Advances in Catalytic Decomposition of N2O by Noble Metal Catalysts
Abstract
:1. Introduction
2. Utilization of Noble Metal Catalysts
2.1. Rh-Based Catalysts
2.1.1. The Effect of Support
2.1.2. The Effect of Particle Size and Valence States of Rh
2.1.3. The Effect of Catalyst Preparation Methods
2.2. Ru-Based Catalysts
2.2.1. The Effect of Support
2.2.2. The Effect of Particle Size and the Chemical Valences of Ru
2.2.3. The Effect of Preparation Methods
2.3. Pd-Based Catalysts
2.4. Pt-Based Catalysts
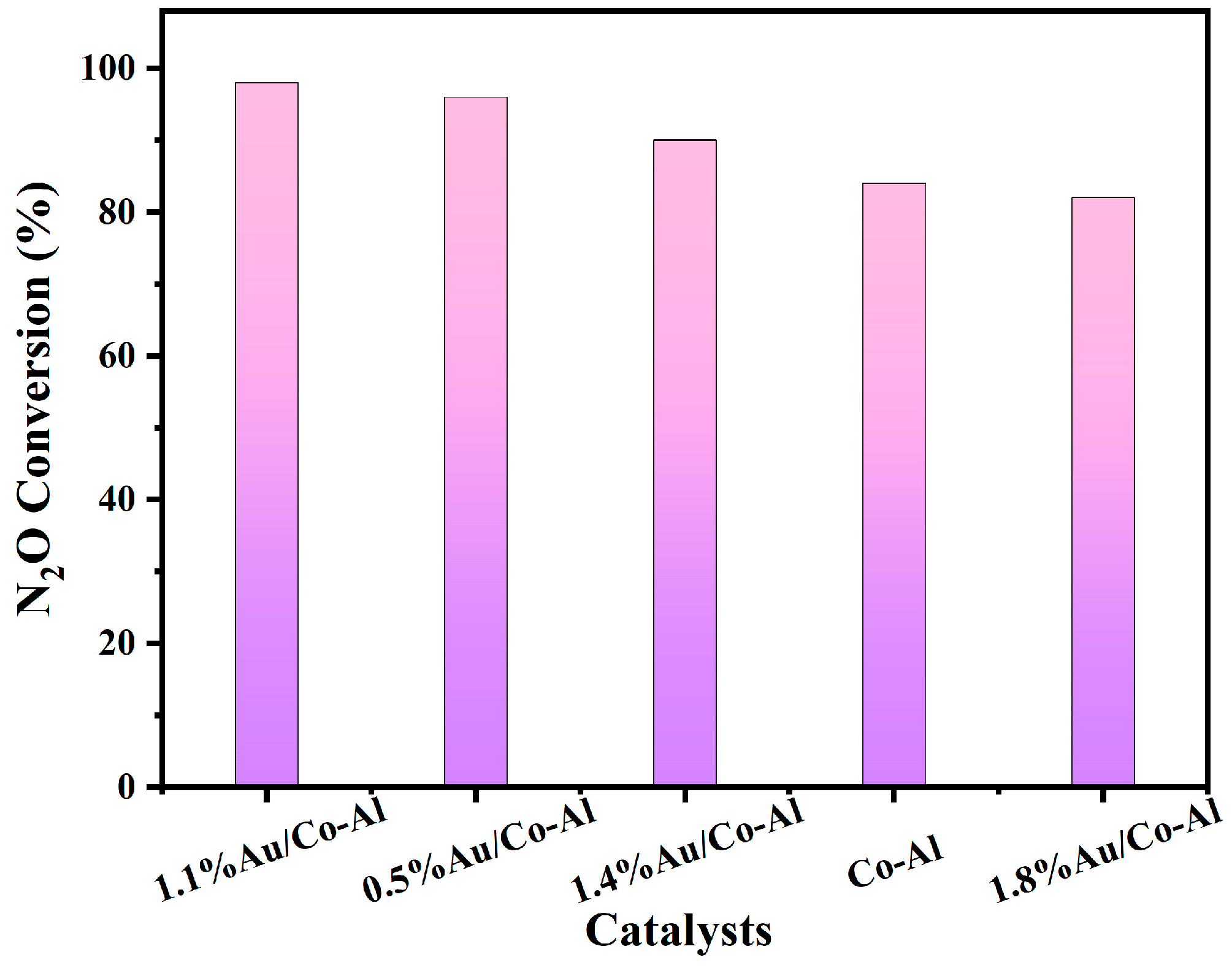
2.5. Au-Based Catalysts
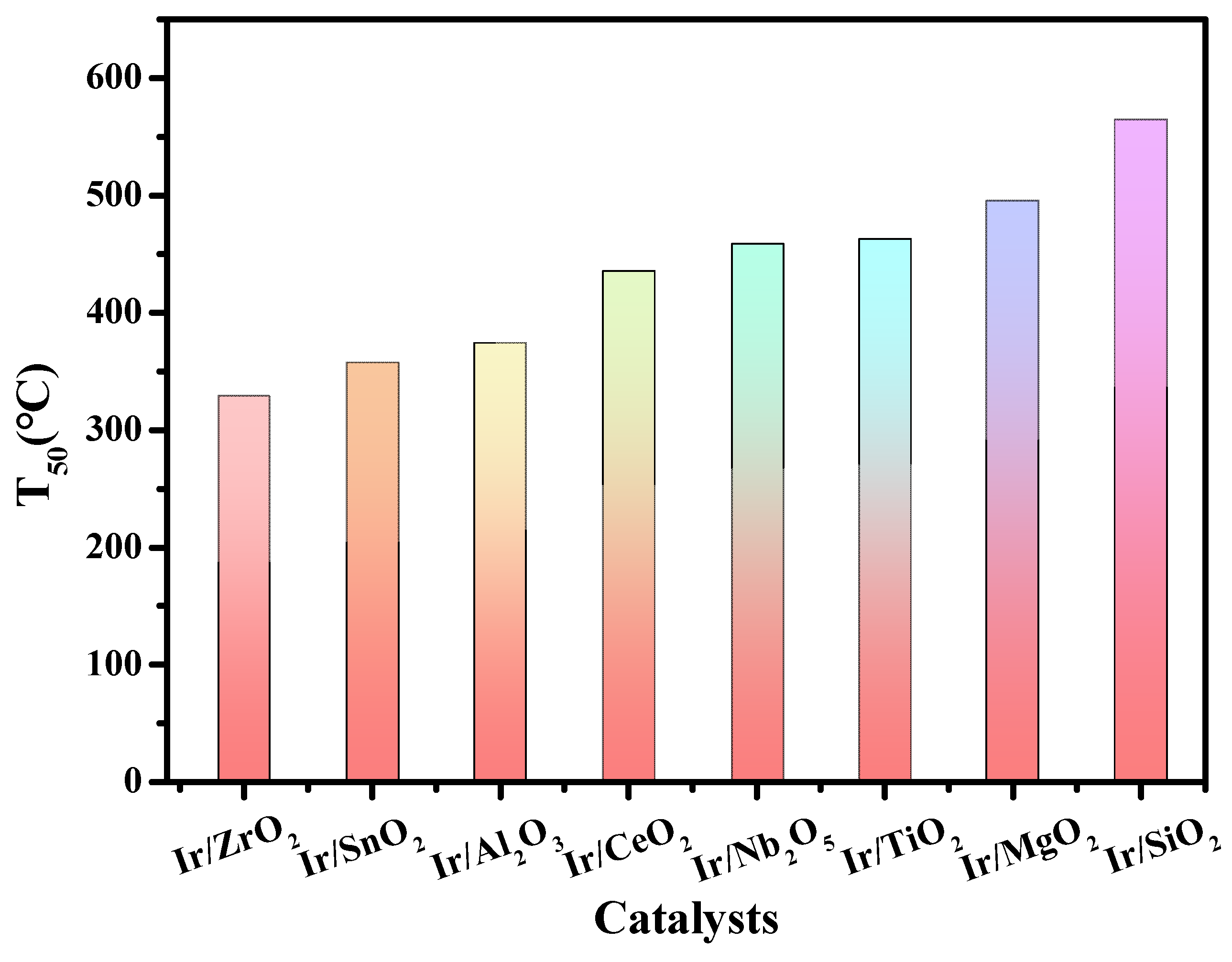
2.6. Ir-Based Catalysts
3. The Effect of Additives and Impurity Gases
3.1. The Effect of Additives
3.2. The Effect of Impurity Gases
3.2.1. The Effect of O2
3.2.2. The Effect of H2O
3.2.3. The Effect of SO2 and CO2
3.2.4. The Effect of NO
4. Catalytic Mechanisms
5. Industrial Applications of Noble Metal Catalysts
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Yun, J.G.; Lee, H.M.; Kim, J.Y.; Yun, J.H.; Hong, J.G. Dependence of N2O/NO Decomposition and Formation on Temperature and Residence Time in Thermal Reactor. Energies 2021, 14, 1153. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, G. Potentials of Using Dietary Plant Secondary Metabolites to Mitigate Nitrous Oxide Emissions from Excreta of Cattle: Impacts, Mechanisms and Perspectives. Anim. Nutr. 2022, 9, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, J.; Hu, J.; Han, J. Reducing Nitrous Oxide Emissions to Mitigate Climate Change and Protect the Ozone Layer. Environ. Sci. Technol. 2014, 48, 5290–5297. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Kanter, D. Inventories and Scenarios of Nitrous Oxide Emissions. Environ. Res. Lett. 2014, 9, 105012. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Russo, N.; Mescia, D.; Fino, D.; Saracco, G.; Specchia, V. N2O Decomposition over Perovskite Catalysts. Ind. Eng. Chem. Res. 2007, 46, 4226–4231. [Google Scholar] [CrossRef]
- Lee, S.J.; Ryu, I.S.; Kim, B.M.; Moon, S.H. A Review of the Current Application of N2O Emission Reduction in CDM Projects. Int. J. Greenhouse Gas Control 2011, 5, 167–176. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, C.; Tu, T.R.; Ma, R.; Hao, Q. Changes in soil greenhouse gas concentrations induced by plastic film mulching in a hot pepper–radish rotation. Int. J. Environ. Sci. Technol. 2022, 1–14. [Google Scholar] [CrossRef]
- Hinokuma, S.; Iwasa, T.; Kon, Y.; Taketsugu, T.; Sato, K. N2O Decomposition Properties of Ru Catalysts Supported on Various Oxide Materials and SnO2. Sci. Rep. 2020, 10, 21605. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J. Prospects of N2O Emission Regulations in the European Fertilizer Industry. Appl. Catal. B Environ. 2007, 70, 31–35. [Google Scholar] [CrossRef]
- Lin, F.; Andana, T.; Wu, Y.; Szanyi, J.; Wang, Y.; Gao, F. Catalytic Site Requirements for N2O Decomposition on Cu-, Co-, and Fe-SSZ-13 Zeolites. J. Catal. 2021, 401, 70–80. [Google Scholar] [CrossRef]
- Li, Y.; Sundermann, A.; Gerlach, O.; Low, K.; Zhang, C.; Zheng, X.; Zhu, H.; Axnanda, S. Catalytic decomposition of N2O on supported Rh catalysts. Catal. Today 2020, 355, 608–619. [Google Scholar] [CrossRef]
- Tran, K.Q.; Kilpinen, P.; Kumar, N. In-situ Catalytic Abatement of NOx During Fluidized Bed Combustion-A Literature Study. Appl. Catal. B Environ. 2008, 78, 129–138. [Google Scholar] [CrossRef]
- Zabilskiy, E. In-situ XAS Study of Catalytic N2O Decomposition over CuO/CeO2 Catalysts. ChemCatChem 2021, 13, 1814–1823. [Google Scholar] [CrossRef]
- Zhang, F.F.; Wang, X.P.; Zhang, X.X.; Turxun, M.; Yu, H.B.; Zhao, J.J. The Catalytic Activity of NiO for N2O Decomposition Doubly Promoted by Barium and Cerium. Chem. Eng. J. 2014, 256, 365–371. [Google Scholar] [CrossRef]
- Komvokis, V.G.; Marnellos, G.E.; Vasalos, I.A.; Triantafyllidis, K.S. Effect of Pretreatment and Regeneration Conditions of Ru/γ-Al2O3 Catalysts for N2O Decomposition and/or Reduction in O2-rich Atmospheres and in the Presence of NOX, SO2 and H2O. Appl. Catal. B Environ. 2009, 89, 627–634. [Google Scholar] [CrossRef]
- Hermes, A.C.; Hamilton, S.M.; Hopkins, W.S.; Harding, D.J.; Kerpal, C.; Meijer, G.; Fielicke, A.; Mackenzie, S.R. Effects of Coadsorbed Oxygen on the Infrared Driven Decomposition of N2O on Isolated Rh5+ Clusters. J. Phys. Chem. Lett. 2011, 2, 3053–3057. [Google Scholar] [CrossRef]
- Xie, P.; Luo, Y.; Ma, Z.; Wang, L.; Huang, C.; Yue, Y.; Hua, W.; Gao, Z. CoZSM-11 Catalysts for N2O Decomposition: Effect of Preparation Methods and Nature of Active Sites. Appl. Catal. B Environ. 2015, 170, 34–42. [Google Scholar] [CrossRef]
- Farhan, K.M.; Thabassum, A.; Ismail, T.M.; Sajith, P.K. Theoretical Investigation into the Effect of Water on the N2O Decomposition Reaction over the Cu-ZSM-5 Catalyst. Catal. Sci. Technol. 2022, 12, 1466–1475. [Google Scholar] [CrossRef]
- Jing, Y.; Taketoshi, K.; Zhang, N.; He, C.; Toyao, T.; Maeno, Z.; Ohori, T.; Ishikawa, N.; Shimizu, K. Catalytic Decomposition of N2O in the Presence of O2 through Redox of Rh Oxide in a RhOx/ZrO2 Catalyst. ACS Catal. 2022, 12, 6325–6333. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Larimi, A.; Rezaei, M. Preparation and Evaluation of A/BaO-MnOx Catalysts (A: Rh, Pt, Pd, Ru) in Lean Methane Catalytic Combustion at Low Temperature. Int. J. Energy Res. 2022, 46, 6292–6313. [Google Scholar] [CrossRef]
- Pinna, F.; Scarpa, M.; Strukul, G.; Guglielminotti, E.; Boccuzzi, F.; Manzoli, M. Ru/ZrO2 Catalysts: II. N2O Adsorption and Decomposition. J. Catal. 2000, 192, 158–162. [Google Scholar] [CrossRef]
- Jo, J.O.; Trinh, Q.H.; Kim, S.H.; Mok, Y.S. Plasma-Catalytic Decomposition of Nitrous Oxide over Gamma-Alumina-Supported Metal Oxides. Catal. Today 2018, 310, 42–48. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.F.; Wang, A.Q.; Li, L.; Liu, X.Y.; Zhang, T.; Mou, C.Y.; Li, J. Bimetallic Au–Pd Alloy Catalysts for N2O Decomposition: Effects of Surface Structures on Catalytic Activity. J. Phys. Chem. C 2012, 116, 6222–6232. [Google Scholar] [CrossRef]
- Kim, K.; Baek, S.; Kim, J.J.; Han, J.W. Catalytic Decomposition of N2O on PdxCuy Alloy Catalysts: A Density Functional Theory Study. Appl. Surf. Sci. 2020, 510, 145349. [Google Scholar] [CrossRef]
- Long, R.; Yang, R.T. Pt/MCM-41 Catalyst for Selective Catalytic Reduction of Nitric Oxide with Hydrocarbons in the Presence of Excess Oxygen. Catal. Lett. 1998, 52, 91–96. [Google Scholar] [CrossRef]
- Arenas-Alatorre, J.; Gómez-Cortés, A.; Avalos-Borja, M.; Díaz, G. Surface Properties of Ni-Pt/SiO2 Catalysts for N2O Decomposition and Reduction by H2. J. Phys. Chem. B 2005, 109, 2371–2376. [Google Scholar] [CrossRef]
- Xu, X.L.; Xu, X.F.; Zhang, G.T.; Niu, X.J. Preparation of Co-Al Mixed Oxide-Supported Gold Catalysts and Their Catalytic Activity for N2O Decomposition. J. Fuel Chem. Technol. 2009, 37, 595–600. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Papista, E.; Marnellos, G.E.; Tavares, P.B.; Maldonado-Hódar, F.J.; Konsolakis, M. Catalytic Decomposition of N2O on Inorganic Oxides: Εffect of Doping with Au Nanoparticles. Mol. Catal. 2017, 436, 78–89. [Google Scholar] [CrossRef]
- Ohnishi, C.; Iwamoto, S.; Inoue, M. Direct Decomposition of Nitrous Oxide in the Presence of Oxygen over Iridium Catalyst Supported on Alumina. Chem. Eng. Sci. 2008, 63, 5076–5082. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, X.; Wang, A.; Cong, Y.; Zhang, T. A Novel Ir-hexaaluminate Catalyst for N2O as a Propellant. Chem. Commun. 2007, 17, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Wu, Y.Y.; Takeda, R.; Matsunami, A.; Arai, N.; Tagawa, T.; Goto, S. Catalytic Decomposition of N2O in Medical Operating Rooms over Rh/Al2O3, Pd/Al2O3, and Pt/Al2O3. Appl. Catal. B Environ. 2001, 35, 43–51. [Google Scholar] [CrossRef]
- Parres-Esclapez, S.; Illán-Gómez, M.J.; de Lecea, C.S.M.; Bueno-López, A. On the Importance of the Catalyst Redox Properties in the N2O Decomposition over Alumina and Ceria Supported Rh, Pd and Pt. Appl. Catal. B Environ. 2010, 96, 370–378. [Google Scholar] [CrossRef]
- Yuzaki, K.; Yarimizu, T.; Ito, S.I.; Kunimori, K.K. Catalytic Decomposition of N2O over Supported Rhodium Catalysts: High Activities of Rh/USY and Rh/Al2O3 and the Effect of Rh Precursors. Catal. Lett. 1997, 47, 173–175. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, S.J.; Hong, S.C. Effect of CeO2 Addition to Rh/Al2O3 Catalyst on N2O Decomposition. Chem. Eng. J. 2011, 169, 173–179. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Drozdek, M.; Rutkowska, M.; Dziembaj, R.; Michalik, M.; Cool, P.; Vansant, E.F. SBA-15 Mesoporous Silica Modified with Rhodium by MDD Method and its Catalytic Role for N2O Decomposition Reaction. J. Porous Mater. 2010, 18, 483–491. [Google Scholar] [CrossRef]
- Xu, X.; Xu, H.; Kapteijn, F.; Moulijn, J.A. SBA-15 based catalysts in catalytic N2O decomposition in a model tail-gas from nitric acid plants. Appl. Catal. B Environ. 2004, 53, 265–274. [Google Scholar] [CrossRef]
- Hussain, M.; Fino, D.; Russo, N. N2O Decomposition by Mesoporous Silica Supported Rh Catalysts. J. Hazard. Mater. 2012, 211, 255–265. [Google Scholar] [CrossRef]
- Piumetti, M.; Hussain, M.; Fino, D.; Russo, N. Mesoporous Silica Supported Rh catalysts for High Concentration N2O Decomposition. Appl. Catal. B Environ. 2015, 165, 158–168. [Google Scholar] [CrossRef]
- Liu, H.; Lin, Y.; Ma, Z. Rh2O3/mesoporous MOx-Al2O3 (M = Mn, Fe, Co, Ni, Cu, Ba) Catalysts: Synthesis, Characterization, and Catalytic Applications. Chin. J. Catal. 2016, 37, 73–82. [Google Scholar] [CrossRef]
- Huang, C.Y.; Ma, Z.; Xie, P.F.; Yue, Y.H.; Hua, W.M.; Gao, Z. Hydroxyapatite-supported Rhodium Catalysts for N2O Decomposition. J. Mol. Catal. A Chem. 2015, 400, 90–94. [Google Scholar] [CrossRef]
- Lin, Y.; Meng, T.; Ma, Z. Catalytic Decomposition of N2O over RhOx Supported on Metal Phosphates. J. Inst. Chem. Eng. 2015, 28, 138–146. [Google Scholar]
- Liu, H.; Ma, Z. Effect of Different LaPO4 Supports on the Catalytic Performance of Rh2O3/LaPO4 in N2O Decomposition and CO Oxidation. J. Taiwan Inst. Chem. Eng. 2017, 71, 373–380. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Z. Rh2O3 /monoclinic CePO4 Composite Catalysts for N2O Decomposition and CO Oxidation. Chin. J. Chem. Eng. 2018, 26, 109–115. [Google Scholar] [CrossRef]
- Parres-Esclapez, S.; López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J.; Ura, B.; Trawczynski, J. Rh–Sr/Al2O3 Catalyst for N2O Decomposition in the Presence of O2. Top. Catal. 2009, 52, 1832–1836. [Google Scholar] [CrossRef]
- Beyer, H.; Emmerich, J.; Chatziapostolou, K.; Köhler, K. Decomposition of Nitrous Oxide by Rhodium Catalysts: Effect of Rhodium Particle Size and Metal Oxide Support. Appl. Catal. A 2011, 391, 411–416. [Google Scholar] [CrossRef]
- Xie, S.; Kim, D.; Ye, K.; Tetard, L.; Liu, F. Regulating local coordination environment of rhodium single atoms in Rh/CeO2 catalysts for N2O decomposition. J. Rare Earths 2023, 41, 941–951. [Google Scholar] [CrossRef]
- Wang, S.W.; Yan, B.; Chai, J.; Li, T.H.; Yu, H.B.; Li, T.; Cao, P.; Yang, F.; Yuan, X.M.; Yin, H.F. Rhodium Encapsulated within Silicalite-1 Zeolite as Highly Efficient Catalyst for Nitrous Oxide Decomposition: From Single Atoms to Nanoclusters and Nanoparticles. Eur. J. Inorg. Chem. 2021, 2021, 2201–2210. [Google Scholar] [CrossRef]
- Ho, P.H.; Jablonska, M.; Palkovits, R.; Rodriguez-Castellon, E.; Ospitali, F.; Fornasari, G.; Vaccari, A.; Benito, P. N2O catalytic decomposition on electrodeposited Rh-based open-cell metallic foams. Chem. Eng. J. 2020, 379, 122259. [Google Scholar] [CrossRef]
- Zheng, J.; Meyer, S.; Köhler, K. Abatement of Nitrous Oxide by Ruthenium Catalysts: Influence of the Support. Appl. Catal. A 2015, 505, 44–51. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, Y.; Wang, Y.; Li, L.; Liu, X.Y.; Lv, F.; Wang, A.; Li, W.C.; Zhang, T. RuO2/rutile-TiO2: A Superior Catalyst for N2O Decomposition. J. Mater. Chem. A 2014, 2, 5178–5781. [Google Scholar] [CrossRef]
- Hussain, M.; Akhter, P.; Fino, D.; Russo, N. Modified KIT-6 and SBA-15-spherical Supported Metal Catalysts for N2O Decomposition. J. Environ. Chem. Eng. 2013, 1, 164–174. [Google Scholar] [CrossRef]
- Kawi, S.; Liu, S.Y.; Shen, S.C. Catalytic Decomposition and Reduction of N2O on Ru/MCM-41 Catalyst. Catal. Today 2001, 68, 237–244. [Google Scholar] [CrossRef]
- Cui, Y.W.; Liu, H.; Lin, Y.; Ma, Z. Metal Phosphate-supported RuOx Catalysts for N2O Decomposition. J. Taiwan Inst. Chem. Eng. 2016, 67, 254–262. [Google Scholar] [CrossRef]
- Christoforou, S.C.; Efthimiadis, E.A.; Vasalos, I.A. Catalytic Conversion of N2O to N2 over Metal-based Catalysts in the Presence of Hydrocarbons and Oxygen. Catal. Lett. 2002, 79, 137–147. [Google Scholar] [CrossRef]
- Chang, Y.F.; McCarty, J.G.; Wachsman, E.D. Effect of Ruthenium-loading on the Catalytic Activity of Ru-NaZSM-5 Zeolites for Nitrous Oxide Decomposition. Appl. Catal. B Environ. 1995, 6, 21–33. [Google Scholar] [CrossRef]
- Sui, C.; Yuan, F.L.; Zhang, Z.P.; Zhang, C.; Niu, X.Y.; Zhu, Y.J. Effect of Ru Species on N2O Decomposition over Ru/Al2O3 Catalysts. Catalysts 2016, 6, 173. [Google Scholar] [CrossRef]
- Komvokis, V.G.; Marti, M.; Delimitis, A.; Vasalos, I.A.; Triantafyllidis, K.S. Catalytic Decomposition of N2O over Highly Active Supported Ru Nanoparticles (≤3 nm) Prepared by Chemical Reduction with Ethylene Glycol. Appl. Catal. B Environ. 2011, 103, 62–71. [Google Scholar] [CrossRef]
- Reddy, P.S.S.; Pasha, N.; Rao, M.G.V.C.; Lingaiah, N.; Suryanarayana, I.; Prasad, P.S.S. Direct Decomposition of Nitrous Oxide over Ru/Al2O3 Catalysts Prepared by Deposition–precipitation Method. Catal. Commun. 2007, 8, 1406–1410. [Google Scholar] [CrossRef]
- Cheng, D.G.; Zhu, C.; Zhao, X.; Chen, F.; Zhan, X. Influence of Pd on FeAlPO-5 Zeolite in the Catalytic Reduction of N2O with Methane. React. Kinet. Mech. Catal. 2011, 103, 219–226. [Google Scholar] [CrossRef]
- Dacquin, J.; Dujardin, C.; Granger, P. Surface Reconstruction of Supported Pd on LaCoO3: Consequences on the Catalytic Properties in the Decomposition of N2O. J. Catal. 2008, 253, 37–49. [Google Scholar] [CrossRef]
- Richards, N.; Carter, J.H.; Nowicka, E.; Parker, L.A.; Pattisson, S.; He, Q.; Dummer, N.F.; Golunski, S.; Hutchings, G.J. Structure-sensitivity of alumina supported palladium catalysts for N2O decomposition. Appl. Catal. B Environ. 2020, 264, 118501. [Google Scholar] [CrossRef]
- Konsolakis, M.; Drosou, C.; Yentekakis, I.V. Support Mediated Promotional Effects of Rare Earth oxides (CeO2 and La2O3) on N2O Decomposition and N2O Reduction by CO or C3H6 over Pt/Al2O3 Structured Catalysts. Appl. Catal. B Environ. 2012, 123, 405–413. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhu, J.; Yin, J.; Wang, H. Experimental Research on Catalytic Decomposition of Nitrous Oxide on Supported Catalysts. Energy Convers. Manag. 2009, 50, 1304–1307. [Google Scholar] [CrossRef]
- Haruta, M.; Daté, M. Advances in the Catalysis of Au Nanoparticles. Appl. Catal. A Gen. 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Hvolbæk, B.; Janssens, T.V.; Clausen, B.S.; Falsig, H.; Christensen, C.H.; Nørskov, J.K. Catalytic Activity of Au Nanoparticles. Nano Today 2007, 2, 14–18. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, X.; Ren, T.; Zhang, H.; Wang, X.; Suo, J. Superior Performance of Nano-Au Supported over Co3O4 Catalyst in Direct N2O Decomposition. Chem. Commun. 2002, 1, 860–861. [Google Scholar] [CrossRef]
- Maihom, T.; Wannakao, S.; Boekfa, B.; Limtrakul, J. Density Functional Study of the Activity of Gold-supported ZSM-5 Zeolites for Nitrous Oxide Decomposition. Chem. Phys. Lett. 2013, 556, 217–224. [Google Scholar] [CrossRef]
- Wu, L.Y.; Chen, C.; Luo, L.; Wang, Y.C.; Yin, B. DFT Study of the Reaction Mechanism of N2O Decomposition on Au3(+/0/−) Clusters. ChemistrySelect 2020, 5, 5391–5399. [Google Scholar] [CrossRef]
- Liu, S.; Cong, Y.; Huang, Y.; Zhao, X.; Zhang, T. TiO2 Promoted Ir/Al2O3 Catalysts for Direct Decomposition of N2O. Catal. Today 2011, 175, 264–270. [Google Scholar] [CrossRef]
- Shen, Q.; Li, L.D.; Hao, Z.P.; Xu, Z.P. Highly Active and Stable Bimetallic Ir/Fe-USY Catalysts for Direct and NO-Assisted N2O Decomposition. Appl. Catal. B Environ. 2008, 84, 734–741. [Google Scholar]
- Hinokuma, S.; Iwasa, T.; Kon, Y.; Taketsugu, T.; Sato, K. Effects of support materials and Ir loading on catalytic N2O decomposition properties. Catal. Commun. 2021, 149, 106208. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Kampouri, S.; Betsi-Argyropoulou, I.; Panagiotopoulou, P.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Ir-Catalysed Nitrous oxide (N2O) Decomposition: Effect of Ir Particle Size and Metal-Support Interactions. Catal. Lett. 2018, 148, 341–347. [Google Scholar] [CrossRef]
- Pachatouridou, E.; Papista, E.; Iliopoulou, E.F.; Delimitis, A.; Goula, G.; Yentekakis, I.V.; Marnellos, G.E.; Konsolakis, M. Nitrous Oxide Decomposition over Al2O3 Supported Noble Metals (Pt, Pd, Ir): Effect of Metal Loading and Feed Composition. J. Environ. Chem. Eng. 2015, 3, 815–821. [Google Scholar] [CrossRef]
- Pachatouridou, E.; Papista, E.; Delimitis, A.; Vasiliades, M.A.; Efstathiou, A.M.; Amiridis, M.D.; Alexeev, O.S.; Bloom, D.; Marnellos, G.E.; Konsolakis, M.; et al. N2O Decomposition over Ceria-promoted Ir/Al2O3 Catalysts: The Role of Ceria. Appl. Catal. B Environ. 2016, 187, 259–268. [Google Scholar] [CrossRef]
- Haber, J.; Nattich, M.; Machej, T. Alkali-metal Promoted Rhodium-on-alumina Catalysts for Nitrous Oxide Decomposition. Appl. Catal. B Environ. 2008, 77, 278–283. [Google Scholar] [CrossRef]
- Bozorgi, B.; Karimi-Sabet, J.; Khadiv-Parsi, P. The removal of N2O from gas stream by catalytic decomposition over Pt-alkali metal/SiO2. Environ. Technol. Innov. 2022, 26, 102344. [Google Scholar] [CrossRef]
- Konsolakis, M.; Aligizou, F.; Goula, G.; Yentekakis, I.V. N2O Decomposition over Doubly-promoted Pt(K)/Al2O3–(CeO2–La2O3) Structured Catalysts: On the Combined Effects of Promotion and Feed Composition. Chem. Eng. J. 2013, 230, 286–295. [Google Scholar] [CrossRef]
- Konsolakis, M.; Yentekakis, I.V.; Pekridis, G.; Kaklidis, N.; Psarras, A.C.; Marnellos, G.E. Insights into the Role of SO2 and H2O on the Surface Characteristics and de-N2O Efficiency of Pd/Al2O3 Catalysts during N2O Decomposition in the Presence of CH4 and O2 Excess. Appl. Catal. B Environ. 2013, 13, 191–198. [Google Scholar] [CrossRef]
- Marnellos, G.E.; Efthimiadis, E.A.; Vasalos, I.A. Effect of SO2 and H2O on the N2O Decomposition in the Presence of O2 over Ru/Al2O3. Appl. Catal. B Environ. 2003, 46, 523–539. [Google Scholar] [CrossRef]
- Huang, C.Y.; Jiang, Y.X.; Ma, Z.; Xie, P.F.; Lin, Y.; Meng, T.; Miao, C.X.; Yue, Y.H.; Hua, W.M.; Gao, Z. Correlation among Preparation Methods/conditions, Physicochemical Properties, and Catalytic Performance of Rh/hydroxyapatite Catalysts in N2O Decomposition. J. Mol. Catal. A Chem. 2016, 420, 73–81. [Google Scholar] [CrossRef]
- Pieterse, J.A.Z.; Mul, G.; Melian-Cabrera, I.; van den Brink, R.W. Synergy between metals in bimetallic zeolite supported catalyst for NO-promoted N2O decomposition. Catal. Lett. 2005, 99, 41–44. [Google Scholar] [CrossRef]
- Sobalik, Z.; Jisa, K.; Kaucky, D.; Vondrova, A.; Tvaruzkova, Z.; Novakova, J. Effect of noble metals in the decomposition of nitrous oxide over Fe-ferrierites. Catal. Lett. 2007, 113, 124–129. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, Y.J.; Lee, S.J.; Ryu, I.S.; Kim, H.J.; Kim, Y.; Ko, C.H.; Jeon, S.G. Enhanced Catalytic Activity of the Rh/-Al2O3 pellet catalyst for N2O Decomposition using High Rh Dispersion Induced by Citric Acid. Chem. Eng. Res. Des. 2019, 141, 455–463. [Google Scholar] [CrossRef]
- Miao, M.; Zhang, M.; Kong, H.; Zhou, T.; Yang, X.; Yang, H. Progress in Catalytic Decomposition and Removal of N2O in Fluidized Bed. Energies 2021, 14, 6148. [Google Scholar] [CrossRef]
- Liu, Z.M.; He, F.; Ma, L.L.; Peng, S. Recent Advances in Catalytic Decomposition of N2O on Noble Metal and Metal Oxide Catalysts. Catal. Surv. Asia 2016, 20, 121–132. [Google Scholar] [CrossRef]
- Burch, R.; Daniells, S.T.; Breen, J.P.; Hu, P. A combined transient and computational study of the dissociation of N2O on platinum catalysts. J. Catal. 2004, 224, 252–260. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, B.S. Catalytic removal of nitrogen oxides (NO, NO2, N2O) from ammonia-fueled combustion exhaust: A review of applicable technologies. Chem. Eng. J. 2023, 461, 141958. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tian, Z.; Huang, L.; Fan, H.; Hou, Q.; Cui, P.; Wang, W. Advances in Catalytic Decomposition of N2O by Noble Metal Catalysts. Catalysts 2023, 13, 943. https://doi.org/10.3390/catal13060943
Zhang Y, Tian Z, Huang L, Fan H, Hou Q, Cui P, Wang W. Advances in Catalytic Decomposition of N2O by Noble Metal Catalysts. Catalysts. 2023; 13(6):943. https://doi.org/10.3390/catal13060943
Chicago/Turabian StyleZhang, Yong, Zhigao Tian, Lin Huang, Honghong Fan, Qiufei Hou, Ping Cui, and Wanqiang Wang. 2023. "Advances in Catalytic Decomposition of N2O by Noble Metal Catalysts" Catalysts 13, no. 6: 943. https://doi.org/10.3390/catal13060943




