One-Pot Fabrication of 2D/2D CdIn2S4/In2S3 Heterojunction for Boosting Photocatalytic Cr(VI) Reduction
Abstract
:1. Introduction
2. Results
2.1. Synthesis of In2S3 (IS)
2.2. Synthesis of CdIn2S4 (CIS)
2.3. Synthesis of CdIn2S4/In2S3 (CISI) Composites
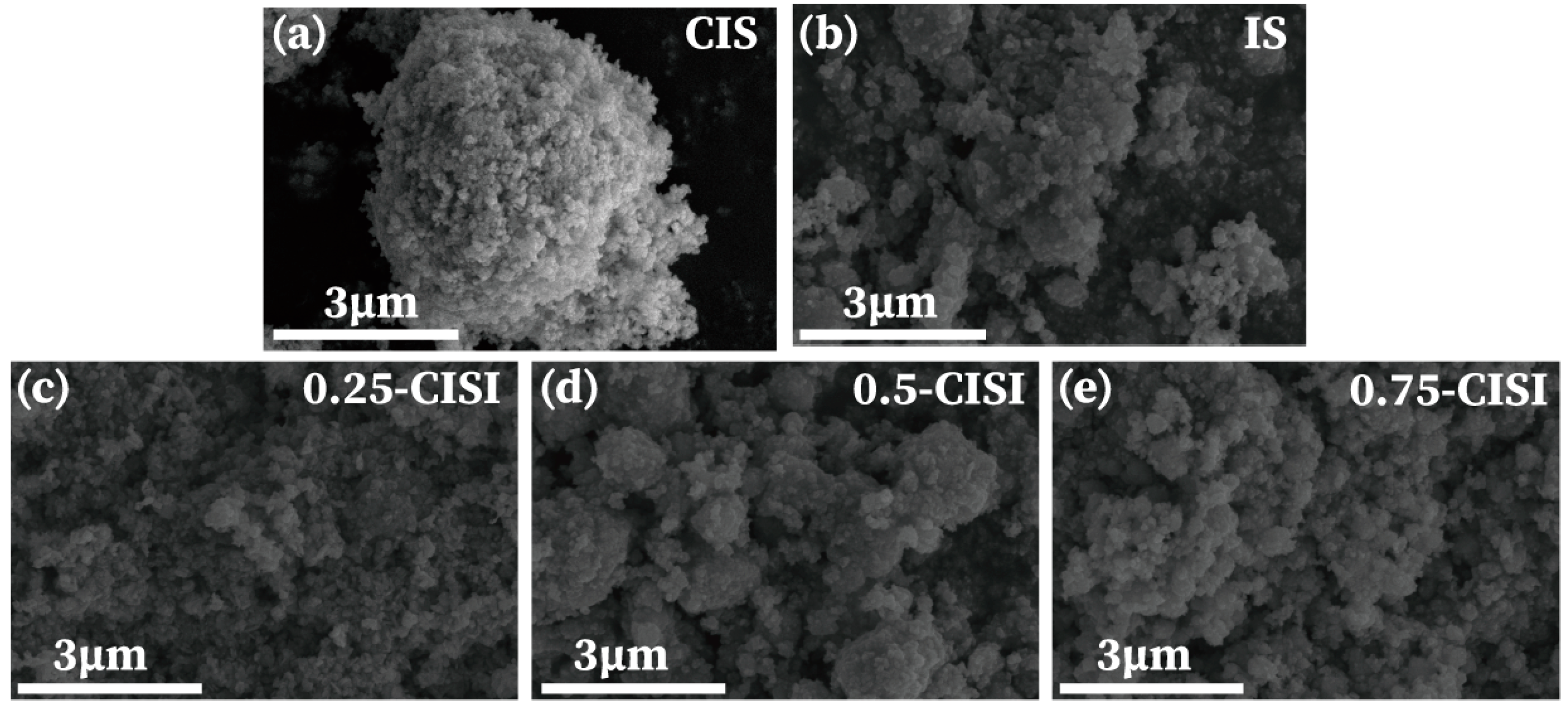
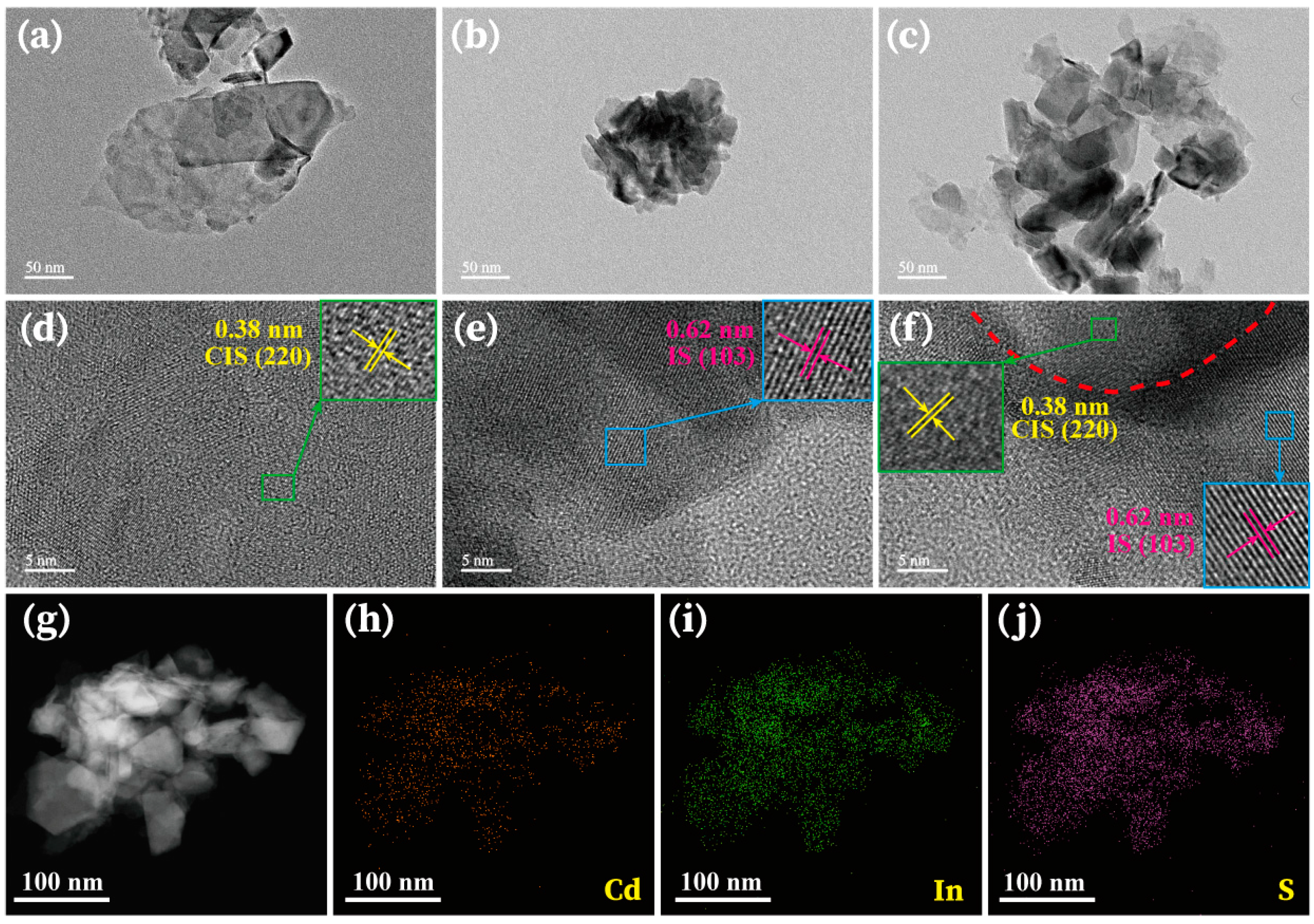
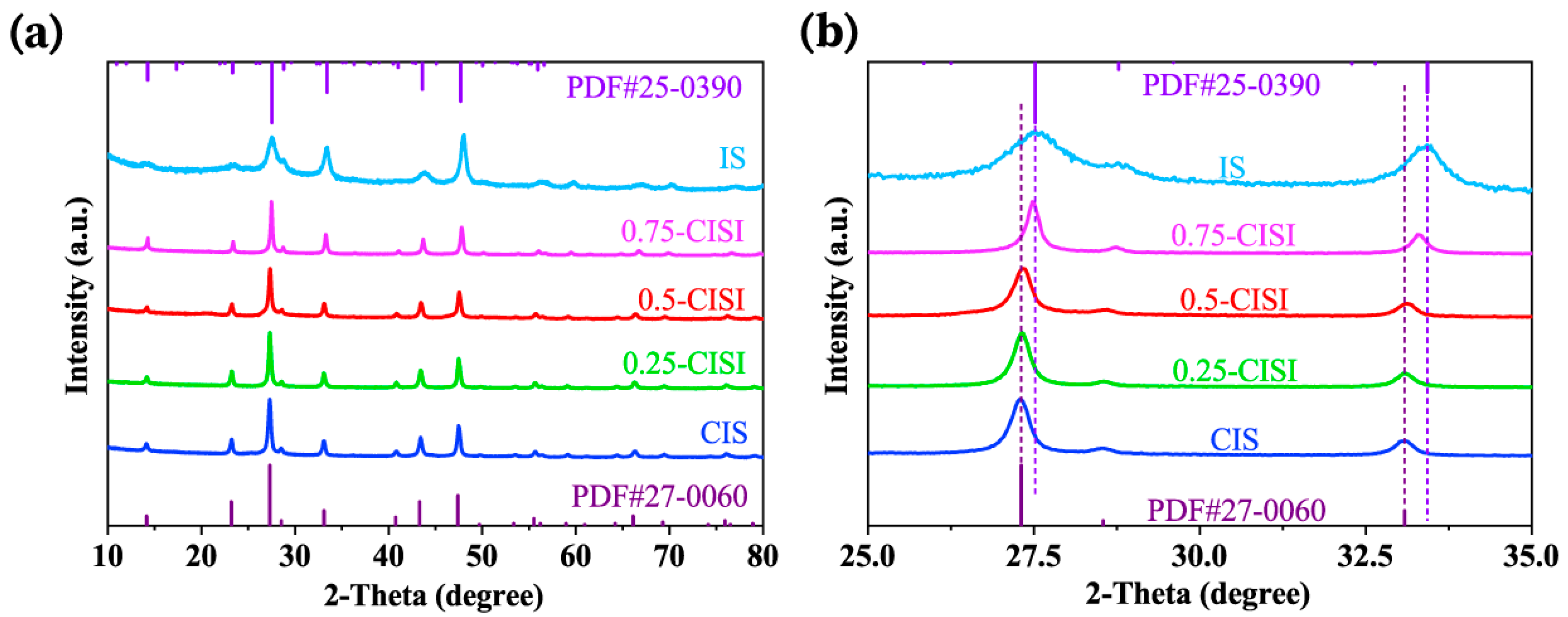
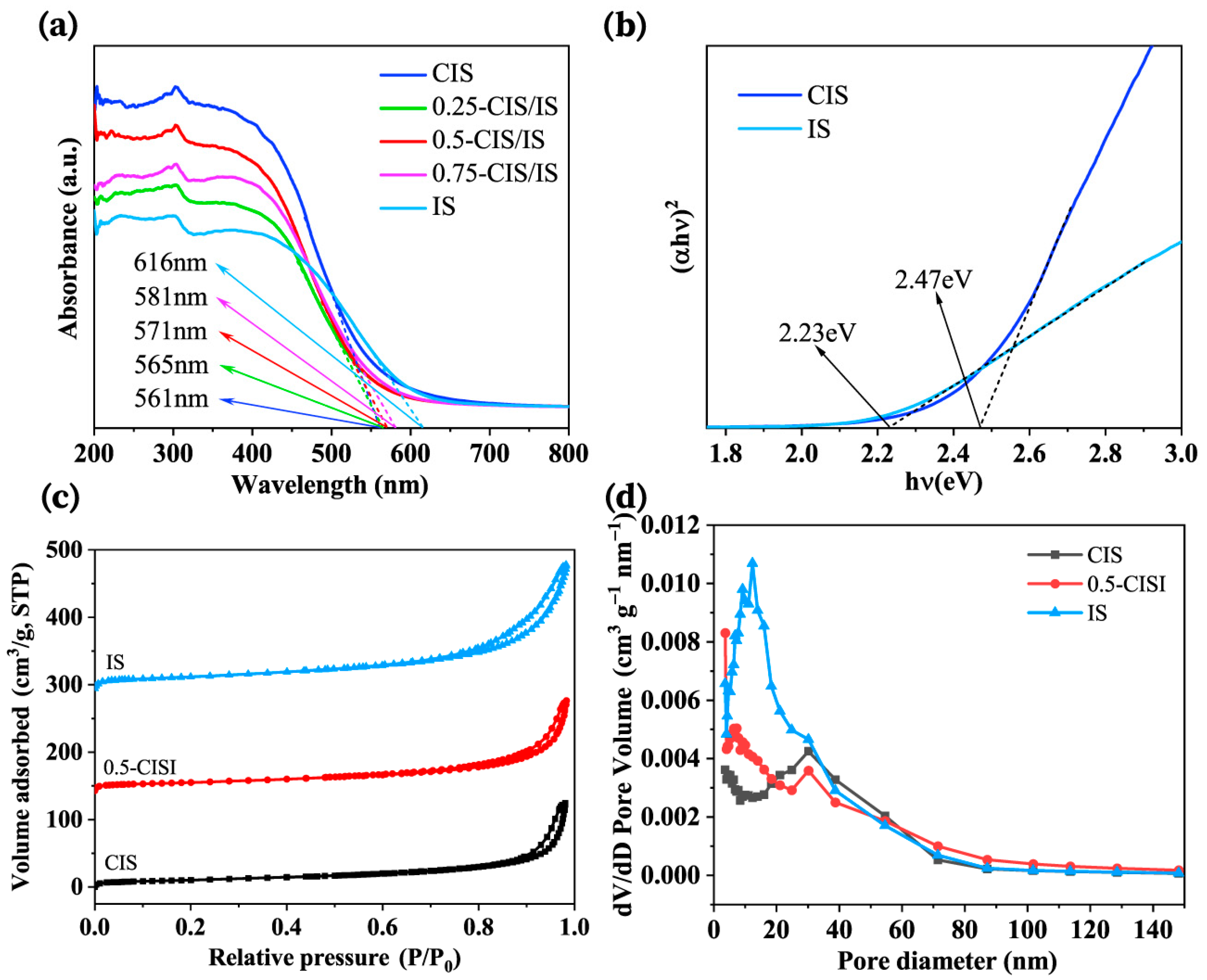
2.4. Characterization
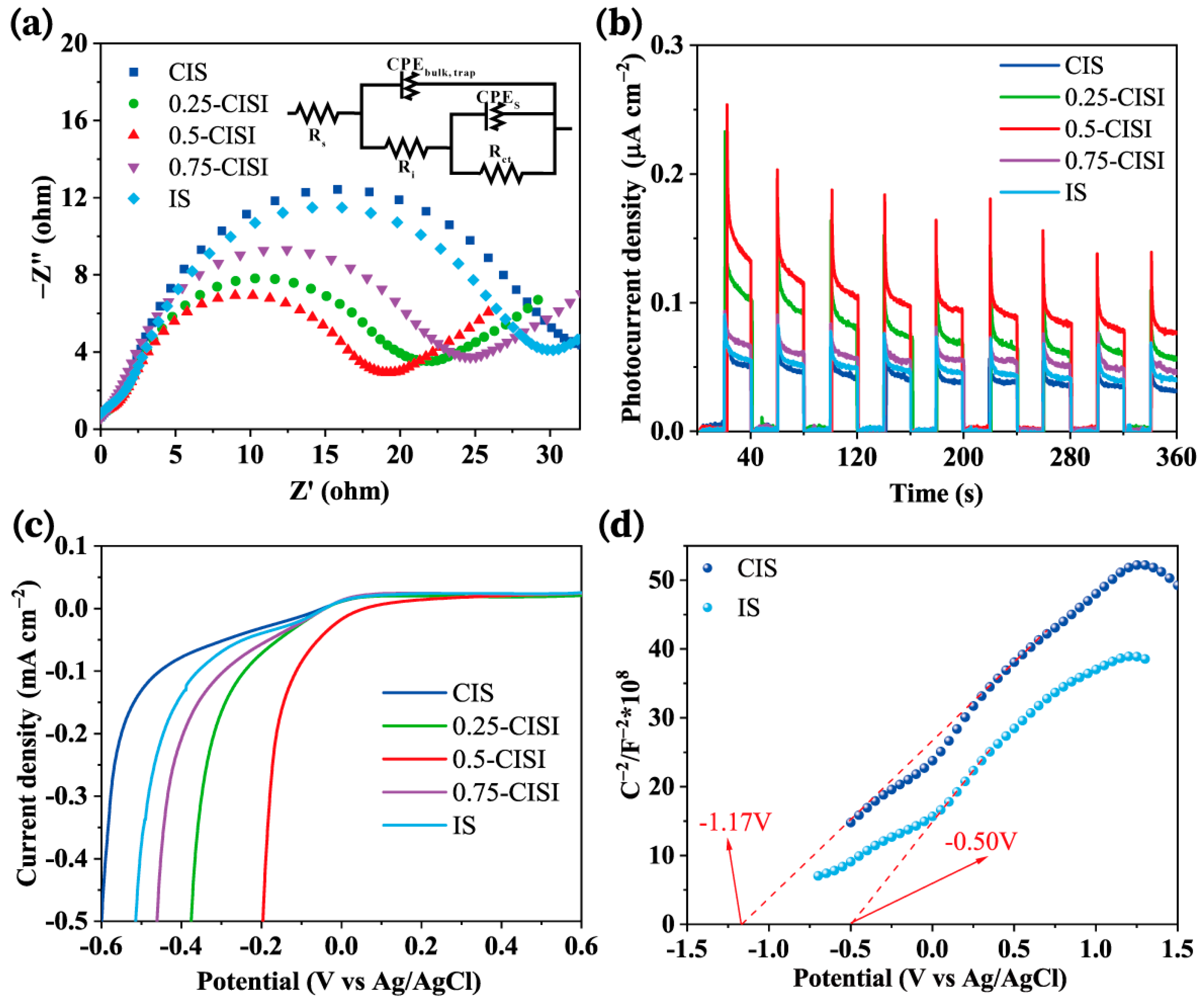
2.5. Photo-Electrochemical Measurements
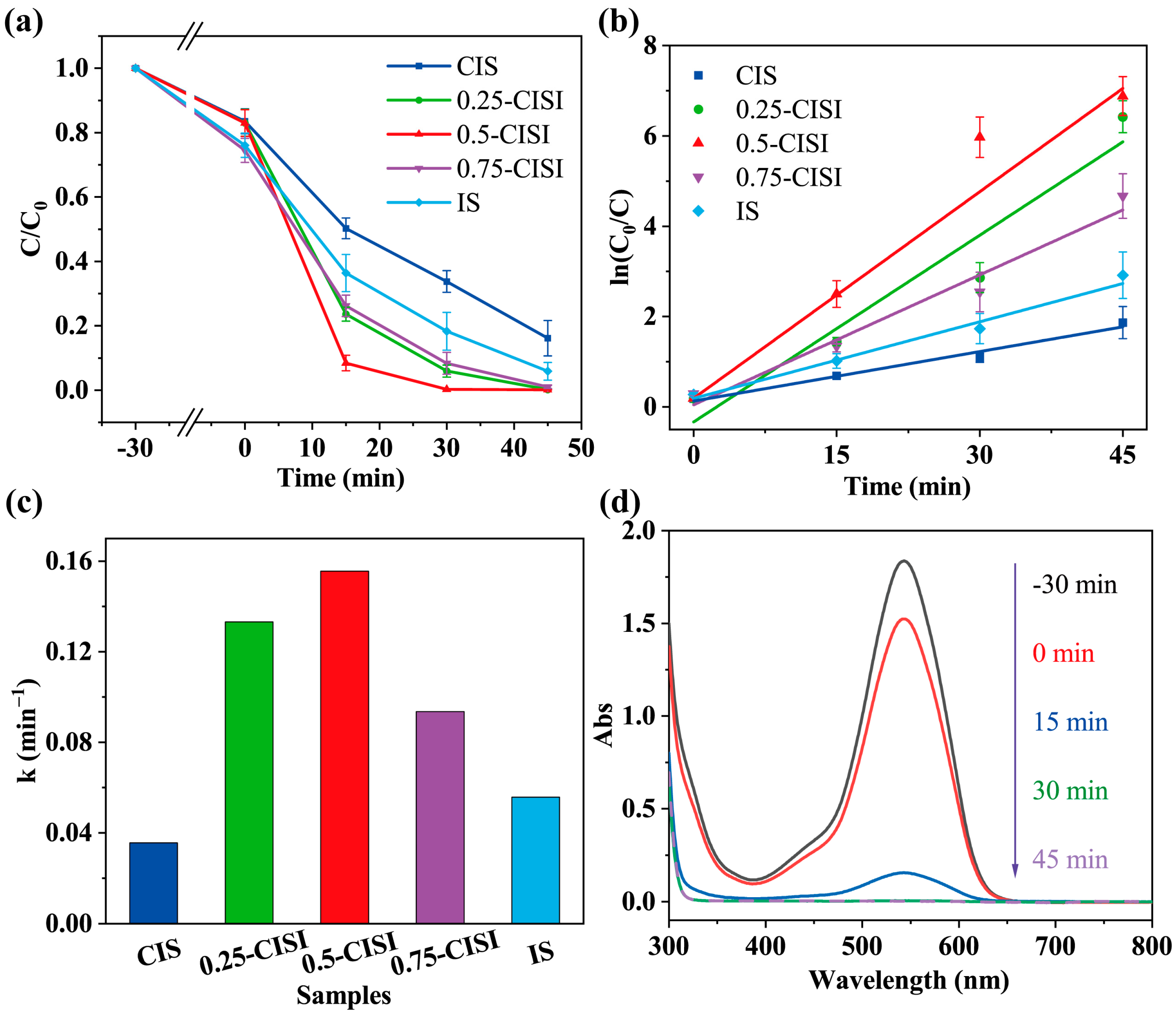
2.6. Photocatalytic Cr(VI) Reduction
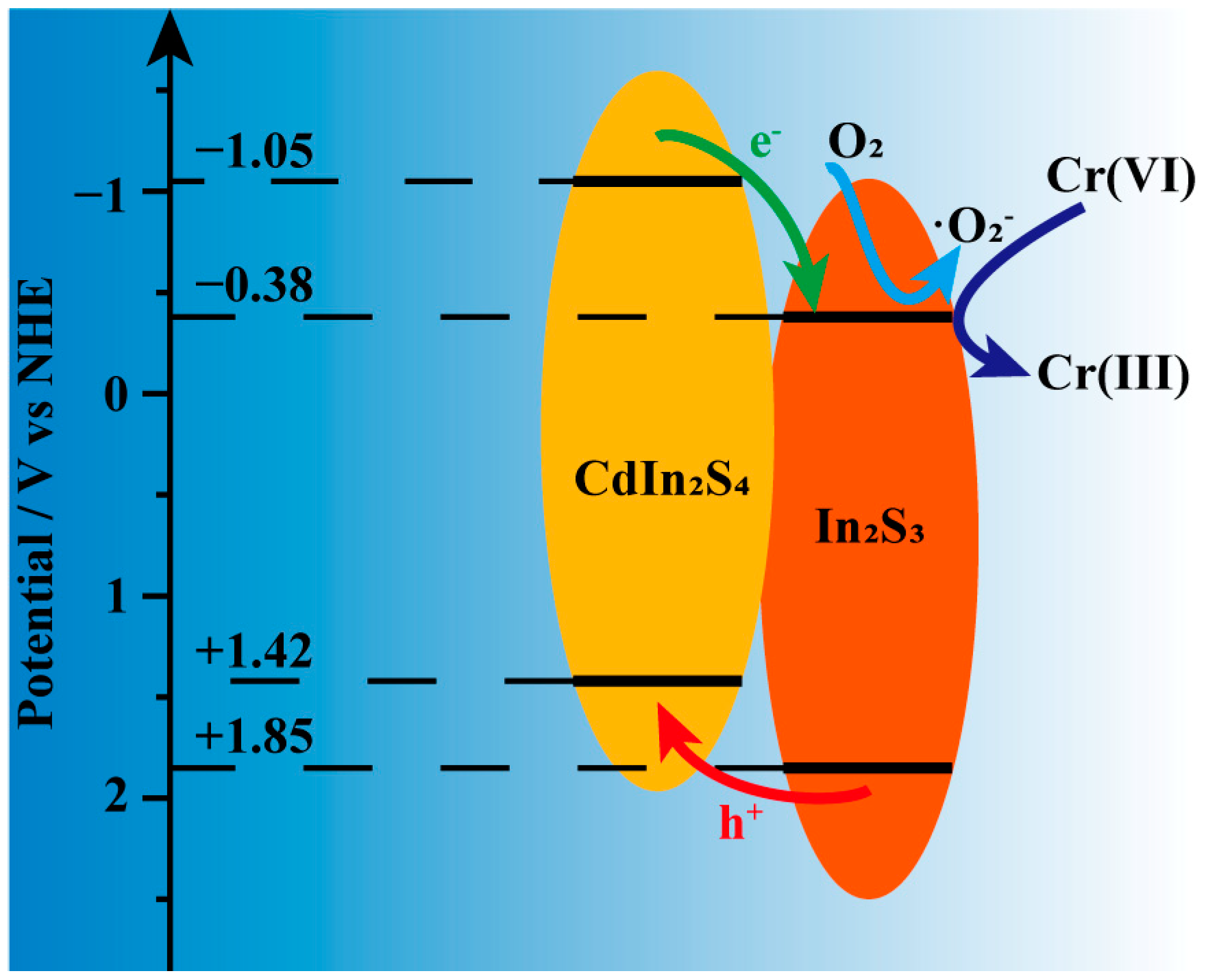
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, C.-C.; Ren, X.; Wang, P.; Chang, C. The state of the art review on photocatalytic Cr(VI) reduction over MOFs-based photocatalysts: From batch experiment to continuous operation. Chemosphere 2022, 303, 134949. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, M. Recent progress in the development of MOF-based photocatalysts for the photoreduction of Cr(VI). ACS Appl. Mater. Interfaces 2022, 14, 24993–25024. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, M.; Wang, C.; Liu, Y. Ta3N5/CdS core-shell S-scheme heterojunction nanofibers for efficient photocatalytic removal of antibiotic tetracycline and Cr(VI): Performance and mechanism insights. Adv. Fiber Mater. 2023. [CrossRef]
- Liu, C.; Xiao, W.; Yu, G.; Wang, Q.; Hu, J.; Xu, C.; Du, X.; Xu, J.; Zhang, Q.; Zou, Z. Interfacial engineering of Ti3C2 MXene/CdIn2S4 Schottky heterojunctions for boosting visible-light H2 evolution and Cr(VI) reduction. J. Colloid Interface Sci. 2023, 640, 851–863. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.; Kang, S.-W.; Kim, H.; Fujishima, A.; Terashima, C. Nanoflakes-like nickel cobaltite as active electrode material for 4-nitrophenol reduction and supercapacitor applications. J. Hazard. Mater. 2021, 419, 126453. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Mohite, B. Role of Nanotechnology in Photocatalysis Application. Recent Pat. Nanotechnol. 2023, 17, 5–7. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Kim, H. Facile synthesis of multitasking composite of Silver nanoparticle with Zinc oxide for 4-nitrophenol reduction, photocatalytic hydrogen production, and 4-chlorophenol degradation. J. Alloys Compd. 2022, 928, 167133. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Hou, W.; Zou, Z. Two-dimensional titanium/niobium metal oxide-based materials for photocatalytic application. Sol. RRL 2020, 4, 2000070. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, B.; Cheng, B.; Zhang, J.; Zhang, L.; Yu, J. In-situ preparation of TiO2/N-doped graphene hollow sphere photocatalyst with enhanced photocatalytic CO2 reduction performance. Chin. J. Catal. 2021, 42, 1648–1658. [Google Scholar] [CrossRef]
- Boumeriame, H.; Da Silva, E.S.; Cherevan, A.S.; Chafik, T.; Faria, J.L.; Eder, D. Layered double hydroxide (LDH)-based materials: A mini-review on strategies to improve the performance for photocatalytic water splitting. J. Energy Chem. 2022, 64, 406–431. [Google Scholar] [CrossRef]
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B. Properties, optimized morphologies, and advanced strategies for photocatalytic applications of WO3 based photocatalysts. J. Hazard. Mater. 2022, 428, 128218. [Google Scholar] [CrossRef]
- Pan, C.; Bian, G.; Zhang, Y.; Lou, Y.; Dong, Y.; Xu, J.; Zhu, Y. Efficient and stable H2O2 production from H2O and O2 on BiPO4 photocatalyst. Appl. Catal. B Environ. 2022, 316, 121675. [Google Scholar] [CrossRef]
- Zhang, G.; Guan, Z.; Yang, J.; Li, Q.; Zhou, Y.; Zou, Z. Metal sulfides for photocatalytic hydrogen production: Current development and future challenges. Sol. RRL 2022, 6, 2200587. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zou, Z. Recent advances in designing ZnIn2S4-based heterostructured photocatalysts for hydrogen evolution. J. Mater. Sci. Technol. 2023, 139, 167–188. [Google Scholar] [CrossRef]
- Zhao, S.; Li, K.; Wu, J.; Zhang, J.; Li, X.; Guo, X.; Song, C. Metal-Organic Framework-Derived Tubular In2O3-C/CdIn2S4 Heterojunction for Efficient Solar-Driven CO2 Conversion. ACS Appl. Mater. Interfaces 2022, 14, 20375–20384. [Google Scholar] [CrossRef]
- Nagappagari, L.R.; Patil, S.S.; Lee, J.; Park, E.; Yu, Y.-T.; Lee, K. Upgraded charge transport in g-C3N4 nanosheets by boron doping and their heterojunction with 3D CdIn2S4 for efficient photodegradation of azo dye. Mater. Today Chem. 2022, 24, 100857. [Google Scholar] [CrossRef]
- Ma, X.; Li, W.; Ren, C.; Dong, M.; Geng, L.; Fan, H.; Li, Y.; Qiu, H.; Wang, T. Construction of novel noble-metal-free MoP/CdIn2S4 heterojunction photocatalysts: Effective carrier separation, accelerating dynamically H2 release and increased active sites for enhanced photocatalytic H2 evolution. J. Colloid Interface Sci. 2022, 628, 368–377. [Google Scholar] [CrossRef]
- Liu, R.Y.; Minh Man, T.; Chang, M.B. Photocatalytic removal of toluene with CdIn2S4/CNFs catalyst: Effect of ozone addition. Sustain. Environ. Res. 2022, 32, 10. [Google Scholar] [CrossRef]
- Li, C.; Ding, G.; Liu, X.; Huo, P.; Yan, Y.; Yan, Y.; Liao, G. Photocatalysis over NH2-UiO-66/CoFe2O4/CdIn2S4 double p-n junction: Significantly promoting photocatalytic performance by double internal electric fields. Chem. Eng. J. 2022, 435, 134740. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Xu, S.; Fan, T.; Yi, X.; Chen, Z. Photocatalytic oxidative coupling of benzylamine to schiff base over 0D/2D CdS/CdIn2S4 heterojunction. Energy Technol. 2022, 10, 2200362. [Google Scholar] [CrossRef]
- Jiang, W.; Yin, X.; Xin, F.; Bi, Y.; Liu, Y.; Li, X. Preparation of CdIn2S4 microspheres and application for photocatalytic reduction of carbon dioxide. Appl. Surf. Sci. 2014, 288, 138–142. [Google Scholar] [CrossRef]
- Wang, W.; Ng, T.W.; Ho, W.; Huang, J.; Liang, S.; An, T.; Li, G.; Yu, J.C.; Wong, P.K. CdIn2S4 microsphere as an efficient visible-light-driven photocatalyst for bacterial inactivation: Synthesis, characterizations and photocatalytic inactivation mechanisms. Appl. Catal. B Environ. 2013, 129, 482–490. [Google Scholar] [CrossRef]
- Gao, L.; Chen, Z.; Zheng, H.; Hu, J. A distinct hollow spindle-like CdIn2S4 photocatalyst for high-efficiency tetracycline removal. Mater. Today Chem. 2022, 24, 100800. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Y.; Han, Z.; Sun, Y.; Wang, X.; Zhang, Q.; Zou, Z. Z-scheme N-doped K4Nb6O17/g-C3N4 heterojunction with superior visible-light-driven photocatalytic activity for organic pollutant removal and hydrogen production. Chin. J. Catal. 2021, 42, 164–174. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, H.; Zhu, Y.; Dong, P.; Hou, H.; Xu, Q.; Chen, X.; Xi, X.; Hou, W. Ordered layered N-doped KTiNbO5/g-C3N4 heterojunction with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2018, 228, 54–63. [Google Scholar] [CrossRef]
- Manh-Hiep, V.; Nguyen, C.-C.; Sakar, M.; Do, T.-O. Ni supported CdIn2S4 spongy-like spheres: A noble metal free high-performance sunlight driven photocatalyst for hydrogen production. Phys. Chem. Chem. Phys. 2017, 19, 29429–29437. [Google Scholar]
- Zhang, P.; Yin, L.; Yang, X.; Wang, J.; Chi, M.; Qiu, J. Cotton-derived 3D carbon fiber aerogel to in situ support Bi2O3 nanoparticles as a separation-free photocatalyst for antibiotic removal. Carbon 2023, 201, 110–119. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; He, T.; Wu, J.; Zhang, J.; Wu, J. Rationally design and in-situ fabrication of ultrasmall pomegranate-like CdIn2S4/ZnIn2S4 Z-scheme heterojunction with abundant vacancies for improving CO2 reduction and water splitting. Chem. Eng. J. 2022, 442, 136309. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, T.; Hou, J.; Zhang, T.; Zhang, G.; Zhang, Y.; Wang, X. Oxygen vacancies induced narrow band gap of BiOCl for efficient visible-light catalytic performance from double radicals. J. Mater. Sci. Technol. 2022, 114, 240–248. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Jiang, Y.; Liu, Y.; Zhang, W.; Zhang, J.; Macauley, A.L.O. Morphology-engineered carbon quantum dots embedded on octahedral CdIn2S4 for enhanced photocatalytic activity towards pollutant degradation and hydrogen evolution. Environ. Res. 2022, 209, 112800. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Liang, Q.; Zhou, M.; Li, Z.; Xu, S. Coupling MOF-derived titanium oxide with CdIn2S4 formed 2D/3D core–shell heterojunctions with enhanced photocatalytic performance. Sep. Purif. Technol. 2021, 279, 119765. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Zhang, F.; Li, W.; Li, Y.; Yu, H.; Wang, M.; Yu, H. Tungsten oxide quantum dots deposited onto ultrathin CdIn2S4 nanosheets for efficient S-scheme photocatalytic CO2 reduction via cascade charge transfer. Chem. Eng. J. 2022, 428, 131218. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Noble-metal-free ultrathin MXene coupled with In2S3 nanoflakes for ultrafast photocatalytic reduction of hexavalent chromium. Appl. Catal. B Environ. 2021, 284, 119754. [Google Scholar] [CrossRef]
- Liu, C.; Han, Z.; Feng, Y.; Dai, H.; Zhao, Y.; Han, N.; Zhang, Q.; Zou, Z. Ultrathin Z-scheme 2D/2D N-doped HTiNbO5 nanosheets/g-C3N4 porous composites for efficient photocatalytic degradation and H2 generation under visible light. J. Colloid Interface Sci. 2021, 583, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Hu, F.; Cheng, W.; Han, Z.; Liu, C.; Dai, Y. ZnCuAl-LDH/Bi2MoO6 nanocomposites with improved visible light-driven photocatalytic degradation. Acta Phys.-Chim. Sin. 2020, 36, 1911016. [Google Scholar]
- Liu, C.; Zhang, Y.; Wu, J.; Dai, H.; Ma, C.; Zhang, Q.; Zou, Z. Ag-Pd alloy decorated ZnIn2S4 microspheres with optimal Schottky barrier height for boosting visible-light-driven hydrogen evolution. J. Mater. Sci. Technol. 2022, 114, 81–89. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Y.; Du, X.; Wu, J.; Liu, C.; Zou, Z. In-situ synthesis of nickel/palladium bimetal/ZnIn2S4 Schottky heterojunction for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 623, 205–215. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, S.; Xing, Y.; Qin, H.; Zheng, Q.; Zhang, P.; Zhang, S.; Xu, X. Sandwich-like P-doped h-BN/ZnIn2S4 nanocomposite with direct Z-scheme heterojunction for efficient photocatalytic H2 and H2O2 evolution. Chem. Eng. J. 2022, 442, 136151. [Google Scholar] [CrossRef]
- Meng, S.; Chen, C.; Gu, X.; Wu, H.; Meng, Q.; Zhang, J.; Chen, S.; Fu, X.; Liu, D.; Lei, W. Efficient photocatalytic H2 evolution, CO2 reduction and N2 fixation coupled with organic synthesis by cocatalyst and vacancies engineering. Appl. Catal. B Environ. 2021, 285, 119789. [Google Scholar] [CrossRef]
- Huang, L.; Li, B.; Su, B.; Xiong, Z.; Zhang, C.; Hou, Y.; Ding, Z.; Wang, S. Fabrication of hierarchical Co3O4@CdIn2S4 p–n heterojunction photocatalysts for improved CO2 reduction with visible light. J. Mater. Chem. A 2020, 8, 7177–7183. [Google Scholar] [CrossRef]
- Bai, J.; Chen, W.; Shen, R.; Jiang, Z.; Zhang, P.; Liu, W.; Li, X. Regulating interfacial morphology and charge-carrier utilization of Ti3C2 modified all-sulfide CdS/ZnIn2S4 S-scheme heterojunctions for effective photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 112, 85–95. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Liu, X.; Huo, P.; Yan, Y.; Wang, L.; Liao, G.; Liu, C. Interface engineering of Co9S8/CdIn2S4 ohmic junction for efficient photocatalytic H2 evolution under visible light. J. Colloid Interface Sci. 2021, 600, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xie, A.; Guo, Y.; Dai, Y.; Zhou, W.; Jana, D.; Xian, Q.; Dong, W.; et al. Enhanced photocatalytic water oxidation by hierarchical 2D-Bi2MoO6@2D-MXene Schottky junction nanohybrid. Chem. Eng. J. 2021, 403, 126328. [Google Scholar] [CrossRef]
- Wang, C.; Yan, R.; Cai, M.; Liu, Y.; Li, S. A novel organic/inorganic S-scheme heterostructure of TCPP/Bi12O17Cl2 for boosting photodegradation of tetracycline hydrochloride: Kinetic, degradation mechanism, and toxic assessment. Appl. Surf. Sci. 2023, 610, 155346. [Google Scholar] [CrossRef]
- Liu, C.; Gao, X.; Zhan, C.; Cheng, T.; Wang, Y.; Zhang, B.; Dong, P.; Chen, X.; Xi, X.; Zou, Z. Layered BiOCl/H+TiNbO5− heterojunctions for boosting visible-light-driven photocatalytic RhB degradation. Sustain. Energy Fuels 2021, 5, 4680–4689. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Q.; Zhang, Q.; Zhu, Y.; Ji, M.; Tong, Z.; Hou, W.; Zhang, Y.; Xu, J. Layered BiOBr/Ti3C2 MXene composite with improved visible-light photocatalytic activity. J. Mater. Sci. 2019, 54, 2458–2471. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Dou, Q.; Chen, Q.; Wang, X.; Hu, C.; Paul, R. Defect engineering in polymeric carbon nitride with accordion structure for efficient photocatalytic CO2 reduction and H2 production. Chem. Eng. J. 2022, 450, 138425. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Liu, Y.; Cai, M.; Zhao, W.; Duan, X. S-scheme MIL-101(Fe) octahedrons modified Bi2WO6 microspheres for photocatalytic decontamination of Cr(VI) and tetracycline hydrochloride: Synergistic insights, reaction pathways, and toxicity analysis. Chem. Eng. J. 2023, 455, 140943. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Zhang, Y.; Liu, C.; Xia, J.; Li, H. Ionic Liquid-Assisted Synthesis of Ag3PO4 Spheres for Boosting Photodegradation Activity under Visible Light. Catalysts 2021, 11, 788. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Q.; Ji, M.; Zhu, H.; Hou, H.; Yang, Q.; Jiang, C.; Wang, J.; Tian, L.; Chen, J.; et al. Constructing Z-scheme charge separation in 2D layered porous BiOBr/graphitic C3N4 nanosheets nanojunction with enhanced photocatalytic activity. J. Alloys Compd. 2017, 723, 1121–1131. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Yan, R.; Chen, X. Constructing Cd0.5Zn0.5S/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic oxidation and Cr(VI) reduction. Adv. Powder Mater. 2023, 2, 100073. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Lv, K.; Chen, X. S-scheme photocatalyst TaON/Bi2WO6 nanofibers with oxygen vacancies for efficient abatement of antibiotics and Cr(VI): Intermediate eco-toxicity analysis and mechanistic insights. Chin. J. Catal. 2022, 43, 2652–2664. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, Q.; Bai, B.; Li, Y.; He, C. Novel all-solid-state Z-scheme SnO2/Pt/In2O3 photocatalyst with boosted photocatalytic performance on water splitting and 2,4-dichlorophenol degradation under visible light. Chem. Eng. J. 2020, 390, 124518. [Google Scholar] [CrossRef]
- Yang, W.; Ma, G.; Fu, Y.; Peng, K.; Yang, H.; Zhan, X.; Yang, W.; Wang, L.; Hou, H. Rationally designed Ti3C2 MXene@TiO2/CuInS2 Schottky/S-scheme integrated heterojunction for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 429, 132381. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Xu, J.; He, Y.; Xiao, G. Hydrothermal synthesis of Cu (X)–CdIn2S4 microspheres and photocatalytic reduction of aqueous Cr (VI) under visible light irradiation. Mater. Lett. 2014, 117, 17–20. [Google Scholar] [CrossRef]
- Fu, R.; Gong, Y.; Li, C.; Niu, L.; Liu, X. CdIn2S4/In (OH)3/NiCr-LDH Multi-Interface Heterostructure Photocatalyst for Enhanced Photocatalytic H2 Evolution and Cr (VI) Reduction. Nanomaterials 2021, 11, 3122. [Google Scholar] [CrossRef]
- He, J.; Hu, J.; Hu, Y.; Guo, S.; Huang, Q.; Li, Y.; Zhou, G.; Gui, T.; Hu, N.; Chen, X. Hierarchical S-Scheme Heterostructure of CdIn2S4@ UiO-66-NH2 toward Synchronously Boosting Photocatalytic Removal of Cr (VI) and Tetracycline. Inorg. Chem. 2022, 61, 19961–19973. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, T.; Wen, J.; Liu, X. Flower-like Bi2S3–In2S3 heterojunction for efficient solar light induced photoreduction of Cr (VI). Chemosphere 2021, 278, 130422. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Shao, C.; Li, J.; Zhang, M.; Zhang, P.; Wang, K.; Lu, N.; Liu, Y. One-dimensional hierarchical heterostructures of In2S3 nanosheets on electrospun TiO2 nanofibers with enhanced visible photocatalytic activity. J. Hazard. Mater. 2013, 260, 892–900. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, F.; Qiu, L.; Gao, M.; Yang, W.; Liu, Y.; Yu, Y. Z-Scheme Mo2C/MoS2/In2S3 dual-heterojunctions for the photocatalytic reduction of Cr (vi). J. Mater. Chem. A 2021, 9, 10297–10303. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G.; Wei, X.; Xu, H.; Jin, S. One-pot soft integration fabrication of graphene-induced phase transition to form dimension control contact In2S3/G heterojunction hybrids for enhancing visible photocatalytic purification performance. J. Alloys Compd. 2022, 895, 162589. [Google Scholar] [CrossRef]
- Fu, X.; Tao, J.; He, Z.; Gao, Y.; Xia, Y.; Zhao, Z. Synergy of Z-scheme heterostructure with interfacial S−O bonding in In2S3/BiOBr for efficient tetracycline hydrochloride degradation and Cr (VI) reduction. J. Alloys Compd. 2023, 936, 168202. [Google Scholar] [CrossRef]



| Catalyst | Catalyst (mg) | Cr(VI) (mg/L) | Solution (mL) | Time (min) | Refs. |
|---|---|---|---|---|---|
| Cu(X)–CdIn2S4 | 20 | 20 | 40 | 110 | [55] |
| CdIn2S4/In(OH)3/NiCr-LDH | 30 | 50 | 50 | 120 | [56] |
| CdIn2S4@UiO-66-NH2 | 50 | 20 | 100 | 120 | [57] |
| Bi2S3-In2S3 | 20 | 70 | 100 | 140 | [58] |
| In2S3/TiO2 | 50 | 20 | 100 | 80 | [59] |
| Mo2C/MoS2/In2S3 | 50 | 40 | 50 | 90 | [60] |
| In2S3/G | 15 | 10 | 30 | 50 | [61] |
| In2S3/BiOBr | 200 | 50 | 500 | 180 | [62] |
| This work | 10 | 20 | 100 | 45 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wu, J.; Zhang, S.; Chen, W.; Xiao, W.; Hou, H.; Lu, X.; Liu, C.; Zhang, Q. One-Pot Fabrication of 2D/2D CdIn2S4/In2S3 Heterojunction for Boosting Photocatalytic Cr(VI) Reduction. Catalysts 2023, 13, 826. https://doi.org/10.3390/catal13050826
Hu J, Wu J, Zhang S, Chen W, Xiao W, Hou H, Lu X, Liu C, Zhang Q. One-Pot Fabrication of 2D/2D CdIn2S4/In2S3 Heterojunction for Boosting Photocatalytic Cr(VI) Reduction. Catalysts. 2023; 13(5):826. https://doi.org/10.3390/catal13050826
Chicago/Turabian StyleHu, Jiawei, Jiaxin Wu, Siyuan Zhang, Wenxuan Chen, Wen Xiao, Haijun Hou, Xiaowang Lu, Chao Liu, and Qinfang Zhang. 2023. "One-Pot Fabrication of 2D/2D CdIn2S4/In2S3 Heterojunction for Boosting Photocatalytic Cr(VI) Reduction" Catalysts 13, no. 5: 826. https://doi.org/10.3390/catal13050826





