Catalytic Hydrogenation of Anthracene on Binary (Bimetallic) Composite Catalysts
Abstract
:1. Introduction
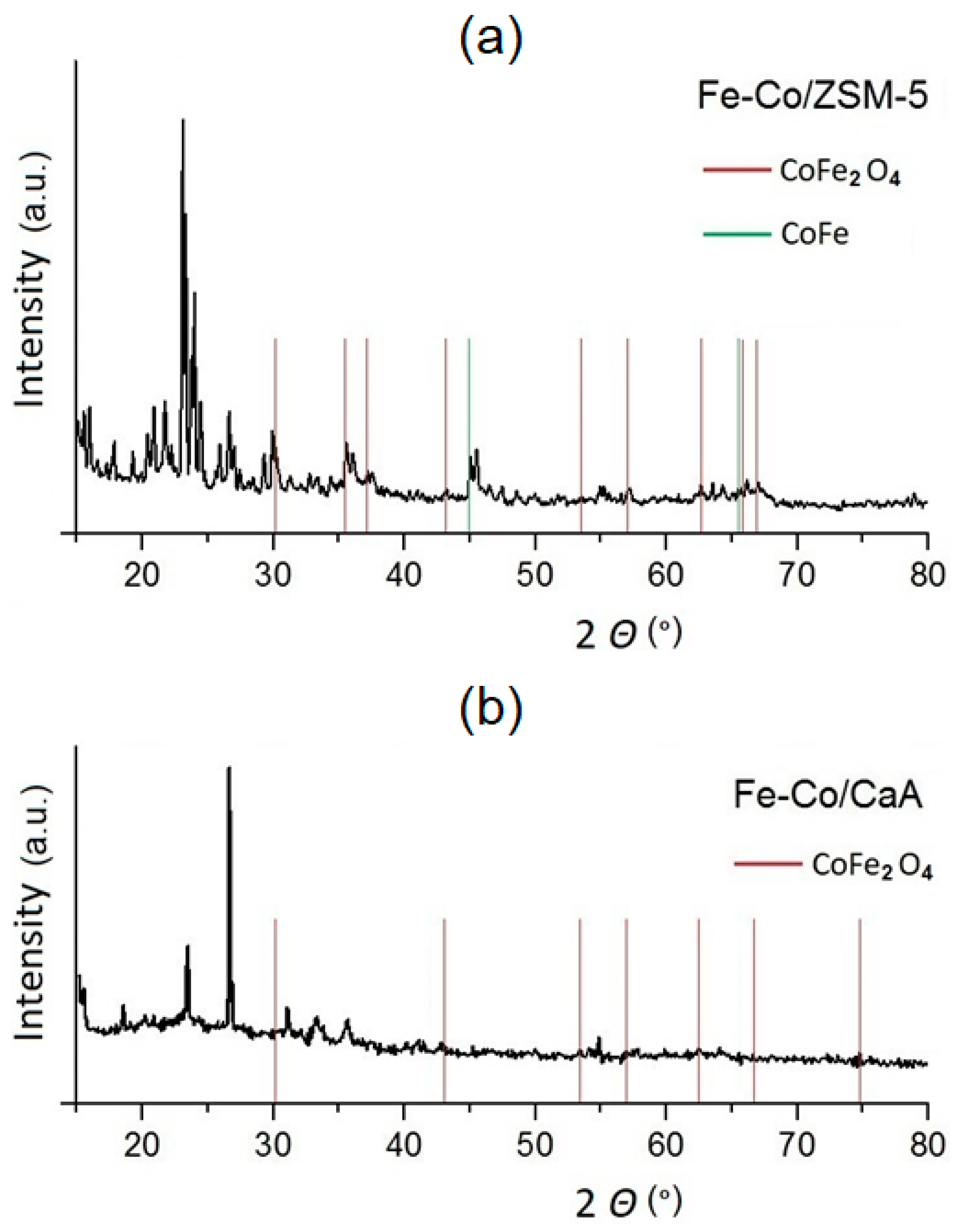
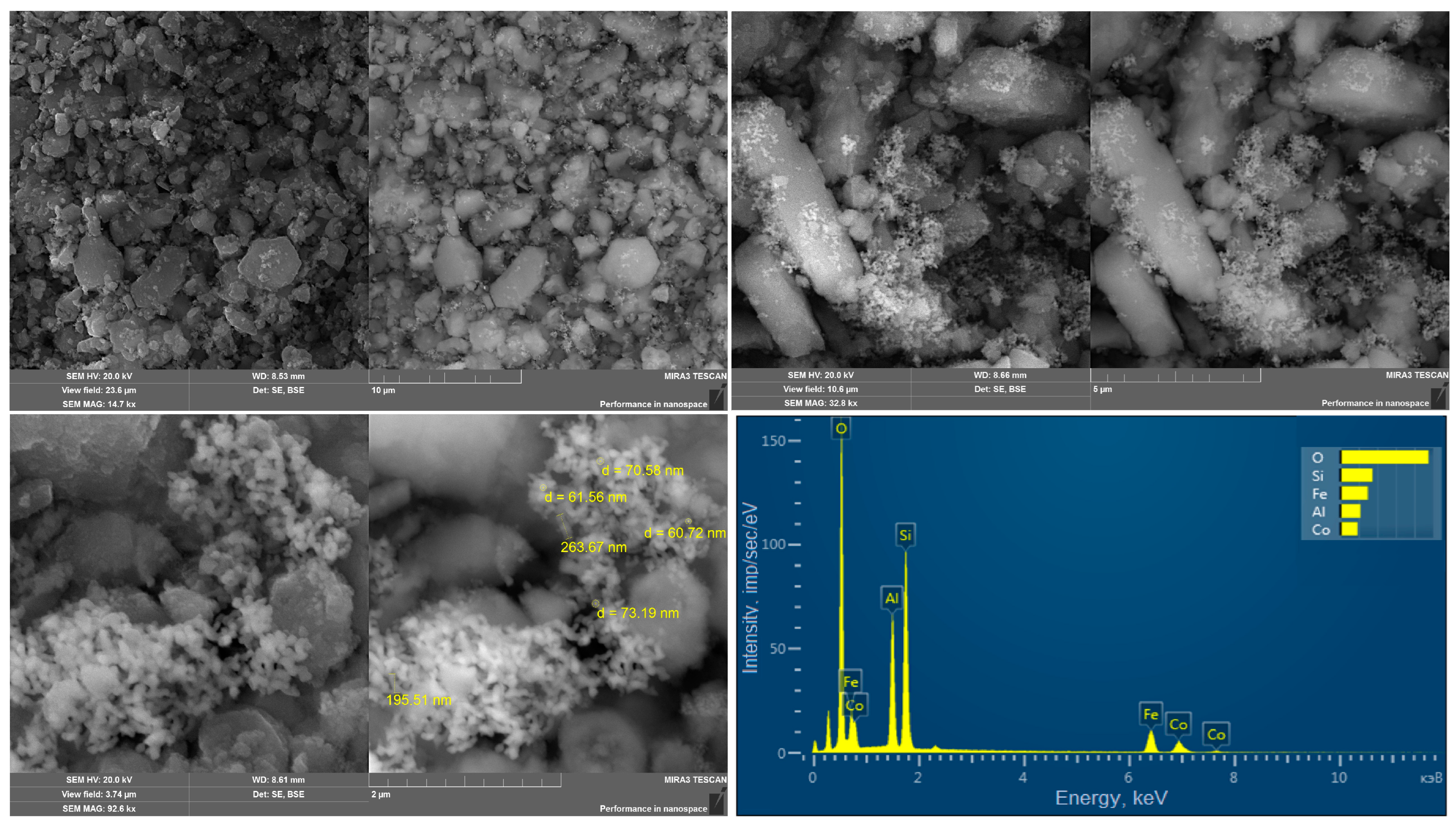
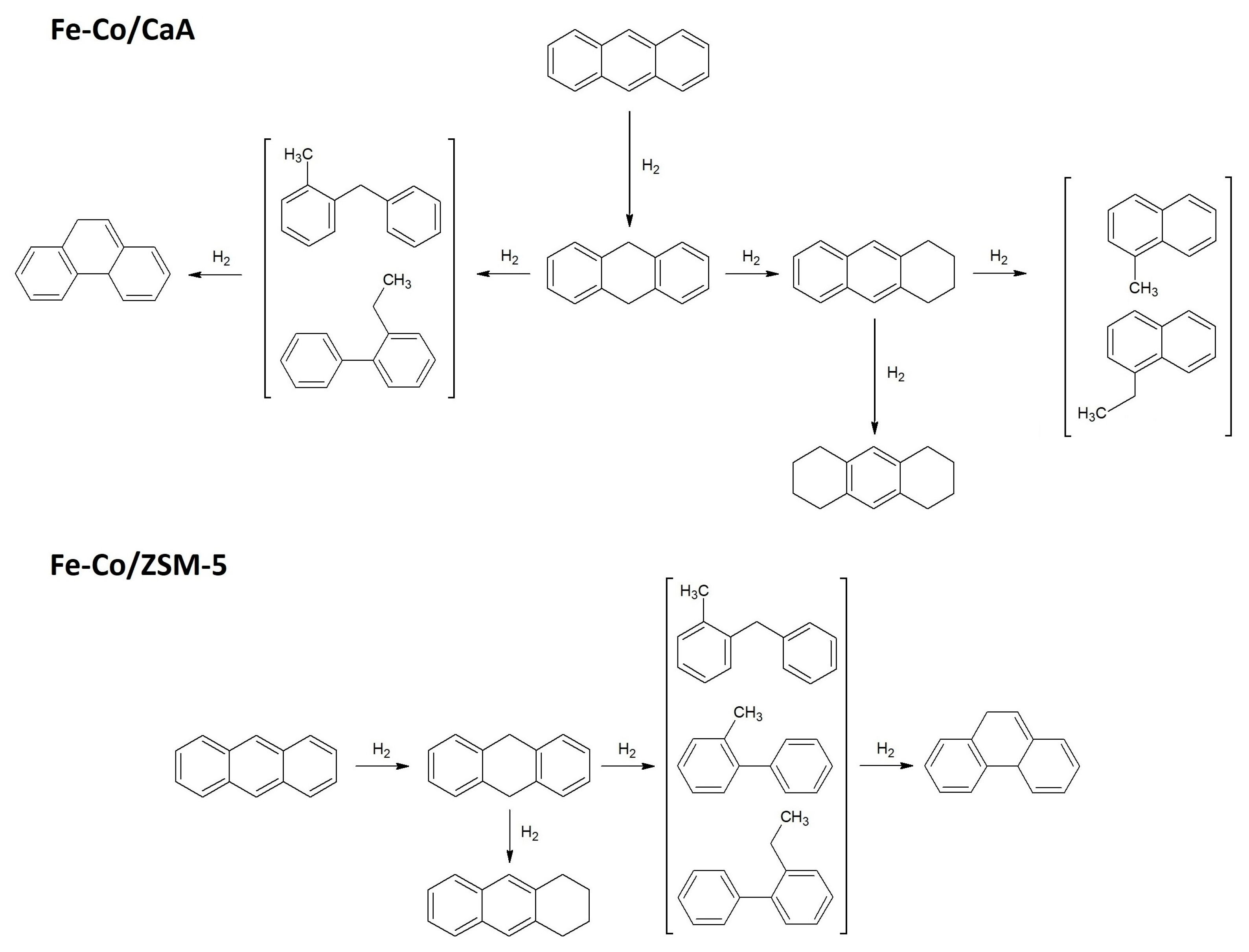
2. Results
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fe-Co/CaA and Fe-Co/ZSM-5 Catalysts
3.3. Hydrogenation of Anthracene
3.4. Physical and Chemical Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shafigulin, R.V.; Filippova, E.O.; Shmelev, A.A.; Bulanova, A.V. Mesoporous silica doped with dysprosium and modified with nickel: A highly efficient and heterogeneous catalyst for the hydrogenation of benzene, ethylbenzene and xylenes. Catal. Lett. 2019, 149, 916–928. [Google Scholar] [CrossRef]
- Su, B.; Cao, Z.-C.; Shi, Z.-J. Exploration of earth-abundant transition metals (Fe, Co, and Ni) as catalysts in unreactive chemical bond activations. Acc. Chem. Res. 2015, 48, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Kaplaneris, N.; Ackermann, L. Earth-abundant 3d transition metals on the rise in catalysis. Beilstein J. Org. Chem. 2022, 18, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Patra, T.; Sonal Upadhayayula, S. Synergistic effect of bifunctional mesoporous ZSM-5 supported Fe-Co catalyst for selective conversion of syngas with low Ribblet ratio into synthetic fuel. Microporous Mesoporous Mater. 2019, 275, 1–13. [Google Scholar] [CrossRef]
- Bueno, A.C.; Mayer, M.; Weber, M.; Bechelany, M.; Klotz, M.; Farrusseng, D. Impregnation protocols on alumina beads for controlling the preparation of supported metal catalysts. Catalysts 2019, 9, 577. [Google Scholar] [CrossRef]
- Xie, X.; Xie, R.; Suo, Z.; Huang, H.; Xing, M.; Lei, D. A highly dispersed Co–Fe bimetallic catalyst to activate peroxymonosulfate for VOC degradation in a wet scrubber. Environ. Sci. Nano 2021, 8, 2976–2987. [Google Scholar] [CrossRef]
- Leyva, C.; Ancheyta, J.; Travert, A.; Mauge, F.; Mariey, L.; Ramirez, J.; Rana, M.S. Activity and surface properties of NiMo/SiO2–Al2O3 catalysts for hydroprocessing of heavy oils. Appl. Catal. A Gen. 2012, 425, 1–12. [Google Scholar] [CrossRef]
- Przydacz, M.; Jędrzejczyk, M.; Rogowski, J.; Szynkowska-Jóźwik, M.; Ruppert, A.M. Highly efficient production of DMF from biomass-derived HMF on recyclable Ni-Fe/TiO2 catalysts. Energies 2020, 13, 4660. [Google Scholar] [CrossRef]
- Huang, C.; Li, H.; Yang, J.; Wang, C.; Hu, F.; Wang, X.; Lu, Z.-H.; Feng, G.; Zhang, R. Ce0.6Zr0.3Y0.1O2 solid solutions-supported Ni-Co bimetal nanocatalysts for NH3 decomposition. Appl. Surf. Sci. 2019, 478, 708. [Google Scholar] [CrossRef]
- Eswaramoorthi, I.; Sundaramurthy, V.; Das, N.; Dalai, A.K.; Adjaye, J. Application of multi-walled carbon nanotubes as efficient support to NiMo hydrotreating catalyst. Appl. Catal. Gen. 2008, 339, 187–195. [Google Scholar] [CrossRef]
- Kantarelis, E.; Yang, W.; Blasiak, W. Effect of zeolite to binder ratio on product yields and composition during catalytic steam pyrolysis of biomass over transition metal modified HZSM5. Fuel 2014, 122, 119–125. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, C.; Wu, M.; Ye, R.; Shi, D.; Li, M.; Zhao, P.; Zhang, R.; Feng, G. Semi-Hydrogenation of Acetylene to Ethylene Catalyzed by Bimetallic CuNi/ZSM-12 Catalysts. Catalysts 2022, 12, 1072. [Google Scholar] [CrossRef]
- Aziz, A.; Bae, J.; Kim, K.S. Preparation of Fe-Co-ZSM-5 Bimetal catalysts with different alternate metal inclusions for catalytic removal of VOCs from air. Water Air Soil Pollut. 2020, 231, 155. [Google Scholar] [CrossRef]
- Fattahi, N.; Triantafyllidis, K.; Luque, R.; Ramazani, A. Zeolite-Based Catalysts: A Valuable Approach toward Ester Bond Formation. Catalysts 2019, 9, 758. [Google Scholar] [CrossRef]
- Degnan, T.F. Applications of zeolites in petroleum refining. Top. Catal. 2000, 13, 349–356. [Google Scholar] [CrossRef]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Role of zeolite catalysts for benzene removal from gasoline via alkylation: A review. Microporous Mesoporous Mater. 2015, 213, 169–180. [Google Scholar] [CrossRef]
- Verdoliva, V.; Saviano, M.; De Luca, S. Zeolites as acid/basic solid catalysts: Recent synthetic developments. Catalysts 2019, 9, 248. [Google Scholar] [CrossRef]
- Yilmaz, B.; Muller, U. Catalytic applications of zeolites in chemical industry. Top Catal. 2009, 52, 888–895. [Google Scholar] [CrossRef]
- Ojeda, M.; Grau-Atienza, A.; Campos, R.; Romero, A.A.; Serrano, E.; Marinas, J.M.; Martinez, J.G.; Luque, R. Hierarchical zeolites and their catalytic performance in selective oxidative processes. ChemSusChem 2015, 8, 1328–1333. [Google Scholar] [CrossRef]
- Ennaert, T.; Van Aelst, J.; Dijkmans, J.; De Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, J.; Deng, F. Solid-state NMR for metal-containing zeolites: From active sites to reaction mechanism. Front. Chem. Sci. Eng. 2019, 14, 159–187. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Yocupicio-Gaxiola, R.I.; Antúnez-García, J.; Chowdari, R.K.; Petranovskii, V.; Fuentes-Moyado, S. Recent advances in catalysis based on transition metals supported on zeolites. Front. Chem. 2021, 9, 716745. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Yu, B.; Zheng, G.; Zhao, J.; Hong, M. Amino Acid Mediated Mesopore Formation in LTA Zeolite. J. Mater. Chem. A 2016, 4, 2305–2313. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, F.; Pan, D.; Ma, J. A Hierarchically micro-meso-macroporous zeolite CaA for methanol conversion to dimethyl ether. Crystals 2016, 6, 155. [Google Scholar] [CrossRef]
- Sanhoob, M.A.; Khan, A.; Ummer, A.C. ZSM-5 Catalysts for MTO: Effect and optimization of the tetrapropylammonium hydroxide concentration on synthesis and performance. ACS Omega 2022, 7, 21654–21663. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Okada, K.; Yamamoto, M.; Yoshida, T. Hydrogenation of anthracene over active carbon-supported nickel catalyst. Catal. Today 1998, 45, 361–366. [Google Scholar] [CrossRef]
- Mfincnerovfi, D.; Augustin, J. Fungal metabolism and detoxification of polycyclic aromatic hydrocarbons: A review. Bioresour. Technol. 1994, 48, 97–106. [Google Scholar]
- Nkansah, M.A.; Christy, A.A.; Barth, T. The use of anthracene as a model compound in a comparative study of hydrous pyrolysis methods for industrial waste remediation. Chemosphere 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Reina, T.R.; Yeletsky, P.; Bermúdez, J.M.; Arcelus-Arrillaga, P.; Yakovlev, V.A.; Millan, M. Anthracene aquacracking using NiMo/SiO2 catalysts in supercritical water conditions. Fuel 2016, 182, 740–748. [Google Scholar] [CrossRef]
- Tao, S.; Li, X.; Yang, Y.; Coveney, R.M.; Lu, X.; Chen, H.; Shen, W. Dispersion modeling of polycyclic aromatic hydrocarbons from combustion of biomass and fossil fuels and production of coke in Tianjin, China. Environ. Sci. Technol. 2006, 40, 4586–4591. [Google Scholar] [CrossRef]
- Zanato, A.F.S.; Silva, V.C.; Lima, D.A.; Jacinto, M.J. Bimetallic magnetic PtPd-nanoparticles as efficient catalyst for PAH removal from liquid media. Appl. Nanosci. 2017, 7, 781. [Google Scholar] [CrossRef]
- Fang, Y.; Li, J.; Sheng, N.; Wang, X.; Chen Di Cai, M.; An, Y.; Chen, Y.; Dai, L. Enhanced catalytic oxidation of anthracene by deposition of MoO3 and WO3 nanoparticles on MCM-41. Mol. Catal. 2020, 497, 111209. [Google Scholar] [CrossRef]
- Liu, H.; Fan, S.; Gong, X.; Wang, J.; Guo, A.; Chen, K.; Wang, Z. Partial hydrogenation of anthracene with in situ hydrogen produced from water-gas shift reaction over Fe-based catalysts. Catalysts 2020, 10, 1379. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Chen, X.; Meng, L.; Liang, C. Selective Hydrogenation of Anthracene to Symmetrical Octahydroanthracene over Al2O3-Supported Pt and Rh Catalysts Prepared by Strong Electrostatic Adsorption. Energy Fuels 2022, 36, 2775. [Google Scholar] [CrossRef]
- Kustov, L.M.; Kalenchuk, A.N. The Role of the metal in the catalytic reactions of hydrogenation–dehydrogenation of polycyclic hydrocarbons for hydrogen Storage. Metals 2022, 12, 2002. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Wei, X.-Y.; Liu, F.-J.; Wang, B.-J.; Zong, Z.-M. Temperature-controlled hydrogenation of anthracene over nickel nanoparticles supported on attapulgite powder. Fuel 2018, 223, 222. [Google Scholar] [CrossRef]
- Nelkenbaum, E.; Dror, I.; Berkowitz, B. Reductive hydrogenation of polycyclic aromatic hydrocarbons catalyzed by metalloporphyrins. Chemosphere 2007, 68, 210. [Google Scholar] [CrossRef]
- Cataldo, F.; Angelini, G.; Garcia-Hernandez, D.A.; Manchado, A. Far infrared (terahertz) spectroscopy of a series of polycyclic aromatic hydrocarbons and application to structure interpretation of asphaltenes and related compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 111, 68. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Yoshida, T. Behavior of hydrogen transfer in the hydrogenation of anthracene over activated carbon. Energy Fuels 2001, 15, 708. [Google Scholar] [CrossRef]
- Baikenov, M.I.; Baikenova, G.G.; Sarsembayev, B.S.; Tateeva, A.B.; Tusipkhan, A.; Matayeva, A.Z. Influence of catalytic systems on process of model object hydrogenation. Int. J. Coal Sci. Technol. 2014, 1, 88. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; Li, J.; Pan, Z.; Ma, H.; Jiang, Y.; Tian, Z. Facile hydrothermal synthesis of MoS2 nano-sheets with controllable structures and enhanced catalytic performance for anthracene hydrogenation. RSC Adv. 2016, 6, 71534. [Google Scholar] [CrossRef]
- Nador, F.; Moglie, Y.; Vitale, C.; Yus, M.; Alonso, F.; Radivoy, G. Reduction of polycyclic aromatic hydrocarbons promoted by cobalt or manganese nanoparticles. Tetrahedron 2010, 66, 4318. [Google Scholar] [CrossRef]
- Mougnol, J.B.B.; Waanders, F.; Fosso-Kankeu, E.; Al Alili, A.R. Leaching of polycyclic aromatic hydrocarbons from the coal tar in sewage wastewater, acidic and alkaline mine drainage. Int. J. Environ. Res. Public Health 2022, 19, 4791. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Shaikh, S.; Jain, K.J.; Desai, C.; Madamwar, D. Polycyclic aromatic hydrocarbons: Sources, Toxicity, and Remediation approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.; Hutchinson, E.; Unwin, J.; Fletcher, T. Lung Cancer Risk after Exposure to Polycyclic Aromatic Hydrocarbons: A Review and Meta-Analysis. Environ. Health Perspect. 2004, 112, 970. [Google Scholar] [CrossRef]
- Meiramov, M.G. Angular–linear isomerization on the hydrogenation of phenanthrene in the presence of iron-containing catalysts. Solid Fuel Chem. 2017, 51, 107–110. [Google Scholar] [CrossRef]
- Yilmaz, B.; Trukhan, N.; Muller, U. Industrial Outlook on Zeolites and Metal Organic Frameworks. Chin. J. Catal. 2012, 33, 3–10. [Google Scholar] [CrossRef]
- Santos, M.B.; Vianna, K.C.; Pastore, H.O.; Andrade, H.M.C.; Mascarenhas, A.J.S. Studies on the synthesis of ZSM-5 by interzeolite transformation from zeolite Y without using organic structure directing agents. Microporous Mesoporous Mater. 2020, 306, 110413. [Google Scholar] [CrossRef]
- Singh, J.; White, R.L. A variable temperature infrared spectroscopy study of CaA zeolite dehydration and carbonate formation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 207, 189–196. [Google Scholar] [CrossRef]
- Julbe, A.; Drobek, M. Zeolite A Type. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Tišler, Z.; Klegová, A.; Svobodová, E.; Šafár, J.; Strejcová, K.; Kohout, J.; Šlang, S.; Pacultová, K.; Daily Rodríguez-Padrón, D.; Bulánek, R. Cobalt based catalysts on alkali-activated zeolite foams for N2O decomposition. Catalysts 2020, 10, 1398. [Google Scholar] [CrossRef]
- Otto, T.; Zones, S.I.; Hong, Y.; Iglesia, E. Synthesis of highly dispersed cobalt oxide clusters encapsulated within LTA zeolites. J. Catal. 2017, 356, 173–185. [Google Scholar] [CrossRef]
- Kosinov, N.; Liu, C.; Hensen, E.J.M.; Pidko, E. Engineering of transition metal catalysts confined in zeolites. Chem. Mater. 2018, 30, 3177–3198. [Google Scholar] [CrossRef]
- Nejad-Darzi, S.K.H.; Samadi-Maybodi, A.; Ghobakhluo, M. Synthesis and characterization of modified ZSM-5 nanozeolite and their applications in adsorption of Acridine Orange dye from aqueous solution. J. Porous Mater. 2013, 20, 909–916. [Google Scholar] [CrossRef]
- Tuli, L.B.; Mebel, A.M. Formation of phenanthrene via H-assisted isomerization of 2-ethynylbiphenyl produced in the reaction of phenyl with phenylacetylene. Int. Journ. Chem Kinet. 2020, 52, 875–883. [Google Scholar] [CrossRef]


| CaA | Fe-Co/CaA | ZSM-5 | Fe-Co/ZSM-5 |
|---|---|---|---|
| 3472.29 | 3443.35 | 3454.93 | 3427.92 |
| - | 3142.42 | 3122.36 | 3169.43 |
| 2835.70 | 2833.78 | 2829.92 | 2833.78 |
| 2716.10 | 2716.10 | 2716.10 | 2716.10 |
| 2361.16 | 2363.09 | 2342.07 | 2359.23 |
| 1628.12 | 1604.97 | 1878.90 | 1882.75 |
| 1400.49 | 1398.56 | 1612.69 | 1604.97 |
| 1361.91 | 1361.91 | 1402.42 | 1396.63 |
| 1100.96 | 1113.06 | 1365.77 | 1361.91 |
| 976.10 | 981.89 | 1224.95 | 1224.95 |
| 763.90 | 773.55 | 1101.49 | 1099.56 |
| 682.89 | 625.01 | 792.84 | 800.56 |
| 567.14 | 561.35 | - | 773.55 |
| 462.97 | 462.97 | 621.15 | 621.15 |
| - | - | 545.92 | 453.33 |
| - | - | 453.33 | 549.78 |
| Fe2O3-CoO/CaA t = 400 °C, τ = 60 min, P = 6 MPa | Fe2O3-CoO/ZSM-5 t = 400 °C, τ = 60 min, P = 6 MPa | ||||
|---|---|---|---|---|---|
| No. | Compound | Yield, % | No. | Compound | Yield, % |
| 1 | 1-methylnaphthalene | 0.17 | 1 | Benzene,1-methyl-2-phenylmethyl | 2.86 |
| 2 | 1-ethylnaphthalene | 0.48 | 2 | 2-ethylbiphenyl | 11.98 |
| 3 | 1-methyl-2-phenylmethylbenzene | 0.92 | 3 | Dihydroanthracene | 35.37 |
| 4 | 2-ethylbiphenyl | 0.67 | 4 | Octahydroanthracene | 1.7 |
| 5 | Dihydroanthracene | 60.67 | 5 | Tetrahydroanthracene | 33.94 |
| 6 | Octahydroanthracene | 0.59 | 6 | Phenanthrene | 4.94 |
| 7 | Tetrahydroanthracene | 22.89 | 7 | Anthracene | 9.21 |
| 8 | Phenanthrene | 0.40 | 8 | - | - |
| 9 | Anthracene | 13.21 | 9 | - | - |
| Anthracene conversion | ~87 | Anthracene conversion | ~91 | ||
| Catalyst | Time (h) | Temperature (°C) | Pressure (MP) | DHA Yield (%) | THA Yield (%) | OHA yield (%) | Anthracene Conversion (%) |
|---|---|---|---|---|---|---|---|
| Fe-Co/CaA | 1 | 400 | 6 | 60.7 | 22.9 | 0.6 | 86.8 |
| Fe-Co/ZSM-5 | 1 | 400 | 6 | 35.37 | 33.94 | 1.7 | 90.8 |
| Pt/Al2O3-SEA [37] | 1 | 240 | 7 | 63.2 | 29.8 | 7.1 | 100 |
| Rh/Al2O3-SEA [37] | 1 | 240 | 7 | 31.5 | 43.4 | 22.7 | 99.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muldakhmetov, Z.M.; Ordabaeva, A.T.; Meiramov, M.G.; Gazaliev, A.M.; Kim, S.V. Catalytic Hydrogenation of Anthracene on Binary (Bimetallic) Composite Catalysts. Catalysts 2023, 13, 957. https://doi.org/10.3390/catal13060957
Muldakhmetov ZM, Ordabaeva AT, Meiramov MG, Gazaliev AM, Kim SV. Catalytic Hydrogenation of Anthracene on Binary (Bimetallic) Composite Catalysts. Catalysts. 2023; 13(6):957. https://doi.org/10.3390/catal13060957
Chicago/Turabian StyleMuldakhmetov, Zainulla M., Aigul T. Ordabaeva, Majit G. Meiramov, Arstan M. Gazaliev, and Sergey V. Kim. 2023. "Catalytic Hydrogenation of Anthracene on Binary (Bimetallic) Composite Catalysts" Catalysts 13, no. 6: 957. https://doi.org/10.3390/catal13060957





