic framework encapsulated Zr-doped polyoxometalate nano-hybrids. RSC Adv. 2020, 10, 8766–8772. [Google Scholar] [CrossRef] [PubMed]
- Badruzzaman, A.; Yuda, A.; Ashok, A.; Kumar, A. Recent advances in cobalt based heterogeneous catalysts for oxygen evolution reaction. Inorg. Chim. Acta 2020, 511, 119854. [Google Scholar] [CrossRef]
- Farabi, M.S.A.; Ibrahim, M.L.; Rashid, U.; Taufiq-Yap, Y.H. Esterification of palm fatty acid distillate using sulfonated carbon-based catalyst derived from palm kernel shell and bamboo. Energy Convers. Manag. 2019, 181, 562–570. [Google Scholar] [CrossRef]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Inorganic heterogeneous catalysts for biodiesel production from vegetable oils. Biomass Bioenergy 2011, 35, 3787–3809. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, H.; Huang, J.; Li, Y.; Zhang, H. A Highly Effective Biomass-Derived Solid Acid Catalyst for Biodiesel Synthesis Through Esterification. Front. Chem. 2022, 10, 882235. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Zaman, S.; Farooq, M.; Khan, I.W.; Ghazi, Z.A.; Saeed, T.; Hamayun, M. Biodiesel production from waste cooking oil employing natural bentonite supported heterogeneous catalyst: Waste to biodiesel. Korean J. Chem. Eng. 2022, 39, 1450–1459. [Google Scholar] [CrossRef]
- Maafa, I.M. Biodiesel Synthesis from High Free-Fatty-Acid Chicken Fat using a Scrap-Tire Derived Solid Acid Catalyst and KOH. Polymers 2022, 14, 643. [Google Scholar] [CrossRef] [PubMed]
- da Conceicao, L.R.V.; Reis, C.E.R.; De Lima, R.; Cortez, D.V.; De Castro, H.F. Keggin-structure heteropolyacid supported on alumina to be used in trans/esterification of high-acid feedstocks. RSC Adv. 2019, 9, 23450–23458. [Google Scholar] [CrossRef] [PubMed]
- Kurhade, A.; Dalai, A.K. Physiochemical characterization and support interaction of alumina-supported heteropolyacid catalyst for biodiesel production. Asia-Pac. J. Chem. Eng. 2018, 13, 2249. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Biodiesel production from microalgal biomass using CaO catalyst synthesized from natural waste material. Renew. Energy 2019, 136, 837–845. [Google Scholar] [CrossRef]
- Bharti, R.; Singh, B.; Oraon, R. Synthesis of Sn-CaO as a bifunctional catalyst and its application for biodiesel production from waste cooking oil. Biofuels 2023, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, S.; Han, K.; Li, Y.; Lu, C. Synthesis of the SrO–CaO–Al2O3 trimetallic oxide catalyst for transesterification to produce biodiesel. Renew. Energy 2021, 168, 981–990. [Google Scholar] [CrossRef]
- Amesho, K.T.T.; Lin, Y.-C.; Chen, C.-E.; Cheng, P.-C.; Shangdiar, S. Kinetics studies of sustainable biodiesel synthesis from Jatropha curcas oil by exploiting bio-waste derived CaO-based heterogeneous catalyst via microwave heating system as a green chemistry technique. Fuel 2022, 323, 123876. [Google Scholar] [CrossRef]
- Hameed, A.; Naqvi, S.R.; Sikandar, U.; Chen, W.-H. One-Step Biodiesel Production from Waste Cooking Oil Using CaO Promoted Activated Carbon Catalyst from Prunus persica Seeds. Catalysts 2022, 12, 592. [Google Scholar] [CrossRef]
- Zhou, G.; Liang, Y.; Zheng, Z.; Ju, L. Application of dewatered paper sludge-derived porous solid base catalyst for biodiesel production: Physicochemical properties, reaction kinetics and thermodynamic studies. Env. Technol. 2022, 1–18. [Google Scholar] [CrossRef]
- Kim, J.; Jia, H.; Wang, P. Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol. Adv. 2006, 24, 296–308. [Google Scholar] [CrossRef]
- Asri, N.P.; Saraswati, R.; Hindarso, H.; Puspitasari, D.A.; Suprapto. Synthesis of biodiesel from kesambi (Schleichera oleosa L.) oil using carbon nanotube-supported zinc oxide heterogeneous catalyst. IOP Conf. Ser. Earth Environ. Sci. 2021, 749, 012048. [Google Scholar] [CrossRef]
- Asri, N.P.; Yuniati, Y.; Hindarso, H.; Suprapto; Yogaswara, R.R. Preparation of Multi-Walled Carbon Nanotubes Supported Zinc Oxide Catalyst for Transesterification of Kesambi (Schleichera oleosa) Oil. IOP Conf. Ser. Mater. Sci. Eng. 2020, 742, 012034. [Google Scholar] [CrossRef]
- Saeedi, M.; Fazaeli, R.; Aliyan, H. Nanostructured sodium–zeolite imidazolate framework (ZIF-8) doped with potassium by sol–gel processing for biodiesel production from soybean oil. J. Sol-Gel Sci. Technol. 2015, 77, 404–415. [Google Scholar] [CrossRef]
- Mittal, V.; Kumar Ghosh, U. Comparative analysis of two different nanocatalysts for producing biodiesel from microalgae. Mater. Today Proc. 2022, 63, 515–519. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, L.; Esmaeili, H. A review on biodiesel production using various heterogeneous nanocatalysts: Operation mechanisms and performances. Biomass Bioenergy 2022, 158, 106356. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N. Highly efficient CaO–ZSM-5 zeolite/Fe3O4 as a magnetic acid–base catalyst upon biodiesel production from used cooking oil. Appl. Nanosci. 2022, 12, 3755–3769. [Google Scholar] [CrossRef]
- Arrais Gonçalves, M.; Karine Lourenço Mares, E.; Roberto Zamian, J.; Narciso da Rocha Filho, G.; Rafael Vieira da Conceição, L. Statistical optimization of biodiesel production from waste cooking oil using magnetic acid heterogeneous catalyst MoO3/SrFe2O4. Fuel 2021, 304, 121463. [Google Scholar] [CrossRef]
- Ghasemzadeh, B.; Matin, A.A.; Habibi, B.; Ebadi, M. Cotton/Fe3O4@SiO2@H3PW12O40 a magnetic heterogeneous catalyst for biodiesel production: Process optimization through response surface methodology. Ind. Crops Prod. 2022, 181, 114806. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Panchal, B.; Zhu, Z.; Qin, S.; Chang, T.; Zhao, Q.; Sun, Y.; Zhao, C.; Wang, J.; Bian, K.; Rankhamb, S. The current state applications of ethyl carbonate with ionic liquid in sustainable biodiesel production: A review. Renew. Energy 2022, 181, 341–354. [Google Scholar] [CrossRef]
- Guo, F.; Fang, Z.; Xu, C.C.; Smith, R.L. Solid acid mediated hydrolysis of biomass for producing biofuels. Prog. Energy Combust. Sci. 2012, 38, 672–690. [Google Scholar] [CrossRef]
- Nazloo, E.K.; Moheimani, N.R.; Ennaceri, H. Graphene-based catalysts for biodiesel production: Characteristics and performance. Sci. Total Environ. 2023, 859 (Pt 1), 160000. [Google Scholar] [CrossRef]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Orege, J.I.; Oderinde, O.; Kifle, G.A.; Ibikunle, A.A.; Raheem, S.A.; Ejeromedoghene, O.; Okeke, E.S.; Olukowi, O.M.; Orege, O.B.; Fagbohun, E.O.; et al. Recent advances in heterogeneous catalysis for green biodiesel production by transesterification. Energy Convers. Manag. 2022, 258, 115406. [Google Scholar] [CrossRef]
- Lin, L.; Cunshan, Z.; Vittayapadung, S.; Xiangqian, S.; Mingdong, D. Opportunities and challenges for biodiesel fuel. Appl. Energy 2011, 88, 1020–1031. [Google Scholar] [CrossRef]
- Haas, M.J.; McAloon, A.J.; Yee, W.C.; Foglia, T.A. A process model to estimate biodiesel production costs. Bioresour. Technol. 2006, 97, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Apostolakou, A.A.; Kookos, I.K.; Marazioti, C.; Angelopoulos, K.C. Techno-economic analysis of a biodiesel production process from vegetable oils. Fuel Process. Technol. 2009, 90, 1023–1031. [Google Scholar] [CrossRef]
- Baddour, F.G.; Snowden-Swan, L.; Super, J.D.; Van Allsburg, K.M. Estimating Precommercial Heterogeneous Catalyst Price: A Simple Step-Based Method. Org. Process Res. Dev. 2018, 22, 1599–1605. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of biodiesel production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Techno-economic performance of a bio-refinery for the production of fuel-grade biofuel using a green catalyst. Biofuels Bioprod. Biorefining 2019, 13, 936–949. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Biodiesel production process using solid acid catalyst: Influence of market variables on the process’s economic feasibility. Biofuels Bioprod. Biorefining 2021, 15, 815–824. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Errazu, A.F. Comparison of different heterogeneous catalysts and different alcohols for the esterification reaction of oleic acid. Fuel 2008, 87, 3477–3480. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Baskar, G. Optimization and techno-economic analysis of biodiesel production from Calophyllum inophyllum oil using heterogeneous nanocatalyst. Bioresour. Technol. 2020, 315, 123852. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Baskar, G. Process optimization, green chemistry balance and technoeconomic analysis of biodiesel production from castor oil using heterogeneous nanocatalyst. Bioresour. Technol. 2021, 320 (Pt A), 124347. [Google Scholar] [CrossRef]
- Mustapha, S.I.; Bux, F.; Isa, Y.M. Techno-economic analysis of biodiesel production over lipid extracted algae derived catalyst. Biofuels 2021, 13, 663–674. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, B.; Ok, Y.S.; Lim, H. Preliminary techno-economic analysis of biodiesel production over solid-biochar. Bioresour. Technol. 2020, 306, 123086. [Google Scholar] [CrossRef] [PubMed]
| Type | Title of the Work | References |
|---|---|---|
| Homogeneous catalyst | The effects of catalysts in biodiesel production: a review | [28] |
| Heterogeneous catalysts | Advances in solid-catalytic and non-catalytic technologies for biodiesel production | [29] |
| Heterogeneous catalysts | Review on latest developments in biodiesel production using carbon-based catalysts | [30] |
| Heterogeneous catalysts | Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification | [31] |
| Heterogeneous catalysts | State of the art of biodiesel production process: a review of the heterogeneous catalyst | [32] |
| Heterogeneous catalysts | Heterogeneous basic catalysts for biodiesel production | [24] |
| Homogeneous catalyst | Application of ILs and DES in biodiesel production: a review | [33] |
| Heterogeneous catalyst | A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production | [34] |
| Heterogeneous catalyst | A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils | [35] |
| Heterogeneous catalyst | Catalysts from renewable resources for biodiesel production | [36] |
| Enzymatic catalyst | Industrial applications of enzymes: recent advances, techniques, and outlooks | [37] |
| Heterogeneous catalyst | Biochars and their use as Transesterification catalysts for biodiesel production: a short review | [38] |
| Heterogeneous catalyst | Production of biodiesel from microalgae via nanocatalyzed transesterification process: a review | [39] |
| Heterogeneous catalyst | A review of heterogeneous calcium oxide based catalyst from waste for biodiesel synthesis | [26] |
| Homogeneous catalyst | Advances in production of bio-based ester fuels with heterogeneous bifunctional catalysts | [40] |
| Heterogeneous catalyst | Application of heterogeneous catalysts for biodiesel production from microalgal oil—a review | [41] |
| Heterogeneous catalyst | An overview on the recent advancements of sustainable heterogeneous catalysts and prominent continuous reactor for biodiesel production | [42] |
| Heterogeneous and homogeneous catalyst | A review on the waste biomass derived catalysts for biodiesel production | [43] |
| Heterogeneous, homogeneous, and enzymatic catalyst | Bio-derived catalysts: a current trend of catalysts used in biodiesel production | [23] |
| Heterogeneous catalyst | Heterogeneous base catalysts: synthesis and application for biodiesel production—a review | [44] |
| Heterogeneous catalyst | Heteropoly acids as supported solid acid catalysts for sustainable biodiesel production using vegetable oils: a review | [3] |
| Homogeneous catalyst | A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts | [45] |
| Enzymatic catalyst | Enzymatic catalysis as a tool in biofuels production in Brazil: current status and perspectives | [46] |
| Heterogeneous catalyst | State-of-the-art catalysts for clean fuel (methyl esters) production—a comprehensive review | [47] |
| Enzymatic catalyst | Moving towards the application of biocatalysis in food waste biorefinery | [48] |
| Heterogeneous catalyst | Magnetic solid catalysts for sustainable and cleaner biodiesel production: a comprehensive review | [49] |
| Catalyst Type | Biodiesel Physicochemical Characteristics | Methanol to Oil/FFA Molar Ratio | Catalyst Dosage (wt%) | Reaction Temperature (°C) | Duration (min) | Yield (wt%) | Reusability (Cycle) | References |
|---|---|---|---|---|---|---|---|---|
| KOH | - | 8:1 | 1 | 55 | 60 | 51–87 | N/A | [54] |
| NaOH | Viscosity = 2.25–3.10 mm2·s−1 | 8:1 | 3 | 50 | 60 | 93 | N/A | [55] |
| HCl | - | - | 1.85 | 100 | 60 | 95.2 | N/A | [56] |
| H2SO4 | - | 245:1 | 41.8 | 70 | 240 | 99 | N/A | [27] |
| H2SO4 | - | 6:1 | 2.5 | 60 | 60 | 96 | N/A | [57] |
| NaOH | - | 6:1 | 1.35 | 60 | 30 | 90.19 | N/A | [58] |
| KOH | Density = 0.864 g·mL−1, viscosity = 12.8 mm2·s−1 | 20:1 | 1.5 | 60 | 60 | 93 | N/A | [59] |
| NaOH | - | 6:1 | 0.6 | 60 | 60 | 97 | N/A | [60] |
| NaOCH3 | Density = 869.3 Kg·m−3, viscosity = 4.75 cSt | 3:1 | 0.04 | 65 | 70 | 84 | N/A | [61] |
| P-DES (ATPB: PTSA) | - | 10:1 | 3.5 | 60 | 30 | 96 | 4 | [62] |
| ChCl-PTSA | - | 10:1 | 5 | 60 | 30 | 97 | 2 | [63] |
| PIL-3 | - | 6:1 | 3 | 65 | 480 | 91.6 | 5 | [64] |
| FS-B-L-IL | Density = 874.3 Kg·m−3, viscosity = 5.018 mm2·s−1 | 40:1 | 10 | 160 | 600 | 93.7 | 5 | [65] |
| p-TsOH(DES) | - | 12.5:1 | 24.6 | 70.5 | 180 | 99.2 | N/A | [66] |
| FnmS-PIL | - | 18:1 | 5 | 120 | 360 | 91.75 | N/A | [67] |
| [DSI][FeCl4] | - | 15:1 | 5 | 120 | 480 | 98.7 | 4 | [68] |
| Catalyst Type | Biodiesel Physicochemical Characteristics | Methanol to Oil/FFA Molar Ratio | Catalyst Dosage (wt%) | Reaction Temperature (°C) | Duration (h) | Yield (%) | Reusability (Cycle) | References |
|---|---|---|---|---|---|---|---|---|
| Fe-Mn-SO4/ZrO2 | Density = 879 Kg·m−3, viscosity = 5.6 mm2·s−1, acid number = 0.4 mg KOH·g−1 | 15:1 | 5 | 65 | 5 | 98.7 | 5 | [96] |
| La-PW-SiO2/SWCNTs | - | 15:1 | 1.5 | 65 | 8 | 93.1 | 6 | [97] |
| Ti0.6H0.6PW | - | 7:1 | 5 | 50 | 0.5 | 94.7 | 5 | [98] |
| Pillared MCM-36 | - | 30:1 | 25.6 | 80 | 6 | 100 | 4 | [99] |
| WO3/ZrO2 | Calorific value = 38.44 MJ·kg−1, acid number = 0.46 mg KOH·g−1 | 12:1 | 15 | 100 | 3 | 94.58 | - | [100] |
| Zn1.2H0.6PW12O40 nanotubes | - | 28:1 | 2.5 | 65 | 12 | 97.2 | 5 | [101] |
| Zr30-MCM | - | 12:1 | 14.6 | 200 | 6 | 91.5 | 3 | [102] |
| CaO | Density = 859 Kg·m−3, viscosity = 3.11 mm2·s−1, saponification number = 188.57 mg KOH·g−1 | 12:1 | 7 | 65 | 0.67 | 98.9 | 3 | [103] |
| MCM-HPW | Density = 879 Kg·m−3, viscosity = 4.7 mm2·s−1, acid number = 0.36 mg KOH·g−1 | 10:1 | 10 | 60 | 1.3 | 93.1 | 4 | [104] |
| CaO | Specific gravity = 0.86, viscosity = 4.35 mm2·s−1, acid number = 0.23 mg KOH·g−1 | 9:1 | 5 | 65 | 4 | 97.84 | - | [105] |
| Fe3O4-SBA-15-SO3H | - | 10:1 | 3 | 60 | 6 | 75 | 5 | [106] |
| MgO/MgFe2O4 | - | 12:1 | 4 | 110 | 4 | 91.2 | 5 | [107] |
| KOH/Fe3O4@Al2O3 | - | 12:1 | 4 | 65 | 6 | 98.8 | 2 | [108] |
| Fe3O4-ZIF-8-H6PV3MoW8O40 | - | 30:1 | 6 | 160 | 10 | 92.6 | 5 | [109] |
| CaO | Density = 865 Kg·m−3, viscosity = 4.18 mm2·s−1, acid number = 0.302 mg KOH·g−1 | 15:1 | 3.5 | 65 | 2.5 | 97.3 | 10 | [110] |
| MgO | - | 10:1 | 2 | 50 | 2 | 91.6 | 14 | [111] |
| ZnO | - | 10:1 | 2 | 65 | 3 | 94.7 | - | [112] |
| SO4/Fe-Al-TiO2 | - | 10:1 | 3 | 90 | 2.5 | 95.6 | 10 | [113] |
| NaAlO2/γ-Al2O3 | Density = 870 Kg·m−3, viscosity = 2.69 mm2·s−1 | 20:1 | 10 | 65 | 3 | 97.65 | 6 | [114] |
| CaO/CuFe2O4 | Density = 833–887 Kg·m−3, viscosity = 3.7–5.3 mm2·s−1 | 15:1 | 3 | 70 | 4 | 94.52 | - | [115] |
| GO/CM-NH2@Fe3O4-HPW | Density = 870 Kg·m−3, viscosity = 4.3 mm2·s−1, acid number = 0.4 mg KOH·g−1 | 12:1 | 15 | 80 | 8 | 94 | 6 | [116] |
| Catalysts Type | Advantages | Disadvantages | References |
|---|---|---|---|
| Homogeneous acid catalysts |
|
| [32] |
| Homogeneous base catalysts |
|
| [43,143] |
| ILs/DESs |
|
| [147,148] |
| Heterogenous acid catalysts |
|
| [147,149] |
| Heterogenous base catalysts |
|
| [32,150] |
| Nanocatalysts/magnetic catalysts |
|
| [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Wang, B.; Shukla, S.K.; Wang, R. Enabling Catalysts for Biodiesel Production via Transesterification. Catalysts 2023, 13, 740. https://doi.org/10.3390/catal13040740
Wang B, Wang B, Shukla SK, Wang R. Enabling Catalysts for Biodiesel Production via Transesterification. Catalysts. 2023; 13(4):740. https://doi.org/10.3390/catal13040740
Chicago/Turabian StyleWang, Baohua, Bingquan Wang, Sudheesh K. Shukla, and Rui Wang. 2023. "Enabling Catalysts for Biodiesel Production via Transesterification" Catalysts 13, no. 4: 740. https://doi.org/10.3390/catal13040740
APA StyleWang, B., Wang, B., Shukla, S. K., & Wang, R. (2023). Enabling Catalysts for Biodiesel Production via Transesterification. Catalysts, 13(4), 740. https://doi.org/10.3390/catal13040740








