Functionalized Graphene-Incorporated Cupric Oxide Charge-Transport Layer for Enhanced Photoelectrochemical Performance and Hydrogen Evolution
Abstract
:1. Introduction
2. Results and Discussion
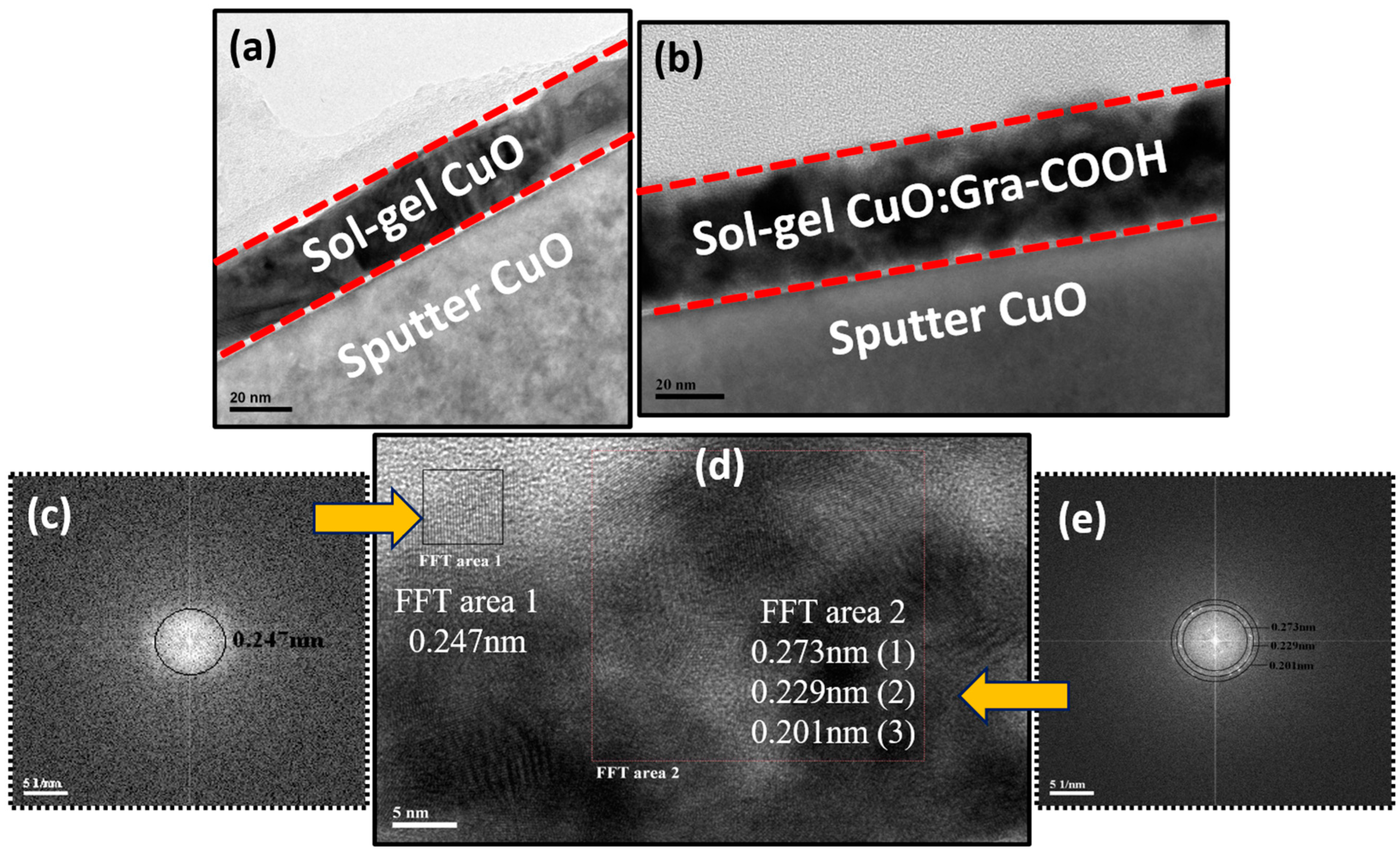
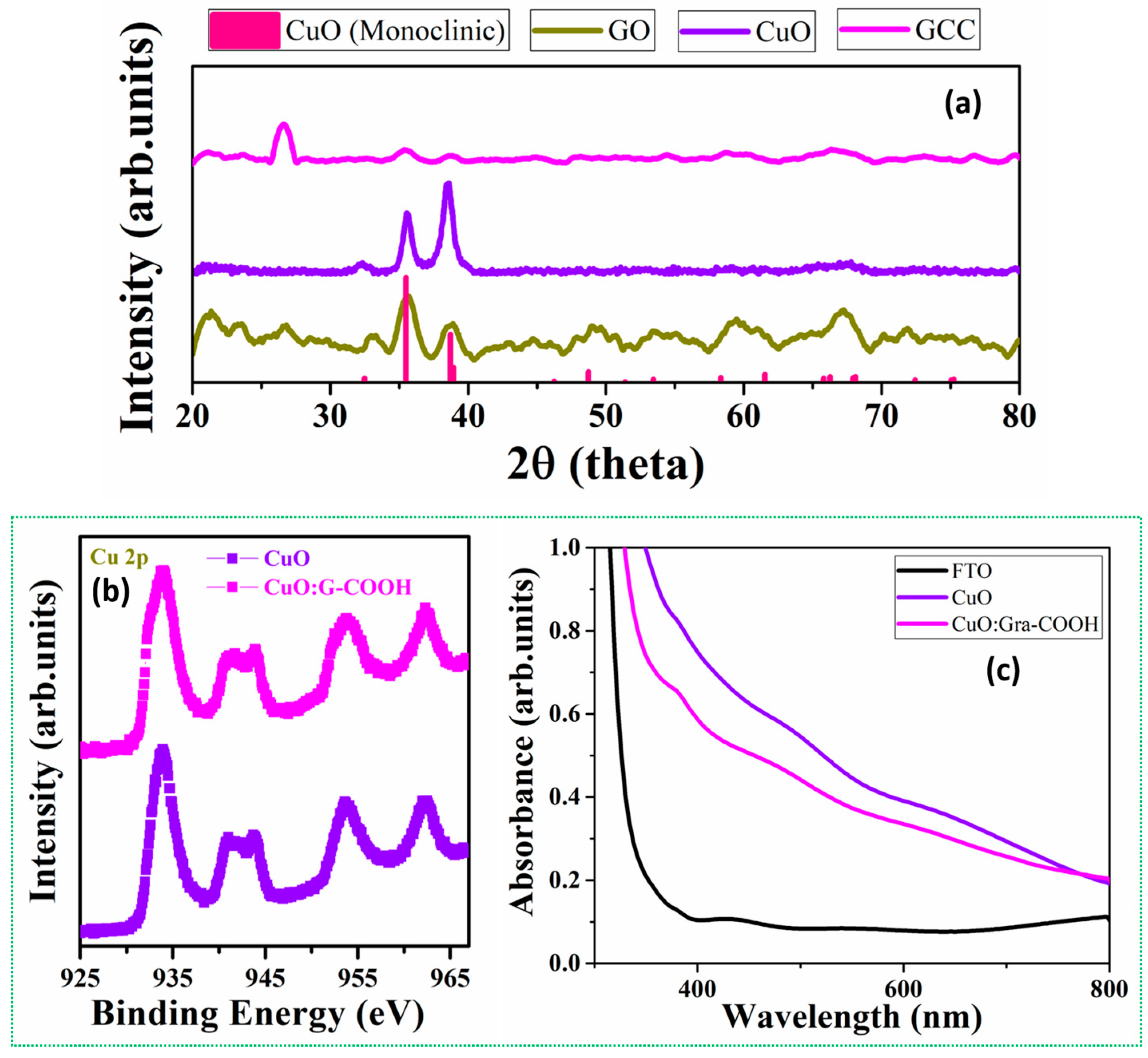
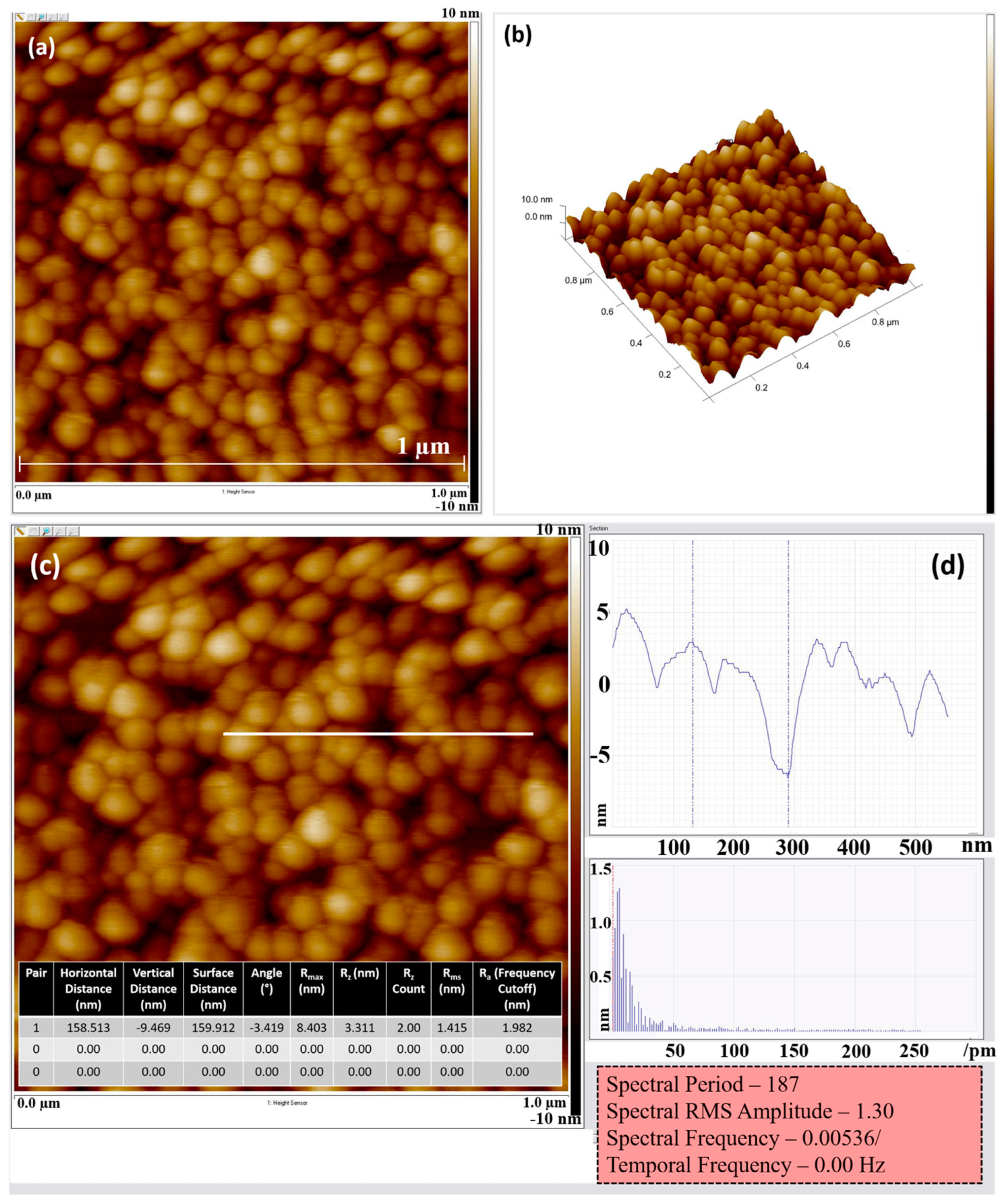
2.1. Structure Elucidation of Active Thin Film
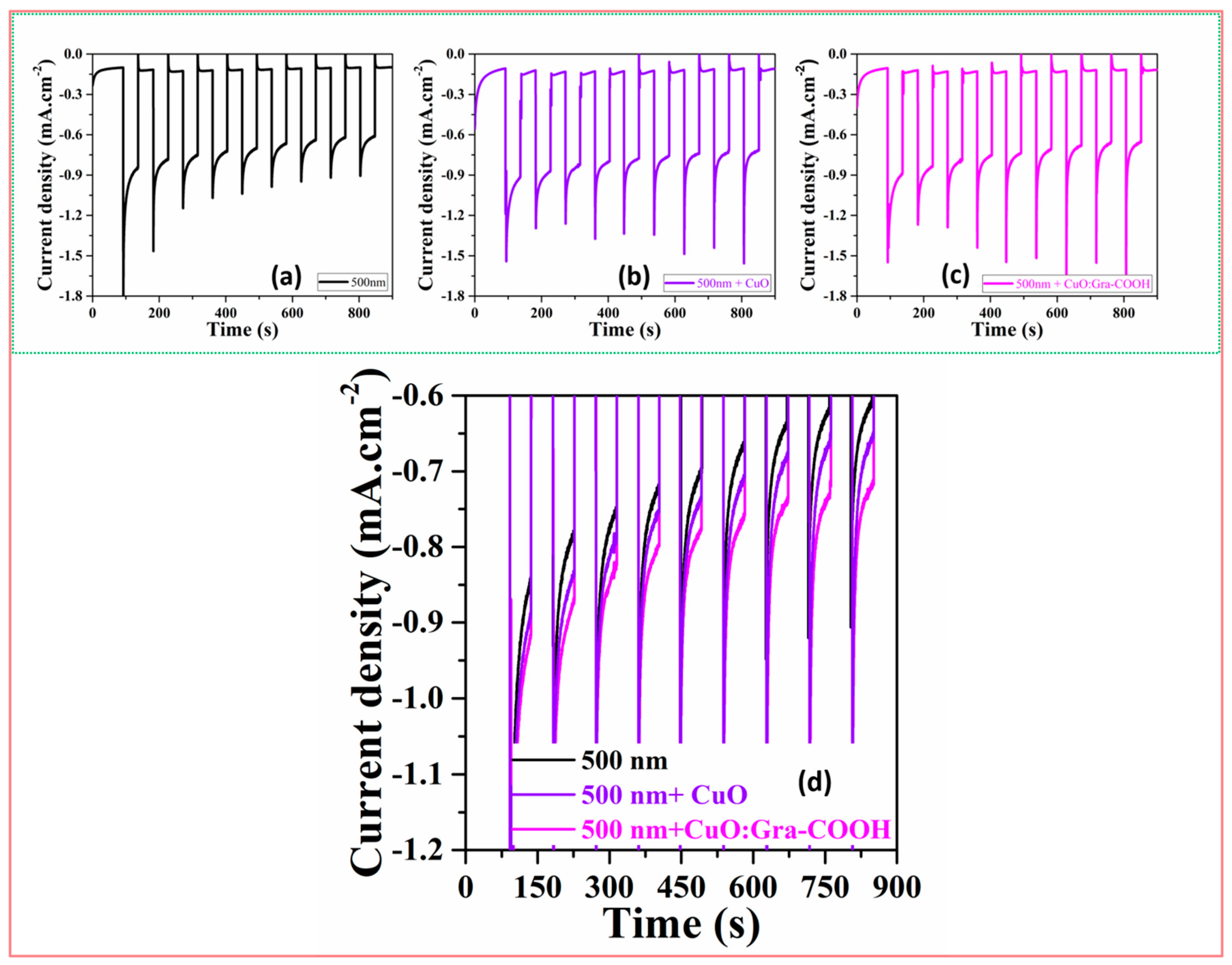
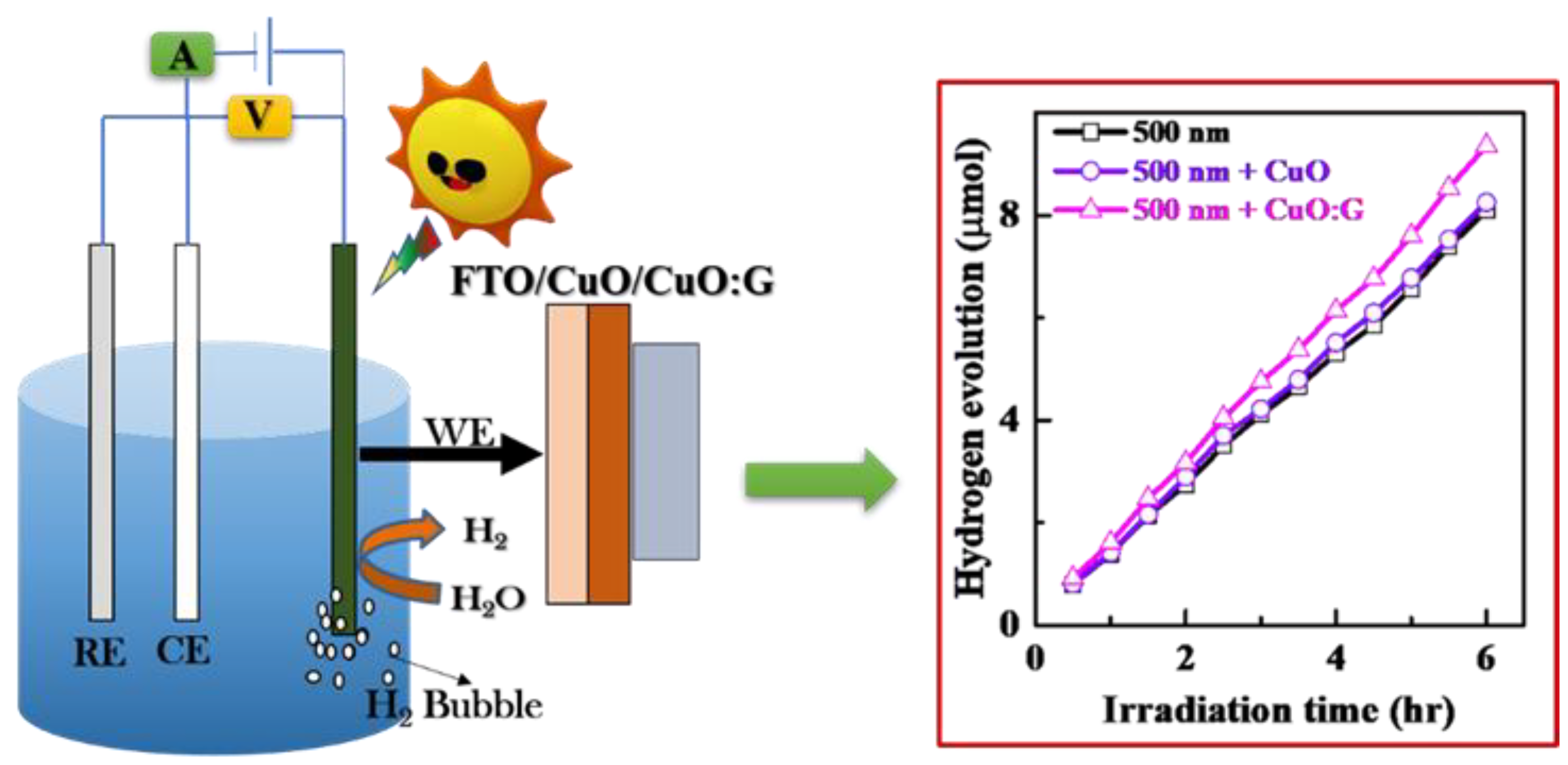
2.2. PEC Property Comparison of CuO and Incorporated Graphene CuO Thin Film
3. Experimental Section
3.1. Thin-Film Preparation on FTO-Coated Glass Substrate
3.2. Material Characterization
3.3. Photoelectrochemical Measurement (PEC)
3.4. Computational Methodologies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernandez, R.R.; Armstrong, A.; Burney, J.; Ryan, G.; Moore-O’leary, K.; Diédhiou, I.; Grodsky, S.M.; Saul-Gershenz, L.; Davis, R.; Macknick, J.; et al. Techno–ecological synergies of solar energy for global sustainability. Nat. Sustain. 2019, 2, 560–568. [Google Scholar] [CrossRef]
- Halabi, M.A.; Al-Qattan, A.; Al-Otaibi, A. Application of solar energy in the oil industry—Current status and future prospects. Renew. Sustain. Energy Rev. 2015, 43, 296–314. [Google Scholar] [CrossRef]
- Rosen, M.A. Engineering Sustainability: A Technical Approach to Sustainability. Sustainability 2012, 4, 2270–2292. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Patnaik, S.; Rath, D.; Nanda, B.; Parida, K. Cu@CuO promoted g-C3N4/MCM-41: An efficient photocatalyst with tunable valence transition for visible light induced hydrogen generation. RSC Adv. 2016, 6, 112602–112613. [Google Scholar] [CrossRef]
- Dangelico, R.M.; Pujari, D. Mainstreaming Green Product Innovation: Why and How Companies Integrate Environmental Sustainability. J. Bus. Ethic 2010, 95, 471–486. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Masudy-Panah, S.; Kumar, A.; Tan, C.C.; Tan, H.R.; Chi, D. Aluminium alloyed iron-silicide/silicon solar cells: A simple approach for low cost environmental-friendly photovoltaic technology. Sci. Rep. 2015, 5, 17810. [Google Scholar] [CrossRef]
- Creissen, C.E.; Fontecave, M. Solar-Driven Electrochemical CO2 Reduction with Heterogeneous Catalysts. Adv. Energy Mater. 2021, 11, 2002652. [Google Scholar] [CrossRef]
- Tawfik, W.Z.; Hassan, M.A.; Johar, M.A.; Ryu, S.-W.; Lee, J.K. Highly conversion efficiency of solar water splitting over p-Cu2O/ZnO photocatalyst grown on a metallic substrate. J. Catal. 2019, 374, 276–283. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical Cells. In Materials for Sustainable Energy; Co-Published with Macmillan Publishers Ltd.: London, UK, 2010; pp. 26–32. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Liu, R.-S.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Nano-architecture and material designs for water splitting photoelectrodes. Chem. Soc. Rev. 2012, 41, 5654–5671. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, G.K.; Chua, C.S.; Kushwaha, A.; Liew, S.L.; Suresh, V.; Chi, D. All earth abundant materials for low cost solar-driven hydrogen production. Mater. Lett. 2016, 183, 183–186. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Dalapati, G.K.; Radhakrishnan, K.; Kumar, A.; Tan, H.R.; Kumar, E.N.; Vijila, C.; Tan, C.C.; Chi, D. p-CuO/n-Si heterojunction solar cells with high open circuit voltage and photocurrent through interfacial engineering. Prog. Photovolt. Res. Appl. 2015, 23, 637–645. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Abdalla, E.M.; Shaban, M. Simple and Low-Cost Synthesis of Ba-Doped CuO Thin Films for Highly Efficient Solar Generation of Hydrogen. J. Phys. Chem. C 2020, 124, 22347–22356. [Google Scholar] [CrossRef]
- Sultana, J.; Paul, S.; Karmakar, A.; Dalapati, G.K.; Chattopadhyay, S. Optimizing the thermal annealing temperature: Technological route for tuning the photo-detecting property of p-CuO thin films grown by chemical bath deposition method. J. Mater. Sci. Mater. Electron. 2018, 29, 12878–12887. [Google Scholar] [CrossRef]
- Dimopoulos, T.; Peić, A.; Müllner, P.; Neuschitzer, M.; Resel, R.; Abermann, S.; Postl, M.; List, E.J.W.; Yakunin, S.; Heiss, W.; et al. Photovoltaic properties of thin film heterojunctions with cupric oxide absorber. J. Renew. Sustain. Energy 2013, 5, 011205. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Epstein, J.; Brown, A.; Munday, J.N.; Culver, J.N.; Ehrman, S. Biological Templates for Antireflective Current Collectors for Photoelectrochemical Cell Applications. Nano Lett. 2012, 12, 6005–6011. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef]
- Koffyberg, F.P.; Benko, F.A. A photoelectrochemical determination of the position of the conduction and valence band edges of p-type CuO. J. Appl. Phys. 1982, 53, 1173–1177. [Google Scholar] [CrossRef]
- Wadia, C.; Alivisatos, A.P.; Kammen, D.M. Materials Availability Expands the Opportunity for Large-Scale Photovoltaics Deployment. Environ. Sci. Technol. 2009, 43, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Manojkumar, K.; Kandeeban, R.; Brindha, R.; Sangeetha, V.; Saminathan, K. Non-precious metal-based integrated electrodes for overall alkaline water splitting. J. Indian Chem. Soc. 2022, 99, 100775. [Google Scholar] [CrossRef]
- Trang, T.; Tu, L.; Man, T.; Mathesh, M.; Nam, N.; Thu, V. A high-efficiency photoelectrochemistry of Cu2O/TiO2 nanotubes based composite for hydrogen evolution under sunlight. Compos. Part B Eng. 2019, 174, 106969. [Google Scholar] [CrossRef]
- Quyen, V.T.; Jitae, K.; Huong, P.T.; Ha, L.T.T.; Thanh, D.M.; Viet, N.M.; Thang, P.Q. Copper doped titanium dioxide as a low-cost visible light photocatalyst for water splitting. Sol. Energy 2021, 218, 150–156. [Google Scholar] [CrossRef]
- Negi, C.; Kandwal, P.; Rawat, J.; Sharma, M.; Sharma, H.; Dalapati, G.; Dwivedi, C. Carbon-doped titanium dioxide nanoparticles for visible light driven photocatalytic activity. Appl. Surf. Sci. 2021, 554, 149553. [Google Scholar] [CrossRef]
- Chatterjee, P.; Ambati, M.S.K.; Chakraborty, A.K.; Chakrabortty, S.; Biring, S.; Ramakrishna, S.; Wong, T.K.S.; Kumar, A.; Lawaniya, R.; Dalapati, G.K. Photovoltaic/photo-electrocatalysis integration for green hydrogen: A review. Energy Convers. Manag. 2022, 261, 115648. [Google Scholar] [CrossRef]
- Bamola, P.; Sharma, M.; Dwivedi, C.; Singh, B.; Ramakrishna, S.; Dalapati, G.K.; Sharma, H. Interfacial interaction of plasmonic nanoparticles (Ag, Au) decorated floweret TiO2 nanorod hybrids for enhanced visible light driven photocatalytic activity. Mater. Sci. Eng. B 2021, 273, 115403. [Google Scholar] [CrossRef]
- Dey, A.; Chandrabose, G.; Damptey, L.A.; Erakulan, E.; Thapa, R.; Zhuk, S.; Dalapati, G.K.; Ramakrishna, S.; Braithwaite, N.S.J.; Shirzadi, A.; et al. Cu2O/CuO heterojunction catalysts through atmospheric pressure plasma induced defect passivation. Appl. Surf. Sci. 2021, 541, 148571. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Benck, J.D.; Seitz, L.C.; Forman, A.J.; Chen, Z.; Deutsch, T.G.; James, B.D.; Baum, K.N.; Baum, G.N.; Ardo, S.; et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013, 6, 1983–2002. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Masudy-Panah, S.; Moakhar, R.S.; Chakrabortty, S.; Ghosh, S.; Kushwaha, A.; Katal, R.; Chua, C.S.; Xiao, G.; Tripathy, S.; et al. Nanoengineered Advanced Materials for Enabling Hydrogen Economy: Functionalized Graphene–Incorporated Cupric Oxide Catalyst for Efficient Solar Hydrogen Production. Glob. Chall. 2020, 4, 1900087. [Google Scholar] [CrossRef]
- Lim, Y.-F.; Chua, C.S.; Lee, C.J.J.; Chi, D. sol-gel deposited Cu2O and CuO thin films for photocatalytic water splitting. Phys. Chem. Chem. Phys. 2014, 16, 25928–25934. [Google Scholar] [CrossRef] [PubMed]
- Masudy-Panah, S.; Radhakrishnan, K.; Kumar, A.; Wong, T.I.; Yi, R.; Dalapati, G.K. Optical bandgap widening and phase transformation of nitrogen doped cupric oxide. J. Appl. Phys. 2015, 118, 225301. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Radhakrishnan, K.; Tan, H.R.; Yi, R.; Wong, T.I.; Dalapati, G.K. Titanium doped cupric oxide for photovoltaic application. Sol. Energy Mater. Sol. Cells 2015, 140, 266–274. [Google Scholar] [CrossRef]
- Hasan, R.; Hamid, S.B.A.; Basirun, W.J.; Suhaimy, S.H.M.; Mat, A.N.C. A sol-gel derived, copper-doped, titanium dioxide–reduced graphene oxide nanocomposite electrode for the photoelectrocatalytic reduction of CO2 to methanol and formic acid. RSC Adv. 2015, 5, 77803–77813. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Liu, Y.; Li, J. Graphene and its derivatives for the development of solar cells, photoelectrochemical, and photocatalytic applications. Energy Environ. Sci. 2013, 6, 1362–1387. [Google Scholar] [CrossRef]
- Fan, W.; Yu, X.; Lu, H.-C.; Bai, H.; Zhang, C.; Shi, W. Fabrication of TiO2/RGO/Cu2O heterostructure for photoelectrochemical hydrogen production. Appl. Catal. B Environ. 2016, 181, 7–15. [Google Scholar] [CrossRef]
- Mateo, D.; Esteve-Adell, I.; Albero, J.; Primo, A.; García, H. Oriented 2.0.0 Cu2O nanoplatelets supported on few-layers graphene as efficient visible light photocatalyst for overall water splitting. Appl. Catal. B Environ. 2017, 201, 582–590. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Beitollahi, H.; Garkani-Nejad, F.; Tajik, S.; Ganjali, M.R. Voltammetric determination of acetaminophen and tryptophan using a graphite screen printed electrode modified with functionalized graphene oxide nanosheets within a Fe3O4@SiO2 nanocomposite. Iran. J. Pharm. Res. 2019, 18, 80–90. [Google Scholar] [CrossRef]
- El-Hout, S.; El-Sheikh, S.; Hassan, H.M.; Harraz, F.A.; Ibrahim, I.; El-Sharkawy, E. A green chemical route for synthesis of graphene supported palladium nanoparticles: A highly active and recyclable catalyst for reduction of nitrobenzene. Appl. Catal. A Gen. 2015, 503, 176–185. [Google Scholar] [CrossRef]
- Cheng, Y.; Fan, Y.; Pei, Y.; Qiao, M. Graphene-supported metal/metal oxide nanohybrids: Synthesis and applications in heterogeneous catalysis. Catal. Sci. Technol. 2015, 5, 3903–3916. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Tong, X.; Guo, X.; Zheng, Z.; Guo, X. Graphene-supported CoS2 particles: An efficient photocatalyst for selective hydrogenation of nitroaromatics in visible light. Catal. Sci. Technol. 2017, 7, 2805–2812. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, K.; Guo, B.; Liu, Q.; Fang, L.; Gong, J.R. Graphene-Based Materials for Hydrogen Generation from Light-Driven Water Splitting. Adv. Mater. 2013, 25, 3820–3839. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Ragupathy, M.; Ramasubramanian, B.; Rajagopalan, K.; Ganesan, A. Electrocatalytic response of the modified ZnO-G electrodes towards the oxidation of serotonin with multi metallic corrosion protection. J. Indian Chem. Soc. 2022, 99, 100768. [Google Scholar] [CrossRef]
- Kou, R.; Shao, Y.; Mei, D.; Nie, Z.; Wang, D.; Wang, C.; Viswanathan, V.V.; Park, S.; Aksay, I.A.; Lin, Y.; et al. Stabilization of Electrocatalytic Metal Nanoparticles at Metal−Metal Oxide−Graphene Triple Junction Points. J. Am. Chem. Soc. 2011, 133, 2541–2547. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, R.; Chen, W. Graphene-Supported Nanoelectrocatalysts for Fuel Cells: Synthesis, Properties, and Applications. Chem. Rev. 2014, 114, 5117–5160. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Siddiq, M.; Ali, M.U.; Ali, A.; Shabbir, H.; Bin Nazeer, U.; Saleem, M.S. Solar light triggered catalytic performance of graphene-CuO nanocomposite for waste water treatment. Ceram. Int. 2017, 43, 10654–10660. [Google Scholar] [CrossRef]
- Burke, K. Perspective on density functional theory. J. Chem. Phys. 2012, 136, 150901. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, P.; De Proft, A.F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Harvey, J.N. On the accuracy of density functional theory in transition metal chemistry. Annu. Rep. Sect. C Phys. Chem. 2006, 102, 203–226. [Google Scholar] [CrossRef]
- Minenkov, Y.; Singstad, Å.; Occhipinti, G.; Jensen, V.R. The accuracy of DFT-optimized geometries of functional transition metal compounds: A validation study of catalysts for olefin metathesis and other reactions in the homogeneous phase. Dalton Trans. 2012, 41, 5526–5541. [Google Scholar] [CrossRef] [PubMed]
- Raji, M.; Essabir, H.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E.K. Influence of graphene oxide and graphene nanosheet on the properties of polyvinylidene fluoride nanocomposites. Polym. Compos. 2017, 39, 2932–2941. [Google Scholar] [CrossRef]
- Ain, Q.T.; Haq, S.H.; Alshammari, A.; Al-Mutlaq, M.A.; Anjum, M.N. The systemic effect of PEG-nGO-induced oxidative stress in vivo in a rodent model. Beilstein J. Nanotechnol. 2019, 10, 901–911. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Y.; Cui, X.; Cheng, P.; Wang, B.; Gao, Y.; Li, X.; Yang, T.; Zhang, T.; Lu, G. Humidity-Sensing Properties of Urchinlike CuO Nanostructures Modified by Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2014, 6, 3888–3895. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, M.; Manivel, S.; Jeyavijayan, S.; Meenakshi, R. Vibrational spectroscopic (FTIR and FT-Raman), first-order hyperpolarizablity, HOMO, LUMO, NBO, Mulliken charge analyses of 2-ethylimidazole based on Hartree–Fock and DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 493–501. [Google Scholar] [CrossRef]
- Bell, N.J.; Ng, Y.H.; Du, A.; Coster, H.; Smith, S.C.; Amal, R. Understanding the Enhancement in Photoelectrochemical Properties of Photocatalytically Prepared TiO2-Reduced Graphene Oxide Composite. J. Phys. Chem. C 2011, 115, 6004–6009. [Google Scholar] [CrossRef]
- Tian, G.; Li, H.; Ma, W.; Wang, Y. Substituent effects in π-stacking of histidine on functionalized-SWNT and graphene. Comput. Theor. Chem. 2015, 1062, 44–49. [Google Scholar] [CrossRef]
- Ionita, M.; Crica, L.E.; Vasile, E.; Dinescu, S.; Pandele, M.A.; Costache, M.; Haugen, H.J.; Iovu, H. Effect of carboxylic acid functionalized graphene on physical-chemical and biological performances of polysulfone porous films. Polymer 2016, 92, 1–12. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Huang, D.; Nguyen, T.; Jiang, Y.; Yu, H.; Ding, N.; Ding, G.; Jiao, Z. Facile synthesis of size-tunable CuO/graphene composites and their high photocatalytic performance. Mater. Res. Bull. 2015, 61, 409–414. [Google Scholar] [CrossRef]
- Fampiou, I.; Ramasubramaniam, A. Binding of Pt Nanoclusters to Point Defects in Graphene: Adsorption, Morphology, and Electronic Structure. J. Phys. Chem. C 2012, 116, 6543–6555. [Google Scholar] [CrossRef]
- Zhen, Y.; Reddy, V.S.; Ramasubramanian, B.; Ramakrishna, S. Three-Dimensional AgNps@Mxene@PEDOT:PSS Composite Hybrid Foam as a Piezoresistive Pressure Sensor with Ultra-Broad Working Range. J. Mater. Sci. 2022, 57, 21960–21979. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-H.; Chen, Y.-P.; Cheng, Z. Microwave-assisted synthesis of rod-like CuO/TiO2 for high-efficiency photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2015, 40, 15994–16000. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Han, X.; Biset-Peiró, M.; Yang, Y.; Imaz, I.; Maspoch, D.; Yang, B.; Morante, J.R.; Arbiol, J. Improvement of carbon dioxide electroreduction by crystal surface modification of ZIF-8. Dalton Trans. 2023, 11, 5460–5475. [Google Scholar] [CrossRef] [PubMed]
- Masudy-Panah, S.; Moakhar, R.S.; Chua, C.S.; Tan, H.R.; Wong, T.I.; Chi, D.; Dalapati, G.K. Nanocrystal Engineering of Sputter-Grown CuO Photocathode for Visible-Light-Driven Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 1206–1213. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
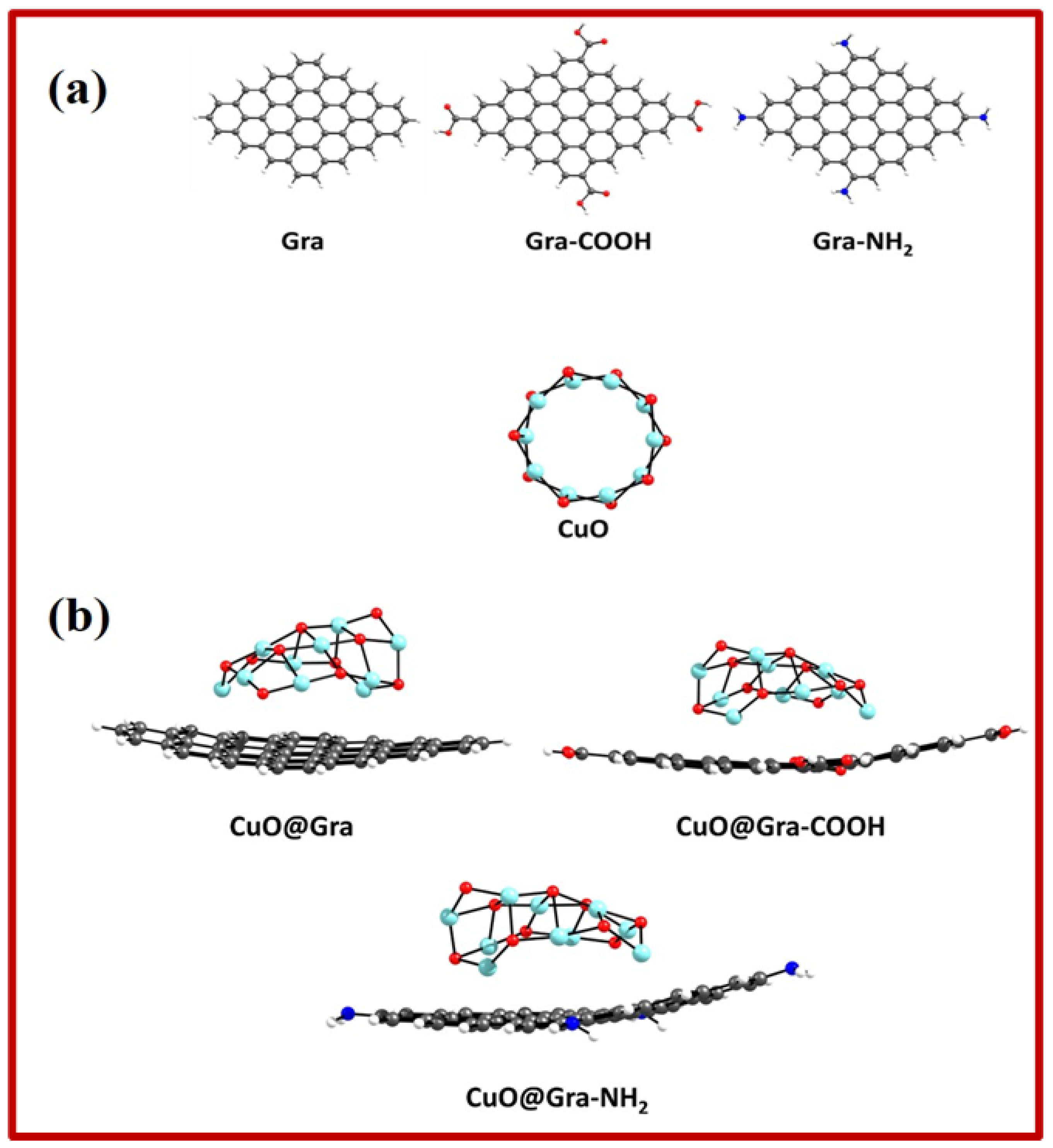
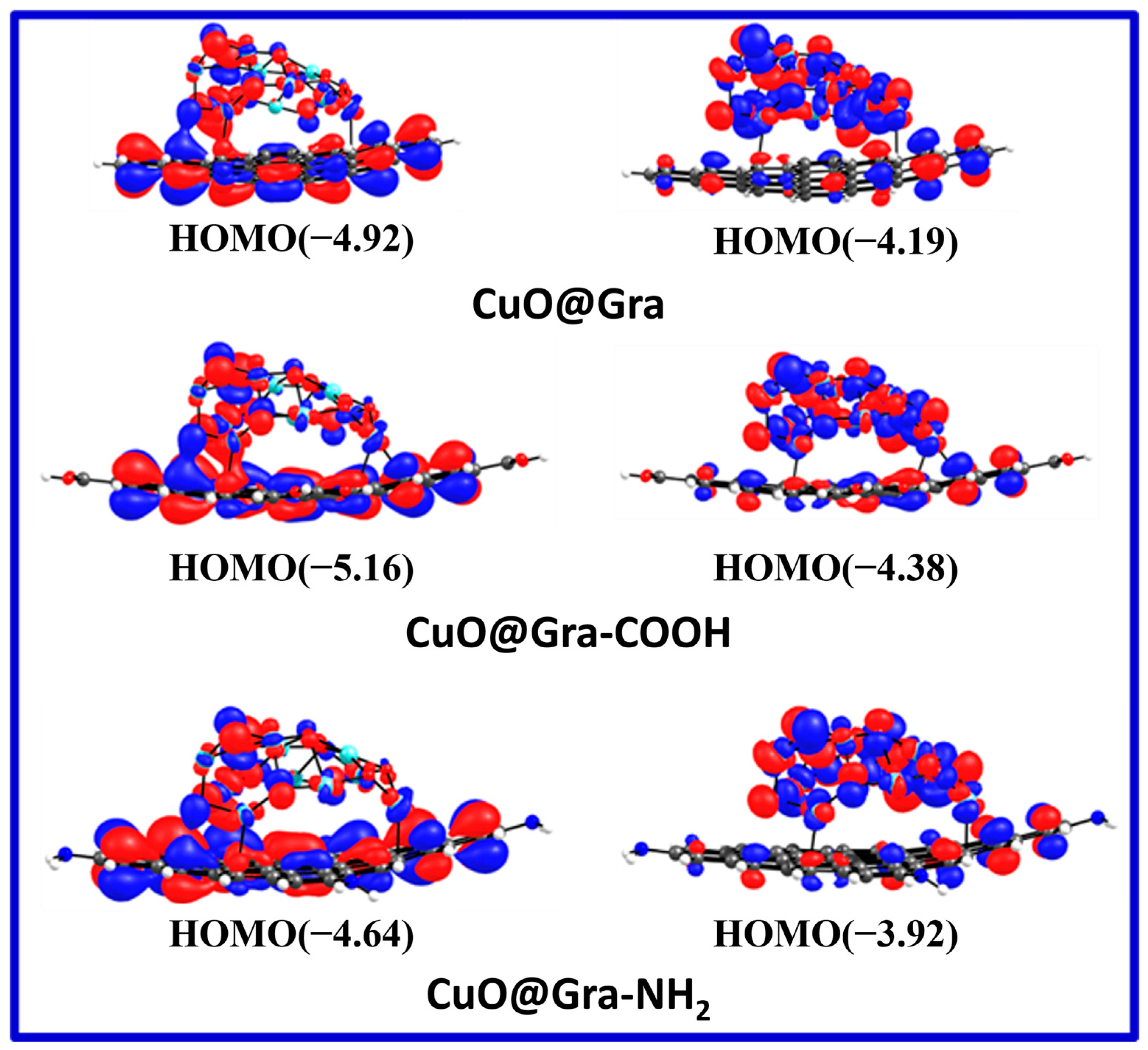

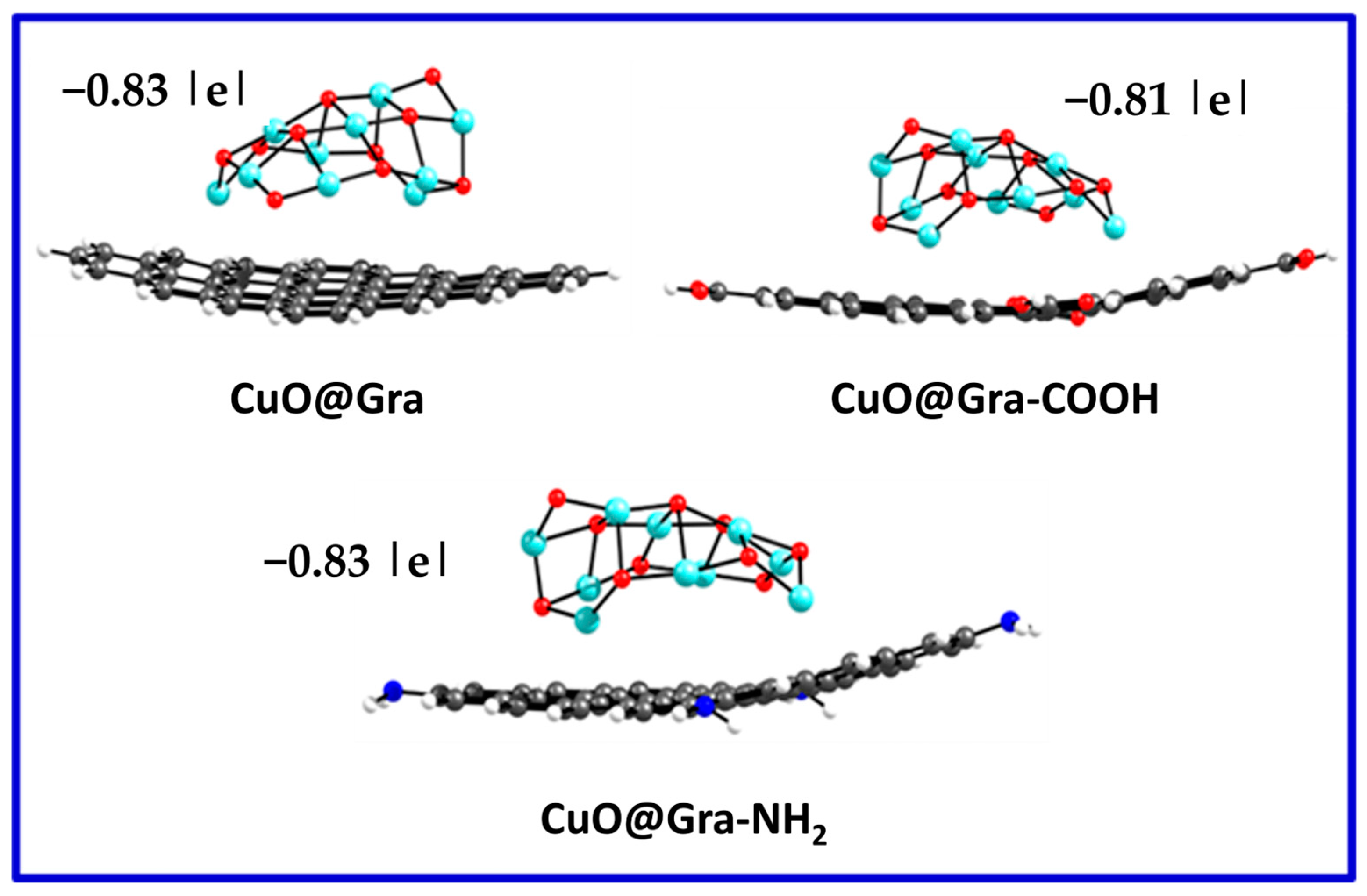


| S. No. | Sample Name | EHOMO | ELUMO | ΔE |
|---|---|---|---|---|
| 1 | CuO@Gra | −4.92 eV | −4.19 eV | 0.73 |
| 2 | CuO@Gra-COOH | −5.16 eV | −4.38 eV | 0.78 |
| 3 | CuO@Gra-NH2 | −4.64 eV | −3.92 eV | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishna, A.M.S.; Ramasubramanian, B.; Haseena, S.; Bamola, P.; Sharma, H.; Mahata, C.; Chroneos, A.; Krishnamurthy, S.; Ravva, M.K.; Chandu, B.; et al. Functionalized Graphene-Incorporated Cupric Oxide Charge-Transport Layer for Enhanced Photoelectrochemical Performance and Hydrogen Evolution. Catalysts 2023, 13, 785. https://doi.org/10.3390/catal13040785
Krishna AMS, Ramasubramanian B, Haseena S, Bamola P, Sharma H, Mahata C, Chroneos A, Krishnamurthy S, Ravva MK, Chandu B, et al. Functionalized Graphene-Incorporated Cupric Oxide Charge-Transport Layer for Enhanced Photoelectrochemical Performance and Hydrogen Evolution. Catalysts. 2023; 13(4):785. https://doi.org/10.3390/catal13040785
Chicago/Turabian StyleKrishna, Ambati Mounika Sai, Brindha Ramasubramanian, Sheik Haseena, Priyanka Bamola, Himani Sharma, Chandreswar Mahata, Alexander Chroneos, Satheesh Krishnamurthy, Mahesh Kumar Ravva, Basavaiah Chandu, and et al. 2023. "Functionalized Graphene-Incorporated Cupric Oxide Charge-Transport Layer for Enhanced Photoelectrochemical Performance and Hydrogen Evolution" Catalysts 13, no. 4: 785. https://doi.org/10.3390/catal13040785
APA StyleKrishna, A. M. S., Ramasubramanian, B., Haseena, S., Bamola, P., Sharma, H., Mahata, C., Chroneos, A., Krishnamurthy, S., Ravva, M. K., Chandu, B., Lim, Y. -F., Kumar, A., Ramakrishna, S., Biring, S., Chakrabortty, S., & Dalapati, G. K. (2023). Functionalized Graphene-Incorporated Cupric Oxide Charge-Transport Layer for Enhanced Photoelectrochemical Performance and Hydrogen Evolution. Catalysts, 13(4), 785. https://doi.org/10.3390/catal13040785













