Abstract
The characteristics and preparation methods of zeolite-based adsorbents and membranes were reviewed and their applications in gas separation and purification were introduced according to classification. The effects of framework structure, equilibrium cations and pore size of zeolites as well as temperature and pressure of the system on gas adsorption and separation were discussed, and the separation mechanisms were also summarized. The main defects and improved methods of zeolite-based adsorbents and membranes were briefly described, and their future trend for gas separation and purification was finally prospected.
1. Introduction
Nitrogen (N2), oxygen (O2), carbon dioxide (CO2), hydrogen (H2), light hydrocarbons (CxHy, x ≤ 4) and other common industrial gases are mainly derived from petrochemical and natural gas processing industries, and these are the basic energy resources and raw materials for the production of important industrial chemicals [1]. However, the separation and purification of these gases, which usually exist in the form of mixtures, is a very critical and challenging industrial process. With people’s attention turned towards green production, the living environment and their own health, the importance of adsorption and separation technology has become increasingly apparent.
At present, the main separation technologies for common light gases (H2, CO2, O2, N2, lower olefins and alkynes) include cryogenic distillation, pressure swing adsorption (PSA) and membrane separation, among which the most effective and traditional method is cryogenic distillation process with phase change and high energy consumption [2]. In order to save energy and reduce consumption, PSA and membrane technologies without phase change gradually expanded their markets to become the technology leader, and these are also characterized by simple operation and less investment for facilities. The key of these two technologies for gas separation, furthermore, lies in the research and development of high-performance adsorbents and membrane materials.
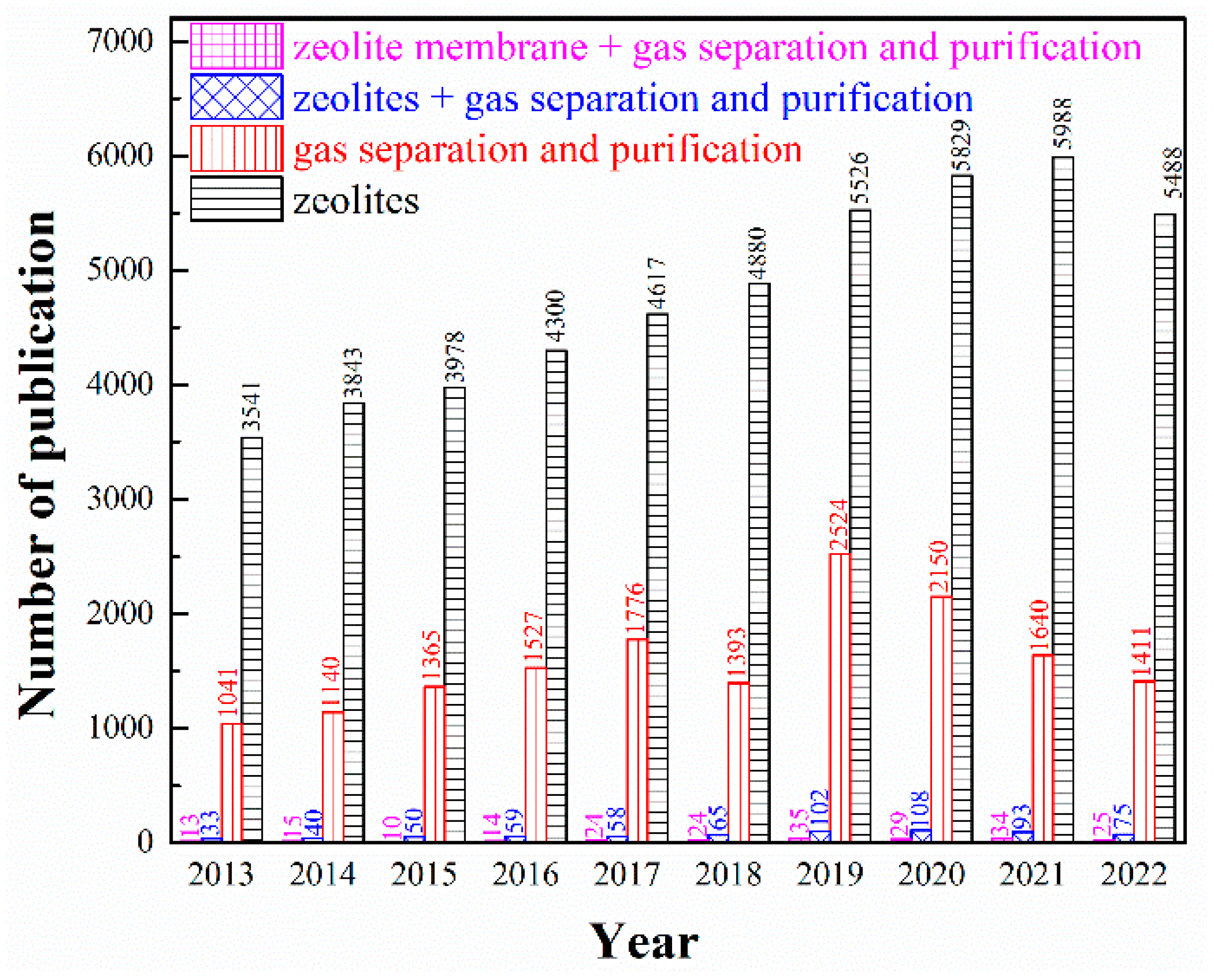
In recent years, zeolites as advanced functional materials have attracted extensive attention in more and more research fields, especially in the petroleum and chemical industries. Different from disordered porous materials such as activated carbon (including carbon molecular sieve), silica gel, porous alumina and diatomite, zeolites are ordered crystalline and porous materials constructed by TO4 tetrahedrons (T = Al, Si, P, Ti, etc.) as building blocks connected to each other by oxygen atoms, with regular pore structure, high micropore volume and specific surface area, as well as outstanding thermal, mechanical and chemical stability [3]. The negative charged TO4 tetrahedrons which are balanced by counter-ions (such as Na+, K+, or Ca2+) make the surface of zeolites highly polarized to easily adsorb molecules with strong polarity. Moreover, the channel dimensions of zeolites generally fall in the sub-nanometric scale, comparable with the kinetic diameters of small molecules, which can be adjusted by ion-exchange properties and by modifying the Si/Al ratio during synthesis [4]. Moreover, artificially-synthesized zeolites made with cheap and easily available raw materials also ensure high purity and consistency of products [5]. Zeolitic membrane is a kind of inorganic porous membrane, which refers to the continuous defect-free membrane formed by the growth of zeolites. It has the unique properties of zeolite itself, such as pore size uniformity, ion exchange, acid-base resistance and high-temperature resistance, which has been widely adopted by virtue of its advantages over organic polymer membrane in high-temperature resistance and easy control of microporous structure in nanoscale. Thereupon, zeolite-based adsorbents and membranes have extensive application value for gas separation. As emphasized in Figure 1, the number of publications concerning on zeolite-based adsorbents or membranes in gas separation and purification has experienced a considerable growth in recent decades. Although its overall research is still in its infancy and in its rapid development stage, zeolite-based adsorbents or membranes exhibit great potential in the field of gas separation and purification from the perspective of a very limited number of research results showing excellent separation effects.
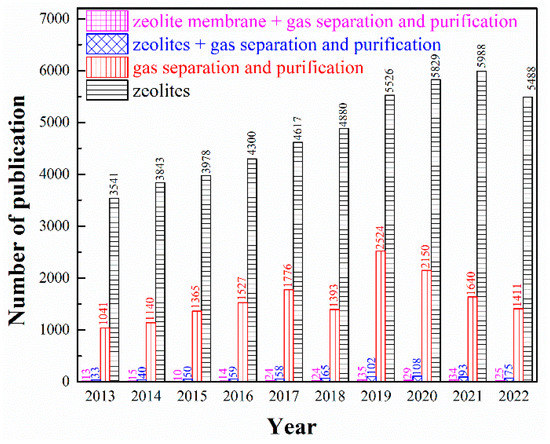
Figure 1.
Number of publications per year for the period of 2012–2022 based on Web of Science database using the keywords: (Black columns) zeolites; (Red columns) gas separation and purification; (Blue columns) zeolites, gas separation and purification; (Pink columns) zeolite membrane, gas separation and purification.
2. Synthesis Strategies toward Zeolite-Based Adsorbents and Membranes
The early discovery and use of zeolites began with natural zeolites, of which clinoptilolite, chabazite, erionite, mordenite, phillipsite and ferrierite are suitable types for gas adsorption and separation [6]. However, due to the limitations of the pore structure of natural zeolites, they are far from meeting the industrial demands with the increase of their application volume and scope. Therefore, people began to research and synthesize artificial zeolites. In recent years, with the advancement of techniques, synthesis methods have become increasingly diverse so that zeolites with complex structures, stable performance and high catalytic efficiency have been extensively synthesized, greatly expanding their application fields.
2.1. Synthesis Methods of Powder Zeolites
The synthetic chemistry of zeolites is extremely complex. After long-term exploration, researchers at home and abroad have continuously improved and innovated on traditional methods. At present, the main synthesis methods include: hydrothermal synthesis, solvothermal synthesis, ionothermal synthesis, vapor-phase transport (VPT), dry gel conversion, green synthesis, etc. [7,8,9]. Various synthesis methods have their own advantages and disadvantages. A broad prospect will surely be opened for the green and efficient synthesis of zeolites by integrating several different synthesis routes.
Hydrothermal synthesis is designed to fully mix alkali (NaOH, KOH, etc.), Al2O3, SiO2 and H2O in a certain proportion under heating in a closed vessel, followed by nucleation and growth to form zeolites. As the most traditional synthesis method, it has the advantages of uniform dissolution of reactants, mild reaction conditions, simple operation, low cost and pollution. Different from hydrothermal synthesis, non-aqueous solvent instead of water is applied in solvothermal synthesis to avoid the interference of H2O and break the boundary of water solvent. However, the large use of organic solvent causes the problem of high cost in actual production and greatly increases the production risk. Among them, ion-thermal synthesis, which uses ionic liquids with low steam pressure, low volatility and strong designability as both solvent and template, has gradually developed. However, the high cost of ionic liquids is not suitable for the large-scale production of zeolites.
In vapor-phase transport (VPT), the gel reactants are heated at a certain temperature in a specific reactor, without contact with organic amine and water in liquid phase. Derived from VPT, dry gel conversion mixes the non-volatile directing agent (e.g., quaternary ammonium base/salt) in gel reactants rather than in aqueous solvent, which has the advantages of less pollution, saving raw materials and reducing costs, but limited practical application due to the fussy operation process and impure products.
As the concept of “green chemistry” has taken root, the shortcomings of traditional synthesis methods have also been exposed. Researchers have studied greening the synthesis process of zeolites from various perspectives, including the application of natural minerals (e.g., kaolinite and diatomite) and green organic templates as well as novel green synthesis methods. Among them, zeolites synthesized by seeding growth which employs crystal seeds instead of organic templates occupies a significant part, which plays an important role in effectively shortening the nucleation and growth period, inhibiting the generation of hybrid crystals and regulating the grain size. In solventless synthesis, raw materials in solid phase are ground and mixed uniformly for high-temperature crystallization during the reaction process without adding other solvents, which significantly increases available space of reactor and reduces environmental pollution and resource waste with simple operating procedures and lower reaction pressure. However, this method is still in the exploration and research stage, and has not yet achieved large-scale industrial production. Microwave-assisted synthesis can quickly transfer energy to the reaction system and accelerate nucleation of microcrystalline and dissolution of reaction gel, however, harmful radiation and high pressure generated under closed conditions should be highly emphasized due to high microwave energy. Hydroxyl radical-assisted synthesis can also effectively accelerate the crystallization of zeolites, reduce the amount of alkali and organic templates, and minimize energy consumption by promoting the depolymerization and polymerization of Si-O-Si bonds in initial aluminosilicate gel.
2.2. Fabrications of Zeolite Membranes
For zeolitic membranes, the main synthesis methods include hydrothermal synthesis (including in-situ and secondary growth), pore plugging, microwave heating, pulsed laser deposition, and electrophoretic deposition [10]. Different from in-situ hydrothermal synthesis in which zeolitic membranes nucleate and grow directly on the surface of support, zeolitic membranes synthesized by secondary growth are formed based on crystal seeds precoated on the surface of support, showing the characteristics of easy control of crystal growth and membrane microstructure, as well as excellent permeability. Currently, most research has focused on the synthesis of zeolitic membranes on porous carriers (e.g., Al2O3, stainless steel, TiO2, SiO2, mullite, glass, etc.) for conferring mechanical strength using in-situ hydrothermal synthesis or secondary growth, because the self-standing layer is very brittle. The secondary growth, as mentioned above, is more reproducible than the one-step method.
During the synthesis of zeolitic membranes, different methods of seed laying, hydrothermal and calcination conditions will have impacts on structure, density, gas permeability, and separation selectivity of the prepared membranes to varying degrees [11]. Currently, the main methods of seed laying include pull-up, wetting-rubbing seeding, vacuum seeding, electrophoretic deposition, spray coating, electrostatic adsorption, cross flow filtration, pulsed laser deposition, graded seeds dip-coating, steam-assisted conversion seeding, Langmuir-Blogett (L-B) assembly, etc. With the innovation of the crystallization process, various novel methods such as dynamic heating, variable solution synthesis, variable temperature synthesis, electric field-assisted crystallization, oil-bath heating, gel-free steam-assisted conversion, two-step hydrothermal synthesis plus dry gel conversion, and ultrasonic-assisted crystallization are gradually emerging in terms of accelerating nucleation and crystallization, shortening synthesis time, reducing film thickness, improving film flux, and reducing synthesis costs. Furthermore, recent developments designed to avoid the formation of thermally induced defects include multi-stage calcination, ozone oxidation, plasma-assisted calcination, rapid thermal processing (RTP), optimized rapid thermal processing (O-RTP), etc. In the RTP or O-RTP, a composite membrane is instantly heated by IR illumination to 600–900 °C without programmed heating for several minutes and then quickly cooled to room temperature to yield a zeolite membrane with far less inter-crystalline defects, thus improving separation performance as compared with membranes calcined using conventional ramp rates. In addition, post treatment of the synthesized zeolitic films can not only repair the film defects, but also improve the surface properties. For example, chemical vapor deposition (CVD) of silane compounds (usually tetraethoxysilane) enables plugging of nanometer-scale defects, but it is inefficient for big defects because a large quantity of silane compounds is required. Sol-gel or a polymeric solution (e.g., silicon rubbers, mainly polydimethylsiloxane (PDMS)) can also be used for plugging the defects of the membranes.
Over the years, some research groups have prepared membranes with oriented crystals for improving the molecular sieving effect. Taking a zeolitic membrane with an MFI-type topological structure (sinusoidal pore structure (a-axis orientation, 0.51 nm × 0.55 nm) and straight channels (b-axis orientation, 0.53 nm × 0.56 nm)) as an example, the pore structure within membrane varies greatly when crystals grow in different directions, which will have a significant impact on its mass transfer characteristics [12]. Compared to MFI zeolitic membranes with arbitrary orientation, the inter-crystalline defects within the membrane will be significantly reduced alongside the membrane thickness and mass transfer resistance when crystals grow perpendicular to the support surface in b-axis direction, which would play a key role in improving permeability and separation selectivity [13]. Another route explored for reducing defects is using very thin zeolite nanosheets during the seeding step. Two-dimensional zeolitic nanosheets have unique structural advantages in constructing high-performance oriented zeolitic membranes due to their nanoscale thickness and high aspect ratio. Dakhchoune et al. reported the fabrication of high-performance H2/CO2 separation by zeolitic membranes prepared by a reactive assembly of sodalite nanosheets that host hydrogen-sieving six-membered rings (6-MRs) of SiO4 tetrahedra, which effectively blocked CO2 transport and led to a H2/CO2 ideal selectivity of above 100 at 250–300 °C [14].
2.3. General Modification Strategies of Zeolite-Based Adsorbents and Membranes
Several common modification strategies for improving the adsorption and separation performance of zeolite-based adsorbents and membranes are as follows:
(1) Adjustment of Si/Al ratios. To promote gas adsorption and separation of zeolites/zeolitic membranes by adjusting Si/Al ratios, there are two primary aspects, namely, enhancing surface adsorption and internal diffusion. In terms of enhancing surface adsorption, the lower the Si/Al ratios are, the more charge-compensated the cations will be, which is more conducive to the adsorption of gas molecules with large dipole or quadrupole moments and can promote the separation of gas mixtures with large polarity differences (e.g., CO2/CH4); In terms of enhancing internal diffusion, the higher the Si/Al ratios, the less the cations, which can reduce the steric hindrance of internal diffusion and promote the separation of gas mixtures with large dynamic diameter differences (e.g., N2/CH4). The study by Sun et al. demonstrated that the combination of X zeolites with a lower Si/Al ratio and a lower number of binders is significantly effective in improving the adsorption and separation properties of X zeolites in purification for LNG production with low CO2 concentration in the feed gas and at high pressure [15]. Guo et al. successfully synthesized ZSM-11 zeolites with high Si/Al ratios (n(Si/Al) = 100, 500), the adsorption capacity of the two samples for CH4 was higher than that of N2, and the selectivity of CH4/N2 reached more than 4.0 at 25 °C and 500 kPa, which is much higher than that of commercial low-silica zeolites [16].
(2) Cation exchange. The exchange of various cations can not only change the adsorption behaviors of gas molecules on/in the surface/pore of zeolites/zeolitic membranes, but also affect the permeation behavior of different gases. The higher the basicity of the cations (low electronegativity), the stronger the binding force with weakly-acidic molecules; the higher the charge density of the cations, the stronger the electrostatic interaction with gases. In addition, the pore characteristics of zeolites will also change after cation exchange, thus affecting the internal diffusion of gas molecules. The Li+, K+, Mg2+ and Ca2+-exchanged X zeolites were prepared by Sun et al. as efficient adsorbents for purification of liquefied natural gas (LNG), and the interaction between CO2 and zeolites were reported to not only depend on the types of cations, but also on the amounts of cations, which significantly affected the adsorption at low pressure [17].
(3) Heteroatom substitution. Taking the substitution of Al by Ti as an example, the substitution of Al atom in the framework of zeolites by Ti atom can make the zeolites/zeolitic membranes have higher thermal stability, acidic stability and hydrophobicity, thus promoting the gas adsorption and separation under wet conditions. CHA-type titanosilicate (Ti-CHA) zeolite prepared by Araki et al. exhibited a relatively high CO2 permeance compared with that of previously reported CHA-type zeolite membranes, and the CO2 permeance and selectivity were only marginally reduced as a result of the highly hydrophobic pore structure in the presence of 1 vol.% H2O [18].
(4) Surface/pore modification. Based on the covalent bonding of Si-O-Si groups, specific functional organic groups (including amino, hydroxy, hydrophobic groups, etc.) are grafted on/in the surface or pore of zeolites/zeolitic membranes by covalent grafting method to enhance the surface/pore polarity or acid-base properties, further promoting adsorption and separation. The study by Ilyas et al. focused on achieving highly selective membranes for CO2 by chemically grafting 4A zeolites with methoxy groups containing cation and acetate anion based ionic liquid in order to enhance the CO2 solubility owing to the molecular interaction of CO2 with methoxy moieties and acetate as anion due to its hydrogen bond basicity [19].
(5) Advance techniques utilization. Other than commonly using chemical and/or physical modification methods, various advance techniques (i.e plasma technology, microwave, irradiation, ultrasonic, etc.) have also been proven to be used to make zeolitic modifications potentially for gas separation/adsorption. Wahono et al. prepared amine adsorbent through a simple plasma polymerization and deposition on physicochemically modified natural mordenite-clinoptilolite zeolite, resulting in a significant increase in surface area-weighted CO2 adsorption capacity [20]. Tang et al. reported that ultrasound-assisted method represents a rapid and controllable means of synthesizing nano/micro-scale zeolites with enhanced mass transfer and adsorption capacity of CO2, CH4, N2, and O2 [21]. The conjunction of e fastvolumetric microwave heating with a unique counter diffusion of metal and linker solutions by Hillman et al. enables the unprecedented rapid synthesis of wellintergrown ZIF-7-8 membranes with tunable molecular sieving properties, showing prospects for the commercial gas separation applications of ZIF membranes [22].
3. The Applications in Gas Separation and Purification
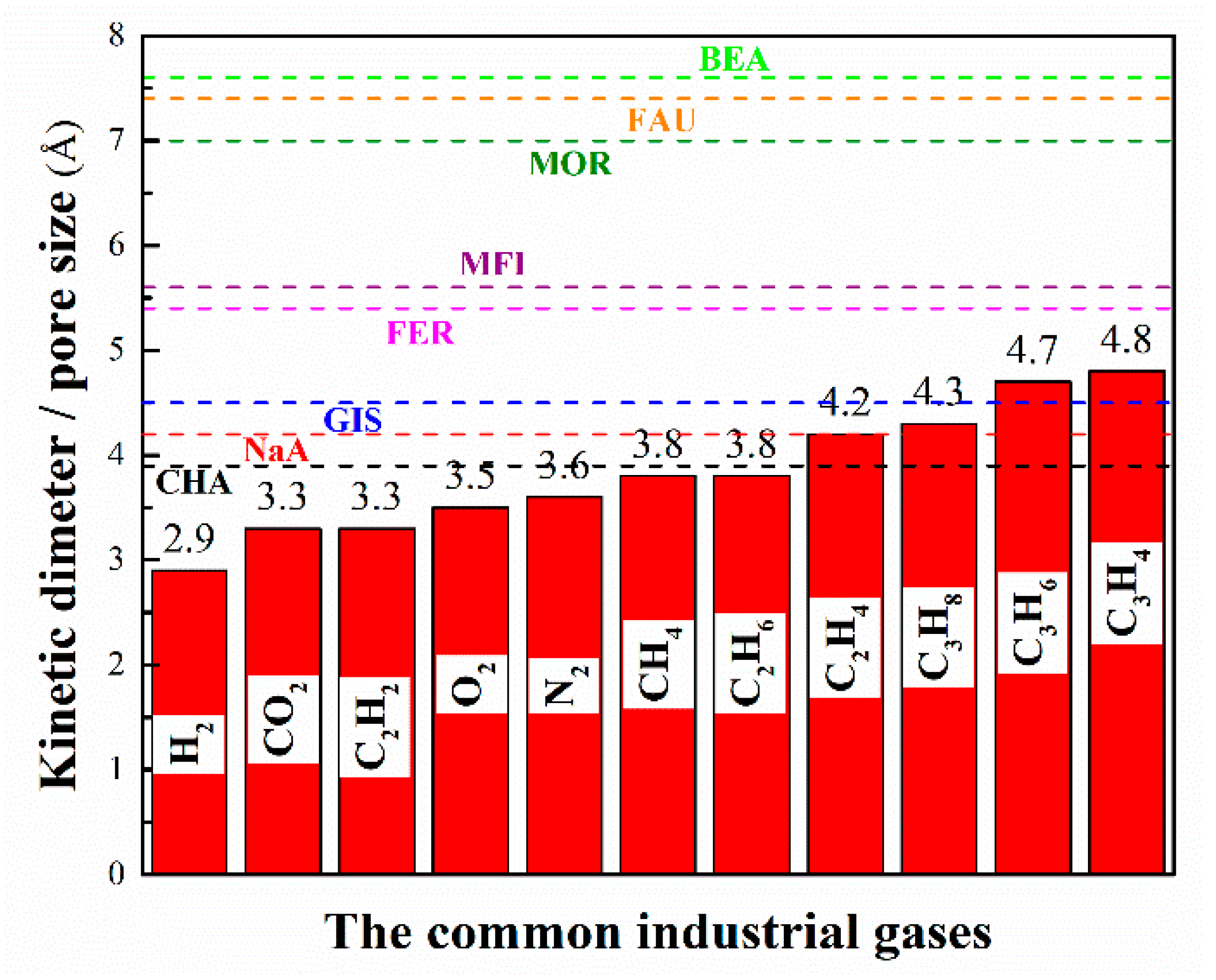
As a matter of fact, zeolites/zeolitic membranes have been employed in various separation processes for over two centuries and they are still the most important candidates for material-based separations. Generally, micropores play a decisive role in gas separation and adsorption, while mesopores and macropores mainly serve as transport channels for gas molecules. The comparison of pore size of typical zeolites with kinetic diameters of common industrial gases is shown in Figure 2.
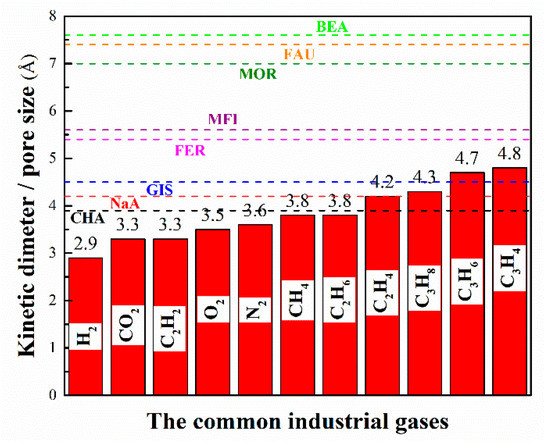
Figure 2.
The comparison of pore size of typical zeolites with kinetic diameters of common industrial gases.
3.1. Air Separation
Oxygen (O2) is not only known as the source of life, but also very important in industrial production and scientific research. Oxygen-enriched combustion is considered an effective method to improve energy efficiency, so O2/N2 separation is of great significance. N2 with a lone pair of electrons has a larger quadrupole moment compared to O2, inducing stronger interaction with cations in zeolites/zeolitic membranes and enriched O2 in outflow gases. Researchers have carried out a large amount of research on O2/N2 separation based on the interaction between N2 and zeolitic cations. In addition, the Si/Al ratio is also an important factor affecting O2/N2 adsorption and separation. Due to low Si/Al ratio of A-type and X-type zeolites with a large number of ion-exchange sites, research on O2/N2 separation mainly focuses on A- and X-type zeolites/zeolitic membranes (Table 1).

Table 1.
O2/N2 separation performances for various zeolites/zeolitic membranes.
3.2. Hydrogen Purifification
Hydrogen (H2), which is mainly generated from natural gas reforming, coal gasification and industrial by-products (e.g., coke oven gas (COG, 54–59% H2)), is widely used as an important industrial raw material and zero-emission energy resource, while attracting more and more attention for acquiring sufficient pure hydrogen in continuous long-term industrial reprocessing (hydrogen production, purification and separation). In Table 2, H2 separation performances for various zeolites/zeolitic membranes are given. Taken together, it is highly desired to prepare small pore zeolites/zeolitic membranes (e.g., SAPO-34) based on the view of size-exclusion.

Table 2.
H2 separation performances for various zeolites/zeolitic membranes.
3.3. Separation of Light Hydrocarbons
Light hydrocarbons are basic organic chemical raw materials, and their production capacity is an important standard to measure a country’s chemical level. Generally, light hydrocarbons are mostly obtained through naphtha cracking and methanol to olefins, among which there are many by-products (e.g., acetylene in trace amounts) seriously affecting their further processing and utilization. Therefore, the separation and purification of light hydrocarbons plays an important role in industry. As shown in Table 3, various zeolites/zeolitic membranes are constantly reported for light hydrocarbons separation in recent years, among which unsaturated hydrocarbon-selective adsorbents basically realized with thermodynamic, kinetic, equilibrium-kinetic synergetic, gate-opening, and molecular sieving separations are the most common. However, the development of alkane-selective adsorbents is highly desired for industrial purification of unsaturated hydrocarbons in an effort to simplify the separation process and save energy consumption.

Table 3.
Separation performances of light hydrocarbons for various zeolites/zeolitic membranes.
3.4. Capture and Separation of Carbon Dioxide
Carbon dioxide (CO2) is a common gas that is also a major greenhouse gas. The large amount of CO2 released into the atmosphere brings about more than 60% of global warming changes. About 80% this carbon dioxide comes from fuel combustion, industrial processes and biogas/natural/shale gas processing. Capturing and separating CO2 effectively from flue gas is of great importance to mitigate climate change resulting from greenhouse gas emissions, which has attracted wide attention all over the world. Table 4 lists the CO2 separation performances for various zeolites/zeolitic membranes. In the adsorption process, high adsorption capacity and selectivity are two key parameters for effective removal of carbon dioxide from gas stream. While in membrane separation, size screening plays a major role, which allows the passing of CO2 and exclude other components from the mixtures to be separated, and kinetic separation based on differences in the diffusion rates also accounts for a portion. Generally, introduction of amine containing groups including ionic liquids to enhance CO2 affinity is usually adopted.

Table 4.
CO2 separation performances for various zeolites/zeolitic membranes.
3.5. Denitration for Flue Gas Purification
Nitrogen oxides (NOx) exhausted from stationary engines (thermal power, cement, and steel industries, etc.) are one of the major sources of air contamination. At present, selective catalytic reduction of NOx (NH3-SCR) technology using NH3 as a reductant is considered to be the most effective NOx purification method, and catalyst is the key of NH3-SCR process. Among various catalysts, zeolites with large specific area, strong gas adsorption on abundant acid sites, wide temperature window, high thermal stability and good SO2 durability play an indispensable role as efficient and stable catalysts in the petrochemical, energy and environmental fields. As shown in Table 5, the NH3-SCR performances of various zeolites/zeolitic membranes are listed. Nowadays, the most studied denitration catalysts are Fe, Cu zeolites, Mn, Ce zeolites and ZSM-5, BEA, SAPO-n, SSZ-13 topological zeolites are also the current research hotspots due to their excellent catalytic activity, selectivity and hydrothermal stability.

Table 5.
NH3-SCR performances for NOx removal by various zeolites/zeolitic membranes.
3.6. Water Vapor Adsorption
The water adsorption/moisture removal is essential in many aspectss (e.g., adsorption heat pumps (AHPs), electric dehumidifiers, dehydration of organic solvents and gaseous industrial streams including hydrogen and natural gas), even in the storage of processed foods and moisture-sensitive materials. As shown in Table 6, water vapor adsorption of various zeolites/zeolitic membranes are displayed. On the whole, water vapor adsorption on zeolitic materials dominantly depends on specific surface area and the hydrophilicity/hydrophobicity which directly dependent on the Si/Al ratio and surface hydroxyl groups. Lower Si/Al ratio and higher surface hydroxyl groups can render the surface of zeolites to be hydrophilic and vice versa.

Table 6.
Water vapor adsorption by various zeolites/zeolitic membranes.
4. Separation Mechanisms
The gas separation with zeolites/zeolitic membranes is generally divided into two processes: surface adsorption and pore diffusion. Firstly, gas molecules are adsorbed on the surface of zeolites/zeolitic membranes; then, under the action of chemical potential, adsorbed molecules transition from one adsorption site to the next adsorption site or vacancy, thereby entering the zeolite channel and diffusing to the permeation side. The study of separation rules helps to determine process conditions in practical applications, while the separation mechanisms study helps to explain the differences generated by different gas separation on various zeolites/zeolitic membranes, thereby selectively selecting separation objects and types of zeolites/zeolitic membranes. The following is a brief overview of several common separation mechanisms for zeolites/zeolitic membranes:
Molecular sieving effect, also known as the steric hindrance effect, is the most common and easily-understood mechanism in adsorption and separation. It involves using the difference of kinetic diameters to achieve screening through regular pore structures at the level of kinetic diameters [58]. Two major categories in this method can be divided: one is that gas molecules with kinetic diameters smaller than the pore size of zeolites/zeolitic membranes can diffuse into the channels, while larger gas molecules are excluded; the other is that the separation of gas molecules with kinetic diameters smaller than the pore size of zeolites/zeolitic membranes may be related not only to the kinetic diameter but also to the shape and size of molecules.
For two gases with a very small size difference, the energy potential barriers of the two gases when passing through the pore of zeolites/zeolitic membranes may differ greatly due to the different diffusion rates, which is called “kinetic effect” [59]. Usually, diffusion selectivity takes place when molecules of one component are smaller and their diffusivity in zeolite micropores is much faster than that of the larger component (e.g., H2/CH4 in many 8MR zeolite membranes). Moreover the contribution of diffusion selectivity increases with temperature.
Guest-host interactions with different adsorption strengths, including the well-known physisorption (van der Waals interaction and electrostatic interaction) and chemisorption (electron transfer, exchange or sharing between the adsorbates and adsorbents, irreversible) have also been adopted to achieve separation. Generally, the highly-polarized pore environments originating from the local electric field make zeolites/zeolitic membranes preferentially adsorb molecules with greater quadruple and dipole moments, thereby achieving adsorptive separations. However, powerful and irreversible chemisorption does not meet the requirements of adsorption and separation, while weak chemical bonds stronger than van der Waals interactions but still in the range of reversible adsorption are also popular in adsorption and separation. Specifically, Π-complexation stronger than van der Waals can be formed among molecules with Π-electrons and adsorbents or membranes containing transition metals, resulting in higher adsorption selectivity, and is more suitable for gas purification and separation with low target gas concentrations [60].
Interestingly, the extraframework cations in zeolites/zeolitic membranes can be induced temporarily and reversibly deviating from the intrinsic position of channels by some specific guest molecules which are subsequently allowed to enter the pores of zeolites/zeolitic membranes, and the according phenomenon is called “molecular trapdoor effect” [61].
Different from molecular trapdoor effect, “breathing effect” is generally caused by the flexibility of zeolite framework, which has been frequently reported in MOFs [62]. The adsorbent hardly adsorbs the adsorbate or the adsorption amount is very low at low pressures, but specific guest molecules can enter the interior as the pressure increases to a certain extent, resulting in a rapid increase in adsorption capacity. Distinctively, the adsorption isotherm of “molecular trapdoor effect” shows a typical type-I isotherm, while for “breathing effect”, a rapid upward trend can be viewed after breaking a certain pressure.
When the difference between the size of gas molecules and the micropore diameter of zeolites/zeolitic membranes is comparable with the de Broglie wavelength of gas molecules, an uncertain quantum effect occurs at low temperatures, leading to different diffusion rates of molecules [63]. At present, hydrogen isotope separation has already been achieved based on “quantum effect”.
In actual adsorption and separation, the above mechanisms are often not separate existence. Compatibility of multiple mechanisms in one class of adsorption or membrane materials can make the adsorbents or membranes have better adsorption capacity and selectivity, but it also adds difficulty to the study of adsorption and separation mechanisms.
5. Challenges and Future Perspectives
A good adsorbent or membrane for separation should not only have high selectivity, but also have excellent adsorption capacity or permeability and mass transfer rate. The selectivity determines whether the adsorbent or membrane can effectively separate gases, while the adsorption capacity or permeability and mass transfer rate determine the capacity to handle gases. In a general sense, homogeneous and thin films are more favorable to harvest both high flux and high separation selectivity. The ordered microporous structure in molecular scale qualifies zeolites with valuable characteristics in dealing with small molecules and great potential for gas separations thereof. So far, zeolites/zeolitic membranes have been successfully adopted as promising candidates in various separation and purification processes like air separation, hydrogen purification, separation of light hydrocarbons, CO2 capture and separation as well as gas drying. Zeolite separations, including membrane separation and adsorptive separation, have become a research hotspot with a bright industrial future.
At present, the research on zeolite-based adsorbents and membranes for gas separation and purification is generally developing in the direction of high selectivity, high adsorption capacity or high permeability. Although certain progress has been made and great application prospects have been shown, it still faces many difficulties and challenges. The research interests and developing trends of zeolites/zeolitic membranes in the application of gas separation and purification are prospected as follows:
- (1)
- Small-pore zeolites with pore size close to the kinetic diameter of gas molecules will still be research hotspots;
- (2)
- Research on hierarchical porous zeolites whereby the introduction of mesopores can significantly improve the mass transfer rate and adsorption capacity;
- (3)
- Preparation of new zeolites and zeolitic membranes;
- (4)
- Simplifying the preparation methods, enhancing the repeatability, reducing the preparation cost and realizing large-scale production are the common problems faced by zeolite-based adsorbents and membranes.
Author Contributions
Conceptualization, K.Q. and F.H.; methodology, K.Q.; software, K.Q.; validation, L.G., X.L. and F.H.; formal analysis, K.Q.; investigation, K.Q.; resources, K.Q.; data curation, K.Q.; writing—original draft preparation, K.Q.; writing—review and editing, L.G.; visualization, X.L.; supervision, F.H.; project administration, K.Q.; funding acquisition, K.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Fundamental Research Program of Shanxi Province (20210302124003)”.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, X.Q.; Chang, Z.Y.; Li, L.B.; Yang, J.F.; Li, J.P. Progress in metal-organic frameworks for efficient separation of gaseous light hydrocarbon. Chem. Ind. Eng. Prog. 2020, 39, 2218–2234. [Google Scholar]
- Zhao, X.; Wang, Y.; Li, D.S.; Bu, X.; Feng, P. Metal-organic frameworks for separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef] [PubMed]
- Burton, A. Recent trends in the synthesis of high-silica zeolites. Catal. Rev.-Sci. Eng. 2018, 60, 132–175. [Google Scholar] [CrossRef]
- Algieri, C.; Barbieri, G.; Drioli, E. Zeolite membranes for gas separation. In Membrane Engineering for the Treatment of Gases: Gas-Separation Problems Combined with Membrane Reactors; Drioli, E., Barbieri, G., Eds.; Royal Society of Chemistry: London, UK, 2011; Volume 2, pp. 223–252. [Google Scholar]
- Feng, C.; Khulbe, K.C.; Matsuura, T.; Farnood, R.; Ismail, A. Recent progress in zeolite/zeotype membranes. J. Membr. Sci. Res. 2015, 1, 49–72. [Google Scholar]
- Wang, S.B. Utilisation of natural zeolites for air separation and pollution control. In Handbook of Natural Zeolites; Bentham Science Publishers: Soest, The Netherlands, 2012; Volume 481, pp. 569–587. [Google Scholar]
- Li, K.; Cheng, H.F. Progress in synthesis and application of zeolite molecular sieves. China Non-Met. Miner. Ind. 2019, 3, 38–41. [Google Scholar]
- Chen, Y.H.; Ren, T.H.; Liu, G.Q.; Liu, X.X.; Ni, X.; Yin, X.L.; Li, G.Z. Development of green routes for synthesis of zeolite. Ind. Catal. 2020, 28, 17–21. [Google Scholar]
- Wu, C.Y.; Wang, Z.W.; Zhao, Y.; Zhang, H.D.; Cheng, P. Research progress of green and efficient synthesis of zeolite. Chem. Reag. 2022, 44, 1543–1550. [Google Scholar]
- Algieri, C.; Drioli, E. Zeolite membranes: Synthesis and applications. Sep. Purif. Technol. 2022, 278, 119295. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, C.M.; Zhu, L.; Wang, W.L.; Li, Z.Y.; Bai, Y. Recent synthesis methods of zeolite membranes. Membr. Sci. Technol. 2020, 40, 145–150. [Google Scholar]
- Lai, Z.; Tsapatsis, M.; Nicolich, J.P. Siliceous ZSM-5 membranes by secondary growth of b-oriented seed layers. Adv. Funct. Mater. 2004, 14, 716–729. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Z.B. Zeolite MFI membranes for separation of ethanol/water mixture. Prog. Chem. 2013, 25, 2178–2188. [Google Scholar]
- Dakhchoune, M.; Villalobos, L.F.; Semino, R.; Liu, L.M.; Rezaei, M.; Schouwink, P.; Avalos, C.E.; Baade, P.; Wood, V.; Han, Y. Gas-sieving zeolitic membranes fabricated by condensation of precursor nanosheets. Nat. Mater. 2021, 20, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.B.; Tang, J.F.; Li, G.Y.; Hua, Y.H.; Li, H.; Hu, S.Y. Experimental investigation of adsorption and CO2/CH4 separation properties of 13X and JLOX-500 zeolites during the purification of liquefied natural gas. ACS Omega 2022, 7, 18542–18551. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.J.; Li, Y.; Li, S.S.; Wu, X.L.; Tan, J.C.; Li, W.; Yang, J.F.; Li, J.P. Study on adsorption and separation performance of CH4/N2 by high-silica ZSM-11 zeolite. Nat. Gas Chem. Ind. 2022, 47, 67–72. [Google Scholar]
- Sun, Y.B.; Tang, J.F.; Li, G.Y.; Hua, Y.H.; Sun, Y.B.; Hu, S.Y.; Wen, X.J. Adsorption, separation and regeneration of cation-exchanged X zeolites for LNG purification: Li+, K+, Mg2+ and Ca2+. Microp. Mesopor. Mat. 2022, 340, 112032. [Google Scholar] [CrossRef]
- Araki, S.; Ishii, H.; Imasaka, S.; Yamamoto, H. Synthesis and gas permeation properties of chabazite-type titanosilicate membranes synthesized using nano-sized seed crystals. Microp. Mesopor. Mat. 2020, 292, 109798. [Google Scholar] [CrossRef]
- Ilyas, A.; Muhammad, N.; Gilani, M.A.; Vankelecom, I.F.J.; Khan, A.L. Effect of zeolite surface modification with ionic liquid [APTMS][Ac] on gas separation performance of mixed matrix membranes. Sep. Purif. Technol. 2018, 205, 176–183. [Google Scholar] [CrossRef]
- Wahono, S.K.; Dwiatmoko, A.A.; Cavallaro, A.; Indirathankam, S.C.; Addai-Mensah, J.; Skinner, W.; Vinu, A.; Vasilev, K. Amine-functionalized natural zeolites prepared through plasma polymerization for enhanced carbon dioxide adsorption. Plasma Process. Polym. 2021, 18, 2100028. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Shang, H.; Wu, L.; Yang, J. Gas diffusion and adsorption capacity enhancement via ultrasonic pretreatment for hydrothermal synthesis of K-KFI zeolite with nano/micro-scale crystals. Micropor. Mesopor. Mater. 2020, 297, 110036. [Google Scholar] [CrossRef]
- Hillman, F.; Brito, J.; Jeong, H.K. Rapid one-pot microwave synthesis of mixed-linker hybrid zeolitic-imidazolate framework membranes for tunable gas separations. ACS Appl. Mater. Interfaces 2018, 10, 5586–5593. [Google Scholar] [CrossRef]
- Prajapati, P.K.; Kansara, A.M.; Aswal, V.K.; Singh, P.S. High oxygen permeable Zeolite-4A poly(dimethylsiloxane) membrane for air separation. J. Appl. Polym. Sci. 2019, 136, 48047. [Google Scholar] [CrossRef]
- Liu, H.B.; Yuan, D.H.; Yang, L.P.; Xing, J.C.; Zeng, S.; Xu, S.T.; Xu, Y.P.; Liu, Z.M. Directly decorated CeY zeolite for O2-selective adsorption in O2/N2 separation at ambient temperature. Mater. Horiz. 2022, 9, 688–693. [Google Scholar] [CrossRef]
- Xia, H.Y.; Hu, Y.F.; Bao, Q.; Zhang, J.; Sun, P.L.; Liang, D.; Wang, B.X.; Qiao, X.; Wang, X.Y. Adsorption separation of O2/N2 by Li-RHO zeolite with high oxygen selectivity. Micropor. Mesopor. Mater. 2023, 350, 112442. [Google Scholar] [CrossRef]
- Jiang, M.M.; Zhu, M.F.; Deng, C.; Zhao, L.; Ma, J.; Shi, M.S.; Gao, W.Y. Modification of LiX zeolite molecular sieve via cerium ions. Appl. Chem. Ind. 2017, 46, 332–334, 346. [Google Scholar]
- Marani, H.T.; Sadeghi, M.; Moheb, A.; Esfahani, E.N. Optimization of the gas separation performance of polyurethane-zeolite 3A and ZSM-5 mixed matrix membranes using response surface methodology. Chin. J. Chem. Eng. 2019, 927, 110–129. [Google Scholar]
- Hu, Y.D. Fabrication and Properties of NaA Zeolite Membranes on Porous Si3N4 Supports. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2021. [Google Scholar]
- Wang, B.; Zheng, Y.H.; Zhang, J.F.; Zhang, W.J.; Zhang, F.; Xing, W.H.; Zhou, R.F. Separation of light gas mixtures using zeolite SSZ-13 membranes. Micropor. Mesopor. Mater. 2019, 275, 191–199. [Google Scholar] [CrossRef]
- Kida, K.; Maeta, Y.; Yogo, K. Preparation and gas permeation properties on pure silica CHA-type zeolite membranes. J. Membr. Sci. 2017, 522, 363–370. [Google Scholar] [CrossRef]
- Xu, C.; Wei, W.C.; He, Y. Enhanced hydrogen separation performance of Linde Type-A zeolite molecular sieving membrane by cesium ion exchange. Mater. Lett. 2022, 324, 132680. [Google Scholar] [CrossRef]
- Zhou, T.; Shi, M.Y.; Chen, L.J.; Gong, C.; Zhang, P.; Xie, J.X.; Wang, X.R.; Gu, X.H. Fluorine-free synthesis of all-silica STT zeolite membranes for H2/CH4 separation. Chem. Eng. J. 2022, 433, 133567. [Google Scholar] [CrossRef]
- Wang, Z.G.; Xu, J.; Pati, S.; Chen, T.J.; Deng, Y.Z.; Dewangan, N.; Meng, L.; Lin, J.Y.S.; Kawi, S. High H2 permeable SAPO-34 hollow fiber membrane for high temperature propane dehydrogenation application. AIChE J. 2020, 66, e16278. [Google Scholar] [CrossRef]
- Mei, W.L.; Du, Y.; Wu, T.Y.; Gao, F.; Wang, B.; Duan, J.G.; Zhou, J.J.; Zhou, R.F. High-flux CHA zeolite membranes for H2 separations. J. Membr. Sci. 2018, 565, 358–369. [Google Scholar] [CrossRef]
- Shao, G.Q.; Zhu, H.; Ma, W.; Yan, P.; Ma, J.L. Synthesis of high-performance AlPO4-14 zeolite membranes for gas separation. Chem. J. Chin. Univ. 2019, 40, 2265–2273. [Google Scholar]
- Ünügül, T.; Nigiz, F.U. Hydrogen purification using natural zeolite-loaded hydroxyethyl cellulose membrane. Int. J. Energ. Res. 2022, 46, 1826–1836. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Hu, N.; Wang, H.M.; Bu, N.; Zhang, F.; Zhou, R.F. Preparation of steam-stable high-silica CHA (SSZ-13) membranes for CO2/CH4 and C2H4/C2H6 separation. J. Membr. Sci. 2015, 475, 303–310. [Google Scholar] [CrossRef]
- Yang, J.F.; Liu, J.Q.; Liu, P.X.; Li, L.B.; Tang, X.; Shang, H.; Li, J.P.; Chen, B.L. K-Chabazite zeolite nanocrystal aggregates for highly efficient methane separation. Angew. Chem. Int. Ed. 2022, 61, e202116850. [Google Scholar]
- Chai, Y.C.; Han, X.; Li, W.Y.; Liu, S.S.; Yao, S.K.; Wang, C.; Shi, W.; Da-silva, I.; Manuel, P.; Cheng, Y.Q.; et al. Control of zeolite pore interior for chemoselective alkyne/olefin separations. Science 2020, 368, 1002–1006. [Google Scholar] [CrossRef]
- Kencana, K.S.; Min, J.G.; Kemp, K.C.; Hong, S.B. Nanocrystalline Ag-ZK-5 zeolite for selective CH4/N2 separation. Sep. Purif. Technol. 2022, 282, 120027. [Google Scholar] [CrossRef]
- Qiu, H.E.; Zhang, Y.; Kong, L.; Kong, X.; Tang, X.X.; Meng, D.N.; Xu, N.; Wang, M.Q.; Zhang, Y.F. High performance SSZ-13 membranes prepared at low temperature. J. Membr. Sci. 2020, 603, 118023. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.Q.; Qiu, H.E.; Kong, L.; Xu, N.; Tang, X.X.; Meng, D.N.; Kong, X.; Zhang, Y.F. Synthesis of thin SAPO-34 zeolite membranes in concentrated gel. J. Membr. Sci. 2020, 603, 118451. [Google Scholar] [CrossRef]
- Imasaka, S.; Itakura, M.; Yano, K.; Fujita, S.; Okada, M.; Hasegawa, Y.; Abe, C.; Araki, S.; Yamamoto, H. Rapid preparation of high-silica CHA-type zeolite membranes and their separation properties. Sep. Purif. Technol. 2018, 199, 298–303. [Google Scholar] [CrossRef]
- Mendes, P.A.P.; Ribeiro, A.M.; Gleichmann, K.; Ferreira, A.F.P.; Rodrigues, A.E. Separation of CO2/N2 on binderless 5A zeolite. J. CO2 Util. 2017, 20, 224–233. [Google Scholar] [CrossRef]
- Wilson, S.M.W. High purity CO2 from direct air capture using a single TVSA cycle with Na-X zeolites. Sep. Purif. Technol. 2022, 294, 121186. [Google Scholar] [CrossRef]
- Castruita-de, L.G.; Yeverino-Miranda, C.Y.; Montes-Luna, A.D.J.; Meléndez-Ortiz, H.I.; Alvarado-Tenorio, G.; García-Cerda, L.A. Amine-impregnated natural zeolite as filler in mixed matrix membranes for CO2/CH4 separation. J. Appl. Polym. Sci. 2020, 137, 48286. [Google Scholar]
- Wahono, S.K.; Stalin, J.; Addai-Mensah, J.; Skinner, W.; Vinu, A.; Vasilev, K. Physicochemical modification of natural mordenite-clinoptilolite zeolites and their enhanced CO2 adsorption capacity. Micropor. Mesopor. Mater. 2020, 294, 109871. [Google Scholar] [CrossRef]
- Tarach, K.A.; Jabłońska, M.; Pyra, K.; Liebau, M.; Reiprich, B.; Gläser, R.; Góra-Marek, K. Effect of zeolite topology on NH3-SCR activity and stability of Cu-exchanged zeolites. Appl. Catal. B-Environ. 2021, 284, 119752. [Google Scholar] [CrossRef]
- Wei, Y.Z.; Chen, M.Y.; Ren, X.Y.; Wang, Q.F.; Han, J.F.; Wu, W.Z.; Yang, X.G.; Wang, S.; Yu, J.H. One-pot three-dimensional printing robust self-supporting MnOx/Cu-SSZ-13 zeolite monolithic catalysts for NH3-SCR. CCS Chem. 2022, 4, 1708–1719. [Google Scholar] [CrossRef]
- Wan, J.; Chen, J.W.; Zhao, R.; Zhou, R.X. One-pot synthesis of Fe/Cu-SSZ-13 catalyst and its highly efficient performance for the selective catalytic reduction of nitrogen oxide with ammonia. J. Environ. Sci. 2021, 100, 306–316. [Google Scholar] [CrossRef]
- Xiao, Y.D.; Jia, X.H.; He, D.; Huang, B.C.; Yang, Y.X.; Zhang, Y.; Lu, M.J.; Tu, X.; Yu, C.L. Controllable synthesis, characterization and low-temperature NH3-SCR activity of FeSAPO-34 and CuFeSAPO-34 molecular sieves. Acta Sci. Circumstantiae 2022, 42, 357–368. [Google Scholar]
- Qi, K.; Yi, Q.; Fang, D.; Gong, P.J.; Shi, L.J.; Gao, L.L.; Li, X.L.; He, F.; Xie, J.L. Temperature dependence of reaction mechanisms and SO2 tolerance over a promising monolithic CuY catalyst for NO removal. Appl. Surf. Sci. 2023, 615, 156473. [Google Scholar] [CrossRef]
- Chen, J.H.; Pan, B.; Wang, B.; Ling, Y.J.; Fu, K.; Zhou, R.F.; Zhong, Z.X.; Xing, W.H. Hydrothermal synthesis of a Pt/SAPO-34@SiC catalytic membrane for the simultaneous removal of NO and particulate matter. Ind. Eng. Chem. Res. 2020, 59, 4302–4312. [Google Scholar] [CrossRef]
- Feng, S.S.; Zhou, M.D.; Han, F.; Zhong, Z.X.; Xing, W.H. A bifunctional MnOx@PTFE catalytic membrane for efficient low temperature NOx-SCR and dust removal. Chin. J. Chem. Eng. 2020, 28, 1260–1267. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Kumar-Reddy, K.S.; Mittal, H.; Al-Wahedi, Y.; Vaithilingam, B.V.; Karanikolos, G.N.; Singaravel, G.; Morin, S.; Berthod, M.; Alhassan, S.M. Water vapor adsorption on metal-exchanged hierarchical porous zeolite-Y. Micropor. Mesopor. Mater. 2021, 326, 111380. [Google Scholar] [CrossRef]
- Wahono, S.K.; Suwanto, A.; Prasetyo, D.J.; Jatmiko, T.H.; Vasilev, K. Plasma activation on natural mordenite-clinoptilolite zeolite for water vapor adsorption enhancement. Appl. Surf. Sci. 2019, 483, 940–946. [Google Scholar] [CrossRef]
- Henao-Sierra, W.; Romero-Sáez, M.; Gracia, F.; Cacua, K.; Buitrago-Sierra, R. Water vapor adsorption performance of Ag and Ni modified 5A zeolite. Micropor. Mesopor. Mater. 2018, 265, 250–257. [Google Scholar] [CrossRef]
- Lin, J.Y.S. Molecular sieves for gas separation. Science 2016, 353, 8–10. [Google Scholar] [CrossRef]
- Li, K.; Olson, D.H.; Seidel, J.; Emge, T.J.; Gong, H.; Zeng, H.; Li, J. Zeolitic imidazolate frameworks for kinetic separation of propane and propene. J. Am. Chem. Soc. 2009, 131, 10368–10369. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.Q.; Wang, S.H. Selective adsorption of CO on CuCl/Y adsorbent prepared using CuCl2 as precursor: Equilibrium and thermodynamics. Chem. Eng. J. 2016, 290, 418–427. [Google Scholar] [CrossRef]
- Shang, J.; Li, G.; Gu, Q.F.; Singh, R.; Xiao, P.; Liu, J.Z.; Webley, P.A. Temperature controlled invertible selectivity for adsorption of N2 and CH4 by molecular trapdoor chabazites. Chem. Commun. 2014, 50, 4544–4546. [Google Scholar] [CrossRef]
- Georgieva, V.M.; Bruce, E.L.; Verbraeken, M.C.; Scott, A.R.; Casteel, W.J.; Brandani, S.; Wright, P.A. Triggered gate opening and breathing effects during selective CO2 adsorption by merlinoite zeolite. J. Am. Chem. Soc. 2019, 141, 12744–12759. [Google Scholar] [CrossRef]
- Salazar, J.M.; Lectez, S.; Gauvin, C.; Macaud, M.; Bellat, J.P.; Weber, G.; Bezverkhyy, I.; Simon, J.M. Adsorption of hydrogen isotopes in the zeolite NaX: Experiments and simulations. Int. J. Hydrog. Energ. 2017, 42, 13099–13110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).