Discrete Au1(0) Stabilized by 15-Crown-5 for High-Efficiency Catalytic Reduction of Nitrophenol and Nitroaniline
Abstract
1. Introduction
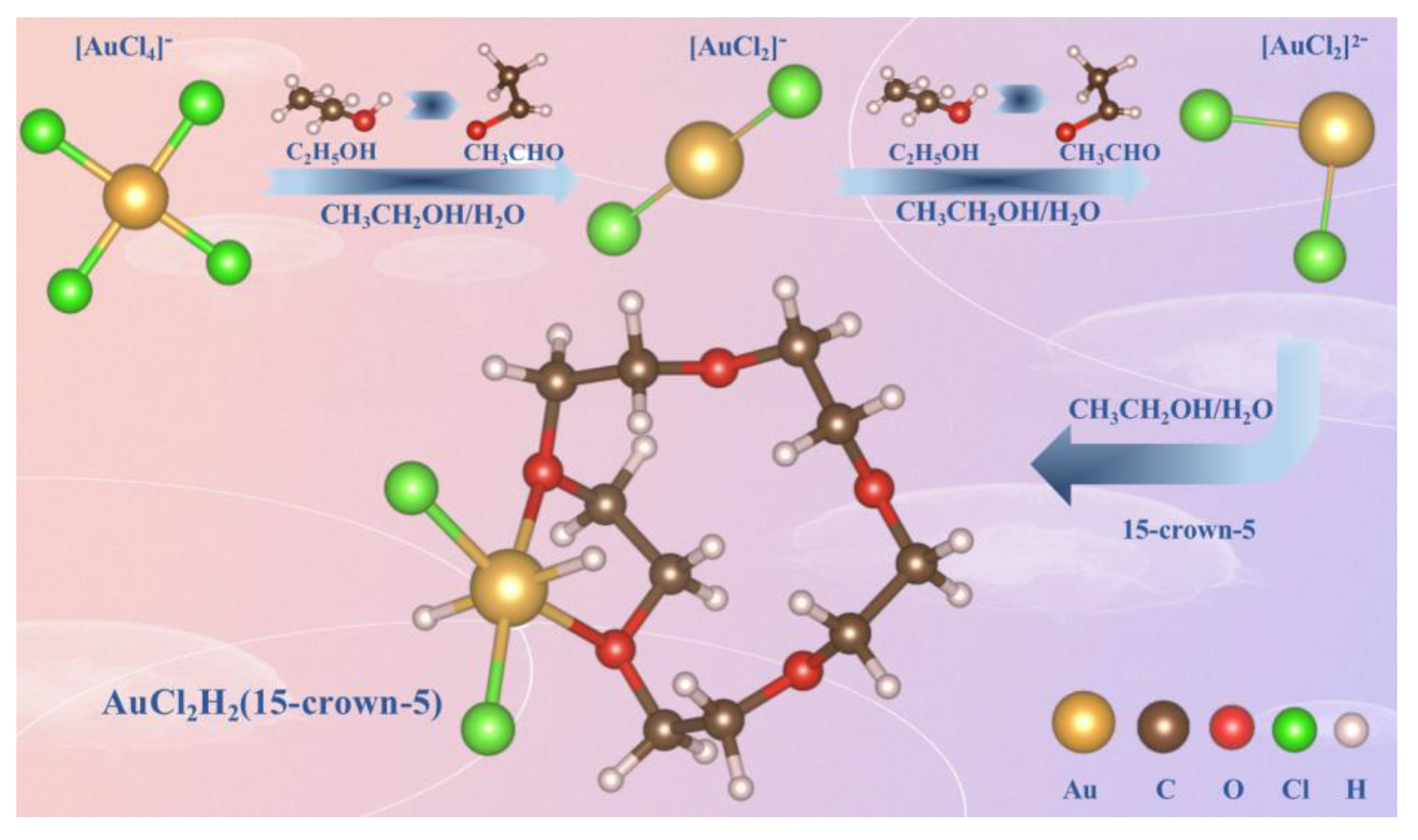
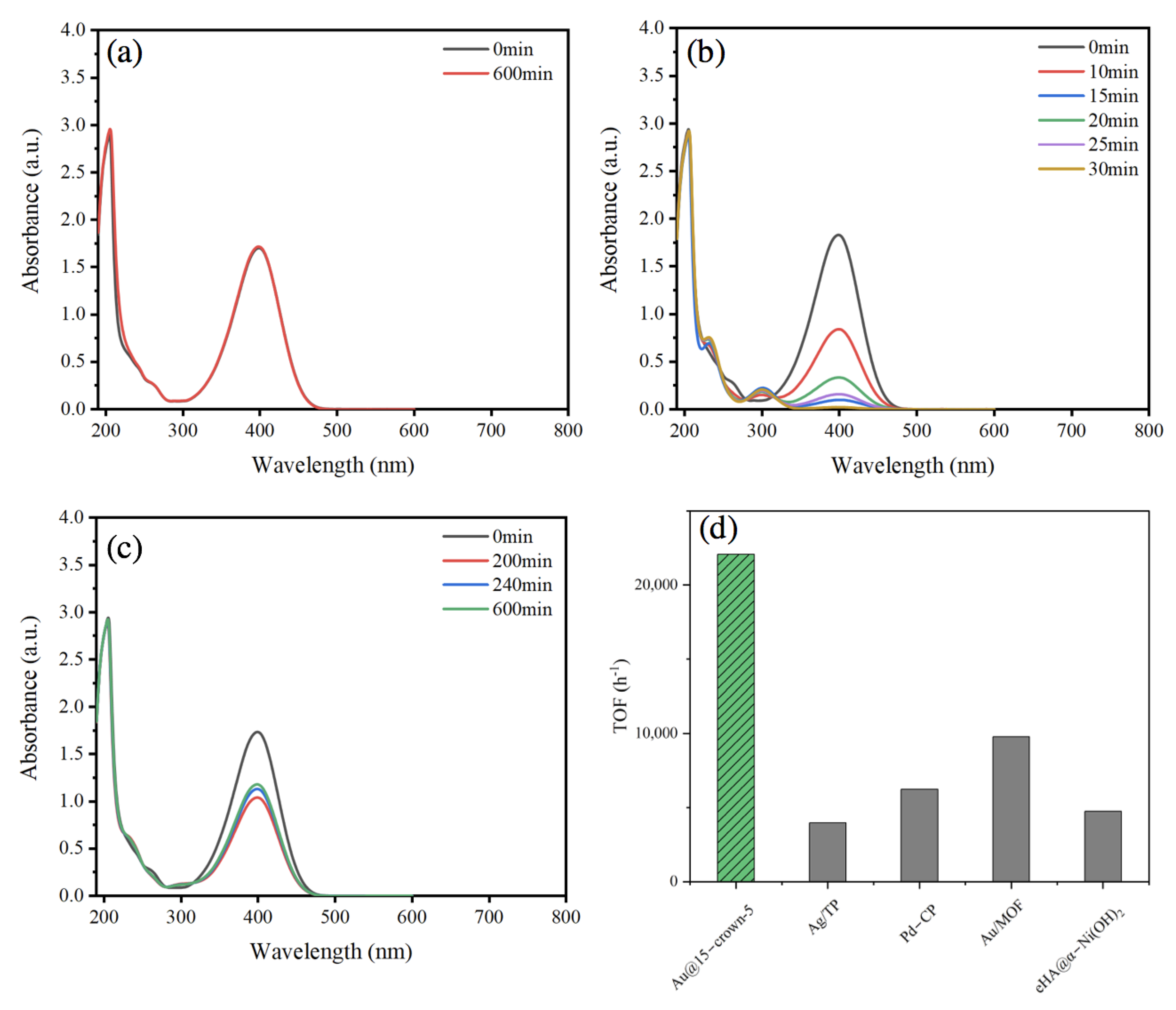
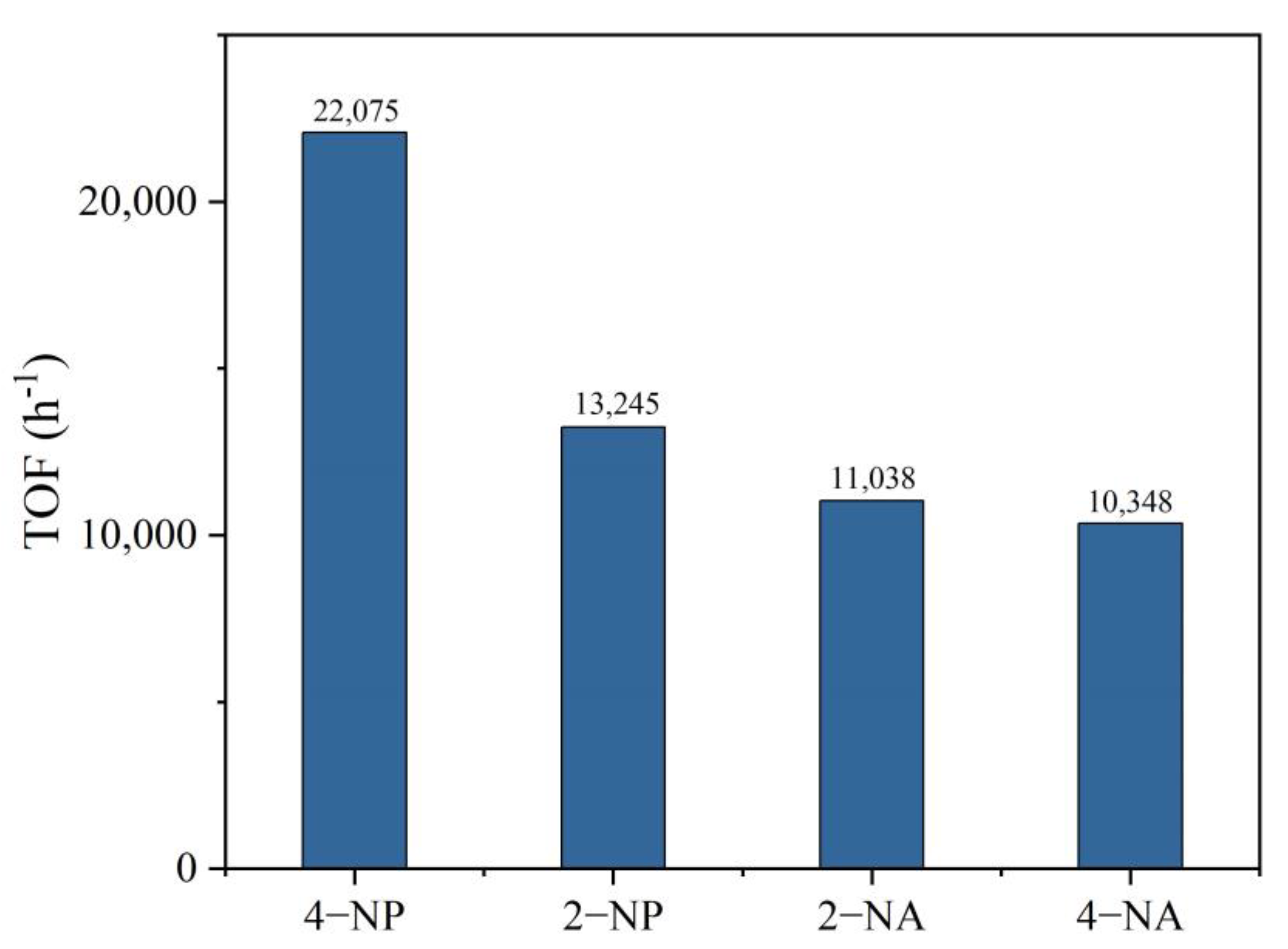
2. Result and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Catalyst
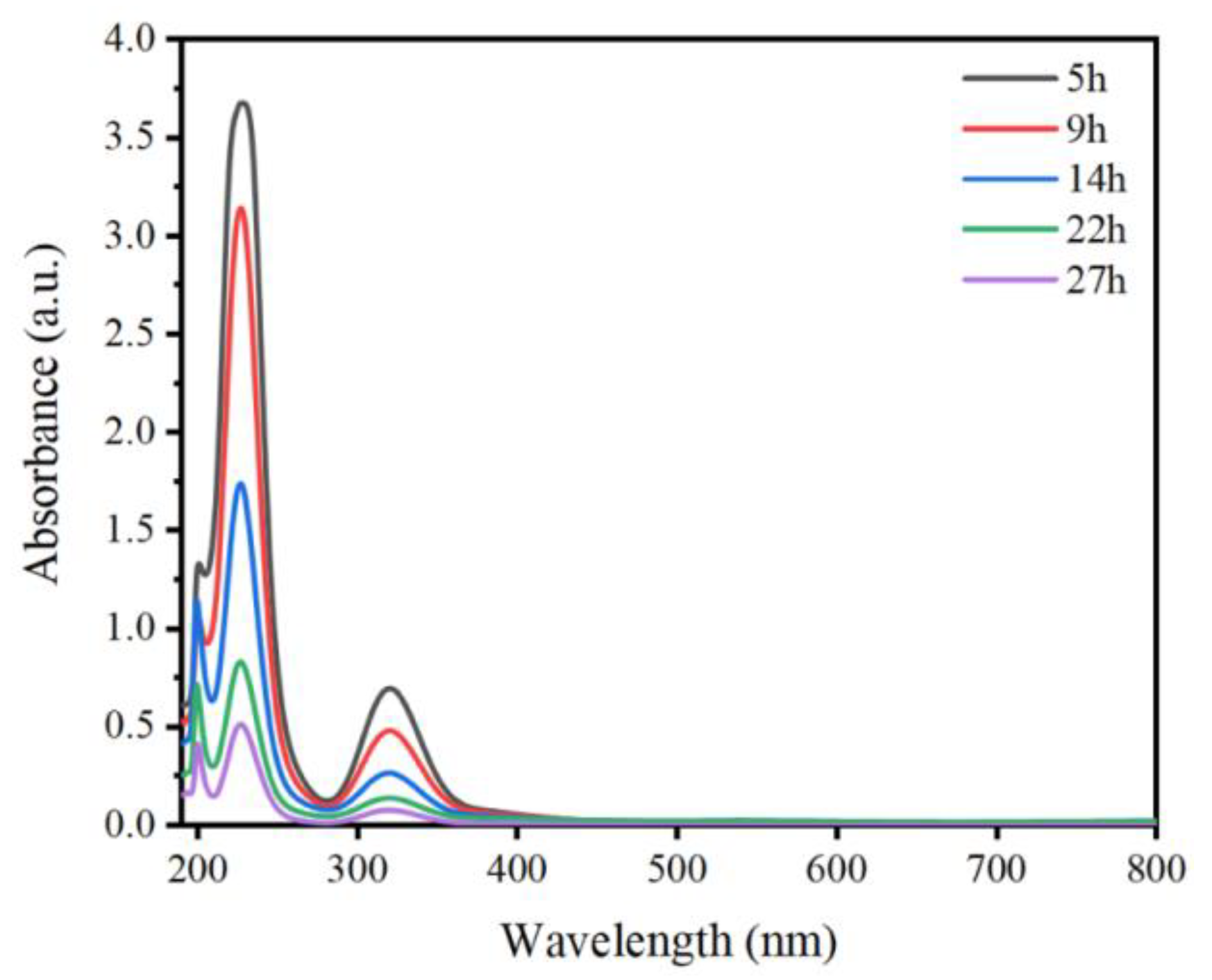
3.3. UV-Vis Absorption Spectroscopy
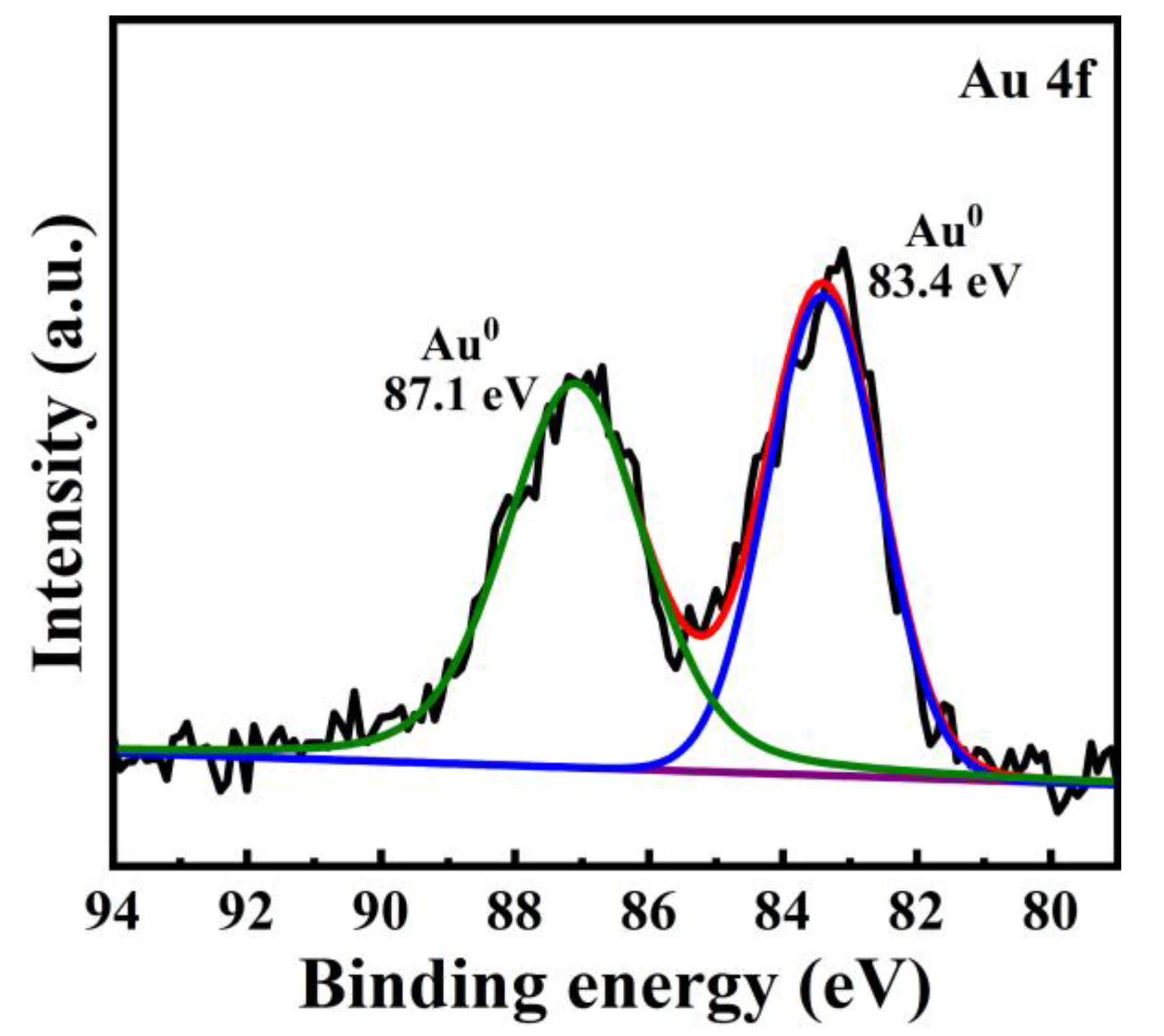
3.4. X-ray Photoelectron Spectroscopy (XPS) Analysis
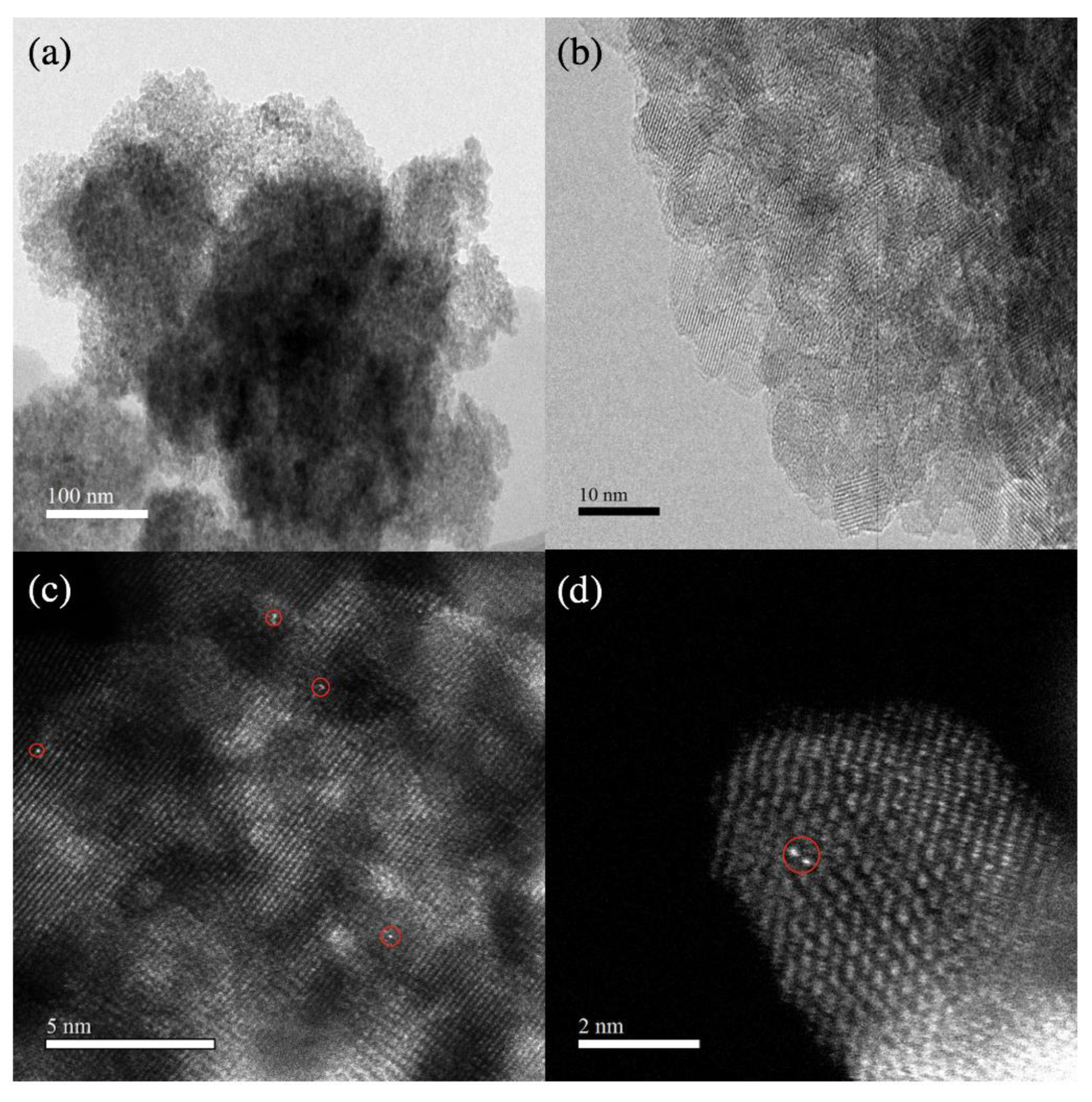
3.5. Transmission Electron Microscopic Analysis (TEM)
3.6. Spherical Aberration Electron Microscopic Analysis
3.7. X-ray Absorption Spectroscopy (XAS) Analysis
3.8. Catalytic Efficiency Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gorin, D.J.; Toste, F.D. Relativistic effects in homogeneous gold catalysis. Nature 2007, 446, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Guan, P.; McKenna, K.; Lang, X.; Hirata, A.; Zhang, L.; Chen, M. Atomic origins of the high catalytic activity of nanoporous gold. Nat. Mater. 2012, 11, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K. Gold-catalyzed organic reactions. Chem. Rev. 2007, 107, 3180–3211. [Google Scholar] [CrossRef] [PubMed]
- Sennewald, K.; Vogt, W.; Erpenbach, H.; Glaser, H.; Dyrschka, H. Carrier Catalyst for the Manufacture of Unsaturated Esters of Carboxylic Acids. U.S. Patent 3,650,986, 21 March 1968. [Google Scholar]
- Qiao, B.; Liang, J.-X.; Wang, A.; Liu, J.; Zhang, T. Single atom gold catalysts for low-temperature CO oxidation. Chin. J. Catal. 2016, 37, 1580–1587. [Google Scholar] [CrossRef]
- Cisneros, L.; Serna, P.; Corma, A. Selective reductive coupling of nitro aliphatic compounds with aldehydes in hydrogen using gold catalyst. Chin. J. Catal. 2016, 37, 1756–1763. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, M.; Zhang, G.; Liu, Y.; Cao, Y.; Liu, S.; Wang, Y. Highly selective supported gold catalyst for CO-driven reduction of furfural in aqueous media. Chin. J. Catal. 2016, 37, 1669–1675. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, X.; Zhang, L.; Lang, R.; Qiao, B. Single-atom catalysis: Bridging the homo- and heterogeneous catalysis. Chin. J. Catal. 2018, 39, 893–898. [Google Scholar] [CrossRef]
- Zhang, A.; Zhou, M.; Liu, S.; Chai, M.; Jiang, S. Synthesis of single-atom catalysts through top-down atomization approaches. Front. Chem. 2021, 1, 754167. [Google Scholar] [CrossRef]
- Liu, K.; Hou, G.; Mao, J.; Xu, Z.; Yan, P.; Li, H.; Guo, X.; Bai, S.; Zhang, Z.C. Genesis of electron deficient Pt1(0) in PDMS-PEG aggregates. Nat. Commun. 2019, 10, 996. [Google Scholar] [CrossRef]
- Liu, K.; Shen, X.; Bai, S.; Zhang, Z.C. Stable discrete Pt1(0) in crown ether with ultra-high hydrosilylation activity. ChemCatChem 2020, 12, 267–272. [Google Scholar] [CrossRef]
- Li, H.X.; Liang, C.H.; Liu, K.R.; Hou, G.J.; Bai, S.; Zhang, Z.C. Bi/trinuclear Pt1.2Cu cluster assembly from isolated metal atoms. Chem. Commun. 2022, 58, 4176–4179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, L.; Sadeghzadeh, S.M. Reduction of 4-nitrophenol and 2-nitroaniline using immobilized CoMn2O4 NPs on lignin supported on FPS. RSC. Adv. 2020, 10, 19553–19561. [Google Scholar] [CrossRef]
- Alshammari, K.; Niu, Y.; Palmer, R.E.; Dimitratos, N. Optimization of sol-immobilized bimetallic Au-Pd/TiO2 catalysts: Reduction of 4-nitrophenol to 4-aminophenol for wastewater remediation. Philos. Trans. R. Soc. A 2020, 378, 20200057. [Google Scholar] [CrossRef] [PubMed]
- Din, M.I.; Khalid, R.; Hussain, Z.; Hussain, T.; Mujahid, A.; Najeeb, J.; Izhar, F. Nanocatalytic assemblies for catalytic reduction of nitrophenols: A critical review. Crit. Rev. Anal. Chem. 2020, 50, 322–338. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Zhai, S.R.; Zhai, B.; Xiao, Z.Y.; An, Q.D. Preparation and catalytic properties of nano-Au catalytic materials based on the reduction of 4-Nitrophenol. Prog. Chem. 2014, 26, 234–247. [Google Scholar]
- Wen, F.; Wang, D.; Zhang, X.; Qiao, N.; Sun, Y.; Zhang, H. Electro-Fenton degradation of p-nitrophenol using fe/chi-mwnts modified graphite cathode. Technol. Water Treat. 2014, 40, 62–65,69. [Google Scholar]
- Osin, O.A.; Yu, T.; Cai, X.; Jiang, Y.; Peng, G.; Cheng, X.; Li, R.; Qin, Y.; Lin, S. Photocatalytic degradation of 4-Nitrophenol by C, N-TiO2: Degradation efficiency vs. embryonic toxicity of the resulting compounds. Front. Chem. 2018, 6, 192. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Wang, H.; Lu, J.; He, M. Electrochemical reduction of m-Nitrophenol. Fine Chem. 2003, 20, 673–675. [Google Scholar]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, P.; Zhang, X.; Zhuo, R. Gold nanoparticles stabilized by amphiphilic hyperbranched polymers for catalytic reduction of 4-Nitrophenol. J. Catal. 2016, 337, 65–71. [Google Scholar] [CrossRef]
- Shang, Y.; Min, C.; Hu, J.; Wang, T.; Liu, H.; Hu, Y. Synthesis of gold nanoparticles by reduction of HAuCl4 under UV irradiation. Solid State Sci. 2013, 15, 17–23. [Google Scholar] [CrossRef]
- Sakamoto, M.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Acceleration of laser-induced formation of gold nanoparticles in a poly(vinyl alcohol) film. Langmuir 2006, 22, 6361–6366. [Google Scholar] [CrossRef]
- Turner, N.H.; Single, A.M. Determination of peak positions and areas from wide-scan XPS spectra. Surf. Interface Anal. 1990, 15, 215–222. [Google Scholar] [CrossRef]
- Li, Y.g.; Quan, X.; Hu, C.; Li, C. Effective Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol over Etched Halloysite Nanotubes@α-Ni(OH)2. ACS Appl. Energy Mater. 2020, 3, 4756–4766. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, G.; Li, S.; Hao, W.; Yu, R. Facile one-pot synthesis of MOF supported gold pseudo-single-atom catalysts for hydrogenation reactions. R. Soc. Chem. 2018, 2, 1024–1030. [Google Scholar] [CrossRef]
- Choi, Y.; Bae, H.S.; Seo, E.; Jang, S.; Kim, B.S. Hybrid gold nanoparticle-reduced graphene oxide nanosheets as active catalysts for highly efficient reduction of nitroarenes. J. Mater. Chem. 2011, 21, 15431–15436. [Google Scholar] [CrossRef]
- Xi, J.; Sun, H.; Wang, D.; Zhang, Z.; Duan, X.; Xiao, J.; Xiao, F.; Liu, L.; Wang, S. Confined-interface-directed synthesis of Palladium single-atom catalysts on graphene/amorphous carbon. Appl. Catal. B Environ. 2018, 225, 291–297. [Google Scholar] [CrossRef]
- Li, J.; Zhong, L.; Tong, L.; Yu, Y.; Zhang, J. Atomic Pd on graphdiyne/graphene heterostructure as efficient catalyst for aromatic nitroreduction. Adv. Funct. Mater. 2019, 29, 1905423. [Google Scholar] [CrossRef]
- Qin, L.; Yi, H.; Zeng, G.; Lai, C.; Huang, D.; Xu, P.; Fu, Y.; He, J.; Li, B.; Zhang, C.; et al. Hierarchical porous carbon material restricted Au catalyst for highly catalytic reduction of nitroaromatics. J. Hazard. Mater. 2019, 380, 120864. [Google Scholar] [CrossRef]
- Liu, L.; Chen, R.; Liu, W.; Wu, J.; Gao, D. Catalytic reduction of 4-nitrophenol over Ni-Pd nanodimers supported on nitrogen-doped reduced graphene oxide. J. Hazard. Mater. 2016, 320, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Ellis, P.R.; Yin, J.; Liu, J.; Brown, C.M.; Griffin, R.; Chang, G.; Yang, D.; Ren, J.; Cooke, K. Performance of preformed Au/Cu nanoclusters deposited on MgO powders in the catalytic reduction of 4-Nitrophenol in solution. Small 2018, 14, 1703734. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.M.; Rocha, M.; Rivera-Cárcamo, C.; Serp, P.; Freire, C. Ru single atoms and nanoparticles on carbon nanotubes as multifunctional catalysts. Dalton Trans. 2020, 49, 10250–10260. [Google Scholar] [CrossRef]
- Liang, J.; Song, Q.; Wu, J.; Lei, Q.; Lee, C.S. Anchoring Copper Single Atoms on Porous Boron Nitride Nanofiber to Boost Selective Reduction of Nitroaromatics. ACS Nano 2021, 16, 4152–4161. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Deng, X.; He, P.; Wang, G. Atomic Pt anchored on hierarchically porous monolithic carbon nanowires as high-performance catalyst for liquid hydrogenation. Nano Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Du, Z.; Qin, J.; Zhang, K.; Jia, L.; Tian, K.; Zhang, J.; Xie, H. N-doped carbon nanosheets supported-single Fe atom for p-nitrophenol degradation via peroxymonosulfate activation. Appl. Surf. Sci. 2022, 591, 153124. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Zhao, H.; Tao, J.; Zhai, Y. Pd Anchored on a phytic acid/thiourea polymer as a highly active and stable catalyst for the reduction of nitroarene. ACS Appl. Mater. Interfaces 2021, 13, 19904–19914. [Google Scholar] [CrossRef]
- Muhammad, I.; Khan, M.I.; Bahadar, K.S.; Kalsoom, A.; Ali, K.M.; Asiri, A.M. Catalytic reduction of picric acid, nitrophenols and organic azo dyes via green synthesized plant supported Ag nanoparticles. J. Mol. Liq. 2018, 268, 87–101. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Liu, K. Discrete Au1(0) Stabilized by 15-Crown-5 for High-Efficiency Catalytic Reduction of Nitrophenol and Nitroaniline. Catalysts 2023, 13, 776. https://doi.org/10.3390/catal13040776
Shen X, Liu K. Discrete Au1(0) Stabilized by 15-Crown-5 for High-Efficiency Catalytic Reduction of Nitrophenol and Nitroaniline. Catalysts. 2023; 13(4):776. https://doi.org/10.3390/catal13040776
Chicago/Turabian StyleShen, Xing, and Kairui Liu. 2023. "Discrete Au1(0) Stabilized by 15-Crown-5 for High-Efficiency Catalytic Reduction of Nitrophenol and Nitroaniline" Catalysts 13, no. 4: 776. https://doi.org/10.3390/catal13040776
APA StyleShen, X., & Liu, K. (2023). Discrete Au1(0) Stabilized by 15-Crown-5 for High-Efficiency Catalytic Reduction of Nitrophenol and Nitroaniline. Catalysts, 13(4), 776. https://doi.org/10.3390/catal13040776





