Production of Human Milk Fat Substitutes by Lipase-Catalyzed Acidolysis: Immobilization, Synthesis, Molecular Docking and Optimization Studies
Abstract
:1. Introduction
2. Results and Discussion
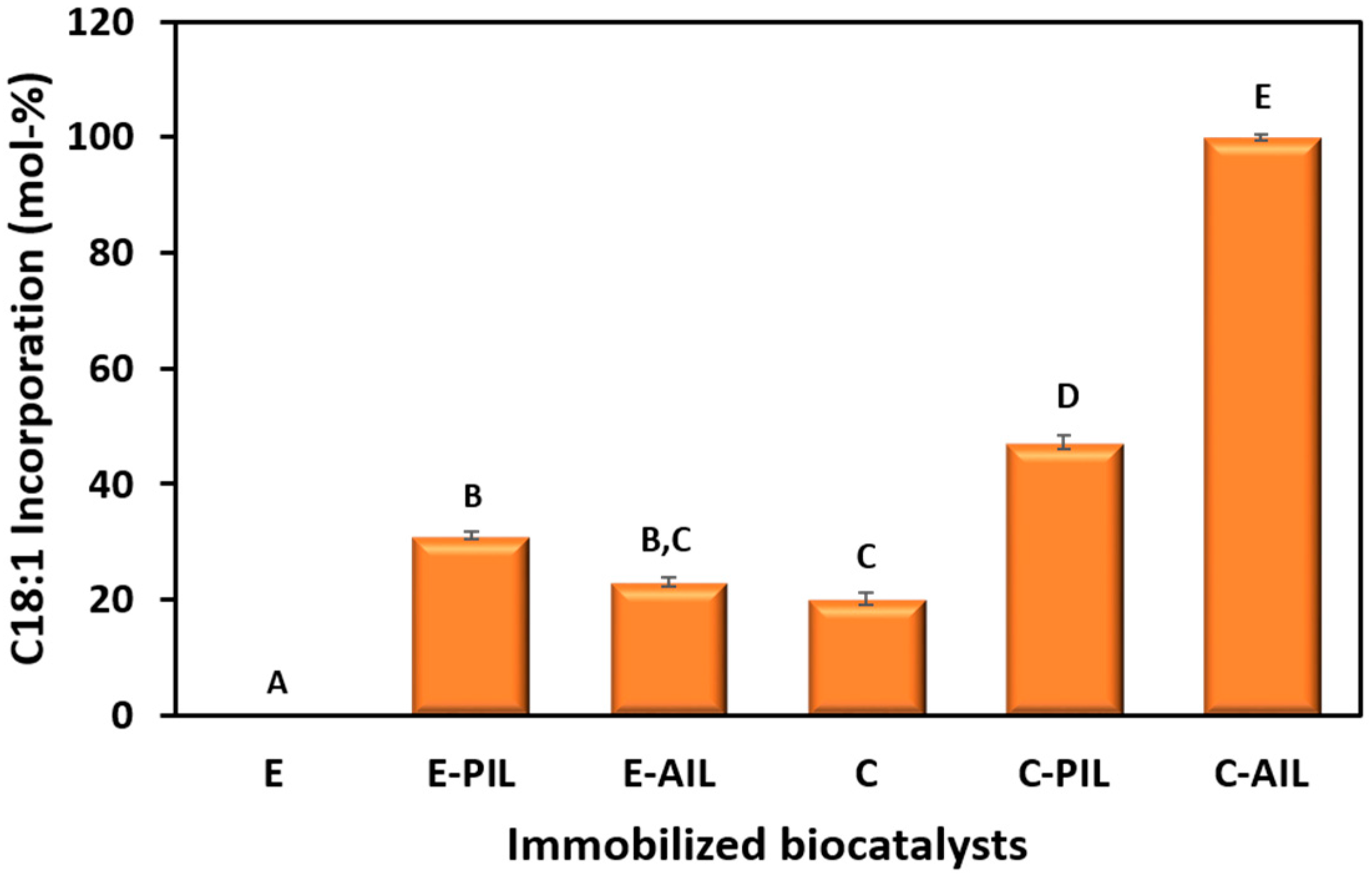
2.1. Selection of Immobilization Strategies
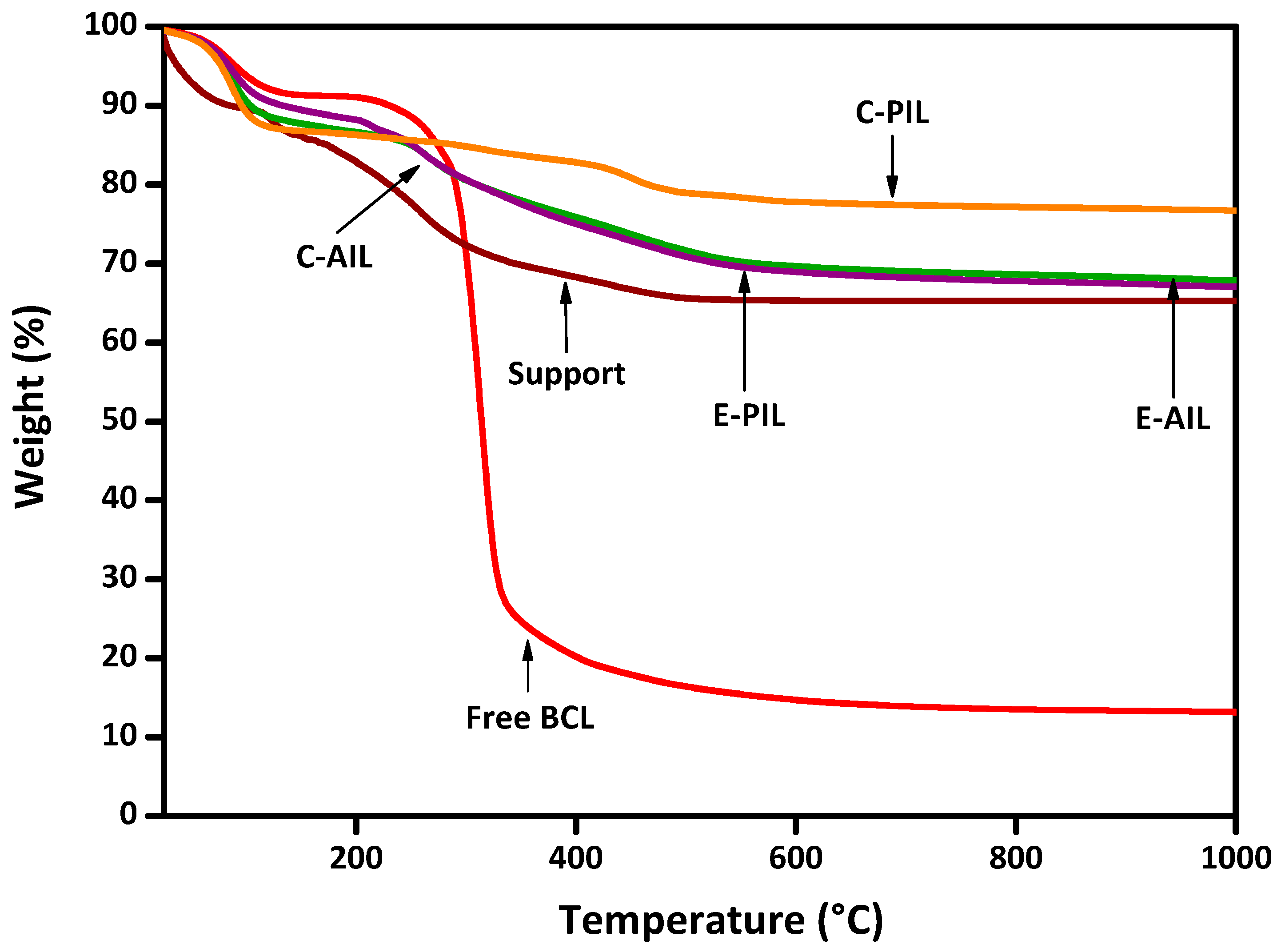
2.2. Physicochemical and Morphological Characterization of the Immobilized Biocatalysts
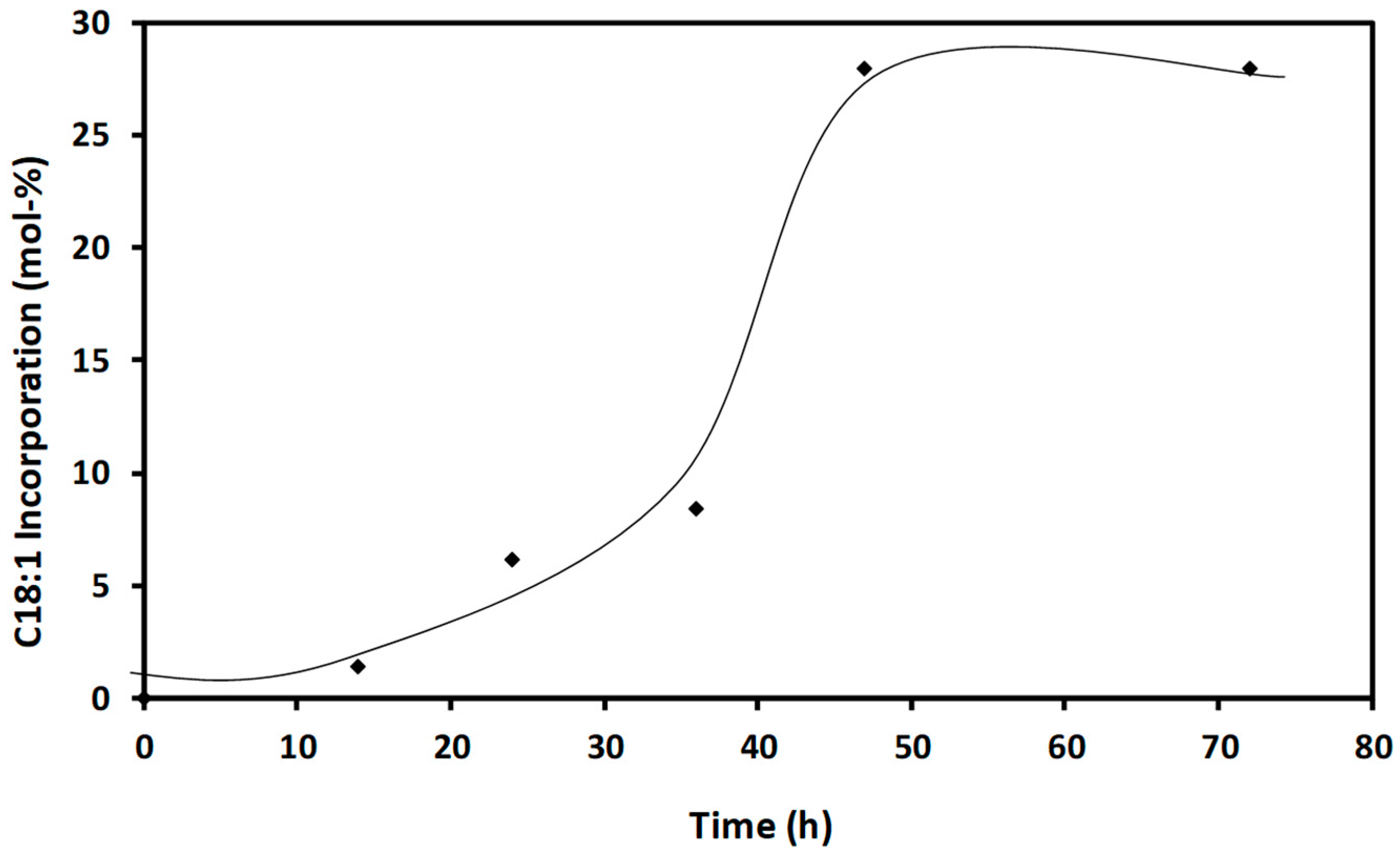
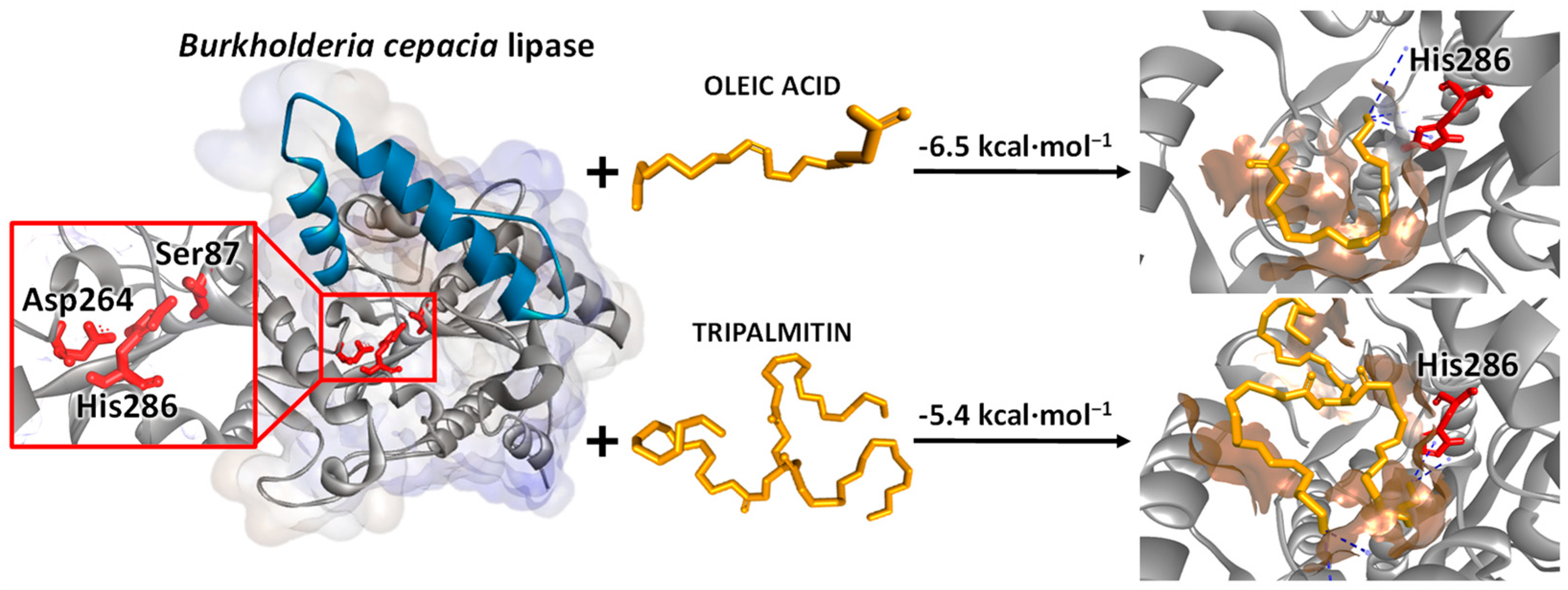
2.3. Time-Course of Acidolysis and Molecular Docking Study
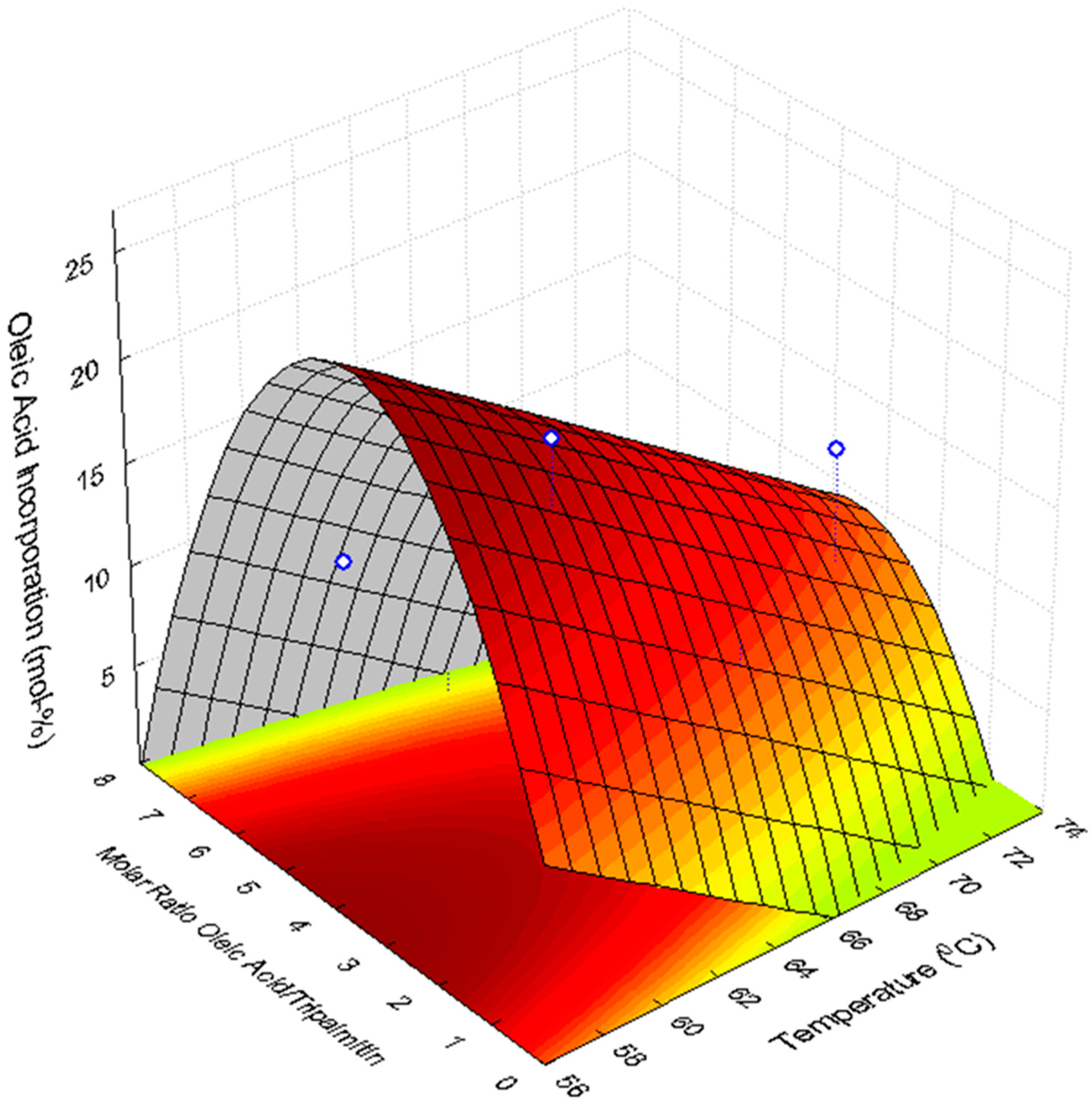
2.4. Optimization of the Enzymatic Acidolysis
3. Materials and Methods
3.1. Materials
3.2. BCL Immobilization by Covalent Binding and Encapsulation
3.3. Morphological and Physicochemical Properties
3.4. Synthesis of HMFS by Enzymatic Acidolysis
3.5. Experimental Design Experiments
3.6. Evaluation of Incorporation Degree
3.7. Molecular Docking Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, W.; Yongfang, F.; Zhang, X.; Cao, X.; Feng, F. Synthesis of Structured Lipid 1,3-Dioleoyl-2-Palmitoylglycerol in Both Solvent and Solvent-Free System. LWT-Food Sci. Technol. 2015, 12, 1187–1194. [Google Scholar] [CrossRef]
- Ferreira-Dias, S.; Osório, N.M.; Rodrigues, J.; Tecelão, C. Structured Lipids for Foods. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 357–369. ISBN 2050011504. [Google Scholar]
- Ferreira-Dias, S.; Osório, N.; Tecelão, C. Bioprocess Technologies for Production of Structured Lipids as Nutraceuticals. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 209–237. ISBN 9780128235065. [Google Scholar]
- Ferreira-Dias, S.; Tecelão, C. Human Milk Fat Substitutes: Advances and Constraints of Enzyme-Catalyzed Production. Lipid Technol. 2014, 26, 183–186. [Google Scholar] [CrossRef]
- Şahin-Yeşilçubuk, N.; Akoh, C.C. Biotechnological and Novel Approaches for Designing Structured Lipids Intended for Infant Nutrition. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 1005–1034. [Google Scholar] [CrossRef]
- Wei, W.; Jin, Q.; Wang, X. Human Milk Fat Substitutes: Past Achievements and Current Trends. Prog. Lipid Res. 2019, 74, 69–86. [Google Scholar] [CrossRef]
- Annapure, U.S.; Jadhav, H.B. A Brief Overview on the Role of Human Milk Fat Substitutes in the Growth of Infants. 2022. Available online: https://www.foodinfotech.com/role-of-human-milk-fat-substitutes-in-the-growth-of-infants-an-overview/ (accessed on 23 March 2023).
- Jiang, X.; Zou, X.; Chao, Z.; Xu, X. Preparation of Human Milk Fat Substitutes: A Review. Life 2022, 12, 187. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, L.; Liu, D.; Du, W. Progress and Perspectives of Enzymatic Preparation of Human Milk Fat Substitutes. Biotechnol. Biofuels Bioprod. 2022, 15, 118. [Google Scholar] [CrossRef]
- Mu, H. Production and Nutritional Aspects of Human Milk Fat Substitutes. Lipid Technol. 2010, 22, 126–129. [Google Scholar] [CrossRef]
- Ghide, M.K.; Yan, Y. 1,3-Dioleoyl-2-Palmitoyl Glycerol (OPO)—Enzymatic Synthesis and Use as an Important Supplement in Infant Formulas. J. Food Biochem. 2021, 45, e13799. [Google Scholar] [CrossRef]
- Robles, A.; Jiménez, M.J.; Esteban, L.; González, P.A.; Martín, L.; Rodríguez, A.; Molina, E. Enzymatic Production of Human Milk Fat Substitutes Containing Palmitic and Docosahexaenoic Acids at Sn-2 Position and Oleic Acid at Sn-1,3 Positions. LWT-Food Sci. Technol. 2011, 44, 1986–1992. [Google Scholar] [CrossRef]
- Tecelão, C.; Silva, J.; Dubreucq, E.; Ribeiro, M.H.; Ferreira-Dias, S. Production of Human Milk Fat Substitutes Enriched in Omega-3 Polyunsaturated Fatty Acids Using Immobilized Commercial Lipases and Candida parapsilosis Lipase/Acyltransferase. J. Mol. Catal. B Enzym. 2010, 65, 122–127. [Google Scholar] [CrossRef]
- Tecelão, C.; Rivera, I.; Sandoval, G.; Ferreira-Dias, S. Carica papaya Latex: A Low-Cost Biocatalyst for Human Milk Fat Substitutes Production. Eur. J. Lipid Sci. Technol. 2012, 114, 266–276. [Google Scholar] [CrossRef]
- Simões, T.; Valero, F.; Tecelão, C.; Ferreira-Dias, S. Production of Human Milk Fat Substitutes Catalyzed by a Heterologous Rhizopus oryzae Lipase and Commercial Lipases. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 411–419. [Google Scholar] [CrossRef]
- Tecelão, C.; Perrier, V.; Dubreucq, E.; Ferreira-Dias, S. Production of Human Milk Fat Substitutes by Interesterification of Tripalmitin with Ethyl Oleate Catalyzed by Candida parapsilosis Lipase/Acyltransferase. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 777–787. [Google Scholar] [CrossRef]
- Available online: https://europe.bungeloders.com/en/brand/betapol-3 (accessed on 23 March 2023).
- Available online: https://advancedlipids.com/products/ (accessed on 23 March 2023).
- Available online: https://www.wilmar-international.com/specialty-fats/products/nutritional-lipids (accessed on 23 March 2023).
- Sheldon, R.A.; van Pelt, S. Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Barbosa, M.; Santos, A.; Carvalho, N.B.; Figueiredo, R.; Pereira, M.M.; Lima, Á.S.; Freire, M.G.; Cabrera-Padilla, R.; Soares, C.M.F. Enhanced Activity of Immobilized Lipase by Phosphonium-Based Ionic Liquids Used in the Supports Preparation and Immobilization Process. ACS Sustain. Chem. Eng. 2019, 7, 15648–15659. [Google Scholar] [CrossRef]
- da Silva, V.G.; de Castro, R.J.S. Biocatalytic Action of Proteases in Ionic Liquids: Improvements on Their Enzymatic Activity, Thermal Stability and Kinetic Parameters. Int. J. Biol. Macromol. 2018, 114, 124–129. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Freire, C.C.C.; Souza, R.L.; Cabrera-, R.Y. Effects of Phosphonium-Based Ionic Liquids on the Lipase Activity Evaluated by Experimental Results and Molecular Docking. Biotechnol. Prog. 2019, 35, e2816. [Google Scholar] [CrossRef] [PubMed]
- Cull, S.G.; Holbrey, J.D.; Vargas-Mora, V.; Seddon, K.R.; Lye, G.J. Room-Temperature Ionic Liquids as Replacements for Organic Solvents in Multiphase Bioprocess Operations. Biotechnol. Bioeng. 2000, 69, 227–233. [Google Scholar] [CrossRef]
- Rebelo, L.P.N.; Lopes, N.C.; Esperanc, M.S.S. On the Critical Temperature, Normal Boiling Point, and Vapor Pressure of Ionic Liquids. J. Phys. Chem. B 2005, 109, 6040–6043. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Toledo Hijo, A.A.C.; Maximo, G.J.; Costa, M.C.; Batista, E.A.C.; Meirelles, A.J.A. Applications of Ionic Liquids in the Food and Bioproducts Industries. ACS Sustain. Chem. Eng. 2016, 4, 5347–5369. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K.R.; Seddon, K. Assessing the Greenness of Some Typical Laboratory Ionic Liquid Preparations. Green Chem. 2010, 12, 17–30. [Google Scholar] [CrossRef]
- Souza, R.L.; Faria, E.L.P.; Figueiredo, R.T.; Freitas, L.S.; Iglesias, M.; Mattedi, S.; Zanin, G.M.; Dos Santos, O.A.A.; Coutinho, J.A.P.; Lima, A.S.; et al. Protic Ionic Liquid as Additive on Lipase Immobilization Using Silica Sol-Gel. Enzyme Microb. Technol. 2013, 52, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Haaland, P.D. Experimental Design in Biotechnology, Statistics: Textbooks and Monographs; CRC Press: Boca Raton, FL, USA, 1989; Volume 1, ISBN 0824778812. [Google Scholar]
- Faustino, A.R.; Osório, N.M.; Tecelão, C.; Canet, A.; Valero, F.; Ferreira-Dias, S. Camelina Oil as a Source of Polyunsaturated Fatty Acids for the Production of Human Milk Fat Substitutes Catalyzed by a Heterologous Rhizopus oryzae Lipase. Eur. J. Lipid Sci. Technol. 2016, 118, 532–544. [Google Scholar] [CrossRef]
- Carvalho, N.B.; Vidal, B.T.; Barbosa, A.S.; Pereira, M.M.; Mattedi, S.; Freitas, L.D.S.; Lima, Á.S.; Soares, C.M.F. Lipase Immobilization on Silica Xerogel Treated with Protic Ionic Liquid and Its Application in Biodiesel Production from Different Oils. Int. J. Mol. Sci. 2018, 19, 1829. [Google Scholar] [CrossRef]
- Portaccio, M.; Della Ventura, B.; Mita, D.G.; Manolova, N.; Stoilova, O.; Rashkov, I.; Lepore, M. FT-IR Microscopy Characterization of Sol-Gel Layers Prior and after Glucose Oxidase Immobilization for Biosensing Applications. J. Sol-Gel Sci. Technol. 2011, 57, 204–211. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, S.; Jiang, L.; Zou, B.; Yang, J.; Huang, H. Immobilization of Burkholderia cepacia Lipase on Functionalized Ionic Liquids Modified Mesoporous Silica SBA-15. Process Biochem. 2012, 47, 2291–2299. [Google Scholar] [CrossRef]
- You, P.; Su, E.; Yang, X.; Mao, D.; Wei, D. Carica papaya Lipase-Catalyzed Synthesis of Terpene Esters. J. Mol. Catal. B Enzym. 2011, 71, 152–158. [Google Scholar] [CrossRef]
- Mukherjee, I.; Mylonakis, A.; Guo, Y.; Samuel, S.P.; Li, S.; Wei, R.Y.; Kojtari, A.; Wei, Y. Effect of Nonsurfactant Template Content on the Particle Size and Surface Area of Monodisperse Mesoporous Silica Nanospheres. Microporous Mesoporous Mater. 2009, 122, 168–174. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, J.; Dong, H.; Dong, J.H.; Qiu, K.; Jansen-Varnum, S.A. Preparation and Physisorption Characterization of D-Glucose-Templated Mesoporous Silica Sol-Gel Materials. Chem. Mater. 1999, 11, 2023–2029. [Google Scholar] [CrossRef]
- Soares, C.M.F.; Dos Santos, O.A.; De Castro, H.F.; De Moraes, F.F.; Zanin, G.M. Studies on Lipase Immobilization in Hydrophobic Sol-Gel Matrix. Appl. Biochem. Biotechnol. 2004, 113, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Song, C.; Xu, X.; Xia, J.; Huo, S.; Cui, F. Enhancing Stabilities of Lipase by Enzyme Aggregate Coating Immobilized onto Ionic Liquid Modified Mesoporous Materials. Appl. Surf. Sci. 2014, 311, 62–67. [Google Scholar] [CrossRef]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The Lid Domain in Lipases: Structural and Functional Determinant of Enzymatic Properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.A.; Tonetto, G.M.; Ferreira, M.L. Burkholderia cepacia Lipase: A Versatile Catalyst in Synthesis Reactions. Biotechnol. Bioeng. 2018, 115, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Barbe, S.; Lafaquière, V.; Guieysse, D.; Monsan, P.; Remaud-Siméon, M.; André, I. Insights into Lid Movements of Burkholderia cepacia Lipase Inferred from Molecular Dynamics Simulations. Proteins Struct. Funct. Bioinform. 2009, 77, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.L.; Oliveira, J.M.; Nascimento, J.S.; Mattedi, S.; Krause, L.C.; Freitas, L.S.; Cavalcanti, E.B.; Pereira, M.M.; Lima, A.S.; Soares, C.M.F. Continuous Flow Reactor Based with an Immobilised Biocatalyst for the Continuous Enzymatic Transesterification of Crude Coconut Oil. Biotechnol. Appl. Biochem. 2020, 55, 404–413. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Freire, C.C.C.; Almeida, L.C.; Freitas, L.S.; Souza, R.L.; Pereira, E.B.; Mendes, A.A.; Pereira, M.M.; Lima, Á.S.; Soares, C.M.F. Optimization of the Enzymatic Hydrolysis of Moringa oleifera Lam Oil Using Molecular Docking Analysis for Fatty Acid Specificity. Biotechnol. Appl. Biochem. 2019, 66, 823–832. [Google Scholar] [CrossRef]
- Brandão, L.M.d.S.; Barbosa, M.S.; Souza, R.L.; Pereira, M.M.; Lima, Á.S.; Soares, C.M.F. Lipase Activation by Molecular Bioimprinting: The Role of Interactions between Fatty Acids and Enzyme Active Site. Biotechnol. Prog. 2021, 37, e3064. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Barbosa, M.S.; Santos, J.C.B.; Lisboa, M.C.; Souza, R.L.; Pereira, M.M.; Lima, Á.S.; Soares, C.M.F. Computational and Experimental Analysis on the Preferential Selectivity of Lipases for Triglycerides in Licuri Oil. Bioprocess Biosyst. Eng. 2021, 44, 2141–2151. [Google Scholar] [CrossRef]
- Gao, W.W.; Zhang, F.X.; Zhang, G.X.; Zhou, C.H. Key Factors Affecting the Activity and Stability of Enzymes in Ionic Liquids and Novel Applications in Biocatalysis. Biochem. Eng. J. 2015, 99, 67–84. [Google Scholar] [CrossRef]
- Zheng, M.; Xiang, X.; Wang, S.; Shi, J.; Deng, Q.; Huang, F.; Cong, R. Lipase Immobilized in Ordered Mesoporous Silica: A Powerful Biocatalyst for Ultrafast Kinetic Resolution of Racemic Secondary Alcohols. Process Biochem. 2017, 53, 102–108. [Google Scholar] [CrossRef]
- Rodrigues, M.I.; Iemma, A.F. Experimental Design and Process Optimization; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]


| Data Set | Experiments | Coded Matrix | Decoded Matrix | Oleic Acid Incorporation (mol.%) | ||
|---|---|---|---|---|---|---|
| T (°C) | MR | T (°C) | MR | |||
| 1 | −1 | −1 | 60 | 2:1 | 23.5 | |
| 2 | −1 | 1 | 60 | 6:1 | 11.0 | |
| Full factorial points | 3 | 1 | −1 | 70 | 2:1 | 17.0 |
| 4 | 1 | 1 | 70 | 6:1 | 6.3 | |
| 5 | −1.41 | 0 | 58 | 4:1 | 27.6 | |
| 6 | 1.41 | 0 | 72 | 4:1 | 8.6 | |
| Star-points | 7 | 0 | −1.41 | 65 | 1.2:1 | 3.7 |
| 8 | 0 | 1.41 | 65 | 6.8:1 | 6.3 | |
| 9 | 0 | 0 | 65 | 4:1 | 16.2 | |
| Center points | 10 | 0 | 0 | 65 | 4:1 | 15.7 |
| 11 | 0 | 0 | 65 | 4:1 | 16.4 | |
| Factor | Effect | p-Value |
|---|---|---|
| T (linear term) | −9.54 | 0.051 |
| T (quadratic term) | 3.62 | 0.458 |
| MR (linear term) | −4.95 | 0.244 |
| MR (quadratic term) | −9.80 | 0.082 |
| T × MR | 0.895 | 0.872 |
| Variation Source | Sum of Squares | Degree of Freedom | Mean Square | Fcalc | p-Value |
|---|---|---|---|---|---|
| Regression | 378.29 | 10 | 37.83 | 5.27 | 0.00005 |
| Residuals | 43.09 | 6 | 7.18 | ||
| Lack of Fit | 560.27 | 1 | 560.27 | ||
| Pure Error | 138.89 | 5 | 27.78 | ||
| Total | 560.27 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, C.M.F.; Barbosa, M.S.; Santos, S.B.; Mattedi, S.; Lima, Á.S.; Pereira, M.M.; Tecelão, C.; Ferreira-Dias, S. Production of Human Milk Fat Substitutes by Lipase-Catalyzed Acidolysis: Immobilization, Synthesis, Molecular Docking and Optimization Studies. Catalysts 2023, 13, 825. https://doi.org/10.3390/catal13050825
Soares CMF, Barbosa MS, Santos SB, Mattedi S, Lima ÁS, Pereira MM, Tecelão C, Ferreira-Dias S. Production of Human Milk Fat Substitutes by Lipase-Catalyzed Acidolysis: Immobilization, Synthesis, Molecular Docking and Optimization Studies. Catalysts. 2023; 13(5):825. https://doi.org/10.3390/catal13050825
Chicago/Turabian StyleSoares, Cleide M. F., Milson S. Barbosa, Samuel B. Santos, Silvana Mattedi, Álvaro S. Lima, Matheus M. Pereira, Carla Tecelão, and Suzana Ferreira-Dias. 2023. "Production of Human Milk Fat Substitutes by Lipase-Catalyzed Acidolysis: Immobilization, Synthesis, Molecular Docking and Optimization Studies" Catalysts 13, no. 5: 825. https://doi.org/10.3390/catal13050825
APA StyleSoares, C. M. F., Barbosa, M. S., Santos, S. B., Mattedi, S., Lima, Á. S., Pereira, M. M., Tecelão, C., & Ferreira-Dias, S. (2023). Production of Human Milk Fat Substitutes by Lipase-Catalyzed Acidolysis: Immobilization, Synthesis, Molecular Docking and Optimization Studies. Catalysts, 13(5), 825. https://doi.org/10.3390/catal13050825







