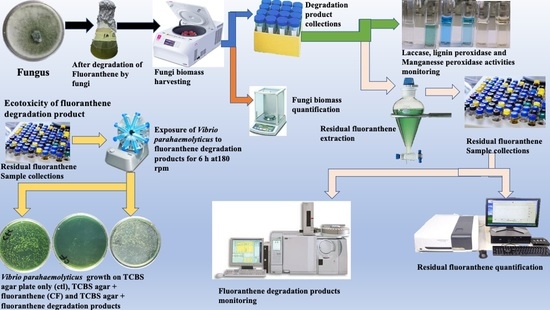
Metabolic Biodegradation Pathway of Fluoranthene by Indigenous Trichoderma lixii and Talaromyces pinophilus spp.
Abstract
:1. Introduction
2. Results
2.1. Fungal Cultural Morphological Characteristics and Molecular Analyses
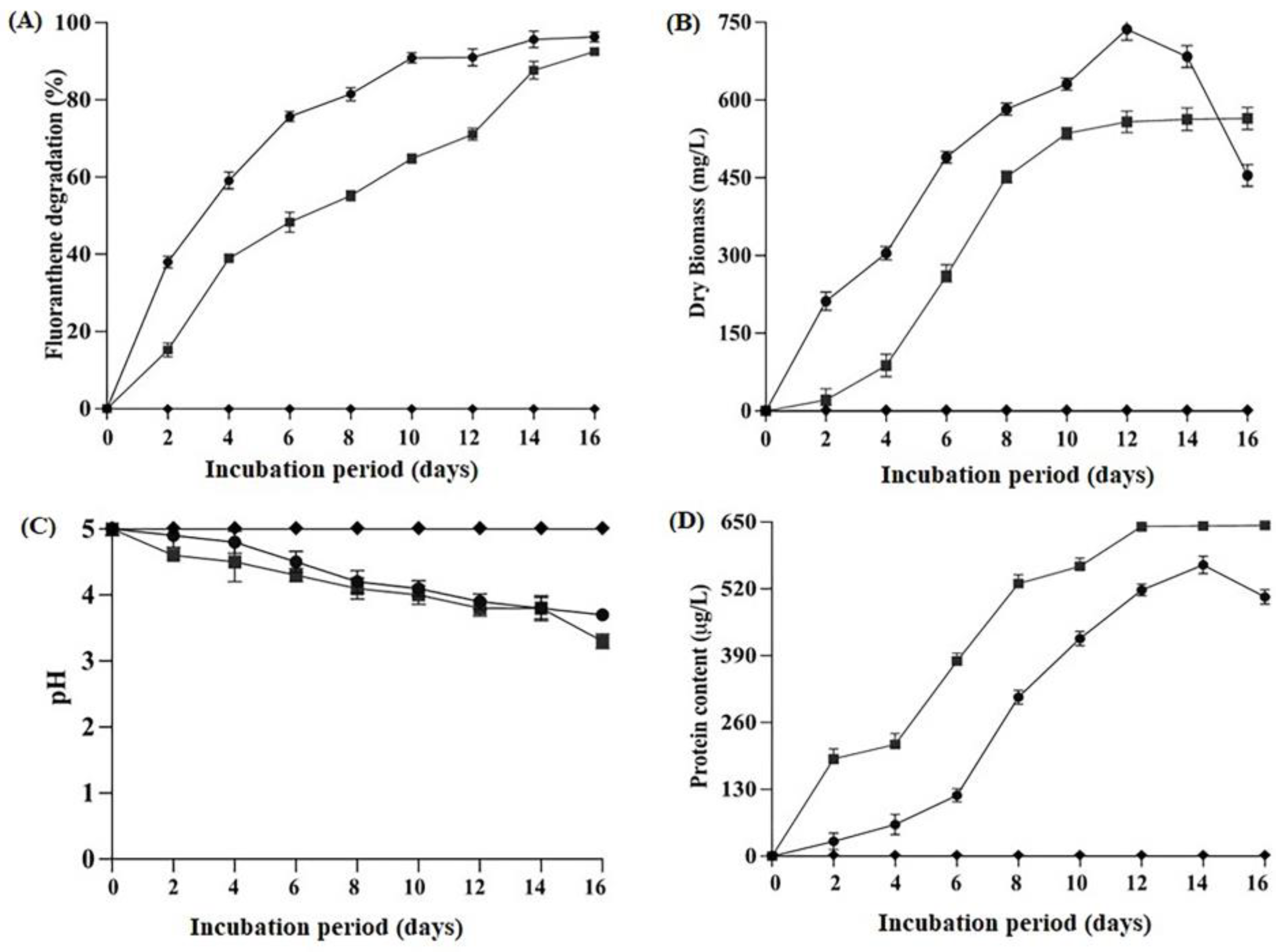
2.2. Degradation Efficiency, Biomass Production, Protein Content and Change in pH Condition
2.3. Degradation Kinetics Study
2.4. Fluoranthene Degradation Metabolites with Its Pathway
2.5. Analysis of the Transformed Fluoranthene Functional Groups
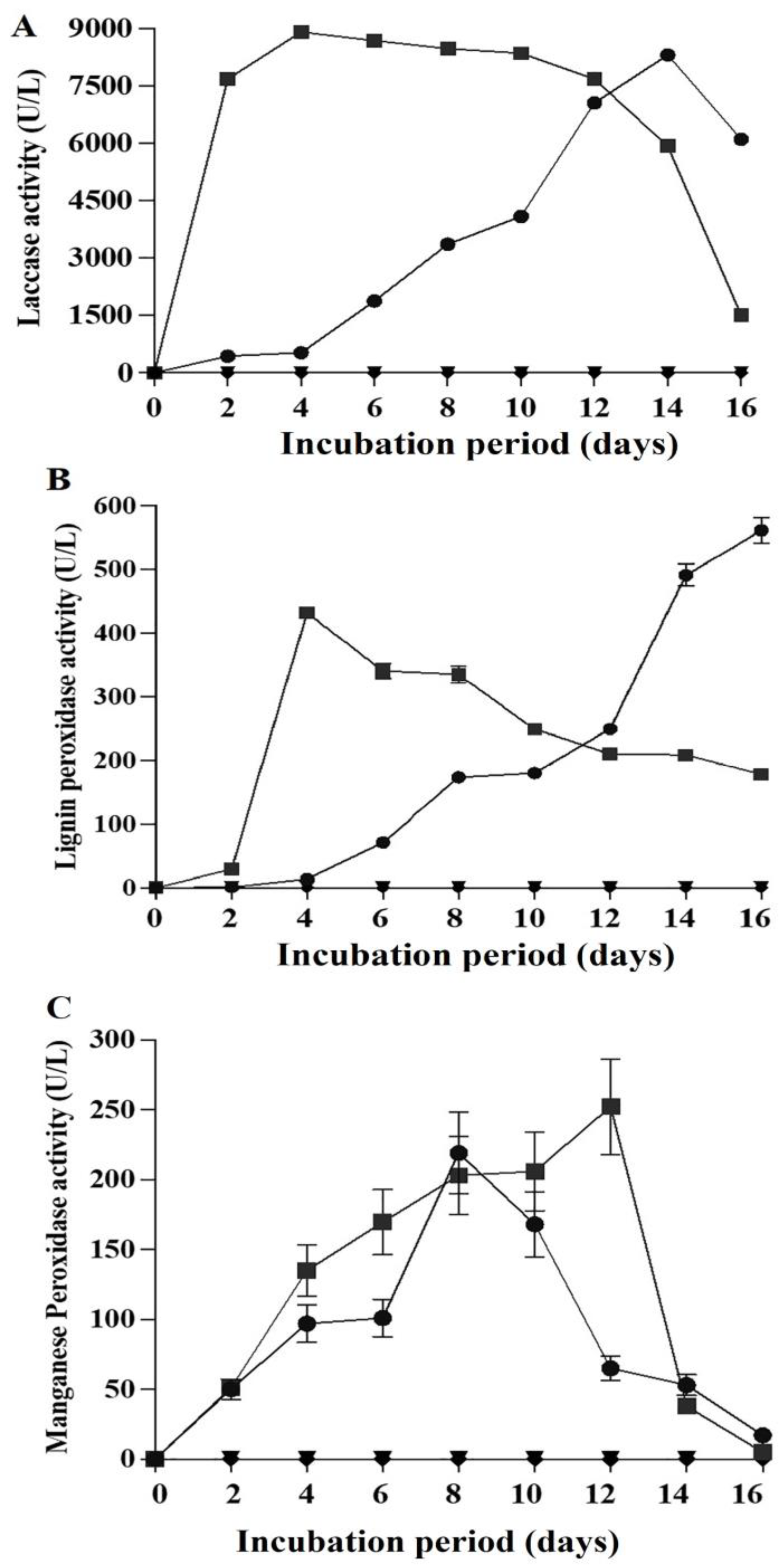
2.6. Ligninolytic Activities in the Presence of Fluoranthene
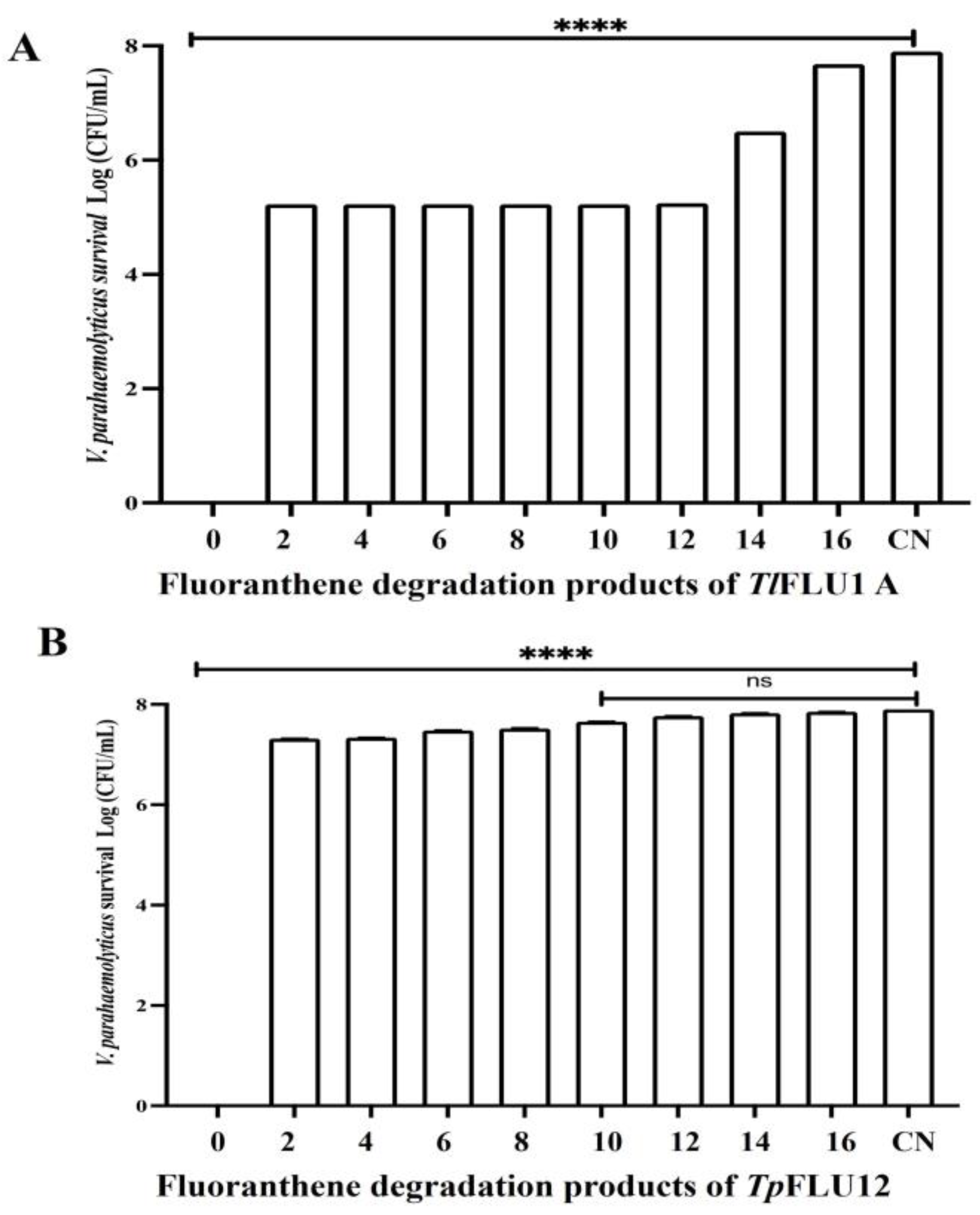
2.7. Ecotoxicity Test
3. Discussion
4. Materials and Methods
4.1. Chemicals and Media
4.2. Preparation of Standard Solutions
4.3. Fungal Strains and Molecular Identification
4.4. Degradation of Fluoranthene
4.5. Fungal Strains Biomass Estimation
4.6. Extraction, Quantification of Residual Fluoranthene and Kinetics of Degradation
4.7. Determination of Intermediates Compounds by GC-MS Analysis
4.8. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
4.9. Quantitative Estimation of Ligninolytic Enzymes Activities
4.9.1. Laccase Assay
4.9.2. Lignin Peroxidase Assay
4.9.3. Manganese Peroxidase Assay
4.10. Ecotoxicity Test
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masiol, M.; Hofer, A.; Squizzato, S.; Piazza, R.; Rampazzo, G.; Pavoni, B. Carcinogenic and mutagenic risk associated to airborne particle-phase polycyclic aromatic hydrocarbons: A source apportionment. Atmos. Environ. 2012, 60, 375–382. [Google Scholar] [CrossRef]
- Zafra, G.; Cortés-Espinosa, D.V. Biodegradation of polycyclic aromatic hydrocarbons by Trichoderma species: A mini review. Environ. Sci. Pollut. Res. 2015, 22, 19426–19433. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Zhang, C.; Zhang, C.; Zhu, Y.; He, H.; Wu, M.; Tang, T.; Li, Z.; Zhao, H. Isolation and characterization of pyrene and benzo[a]pyrene-degrading Klebsiella pneumonia PL1 and its potential use in bioremediation. Appl. Microbiol. Biotechnol. 2014, 98, 3819–3828. [Google Scholar] [CrossRef]
- Haneef, T.; Ul Mustafa, M.R.; Yusof, K.W.; Isa, M.H.; Bashir, M.J.K.; Ahmad, M.; Zafar, M. Removal of polycyclic aromatic hydrocarbons (PAHs) from produced water by Ferrate (VI) oxidation. Water 2020, 12, 3132. [Google Scholar] [CrossRef]
- Aranda, E.; Godoy, P.; Reina, R.; Badia-Fabregat, M.; Rosell, M.; Marco-Urrea, E.; García-Romera, I. Isolation of Ascomycota fungi with capability to transform PAHs: Insights into the biodegradation mechanisms of Penicillium oxalicum. Int. Biodeterior. Biodegrad. 2017, 122, 141–150. [Google Scholar] [CrossRef]
- Moghadam, S.; Ebrahimipour, M.; Abtahi, G.; Ghassempour, B.; Hashtroudi, A.; Seyed, M. Biodegradation of polycyclic aromatic hydrocarbons by a bacterial consortium enriched from mangrove sediments. J. Environ. Health Sci. Eng. 2014, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-M.; Chen, C.-W.; Huang, C.-P.; Tsai, M.-L.; Dong, C.-D. Metal-free carbocatalysts derived from macroalga biomass (Ulva lactuca) for the activation of peroxymonosulfate toward the remediation of polycyclic aromatic hydrocarbons laden marine sediments and its impacts on microbial community. Environ. Res. 2022, 208, 112782. [Google Scholar] [CrossRef] [PubMed]
- Maharaj Kumari, K.; Lakhani, A. PAHs in gas and particulate phases: Measurement and control. In Environmental Contaminants: Measurement, Modelling and Control; Gupta, T., Agarwal, A.K., Agarwal, R.A., Labhsetwar, N.K., Eds.; Springer: Singapore, 2018; pp. 43–75. [Google Scholar] [CrossRef]
- Kumar, S.; Upadhayay, S.K.; Kumari, B.; Tiwari, S.; Singh, S.N.; Singh, P.K. In vitro degradation of fluoranthene by bacteria isolated from petroleum sludge. Bioresour. Technol. 2011, 102, 3709–3715. [Google Scholar] [CrossRef]
- Bhushan, K.; Chouhan, S.; Tandey, R.; Mandal, V. Central effect of polycyclic aromatic hydrocarbons liberated from thermal power units on the metabolic and oxidative status of Raphanus sativus. JSM Environ. Sci. Ecol. 2020, 8, 1071. [Google Scholar]
- Tomar, R.S.; Jajoo, A. PSI becomes more tolerant to fluoranthene through the initiation of cyclic electron flow. Funct. Plant Biol. 2017, 44, 978–984. [Google Scholar] [CrossRef]
- Šepič, E.; Bricelj, M.; Leskovšek, H. Degradation of fluoranthene by Pasteurella sp. IFA and Mycobacterium sp. PYR-1: Isolation and identification of metabolites. J. Appl. Microbiol. 1998, 85, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Spehar, R.L.; Poucher, S.; Brooke, L.T.; Hansen, D.J.; Champlin, D.; Cox, D.A. Comparative toxicity of fluoranthene to freshwater and saltwater species under fluorescent and ultraviolet light. Arch. Environ. Contam. Toxicol. 1999, 37, 496–502. [Google Scholar] [CrossRef]
- Saunders, C.R.; Shockley, D.C.; Knuckles, M.E. Fluoranthene-induced neurobehavioral toxicity in F-344 rats. Int. J. Toxicol. 2003, 22, 263–276. [Google Scholar] [CrossRef]
- Mallah, M.A.; Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Liu, F.; Zhang, Q.; Ali Mallah, M.; Ali Mallah, M.; Liu, Y.; Xi, H.; Wang, W.; Feng, F. Relationship between polycyclic aromatic hydrocarbons and cardiovascular diseases: A systematic review. Front. Public Health 2021, 9, 763706. [Google Scholar] [CrossRef]
- Kubincová, P.; Sychrová, E.; Raška, J.; Basu, A.; Yawer, A.; Dydowiczová, A.; Babica, P.; Sovadinová, I. Polycyclic aromatic hydrocarbons and endocrine disruption: Role of testicular gap junctional intercellular communication and connexins. Toxicol. Sci. 2019, 169, 70–83. [Google Scholar] [CrossRef]
- Nur-Aainaa-Syafini, M.R.; Tay, K.S.; Abu Bakar, N.K.; Uche Emenike, C.; Krishnan, S.; Shahul Hamid, F.; Abas, M.R. Degradation of polycyclic aromatic hydrocarbons (pyrene and fluoranthene) by bacterial consortium isolated from contaminated road side soil and soil termite fungal comb. Environ. Earth Sci. 2015, 74, 5383–5391. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V. Bioremediation of polycyclic aromatic hydrocarbon (PAH) compounds: (acenaphthene and fluorene) in water using indigenous bacterial species isolated from the Diep and Plankenburg rivers, Western Cape, South Africa. Braz. J. Microbiol. 2017, 48, 314–325. [Google Scholar] [CrossRef]
- Jain, P.K.; Gupta, V.K.; Gaur, R.K.; Lowry, M.; Jaroli, D.P.; Chauhan, U.K. Bioremediation of petroleum oil contaminated soil and water. Res. J. Environ. Toxicol. 2011, 5, 1–26. [Google Scholar] [CrossRef]
- Ipeaiyeda, A.R.; Nwauzor, G.O.; Akporido, S.O. Biodegradation of polycyclic aromatic hydrocarbons in agricultural soil contaminated with crude oil from Nigeria refinery using Pleurotus sajor-caju. J. Bioremediat. Biodegrad. 2015, 6, 1000301. [Google Scholar] [CrossRef]
- Nasrawi, H.A. Biodegradation of crude oil by fungi isolated from Gulf of Mexico. J. Bioremediat. Biodegrad. 2012, 3, 1000147. [Google Scholar] [CrossRef]
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H.J. Diverse metabolic capacities of fungi for bioremediation. Indian J. Microbiol. 2016, 56, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-S.; Yin, H.; Qiang, J.; Peng, H.; Qin, H.-M. Biodegradation of anthracene by Aspergillus fumigatus. J. Hazard. Mater. 2011, 185, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Lahkar, J.; Deka, H. Isolation of polycyclic aromatic hydrocarbons (PAHs) degrading fungal candidate from oil-contaminated soil and degradation potentiality study on anthracene. Polycycl. Aromat. Comp. 2017, 37, 141–147. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, S.B.; Sharma, R.; Chaudhary, P.; Pandey, A.K.; Ansari, R.; Vasudevan, V.; Arora, A.; Singh, S.; Saha, S.; et al. Enhanced biodegradation of PAHs by microbial consortium with different amendment and their fate in in-situ condition. J. Environ. Manag. 2016, 181, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Teng, Y.; Luo, Y.; Christie, P.; Ma, W.; Liu, F.; Wu, Y.; Luo, Y.; Li, Z. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by Trichoderma reesei FS10-C and effect of bioaugmentation on an aged PAH-contaminated soil. Bioremediat. J. 2015, 19, 9–17. [Google Scholar] [CrossRef]
- Agrawal, N.; Shahi, S.K. Degradation of polycyclic aromatic hydrocarbon (pyrene) using novel fungal strain Coriolopsis byrsina strain APC5. Int. Biodeterior. Biodegrad. 2017, 122, 69–81. [Google Scholar] [CrossRef]
- Sack, U.; Hofrichter, M.; Fritsche, W. Degradation of polycyclic aromatic hydrocarbons by Manganese peroxidase of Nematoloma frowardii. FEMS Microbiol. Lett. 1997, 152, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Teh, Z.C.; Hadibarata, T. Enhanced degradation of pyrene and metabolite identification by Pleurotus eryngii F032. Water Air Soil Pollut. 2014, 225, 1–8. [Google Scholar] [CrossRef]
- Hadibarata, T.; Kristanti, R.A.; Hamdzah, M. Biosorption and biotransformation of fluoranthene by the white-rot fungus Pleurotus eryngii F032. Biotechnol. Appl. Biochem. 2014, 61, 126–133. [Google Scholar] [CrossRef]
- Lin, Z.; Du, J.; Yin, K.; Wang, L.; Yu, H. Mechanism of concentration addition toxicity: They are different for nonpolar narcotic chemicals, polar narcotic chemicals and reactive chemicals. Chemosphere 2004, 54, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Hesham, A.E.-L.; Mohamed, E.A.; Mawad, A.M.M.; Elfarash, A.; Abd El-Fattah, B.S.; El-Rawy, M.A. Molecular characterization of Fusarium Solani degrades a mixture of low and high molecular weight polycyclic aromatic hydrocarbons. Open Biotechnol. J. 2017, 11, 27–35. [Google Scholar] [CrossRef]
- Govarthanan, M.; Fuzisawa, S. Biodegradation of aliphatic and aromatic hydrocarbons using the filamentous fungus Penicillium sp. CHY-2 and characterization of its manganese peroxidase activity. RSC Adv. 2017, 7, 20716–20723. [Google Scholar] [CrossRef]
- Zafra, G.; Absalon, A.E.; Cuevas, M.D.C.; Cortes-Espinosa, D.V. Isolation and selection of a highly tolerant microbial consortium with potential for PAH biodegradation from heavy crude oil-contaminated soils. Water Air Soil Pollut. 2014, 225, 1–18. [Google Scholar] [CrossRef]
- Asemoloye, M.D.; Ahmad, R.; Jonathan, S.G. Transcriptomic responses of catalase, peroxidase and laccase encoding genes and enzymatic activities of oil spill inhabiting rhizospheric fungal strains. Environ. Pollut. 2018, 235, 55–64. [Google Scholar] [CrossRef]
- Álvarez-Barragán, J.; Cravo-Laureau, C.; Wick, L.Y.; Duran, R. Fungi in PAH-contaminated marine sediments: Cultivable diversity and tolerance capacity towards PAH. Mar. Pollut. Bull. 2021, 164, 112082. [Google Scholar] [CrossRef]
- Reyes-César, A.; Absalón, Á.E.; Fernández, F.J.; González, J.M.; Cortés-Espinosa, D.V. Biodegradation of a mixture of PAHs by non-ligninolytic fungal strains isolated from crude oil-contaminated soil. World J. Microbiol. Biotechnol. 2014, 30, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Memić, M.; Vrtačnik, M.; Boh, B.; Pohleven, F.; Mahmutović, O. Biodegradation of PAHs by ligninolytic fungi Hypoxylon Fragiforme and Coniophora Puteana. Polycycl. Aromat. Compd. 2017, 6638, 1–8. [Google Scholar] [CrossRef]
- Bankole, P.O.; Semple, K.T.; Jeon, B.H.; Govindwar, S.P. Enhanced enzymatic removal of anthracene by the mangrove soil-derived fungus, Aspergillus sydowii BPOI. Front. Environ. Sci. Eng. 2020, 14, 113. [Google Scholar] [CrossRef]
- Saraswathy, A.; Hallberg, R. Degradation of pyrene by indigenous fungi from a former gasworks site. FEMS Microbiol. Lett. 2002, 210, 227–232. [Google Scholar] [CrossRef]
- Mandal, S.K.; Das, N. Biodegradation of perylene and benzo [ghi] perylene (5-6 rings) using yeast consortium: Kinetic study, enzyme analysis and degradation pathway. J. Environ. Biol. 2018, 39, 5–15. [Google Scholar] [CrossRef]
- Lee, H.; Jang, Y.; Lee, Y.M.; Lee, H.; Kim, G.H.; Kim, J.J. Enhanced removal of PAHs by Peniophora incarnata and ascertainment of its novel ligninolytic enzyme genes. J. Environ. Manag. 2015, 164, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Asemoloye, M.D.; Ahmad, R.; Jonathan, S.G. Synergistic action of rhizospheric fungi with Megathyrsus maximus root speeds up hydrocarbon degradation kinetics in oil polluted soil. Chemosphere 2017, 187, 1–10. [Google Scholar] [CrossRef]
- Lu, X.Y.; Li, B.; Zhang, T.; Fang, H.H.P. Enhanced anoxic bioremediation of PAHs-contaminated sediment. Bioresour. Technol. 2012, 104, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hadibarata, T.; Kristanti, R.A. Biotransformation studies on fluoranthene, a four-ring polycylic aromatic hydrocarbon, by white-rot fungus Armillaria sp. F022. Agric. Agric. Sci. Procedia 2015, 3, 45–50. [Google Scholar] [CrossRef]
- Jin, J.; Yao, J.; Liu, W.; Zhang, Q.; Liu, J. Fluoranthene degradation and binding mechanism study based on the active-site structure of ring-hydroxylating dioxygenase in Microbacterium paraoxydans JPM1. Environ. Sci. Pollut. Res. 2017, 24, 363–371. [Google Scholar] [CrossRef]
- Bankole, P.O.; Semple, K.T.; Jeon, B.H.; Govindwar, S.P. Biodegradation of fluorene by the newly isolated marine-derived fungus, Mucor irregularis strain bpo1 using response surface methodology. Ecotoxicol. Environ. Saf. 2021, 208, 111619. [Google Scholar] [CrossRef]
- Casellas, M.; Grifoll, M.; Bayona, J.M.; Solanas, A.M. New metabolites in the degradation of fluorene by Arthrobacter sp. strain F101. Appl. Environ. Microbiol. 1997, 63, 819–826. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; de Oliveira, F.C.S.; Passos, T.M.; Quilty, B.; Thiré, R.M.d.S.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Rev. Mater. 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, S.; Pandey, O.P. Study of energy transfer from capping agents to intrinsic vacancies/defects in passivated ZnS nanoparticles. J. Nanopart. Res. 2010, 12, 2655–2666. [Google Scholar] [CrossRef]
- Mecozzi, M.; Sturchio, E. Computer assisted examination of infrared and near infrared spectra to assess structural and molecular changes in biological samples exposed to pollutants: A case of study. J. Imaging 2017, 3, 11. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z.; Skic, K. Use of thermal analysis coupled with differential scanning calorimetry, quadrupole mass spectrometry and infrared spectroscopy (TG-DSC-QMS-FTIR) to monitor chemical properties and thermal stability of fulvic and humic acids. PLoS ONE 2017, 12, e0189653. [Google Scholar] [CrossRef] [PubMed]
- Balogun, A.O.; Lasode, O.A.; Li, H.; McDonald, A.G. Fourier transform infrared (FTIR) study and thermal decomposition kinetics of Sorghum bicolour glume and Albizia pedicellaris residues. Waste Biomass Valorization 2015, 6, 109–116. [Google Scholar] [CrossRef]
- Auta, R.; Adamus, G.; Kwiecien, M.; Radecka, I.; Hooley, P. Production and characterization of bacterial cellulose before and after enzymatic hydrolysis. Afr. J. Biotechnol. 2017, 16, 470–482. [Google Scholar]
- Mostafa, Y.S.; Alrumman, S.A.; Alamri, S.A.; Otaif, K.A.; Mostafa, M.S.; Alfaify, A.M. Bioplastic (poly-3-hydroxybutyrate) production by the marine bacterium Pseudodonghicola xiamenensis through date syrup valorization and structural assessment of the biopolymer. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Teerapatsakul, C.; Pothiratana, C.; Chitradon, L.; Thachepan, S. Biodegradation of polycyclic aromatic hydrocarbons by a thermotolerant white rot fungus Trametes polyzona RYNF13. J. Gen. Appl. Microbiol. 2016, 62, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Zafra, G.; Moreno-montaño, A.; Absalón, Á.E.; Cortés-espinosa, D.V. Degradation of polycyclic aromatic hydrocarbons in soil by a tolerant strain of Trichoderma asperellum. Environ. Sci. Pollut. Res. 2015, 22, 1034–1042. [Google Scholar] [CrossRef]
- Mtibaà, R.; Olicón-Hernández, D.R.; Pozo, C.; Nasri, M.; Mechichi, T.; González, J.; Aranda, E. Degradation of bisphenol A and acute toxicity reduction by different thermo-tolerant ascomycete strains isolated from arid soils. Ecotoxicol. Environ. Saf. 2018, 156, 87–96. [Google Scholar] [CrossRef]
- Kim, Y.C.; Lee, S.R.; Jeon, H.J.; Kim, K.; Kim, M.J.; Choi, S.D.; Lee, S.E. Acute toxicities of fluorene, fluorene-1-carboxylic acid, and fluorene-9-carboxylic acid on zebrafish embryos (Danio rerio): Molecular mechanisms of developmental toxicities of fluorene-1-carboxylic acid. Chemosphere 2020, 260, 127622. [Google Scholar] [CrossRef]
- Kamaya, Y.; Fukaya, Y.; Suzuki, K. Acute toxicity of benzoic acids to the crustacean Daphnia magna. Chemosphere 2005, 59, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Tuvikene, A. Aquatic ecotoxicology-study of freshwater systems stressed by toxic chemical. Ann. Zool. Fenn. 1995, 32, 295–309. [Google Scholar]
- So-Young, L.; Kwon, J.-H. Enhancement of toxic efficacy of alkylated polycyclic aromatic hydrocarbons transformed by Sphingobium quisquiliarum. Int. J. Environ. Res. Public Health 2020, 17, 6416. [Google Scholar] [CrossRef]
- Arunprasath, T.; Sudalai, S.; Meenatchi, R.; Jeyavishnu, K.; Arumugam, A. Biodegradation of triphenylmethane dye malachite green by a newly isolated fungus strain. Biocatal. Agric. Biotechnol. 2019, 17, 672–679. [Google Scholar] [CrossRef]
- Strotmann, U.; Flores, D.P.; Konrad, O.; Gendig, C. Bacterial toxicity testing: Modification and evaluation of the luminescent bacteria test and the respiration inhibition test. Processes 2020, 8, 1349. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Bio. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ortega-González, D.K.; Cristiani-Urbina, E.; Flores-Ortíz, C.M.; Cruz-Maya, J.A.; Cancino-Díaz, J.C.; Jan-Roblero, J. Evaluation of the removal of pyrene and fluoranthene by Ochrobactrum anthropi, fusarium sp. and their coculture. Appl. Biochem. Biotechnol. 2015, 175, 1123–1138. [Google Scholar] [CrossRef]
- Patel, H.; Gupte, A.; Gupte, S. Effect of different culture conditions and inducers on production of laccase by a basidiomycete fungal isolate Pleurotus ostreatus HP-1 under solid state fermentation. BioResour 2009, 4, 268–284. [Google Scholar]
- Rotini, A.; Tornambè, A.; Cossi, R.; Iamunno, F.; Benvenuto, G.; Berducci, M.T.; Maggi, C.; Thaller, M.C.; Cicero, A.M.; Manfra, L.; et al. Salinity-based toxicity of CuO nanoparticles, CuO-bulk and Cu ion to Vibrio anguillarum. Front. Microbiol. 2017, 8, 2076. [Google Scholar] [CrossRef]
- Czech, B.; Jośko, I.; Oleszczuk, P. Ecotoxicological evaluation of selected pharmaceuticals to Vibrio fischeri and Daphnia magna before and after photooxidation process. Ecotoxicol. Environ. Saf. 2014, 104, 247–253. [Google Scholar] [CrossRef]

| Kinetic Model | Parameter | Strains | |
|---|---|---|---|
| TlFLU1 | TpFLU12 | ||
| Zero order | Regression Equation | Cd = −21.24 d + 290.7 | Cd = −22.28 d + 367.7 |
| Cd − C0 = Kd | K (d−1) | 21.24 | 22.28 |
| D1/2 = C0/2K0 | D1/2 | 9.412 | 8.969 |
| R2 | 0.802 | 0.968 | |
| First order | Regression Equation | lnCd = −0.2100 d + 5.924 | lnCd = −0.1503 d + 6.184 |
| lnCd= K1d + lnCd | K (d−1) | 0.210 | 0.1503 |
| D1/2 = ln2/Kd | DT50 | 2.254 | 2.588 |
| R2 | 0.987 | 0.919 | |
| Second order | Regression Equation | 1/Cd = 0.0040 d − 0.008 | 1/Cd = 0.0109 d − 0.03229 |
| 1/Ct = 1/C0 + K2d | K (d−1) | 0.0040 | 0.0109 |
| D1/2 =1/C0K2 | DT50 | 0.625 | 0.229 |
| R2 | 0.849 | 0.749 | |
| Functional Group | Frequency (cm−1) | Assignment | ||
|---|---|---|---|---|
| Control | TlFLU1 | TpFLU12 | ||
| i | 3202–3340 | O–H stretch of the alcohol rings | ||
| ii | 1610–1730 | C=C and C=O asymmetric vibration stretching of the aromatic, carbonyl and quinone compounds | ||
| iii | ND | ND | 1421 | Plane bending of C–O–H and aromatic ring vibrations |
| iv | ND | ND | 1385 | Aliphatic bending of CH and CH2 |
| v | ND | 1030 | ND | C–C, C–OH and O–CH3 stretching |
| vi | 600–610 | –C≡C–H: C–H bend in alkynes | ||
| Acute Toxicity | Strains | |
|---|---|---|
| Profile | TlFLU1 | TpFLU12 |
| EC50 (mg/L) | 14.25 b | 197.1 a |
| TU (%) | 7.018 a | 0.05 b |
| Class | Harmful | non-toxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egbewale, S.O.; Kumar, A.; Mokoena, M.P.; Olaniran, A.O. Metabolic Biodegradation Pathway of Fluoranthene by Indigenous Trichoderma lixii and Talaromyces pinophilus spp. Catalysts 2023, 13, 791. https://doi.org/10.3390/catal13050791
Egbewale SO, Kumar A, Mokoena MP, Olaniran AO. Metabolic Biodegradation Pathway of Fluoranthene by Indigenous Trichoderma lixii and Talaromyces pinophilus spp. Catalysts. 2023; 13(5):791. https://doi.org/10.3390/catal13050791
Chicago/Turabian StyleEgbewale, Samson O., Ajit Kumar, Mduduzi P. Mokoena, and Ademola O. Olaniran. 2023. "Metabolic Biodegradation Pathway of Fluoranthene by Indigenous Trichoderma lixii and Talaromyces pinophilus spp." Catalysts 13, no. 5: 791. https://doi.org/10.3390/catal13050791
APA StyleEgbewale, S. O., Kumar, A., Mokoena, M. P., & Olaniran, A. O. (2023). Metabolic Biodegradation Pathway of Fluoranthene by Indigenous Trichoderma lixii and Talaromyces pinophilus spp. Catalysts, 13(5), 791. https://doi.org/10.3390/catal13050791