Abstract
Lignin obtained by hydrogenolysis of lignocellulose biomass is a prospective source of valuable green fuels and chemicals such as monophenols. One of the key factors in the chemical decomposition of lignin to monophenols is an efficient catalyst. Inert porous materials such as hypercrosslinked polymers are suitable catalytic supports for the immobilization of noble and transition metal nanoparticles. However, such polymers do not have acidic properties, which are crucial for catalyzing hydrolysis. In this work, we report novel, efficient catalysts for lignin hydrogenolysis to produce valuable monophenolic compounds. The synthesized catalysts contained Ni, Ru, and Ni–Ru nanoparticles supported on SiO2-coated hypercrosslinked polystyrene (SiO2@HPS). Ni-Ru/SiO2@HPS demonstrated remarkable stability without any loss of the metallic phase and a high yield of monophenols (>42 wt.%) at close to full lignin conversion (>95 wt.%). This result was attributed to the synergy between the two metals and the support’s surface acidity. All catalysts were fully characterized by a series of physico-chemical methods.
1. Introduction
Lignin is considered as a prospective green source to produce valuable aromatic and phenolic compounds [1,2,3]. Lignin’s structure mainly contains covalently bonded phenyl-propanoid units [4,5,6]. Selective cleavage of the C–C and C–O bonds in the lignin polymeric network is one of the ways to obtain aromatic and oxygen-containing monomers, such as phenol, benzene, anisole, guaiacol, syringol, eugenol, p-ethylphenol, vanillin, etc. [7,8,9,10,11].
Nowadays, different techniques for lignin depolymerization are used. Among them are acid-catalyzed, base-catalyzed and oxidative depolymerization; pyrolysis; and hydrogenolysis [9,12,13,14,15,16,17,18,19]. Hydrogenolysis seems to be one of the most promising methods for lignin liquefaction [20]. The choice of a proper solvent is one of the main challenges for hydrogenolysis. The solvent should dissolve both lignin and the depolymerization products while preventing condensation reactions which usually lead to coke formation [21,22,23,24,25]. Water and alcohols are the most frequently used solvents for lignin hydrogenolysis [26]. On one hand, water is cheap and non-toxic, attaining over 80% lignin conversion under mild conditions [27]. However, poor lignin and hydrogen solubility in water require the use of either alkaline or acidic additives and also put certain restrictions on the catalyst design [28]. Thus, alcohols are the solvents of choice in lignin hydrogenolysis, which efficiently dissolve lignin and depolymerization products, inhibit char deposition, and provide in situ hydrogen formation [29,30]. Isopropanol is most frequently used for lignin depolymerization compared to primary alcohols because it has a higher hydrogen supply capacity [31,32,33,34,35].
The catalyst is one of the most important factors in lignin hydrogenolysis. It controls the lignin conversion, product yield, and product nature. Metals and their binary compounds have been reported to be effective catalysts. Different metals such as Ru, Rh, Cu, Ni, Pt, and Pd are able to promote the cleavage of different C–O bonds in lignin, resulting in up to a 60 wt.% yield of monomers [36,37,38,39,40,41,42,43,44,45]. For example, highly dispersed Ni allowed up to a 15 wt.% monophenol yield and a high lignin conversion (over 93 wt.%) [46]. However, Ni also tends to catalyze the hydrodeoxygenation of phenols, leading to the formation of aromatic compounds [47]. Pt exhibits a high activity in dehydrogenation reactions, resulting in about a 17 wt.% monophenol yield [48]. Pd is known to effectively catalyze C–O cleavage, resulting in the formation of aromatics [49]. Ru shows a moderate activity in lignin depolymerization but exhibits a high selectivity to monophenols and aromatics due to its defect-rich structure [50,51].
From mechanistic insights, lignin hydrogenolysis into aromatic and phenolic monomers mainly proceeds through the homolytic cleavage of Cα–O, O–CH3, and Cα–Cβ bonds [52,53] or through a pyrolysis mechanism via breaking of β-O-4, β-1, and β-5 bonds [54,55,56]. It was concluded that the homolytic cleavage of C–O bonds is more preferable because of the lower dissociation energy according to DFT calculations [57,58]. The homolytic mechanism depends on the presence of hydrogen, the catalyst’s nature, and the use of hydrogen donor solvents. For example, in an inert solvent, H2 dissociation plays an important role in C–O cleavage [59,60]. The alcohols used as solvents easily form H species on the catalyst surface due to dehydrogenation processes [60,61]. Catalysts containing a metallic active phase are suitable in both cases because of their activity in hydrogen dissociation and solvent dehydration.
The catalytic support also plays an important role in depolymerization processes. Strong Brønsted and Lewis acid sites on the surface of the support promote the activation of C–O bonds and facilitate their cleavage. Acid sites also influence the electron state of the active metal phase through metal–support interactions [62,63,64,65,66,67]. In particular, Ni, Pd, and Ru have been reported to have strong interactions with acidic supports, resulting in a high number of electron-rich sites and a defect-rich crystalline structure [68,69,70]. Moreover, the acidic support can serve as a structural and energetic promoter, facilitating the reduction of the metal active phase [71,72].
Inert porous materials such as hypercrosslinked polymers are promising catalytic supports for the immobilization of noble metals, transition metals, and metal nano-particles. In our recent work, it was shown that the aromatic polymeric matrix can effectively stabilize the metal particles in the pores, providing high stability against aggregation [73,74,75]. However, such polymers do not have acidic sites, which are crucial for hydrogenolysis. Recently, we found that Ni nanoparticles supported on polymers modified by SiO2 effectively catalyze the conversion of polyaromatic compounds into mono-aromatics through the hydrogenolysis of C–C bonds [76].
In this work, we report for the first time the structural properties of Ni, Ru, and bimetallic Ni–Ru nanoparticles supported on hypercrosslinked polystyrene coated by SiO2 (SiO2@HPS) and their catalytic behavior in lignin depolymerization to phenolic monomers. Newly developed Ni-Ru/SiO2@HPS demonstrated a remarkable stability without any loss of the metallic phase and a high yield of monophenols (42 wt.%) at close to full lignin conversion (up to 95%) under optimized reaction conditions.
2. Results and Discussion
2.1. Catalyst Characterisation
To study the structure and morphology of the synthesized SiO2@HPS support, low-temperature nitrogen physisorption (BET), X-ray photoelectron spectroscopy (XPS), and X-ray powder diffraction (XRD) was carried out.
During preparation of the SiO2@HPS sample, APTES is hydrolyzed by the subcritical water, leading to the formation of silica gel on the polymer surface. Moreover, some covalent and van der Waals interactions between the tert-amino groups of HPS and APTES can also take place, as shown previously [77]. The results of the low-temperature nitrogen physisorption showed that the deposition of the silicon-containing phase on the surface of HPS leads to a decrease in the total specific surface area and the surface area of micropores by about 200 m2/g compared to the as-synthesized sample. This is due to the partial HPS pore blockage while using the APTES as a surface modifier [78]. Moreover, during SiO2@HPS synthesis, APTES partially undergoes hydrolysis with water, forming an amorphous polysiloxane, as already reported [77,79]. Heating under a nitrogen flow, in contrast, increases the surface area, particularly by increasing the mesopores. Such an increase can be correlated with the formation of a SiO2 phase during the calcination and additional decomposition of non-hydrolyzed APTES (see Table 1). The formation of xerogel-like structures with an additional porosity on the inert support while using ethoxysilane as a modifier has also been discussed elsewhere [76,80].

Table 1.
BET study of supports.
To analyze the changes in the textural properties of the sample after heating, a comparison of the nitrogen adsorption–desorption isotherms was carried out (Figure 1). The presented isotherms can be associated with the Via type [81] for both samples. An increase in the adsorbed nitrogen volume can be observed at high Ps/P0 ratios. This is correlated with an increase in the capillary absorption process in pores with a diameter of 6–8 nm. For the sample obtained after heating at 300 °C, the hysteresis loop is more significant, indicating the intensification of capillary condensation in mesopores.
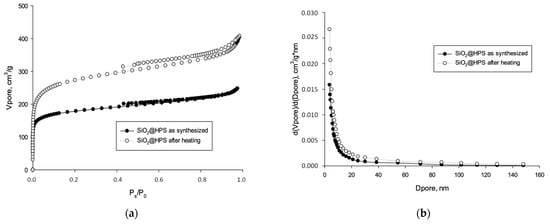
Figure 1.
BET study of SiO2@HPS support: (a) adsorption isotherms; (b) pore size distribution.
An XPS study of the SiO2@HPS samples showed the presence of C, O, Cl, and Si on the surface of the synthesized support (see Figure S1). The initial polymer possesses an aromatic polymeric matrix containing about 4–5 wt.% of O and 0.05–0.1 wt.% of Cl. The presence of N (about 1–2 at.%) is due to the tert-amino groups in the polymer structure. The surface composition of SiO2@HPS samples is presented in Table 2. Heating of the SiO2@HPS sample at 300 °C leads to a two-fold increase in the surface concentration of Si and a 1.3-fold increase in the surface concentration of O in comparison with the as-synthesized one. This is related to SiO2∙xH2O migration from the polymer volume to the surface with the capillary water during its evaporation, as well as the decomposition of SiO2∙xH2O leading to SiO2 shell formation [80]. An analysis of the high-resolution XPS spectra in the Si and O region showed that in the as-synthesized sample, the silica-containing phase is presented by SiOx, and in the sample after heating, the formation of SiO2 can be observed (Figure S2) [82]. However, a shift in the binding energies due to the differential charging of the polymer surface should be noted.

Table 2.
Elemental composition of SiO2@HPS samples.
An XRD analysis of the sample (Figure 2) shows that in the as-synthesized SiO2@HPS sample, SiO2 was found to be amorphous, with a wide peak at ca. 22 2θ. For the sample after heating at 300 °C, XRD peaks at ca. 23, 29, 32, 36, 43, 45, and 48 2θ are observed and are associated with the developed tridymite structure [83]. The possibility of low-temperature calcination of ethoxysilanes leading to the formation of a crystalline phase of SiO2 was studied by Ayu Lestari et al. in [80]. This confirms the results obtained in the current study, showing the change in crystallinity of the silica shell after heating the SiO2@HPS sample at 300 °C. However, the low peak intensity, probably due to a high dispersion of SiO2, should be mentioned [84,85,86].
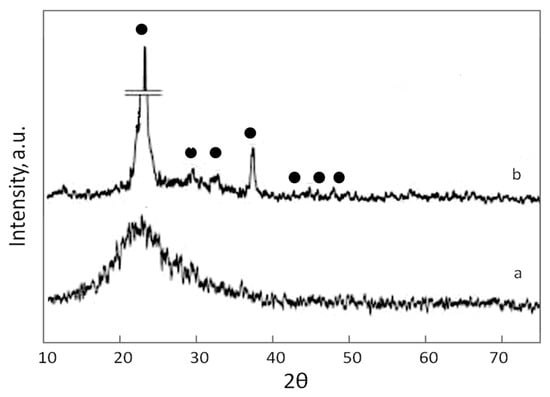
Figure 2.
XRD pattern for as-synthesized SiO2@HPS (a) and SiO2@HPS after heating (b): ●—literature data.
Three catalysts (Ni, Ru, and Ni-Ru) using a SiO2@HPS support were synthesized according to the procedure described in Section 3.3. The catalysts were studied by low-temperature nitrogen physisorption (BET), X-ray photoelectron spectroscopy (XPS), small-angle X-ray scattering (SAXS), and NH3 chemisorption. The results are reported in Table 3. The deposition of the metal-containing phase on the SiO2@HPS surface leads to a decrease in the specific surface area caused by the blockage of micropores by active phase formation. After the reaction, the surface area of the catalysts slightly decreased due to the adsorption of the reaction products on the surface. The adsorption of reaction components is also indicated by a decrease in acidity. The acidity of the initial support was found to be 1012 and 986 μmol/g for the as-synthesized SiO2@HPS and SiO2@HPS after heating, respectively. Metal deposition on the support slightly decreases the acidity due to formation of the metal-containing phase on both the polymer and SiO2 surface. For the catalysts after the reaction, the acidity was found to be 0.7–0.8 mmol/g, which is probably related to the adsorption of phenolic and aromatic products on acid sites, along with their further condensation [87]. Coke formation can lead to catalyst deactivation; however, as no metal carbonization was found on the catalyst after the reaction (see Figure 3), the active phase seems to be stable. One can suppose that the coke is mostly formed on surface acid sites.

Table 3.
Catalyst characterization.
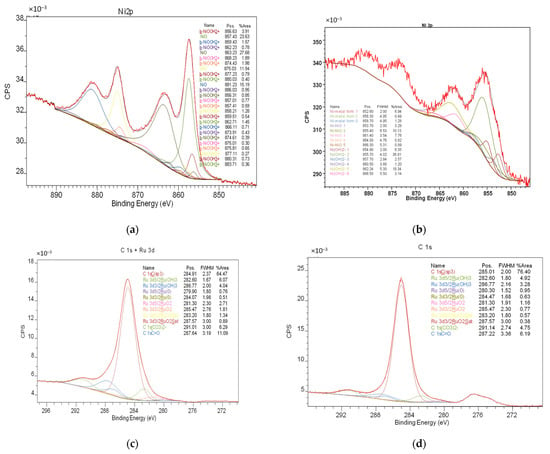
Figure 3.
High-resolution Ni 2p and C 1s XPS spectra of the Ni-Ru-SiO2@HPS catalyst before (a,c) and after (b,d) reaction.
Regarding the active phase particle size (see Table 3, column 4, and Figure S3), it should be noted that for Ni-SiO2@HPS, the formation of large particles was observed. After the reaction, a further increase in particle size of the Ni-containing samples was seen, indicating that aggregation took place. For Ru-SiO2@HPS, smaller particles were formed, while no aggregation during the reaction was noticed. The addition of Ru to Ni leads to a decrease in the particle size as compared to pure Ni, and almost no aggregation during the reaction was observed.
An XPS study of the as-synthesized catalysts confirmed the formation of metal oxide particles on the catalyst surface. However, after the reaction, the presence of a metallic phase due to the partial in situ reduction of the metal oxides was detected (see Figure 3).
2.2. Catalyst Testing
The catalytic performance of the synthesized samples was tested in lignin hydrogenolysis according to the procedure described in Section 3.3. The catalytic performance was characterized by lignin conversion and the total monophenol yield (see Table 4). All synthesized catalysts showed lignin conversion into liquid and gaseous products over 70 wt.%. The highest conversion (over 85 wt.%) was observed when using the bimetallic Ni-Ru-SiO2@HPS sample. For monometallic Ni and Ru catalysts, the conversion was also sufficiently high (>75 wt.%). An increase in the lignin conversion over the bimetallic catalyst can be assigned to synergy between the two metals, leading to an increased electron density on Ni, which facilitates metal reduction due to an increased hydrogen and electron transfer [18,21,28,32,33,35,39]. Ru is known to provide high selectivity to monophenols and aromatics due to its defect-rich structure [50,51].

Table 4.
Catalyst performance in lignin hydrogenolysis.
The gaseous phase contained light hydrocarbons (mainly methane and ethylene), methanol, methyl aldehyde, and CO. As hydrogenolysis mainly targets liquid products, the composition of the gas phase was estimated only qualitatively. Lignin hydrogenolysis results in the formation of a wide range of products, including monophenols (phenol, anisole, guaiacol, syringol, eugenol, p-ethylphenol, and p-hydroxyphenol), arenes (toluene and benzene), cyclohexanes (cyclohexane and methylcyclohexane), and soluble oligomers (see Figure 4). Monophenols were chosen to be the target product in this study as they have a wide range of applications. The yield of monophenols was over 20 wt.% for Ni-containing catalysts, and even exceeded 32 wt.% for Ni-Ru-SiO2@HPS. The chosen catalyst seems to provide an improved monophenol yield in comparison with the literature data for bimetallic Ni-based catalysts, which showed ca. 20–35 wt.% yield of monophenols at 50–80 wt.% lignin conversion [33,34,37,40,41,46,88]. The synergistic effect can be related to the higher Ni dispersion obtained by the addition of Ru [33,89]. In Section 2.1, it was shown that the addition of Ru to Ni leads to a diminished mean particle size of the active phase and a decreased polydispersity (Figure S3). Moreover, the addition of noble metals to Ni is known to increase the number of electron-rich sites, resulting in a higher catalytic activity in C–O and C–C bond cleavage [90,91]. Besides, the high acidity of the catalyst can play an important role because the acid sites can effectively adsorb the isopropanol and polyphenols, facilitating their further transformation.
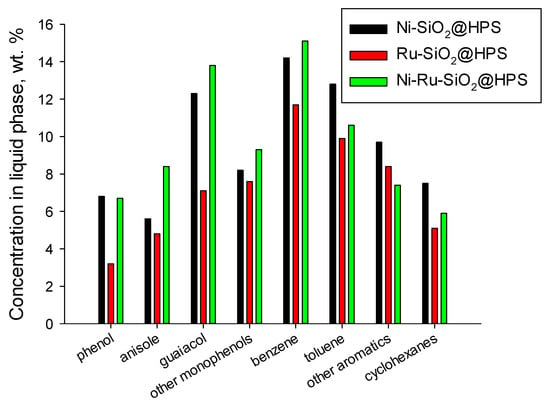
Figure 4.
Product composition of lignin hydrogenolysis over SiO2@HPS-supported catalysts.
To estimate the effect of HPS and SiO2 coating on the lignin hydrogenolysis, the catalysts Ni-Ru-HPS and Ni-Ru-SiO2 were synthesized by the impregnation method and tested in the process at the same conditions (lines 4 and 5 in Table 4). It is seen that the HPS-supported catalyst showed relatively low lignin conversion and monophenol yield in comparison with the developed catalysts. As it is well known that the presence of Lewis acid sites enhances the C–O cleavage and increases both lignin conversion and the product yield [64,65,66,67]. HPS, in turn, does not have acidic sites, thus leading to low lignin conversion in comparison with other supports [92,93,94]. For the SiO2-based catalyst, the lignin conversion was over 50 wt.%; however, the monophenol yield did not exceed 16 wt.%. This catalyst mainly formed oligomers with a molecular weight of 250–500 m/z, which is in a good agreement with other studies [8,10,11,31]. Despite the high acidity of the support, for the indicated catalyst, the specific surface area was significantly lower (ca. 120 m2/g) and aggregation of the active phase was observed (mean particle diameter 31 ± 8 nm), leading to a lower activity in C–O bond cleavage. Moreover, a high carbonization degree (ca. 21 wt.% in one run) was observed for the Ni-Ru-SiO2 catalyst. Thus, HPS coated by SiO2 is a promising support for effective lignin hydrogenolysis catalysts.
2.3. Ni-Ru-SiO2@HPS Stability Tests
The catalyst Ni-Ru-SiO2@HPS showed a higher lignin conversion and monophenol yield and so was tested in several consecutive reaction runs to evaluate its stability during lignin depolymerization. The catalyst was removed from the reaction mixture by filtration and washed twice with chloroform to dissolve the residuals. After washing, the catalyst was used in the next depolymerization experiment with a fresh portion of lignin. Ni-Ru-SiO2@HPS did not show any decrease in lignin conversion or monophenol yield during eight consecutive runs (see Table 5). Afterwards, a slight decrease in both parameters (ca. 2%) was observed (see the 10th cycle). Since no leaching of silica or the active metal was detected, the decrease in the catalyst efficiency can be attributed to a slight aggregation of the metal particles and a reduction in acidity.

Table 5.
Ni-Ru-SiO2@HPS catalyst performance in consecutive cycles.
2.4. Process Conditions Optimization
To increase the monophenol yield, the optimization of lignin hydrogenolysis conditions in the presence of the Ni-Ru-SiO2@HPS catalyst was carried out. Three parameters were varied: temperature (from 260 to 300 °C), hydrogen pressure (from 2.0 to 5.0 MPa), and the lignin/catalyst ratio (from 1000 to 2000 g/g). The results are shown in Table 6.

Table 6.
Influence of hydrogenolysis conditions on the lignin conversion monophenol yield.
An increase in the lignin hydrogenolysis temperature (Table 6, lines 1–5) led to a higher lignin conversion due to the acceleration of the reaction rate. The monophenol yield also increased in the temperature range of 260–280 °C and reached up to 40 wt.%. A further temperature rise resulted in a decrease in the monophenol yield, probably due to the intensification of side reactions such as the deoxygenation and hydrogenation of the products [10,18,19,28]. This was confirmed by an increase in the concentration of aromatic and cyclic hydrocarbons in the reaction mixture. When the partial hydrogen pressure was increased from 2.0 to 3.0 MPa at the optimal reaction temperature (280 °C), rises in both the lignin conversion and the monophenol yield were observed (Table 6, lines 6–9). However, a further increase in hydrogen pressure decreased the monophenol yield, favoring the formation of hydrocarbons (aromatic and cyclic). Besides the lignin depolymerization, the hydrogen pressure also affects the deoxygenation process [40,41,62,88]. Varying the catalyst loading, an augmentation in the lignin conversion was observed when the lignin/catalyst ratio decreased (Table 6, lines 10–12). Meanwhile, the monophenol yield decreased in this case because of the intensification of side reactions. Thus, the highest yield of target monophenols (up to 42 wt.%) was obtained at a temperature of 280 °C, a partial hydrogen pressure of 3.0 MPa, and a lignin/catalyst ratio of 2000 g/g.
3. Materials and Methods
3.1. Materials
Hypercrosslinked polystyrene (HPS) Macronet MN100 (functional groups: tert-amino groups, specific surface area: 790 m2/g) was purchased from Purolite Int. Ltd. (Llantrisant, UK) and used as received as a catalytic support. (3-aminopropyl)triethoxysilane (APTES, 99.9%, Sigma Aldrich, St. Louis, MO, USA) was used for SiO2 shell formation on HPS. Acetates of nickel and ruthenium (Aurat, Moscow, Russia) were used as catalyst active phase precursors. Tetrahydrofuran (chemical grade, Sigma Aldrich, St. Louis, MO, USA) and methanol (chemical grade, Sigma Aldrich, St. Louis, MO, USA) were used as received for catalyst preparation. Kraft lignin alkali (water soluble, 5 wt.% of water, pH 6.5) (Sigma Aldrich, St. Louis, MO, USA) was used for catalyst testing in the hydrogenolysis process. Isopropyl alcohol (IPA) (chemical grade, Kupavna Reactive, Moscow, Russia) was used as a solvent in the hydrogenolysis process. H2 (99.9%, GasProduct, Tver, Russia) and N2 (99.9%, GasProduct, Tver, Russia) were used as received in the hydrogenolysis process.
3.2. SiO2@HPS Preparation
Amounts of 3.0 g of HPS, 1.17 mL of APTES (calculated to get 10 wt.% of SiO2 per 1 g of HPS), and 10.0 mL of distilled water were loaded into a PARR 4307 reactor (Parr Instruments Ltd., Moline, IN, USA). The reactor was sealed and purged with nitrogen to remove air. Then, the reactor was heated up to 200 ± 1 °C under a nitrogen atmosphere. After the temperature reached the set value, the reaction mixture was held for 1 h at constant stirring (500 rpm). Then, the reactor was cooled down to room temperature. The suspension was filtered and washed with 10 mL of distilled water. The sample was dried in air at 105 ± 5 °C for 4 h and heated in a nitrogen flow at 300 ± 5 °C for 5 h. The resulting sample was called SiO2@HPS.
3.3. Catalyst Preparation
The catalyst was prepared by wet impregnation of 3.0 g SiO2@HPS with the calculated amount of metal precursor in a solution containing 5.0 mL of tetrahydrofuran, 1.0 mL of methanol, and 3.0 mL of distilled water. The suspension was continuously stirred for 10 min. Then, the resulting granules were dried at 75 ± 5 °C for 1 h. The catalyst was washed with 10.0 mL of an aqueous solution containing 0.084 g of sodium bicarbonate and dried again at 100 ± 5 °C for 3 h. The dry sample was heated up to 300 ± 5 °C for 5 h under a nitrogen flow to form metal oxides. The synthesized catalysts and their Si and metal contents are listed in Table 7.

Table 7.
List of the synthesized catalysts.
3.4. Catalyst Characterisation
The specific surface area, porosity, and pore size distribution of the catalyst sample (initial, after saturation with hydrogen, and after catalysis) were determined by nitrogen low-temperature adsorption using a Beckman Coulter SA 3100 (Coulter Corporation, Brea, CA, USA) analyzer. Before the analysis, the samples were degassed in a Beckmann Coulter SA-PREP (Coulter Corporation, Brea, CA, USA) apparatus for sample preparation at 120 °C in a vacuum for 1 h. To estimate the specific surface area and total pore volume, t-plots and Brunauer–Emmett–Teller models were used. The pore size distribution was evaluated using the Harkins–Jura equation.
X-ray photoelectron spectroscopy (XPS) analysis was performed using an ES-2403 spectrometer (Institute for Analytical Instrumentation RAS, St. Petersburg, Russia) modified with a PHOIBOS 100 analyzer produced by SPECS GmbH (Berlin, Germany) equipped with a MgKα/AlKα XR-50 X-ray radiation source. The spectra were acquired at an X-ray power of 200 W and an energy step of 0.1 eV. Before the analysis, the samples were degassed for 180 min. Data analyses were performed in CasaXPS (Casa Software Ltd., Teignmouth, UK).
X-ray powder diffraction (XRD) patterns were collected on an Empyrean from PANalytical (Malvern, UK). X-rays were generated from a copper target with a scattering wavelength of 1.54 Å. The step size of the experiment was 0.02.
To obtain the SAXS data, an S3 MICRO diffractometer (Hecus X-Ray Systems GmbH, Graz, Austria) with point collimation and copper radiation (Cu Ka, 50 W) was used. The measurements were performed in the range of vectors q from 0.01 to 0.6 Å−1, where q = 4πsinθ/λ. The samples for the study were placed in a 1.5 mm glass capillary with a wall thickness of 0.01 mm. To exclude the influence of residual scattering from porous hypercrosslinked polystyrene, the sample was impregnated with a contrast agent with a known excess in terms of moisture capacity. Data analyses were performed in ATSAS data analysis software (EMBL, Hamburg, Germany) using a spherical form factor.
NH3 chemisorption was carried out using an AutoChem HP (Micromeritics Ltd., Norcross, GA, USA). The analysis was performed in a temperature range of 30–300 °C with a heating rate of 5 °C/min and following this the temperature was maintained at 300 °C for 1 h. The quantity of the desorbed gas was estimated using calibration curves.
3.5. Lignin Hydrogenolysis Procedure
Lignin hydrogenolysis experiments were carried out in a stainless-steel batch reactor (Parr Series 5000 Multiple Reactor System) (Parr Instrument, Moline, IN, USA) with a cell volume of 50 mL equipped with a magnetic stirrer. In a typical procedure, 1.0 g of lignin, a calculated amount of catalyst, and 30 mL of isopropanol were loaded into the reactor cell. The catalyst loading was calculated as 2000 g of lignin per 1 g of metal in the catalyst. The cell was sealed and the air was replaced by nitrogen by flushing three times. The reactor was heated up to 260 °C under a nitrogen pressure of 2.0 MPa. After the temperature reached 260 °C, nitrogen was replaced by hydrogen. Hydrogenolysis was performed for 3 h at a constant stirring (1200 rpm). Then, the reactor was cooled to room temperature.
The gaseous phase was sampled after cooling of the reactor and analyzed by GC using a Crystallux 4000 M (Meta-Chrom, Yoshkar Ola, Russia) equipped with a flame ionization detector, a katharometer, and a packed column filled with granules of polymer adsorbent MN-270 (Purolight Inc., Llantrisant, UK) with a fraction of 125–250 µm (length: 2.5 m, diameter: 3.0 mm). The analysis was carried out under the following conditions: the initial temperature of the column was 40 °C, which was maintained for 4 min, then the temperature was raised to 250 °C at a heating rate of 15 °C/min; the temperatures of the evaporator and the detector were 260 °C; the carrier gas was helium; and the total flow of He was 30.0 mL/min. A quantitative analysis of the gaseous phase was performed based on calibration curves using standard compounds.
The liquid phase was separated from the solid residue by centrifugation. An analysis of the liquid phase was performed by GCMS using a GC-2010 gas chromatograph and a GCMS-QP2010S mass spectrometer (Shimadzu, Kyoto, Japan) equipped with a HP-1MS chromatographic column (length: 30 m, diameter: 0.25 mm, film thickness: 0.25 µm). An analysis was carried out under the following conditions: an initial temperature of 120 °C, which was maintained for 5 min, then the column was heated up to 250 °C at a rate of 5 °C/min and maintained at 250 °C for 5 min. Helium (volumetric velocity of 20.8 mL/s, pressure of 253.5 kPa) was used as the gas carrier. The injector temperature was 280 °C, the ion source temperature was 260 °C, and the interface temperature was 280 °C. Methylene diamine (Sigma Aldrich, St. Louis, MO, USA) was used as an external standard for the quantitative estimation. For the proper quantitative analysis, calibration regarding the main monophenolic compounds was performed.
Lignin conversion was calculated based on Equation (1). The yield of monophenolic compounds was calculated according to Equation (2).
where mL—the weight of lignin taken for the experiment, g; ms—the weight of residual after the hydrogenolysis, g; and mmono—the total weight of monophenols, g.
4. Conclusions
Catalysts containing Ni, Ru, and Ni–Ru nanoparticles supported on SiO2@HPS were synthesized for the first time and tested in a lignin hydrogenolysis process, targeting catalyst stability and a high yield of monophenols. Coating of HPS with amorphous SiOx and subsequent heating in a nitrogen flow at 300 °C led to the formation of tridymite-type SiO2 on the surface, presenting a high SSA of up to 950 m2/g. The synthesized catalysts contain metal/metal oxide nanoparticles as an active phase and have a surface acidity of ca. 1 mmol/g. The best catalyst was found to be bimetallic Ni-Ru-SiO2@HPS, which showed a high lignin conversion (up to 95%) and a high monophenol yield (42 wt.%) under optimized reaction conditions. Moreover, the catalysts demonstrated a remarkable stability in ten consecutive runs without any loss of the active metal phase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13050856/s1, Figure S1: Survey XPS spectra of the as-synthesized (a) and heated (b) SiO2@HPS; Figure S2: High-resolution XPS spectra of Si 2p for the as-synthesized (a) and heated (b) SiO2@HPS, and O 1s for the as-synthesized (c) and heated (d) SiO2@HPS; Figure S3: Particle size distribution for the as-synthesized Ni-SiO2@HPS, Ru-SiO2@HPS, and Ni-Ru-SiO2@HPS catalysts.
Author Contributions
Conceptualization, L.K.-M. and M.G.S.; methodology, A.I.S.; investigation, M.E.M., A.V.B. and O.V.M.; writing—original draft preparation, A.A.S.; writing—review and editing, A.A.S. and L.K.-M.; project administration, A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support for the work was provided by the Russian Science Foundation (grant 22-79-10096).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, C.; Wang, F. Catalytic Lignin Depolymerization to Aromatic Chemicals. Acc. Chem. Res. 2020, 53, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Duarah, P.; Haldar, D.; Purkait, M.K. Technological advancement in the synthesis and applications of lignin-based nanoparticles derived from agro-industrial waste residues: A review. Int. J. Biol. Macromol. 2020, 163, 1828–1843. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Yi, W.; Fu, P.; Zhang, A.; Bai, X. Renewable aromatic hydrocarbons production from catalytic pyrolysis of lignin with Al-SBA-15 and HZSM-5: Synergistic effect and coke behavior. Renew. Energy 2021, 163, 1673–1681. [Google Scholar] [CrossRef]
- Wang, Z.X.; Li, H.; Xie, W.L.; Hu, B.; Li, K.; Lu, Q. Progress on basic structure, pyrolysis mechanism and characteristics of lignin. Adv. New. Renew. Energy 2020, 8, 6–14. [Google Scholar]
- Tejado, A.; Pena, C.; Labidi, J.; Echeverria, J.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Romero, R.A.; Redondo, A.; Gnanakaran, S. Theoretical study of the remarkably diverse linkages in lignin. J. Phys. Chem. Lett. 2011, 2, 2660–2666. [Google Scholar] [CrossRef]
- Alherech, M.; Omolabake, S.; Holland, C.M.; Klinger, G.E.; Hegg, E.L.; Stahl, S.S. From Lignin to Valuable Aromatic Chemicals: Lignin Depolymerization and Monomer Separation via Centrifugal Partition Chromatography. ACS Cent. Sci. 2021, 7, 1831–1837. [Google Scholar] [CrossRef]
- Lundquist, K. Low-molecular weight lignin hydrolysis products. In Applied Polymer Symposium; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1976; pp. 1393–1407. [Google Scholar]
- Roy, R.; Rahman, M.S.; Amit, T.A.; Jadhav, B. Recent Advances in Lignin Depolymerization Techniques: A Comparative Overview of Traditional and Greener Approaches. Biomass 2022, 2, 130–154. [Google Scholar] [CrossRef]
- Duan, B.; Wang, Q.; Zhao, Y.; Li, N.; Zhang, S.; Du, Y. Effect of catalysts on liquefaction of alkali lignin for production of aromatic phenolic monomer. Biomass Bioenergy 2019, 131, 105413. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Long, J.; Xu, Y.; Wang, T.; Yuan, Z.; Shu, R.; Zhang, Q.; Ma, L. Efficient base-catalyzed decomposition and in situ hydrogenolysis process for lignin depolymerization and char elimination. Appl. Energy 2015, 141, 70–79. [Google Scholar] [CrossRef]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Yunpu, W.; Leilei, D.; Liangliang, F.; Shaoqi, S.; Yuhuan, L.; Roger, R. Review of microwave-assisted lignin conversion for renewable fuels and chemicals. J. Anal. Appl. Pyrolysis 2016, 119, 104–113. [Google Scholar] [CrossRef]
- Yang, W.; Du, X.; Liu, W.; Tricker, A.W.; Dai, H.; Deng, Y. Highly Efficient Lignin Depolymerization via Effective Inhibition of Condensation during Polyoxometalate-Mediated Oxidation. Energy Fuels 2019, 33, 6483–6490. [Google Scholar] [CrossRef]
- Oregui-Bengoechea, M.; Gandarias, I.; Miletić, N.; Simonsen, S.F.; Kronstad, A.; Arias, P.L. Thermocatalytic conversion of lignin in an ethanol/formic acid medium with NiMo catalysts: Role of the metal and acid sites. Appl. Catal. B Environ. 2017, 217, 353–364. [Google Scholar] [CrossRef]
- Radhika, N.L.; Sachdeva, S.; Kumar, M. Lignin depolymerization and biotransformation to industrially important chemicals/biofuels. Fuel 2022, 312, 122935. [Google Scholar] [CrossRef]
- Dou, X.; Li, W.; Zhu, C.; Jiang, X. Catalytic waste Kraft lignin hydrodeoxygenation to liquid fuels over a hollow Ni-Fe catalyst. Appl. Catal. B Environ. 2021, 287, 119975. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Li, H.; Xiao, L.P.; Song, G. Selective hydrogenolysis of catechyl lignin into propenylcatechol over an atomically dispersed ruthenium catalyst. Nat. Commun. 2021, 12, 416. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Liu, Y.; Ruan, X.; Tsang, D.C.W.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Lignin valorization for the production of renewable chemicals: State-of-the art review and future prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Hydrolytic depolymerization of hydrolysis lignin: Effects of catalysts and solvents. Bioresour. Technol. 2015, 190, 416–419. [Google Scholar] [CrossRef]
- Kuznetsov, B.; Sharypov, V.; Chesnokov, N.; Beregovtsova, N.; Baryshnikov, S.; Lavrenov, A.; Vosmerikov, A.; Agabekov, V. Lignin conversion in supercritical ethanol in the presence of solid acid catalysts. Kinet. Catal. 2015, 56, 434–441. [Google Scholar] [CrossRef]
- Cocero, M.J.; Cabeza, A.; Abad, N.; Adamovic, T.; Vaquerizo, L.; Martinez, C.M.; Pazo-Cepeda, M.V. Understanding biomass fractionation in subcritical & supercritical water. J. Supercrit. Fluids 2018, 133, 550–565. [Google Scholar]
- Fan, D.; Xie, X.-A.; Li, Y.; Li, L.; Sun, J. Comparative study about catalytic liquefaction of alkali lignin to aromatics by HZSM-5 in sub-and supercritical ethanol. J. Renew. Sustain. Energy 2018, 10, 013106. [Google Scholar] [CrossRef]
- Yang, T.; Wu, K.; Li, B.; Du, C.; Wang, J.; Li, R. Conversion of lignin into phenolic-rich oil by two-step liquefaction in subsupercritical ethanol system assisted by carbon dioxide. J. Energy Inst. 2021, 94, 329–336. [Google Scholar] [CrossRef]
- Ye, K.; Liu, Y.; Wu, S.; Zhuang, J. A review for lignin valorization: Challenges and perspectives in catalytic hydrogenolysis. Ind. Crops Prod. 2021, 172, 114008. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Li, X.; Chen, L.; Li, G.; Li, X. Lignin valorization: A novel in Situ Catalytic Hydrogenolysis Method in alkaline aqueous solution. Energy Fuels 2018, 32, 7643–7651. [Google Scholar] [CrossRef]
- Scarsella, M.; de Caprariis, B.; Damizia, M.; De Filippis, P. Heterogeneous catalysts for hydrothermal liquefaction of lignocellulosic biomass: A review. Biomass Bioenergy 2020, 140, 105662. [Google Scholar] [CrossRef]
- Barta, K.; Matson, T.D.; Fettig, M.L.; Scott, S.L.; Iretskii, A.V.; Ford, P.C. Catalytic disassembly of an organosolv lignin via hydrogen transfer from supercritical methanol. Green Chem. 2010, 12, 1640–1647. [Google Scholar] [CrossRef]
- Barta, K.; Warner, G.R.; Beach, E.S.; Anastas, P.T. Depolymerization of organosolv lignin to aromatic compounds over Cu-doped porous metal oxides. Green Chem. 2014, 16, 191–196. [Google Scholar] [CrossRef]
- Liu, Q.; Li, P.; Liu, N.; Shen, D. Lignin depolymerization to aromatic monomers and oligomers in isopropanol assisted by microwave heating. Polym. Degrad. Stab. 2017, 135, 54–60. [Google Scholar] [CrossRef]
- Kong, L.; Liu, C.; Gao, J.; Wang, Y.; Dai, L. Efficient and controllable alcoholysis of Kraft lignin catalyzed by porous zeolite-supported nickel-copper catalyst. Bioresour. Technol. 2019, 276, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, L.; Gu, J.; Gou, L.; Xie, L.; Wang, Y.; Dai, L. Catalytic hydrotreatment of kraft lignin into aromatic alcohols over nickel-rhenium supported on niobium oxide catalyst. Bioresour. Technol. 2020, 299, 122582. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, L.; Liu, S.; Wang, Y.; Dai, L. High-quality bio-oil from one-pot catalytic hydrocracking of kraft lignin over supported noble metal catalysts in isopropanol system. Bioresour. Technol. 2016, 212, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, S.; Xiao, R.; Jiang, X.; Wang, Y.; Sun, Y.; Lu, P. Catalytic transfer hydrogenolysis of lignin into monophenols over platinum-rhenium supported on titanium dioxide using isopropanol as in situ hydrogen source. Bioresour. Technol. 2019, 279, 228–233. [Google Scholar] [CrossRef]
- Hita, I.; Deuss, P.J.; Bonura, G.; Frusteri, F.; Heeres, H.J. Biobased chemicals from the catalytic depolymerization of Kraft lignin using supported noble metal-based catalysts. Fuel Process. Technol. 2018, 179, 143–153. [Google Scholar] [CrossRef]
- Hossain, M.A.; Phung, T.K.; Rahaman, M.S.; Tulaphol, S.; Jasinski, J.B.; Sathitsuksanoh, N. Catalytic cleavage of the β-O-4 aryl ether bonds of lignin model compounds by Ru/C catalyst. Appl. Catal. A Gen. 2019, 582, 117100. [Google Scholar] [CrossRef]
- Zhang, S.; Su, L.; Liu, L.; Fang, G. Degradation on hydrogenolysis of soda lignin using CuO/SO42−/ZrO2 as catalyst. Ind. Crops Prod. 2015, 77, 451–457. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Lu, G.-P.; Cai, C. Self-hydrogen transfer hydrogenolysis of β-O-4 linkages in lignin catalyzed by MIL-100(Fe) supported Pd–Ni BMNPs. Green Chem. 2017, 19, 4538–4543. [Google Scholar] [CrossRef]
- Xue, Z.; Yu, H.; He, J.; Zhang, Y.; Lan, X.; Liu, R.; Zhang, L.; Mu, T. Highly efficient cleavage of ether bonds in lignin models by transfer hydrogenolysis over dual functional Ruthenium/Montmorillonite. ChemSusChem 2020, 13, 4579–4586. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation-hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S.; et al. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017, 8, 16104. [Google Scholar] [CrossRef] [PubMed]
- Ayusheev, A.B.; Taran, O.P.; Afinogenova, I.I.; Mishchenko, T.I.; Shashkov, M.V.; Sashkina, A.; Semeikina, V.S.; Parkhomchuk, E.V.; Agabekov, V.E.; Parmon, V.N. Depolymerization of Birch-Wood Organosolv Lignin Over Solid Catalysts in Supercritical Ethanol. J. Sib. Fed. Univ. Chem 2016, 9, 353–370. [Google Scholar] [CrossRef]
- Guo, H.; Qi, Z.; Liu, Y.; Xia, H.; Li, L.; Huang, Q.; Wang, A.; Li, C. Tungsten-based catalysts for lignin depolymerization: The role of tungsten species in C–O bond cleavage. Catal. Sci. Technol. 2019, 9, 2144–2151. [Google Scholar] [CrossRef]
- Fang, Q.; Jiang, Z.; Guo, K.; Liu, X.; Li, Z.; Li, G.; Hu, C. Low temperature catalytic conversion of oligomers derived from lignin in pubescens on Pd/NbOPO4. Appl. Catal. B Environ. 2020, 263, 118325. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Li, H.; Zheng, X.; Ma, H.; Long, J.; Li, X. Selective hydrogenolysis of lignin catalyzed by a cost-effective Ni metal supported on alkaline MgO. ACS Sust. Chem. Eng. 2019, 7, 19750–19760. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, X.; Wang, L.; Liang, J.; Wei, X.; Nong, W. Anchoring Single Ni Atoms on CeO2 Nanospheres as an Efficient Catalyst for the Hydrogenolysis of Lignin to Aromatic Monomers. Fuel 2022, 324, 124499. [Google Scholar] [CrossRef]
- Li, L.; Dong, L.; Li, D.; Guo, Y.; Liu, X.; Wang, Y. Hydrogen-Free Production of 4-Alkylphenols from Lignin via Self-Reforming-Driven Depolymerization and Hydrogenolysis. ACS Catal. 2020, 10, 15197–15206. [Google Scholar] [CrossRef]
- Karnitski, A.; Choi, J.-W.; Suh, D.J.; Yoo, C.-J.; Lee, H.; Kim, K.H.; Kim, C.S.; Kim, K.; Ha, J.-M. Roles of Metal and Acid Sites in the Reductive Depolymerization of Concentrated Lignin over Supported Pd Catalysts. Catal. Today 2022, 411–412, 113844. [Google Scholar] [CrossRef]
- Li, T.; Lin, H.; Ouyang, X.; Qiu, X.; Wan, Z. In Situ Preparation of Ru@N-Doped Carbon Catalyst for the Hydrogenolysis of Lignin to Produce Aromatic Monomers. ACS Catal. 2019, 9, 5828–5836. [Google Scholar] [CrossRef]
- Ding, T.; Wu, Y.; Zhu, X.; Lin, G.; Hu, X.; Sun, H.; Huang, Y.; Zhang, S.; Zhang, H. Promoted Production of Phenolic Monomers from Lignin-First Depolymerization of Lignocellulose over Ru Supported on Biochar by N,P-Co-Doping. ACS Sustain. Chem. Eng. 2022, 10, 2343–2354. [Google Scholar] [CrossRef]
- Huang, J.B.; He, C. Pyrolysis mechanism of α-O-4 linkage lignin dimer: A theoretical study. J. Anal. Appl. Pyrol. 2015, 113, 655–664. [Google Scholar] [CrossRef]
- Zhu, C.; Cao, J.-P.; Feng, X.-B.; Zhao, X.-Y.; Yang, Z.; Li, J.; Zhao, M.; Zhao, Y.-P.; Bai, H.-C. Theoretical insight into the hydrogenolysis mechanism of lignin dimer compounds based on experiments. Renew. Energy 2021, 163, 1831–1837. [Google Scholar] [CrossRef]
- Huang, J.B.; Liu, C.; Wu, D.; Tong, H.; Ren, L. Density functional theory studies on pyrolysis mechanism of β-O-4 type lignin dimer model compound. J. Anal. Appl. Pyrol. 2014, 109, 98–108. [Google Scholar] [CrossRef]
- Huang, J.; He, C.; Liu, C.; Tong, H.; Wu, L.Q.; Wu, S.B. A computational study on thermal decomposition mechanism of β-1 linkage lignin dimer. Comput. Theor. Chem. 2015, 1054, 80–87. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, S.X.; Cao, X.L. Density functional theory investigation of gasification mechanism of a lignin dimer with β-5 linkage. Renew. Energy 2018, 115, 937–945. [Google Scholar] [CrossRef]
- Kim, S.; Chmely, S.C.; Nimlos, M.R.; Bomble, Y.J.; Foust, T.D.; Paton, R.S.; Beckham, G.T. Computational study of bond cleavage enthalpies for a large range of native and modified lignins. J. Phys. Chem. Lett. 2011, 2, 2846–2852. [Google Scholar] [CrossRef]
- Qi, S.-C.; Zhang, L.; Kudo, S.; Norinaga, K.; Hayashi, J. Theoretical Study on Hydrogenolytic Cleavage of Intermonomer Linkages in Lignin. J. Phys. Chem. A 2017, 121, 2868–2877. [Google Scholar] [CrossRef]
- Wu, A.; Patrick, B.O.; Chung, E.; James, B.R. Hydrogenolysis of β-O-4 lignin model dimers by a ruthenium-xantphos catalyst. Dalton Trans. 2012, 41, 11093–11106. [Google Scholar] [CrossRef]
- Atesin, A.C.; Ray, N.A.; Stair, P.C.; Marks, T.J. Etheric C-O Bond Hydrogenolysis Using a Tandem Lanthanide Triflate/Supported Palladium Nanoparticle Catalyst System. J. Am. Chem. Soc. 2012, 134, 14682–14685. [Google Scholar] [CrossRef]
- Sang, Y.; Chen, H.; Khalifeh, M.; Li, Y. Catalysis and chemistry of lignin depolymerization in alcohol solvents–A review. Catal. Today. 2023, 408, 168–181. [Google Scholar] [CrossRef]
- Selvaraj, M.; Shanthi, K.; Maheswari, R.; Ramanathan, A. Hydrodeoxygenation of guaiacol over MoO3-NiO/mesoporous silicates: Effect of incorporated heteroatom. Energy Fuels 2014, 28, 2598–2607. [Google Scholar] [CrossRef]
- Ambursa, M.M.; Sudarsanam, P.; Voon, L.H.; Hamid, S.B.A.; Bhargava, S.K. Bimetallic CuNi catalysts supported on MCM-41 and Ti-MCM-41 porous materials for hydrodeoxygenation of lignin model compound into transportation fuels. Fuel Process Technol. 2017, 162, 87–97. [Google Scholar] [CrossRef]
- Güvenatam, B.; Heeres, E.H.J.; Pidko, E.A.; Hensen, E.J.M. Lewis-acid catalyzed depolymerization of Protobind lignin in supercritical water and ethanol. Catal. Today 2016, 259, 460–466. [Google Scholar] [CrossRef]
- Lohr, T.L.; Li, Z.; Marks, T.J. Thermodynamic strategies for C–O bond formation and cleavage via tandem catalysis. Acc. Chem. Res. 2016, 49, 824–834. [Google Scholar] [CrossRef]
- Jastrzebski, R.; Constant, S.; Lancefield, C.S.; Westwood, N.J.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Tandem catalytic depolymerization of lignin by Water Tolerant Lewis acids and rhodium complexes. ChemSusChem 2016, 9, 2074–2079. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Savage, P.E. Hydrolytic cleavage of C–O linkages in lignin model compounds catalyzed by Water-Tolerant Lewis acids. Ind. Eng. Chem. Res. 2014, 53, 2633–2639. [Google Scholar] [CrossRef]
- Cai, Q.; Gong, T.; Yu, T.; Zhang, S. Comparison of Hydrocracking and Cracking of Pyrolytic Lignin over Different Ni-Based Catalysts for Light Aromatics Production. Fuel Process. Technol. 2023, 240, 107564. [Google Scholar] [CrossRef]
- He, J.; Tang, D.; Hu, C.; Luo, Y.; Kim, C.K.; Su, Z. Mechanistic Study on the Depolymerization of Typical Lignin-Derived Oligomers Catalyzed by Pd/NbOPO4. Mol. Catal. 2022, 528, 112500. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, J.-P.; Yang, Z.; Xie, J.-X.; Zhao, L.; Zhu, C.; Zhang, C.; Zhao, X.-Y.; Zhao, Y.-P.; Zhang, J.-L. Hydrodeoxygenation of Lignin and Its Model Compounds to Hydrocarbon Fuels over a Bifunctional Ga-Doped HZSM-5 Supported Metal Ru Catalyst. Appl. Catal. A Gen. 2022, 633, 118516. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, L.; Sun, L.; Gao, S.; Gao, W.; Cheng, X.; Shang, N.; Gao, Y.; Wang, C. Highly efficient hydrodeoxygenation of lignin-derivatives over Ni-based catalyst. Appl. Catal. B Environ. 2021, 293, 120243. [Google Scholar] [CrossRef]
- Yan, S.; Ding, W.; Lin, X.; Cai, Q.; Zhang, S. Selective hydrogenolysis of lignin for phenolic monomers with a focus on β-O-4 cleavage and C = O hydrodeoxygenation. Fuel 2022, 320, 123732. [Google Scholar] [CrossRef]
- Bykov, A.V.; Demidenko, G.N.; Nikoshvili, L.Z.; Kiwi-Minsker, L. Hyper-Cross-Linked Polystyrene as a Stabilizing Medium for Small Metal Clusters. Molecules 2021, 26, 5294. [Google Scholar] [CrossRef] [PubMed]
- Grigorev, M.E.; Mikhailov, S.P.; Bykov, A.V.; Sidorov, A.I.; Tiamina, I.Y.; Vasiliev, A.L.; Nikoshvili, L.Z.; Matveeva, V.G.; Meneghetti, S.M.P.; Sulman, M.G. Mono-and Bimetallic (Ru-Co) Polymeric Catalysts for Levulinic Acid Hydrogenation. Catal. Today 2021, 378, 167–175. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Markova, M.E.; Bykov, A.V.; Sidorov, A.I.; Sulman, M.G.; Matveeva, V.G.; Sulman, E.M. Ni catalyst synthesized by hydrothermal deposition on the polymeric matrix in the supercritical deoxygenation of fatty acids. React. Kinet. Catal. Lett. 2018, 125, 213–226. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Markova, M.E.; Monzharenko, M.A.; Matveeva, V.G.; Sulman, M.G. Polymer-based bifunctional catalysts for anthracene hydrocracking in the medium of supercritical propanol-2. Catal. Today 2021, 378, 158–166. [Google Scholar] [CrossRef]
- Grebennikova, O.V.; Mikhailova, A.N.; Molchanov, V.P.; Sulman, A.M.; Doluda, V.Y.; Matveeva, V.G. Polymeric supports for enzymes immobilization in synthesis of biologically active compounds. ChemChemTech 2021, 64, 67–72. [Google Scholar] [CrossRef]
- Cueto-Díaz, E.J.; Castro-Muñiz, A.; Suárez-García, F.; Gálvez-Martínez, S.; Torquemada-Vico, M.C.; Valles-González, M.P.; Mateo-Martí, E. APTES-Based Silica Nanoparticles as a Potential Modifier for the Selective Sequestration of CO2 Gas Molecules. Nanomaterials 2021, 11, 2893. [Google Scholar] [CrossRef]
- Smith, E.A.; Chen, W. How to prevent the loss of surface functionality derived from aminosilanes. Langmuir 2008, 24, 12405–12409. [Google Scholar] [CrossRef]
- Ayu Lestari, R.; Elma, M.; AtikaRampun, E.L.; Sumardi, A.; Paramitha, A.; Lestari Eka, A.; Rabiah, S.; Assyaifi, Z.L.; Satriaji, G. Functionalization of Si-C Using TEOS (Tetra Ethyl Ortho Silica) as Precursor and Organic Catalyst. E3S Web Conf. 2020, 148, 07008. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodrigues-Reinoso, F.; Rouquerol, J.; Sing, K.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Logofatu, C.; Negrila, C.-C.; Ghita, R.; Ungureanu, F.; Cotirlan, C.; Ghica, C.; Manea, A.S.; Lazarescu, M.F. Study of SiO2/Si Interface by Surface Techniques. In Crystalline Silicon—Properties and Uses; Basu, S., Ed.; InTech: Rijeka, Croatia, 2011; pp. 23–42. [Google Scholar]
- Ren, S.; Guo, Y.; Zhao, L.; Ma, X.; Xiao, Y.; Yu, Y.; Liu, Y.; Zhao, X.; Wang, Z. The preparation of tridymite crystal by chemical processing. Phase Transit. 2008, 81, 395–402. [Google Scholar] [CrossRef]
- Wen, X.; Su, Y.; Liu, G.; Muller, A.J.; Kumar, S.K.; Wang, D. Direct Relationship between Dispersion and Crystallization Behavior in Poly(ethylene oxide)/Poly(ethylene glycol)-g-Silica Nanocomposites. Macromolecules 2021, 54, 1870–1880. [Google Scholar] [CrossRef]
- Lan, C.-H.; Sun, Y.-M. Dispersion, crystallization behavior, and mechanical properties of poly(3-hydroxybutyrate) nanocomposites with various silica nanoparticles: Effect of surface modifiers. J. Polym. Res. 2018, 25, 121. [Google Scholar] [CrossRef]
- Siot, A.; Longuet, C.; Léger, R.; Otazaghine, B.; Ienny, P.; Caro-Bretelle, A.-S.; Azema, N. Correlation between process and silica dispersion/distribution into composite: Impact on mechanical properties and Weibull statistical analysis. Polym. Test. 2018, 70, 92–101. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Palos, R.; Hita, I.; Arandes, J.M.; Rodriguez-Mirasol, J.; Cordero, T.; Bilbao, J.; Castano, P. Revealing the pathways of catalyst deactivation by coke during the hydrodeoxygenation of raw bio-oil. Appl. Catal. B Environ. 2018, 239, 513–524. [Google Scholar] [CrossRef]
- Zhang, J.G.; Asakura, H.; van Rijn, J.; Yang, J.; Duchesne, P.; Zhang, B.; Chen, X.; Zhang, P.; Saeys, M.; Yan, N. Highly efficient, NiAu-catalyzed hydrogenolysis of lignin into phenolic chemicals. Green Chem. 2014, 16, 2432–2437. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, M.; Jiang, B.; Wu, S.; Lu, P. Catalytic Transfer Hydrogenolysis of Native Lignin to Monomeric Phenols over a Ni–Pd Bimetallic Catalyst. Energy Fuels 2020, 34, 9754–9762. [Google Scholar] [CrossRef]
- Lu, X.; Guo, H.; Wang, D.; Xiu, P.; Qin, Y.; Chen, J.; Xu, C.; Gu, X. A Review on Catalytic Conversion of Lignin into High-Value Chemicals over Ni-Based Catalysts. Biomass Convers. Biorefin. 2021, 1–43. [Google Scholar] [CrossRef]
- Li, F.; France, L.J.; Cai, Z.; Li, Y.; Liu, S.; Lou, H.; Long, J.; Li, X. Catalytic transfer hydrogenation of butyl levulinate to gamma-valerolactone over zirconium phosphates with adjustable lewis and bronsted acid sites. Appl. Catal. B Environ. 2017, 214, 67–77. [Google Scholar] [CrossRef]
- Dong, L.; Lin, L.; Han, X.; Si, X.; Liu, X.; Guo, Y.; Lu, F.; Rudić, S.; Parker, S.F.; Yang, S.; et al. Breaking the limit of lignin monomer production via cleavage of interunit carbon–carbon linkages. Chem 2019, 5, 1521–1536. [Google Scholar] [CrossRef]
- Ma, H.; Li, H.; Zhao, W.; Li, L.; Liu, S.; Long, J.; Li, X. Selective depolymerization of lignin catalyzed by nickel supported on zirconium phosphate. Green Chem. 2019, 21, 658–668. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).