Influence of Y Doping on Catalytic Activity of CeO2, MnOx, and CeMnOx Catalysts for Selective Catalytic Reduction of NO by NH3
Abstract
1. Introduction
2. Results and Discussion
2.1. Textural Characteristics
2.2. XRD Analysis
2.3. Raman Spectroscopy
2.4. H2-TPR
2.5. Catalytic Performance
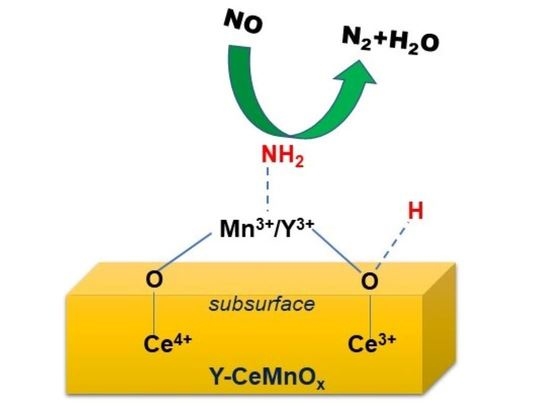
2.6. Role of Redox Properties and Surface Acidity
3. Materials and Methods
3.1. Sample Preparation
3.1.1. Sample Preparation Using Sol-Gel Citrate Method
3.1.2. Sample Modification Using Impregnation Technique
3.2. Characterization
3.2.1. Low-Temperature Nitrogen Adsorption/Desorption
3.2.2. XRD
3.2.3. Raman Spectroscopy
3.2.4. Transmission Electron Microscopy
3.2.5. H2-TPR
3.2.6. NH3-TPD
3.3. Activity Tests in SCR of NO by NH3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-García, M.A.; Pitchon, V.; Kiennemann, A. Pollution by Nitrogen Oxides: An Approach to NOx Abatement by Using Sorbing Catalytic Materials. Environ. Int. 2005, 31, 445–467. [Google Scholar] [CrossRef]
- Reşitoʇlu, I.A.; Altinişik, K.; Keskin, A. The Pollutant Emissions from Diesel-Engine Vehicles and Exhaust Aftertreatment Systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef]
- Flagiello, D.; Esposito, M.; Di Natale, F.; Salo, K. A Novel Approach to Reduce the Environmental Footprint of Maritime Shipping. J. Mar. Sci. Appl. 2021, 20, 229–247. [Google Scholar] [CrossRef]
- Chmielewski, A.G.; Sun, Y.; Zimek, Z.; Bułka, S.; Licki, J. Mechanism of NOx removal by electron beam process in the presence of scavengers. Radiat. Phys. Chem. 2002, 65, 397–403. [Google Scholar] [CrossRef]
- Wang, S.; Xu, S.; Gao, S.; Xiao, P.; Jiang, M.; Zhao, H.; Huang, B.; Liu, L.; Niu, H.; Wang, J.; et al. Simultaneous removal of SO2 and NOx from flue gas by low-temperature adsorption over activated carbon. Sci. Rep. 2021, 11, 11003. [Google Scholar] [CrossRef] [PubMed]
- Flagiello, D.; Di Natale, F.; Erto, A.; Lancia, A. Oxidative Scrubber for NOX Emission Control Using NaClO2 Aqueous Solutions. Chem. Eng. Trans. 2021, 86, 397–402. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Alemany, L.J.; Berti, F.; Busca, G.; Ramis, G.; Robba, D.; Toledo, G.P.; Trombetta, M. Characterization and Composition of Commercial V2O5-WO3-TiO2 SCR Catalysts. Appl. Catal. B Environ. 1996, 10, 299–311. [Google Scholar] [CrossRef]
- Wu, H.; He, M.; Liu, W.; Jiang, L.; Cao, J.; Yang, C.; Yang, J.; Peng, J.; Liu, Y.; Liu, Q. Application of Manganese-Containing Soil as Novel Catalyst for Low-Temperature NH3-SCR of NO. J. Environ. Chem. Eng. 2021, 9, 105426. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Zhao, X.; Liu, Z.; Zhang, T.; Niu, X.; Zhu, Y. Effects of Nb-Modified CeVO4 to Form Surface Ce-O-Nb Bonds on Improving Low-Temperature NH3-SCR DeNOx Activity and Resistance to SO2 & H2O. Fuel 2023, 331, 125799. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Hu, W.; Gao, J.; Yang, J. Vanadium in Soil-Plant System: Source, Fate, Toxicity, and Bioremediation. J. Hazard. Mater. 2021, 405, 124200. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, Y.; Qian, L.; Qi, P.; Xie, M.; Long, H. Flue Gas DeNOxing Spent V2O5-WO3/TiO2 Catalyst: A Review of Deactivation Mechanisms and Current Disposal Status. Fuel 2023, 338, 127268. [Google Scholar] [CrossRef]
- Casapu, M.; Kröcher, O.; Elsener, M. Screening of Doped MnOx-CeO2 Catalysts for Low-Temperature NO-SCR. Appl. Catal. B Environ. 2009, 88, 413–419. [Google Scholar] [CrossRef]
- Shi, J.W.; Gao, C.; Liu, C.; Fan, Z.; Gao, G.; Niu, C. Porous MnOx for Low-Temperature NH3-SCR of NOx: The Intrinsic Relationship between Surface Physicochemical Property and Catalytic Activity. J. Nanoparticle Res. 2017, 19, 194. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and Selectivity of Pure Manganese Oxides in the Selective Catalytic Reduction of Nitric Oxide with Ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-Based Oxides in the Three-Way Catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Sun, H.; Park, S.J. Recent Advances in MnOx/CeO2-Based Ternary Composites for Selective Catalytic Reduction of NOx by NH3: A Review. Catalysts 2021, 11, 1519. [Google Scholar] [CrossRef]
- Wu, X.; Yu, H.; Weng, D.; Liu, S.; Fan, J. Synergistic Effect between MnO and CeO2 in the Physical Mixture: Electronic Interaction and NO Oxidation Activity. J. Rare Earths 2013, 31, 1141–1147. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.; Tang, C.; Zhang, K.; Zhao, D.; Dong, L.; Dai, B. Highly Selective Catalytic Reduction of NOx by MnOx–CeO2–Al2O3 Catalysts Prepared by Self-Propagating High-Temperature Synthesis. J. Environ. Sci. 2019, 75, 124–135. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 Mixed Oxides Prepared by Co-Precipitation for Selective Catalytic Reduction of NO with NH3 at Low Temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.S.; Crocker, M.; Zhang, Z.S.; Zhu, A.M.; Shi, C. A Study of the Mechanism of Low-Temperature SCR of NO with NH3 on MnOx/CeO2. J. Mol. Catal. A Chem. 2013, 378, 82–90. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Xing, X.; Yang, J.; Li, J.; Yang, J.; Liu, Q. Effect of MnO2 crystal types on CeO2@MnO2 oxides catalysts for low-temperature NH3-SCR. J. Environ. Chem. Eng. 2022, 10, 108239. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, S.; Zhou, Y.; Li, X.; Wang, M.; Chen, L. Comparison of Mn doped CeO2 with different exposed facets for NH3-SCR at low temperature. J. Energy Inst. 2022, 105, 114–120. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Liu, Y.; Wang, H. Ceria Modified MnOx/TiO2 as a Superior Catalyst for NO Reduction with NH3 at Low-Temperature. Catal. Commun. 2008, 9, 2217–2220. [Google Scholar] [CrossRef]
- Zhou, B.; Xi, K.; Fan, L.J.; Zhou, Y.; Wang, Y.; Zhu, Q.L.; Lu, H.F. A Comparative Study on Ce–Pr and Ce–Mn Mixed Oxide Catalysts toward Soot Catalytic Combustion. Appl. Catal. A Gen. 2018, 562, 1–10. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, C.; Ma, M.; Guo, Z.; Yu, Y.; He, C. Rare-Earth Element Doping-Promoted Toluene Low-Temperature Combustion over Mesostructured CuMCeO: X (M = Y, Eu, Ho, and Sm) Catalysts: The Indispensable Role of in Situ Generated Oxygen Vacancies. Catal. Sci. Technol. 2018, 8, 5933–5942. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Zhong, Q.; Yao, Y. Effect of y Doping on Oxygen Vacancies of TiO2 Supported MnOX for Selective Catalytic Reduction of NO with NH3 at Low Temperature. Catal. Commun. 2012, 25, 7–11. [Google Scholar] [CrossRef]
- La Greca, E.; Kharlamova, T.S.; Grabchenko, M.V.; Consentino, L.; Savenko, D.Y.; Pantaleo, G.; Kibis, L.S.; Stonkus, O.A.; Vodyankina, O.V.; Liotta, L.F. Ag Catalysts Supported on CeO2, MnO2 and CeMnOx Mixed Oxides for Selective Catalytic Reduction of NO by C3H6. Nanomaterials 2023, 13, 873. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, J.; Sun, P.; Ye, S.; Liu, B. Effects of Mn-Doped Ceria Oxygen-Storage Material on Oxidation Activity of Diesel Soot. RSC Adv. 2017, 7, 7406–7412. [Google Scholar] [CrossRef]
- Murugan, B.; Ramaswamy, A.V.; Srinivas, D.; Gopinath, C.S.; Ramaswamy, V. Nature of Manganese Species in Ce1-xMnxO2-δ Solid Solutions Synthesized by the Solution Combustion Route. Chem. Mater. 2005, 17, 3983–3993. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Zhang, G.; Zheng, J.; Wang, T.; Liu, X.; Shu, C.; Jiang, L.; Wang, C. Enhanced Electrocatalytic Performance for Methanol Oxidation of Pt Nanoparticles on Mn3O4-Modified Multi-Walled Carbon Nanotubes. Int. J. Hydrogen Energy 2012, 37, 11167–11175. [Google Scholar] [CrossRef]
- Larbi, T.; Doll, K.; Manoubi, T. Density Functional Theory Study of Ferromagnetically and Ferrimagnetically Ordered Spinel Oxide Mn3O4. A Quantum Mechanical Simulation of Their IR and Raman Spectra. J. Alloys Compd. 2016, 688, 692–698. [Google Scholar] [CrossRef]
- Gao, T.; Fjellvåg, H.; Norby, P. A Comparison Study on Raman Scattering Properties of α- and β-MnO2. Anal. Chim. Acta 2009, 648, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.M.; Massot, M.; Poinsignon, C. Lattice Vibrations of Manganese Oxides: Part I. Periodic Structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Norby, P.; Krumeich, F.; Okamoto, H.; Nesper, R.; Fjellvåg, H. Synthesis and Properties of Layered-Structured Mn5O8 Nanorods. J. Phys. Chem. C 2010, 114, 922–928. [Google Scholar] [CrossRef]
- Schilling, C.; Hofmann, A.; Hess, C.; Ganduglia-Pirovano, M.V. Raman Spectra of Polycrystalline CeO2: A Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 20834–20849. [Google Scholar] [CrossRef]
- Schilling, C.; Ganduglia-Pirovano, M.V.; Hess, C. Experimental and Theoretical Study on the Nature of Adsorbed Oxygen Species on Shaped Ceria Nanoparticles. J. Phys. Chem. Lett. 2018, 9, 6593–6598. [Google Scholar] [CrossRef]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A.K.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying Defects in Ceria-Based Nanocrystals by UV Resonance Raman Spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. [Google Scholar] [CrossRef]
- Nakajima, A.; Yoshihara, A.; Ishigame, M. Defect-Induced Raman Spectra in Doped CeO2. Phys. Rev. B 1994, 50, 13297–13307. [Google Scholar] [CrossRef]
- Derevyannikova, E.A.; Kardash, T.Y.; Kibis, L.S.; Slavinskaya, E.M.; Svetlichnyi, V.A.; Stonkus, O.A.; Ivanova, A.S.; Boronin, A.I. The Structure and Catalytic Properties of Rh-Doped CeO2 Catalysts. Phys. Chem. Chem. Phys. 2017, 19, 31883–31897. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; La Parola, V.; Liotta, L.F.; Vodyankina, O.V. Design of Ag-CeO2/SiO2 Catalyst for Oxidative Dehydrogenation of Ethanol: Control of Ag–CeO2 Interfacial Interaction. Catal. Today 2019, 333, 2–9. [Google Scholar] [CrossRef]
- Ilieva, L.; Venezia, A.M.; Petrova, P.; Pantaleo, G.; Liotta, L.F.; Zanella, R.; Kaszkur, Z.; Tabakova, T. Effect of Y Modified Ceria Support in Mono and Bimetallic Pd-Au Catalysts for Complete Benzene Oxidation. Catalysts 2018, 8, 283. [Google Scholar] [CrossRef]
- Trawczyński, J.; Bielak, B.; Miśta, W. Oxidation of Ethanol over Supported Manganese Catalysts—Effect of the Carrier. Appl. Catal. B Environ. 2005, 55, 277–285. [Google Scholar] [CrossRef]
- Stobbe, E.R.; De Boer, B.A.; Geus, J.W. The Reduction and Oxidation Behaviour of Manganese Oxides. Catal. Today 1999, 47, 161–167. [Google Scholar] [CrossRef]
- Zhan, S.; Zhu, D.; Qiu, M.; Li, Y. Highly efficient Removal of NO with Ordered Mesoporous Manganese Oxide at Low Temperature. RSC Adv. 2015, 5, 29353–29361. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, J.; Huo, F.; Wang, J.; Cheng, S.; Kang, T.; Dai, H. Nanosized Ag/α-MnO2 Catalysts Highly Active for the Low-Temperature Oxidation of Carbon Monoxide and Benzene. Catal. Today 2011, 175, 603–609. [Google Scholar] [CrossRef]
- Tang, X.; Chen, J.; Li, Y.; Li, Y.; Xu, Y.; Shen, W. Complete Oxidation of Formaldehyde over Ag/MnOx–CeO2 Catalysts. Chem. Eng. J. 2006, 118, 119–125. [Google Scholar] [CrossRef]
- Wang, K.; Liu, X.; Tu, S.; Zhang, L.; Li, W.; Jiang, C.; Ye, D. Low Temperature Catalytic Performance of Manganese and Cerium Complex Oxide Catalyst towards Toluene. IOP Conf. Ser. Mater. Sci. Eng. 2020, 729, 012069. [Google Scholar] [CrossRef]
- Consentino, L.; Pantaleo, G.; Parola, V.L.; Migliore, C.; Greca, E.L.; Liotta, L.F. NH3-NO SCR Catalysts for Engine Exhaust Gases Abatement: Replacement of Toxic V2O5 with MnOx to Improve the Environmental Sustainability. Top. Catal. 2022, 1–10. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.; Sani, Z.; Tang, X.; Yi, H.; Zhao, S.; Yu, Q.; Zhou, Y. Advances in Selective Catalytic Oxidation of Ammonia (NH3-SCO) to Dinitrogen in Excess Oxygen: A Review on Typical Catalysts, Catalytic Performances and Reaction Mechanisms. J. Environ. Chem. Eng. 2021, 9, 104575. [Google Scholar] [CrossRef]
- Yang, S.; Liao, Y.; Xiong, S.; Qi, F.; Dang, H.; Xiao, X.; Li, J. N2 Selectivity of NO Reduction by NH3 over MnOx-CeO2: Mechanism and Key Factors. J. Phys. Chem. C 2014, 118, 21500–21508. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, Z.; Li, Z.; Gao, G.; Ji, L.; Xu, H.; Huang, W.; Qu, Z.; Yan, N. The Unique CO Activation Effects for Boosting NH3 Selective Catalytic Oxidation over CuOx–CeO2. Environ. Sci. Technol. 2022, 56, 10402–10411. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, D.; Yang, L.; Yang, Z.; Ge, H.; Lin, R.; Wang, X. Improving the K resistance effectively of CeO2-TiO2 catalyst by Nb doping for NH3-SCR reaction. Process Saf. Environ. Prot. 2022, 160, 876–886. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, K.H.; Hong, S.C. MnOx/CeO2-TiO2 Mixed Oxide Catalysts for the Selective Catalytic Reduction of NO with NH3 at Low Temperature. Chem. Eng. J. 2012, 195–196, 323–331. [Google Scholar] [CrossRef]
- Kharlamova, T.S.; Timofeev, K.L.; Salaev, M.A.; Svetlichnyi, V.A.; Vodyankina, O.V. Monolayer MgVOx/Al2O3 catalysts for propane oxidative dehydrogenation: Insights into a role of structural, redox, and acid-base properties in catalytic performance. Appl. Catal. A 2020, 598, 117574. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Shi, J.; Zhou, S.; Zhang, Z.; Zhang, S.; Guo, M. Propane Dehydrogenation over PtSnNa/La-Doped Al2O3 Catalyst: Effect of La Content. Fuel Process. Technol. 2013, 111, 94–104. [Google Scholar] [CrossRef]
- Inomata, Y.; Mino, M.; Hata, S.; Kiyonaga, E.; Morita, K.; Hikino, K.; Yoshida, K.; Haruta, M.; Murayama, T. Low-temperature NH3-SCR Activity of Nanoparticulate Gold Supported on a Metal Oxide. J. Japan Pet. Inst. 2019, 62, 234–243. [Google Scholar] [CrossRef]








| Sample | Content (wt.%) | Ce/Mn | SSA (m2/g) | V (cm3/g) | ||
|---|---|---|---|---|---|---|
| Y | Ce | Mn | ||||
| CeO2 | - | 80.6 | - | - | 40 | 0.14 |
| Y/CeO2 | 2.7 | 78.6 | - | - | 32 | 0.13 |
| Y-CeO2 | 1.8 | 79.5 | - | - | 43 | 0.16 |
| MnOx | - | - | 72.7 | - | 14 | 0.12 |
| Y/MnOx | 3.3 | - | 66.8 | - | 9 | 0.10 |
| Y-MnOx | 3.0 | - | 69.3 | - | 19 | 0.16 |
| CeMnOx | - | 58.9 | 22.6 | 1.2 | 59 | 0.24 |
| Y/CeMnOx | 2.8 | 56.8 | 21.8 | 1.2 | 50 | 0.21 |
| Y-CeMnOx | 2.1 | 57.8 | 19.0 | 1.2 | 62 | 0.24 |
| Sample | Phase Composition | Structural Parameters | DXRD, nm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase | wt.% | S.G. | Symmetry | a, Å | b, Å | c, Å | β, ° | ||
| CeO2 | fluorite | 100 | Fm-3m | cubic | 5.41 | 5.41 | 5.41 | 90 | 18 |
| Y/CeO2 | fluorite | 100 | Fm-3m | cubic | 5.40 | 5.40 | 5.40 | 90 | 18 |
| Y-CeO2 | fluorite | 100 | Fm-3m | cubic | 5.41 | 5.41 | 5.41 | 90 | 15 |
| MnOx | Mn3O4 | 75 | I41/amd | tetragonal | 5.76 | 5.76 | 9.46 | 90 | 33 |
| Mn2O3 | 25 | Ia-3 | cubic | 9.41 | 9.41 | 9.41 | 90 | 61 | |
| Y/MnOx | Mn3O4 | 34 | I41/amd | tetragonal | 5.76 | 5.76 | 9.47 | 90 | 48 |
| Mn2O3 | 11 | Ia-3 | cubic | 9.42 | 9.42 | 9.42 | 90 | 64 | |
| Mn5O8 | 55 | C12/m1 | monoclinic | 10.42 | 5.73 | 4.87 | 109.9 | 24 | |
| Y-MnOx | Mn3O4 | 100 | I41/amd | tetragonal | 5.76 | 5.76 | 9.45 | 90 | 28 |
| CeMnOx | fluorite | 100 | Fm-3m | cubic | 5.38 | 5.38 | 5.38 | 90 | 5 |
| Y/CeMnOx | fluorite | 100 | Fm-3m | cubic | 5.38 | 5.38 | 5.38 | 90 | 5 |
| Y-CeMnOx | fluorite | 100 | Fm-3m | cubic | 5.38 | 5.38 | 5.38 | 90 | 4 |
| Sample | T (°C) | NH3 Desorbed (µmol g−1) |
|---|---|---|
| MnOx | 225 | 52 |
| Y-MnOx | 245 | 105 |
| Y/MnOx | 240 | 89 |
| CeMnOx | 235 | 243 |
| Y-CeMnOx | 225 | 370 |
| Y/CeMnOx | 280 | 318 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Greca, E.; Kharlamova, T.S.; Grabchenko, M.V.; Svetlichnyi, V.A.; Pantaleo, G.; Consentino, L.; Stonkus, O.A.; Vodyankina, O.V.; Liotta, L.F. Influence of Y Doping on Catalytic Activity of CeO2, MnOx, and CeMnOx Catalysts for Selective Catalytic Reduction of NO by NH3. Catalysts 2023, 13, 901. https://doi.org/10.3390/catal13050901
La Greca E, Kharlamova TS, Grabchenko MV, Svetlichnyi VA, Pantaleo G, Consentino L, Stonkus OA, Vodyankina OV, Liotta LF. Influence of Y Doping on Catalytic Activity of CeO2, MnOx, and CeMnOx Catalysts for Selective Catalytic Reduction of NO by NH3. Catalysts. 2023; 13(5):901. https://doi.org/10.3390/catal13050901
Chicago/Turabian StyleLa Greca, Eleonora, Tamara S. Kharlamova, Maria V. Grabchenko, Valery A. Svetlichnyi, Giuseppe Pantaleo, Luca Consentino, Olga A. Stonkus, Olga V. Vodyankina, and Leonarda Francesca Liotta. 2023. "Influence of Y Doping on Catalytic Activity of CeO2, MnOx, and CeMnOx Catalysts for Selective Catalytic Reduction of NO by NH3" Catalysts 13, no. 5: 901. https://doi.org/10.3390/catal13050901
APA StyleLa Greca, E., Kharlamova, T. S., Grabchenko, M. V., Svetlichnyi, V. A., Pantaleo, G., Consentino, L., Stonkus, O. A., Vodyankina, O. V., & Liotta, L. F. (2023). Influence of Y Doping on Catalytic Activity of CeO2, MnOx, and CeMnOx Catalysts for Selective Catalytic Reduction of NO by NH3. Catalysts, 13(5), 901. https://doi.org/10.3390/catal13050901