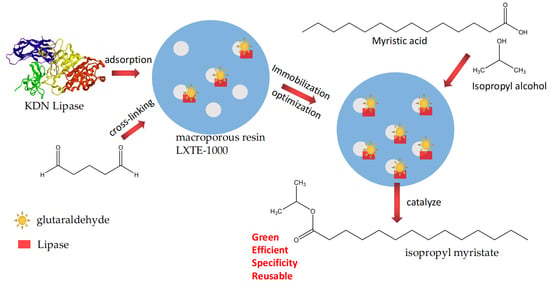
Immobilized KDN Lipase on Macroporous Resin for Isopropyl Myristate Synthesis
Abstract
1. Introduction
2. Results and Discussion
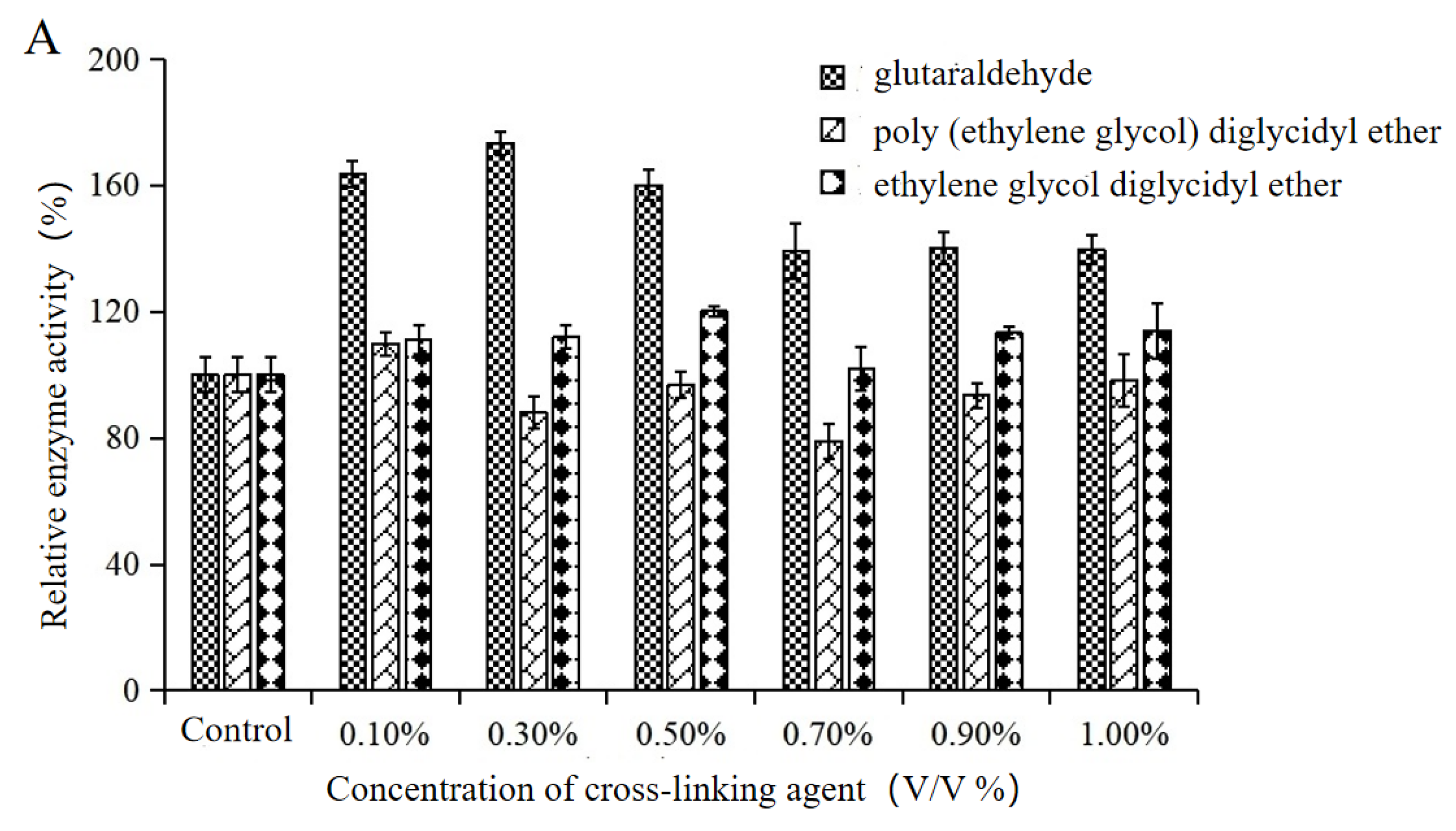
2.1. Optimization of Macroporous Resin Adsorption/Cross-Linking
2.2. Optimization of KDN Lipase Immobilization Using RSM for Immobilization Efficiency
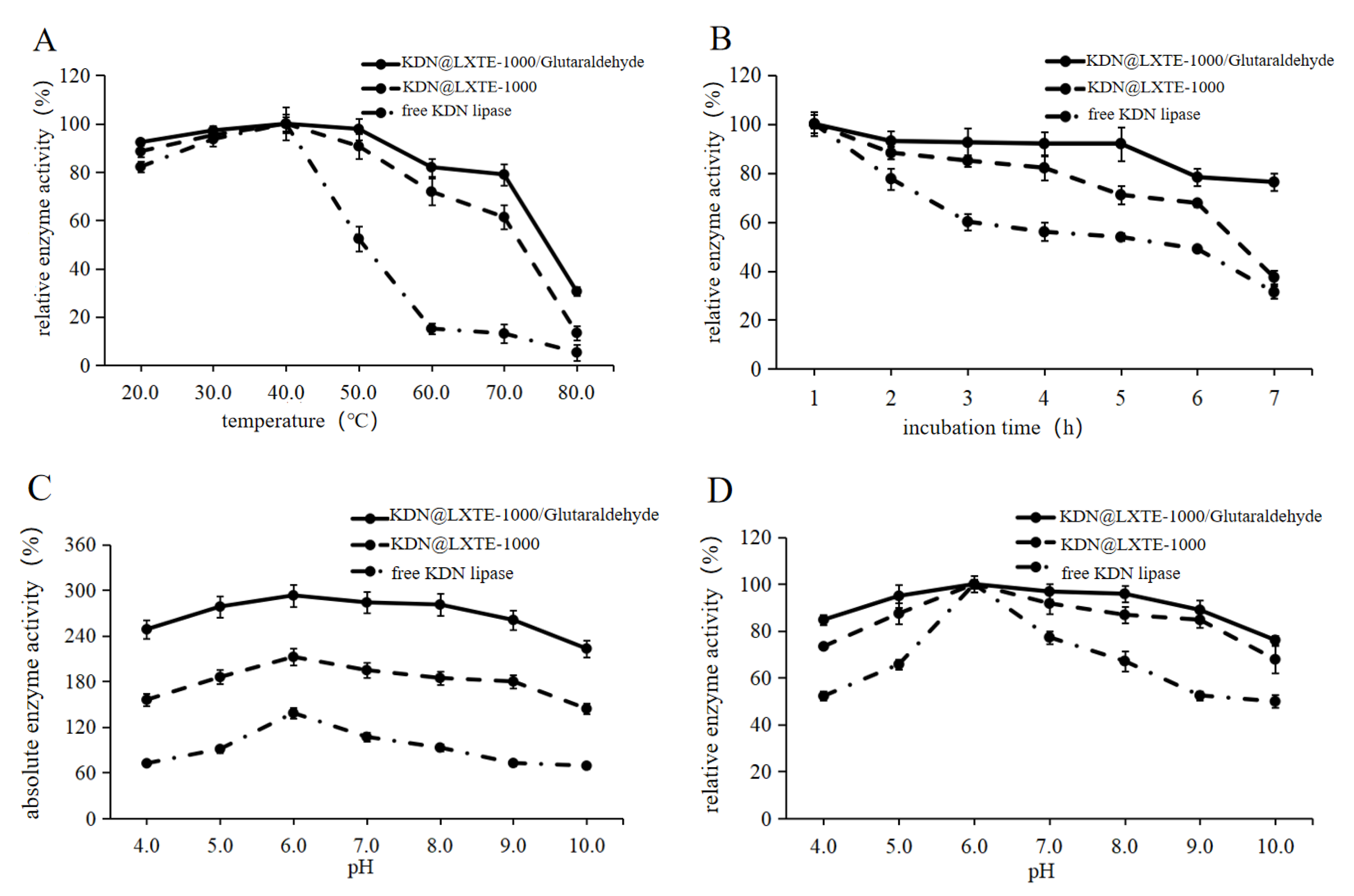
2.3. Biochemical Characterization of KDN@LXTE-1000/Glutaraldehyde and Free KDN Lipase
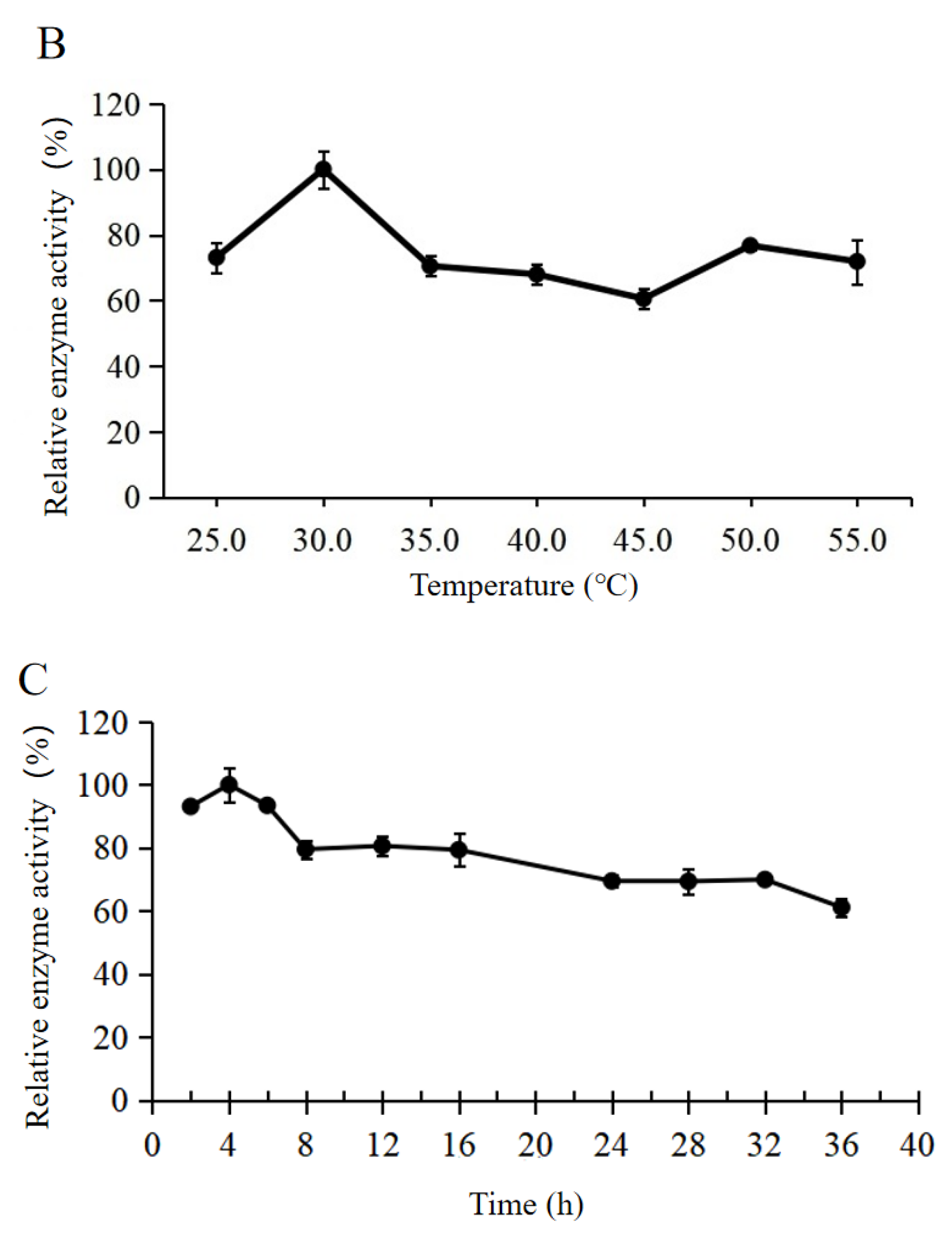
2.3.1. Thermal and pH Stability
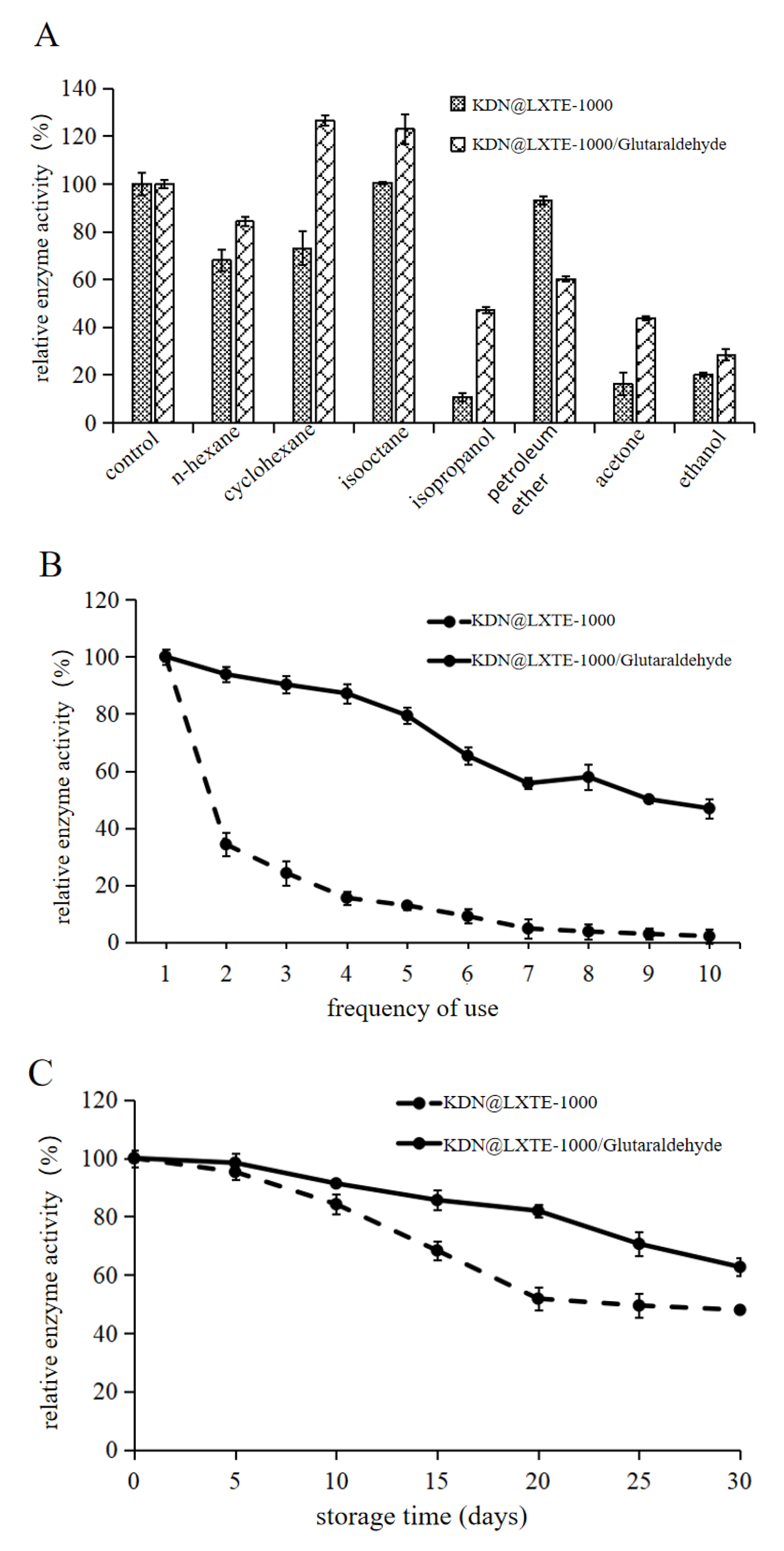
2.3.2. Solvent Tolerance, Reusability, and Storage Time Analysis
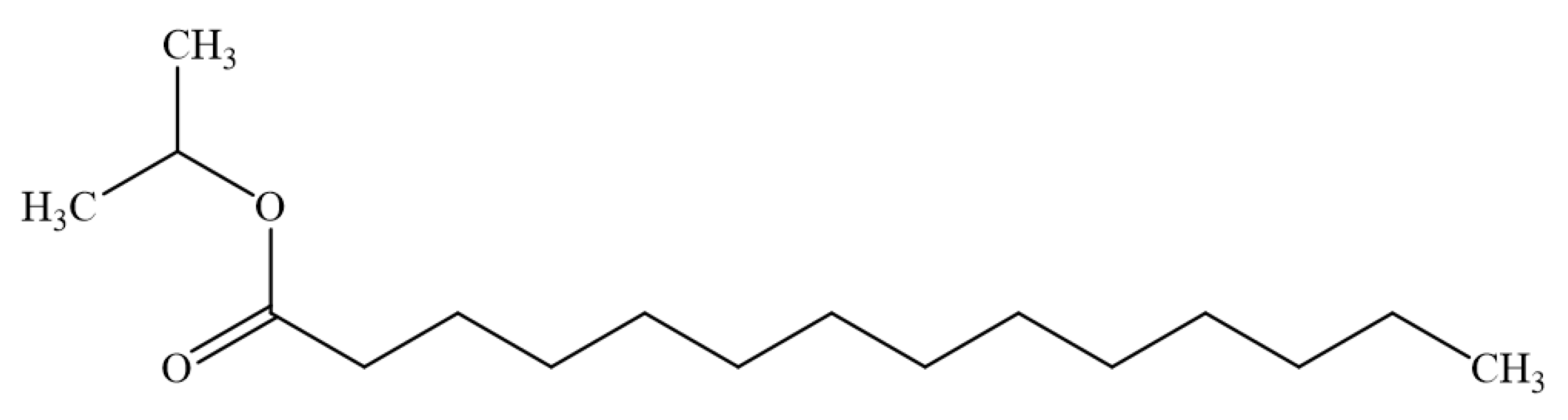
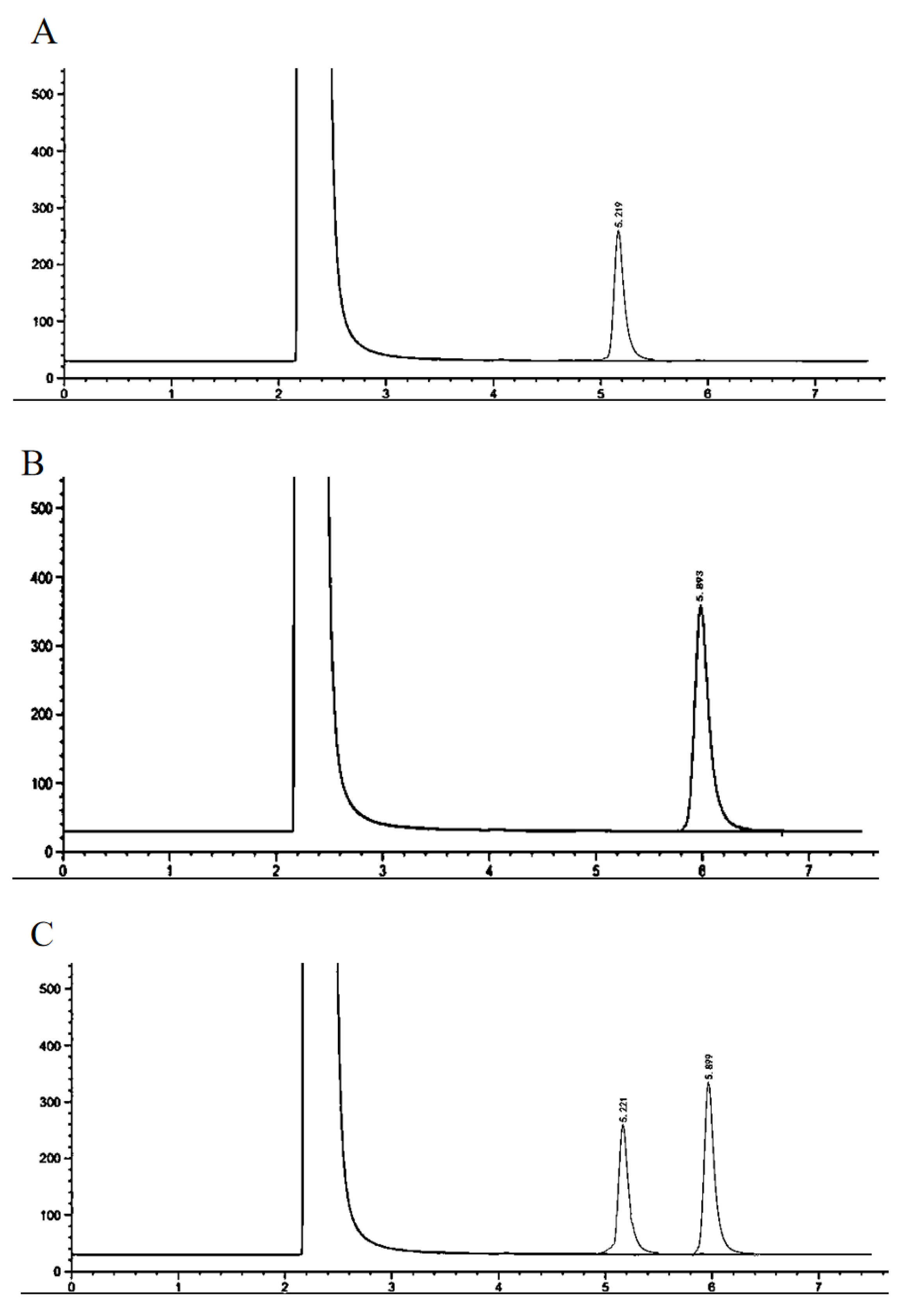
2.4. Determination of Myristic Acid and Isopropyl Myristate by GC
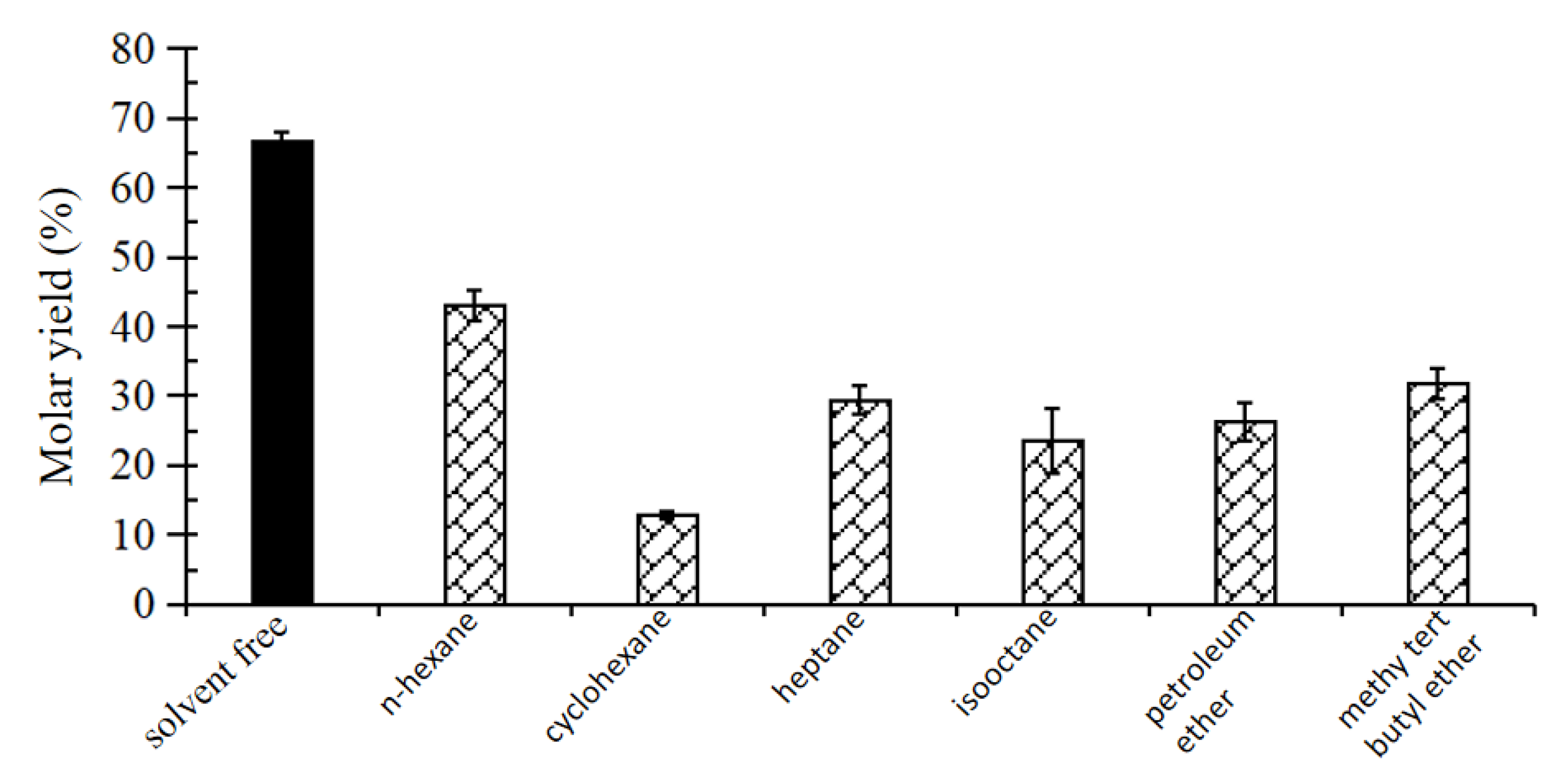
2.5. Establishment of Solvent System
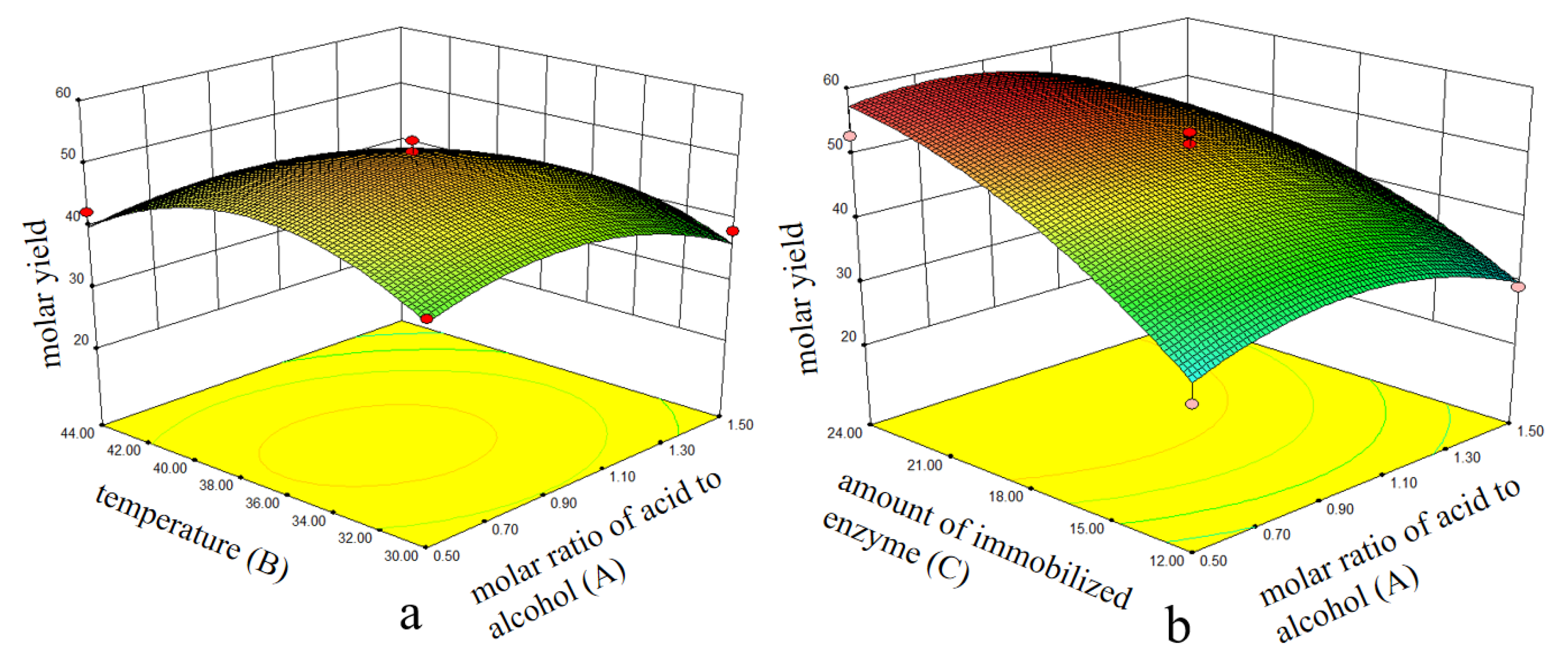
2.6. Optimization of Synthesis of Isopropyl Myristate Using RSM
3. Materials and Methods
3.1. Enzyme and Chemicals
3.2. Two-Step Immobilization of KDN Lipase with LXTE-1000 and Glutaraldehyde
3.3. Determination of Lipase Activity
3.4. Design for Response Surface Methodology (RSM)
3.5. Biochemical Indexes of Free KDN Lipase and Immobilized Lipase
3.6. Analytical Method of Isopropyl Myristate
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siódmiak, T.; Dulęba, J.; Kocot, N.; Wątróbska-Świetlikowska, D.; Marszałł, M.P. The High ‘Lipolytic Jump’ of Immobilized Amano A Lipase from Aspergillus niger in Developed ‘ESS Catalytic Triangles’ Containing Natural Origin Substrates. Catalysts 2022, 12, 853. [Google Scholar] [CrossRef]
- Sandoval, G.; Herrera-López, E.J. Lipase, Phospholipase, and Esterase Biosensors (Review). In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Springer: New York, NY, USA, 2018; pp. 391–425. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Factories 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Guisan, J.M.; López-Gallego, F.; Bolivar, J.M.; Rocha-Martín, J.; Fernandez-Lorente, G. The Science of Enzyme Immobilization. In Immobilization of Enzymes and Cells: Methods and Protocols; Guisan, J.M., Bolivar, J.M., López-Gallego, F., Rocha-Martín, J., Eds.; Springer: New York, NY, USA, 2020; pp. 1–26. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramlee, N.N.; Md Illias, R.A.; Rahman, R.; Toemen, S.; Selvasembian, R.; Ahmad, R.A.A.; Abdul Manas, N.H.; Wan Azelee, N.I. Biochemical and Physical Characterization of Immobilized Candida rugosa Lipase on Metal Oxide Hybrid Support. Catalysts 2022, 12, 854. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Cheng, H.; Yan, Y.; Iqbal, H.M.N. Multi-point enzyme immobilization, surface chemistry, and novel platforms: A paradigm shift in biocatalyst design. Crit. Rev. Biotechnol. 2019, 39, 202–219. [Google Scholar] [CrossRef]
- Sirisha, V.L.; Jain, A.; Jain, A. Chapter Nine—Enzyme Immobilization: An Overview on Methods, Support Material, and Applications of Immobilized Enzymes. In Advances in Food and Nutrition Research; Kim, S.-K., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 79, pp. 179–211. [Google Scholar]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef][Green Version]
- Manoel, E.A.; Pinto, M.; dos Santos, J.C.S.; Tacias-Pascacio, V.G.; Freire, D.M.G.; Pinto, J.C.; Fernandez-Lafuente, R. Design of a core–shell support to improve lipase features by immobilization. RSC Adv. 2016, 6, 62814–62824. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A.M. Enzymatic Catalysis in Organic Media at 100 °C. Science 1984, 224, 1249–1251. [Google Scholar] [CrossRef]
- Kurtovic, I.; Nalder, T.D.; Cleaver, H.; Marshall, S.N. Immobilisation of Candida rugosa lipase on a highly hydrophobic support: A stable immobilised lipase suitable for non-aqueous synthesis. Biotechnol. Rep. 2020, 28, e00535. [Google Scholar] [CrossRef]
- O’Neil, M.J. (Ed.) The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals; Merck and Co., Inc.: Whitehouse Station, NJ, USA, 2006; p. 902. [Google Scholar]
- Vadgama, R.N.; Odaneth, A.A.; Lali, A.M. Green synthesis of isopropyl myristate in novel single phase medium Part II: Packed bed reactor (PBR) studies. Biotechnol. Rep. 2015, 8, 105–109. [Google Scholar] [CrossRef][Green Version]
- Verma, M.; Chauhan, G.; Kanwar, S. Enzymatic synthesis of isopropyl myristate using immobilized lipase from Bacillus cereus MTCC 8372. Acta Microbiol. Immunol. Hung. 2008, 55, 327–342. [Google Scholar] [CrossRef][Green Version]
- Vanti, G.; Grifoni, L.; Bergonzi, M.C.; Antiga, E.; Montefusco, F.; Caproni, M.; Bilia, A.R. Development and optimisation of biopharmaceutical properties of a new microemulgel of cannabidiol for locally-acting dermatological delivery. Int. J. Pharm. 2021, 607, 121036. [Google Scholar] [CrossRef]
- Najjar, A.A.; Hassan, E.A.; Zabermawi, N.M.; Almasaudi, S.B.; Moulay, M.; Harakeh, S.; Abd El-Aal, M. Efficacy of the Immobilized Kocuria flava Lipase on Fe3O4/Cellulose Nanocomposite for Biodiesel Production from Cooking Oil Wastes. Catalysts 2022, 12, 977. [Google Scholar] [CrossRef]
- Pan, Z.; Jin, S.; Zhang, X.; Zheng, S.; Han, S.; Pan, L.; Lin, Y. Biocatalytic behavior of a new Aspergillus niger whole-cell biocatalyst with high operational stability during the synthesis of green biosolvent isopropyl esters. J. Mol. Catal. B Enzym. 2016, 131, 10–17. [Google Scholar] [CrossRef]
- Monteiro, R.R.; Virgen-Ortiz, J.J.; Berenguer-Murcia, A.; da Rocha, T.N.; dos Santos, J.C.; Alcantara, A.R.; Fernandez-Lafuente, R. Biotechnological relevance of the lipase A from Candida antarctica. Catal. Today 2021, 362, 141–154. [Google Scholar] [CrossRef]
- Samira, H.A.; Amalraj, J.; Mohammad, S.S.G. How a crosslinker agent interacts with the β-glucosidase enzyme surface in an aqueous solution: Insight from quantum mechanics calculations and molecular dynamics simulations. Colloids Surf. B Biointerfaces 2021, 203, 111761. [Google Scholar] [CrossRef]
- Modenez, I.A.; Sastre, D.E.; Moraes, F.C.; Marques Netto, C.G. Influence of glutaraldehyde cross-linking modes on the recyclability of immobilized lipase B from Candida antarctica for transesterification of soybean oil. Molecules 2018, 23, 2230. [Google Scholar] [CrossRef][Green Version]
- Danielli, C.; van Langen, L.; Boes, D.; Asaro, F.; Anselmi, S.; Provenza, F.; Renzi, M.; Gardossi, L. 2,5-Furandicarboxaldehyde as a bio-based crosslinking agent replacing glutaraldehyde for covalent enzyme immobilization. RSC. Adv. 2022, 12, 35676–35684. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Syn. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Jun, L.Y.; Mubarak, N.M.; Yon, L.S.; Bing, C.H.; Khalid, M.; Jagadish, P.; Abdullah, E.C. Immobilization of Peroxidase on Functionalized MWCNTs-Buckypaper/Polyvinyl alcohol Nanocomposite Membrane. Sci. Rep. 2019, 9, 2215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, H.; Jin, X.; Long, N.; Zhang, R. Improved biodegradation of synthetic azo dye by horseradish peroxidase cross-linked on nano-composite support. Int. J. Biol. Macromol. 2017, 95, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zheng, B.; Zhang, J.; Huang, X.; Liu, H.; Guo, S.; Zhang, J. Horseradish Peroxidase Immobilized on Graphene Oxide: Physical Properties and Applications in Phenolic Compound Removal. J. Phys. Chem. C 2010, 114, 8469–8473. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Zheng, X.-B.; Zhang, S.-P.; Zheng, Y.-G. Cloning, expression and characterization of a lipase gene from the Candida antarctica ZJB09193 and its application in biosynthesis of vitamin A esters. Microbiol. Res. 2012, 167, 452–460. [Google Scholar] [CrossRef]
- Zisis, T.; Freddolino, P.L.; Turunen, P.; van Teeseling, M.C.F.; Rowan, A.E.; Blank, K.G. Interfacial Activation of Candida antarctica Lipase B: Combined Evidence from Experiment and Simulation. Biochemistry 2015, 54, 5969–5979. [Google Scholar] [CrossRef][Green Version]
- Naef, N.U.; Seeger, S. Immobilization of Candida antarctica Lipase B on Silicone Nanofilaments. J. Nanomater. 2021, 2021, 8812240. [Google Scholar] [CrossRef]


| Model | Sum of Squares | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|
| 11,724.46 | 1302.72 | 6.05 | 0.0135 | ||
| A | 5179.08 | 5179.08 | 24.04 | 0.0017 | very significant |
| B | 160.03 | 160.03 | 0.75 | 0.4173 | |
| C | 8.47 | 8.47 | 0.039 | 0.8485 | |
| AB | 1058.53 | 1058.53 | 4.91 | 0.0622 | |
| AC | 330.88 | 330.88 | 1.54 | 0.2552 | |
| BC | 365.00 | 365.00 | 1.69 | 0.2342 | |
| A2 | 3658.37 | 3658.37 | 16.98 | 0.0045 | |
| B2 | 119.80 | 119.80 | 0.56 | 0.4801 | |
| C2 | 556.50 | 556.50 | 2.58 | 0.1520 | |
| Residual | 1508.07 | 215.44 | |||
| Lack of Fit | 664.26 | 221.42 | 1.05 | 0.3032 | non-significant |
| Pure Error | 843.81 | 210.95 | |||
| Cor Total | 13,232.53 |
| Model | Sum of Squares | Mean Square | F-Value | p-Value | Very Significant |
|---|---|---|---|---|---|
| 2380.09 | 170.01 | 11.66 | <0.0001 | ||
| A | 167.03 | 167.03 | 11.46 | 0.0044 | significant |
| B | 38.84 | 38.84 | 2.66 | 0.1249 | |
| C | 1199.40 | 1199.40 | 82.28 | <0.0001 | very significant |
| D | 210.42 | 210.42 | 14.44 | 0.0020 | significant |
| AB | 6.68 | 6.68 | 0.46 | 0.5094 | |
| AC | 28.89 | 28.89 | 1.98 | 0.1810 | |
| AD | 46.99 | 46.99 | 3.22 | 0.0942 | |
| BC | 10.89 | 10.89 | 0.75 | 0.4020 | |
| BD | 5.15 | 5.15 | 0.35 | 0.5616 | |
| CD | 45.83 | 45.83 | 3.14 | 0.0979 | |
| A2 | 220.43 | 220.43 | 15.12 | 0.0016 | |
| B2 | 468.04 | 468.04 | 32.11 | <0.0001 | |
| C2 | 124.55 | 124.55 | 8.54 | 0.0111 | |
| D2 | 29.00 | 29.00 | 1.99 | 0.1802 | |
| Residual | 204.08 | 14.58 | |||
| Lack of Fit | 179.75 | 17.97 | 2.96 | 0.1539 | non-significant |
| Pure Error | 24.33 | 6.08 | |||
| Cor Total | 2584.17 | 28 |
| Enzyme | Materials | Conversion Rate | Reusability |
|---|---|---|---|
| Lipase from Bacillus cereus MTCC 8372 | A poly (MAc-co-DMA-cl-MBAm) hydrogel | 66% | 38% (3rd cycle) |
| Novozym 435 | A packed bed reactor | 98.5% (Calculated based on acid consumption) | 50 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Xin, Y.; Cai, S.; Xu, W.; Xu, W. Immobilized KDN Lipase on Macroporous Resin for Isopropyl Myristate Synthesis. Catalysts 2023, 13, 772. https://doi.org/10.3390/catal13040772
Song M, Xin Y, Cai S, Xu W, Xu W. Immobilized KDN Lipase on Macroporous Resin for Isopropyl Myristate Synthesis. Catalysts. 2023; 13(4):772. https://doi.org/10.3390/catal13040772
Chicago/Turabian StyleSong, Ming, Yuhan Xin, Sulan Cai, Weizhuo Xu, and Wei Xu. 2023. "Immobilized KDN Lipase on Macroporous Resin for Isopropyl Myristate Synthesis" Catalysts 13, no. 4: 772. https://doi.org/10.3390/catal13040772
APA StyleSong, M., Xin, Y., Cai, S., Xu, W., & Xu, W. (2023). Immobilized KDN Lipase on Macroporous Resin for Isopropyl Myristate Synthesis. Catalysts, 13(4), 772. https://doi.org/10.3390/catal13040772