NO Oxidation on Lanthanum-Doped Ceria Nanoparticles with Controlled Morphology
Abstract
1. Introduction
2. Results and Discussion
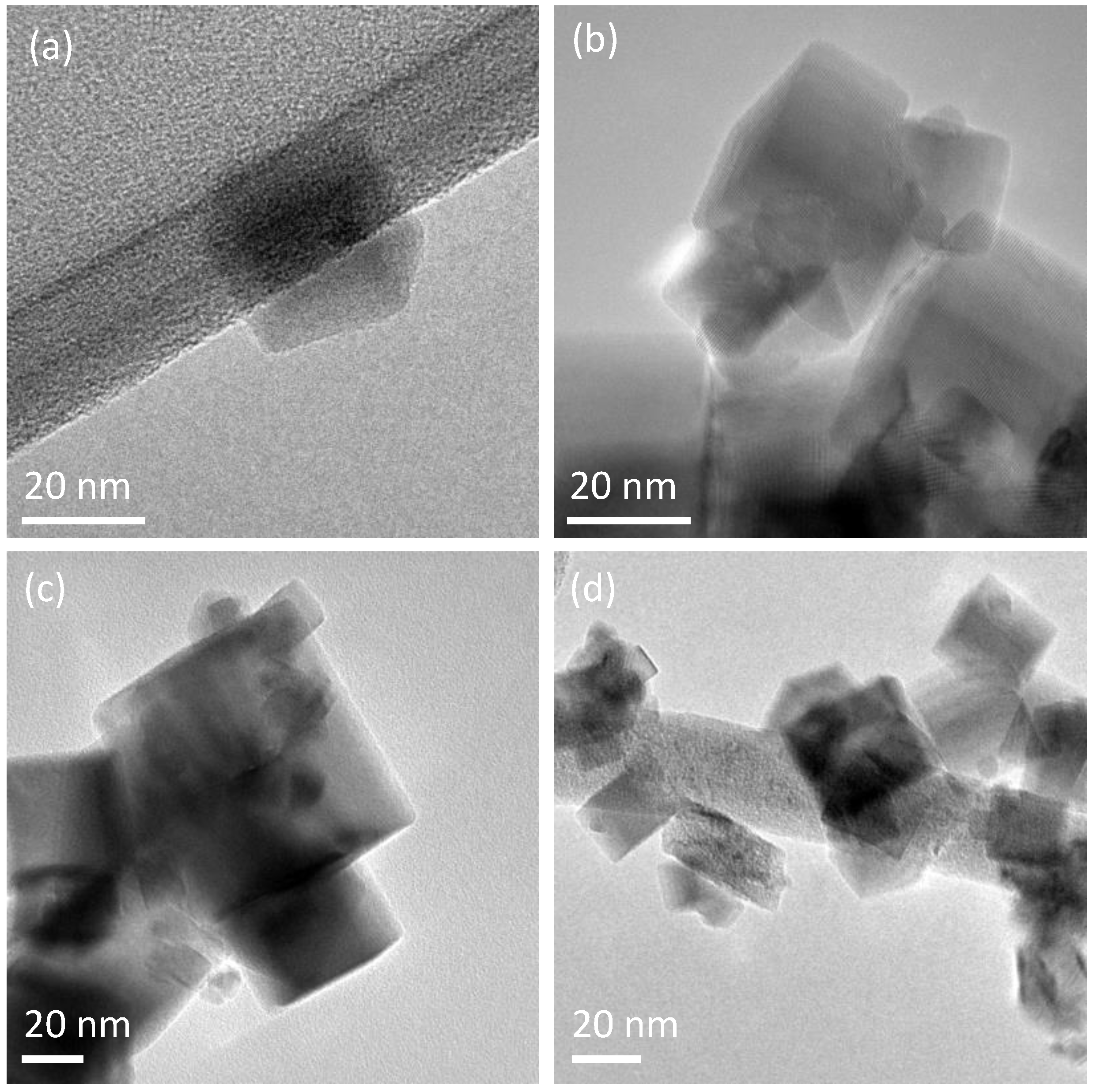
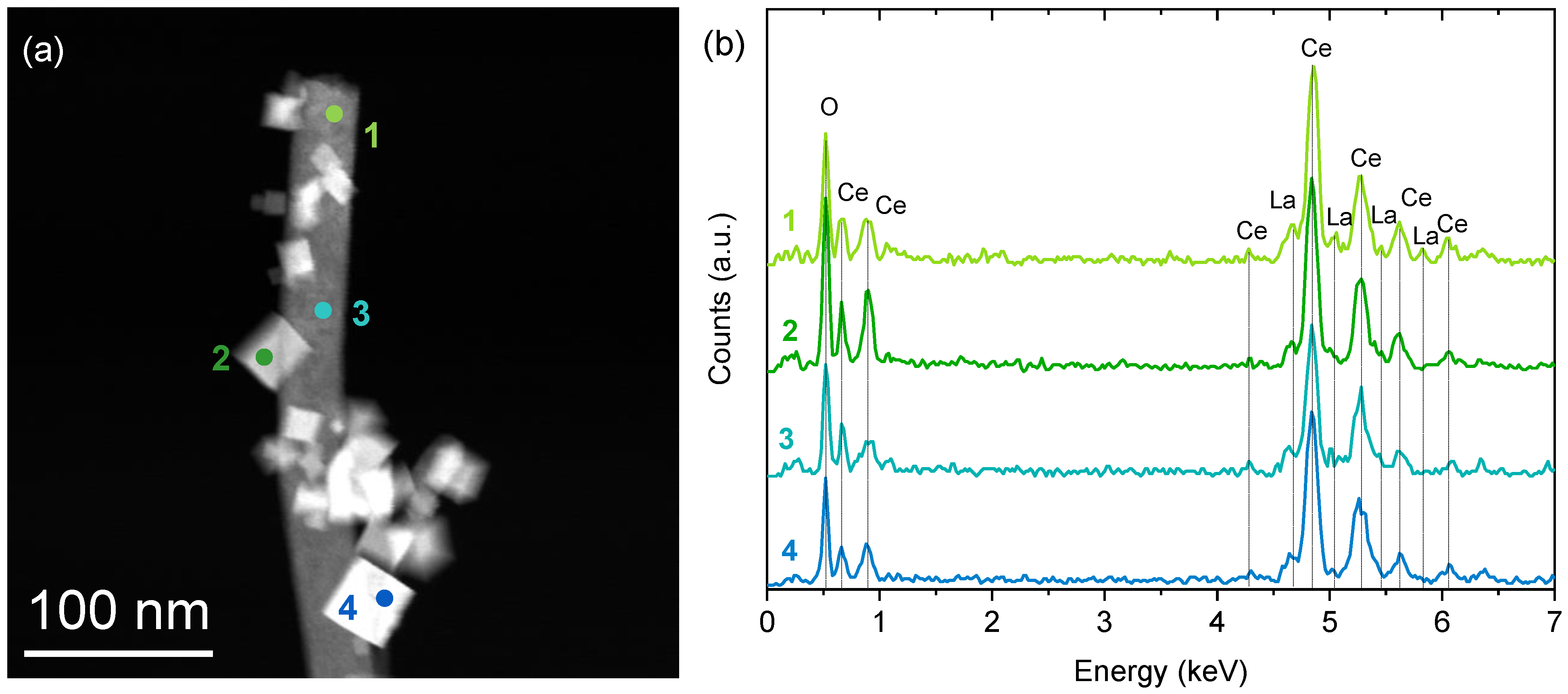
2.1. Structural and Compositional Analysis of the Ce1−xLaxO2−δ Samples
2.2. Redox Properties
2.2.1. H2-TPR Experiments
2.2.2. OSC Measurements
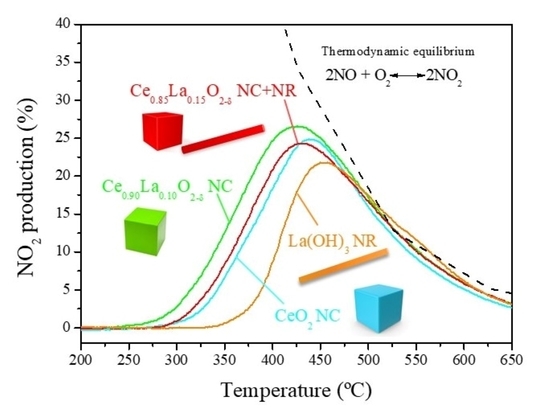
2.3. Catalytic Activity: Oxidation of NO to NO2
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Physical, Compositional, and Redox Characterization
3.3. Catalytic Test: NO Oxidation to NO2
4. Conclusions
- -
- Investigating the reaction mechanism of NO adsorption and oxidation over La-doped ceria catalysts using in situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS);
- -
- Evaluating the catalytic activity of the most active La-ceria catalysts towards NO oxidation under a more real composition of the exhaust gas, analyzing the impact of the presence of CO2, hydrocarbons, and H2O on the catalysts’ performances.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, C.; Wu, T.; Huang, B.; Zhang, J.; Guan, G.; Zhang, Z.; Hou, X.; Hu, C.; Lu, Q. Effects of a-site replacement (Sm, Y, and Pr) on catalytic performances of mullite catalysts for NO oxidation. Fuel 2023, 337, 126838. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Yang, Z.; Cao, F.; Li, L.; Chen, H.; Liu, H.; Xiong, K.; Wu, J.; Hong, Z.; et al. Electrospun YMn2O5 nanofibers: A highly catalytic activity for NO oxidation. Appl. Catal. B 2019, 247, 133–141. [Google Scholar] [CrossRef]
- Jiang, X.-Q.; Mei, X.-D.; Feng, D. Air pollution and chronic airway diseases: What should people know and do? J. Thorac. Dis. 2016, 8, E31. [Google Scholar]
- Ma, S.-j.; Wang, X.-w.; Chen, T.; Yuan, Z.H. Effect of surface morphology on catalytic activity for NO Oxidation of SmMn2O5 nanocrystals. Chem. Eng. J. 2018, 354, 191–196. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.; Guang, J.; Guo, D.; Wang, L.; Li, X. Ceria Modified FeMnOx—Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Atribak, I.; Bueno-López, A.; García-García, A. Combined removal of diesel soot particulates and NOx over CeO2–ZrO2 mixed oxides. J. Catal. 2008, 259, 123–132. [Google Scholar] [CrossRef]
- Atribak, I.; Bueno-López, A.; García-García, A.; Azambre, B. Contributions of surface and bulk heterogeneities to the NO oxidation activities of ceria–zirconia catalysts with composition Ce0.76Zr0.24O2 prepared by different methods. Phys. Chem. Chem. Phys. 2010, 12, 13770–13779. [Google Scholar] [CrossRef]
- Yang, N.; Yu, J.L.; Dou, J.X.; Tahmasebi, A.; Song, H.; Moghtaderi, B.; Lucas, J.; Wall, T. The effects of oxygen and metal oxide catalysts on the reduction reaction of NO with lignite char during combustion flue gas cleaning. Fuel Process. Technol. 2016, 152, 102–107. [Google Scholar] [CrossRef]
- Ali, S.; Chen, L.; Yuan, F.; Li, R.; Zhang, T.; Bakhtiar, S.H.; Leng, X.; Niu, X.; Zhu, Y. Synergistic effect between copper and cerium on the performance of Cux-Ce0.5-x-Zr0.5 (x = 0.1–0.5) oxides catalysts for selective catalytic reduction of NO with ammonia. Appl. Catal. B 2017, 210, 223–234. [Google Scholar] [CrossRef]
- Song, I.; Youn, S.; Lee, H.; Lee, S.G.; Cho, S.J.; Kim, D.H. Effects of Microporous TiO2 support on the catalytic and structural properties of V2O5/Microporous TiO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2017, 210, 421–431. [Google Scholar] [CrossRef]
- Auvray, X.; Olsson, L. Stability and activity of Pd-, Pt- and Pd–Pt catalysts supported on alumina for NO oxidation. Appl. Catal. B 2015, 168–169, 342–352. [Google Scholar] [CrossRef]
- Li, L.; Qu, L.; Cheng, J.; Li, J.; Hao, Z. Oxidation of nitric oxide to nitrogen dioxide over Ru catalysts. Appl. Catal. B 2009, 88, 224–231. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Song, S.; Zhang, H. Pt@CeO2 multicore@Shell self-assembled nanospheres: Clean synthesis, structure optimization, and catalytic applications. J. Am. Chem. Soc. 2013, 135, 15864–15872. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A Review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalysis by Ceria and Related Materials; World Scientific: Singapore, 2002; Volume 2, ISBN 1783261315. [Google Scholar]
- Zhu, Y.; Li, C.; Liang, C.; Li, S.; Liu, X.; Du, X.; Yang, K.; Zhao, J.; Yu, Q.; Zhai, Y.; et al. Regulating CeO2 morphologies on the catalytic oxidation of toluene at lower temperature: A study of the structure–activity relationship. J. Catal. 2023, 418, 151–162. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Aneggi, E.; Wiater, D.; de Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-dependent activity of ceria in soot combustion. ACS Catal. 2013, 4, 172–181. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, D.; Maitarad, P.; Shi, L.; Rungrotmongkol, T.; Li, H.; Zhang, J.; Cao, W. Morphology-dependent properties of MnOx/ZrO2–CeO2 Nanostructures for the selective catalytic reduction of NO with NH3. J. Phys. Chem. C 2013, 117, 10502–10511. [Google Scholar] [CrossRef]
- Tinoco, M.; Fernandez-Garcia, S.; Villa, A.; Gonzalez, J.M.; Blanco, G.; Hungria, A.B.; Jiang, L.; Prati, L.; Calvino, J.J.; Chen, X. Selective oxidation of glycerol on morphology controlled ceria nanomaterials. Catal. Sci. Technol. 2019, 9, 2328–2334. [Google Scholar] [CrossRef]
- Ouyang, B.; Tan, W.; Liu, B. Morphology effect of nanostructure ceria on the Cu/CeO2 catalysts for synthesis of methanol from CO2 hydrogenation. Catal. Commun. 2017, 95, 36–39. [Google Scholar] [CrossRef]
- Tina; Zhang, M.; Li, J.; Li, H.; Li, Y.; Shen, W. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183. [Google Scholar]
- Zhou, K.; Wang, X.; Sun, X.; Peng, Q.; Li, Y. Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J. Catal. 2005, 229, 206–212. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Wu, Z.; Dai, S. Shape-controlled ceria-based nanostructures for catalysis applications. ChemSusChem 2013, 6, 1821–1833. [Google Scholar] [CrossRef]
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef] [PubMed]
- Di Sarli, V.; Landi, G.; Di Benedetto, A.; Lisi, L. Synergy between ceria and metals (Ag or Cu) in catalytic diesel particulate filters: Effect of the metal content and of the preparation method on the regeneration performance. Top. Catal. 2021, 64, 256–269. [Google Scholar] [CrossRef]
- Yeste, M.P.; Primus, P.A.; Alcantara, R.; Cauqui, M.A.; Calvino, J.J.; Pintado, J.M.; Blanco, G. Surface Characterization of Two Ce0.62Zr0.38O2 Mixed Oxides with Different Reducibility. Appl. Surf. Sci. 2020, 503, 144255. [Google Scholar] [CrossRef]
- Mamontov, E.; Brezny, R.; Koranne, M.; Egami, T. Nanoscale heterogeneities and oxygen storage capacity of Ce0.5Zr0.5O2. J. Phys. Chem. B 2003, 107, 13007–13014. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Cauqui, M.A.; Cifredo, G.A.; Pintado, J.M.; Rodriguez-Izquierdo, J.M. Influence of reduction treatment on the structural and redox behaviour of ceria, La/Ce and Y/Ce mixed oxides. Catal. Lett. 1998, 53, 51–57. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Laguna, O.H.; Centeno, M.A.; Odriozola, J.A. Structural and catalytic properties of lanthanide (La, Eu, Gd) doped ceria. J. Solid State Chem. 2011, 184, 3014–3020. [Google Scholar] [CrossRef]
- Giménez-Mañogil, J.; Guillén-Hurtado, N.; Fernández-García, S.; Chen, X.; Calvino-Gámez, J.J.; García-García, A. Ceria-Praseodymia mixed oxides: Relationships between redox properties and catalytic activities towards NO oxidation to NO2 and CO-PROX reactions. Top. Catal. 2016, 59, 1065–1070. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Cauqui, M.A.; Corchado, P.; Pintado, J.M.; Rodríguez-Izquierdo, J.M. Oxygen buffering capacity of mixed cerium terbium oxide: A new material with potential applications in three-way catalysts. Chem. Commun. 1997, 16, 1545–1546. [Google Scholar] [CrossRef]
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B 2014, 146, 1–11. [Google Scholar] [CrossRef]
- Trindade, F.J.; Damasceno, S.; Otubo, L.; Felez, M.R.; De Florio, D.Z.; Fonseca, F.C.; Ferlauto, A.S. Tuning of shape, defects, and disorder in lanthanum-doped ceria nanoparticles: Implications for high-temperature catalysis. ACS Appl. Nano Mater. 2022, 5, 8859–8867. [Google Scholar] [CrossRef]
- Xia, X.; Li, J.; Chen, C.; Lan, Y.-P.; Mao, X.; Chu, Z.; Ning, D.; Zhang, J.; Liu, F. Collaborative influence of morphology tuning and RE (La, Y, and Sm) doping on photocatalytic performance of nanoceria. Environ. Sci. Pollut. Res. 2022, 29, 88866–88881. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, J.; Chen, C.; Lan, Y.-P.; Mao, X.; Bai, F. Optimal rare-earth (La, Y and Sm) doping conditions and enhanced mechanism for photocatalytic application of ceria nanorods. Nanotechnology 2021, 32, 195708. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, E.; Novara, C.; Chiodoni, A.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts doped with La and Nd: How acid-base sites and redox properties determine the oxidation mechanisms. Catal. Today 2022, 390–391, 117–134. [Google Scholar] [CrossRef]
- Mohanty, B.; Nayak, J. Parameters dependent studies of structural, optical and electrical properties of CeO2 nanoparticles prepared via facile one-pot hydrothermal technique. Mater. Res. Express 2017, 4, 115015. [Google Scholar] [CrossRef]
- Loche, D.; Morgan, L.M.; Casu, A.; Mountjoy, G.; O’Regan, C.; Corrias, A.; Falqui, A. Determining the maximum lanthanum incorporation in the fluorite structure of La-doped ceria nanocubes for enhanced redox ability. RSC Adv. 2019, 9, 6745–6751. [Google Scholar] [CrossRef]
- Fernandez-Garcia, S.; Jiang, L.; Tinoco, M.; Hungria, A.B.; Han, J.; Blanco, G.; Calvino, J.J.; Chen, X. Enhanced hydroxyl radical scavenging activity by doping lanthanum in ceria nanocubes. J. Phys. Chem. C 2016, 120, 1891–1901. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry; McGraw-Hill: New York, NY, USA, 1985; Volume 13. [Google Scholar]
- Ryan, K.; McGrath, J.; Farrell, R.; O’Neill, W.; Barnes, C.; Morris, M. Measurements of the lattice constant of Ceria when doped with lanthana and praseodymia-The possibility of local defect ordering and the observation of extensive phase separation. J. Phys. Condens. Matter 2003, 15, L49. [Google Scholar] [CrossRef]
- Liang, S.; Veser, G. Mixed lanthana/ceria nanorod-supported gold catalysts for water–gas-shift. Catal. Lett. 2012, 142, 936–945. [Google Scholar] [CrossRef]
- Deng, W.; Frenkel, A.I.; Si, R.; Flytzani-Stephanopoulos, M. Reaction-relevant gold structures in the low temperature water-gas shift Reaction on Au-CeO2. J. Phys. Chem. C 2008, 112, 12834–12840. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Mallesham, B.; Reddy, P.S.; Großmann, D.; Grünert, W.; Reddy, B.M. Nano-Au/CeO2 catalysts for CO oxidation: Influence of dopants (Fe, La and Zr) on the physicochemical properties and catalytic activity. Appl. Catal. B 2014, 144, 900–908. [Google Scholar] [CrossRef]
- Jiang, L.; Fernandez-Garcia, S.; Tinoco, M.; Yan, Z.; Xue, Q.; Blanco, G.; Calvino, J.J.; Hungria, A.B.; Chen, X. Improved oxidase mimetic activity by praseodymium incorporation into ceria nanocubes. ACS Appl. Mater. Interfaces 2017, 9, 18595–18608. [Google Scholar] [CrossRef]
- Biswas, M.; Bandyopadhyay, S. Synthesis of La3+ doped nanocrystalline ceria powder by urea–formaldehyde gel combustion route. Mater. Res. Bull. 2012, 47, 544–550. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Johnson, M.F.L.; Mooi, J. Cerium dioxide crystallite sizes by temperature-programmed reduction. J. Catal. 1987, 103, 502–505. [Google Scholar] [CrossRef]
- Giordano, F.; Trovarelli, A.; De Leitenburg, C.; Giona, M. A model for the temperature-programmed reduction of low and high surface area ceria. J. Catal. 2000, 193, 273–282. [Google Scholar] [CrossRef]
- Boaro, M.; Vicario, M.; De Leitenburg, C.; Dolcetti, G.; Trovarelli, A. The use of temperature-programmed and dynamic/transient methods in catalysis: Characterization of ceria-based, model three-way catalysts. Catal. Today 2003, 77, 407–417. [Google Scholar] [CrossRef]
- Désaunay, T.; Bonura, G.; Chiodo, V.; Freni, S.; Couzinié, J.P.; Bourgon, J.; Ringuedé, A.; Labat, F.; Adamo, C.; Cassir, M. Surface-dependent oxidation of H2 on CeO2 surfaces. J. Catal. 2013, 297, 193–201. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Sivamohan, R.; Ito, S.; Kasuya, A.; Fukuda, T. Structural study on monosize CeO2-x nano-particles. Nanostruct. Mater. 1999, 11, 141–147. [Google Scholar] [CrossRef]
- Yao, H.C.; Yao, Y.F.Y. Ceria in automotive exhaust catalysts: I. Oxygen storage. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Deganello, F.; Martorana, A. Phase analysis and oxygen storage capacity of ceria-lanthana-based TWC promoters prepared by sol–gel routes. J. Solid State Chem. 2002, 163, 527–533. [Google Scholar] [CrossRef]
- Bray, J.M.; Schneider, W.F. First-principles analysis of structure sensitivity in NO oxidation on Pt. ACS Catal. 2015, 5, 1087–1099. [Google Scholar] [CrossRef]
- Guillén-Hurtado, N.; Atribak, I.; Bueno-López, A.; García-García, A. Influence of the cerium precursor on the physico-chemical features and NO to NO2 oxidation activity of ceria and ceria–zirconia catalysts. J. Mol. Catal. A Chem. 2010, 323, 52–58. [Google Scholar] [CrossRef]
- Atribak, I.; Azambre, B.; Bueno López, A.; García-García, A. Effect of NOx adsorption/desorption over ceria-zirconia catalysts on the catalytic combustion of model soot. Appl. Catal. B 2009, 92, 126–137. [Google Scholar] [CrossRef]
- Azambre, B.; Atribak, I.; Bueno-López, A.; García-García, A. Probing the surface of ceria–zirconia catalysts using NOx adsorption/desorption: A first step toward the investigation of crystallite heterogeneity. J. Phys. Chem. C 2010, 114, 13300–13312. [Google Scholar] [CrossRef]
- Klingenberg, B.; Vannice, M.A. Influence of pretreatment on lanthanum nitrate, carbonate, and oxide powders. Chem. Mater. 1996, 8, 2755–2768. [Google Scholar] [CrossRef]
- Zhang, B.; Li, D.; Wang, X. Catalytic performance of La–Ce–O mixed oxide for combustion of methane. Catal. Today 2010, 158, 348–353. [Google Scholar] [CrossRef]
- Nolan, M. Molecular adsorption on the doped (110) ceria surface. J. Phys. Chem. C 2009, 113, 2425–2432. [Google Scholar] [CrossRef]
- Djerdj, I.; Garnweitner, G.; Sheng Su, D.; Niederberger, M. Morphology-controlled nonaqueous synthesis of anisotropic lanthanum hydroxide nanoparticles. J. Solid State Chem. 2007, 180, 2154–2165. [Google Scholar] [CrossRef]





| Samples | Morphology | Nominal mol.% of La | mol.% of La by ICP | Lattice Parameter (Å) a | τScherrer (nm) b | BET Surface Areas (m2/g) |
|---|---|---|---|---|---|---|
| CeO2 NC | Nanocubes | - | - | 5.413 | 21 | 38 |
| Ce0.95La0.05O2−δ NC | Nanocubes | 5 | 4.4 | 5.437 | 26 | 23 |
| Ce0.90La0.10O2−δ NC | Nanocubes | 10 | 9.7 | 5.440 | 28 | 25 |
| Ce0.85La0.15O2−δ NC+NR | Nanocubes + Nanorods | 15 | 14.4 | 5.443 | 29 | 29 |
| Samples | Ce3+ (%) | |||
|---|---|---|---|---|
| Treduction (°C) | ||||
| 200 | 350 | 500 | 700 | |
| CeO2 NC | 0.2 | 2.0 | 6.2 | 18.1 |
| Ce0.95La0.05O2−δ NC | 0.1 | 1.5 | 6.4 | 25.0 |
| Ce0.90La0.10O2−δ NC | 0.2 | 2.6 | 8.0 | 28.6 |
| Ce0.85La0.15O2−δ NC+NR | 0.4 | 2.6 | 11.2 | 37.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-García, S.; Tinoco, M.; Hungría, A.B.; Chen, X.; Calvino, J.J.; Martínez-Munuera, J.C.; Giménez-Mañogil, J.; García-García, A. NO Oxidation on Lanthanum-Doped Ceria Nanoparticles with Controlled Morphology. Catalysts 2023, 13, 894. https://doi.org/10.3390/catal13050894
Fernández-García S, Tinoco M, Hungría AB, Chen X, Calvino JJ, Martínez-Munuera JC, Giménez-Mañogil J, García-García A. NO Oxidation on Lanthanum-Doped Ceria Nanoparticles with Controlled Morphology. Catalysts. 2023; 13(5):894. https://doi.org/10.3390/catal13050894
Chicago/Turabian StyleFernández-García, Susana, Miguel Tinoco, Ana Belén Hungría, Xiaowei Chen, José Juan Calvino, Juan Carlos Martínez-Munuera, Javier Giménez-Mañogil, and Avelina García-García. 2023. "NO Oxidation on Lanthanum-Doped Ceria Nanoparticles with Controlled Morphology" Catalysts 13, no. 5: 894. https://doi.org/10.3390/catal13050894
APA StyleFernández-García, S., Tinoco, M., Hungría, A. B., Chen, X., Calvino, J. J., Martínez-Munuera, J. C., Giménez-Mañogil, J., & García-García, A. (2023). NO Oxidation on Lanthanum-Doped Ceria Nanoparticles with Controlled Morphology. Catalysts, 13(5), 894. https://doi.org/10.3390/catal13050894