Novel Laser-Assisted Chemical Bath Synthesis of Pure and Silver-Doped Zinc Oxide Nanoparticles with Improved Antimicrobial and Photocatalytic Properties
Abstract
:1. Introduction
2. Results and Discussion
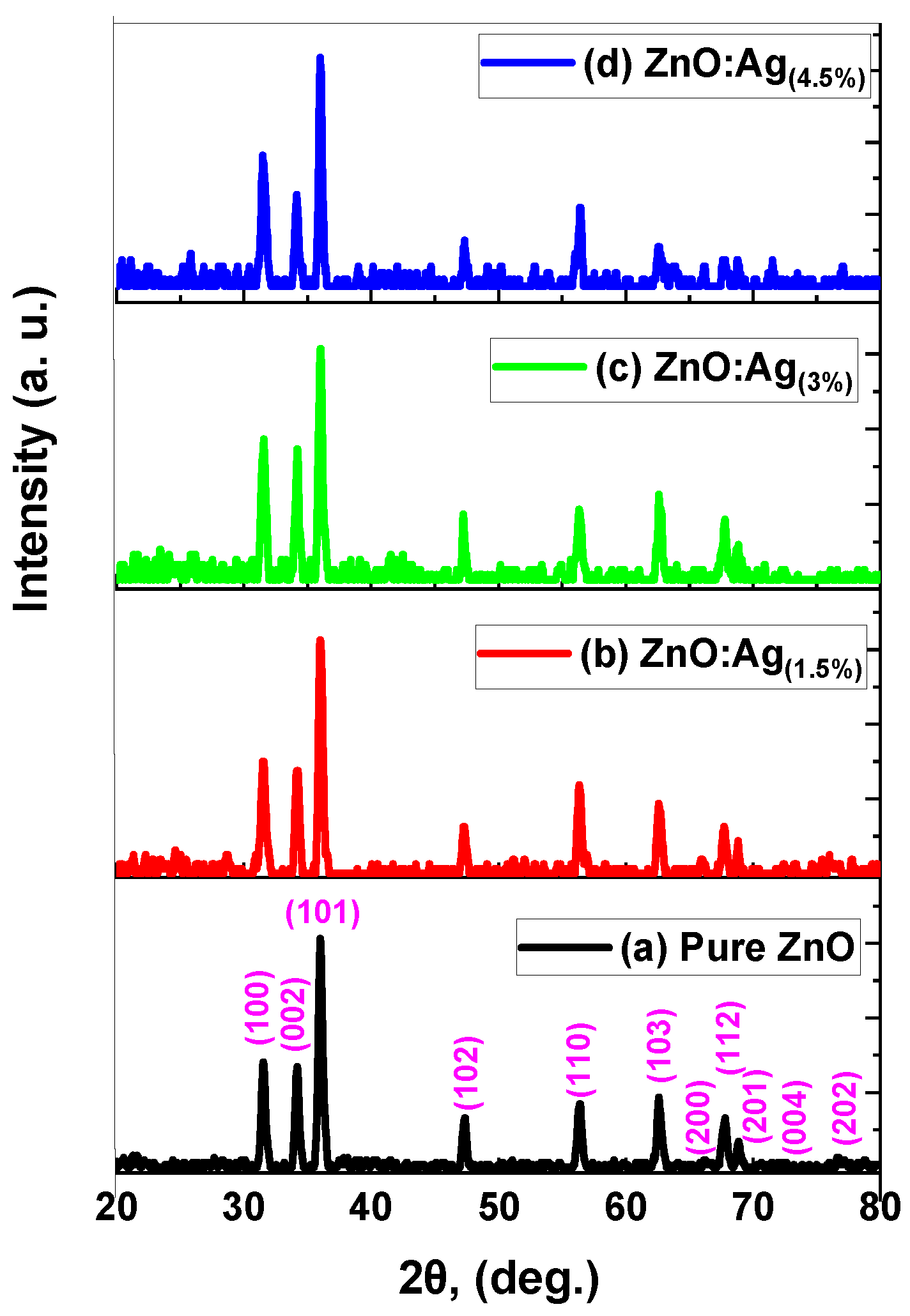
2.1. X-ray Diffraction (XRD)
2.2. Scanning Electron Microscope (SEM)
2.3. Energy Dispersive X-ray Diffractive (EDX)
2.4. UV-Vis Absorption
2.5. Fourier-Transform Infrared Spectra (FTIR)
2.6. Photocatalyst Study
2.6.1. Photocatalytic Degradation under Blue Laser Irradiation/UV–VIS Studies
2.6.2. Photocatalyst Stability
2.6.3. The Mechanism of Photocatalyst
2.7. Antimicrobial Activity of ZnO-NPs
2.7.1. ZOI of the Various Microorganisms
2.7.2. Comparison between Our Findings and Prior Investigations
2.7.3. The Antimicrobial Mechanisms of Zinc Oxide Nanoparticles
3. Materials and Methods
3.1. Starting Materials and Microbial Strain Culture Preparation
3.2. Catalyst Preparation
3.3. Synthesis of Pure and Silver-Doped Zinc Oxide Nanostructures via the LACBS Method
3.4. Photocatalytic Study
3.5. Characterization and Evaluation of Antimicrobial Efficacy of Zinc Oxide Nanostructures
3.6. Disc Diffusion Method and Zone of Inhibition (ZOI)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godman, B.; Egwuenu, A.; Wesangula, E.; Schellack, N.; Kalungia, A.C.; Tiroyakgosi, C.; Kgatlwane, J.; Mwita, J.C.; Patrick, O.; Niba, L.L.; et al. Tackling antimicrobial resistance across sub-Saharan Africa: Current challenges and implications for the future. Expert Opin. Drug Saf. 2022, 21, 1089–1111. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef]
- Di, K.N.; Pham, D.T.; Tee, T.S.; Binh, Q.A.; Nguyen, T.C. Antibiotic usage and resistance in animal production in Vietnam: A review of existing literature. Trop. Anim. Health Prod. 2021, 53, 340. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Hu, Y.; Anes, J.; Devineau, S.; Fanning, S. Klebsiella pneumoniae: Prevalence, Reservoirs, Antimicrobial Resistance, Pathogenicity, and Infection: A Hitherto Unrecognized Zoonotic Bacterium. Foodborne Pathog. Dis. 2021, 18, 63–84. [Google Scholar] [CrossRef]
- Tyrrell, C.; Burgess, C.M.; Brennan, F.P.; Walsh, F. Antibiotic resistance in grass and soil. Biochem. Soc. Trans. 2019, 47, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, G.; Girish, M. Antibiotic Resistance—A Cause for Reemergence of Infections. Indian J. Pediatr. 2020, 87, 937–944. [Google Scholar] [CrossRef]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef]
- Colomb-Cotinat, M.; Lacoste, J.; Brun-Buisson, C.; Jarlier, V.; Coignard, B.; Vaux, S. Estimating the morbidity and mortality associated with infections due to multidrug-resistant bacteria (MDRB), France, 2012. Antimicrob. Resist. Infect. Control 2016, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Drug-Resistant Infections: A Threat to Our Economic Future. 2017. Available online: https://elibrary.worldbank.org/doi/abs/10.1596/26707 (accessed on 4 March 2023).
- Buckley, G.J.; Palmer, G.H. Combating Antimicrobial Resistance and Protecting the Miracle of Modern Medicine; The National Academies Press: Washington, DC, USA, 2022; pp. 1–366. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. CDC 2013, 55–56. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 4 March 2023).
- Toda, M.; Williams, S.R.; Berkow, E.L.; Farley, M.M.; Harrison, L.H.; Bonner, L.; Marceaux, K.M.; Hollick, R.; Zhang, A.Y.; Schaffner, W.; et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia—Four Sites, United States, 2012–2016. MMWR Surveill. Summ. 2020, 68, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Crowe-McAuliffe, C.; Graf, M.; Huter, P.; Takada, H.; Abdelshahid, M.; Nováček, J.; Murina, V.; Atkinson, G.C.; Hauryliuk, V.; Wilson, D.N. Structural basis for antibiotic resistance mediated by the Bacillus subtilis ABCF ATPase VmlR. Proc. Natl. Acad. Sci. USA 2018, 115, 8978–8983. [Google Scholar] [CrossRef]
- Sholeh, M.; Krutova, M.; Forouzesh, M.; Mironov, S.; Sadeghifard, N.; Molaeipour, L.; Maleki, A.; Kouhsari, E. Antimicrobial resistance in Clostridioides (Clostridium) difficile derived from humans: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Koop, S.H.A.; van Leeuwen, C.J. The challenges of water, waste and climate change in cities. Environ. Dev. Sustain. 2017, 19, 385–418. [Google Scholar] [CrossRef]
- Boulkhessaim, S.; Gacem, A.; Khan, S.H.; Amari, A.; Yadav, V.K.; Harharah, H.N.; Elkhaleefa, A.M.; Yadav, K.K.; Rather, S.U.; Ahn, H.J.; et al. Emerging Trends in the Remediation of Persistent Organic Pollutants Using Nanomaterials and Related Processes: A Review. Nanomaterials 2022, 12, 2148. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.R.D.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2019, 17, 170. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef] [PubMed]
- Wise, R. The development of new antimicrobial agents. BMJ 1998, 317, 643–644. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Hariyanto, H.; Yahya, C.Q.; Cucunawangsih, C.; Pertiwi, C.L.P. Antimicrobial resistance and mortality. Afr. J. Infect. Dis. 2022, 16, 13–20. [Google Scholar] [CrossRef]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Yahia, I.S.; Shahwan, M.; Zyoud, A.H.; Zahran, H.Y.; Abdel-wahab, M.S.; Daher, M.G.; Nasor, M.; Makhadmeh, G.N.; Hassan, N.; et al. Fast and Excellent Enhanced Photocatalytic Degradation of Methylene Blue Using Silver-Doped Zinc Oxide Submicron Structures under Blue Laser Irradiation. Crystals 2023, 13, 229. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767. [Google Scholar] [CrossRef]
- Rajendran, R.; Mani, A. Photocatalytic, antibacterial and anticancer activity of silver-doped zinc oxide nanoparticles. J. Saudi Chem. Soc. 2020, 24, 1010–1024. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Qin, L.; Mawignon, F.J.; Hussain, M.; Ange, N.K.; Lu, S.; Hafezi, M.; Dong, G. Economic Friendly ZnO-Based UV Sensors Using Hydrothermal Growth: A Review. Materials 2021, 14, 4083. [Google Scholar] [CrossRef] [PubMed]
- Aftab, S.; Shabir, T.; Shah, A.; Nisar, J.; Shah, I.; Muhammad, H.; Shah, N.S. Highly Efficient Visible Light Active Doped ZnO Photocatalysts for the Treatment of Wastewater Contaminated with Dyes and Pathogens of Emerging Concern. Nanomaterials 2022, 12, 486. [Google Scholar] [CrossRef]
- Lemos, S.C.S.; de Lima Rezende, T.K.; Assis, M.; da Costa Romeiro, F.; Peixoto, D.A.; de Oliveira Gomes, E.; Jacobsen, G.M.; Teodoro, M.D.; Gracia, L.; Ferrari, J.L.; et al. Efficient Ni and Fe doping process in ZnO with enhanced photocatalytic activity: A theoretical and experimental investigation. Mater. Res. Bull. 2022, 152, 111849. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between Structure and Antimicrobial Activity of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.D.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Mirhosseini, F.; Amiri, M.; Daneshkazemi, A.; Zandi, H.; Javadi, Z.S. Antimicrobial Effect of Different Sizes of Nano Zinc Oxide on Oral Microorganisms. Front. Dent. 2019, 16, 105. [Google Scholar] [CrossRef]
- Zanet, V.; Vidic, J.; Auger, S.; Vizzini, P.; Lippe, G.; Iacumin, L.; Comi, G.; Manzano, M. Activity evaluation of pure and doped zinc oxide nanoparticles against bacterial pathogens and Saccharomyces cerevisiae. J. Appl. Microbiol. 2019, 127, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.S.; Shah, N.A.; Tiwari, M. Mechanism of anti-bacterial activity of zinc oxide nanoparticle against Carbapenem-Resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1218. [Google Scholar] [CrossRef]
- Mthana, M.S.; Mthiyane, M.N.; Ekennia, A.C.; Singh, M.; Onwudiwe, D.C. Cytotoxicity and antibacterial effects of silver doped zinc oxide nanoparticles prepared using fruit extract of Capsicum Chinense. Sci. Afr. 2022, 17, e01365. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Z.; Chen, D.; Liu, F.; Yang, Z.; Li, X.; Yu, H.; Liu, H.; Zhou, W. Laser Synthesis and Microfabrication of Micro/Nanostructured Materials Toward Energy Conversion and Storage. Nano-Micro Lett. 2021, 13, 49. [Google Scholar] [CrossRef]
- Palneedi, H.; Park, J.H.; Maurya, D.; Peddigari, M.; Hwang, G.T.; Annapureddy, V.; Kim, J.W.; Choi, J.J.; Hahn, B.D.; Priya, S.; et al. Laser Irradiation of Metal Oxide Films and Nanostructures: Applications and Advances. Adv. Mater. 2018, 30, e1705148. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Ahmed, N.M.; Lahewil, A.S.Z.; Omar, A.F. Micro spot ZnO nanotubes using laser assisted chemical bath deposition: A low-cost approach to UV photodetector fabrication. Sens. Actuators A Phys. 2022, 338, 113485. [Google Scholar] [CrossRef]
- Mutluay Unal, S.; Ozkir, S.E.; Seyfioglu Polat, Z.; Guven, S.; Asutay, H. The Effect of Ytterbium-Doped Fiber Laser with Different Parameters on Physical Properties of Zirconia Surface. Photomed. Laser Surg. 2017, 35, 157–163. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Wang, Z.; Zhang, C.; Wu, Y.; Zheng, H. Enhancement of antibacterial function by incorporation of silver-doped ZnO nanocrystals onto a laser-induced graphene surface. RSC Adv. 2021, 11, 33883–33889. [Google Scholar] [CrossRef]
- Masa, A.; Jehsoh, N.; Dueramae, S.; Hayeemasae, N. Boosting the Antibacterial Performance of Natural Rubber Latex Foam by Introducing Silver-Doped Zinc Oxide. Polymers 2023, 15, 1040. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.M.; Pal, K.; Kodandaram, A.; Manjula, B.L.; Ravishankar, D.K.; Gowtham, H.G.; Murali, M.; Rahdar, A.; Kyzas, G.Z. Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta. Green Process. Synth. 2022, 11, 857–867. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M.; Mahmood, A.; Kejzlar, P. Photocatalytic Behaviour of Zinc Oxide Nanostructures on Surface Activation of Polymeric Fibres. Polymers 2021, 13, 1227. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, E.; Slimani, Y.; Nawaz, M.; Trabelsi, Z.; Yasin, G.; Bilal, M.; Almessiere, M.A.; Baykal, A.; Thakur, A.; Thakur, P. Synthesis, characterization, and evaluation of the photocatalytic properties of zinc oxide co-doped with lanthanides elements. J. Phys. Chem. Solids 2022, 170, 110910. [Google Scholar] [CrossRef]
- Wagner, E.; Maudez, W.; Bagdzevicius, S.; Sandu, S.C.; Benvenuti, G. Chemical beam vapour deposition technique with Sybilla equipment: Review of main results in its 20-year anniversary. Oxide-Based Mater. Devices 2021, 11687, 135–154. [Google Scholar] [CrossRef]
- Pakma, O.; Özaydın, C.; Özden, Ş.; Kariper, I.A.; Güllü, Ö. Synthesis and characterization of vanadium oxide thin films on different substrates. J. Mater. Sci. Mater. Electron. 2017, 28, 10909–10913. [Google Scholar] [CrossRef]
- Wang, X.; Ahmad, M.; Sun, H. Three-Dimensional ZnO Hierarchical Nanostructures: Solution Phase Synthesis and Applications. Materials 2017, 10, 1304. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Popović, Z.; Chen, O.; Cui, J.; Fukumura, D.; Bawendi, M.G.; Jain, R.K. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew. Chem. Int. Ed. Engl. 2011, 50, 11417–11420. [Google Scholar] [CrossRef] [PubMed]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel Green Route of Synthesis of ZnO Nanoparticles by Using Natural Biodegradable Polymer and Its Application as a Catalyst for Oxidation of Aldehydes. J. Macromol. Sci. Part A 2014, 51, 941–947. [Google Scholar] [CrossRef]
- Li, J.; Guo, J.; Dai, H. Probing dissolved CO2(aq) in aqueous solutions for CO2 electroreduction and storage. Sci. Adv. 2022, 8, 399. [Google Scholar] [CrossRef]
- Sabry, R.S.; Rahmah, M.I.; Aziz, W.J. A systematic study to evaluate effects of stearic acid on superhydrophobicity and photocatalytic properties of Ag-doped ZnO nanostructures. J. Mater. Sci. Mater. Electron. 2020, 31, 13382–13391. [Google Scholar] [CrossRef]
- Kwon, D.; Kim, J. Silver-doped ZnO for photocatalytic degradation of methylene blue. Korean J. Chem. Eng. 2020, 37, 1226–1232. [Google Scholar] [CrossRef]
- Mohammadzadeh Kakhki, R.; Tayebee, R.; Ahsani, F. New and highly efficient Ag doped ZnO visible nano photocatalyst for removing of methylene blue. J. Mater. Sci. Mater. Electron. 2017, 28, 5941–5952. [Google Scholar] [CrossRef]
- Chauhan, A.; Verma, R.; Kumari, S.; Sharma, A.; Shandilya, P.; Li, X.; Batoo, K.M.; Imran, A.; Kulshrestha, S.; Kumar, R. Photocatalytic dye degradation and antimicrobial activities of Pure and Ag-doped ZnO using Cannabis sativa leaf extract. Sci. Rep. 2020, 10, 7881. [Google Scholar] [CrossRef]
- Zhang, L.; Mohamed, H.H.; Dillert, R.; Bahnemann, D. Kinetics and mechanisms of charge transfer processes in photocatalytic systems: A review. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 263–276. [Google Scholar] [CrossRef]
- Liu, S.; Huang, G.; Wang, J.; Bao, J.; Wang, M.; Wei, Y.; Zhong, Y.; Bai, F. Noble Metal Nanoparticle-Loaded Porphyrin Hexagonal Submicrowires Composites (M-HW): Photocatalytic Synthesis and Enhanced Photocatalytic Activity. Nanomaterials 2023, 13, 660. [Google Scholar] [CrossRef]
- Ghosh, S.; Goudar, V.S.; Padmalekha, K.G.; Bhat, S.V.; Indi, S.S.; Vasan, H.N. ZnO/Ag nanohybrid: Synthesis, characterization, synergistic antibacterial activity and its mechanism. RSC Adv. 2012, 2, 930–940. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Q.; Liu, J.; Cui, N.; Guan, G.; Huang, W. Electron promoted ZnO for catalytic synthesis of higher alcohols from syngas. Green Energy Environ. 2022, 7, 1390–1400. [Google Scholar] [CrossRef]
- Sun, M.; Kong, W.; Zhao, Y.; Liu, X.; Xuan, J.; Liu, Y.; Jia, F.; Yin, G.; Wang, J.; Zhang, J. Improving Photocatalytic Degradation Activity of Organic Pollutant by Sn4+ Doping of Anatase TiO2 Hierarchical Nanospheres with Dominant {001} Facets. Nanomaterials 2019, 9, 1603. [Google Scholar] [CrossRef]
- Wang, M.; Tan, G.; Feng, S.; Yin, L.; Wang, Y.; Zhang, B.; Lv, L.; Ren, H. Construction of Ag/NaBiO3 with dual active sites for photocatalytic NO deep oxidation and long-lasting organic pollutants degradation in the dark. J. Hazard. Mater. 2021, 416, 125877. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Naskar, A.; Lee, S.; Kim, K.S. Easy One-Pot Low-Temperature Synthesized Ag-ZnO Nanoparticles and Their Activity Against Clinical Isolates of Methicillin-Resistant Staphylococcus aureus. Front. Bioeng. Biotechnol. 2020, 8, 216. [Google Scholar] [CrossRef]
- Elbasuney, S.; El-Sayyad, G.S.; Tantawy, H.; Hashem, A.H. Promising antimicrobial and antibiofilm activities of reduced graphene oxide-metal oxide (RGO-NiO, RGO-AgO, and RGO-ZnO) nanocomposites. RSC Adv. 2021, 11, 25961–25975. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Faruque, M.R.I.; Din, I.U.; Alotaibi, M.A.; Khan, A. Synergistic effects of Cu-doped ZnO nanoantibiotic against Gram-positive bacterial strains. PLoS ONE 2021, 16, e0251082. [Google Scholar] [CrossRef]
- Manoharan, C.; Pavithra, G.; Bououdina, M.; Dhanapandian, S.; Dhamodharan, P. Characterization and study of antibacterial activity of spray pyrolysed ZnO:Al thin films. Appl. Nanosci. 2016, 6, 815–825. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on, E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.B.; Eliopoulos, G.M.; Jenkins, S.G.; James Lewis, F.S., II; Brandi Limbago, P.; Nicolau, D.P.; Robin Patel, F.; Powell, M.; Sandra Richter, F.S.; Jana Swenson, D.M.; et al. Performance Standards for Antimicrobial Susceptibility Testing Performance Standards for Antimicrobial Susceptibility Testing Suggested Citation, 29th ed.; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2019; Volume 39, ISBN 9781684400324. [Google Scholar]














| (h, k, l) | (deg) | (deg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pure ZnO | 101 | 36.16 | 2.47 | 0.78 | 1.87 | 2.87 | 4.97 | 1.73 | −11.75 | −4.56 |
| ZnO:Ag(1.5%) | 101 | 36.19 | 2.49 | 0.79 | 1.85 | 2.87 | 4.96 | 1.73 | −11.82 | −4.63 |
| ZnO:Ag(3%) | 101 | 36.20 | 2.49 | 0.86 | 1.70 | 2.86 | 4.96 | 1.73 | −11.85 | −4.66 |
| ZnO:Ag(4.5%) | 101 | 36.87 | 2.51 | 0.88 | 1.66 | 2.81 | 4.87 | 1.73 | −13.39 | −6.33 |
| Samples | Pure ZnO | ZnO:Ag(1.5%) | ZnO:Ag(3%) | ZnO:Ag(4.5%) | ||||
|---|---|---|---|---|---|---|---|---|
| Elements | wt% | At% | wt% | At% | wt% | At% | wt% | At% |
| O | 19.63 | 49.96 | 18.7 | 48.92 | 19.05 | 49.97 | 17.74 | 48.23 |
| Zn | 80.37 | 50.04 | 77.48 | 49.6 | 73.29 | 47.05 | 70.93 | 47.2 |
| Ag | 0 | 0 | 3.82 | 1.48 | 7.66 | 2.98 | 11.33 | 4.57 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Synthesis Methods | Catalyst Types | Catalyst Amounts | Dyes | Light Sources | % Deg | (min−1) | Time, (min) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Co-precipitation | ZnO:Ag(0%) | 25 mg/10 mg/L | MB (20 ppm) | Visible light | 93 | - | 80 | [59] |
| Hydrothermal | ZnO:Ag(0.05–0.5%) | 50 mg/50 ml | MB (10 ppm) | Sunlight | 92.9 | - | 210 | [60] |
| Co-precipitation | Ag-ZnO(0–6%) | 5 mg/50 ml | MB (10 ppm) | Visible light | 98 | - | 120 | [61] |
| LACBS | ZnO:Ag(0%) | 10 mg/50 mL | MB (20 ppm) | Blue | 85.22 | 0.00934 | 60 | This work |
| LACBS | ZnO:Ag(1.5%) | 10 mg/50 mL | MB (20 ppm) | Blue | 85.98 | 0.02088 | 60 | This work |
| LACBS | ZnO:Ag(3%) | 10 mg/50 mL | MB (20 ppm) | Blue | 98.71 | 0.02878 | 60 | This work |
| LACBS | ZnO:Ag(4.5%) | 10 mg/50 mL | MB (20 ppm) | Blue | 99.54 | 0.06085 | 60 | This work |
| Green combustion method | ZnO:Ag(5%) | - | MO (20 ppm) | Visible light | 94 | - | 80 | [62] |
| LACBS | ZnO:Ag(0%) | 10 mg/50 mL | MO (20 ppm) | Blue | 43.38 | 0.0164 | 60 | This work |
| LACBS | ZnO:Ag(1.5%) | 10 mg/50 mL | MO (20 ppm) | Blue | 73.53 | 0.02819 | 60 | This work |
| LACBS | ZnO:Ag(3%) | 10 mg/50 mL | MO (20 ppm) | Blue | 84.56 | 0.03782 | 60 | This work |
| LACBS | ZnO:Ag(4.5%) | 10 mg/50 mL | MO (20 ppm) | Blue | 97.43 | 0.05968 | 60 | This work |
| Pathogens | Antibiotics a | A b | Pure ZnO | ZnO-Ag(1.5%) | ZnO-Ag(3%) | ZnO-Ag(4.5%) |
|---|---|---|---|---|---|---|
| E. coli | G | 23 | 22 | 25 | 26 | 27 |
| S. aureus | G | 28 | 26 | 32 | 48 | 37 |
| Bacillus subtilis | G | 21 | 14 | 15 | 20 | 21 |
| K. pneumonia | G | 30 | 35 | 34 | 40 | 45 |
| C. albicans | NY | 16 | 32 | 35 | 30 | 40 |
| Pathogens | Materials | ZOI (mm) | Ref. |
|---|---|---|---|
| E. coli | ZnO-Ag(4.5%) | 27 | This study |
| Ag-ZnO-NPs | 21 | [41] | |
| ZnO-Ag(5%) | R a | [70] | |
| S. aureus | ZnO-Ag(4.5%) | 37 | This study |
| Ag-ZnO-NPs | 19 | [41] | |
| ZnO-Ag(5%) | 14 | [70] | |
| Bacillus subtilis | ZnO-Ag(4.5%) | 21 | This study |
| RGO-ZnO | 28.8 | [71] | |
| K. pneumonia | ZnO-Ag(4.5%) | 45 | This study |
| Cu-doped ZnO-NP | 17 | [72] | |
| Al-doped ZnO | 10 | [73] | |
| C. albicans | ZnO-Ag(4.5%) | 40 | This study |
| Pure ZnO | 7.66 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyoud, S.H.; Alalalmeh, S.O.; Hegazi, O.E.; Yahia, I.S.; Zahran, H.Y.; Sara, H.A.; Bloukh, S.H.; Shahwan, M.; Zyoud, A.H.; Hassan, N.; et al. Novel Laser-Assisted Chemical Bath Synthesis of Pure and Silver-Doped Zinc Oxide Nanoparticles with Improved Antimicrobial and Photocatalytic Properties. Catalysts 2023, 13, 900. https://doi.org/10.3390/catal13050900
Zyoud SH, Alalalmeh SO, Hegazi OE, Yahia IS, Zahran HY, Sara HA, Bloukh SH, Shahwan M, Zyoud AH, Hassan N, et al. Novel Laser-Assisted Chemical Bath Synthesis of Pure and Silver-Doped Zinc Oxide Nanoparticles with Improved Antimicrobial and Photocatalytic Properties. Catalysts. 2023; 13(5):900. https://doi.org/10.3390/catal13050900
Chicago/Turabian StyleZyoud, Samer H., Samer O. Alalalmeh, Omar E. Hegazi, Ibrahim S. Yahia, Heba Y. Zahran, Hamed Abu Sara, Samir Haj Bloukh, Moyad Shahwan, Ahed H. Zyoud, Nageeb Hassan, and et al. 2023. "Novel Laser-Assisted Chemical Bath Synthesis of Pure and Silver-Doped Zinc Oxide Nanoparticles with Improved Antimicrobial and Photocatalytic Properties" Catalysts 13, no. 5: 900. https://doi.org/10.3390/catal13050900







