Engineering Surface Properties of CuO/Ce0.6Zr0.4O2 Catalysts for Efficient Low-Temperature Toluene Oxidation
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalytic Activity and Kinetic Study
| Samples | Calcination Temperature, °C | Reaction Mixture | T50, °C | T90, °C | Reference |
|---|---|---|---|---|---|
| Cu0.4Ce0.6Oy | 500 | 500 ppm toluene 20 vol.% O2/N2 GHSV: 50,000 h−1 | 230 | 245 | [15] |
| 5 wt% CuO/CeO2 | 400 | 500 ppm toluene 20vol.% O2/N2 GHSV: 75,000 h−1 | 210 | 235 | [19] |
| Cu1Ce3Oy | 500 | 1000 ppm toluene 21 vol.% O2/N2 GHSV: 60,000 h−1 | 200 | 210 | [40] |
| CuO/CeO2-ZrO2 (nCe/nCu mole ratio = 5 and nCe/nZr mole ratio = 9) | 400 | 1500 ppm toluene GHSV: 24,000 h−1 | 300 | 350 | [44] |
| Cu0.15Ce0.85Oy | 550 | 600 ppm toluene 20 vol.% O2/He GHSV: 50,000 h−1 | 240 | 250 | [45] |
| Cu6Ce4Ox (Cu/Ce atomic ratio = 6:4) | 550 | 1000 ppm toluene 20 vol.% O2/N2 GHSV: 36,000 mL·g−1·h−1 | 240 | 260 | [39] |
| UiO-66-CeCu (nCe/nCu mole ratio = 3) | — | 500 ppm toluene 21 vol.% O2/N2 GHSV: 30,000 mL·g−1·h−1 | — | 220 | [13] |
| 6% CuO/CeO2-ZrO2 | 700 | 1000 ppm toluene 30 vol.% O2/Ar | 277 | 310 | [41] |
| 8 wt% CuO/Ce0.8Zr0.2Oy | 400 | 4400 ppm toluene GHSV: 33,000 mL·g−1 | 210 | 230 | [46] |
| Cu1Ce3Oy (nCe/nCu mole ratio = 3) | 500 | 1.0 vol.% toluene GHSV: 66,000 mL·g−1·h−1 | 223 | 225 | [47] |
| Cu0.13Ce0.87Oy | 600 | 1000 ppm toluene GHSV: 15,000 h−1 | 470 | 490 | [48] |
| 8 wt% CuO/Ce0.6Zr0.4Oy | 550 | 600 ppm toluene 21 vol.% O2/N2 GHSV: 20,000 mL·g−1·h−1 | 220 | 240 | In this study |
| Samples | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) | Average Ce0.6Zr0.4O2 Crystal Sizes a (nm) | Average CuO Crystal Sizes b (nm) | ID/IF2g c | Ea d (kJ·mol−1) |
|---|---|---|---|---|---|---|---|
| Cu/CZ-450 | 57.6 | 0.15 | 8.4 | 4.9 | - e | 0.50 | 105.5 ± 6.0 |
| Cu/CZ-550 | 50.8 | 0.16 | 10.4 | 5.3 | - e | 0.61 | 88.3 ± 4.7 |
| Cu/CZ-650 | 42.0 | 0.18 | 12.8 | 5.7 | - e | 0.55 | 112.3 ± 4.5 |
| Cu/CZ-750 | 9.5 | 0.06 | 13.8 | 10.1 | 22.3 | 0.30 | 146.5 ± 5.4 |
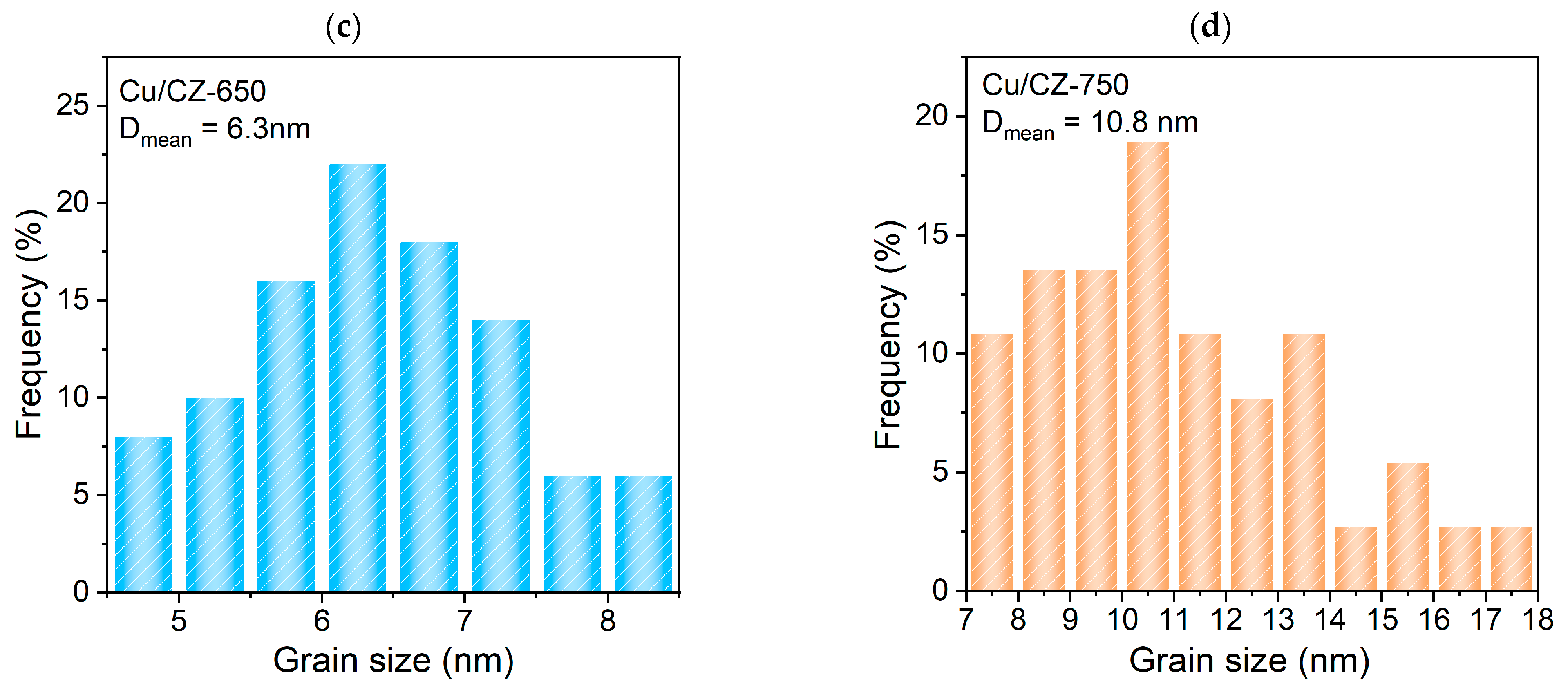
2.2. Textural Characteristics
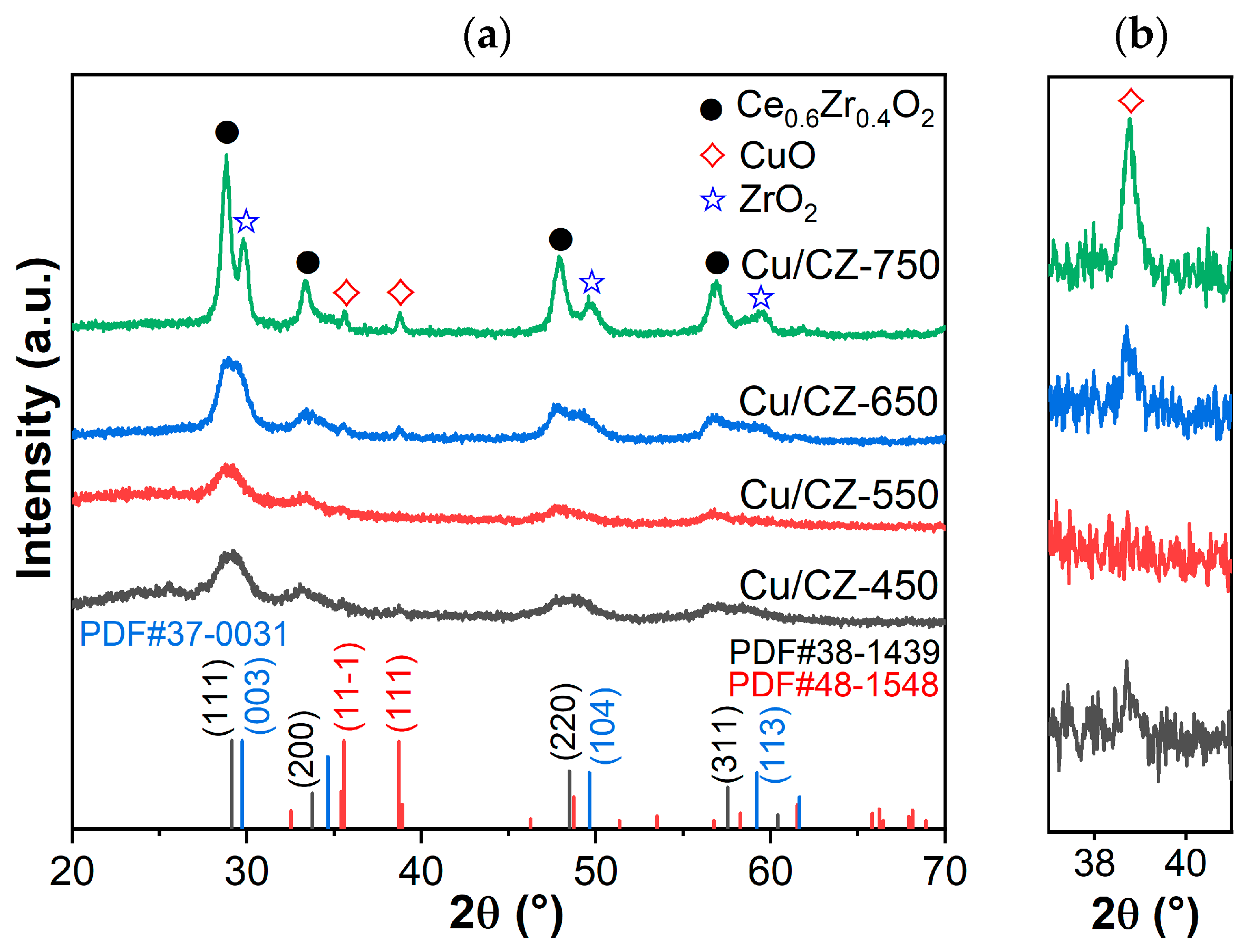
2.3. Structural Characteristics
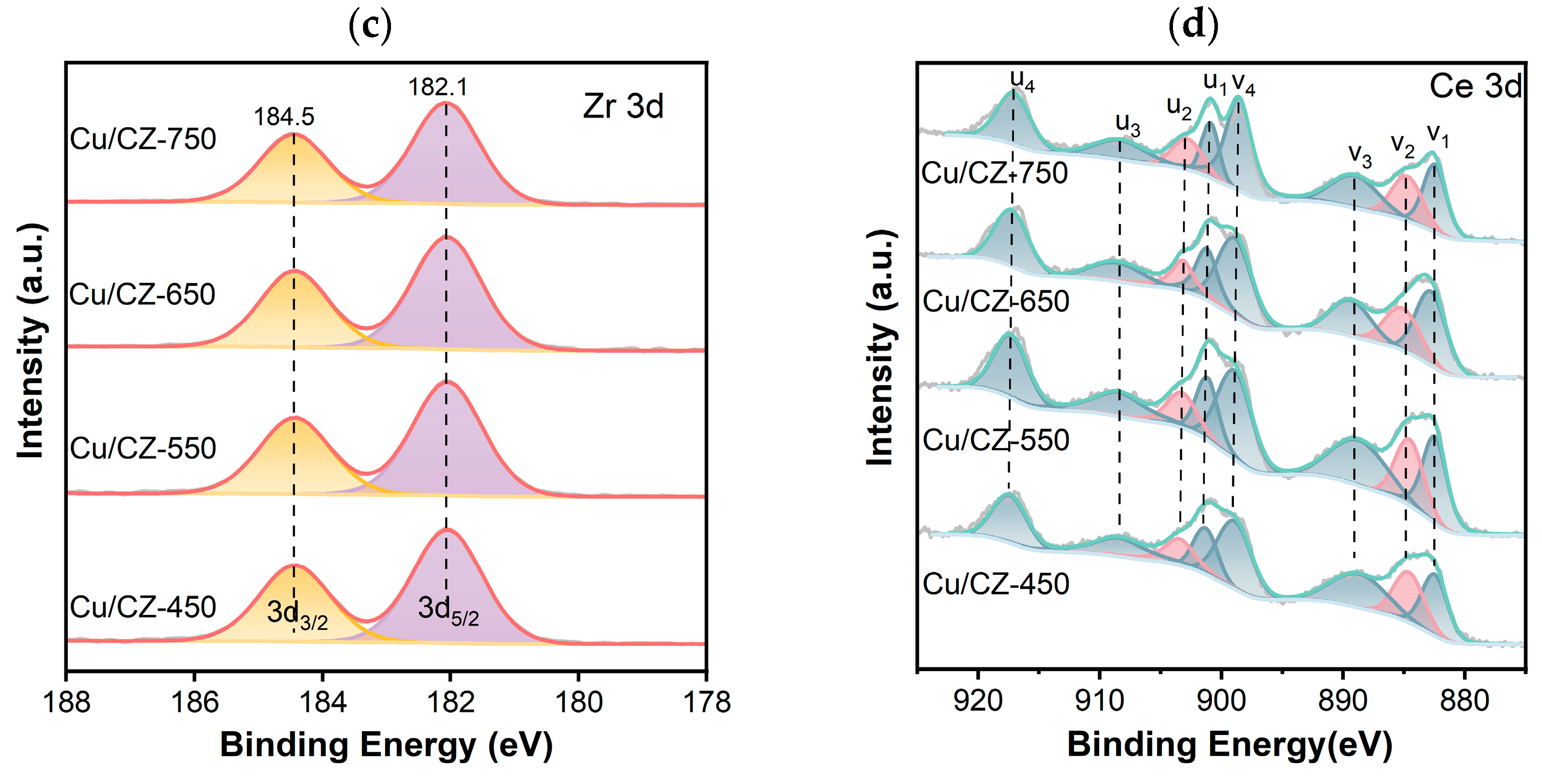
2.4. Chemical State
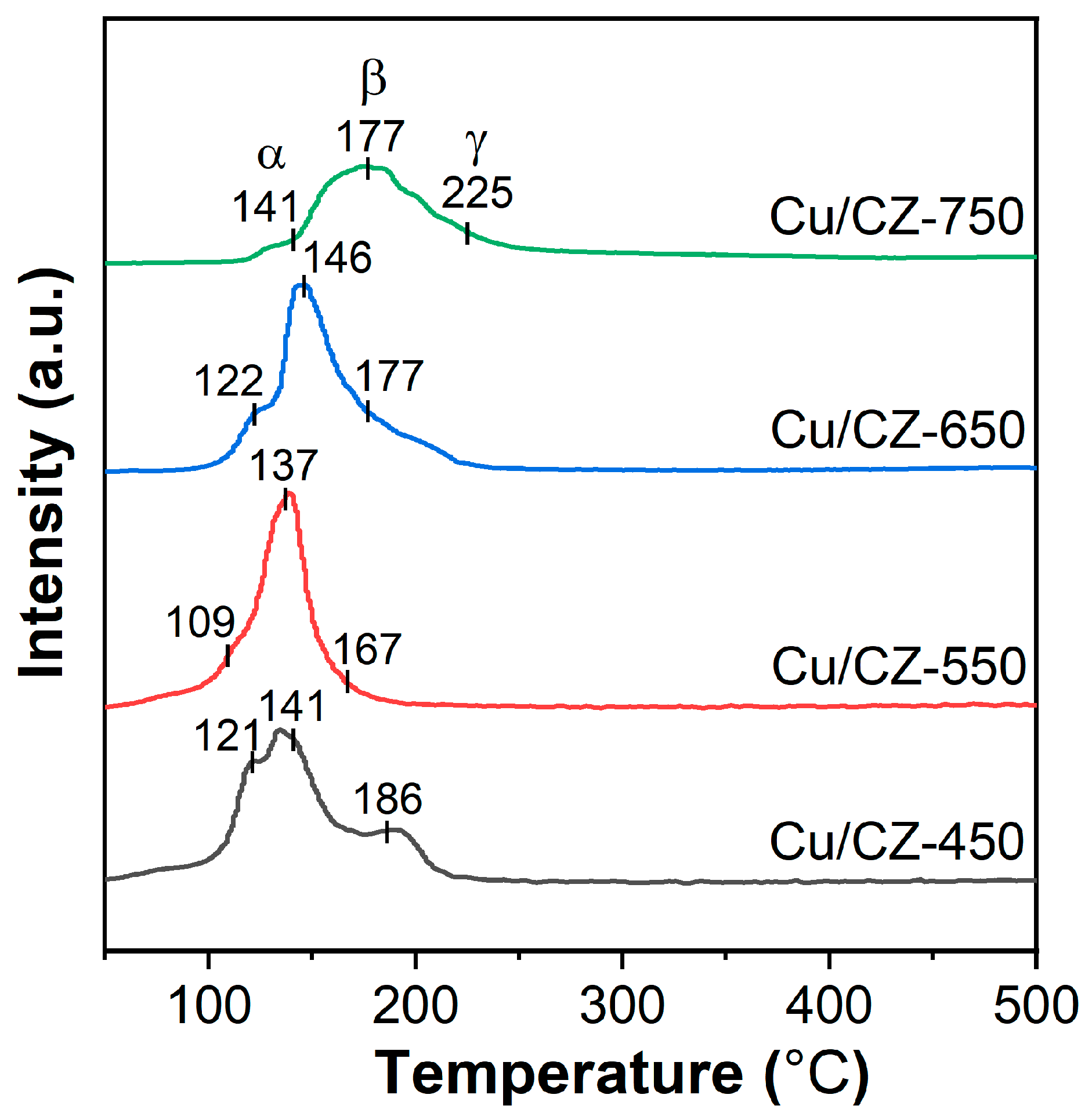
2.5. Reducibility
3. Materials and Methods
3.1. Chemicals
3.2. Preparations of Catalysts
3.2.1. Synthesis of Ce0.6Zr0.4O2 Supports
3.2.2. Synthesis of CuO/Ce0.6Zr0.4O2 Catalysts
3.3. Characterization of Catalysts
3.4. Catalytic Measurements
3.5. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 1003–1004, 403–412. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, Q.; Zhang, D.; Wang, J.; Tang, T.; Wang, H.; Liu, X.; Song, Z.; Ning, P. The influence of annealing temperature on copper-manganese catalyst towards the catalytic combustion of toluene: The mechanism study. Appl. Surf. Sci. 2019, 497, 143777. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Cen, B.H.; Tang, C.; Lu, J.Q.; Chen, J.; Luo, M.F. Different roles of MoO3 and Nb2O5 promotion in short-chain alkane combustion over Pt/ZrO2 catalysts. Chin. J. Catal. 2021, 4212, 2287–2295. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, X.; Peng, R.; Zhao, M.; Ye, D. Catalytic properties of manganese oxide polyhedra with hollow and solid morphologies in toluene removal. Appl. Surf. Sci. 2017, 405, 20–28. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, J.; Cheng, H.; Mo, S.; Ke, Y.; Ren, Q.; Fu, M.; Chen, P.; Wu, J.; Ye, D. Investigation into the roles of different oxygen species in toluene oxidation over manganese-supported platinum catalysts. Mol. Catal. 2021, 507, 111569. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Zhao, S.; Wu, P.; Chong, Y.; Li, A.; Zhao, Y.; Chen, G.; Jin, X.; Qiu, Y.; et al. Engineering Cobalt Oxide with Coexisting Cobalt Defects and Oxygen Vacancies for Enhanced Catalytic Oxidation of Toluene. ACS Catal. 2022, 129, 4906–4917. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, H.; Zhang, Z.; Li, L. Improved reactivity for toluene oxidation on MnOx/CeO2-ZrO2 catalyst by the synthesis of cubic-tetragonal interfaces. Appl. Surf. Sci. 2021, 539, 148188. [Google Scholar] [CrossRef]
- Xu, X.; Li, L.; Yu, F.; Peng, H.; Fang, X.; Wang, X. Mesoporous high surface area NiO synthesized with soft templates: Remarkable for catalytic CH4 deep oxidation. Mol. Catal. 2017, 441, 81–91. [Google Scholar] [CrossRef]
- Ye, Z.; Giraudon, J.M.; Nuns, N.; Simon, P.; De Geyter, N.; Morent, R.; Lamonier, J.F. Influence of the preparation method on the activity of copper-manganese oxides for toluene total oxidation. Appl. Catal. B Environ. 2018, 223, 154–166. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Li, Y.; Leng, X.; Zhang, T.; Yuan, F.; Niu, X.; Zhu, Y. Synthesis of CeaMnOx hollow microsphere with hierarchical structure and its excellent catalytic performance for toluene combustion. Appl. Catal. B Environ. 2019, 245, 502–512. [Google Scholar] [CrossRef]
- Hou, X.; Bian, Y.; Yang, L. One-step synthesis of transition metal modified UiO-66-Ce metal-organic framework: Catalytic oxidation of toluene and investigation of the mechanism. Microporous Mesoporous Mater. 2022, 345, 112214. [Google Scholar] [CrossRef]
- Kondratowicz, T.; Drozdek, M.; Rokicińska, A.; Natkański, P.; Michalik, M.; Kuśtrowski, P. Novel CuO-containing catalysts based on ZrO2 hollow spheres for total oxidation of toluene. Microporous Mesoporous Mater. 2019, 279, 446–455. [Google Scholar] [CrossRef]
- Zeng, Y.; Haw, K.G.; Wang, Z.; Wang, Y.; Zhang, S.; Hongmanorom, P.; Zhong, Q.; Kawi, S. Double redox process to synthesize CuO-CeO2 catalysts with strong Cu-Ce interaction for efficient toluene oxidation. J. Hazard. Mater. 2021, 404 Pt A, 124088. [Google Scholar] [CrossRef]
- Hu, F.; Peng, Y.; Chen, J.; Liu, S.; Song, H.; Li, J. Low content of CoOx supported on nanocrystalline CeO2 for toluene combustion: The importance of interfaces between active sites and supports. Appl. Catal. B Environ. 2019, 240, 329–336. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Wang, Z.; Huang, H.; Peng, H.; Li, X. Fabrication of mesoporous Co3O4 oxides by acid treatment and their catalytic performances for toluene oxidation. Appl. Catal. A Gen. 2018, 550, 67–76. [Google Scholar] [CrossRef]
- Yun, J.; Wu, L.; Hao, Q.; Teng, Z.; Gao, X.; Dou, B.; Bin, F. Non-equilibrium plasma enhanced oxygen vacancies of CuO/CeO2 nanorod catalysts for toluene oxidation. J. Environ. Chem. Eng. 2022, 103, 107847. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Song, F.; Zhang, S.; Zhong, Q. The effect of CuO loading on different method prepared CeO2 catalyst for toluene oxidation. Sci. Total Environ. 2020, 712, 135635. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Li, J.; Sun, Y.; Ren, Q.; Zou, S.; Zhang, Q.; Lu, J.; Fu, M.; Mo, D.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B Environ. 2020, 264, 118464. [Google Scholar] [CrossRef]
- Li, Y.; Peng, H.; Xu, X.; Peng, Y.; Wang, X. Facile preparation of mesoporous Cu–Sn solid solutions as active catalysts for CO oxidation. RSC Adv. 2015, 533, 25755–25764. [Google Scholar] [CrossRef]
- Tang, H.; Wei, J.; Liu, F.; Qiao, B.; Pan, X.; Li, L.; Liu, J.; Wang, J.; Zhang, T. Strong Metal-Support Interactions between Gold Nanoparticles and Nonoxides. J. Am. Chem. Soc. 2016, 1381, 56–59. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Z.; Brosnahan, J.T.; Zhang, S.; Guo, Y.; Guo, Y.; Wang, L.; Wang, Y.; Zhan, W. Ru/CeO2 Catalyst with Optimized CeO2 Support Morphology and Surface Facets for Propane Combustion. Environ. Sci. Technol. 2019, 539, 5349–5358. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, D.; Xue, W.; Dou, B.; Wang, H.; Hao, Z. Investigation of formaldehyde oxidation over Co3O4-CeO2 and Au/Co3O4-CeO2 catalysts at room temperature: Effective removal and determination of reaction mechanism. Environ. Sci. Technol. 2011, 458, 3628–3634. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.; Ran, R.; Si, Z.; Weng, D. IR characterization of propane oxidation on Pt/CeO2–ZrO2: The reaction mechanism and the role of Pt. J. Mol. Catal. A Chem. 2012, 356, 100–105. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Shen, Z.; Zhao, J. Catalytic oxidation of toluene and p-xylene using gold supported on Co3O4 catalyst prepared by colloidal precipitation method. J. Mol. Catal. A Chem. 2011, 351, 188–195. [Google Scholar] [CrossRef]
- Su, Z.; Si, W.; Liu, H.; Xiong, S.; Chu, X.; Yang, W.; Peng, Y.; Chen, J.; Cao, X.; Li, J. Boosting the Catalytic Performance of CeO2 in Toluene Combustion via the Ce-Ce Homogeneous Interface. Environ. Sci. Technol. 2021, 5518, 12630–12639. [Google Scholar] [CrossRef]
- Su, Z.; Yang, W.; Wang, C.; Xiong, S.; Cao, X.; Peng, Y.; Si, W.; Weng, Y.; Xue, M.; Li, J. Roles of Oxygen Vacancies in the Bulk and Surface of CeO2 for Toluene Catalytic Combustion. Environ. Sci. Technol. 2020, 5419, 12684–12692. [Google Scholar] [CrossRef]
- Liu, C.; Xian, H.; Jiang, Z.; Wang, L.; Zhang, J.; Zheng, L.; Tan, Y.; Li, X. Insight into the improvement effect of the Ce doping into the SnO2 catalyst for the catalytic combustion of methane. Appl. Catal. B Environ. 2015, 176–177, 542–552. [Google Scholar] [CrossRef]
- Lou, B.; Shakoor, N.; Adeel, M.; Zhang, P.; Huang, L.; Zhao, Y.; Zhao, W.; Jiang, Y.; Rui, Y. Catalytic oxidation of volatile organic compounds by non-noble metal catalyst: Current advancement and future prospectives. J. Clean. Prod. 2022, 363, 132523. [Google Scholar] [CrossRef]
- Song, W.; Ji, J.; Guo, K.; Wang, X.; Wei, X.; Cai, Y.; Tan, W.; Li, L.; Sun, J.; Tang, C.; et al. Solid-phase impregnation promotes Ce doping in TiO2 for boosted denitration of CeO2/TiO2 catalysts. Chin. Chem. Lett. 2022, 332, 935–938. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Yu, G.; Zhu, L.; Zhang, G.; Qin, G.; Fu, H.; Ji, F.; Zhao, J. Preparation and characterization of the continuous titanium-doped ZrO2 mesoporous fibers with large surface area. J. Porous Mater. 2013, 211, 105–112. [Google Scholar] [CrossRef]
- Shukla, M.K.; Balyan, Y.; Kumar, A.; Bhaskar, T.; Dhar, A. Catalytic oxidation of soot by CeO2–ZrO2 catalysts: Role of Zr. Mater. Chem. Phys. 2022, 286, 126161. [Google Scholar] [CrossRef]
- Wen, J.; Xie, Y.; Ma, Y.; Sun, H.; Wang, H.; Liu, M.; Zhang, Q.; Chen, J. Engineering of surface properties of Ni-CeZrAl catalysts for dry reforming of methane. Fuel 2022, 308, 122008. [Google Scholar] [CrossRef]
- Guo, F.; Li, J.; Zhang, Y.; Yang, X. Enhanced Stability and Catalytic Performance of Active Rh Sites on Al2O3 Via Atomic Layer Deposited ZrO2. J. Phys. Chem. Lett. 2022, 1338, 8825–8832. [Google Scholar] [CrossRef]
- Xu, H.; Sun, M.; Liu, S.; Li, Y.; Wang, J.; Chen, Y. Effect of the calcination temperature of cerium–zirconium mixed oxides on the structure and catalytic performance of WO3/CeZrO2 monolithic catalyst for selective catalytic reduction of NOx with NH3. RSC Adv. 2017, 739, 24177–24187. [Google Scholar] [CrossRef]
- Abdollahzadeh-Ghom, S.; Zamani, C.; Andreu, T.; Epifani, M.; Morante, J.R. Improvement of oxygen storage capacity using mesoporous ceria–zirconia solid solutions. Appl. Catal. B Environ. 2011, 108–109, 32–38. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Chen, F.; Xiang, Y.; Yan, J.; Chu, W. Facile fabrication of hollow structured Cu-Ce binary oxides and their catalytic properties for toluene combustion. Catal. Today 2021, 376, 239–246. [Google Scholar] [CrossRef]
- Song, B.; Li, C.; Du, X.; Li, S.; Zhang, Y.; Lyu, Y.; Zhou, Q. Superior performance of Cu-Ce binary oxides for toluene catalytic oxidation: Cu-Ce synergistic effect and reaction pathways. Fuel 2021, 306, 121654. [Google Scholar] [CrossRef]
- Wang, L.; Yin, G.; Yang, Y.; Zhang, X. Enhanced CO oxidation and toluene oxidation on CuCeZr catalysts derived from UiO-66 metal organic frameworks. React. Kinet. Mech. Catal. 2019, 1281, 193–204. [Google Scholar] [CrossRef]
- Liu, M.; Yang, X.; Tian, Z.; Wang, H.; Yin, L.; Chen, J.; Guan, Q.; Yang, H.; Zhang, Q. Insights into the role of strontium in catalytic combustion of toluene over La1-xSrxCoO3 perovskite catalysts. Phys. Chem. Chem. Phys. 2022, 246, 3686–3694. [Google Scholar] [CrossRef] [PubMed]
- Tathod, A.P.; Hayek, N.; Shpasser, D.; Simakov, D.S.A.; Gazit, O.M. Mediating interaction strength between nickel and zirconia using a mixed oxide nanosheets interlayer for methane dry reforming. Appl. Catal. B Environ. 2019, 249, 106–115. [Google Scholar] [CrossRef]
- Dou, B.; Yang, D.; Kang, T.; Xu, Y.; Hao, Q.; Bin, F.; Xu, X. Morphology effects of CeO2-ZrO2 on the catalytic performance of CuO/CeO2-ZrO2 for toluene oxidation. Carbon Resour. Convers. 2021, 4, 55–60. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. VOC oxidation over CuO–CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2009, 891–892, 295–302. [Google Scholar] [CrossRef]
- Deng, Q.-F.; Ren, T.-Z.; Agula, B.; Liu, Y.; Yuan, Z.-Y. Mesoporous CexZr1−xO2 solid solutions supported CuO nanocatalysts for toluene total oxidation. J. Ind. Eng. Chem. 2014, 205, 3303–3312. [Google Scholar] [CrossRef]
- Zhou, G.; Lan, H.; Yang, X.; Du, Q.; Xie, H.; Fu, M. Effects of the structure of Ce@Cu catalysts on the catalytic combustion of toluene in air. Ceram. Int. 2013, 394, 3677–3683. [Google Scholar] [CrossRef]
- Hu, C. Catalytic combustion kinetics of acetone and toluene over Cu0.13Ce0.87Oy catalyst. Chem. Eng. J. 2011, 1683, 1185–1192. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Chai, S.-H.; Binder, A.; Li, Y.-Y.; Ji, L.-T.; Dai, S. Influence of calcination temperature on the structure and catalytic performance of CuOx-CoOy-CeO2 ternary mixed oxide for CO oxidation. Appl. Catal. A Gen. 2013, 451, 282–288. [Google Scholar] [CrossRef]
- Mi, R.; Li, D.; Hu, Z.; Yang, R.T. Morphology Effects of CeO2 Nanomaterials on the Catalytic Combustion of Toluene: A Combined Kinetics and Diffuse Reflectance Infrared Fourier Transform Spectroscopy Study. ACS Catal. 2021, 1113, 7876–7889. [Google Scholar] [CrossRef]
- Du, Y.; Xiao, G.; Guo, Z.; Lin, B.; Fu, M.; Ye, D.; Hu, Y. A high-performance and stable Cu/Beta for adsorption-catalytic oxidation in-situ destruction of low concentration toluene. Sci. Total Environ. 2022, 833, 155288. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Wang, W.; He, L.; Li, D.; Chen, J.; Luo, M. Novel insights into diethylamine catalytic combustion over CuO catalysts supported by SSZ-13: Undesirable product NOx as a crucial intermediate for N2 generation. Mol. Catal. 2021, 516, 111952. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, S.; Xu, S.; Liu, J.; Xu, H.; Chen, Y.; Dan, Y. Fabricate surface structure-stabilized Cu/BEA with hydrothermal-resistant via si-deposition for NOx abatement. Mol. Catal. 2020, 495, 111153. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Wu, X.; Wen, J.; Zhao, R.; Li, Z.; Tian, G.; Zhang, Q.; Ning, P.; Hao, J. Frustrated Lewis Pairs Boosting Low-Temperature CO2 Methanation Performance over Ni/CeO2 Nanocatalysts. ACS Catal. 2022, 1217, 10587–10602. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, X.; Chu, X.; Chen, M.; Chen, X.; Chen, J.; He, H.; Zhang, C. Tuning Metal-Support Interaction of Pt-CeO2 Catalysts for Enhanced Oxidation Reactivity. Environ. Sci. Technol. 2021, 5524, 16687–16698. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.; Zhang, W.; Liotta, L.F.; Li, M.; Guo, Y.; Giroir-Fendler, A. Total oxidation of propane over Co3O4-based catalysts: Elucidating the influence of Zr dopant. Appl. Catal. B Environ. 2021, 298, 120606. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Cui, X.; Niu, X.; Zhu, Y. Enhancing catalytic activity for toluene and acetone oxidation over ZraCo1-aOx catalysts by doping Zr to improve the oxygen activation capacity due to formation of Zr-O-Co bonds. Chem. Eng. J. 2023, 465, 142857. [Google Scholar] [CrossRef]
- Chen, G.; Hong, D.; Xia, H.; Sun, W.; Shao, S.; Gong, B.; Wang, S.; Wu, J.; Wang, X.; Dai, Q. Amorphous and homogeneously Zr-doped MnOx with enhanced acid and redox properties for catalytic oxidation of 1,2-Dichloroethane. Chem. Eng. J. 2022, 428, 131067. [Google Scholar] [CrossRef]
- Cao, J.-L.; Wang, Y.; Zhang, T.-Y.; Wu, S.-H.; Yuan, Z.-Y. Preparation, characterization and catalytic behavior of nanostructured mesoporous CuO/Ce0.8Zr0.2O2 catalysts for low-temperature CO oxidation. Appl. Catal. B Environ. 2008, 781–782, 120–128. [Google Scholar] [CrossRef]
- Qi, L.; Yu, Q.; Dai, Y.; Tang, C.; Liu, L.; Zhang, H.; Gao, F.; Dong, L.; Chen, Y. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation. Appl. Catal. B Environ. 2012, 119–120, 308–320. [Google Scholar] [CrossRef]
- Yang, X.; Ma, X.; Yu, X.; Ge, M. Exploration of strong metal-support interaction in zirconia supported catalysts for toluene oxidation. Appl. Catal. B Environ. 2020, 263, 118355. [Google Scholar] [CrossRef]






| Samples | Cu2+ BE (eV) | Cu2+/(Cu+ + Cu2+) | Osur/(Osur + Olatt) | Ce3+/(Ce3+ + Ce4+) a | Grain Sizes b (nm) |
|---|---|---|---|---|---|
| Cu/CZ-450 | 935.2 | 0.54 | 0.27 | 0.19 | 5.3 |
| Cu/CZ-550 | 934.8 | 0.58 | 0.28 | 0.22 | 5.9 |
| Cu/CZ-650 | 935.2 | 0.52 | 0.26 | 0.18 | 6.3 |
| Cu/CZ-750 | 935.4 | 0.35 | 0.27 | 0.17 | 10.8 |
| samples | Peak α H2 Consumption a (μmol·gcat−1) | Peak β H2 Consumption a (μmol·gcat−1) | Peak γ H2 Consumption a (μmol·gcat−1) | Total H2 Consumption a (μmol·gcat−1) |
|---|---|---|---|---|
| Cu/CZ-450 | 27.1 | 53.4 | 23.3 | 103.7 |
| Cu/CZ-550 | 14.0 | 100.6 | 6.0 | 120.6 |
| Cu/CZ-650 | 9.5 | 60.4 | 42.0 | 111.9 |
| Cu/CZ-750 | 5.6 | 65.5 | 16.0 | 86.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, Q.; Zou, M.; Wang, J.; Zhu, D.; Liu, J.; Wang, J.; Zuo, Y.; Chen, J.; Ning, P. Engineering Surface Properties of CuO/Ce0.6Zr0.4O2 Catalysts for Efficient Low-Temperature Toluene Oxidation. Catalysts 2023, 13, 866. https://doi.org/10.3390/catal13050866
Wang M, Zhang Q, Zou M, Wang J, Zhu D, Liu J, Wang J, Zuo Y, Chen J, Ning P. Engineering Surface Properties of CuO/Ce0.6Zr0.4O2 Catalysts for Efficient Low-Temperature Toluene Oxidation. Catalysts. 2023; 13(5):866. https://doi.org/10.3390/catal13050866
Chicago/Turabian StyleWang, Mingyue, Qiulin Zhang, Meilin Zou, Jingge Wang, Danrui Zhu, Jiaying Liu, Junwei Wang, Yang Zuo, Jianjun Chen, and Ping Ning. 2023. "Engineering Surface Properties of CuO/Ce0.6Zr0.4O2 Catalysts for Efficient Low-Temperature Toluene Oxidation" Catalysts 13, no. 5: 866. https://doi.org/10.3390/catal13050866
APA StyleWang, M., Zhang, Q., Zou, M., Wang, J., Zhu, D., Liu, J., Wang, J., Zuo, Y., Chen, J., & Ning, P. (2023). Engineering Surface Properties of CuO/Ce0.6Zr0.4O2 Catalysts for Efficient Low-Temperature Toluene Oxidation. Catalysts, 13(5), 866. https://doi.org/10.3390/catal13050866






