Progress in the Preparation of Metal Oxide Electrodes for the Electrochemical Treatment of Organic Wastewater: A Short Review
Abstract
:1. Introduction

2. Preparation of Metal Oxide Electrodes: Fundamentals, Challenges, and Progress
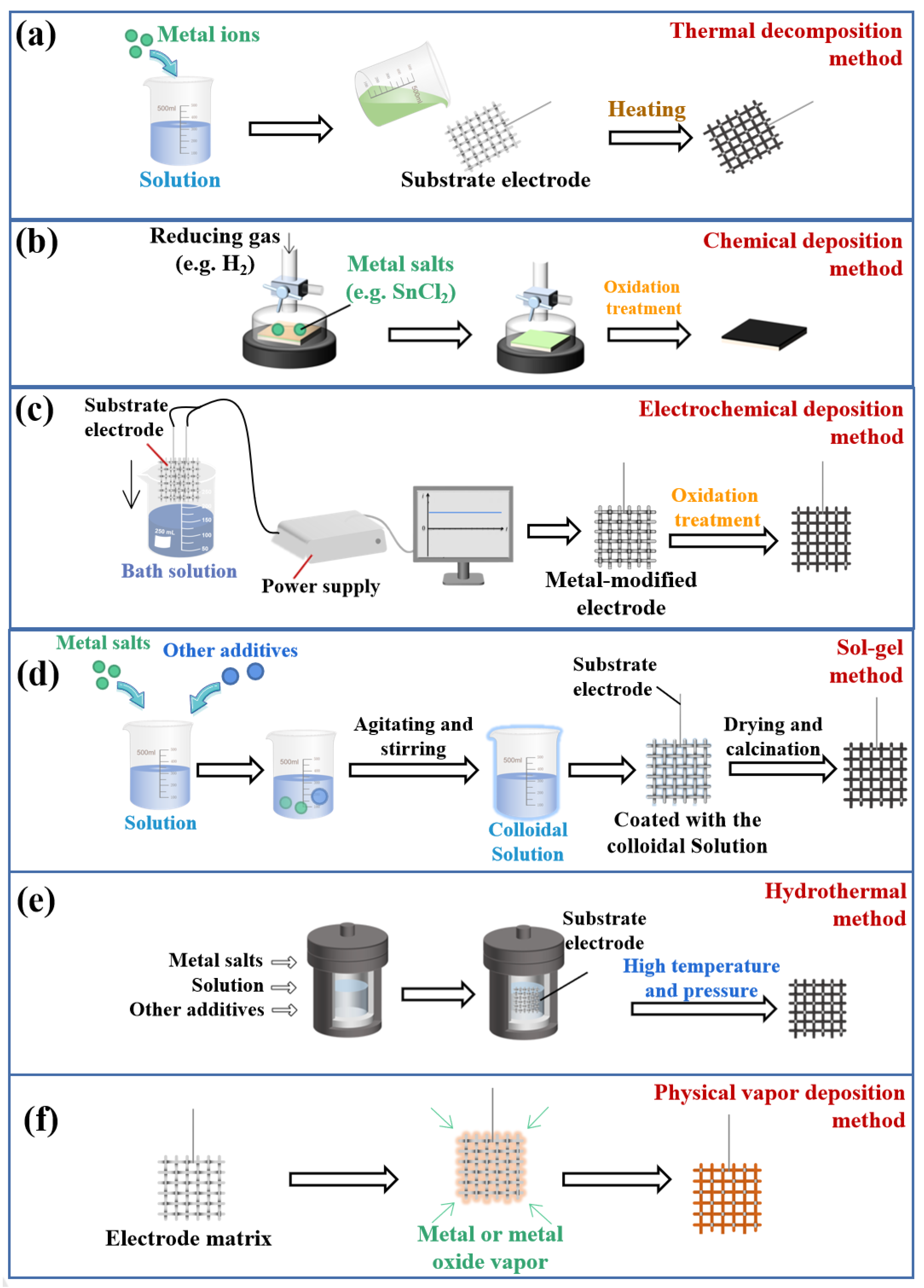
2.1. Fundamentals of Preparing Metal Oxide Electrode Materials
2.2. Challenges and Research Progress in the Preparation of Metal Oxide Electrodes
3. Single-Metal-Oxide Electrodes: Applications and Enhancements in Organic Wastewater Treatment
3.1. Application in Organic Wastewater Treatment
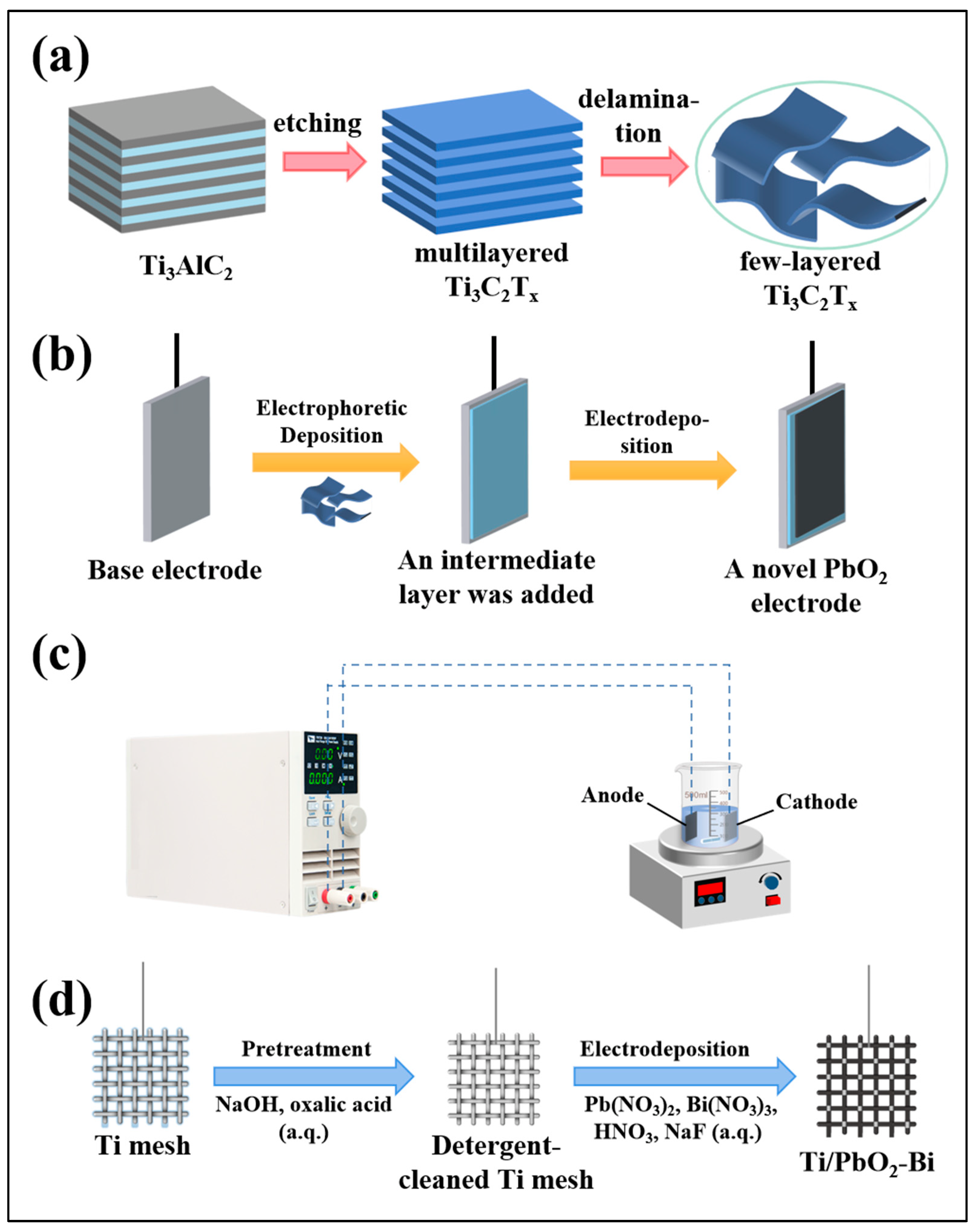
3.2. Optimization Strategy Based on Adding Intermediate Layers
4. Metal Oxide Composite Electrodes: Relative Advantages and Progress in Electrochemical Wastewater Treatment
4.1. Relative Advantages
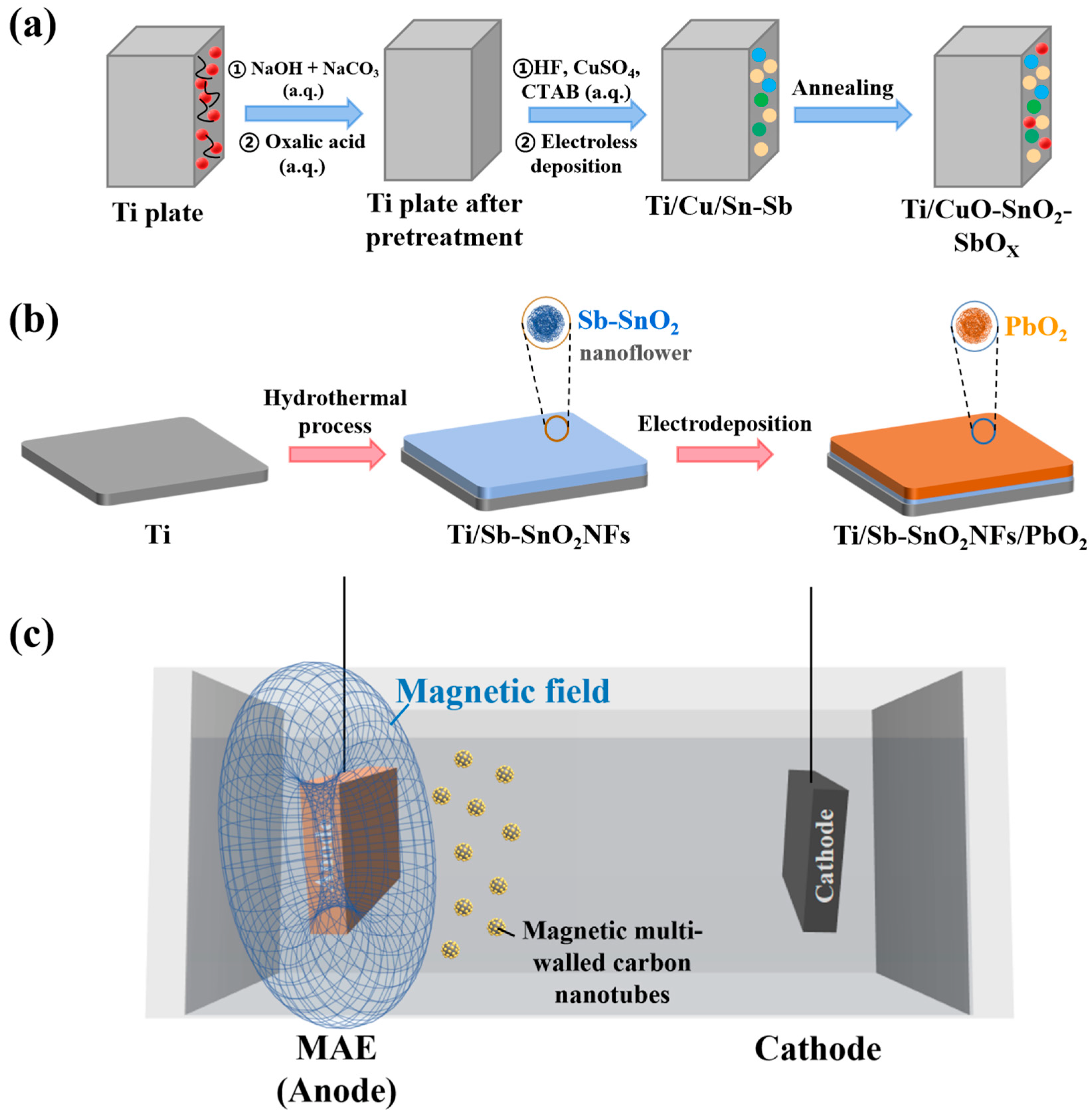
4.2. Progress in the Design of Metal Oxide Composite Electrodes
5. Summary and Prospects
5.1. Summary
5.2. Prospects
5.2.1. Challenges and Potential Solutions in Using Electrocatalytic Oxidation Technology for Organic Wastewater Treatment
5.2.2. The Possibility of Undertaking Electrocatalytic Oxidation Using Renewable Energy
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, C.; Zhong, H.; Zhang, Y.; Li, Y.; Chung, K.H.; Fang, L.; Zhang, W.; Wu, B.; Xu, C.; Shi, Q. Organic matter in delayed coking wastewater: Molecular composition and its effect on emulsification. Fuel 2020, 279, 118432. [Google Scholar] [CrossRef]
- Fukami, K.; Oogi, T.; Motomura, K.; Morita, T.; Sakamoto, M.; Hata, T. Effective Purification of Eutrophic Wastewater from the Beverage Industry by Microbubbles. Water 2021, 13, 3661. [Google Scholar] [CrossRef]
- Ishak, S.A.; Murshed, M.F.; Md Akil, H.; Ismail, N.; Md Rasib, S.Z.; Al-Gheethi, A.A.S. The Application of Modified Natural Polymers in Toxicant Dye Compounds Wastewater: A Review. Water 2020, 12, 2032. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Tang, H.; Khan, I.; Seleiman, M.F.; Rasheed, A.; Nawaz, M.; Rehman, A.; Talha Aslam, M.; et al. Sugarcane Distillery Spent Wash (DSW) as a Bio-Nutrient Supplement: A Win-Win Option for Sustainable Crop Production. Agronomy 2021, 11, 183. [Google Scholar] [CrossRef]
- Hua, T.; Wang, H.; Li, F. Degradation of TBBP-A wastewater by the process of electro-activated sodium persulfate. Environ. Pollut. Control 2020, 42, 539–542. [Google Scholar]
- Wang, B.; Xiong, M.; Wang, P.; Shi, B. Chemical characterization in hydraulic fracturing flowback and produced water (HF-FPW) of shale gas in Sichuan of China. Environ. Sci. Pollut. Res. 2020, 27, 26532–26542. [Google Scholar] [CrossRef]
- Pasmionka, I.B.; Gospodarek, J. Assessment of the Impact of Selected Industrial Wastewater on the Nitrification Process in Short-Term Tests. Int. J. Environ. Res. Public Health 2022, 19, 3014. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, S.Y.; Noh, J.H.; Bae, Y.H.; Lee, J.W.; Maeng, S.K. A shift from chemical oxygen demand to total organic carbon for stringent industrial wastewater regulations: Utilization of organic matter characteristics. J. Environ. Manag. 2022, 305, 114412. [Google Scholar] [CrossRef]
- Meng, S.; Wen, S.; Han, G.; Wang, X.; Feng, Q. Wastewater Treatment in Mineral Processing of Non-Ferrous Metal Resources: A Review. Water 2022, 14, 726. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Pang, C.K.; Affandi, N.A.; Maruja, S.N.; Vijayan, V. Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. ChemEngineering 2022, 6, 58. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, H.; Yan, M.; Jiang, N.; Liu, Y.; Li, Y.; Huang, M. Resources Recycle of Printing and Dyeing Wastewater: UF/FO Hybrid System Optimization and TPA Recovery. ACS EST Water 2022. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, H.; Fu, K.; Cheng, W.; Wu, D.; Luo, C.; Jiang, S.; Li, J.; Zhang, M. Preparation of Mn/Mg/Ce Ternary Ozone Catalyst and Its Mechanism for the Degradation of Printing and Dyeing Wastewater. Front. Energy Res. 2022, 9, 815633. [Google Scholar] [CrossRef]
- Zhou, J.; Geng, C.; Zhao, A. Pilot-scale experimental study on advanced treatment of printing and dyeing wastewater by catalytic ozonation. Water & Wastewater Engineering 2022, 48, 75–80. [Google Scholar]
- Masoner, J.R.; Kolpin, D.W.; Cozzarelli, I.M.; Bradley, P.M.; Arnall, B.B.; Forshay, K.J.; Gray, J.L.; Groves, J.F.; Hladik, M.L.; Hubbard, L.E.; et al. Contaminant Exposure and Transport from Three Potential Reuse Waters within a Single Watershed. Environ. Sci. Technol. 2023, 57, 1353–1365. [Google Scholar] [CrossRef]
- Abou-tammame, D.; Zouhri, A.; Boutarfa, A.; Fathi, J.; Aboutayeb, R. The Effect of Purified Wastewater on the Physicochemical Properties of Agricultural Soils in Chaouia in Morocco. J. Ecol. Eng. 2022, 23, 34–42. [Google Scholar] [CrossRef]
- Akinnawo, S.O.; Ayadi, P.O.; Oluwalope, M.T. Chemical coagulation and biological techniques for wastewater treatment. Ovidius Univ. Ann. Chem. 2023, 34, 14–21. [Google Scholar] [CrossRef]
- Lan, J.; Liu, L.; Wang, X.; Wu, X.; Wang, Z. DOM tracking and prediction of rural domestic sewage with UV-vis and EEM in the Yangtze River Delta, China. Environ. Sci. Pollut. Res. 2022, 29, 74579–74590. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, D.; Karanfil, T. Effect of activated sludge treatment on the formation of N-nitrosamines under different chloramination conditions. J. Environ. Sci. 2022, 117, 242–252. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.; Guo, Y.; Li, J.; Wei, F. Environmental impact, treatment technology and monitoring system of ship domestic sewage: A review. Sci. Total Environ. 2022, 811, 151410. [Google Scholar] [CrossRef]
- Sui, Y.; Al-Huqail, A.A.; Suhatril, M.; Abed, A.M.; Zhao, Y.; Assilzadeh, H.; Khadimallah, M.A.; Ali, H.E. Hydrogen energy of mining waste waters: Extraction and analysis of solving issues. Fuel 2023, 331, 125685. [Google Scholar] [CrossRef]
- Grgas, D.; Stefanac, T.; Baresic, M.; Toromanovic, M.; Ibrahimpasic, J.; Pavicic, T.V.; Habuda-Stanic, M.; Herceg, Z.; Dragicevic, T.L. Co-composting of Sewage Sludge, Green Waste, and Food Waste. J. Sustain. Dev. Energy 2023, 11, 1100415. [Google Scholar] [CrossRef]
- Ng, M.; Dalhatou, S.; Wilson, J.; Kamdem, B.P.; Temitope, M.B.; Paumo, H.K.; Djelal, H.; Assadi, A.A.; Phuong, N.-T.; Kane, A. Characterization of Slaughterhouse Wastewater and Development of Treatment Techniques: A Review. Processes 2022, 10, 1300. [Google Scholar] [CrossRef]
- Balu, S.; Chuaicham, C.; Balakumar, V.; Rajendran, S.; Sasaki, K.; Sekar, K.; Maruthapillai, A. Recent development on core-shell photo(electro)catalysts for elimination of organic compounds from pharmaceutical wastewater. Chemosphere 2022, 298, 134311. [Google Scholar] [CrossRef]
- Quang, H.H.P.; Nguyen, T.P.; Nguyen, D.D.D.; Bao, L.T.N.; Nguyen, D.C.; Nguyen, V.-H. Advanced electro-Fenton degradation of a mixture of pharmaceutical and steel industrial wastewater by pallet-activated-carbon using three-dimensional electrode reactor. Chemosphere 2022, 297, 134074. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Chen, C.; Chen, Y.; Zheng, X. Preparing coal slurry from organic wastewater to achieve resource utilization: Slurrying performance and dispersant suitability. Fuel 2023, 339, 126970. [Google Scholar] [CrossRef]
- Abdollahi, N.; Moussavi, G.; Giannakis, S. A review of heavy metals’ removal from aqueous matrices by Metal-Organic Frameworks (MOFs): State-of-the art and recent advances. J. Environ. Chem. Eng. 2022, 10, 107394. [Google Scholar] [CrossRef]
- Naghdi, S.; Shahrestani, M.M.; Zendehbad, M.; Djahaniani, H.; Kazemian, H.; Eder, D. Recent advances in application of metal-organic frameworks (MOFs) as adsorbent and catalyst in removal of persistent organic pollutants (POPs). J. Hazard. Mater. 2023, 442, 130127. [Google Scholar] [CrossRef]
- Lin, S.H.; Kiang, C.D. Combined physical, chemical and biological treatments of wastewater containing organics from a semiconductor plant. J. Hazard. Mater. 2003, 97, 159–171. [Google Scholar] [CrossRef]
- Türk, O.K.; Zoungrana, A.; Çakmakci, M. Chemical precipitation and membrane distillation process for the treatment of acidic anodic oxidation wastewaters. J. Environ. Chem. Eng. 2022, 10, 108036. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Sun, Z.; Hu, B.; Li, X.; Lan, M.; Guo, H.; Li, B. Mainstream wastewater treatment by polyaluminium ferric chloride (PAFC) flocculation and nitritation-denitritation membrane aerated biofilm reactor (MABR). J. Water Process. Eng. 2023, 52, 103563. [Google Scholar] [CrossRef]
- Wang, S.; Hu, C.; Cheng, F.; Lu, X. Performance of a combined low-consumption biotreatment system with cost-effective ecological treatment technology for rural domestic sewage treatment. J. Water Process. Eng. 2023, 51, 103380. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Hu, H.; Khu, S.-T. Novel Quantitative Evaluation of Biotreatment Suitability of Wastewater. Water 2022, 14, 1038. [Google Scholar] [CrossRef]
- Jiang, P.; Zhou, T.; Bai, J.; Zhang, Y.; Li, J.; Zhou, C.; Zhou, B. Nitrogen-containing wastewater fuel cells for total nitrogen removal and energy recovery based on Cl•/ClO• oxidation of ammonia nitrogen. Water Res. 2023, 235, 119914. [Google Scholar] [CrossRef]
- Manna, M.; Sen, S. Advanced oxidation process: A sustainable technology for treating refractory organic compounds present in industrial wastewater. Environ. Sci. Pollut. Res. 2013, 30, 25477–25505. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, W.; Zhu, H.; Chen, H.; Yan, S.; Zhao, M.; Sun, H.; Zhang, J.; Zhang, S. A Review on N-Doped Biochar for Oxidative Degradation of Organic Contaminants in Wastewater by Persulfate Activation. Int. J. Environ. Res. Public Health 2022, 19, 14805. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, J.; Yu, P.; Wu, Y. Study on degradation effect of pretreatment to cephalosporin pharmaceutical wastewater and removal of organic matter. Ind. Water Treat. 2022, 42, 89–95. [Google Scholar]
- Lin, R.; Li, Y.; Yong, T.; Cao, W.; Wu, J.; Shen, Y. Synergistic effects of oxidation, coagulation and adsorption in the integrated fenton-based process for wastewater treatment: A review. J. Environ. Manag. 2022, 306, 114460. [Google Scholar] [CrossRef]
- Dong, H.; Su, H.; Chen, Z.; Yu, H.; Yu, H. Fabrication of Electrochemically Reduced Graphene Oxide Modified Gas Diffusion Electrode for In-situ Electrochemical Advanced Oxidation Process under Mild Conditions. Electrochim. Acta 2016, 222, 1501–1509. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, J.; Wang, Z.; Yang, Z.; Yang, W.; Yin, Z. Defective MOFs-based electrocatalytic self-cleaning membrane for wastewater reclamation: Enhanced antibiotics removal, membrane fouling control and mechanisms. Water Res. 2022, 220, 118635. [Google Scholar] [CrossRef]
- Cardozo, J.C.; da Silva, D.R.; Martínez-Huitle, C.A.; Quiroz, M.A.; Vieira dos Santos, E. The versatile behavior of diamond electrodes—Electrochemical examination of the anti-psychotic drug olanzapine (OL) oxidation as a model organic aqueous solution. Electrochim. Acta 2022, 411, 140063. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Chiron, S. Solar photo-Fenton like using persulphate for carbamazepine removal from domestic wastewater. Water Res. 2014, 48, 229–236. [Google Scholar] [CrossRef]
- Zhu, J.; Cao, G.; Zhou, Y.; Li, Y.; Zheng, J.; Zhang, D. Influence of the Synthesis Route on the Properties of Hybrid NiO-MnCo2O4-Ni6MnO8 Anode Materials and their Electrochemical Performances. ChemSusChem 2020, 13, 1890–1899. [Google Scholar] [CrossRef]
- Qiao, S.; Huang, N.; Zhang, Y.; Zhang, J.; Gao, Z.; Zhou, S. One-step synthesis of nanoblocks@nanoballs NiMnO3/Ni6MnO8 nanocomposites as electrode material for supercapacitors. Int. J. Hydrogen Energy 2019, 44, 18351–18359. [Google Scholar] [CrossRef]
- Bernaecker, C.I.; Rauscher, T.; Buettner, T.; Kieback, B.; Roentzsch, L. Powder Metallurgy Route to Produce Raney-Nickel Electrodes for Alkaline Water Electrolysis. J. Electrochem. Soc. 2019, 166, F357–F363. [Google Scholar] [CrossRef]
- Duran, S.; Elmaalouf, M.; Odziomek, M.; Piquemal, J.-Y.; Faustini, M.; Giraud, M.; Peron, J.; Tard, C. Electrochemical Active Surface Area Determination of Iridium-Based Mixed Oxides by Mercury Underpotential Deposition. ChemElectroChem 2021, 8, 3519–3524. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, F.; Wu, H. Boosting pH-Universal Hydrogen Evolution of FeP/CC by Anchoring Trace Platinum. Crystals 2022, 12, 37. [Google Scholar] [CrossRef]
- Huang, H.; Shen, B.; Yan, M.; He, H.; Yang, L.; Jiang, Q.; Ying, G. Coupled spinel manganese-cobalt oxide and MXene electrocatalysts towards efficient hydrogen evolution reaction. Fuel 2022, 328, 125234. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, S.; Wang, P.; Shao, X.; Sun, X.; Hu, J.; Shi, X.-R. Bimetallic FeCo phosphide nanoparticles anchored on N-doped carbon foam for wide pH hydrogen evolution reaction. J. Alloys Compd. 2023, 931, 167570. [Google Scholar] [CrossRef]
- Guo, S.; Xu, Z.; Hu, W.; Yang, D.; Wang, X.; Xu, H.; Xu, X.; Long, Z.; Yan, W. Progress in Preparation and Application of Titanium Sub-Oxides Electrode in Electrocatalytic Degradation for Wastewater Treatment. Catalysts 2022, 12, 618. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Wang, B.; Zhang, W.; Wang, J.; Cui, C.; Wang, S. Heterogeneous electro-Fenton using three-dimension Fe-Co-Bi/kaolin particle electrodes for degradation of quinoline in wastewater. Environ. Sci. Pollut. Res. 2013, 30, 1399–1412. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Yang, H.; Hu, C.; Li, H.; Jing, H. Preparation of titanium suboxide electrode and its application in wastewater treatment. Ind. Water Treat. 2022, 42, 56–64. [Google Scholar]
- Chi, C.; Zhou, X.; Wang, Y.; Zhang, H.; Meng, G.; Hu, Y.; Bai, Z. Preparation of needle coke composite cathode and its treatment of RhB wastewater. J. Electroanal. Chem. 2022, 920, 116612. [Google Scholar] [CrossRef]
- Krishna, B.R.; Bhuvaneshwari, S.; Majeed, F.; Aravind, S.P. Development and applicability of Aluminium-Copper alloy electrodes for dairy wastewater treatment. J. Water Process. Eng. 2022, 48, 102915. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Yao, J.; Long, J. Industrial indigo dyeing wastewater purification: Effective COD removal with peroxi-AC electrocoagulation system. Arab. J. Chem. 2023, 16, 104607. [Google Scholar] [CrossRef]
- Siburian, R.; Hutagalung, F.; Silitonga, O.; Paiman, S.; Simatupang, L.; Simanjuntak, C.; Aritonang, S.P.; Alias, Y.; Jing, L.; Goei, R.; et al. The New Materials for Battery Electrode Prototypes. Materials 2023, 16, 555. [Google Scholar] [CrossRef]
- Ferreira de Oliveira, A.E.; Pereira, A.C.; Ferreira, L.F. Fully handwritten electrodes on paper substrate using rollerball pen with silver nanoparticle ink, marker pen with carbon nanotube ink and graphite pencil. Anal. Methods 2022, 14, 1880–1888. [Google Scholar] [CrossRef]
- Bansal, R.; Verduzco, R.; Wong, M.S.; Westerhoff, P.; Garcia-Segura, S. Development of nano boron-doped diamond electrodes for environmental applications. J. Electroanal. Chem. 2022, 907, 116028. [Google Scholar] [CrossRef]
- De Luna, Y.; Bensalah, N. Review on the electrochemical oxidation of endocrine-disrupting chemicals using BDD anodes. Curr. Opin. Electrochem. 2022, 32, 100900. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Z.; Xie, Y.; Huang, G.D.; Peng, G.P. Fabrication of a boron-doped nanocrystalline diamond grown on an WC-Co electrode for degradation of phenol. RSC Adv. 2022, 12, 26580–26587. [Google Scholar] [CrossRef]
- Nagels, M.; Verhoeven, B.; Larché, N.; Dewil, R.; Rossi, B. Corrosion behaviour of lean duplex stainless steel in advanced oxidation process (AOP) based wastewater treatment plants. Eng. Fail. Anal. 2022, 136, 106170. [Google Scholar] [CrossRef]
- Okur, M.C.; Akyol, A.; Nayir, T.Y.; Kara, S.; Ozturk, D.; Civas, A. Performance of Ti/RuO2-IrO2 electrodes and comparison with BDD electrodes in the treatment of textile wastewater by electro-oxidation process. Chem. Eng. Res. Des. 2022, 183, 398–410. [Google Scholar] [CrossRef]
- Kuhl, M.; Henning, A.; Haller, L.; Wagner, L.I.; Jiang, C.-M.; Streibel, V.; Sharp, I.D.; Eichhorn, J. Designing Multifunctional Cobalt Oxide Layers for Efficient and Stable Electrochemical Oxygen Evolution. Adv. Mater. Interfaces 2022, 9, 2200582. [Google Scholar] [CrossRef]
- Khan, S.; Shah, S.S.; Ahmad, A.; Yurtcan, A.B.; Katubi, K.M.; Janjua, N.K. gamma-Alumina Supported Copper Oxide Nanostructures Promoted with Ruthenium Oxide (RuO2-CuO/Al2O3) and Palladium Oxide (PdO-CuO/Al2O3): Efficient Electrodes for Heterogeneous Catalysis of Ammonia Electrooxidation. J. Electrochem. Soc. 2022, 169, 076512. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Tahira, A.; Shah, A.A.; Aftab, U.; Solangi, M.Y.; Leghari, J.A.; Samoon, A.H.; Bhatti, A.L.; Bhatti, M.A.; Mazzaro, R.; et al. NiCo2O4 nanostructures loaded onto pencil graphite rod: An advanced composite material for oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 6650–6665. [Google Scholar] [CrossRef]
- Zou, C.; Ma, C.; Chen, F.; Shao, X.; Cao, L.; Yang, J. Crystal facet controlled stable PbO2 electrode for efficient degradation of tetracycline. J. Electroanal. Chem. 2022, 914, 116330. [Google Scholar] [CrossRef]
- Yan, Y.; Ma, X.; Xia, Y.; Feng, H.; Liu, S.; He, C.; Ding, Y. Mechanism of highly efficient electrochemical degradation of antibiotic sulfadiazine using a layer-by-layer GNPs/PbO2 electrode. Environ. Res. 2023, 217, 114778. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Xi, G.; Zhang, X.; He, Z. Sonoelectrochemical oxidation of aged landfill leachate with high-efficiency Ti/PANI/PDMS-Ce-PbO2 anode. J. Environ. Chem. Eng. 2022, 10, 107499. [Google Scholar] [CrossRef]
- Wen, Z.; Ren, S.; Zhang, Y.; Li, J.; Zhang, Z.; Wang, A. Performance of anode materials in electro-Fenton oxidation of cefoperazone in chloride medium: New insight into simultaneous mineralization and toxic byproducts formation. J. Clean. Prod. 2022, 377, 120793. [Google Scholar] [CrossRef]
- Wang, G.; Fu, R.; Li, X.; Jia, Z.; Qu, J. Study on the anode stability of polyaniline with its pyrolysis products as electroactive components and the experimental study of cobalt electrowinning in chloride system. Energy Rep. 2022, 8, 754–768. [Google Scholar] [CrossRef]
- Eguiluz, K.I.B.; Hernandez-Sanchez, N.K.; Doria, A.R.; Santos, G.O.S.; Salazar-Banda, G.R.; de Leon, C.P. Template-made tailored mesoporous Ti/SnO2-Sb2O5-IrO2 anodes with enhanced activity towards dye removal. J. Electroanal. Chem. 2022, 910, 116153. [Google Scholar] [CrossRef]
- Yudha, C.S.; Rahmawati, M.; Apriliyani, E.; Nisa, S.S.; Jumari, A. Synthesis of Nickel Cobalt Manganese Ternary Transition Metal Oxide from Mixed Hydroxide Precipitate as a Precursor to NCM811. Defect Diffus. Forum 2022, 417, 131–139. [Google Scholar] [CrossRef]
- Huang, Z.; Mai Thanh, N.; Sim, W.J.; Takahashi, M.; Kheawhom, S.; Yonezawa, T. CoxNi1-xO-NiCo2O4/rGO synergistic bifunctional electrocatalysts for high-rate rechargeable zinc-air batteries. Sustain. Energy Fuels 2022, 6, 3931–3943. [Google Scholar] [CrossRef]
- Fan, Z.; Yu, H.; Jiang, G.; Yao, D.; Sun, S.; Chi, J.; Qin, B.; Shao, Z. Low precious metal loading porous transport layer coating and anode catalyst layer for proton exchange membrane water electrolysis. Int. J. Hydrogen Energy 2022, 47, 18963–18971. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Yu, S.H.; Yan, J.; Chua, D.H.C. SnO2-anchored carbon fibers chemical vapor deposition (CVD) synthesis: Effects of growth parameters on morphologies and electrochemical behaviors. J. Mater. Sci. 2020, 55, 15588–15601. [Google Scholar] [CrossRef]
- Mathur, S.; Erdem, A.; Cavelius, C.; Barth, S.; Altmayer, J. Amplified electrochemical DNA-sensing of nanostructured metal oxide films deposited on disposable graphite electrodes functionalized by chemical vapor deposition. Sens. Actuator B-Chem. 2009, 136, 432–437. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Qiu, Y.; Pan, Q.; Hu, P. 3D graphene/ZnO nanorods composite networks as supercapacitor electrodes. J. Alloys Compd. 2015, 620, 31–37. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, Z.; Yuan, S.; Li, H.; Zhang, Y.; Dong, Y. Direct current electrochemical method for removal and recovery of heavy metals from water using straw biochar electrode. J. Clean. Prod. 2022, 339, 130746. [Google Scholar] [CrossRef]
- Pozio, A.; Bozza, F.; Lisi, N.; Chierchia, R.; Migliorini, F.; Donde, R.; De Iuliis, S. Cobalt Oxide Synthesis via Flame Spray Pyrolysis as Anode Electrocatalyst for Alkaline Membrane Water Electrolyzer. Materials 2022, 15, 4626. [Google Scholar] [CrossRef]
- Le Luu, T.; Ngan, P.T.K. Fabrication of high performance Ti/SnO2-Nb2O5 electrodes for electrochemical textile wastewater treatment. Sci. Total Environ. 2023, 860, 160366. [Google Scholar] [CrossRef]
- Peroni, M.B.; Navas, M.B.; Ocsachoque, M.A.; Lick, I.D.; Casella, M.L.; Jaworski, M.A. Elimination of NO3− from water using Pd and PdCu catalysis supported on ZrO2-CeO2 materials: Effect of the support preparation. Mater. Chem. Phys. 2023, 296, 127186. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Li, Y.T.; Yao, Z.H.; Wang, J.; Zhong, Q. Iron-nickel aerogels anchored on GO nanosheets as efficient oxygen evolution reaction catalysts under industrial conditions. Int. J. Hydrogen Energ. 2022, 47, 6996–7004. [Google Scholar] [CrossRef]
- Deng, S.; Dai, Y.; Situ, Y.; Liu, D.; Huang, H. Preparation of nanosheet-based spherical Ti/SnO2-Sb electrode by in-situ hydrothermal method and its performance in the degradation of methylene blue. Electrochim. Acta 2021, 398, 139335. [Google Scholar] [CrossRef]
- Shivakumar, M.; Manjunatha, S.; Nithyayini, K.N.; Dharmaprakash, M.S.; Nagashree, K.L. Electrocatalytic detection of nitrite at NiCo2O4 nanotapes synthesized via microwave-hydrothermal method. J. Electroanal. Chem. 2021, 882, 115016. [Google Scholar] [CrossRef]
- Kalinic, B.; Girardi, L.; Ragonese, P.; Faramawy, A.; Mattei, G.; Frasconi, M.; Baretta, R.; Bogialli, S.; Roverso, M.; Rizzi, G.A.; et al. Diffusion-driven formation of Co3O4 nanopetals layers for photoelectrochemical degradation of organophosphate pesticides. Appl. Surf. Sci. 2022, 596, 153552. [Google Scholar] [CrossRef]
- Xiao, Z.H.; Wang, J.; Liu, C.H.; Wang, B.W.; Zhang, Q.; Xu, Z.L.; Sarwar, M.T.; Tang, A.D.; Yang, H.M. In-situ surface structural reconstruction of NiMoO4 for efficient overall water splitting. Appl. Surf. Sci. 2022, 602, 154314. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Z.; Qiao, D.; Dan, W.; Xu, H.; Yan, W.; Jin, X. Fabrication and characterization of porous titanium-based PbO2 electrode through the pulse electrodeposition method: Deposition condition optimization by orthogonal experiment. Chemosphere 2020, 261, 128157. [Google Scholar] [CrossRef]
- Wang, K.; Xing, X.; Liu, W.; Jiang, Y.; Li, H.; Lu, Y.; Chen, H.; Ren, H. Fabrication of a novel PbO2 electrode with rare earth elements doping for p-nitrophenol degradation. J. Environ. Chem. Eng. 2023, 11, 109513. [Google Scholar] [CrossRef]
- Wu, D.; Wu, X. Research Progress in Electrodeposition Technology of Titanium-Based Iridium Oxide Electrode. J. Electrochem. 2021, 27, 35–44. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhou, X.; Zheng, R.; Liu, Z.; Wang, J. Application of lead oxide electrodes in wastewater treatment: A review. Sci. Total Environ. 2022, 806, 150088. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, G.; Li, P.; Wang, X.; Wang, X.; Zhang, C.; Zhang, Y. Recent progress in engineering approach towards the design of PbO2-based electrodes for the anodic oxidation of organic pollutants. J. Water Process Eng. 2021, 42, 102173. [Google Scholar] [CrossRef]
- Shao, D.; Li, W.; Wang, Z.; Yang, C.; Xu, H.; Yan, W.; Yang, L.; Wang, G.; Yang, J.; Feng, L.; et al. Variable activity and selectivity for electrochemical oxidation wastewater treatment using a magnetically assembled electrode based on Ti/PbO2 and carbon nanotubes. Sep. Purif. Technol. 2022, 301, 122008. [Google Scholar] [CrossRef]
- Man, S.; Luo, D.; Sun, Q.; Yang, H.; Bao, H.; Xu, K.; Zeng, X.; He, M.; Yin, Z.; Wang, L.; et al. When MXene (Ti3C2Tx) meet Ti/PbO2: An improved electrocatalytic activity and stability. J. Hazard. Mater. 2022, 430, 128440. [Google Scholar] [CrossRef]
- Dai, J.; Feng, H.; Shi, K.; Ma, X.; Yan, Y.; Ye, L.; Xia, Y. Electrochemical degradation of antibiotic enoxacin using a novel PbO2 electrode with a graphene nanoplatelets inter-layer: Characteristics, efficiency and mechanism. Chemosphere 2022, 307, 135833. [Google Scholar] [CrossRef]
- You, S.J.; Liu, B.; Gao, Y.F.; Wang, Y.; Tang, C.Y.Y.; Huang, Y.B.; Ren, N.Q. Monolithic Porous Magneli-phase Ti4O7 for Electro-oxidation Treatment of Industrial Wastewater. Electrochim. Acta 2016, 214, 326–335. [Google Scholar] [CrossRef]
- Pei, S.; Zhu, L.; Zhang, Z.; Teng, J.; Liu, X.; You, S. Electrochemical properties of titanium sub-oxide membrane electrode and application for electro-oxidation treatment of dyeing wastewater. Acta Sci. Circumst. 2020, 40, 3658–3665. [Google Scholar]
- Huang, P.; Lei, J.; Sun, Z.; Hu, X. Fabrication of MOF-derivated CuOx-C electrode for electrochemical degradation of ceftazidime from aqueous solution. Chemosphere 2021, 268, 129157. [Google Scholar] [CrossRef]
- Safarvand, D.; Naser, I.; Samipourgiri, M.; Arjmand, M. Efficient Photoelectrocatalytic Degradation of BTEX Using TiO2/CuO/Cu2O Nanorod-Array Film as the Photoanode and MWCNT/GO/Graphite Felt as the Photocathode. Electrocatalysis 2020, 11, 188–202. [Google Scholar] [CrossRef]
- Lei, J.; Zhang, Y.; He, P. An alpha-Fe2O3/Circulating Fluidized Bed Fly Ash Based Geopolymer Composite Anode for Electrocatalytic Degradation of Indigo Carmine Dye Wastewater. J. Renew. Mater. 2021, 9, 2277–2289. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, X.; Dang, Y.; Yu, S.; Chen, S.; Tang, J.; Zhang, L.; Zhou, Y. Electrochemical Acceleration of Redox Reaction Cycles on the Surface of Fe2O3-MnO2 Cathode to Activate the Peroxymonosulfate for the Efficient Removal of Levofloxacin. J. Electrochem. Soc. 2022, 169, 023505. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, D.; Huang, M.; Pan, J.; Lin, J.; Chan, W. Iron oxide nanoparticles wrapped in graphene aerogel composite: Fabrication and application in electro-fenton at a Wide pH. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124269. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Z.; Wang, D.; Chen, S.; Qiao, D.; Wan, D.; Xu, H.; Yan, W.; Jin, X. Evaluation of diclofenac degradation effect in “active” and “non-active” anodes: A new consideration about mineralization inclination. Chemosphere 2022, 286, 131580. [Google Scholar] [CrossRef]
- Jiani, L.; Zhicheng, X.; Hao, X.; Dan, Q.; Zhengwei, L.; Wei, Y.; Yu, W. Pulsed electrochemical oxidation of acid Red G and crystal violet by PbO2 anode. J. Environ. Chem. Eng. 2020, 8, 103773. [Google Scholar] [CrossRef]
- Rong, H.; Zhang, C.; Sun, Y.; Wu, L.; Lian, B.; Wang, Y.; Chen, Y.; Tu, Y.; Waite, T.D. Electrochemical degradation of Ni-EDTA complexes in electroless plating wastewater using PbO2-Bi electrodes. Chem. Eng. J. 2022, 431, 133230. [Google Scholar] [CrossRef]
- Kim, T.; Kim, G.-P.; Lee, D.; Kim, Y.; Shim, S.E.; Baeck, S.-H. Electrochemical Oxidation of Organic Matter in the Presence of Chloride Over Ti/SnO2-Sb2O5 Prepared via Sol-Gel Methods. J. Nanosci. Nanotechnol. 2016, 16, 10892–10897. [Google Scholar] [CrossRef]
- Chen, X.M.; Gao, F.R.; Chen, G.H. Comparison of Ti/BDD and Ti/SnO2-Sb2O5 electrodes for pollutant oxidation. J. Appl. Electrochem. 2005, 35, 185–191. [Google Scholar] [CrossRef]
- Adams, B.; Tian, M.; Chen, A. Design and electrochemical study of SnO2-based mixed oxide electrodes. Electrochim. Acta 2009, 54, 1491–1498. [Google Scholar] [CrossRef]
- Ganzoury, M.A.; Ghasemian, S.; Zhang, N.; Yagar, M.; De Lannoy, C.-F. Mixed metal oxide anodes used for the electrochemical degradation of a real mixed industrial wastewater. Chemosphere 2022, 286, 131600. [Google Scholar] [CrossRef]
- Wei, L.; Mao, X.; Lin, A.; Gan, F. PbO2-SnO2 composite anode with interconnected structure for the electrochemical incineration of phenol. Russ. J. Electrochem. 2011, 47, 1394–1398. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, J.; Song, G.; Tian, Y.; Zhou, M. Anodic oxidation of organic pollutants: Anode fabrication, process hybrid and environmental applications. Curr. Opin. Electrochem. 2021, 26, 100659. [Google Scholar] [CrossRef]
- Duan, X.; Sui, X.; Wang, W.; Bai, W.; Chang, L. Fabrication of PbO2/SnO2 composite anode for electrochemical degradation of 3-chlorophenol in aqueous solution. Appl. Surf. Sci. 2019, 494, 211–222. [Google Scholar] [CrossRef]
- Memar, M.; Rezvani, A.R.; Saheli, S. Synthesis, characterization, and application of CuO nanoparticle 2D doped with Zn2+ against photodegradation of organic dyes (MB & MO) under sunlight. J. Mater. Sci. Mater. Electron. 2021, 32, 2127–2145. [Google Scholar] [CrossRef]
- Sun, G.; Wang, C.; Gu, W.; Song, Q. A facile electroless preparation of Cu, Sn and Sb oxides coated Ti electrode for electrocatalytic degradation of organic pollutants. Sci. Total Environ. 2021, 772, 144908. [Google Scholar] [CrossRef]
- Man, S.; Ge, X.; Xu, K.; Yang, H.; Bao, H.; Sun, Q.; He, M.; Xie, Y.; Li, A.; Mo, Z.; et al. Fabrication of a Ti/PbO2 electrode with Sb doped SnO2 nanoflowers as the middle layer for the degradation of methylene blue, norfloxacin and p-dihydroxybenzene. Sep. Purif. Technol. 2022, 280, 119816. [Google Scholar] [CrossRef]
- Zhang, F.X.; Shao, D.; Yang, C.A.; Xu, H.; Yang, J.; Feng, L.; Wang, S.Z.; Li, Y.; Jia, X.H.; Song, H.J. New Magnetically Assembled Electrode Consisting of Magnetic Activated Carbon Particles and Ti/Sb-SnO2 for a More Flexible and Cost-Effective Electrochemical Oxidation Wastewater Treatment. Catalysts 2023, 13, 7. [Google Scholar] [CrossRef]

| Electrode Types | Main Advantages | Main Drawbacks | Refs. |
|---|---|---|---|
| Lead oxide electrodes (PbO2 electrode, etc.) | Lead oxide electrodes have good electrical conductivity, corrosion resistance, and catalytic activity, and their preparation cost is relatively low. | Lead oxide electrodes have the potential to gradually release lead ions into the water, which can lead to heavy metal contamination. | [90,91,92,93] |
| Titanium sub-oxide electrodes (Ti4O7 and Ti5O9 electrodes, etc.) | They have good electrocatalytic activity and stability, and their potential toxicity is very low. | The manufacturing cost of the electrode is relatively high, and it is easily deactivated by some external pollutants in practical applications. | [49,94,95] |
| Electrodes based on copper oxides | Copper oxides demonstrate remarkable catalytic activity, providing an effective solution to the degradation of some resistant organic substances, particularly antibiotics. | Compared with lead oxide electrodes, their preparation costs are relatively high and the preparation technology is complex. | [96,97] |
| Electrodes based on iron oxides | Iron oxides can enhance the electrode’s capacity to degrade organic wastewater. Furthermore, they can elevate the electrode’s oxygen evolution potential and reduce the electrode’s inherent resistance in certain application environments. | Iron oxides are susceptible to corrosion in highly acidic environments. | [98,99,100] |
| Electrode Types | Main Advantages | Main Drawbacks | Refs. |
|---|---|---|---|
| Ti/SnO2-Sb2O5 electrode | High catalytic activity, excellent corrosion resistance, and stable electrochemical performance. | High price and brittleness. | [104,105,106] |
| Ti/RuO2-IrO2 electrode | High catalytic activity, excellent corrosion resistance, stable electrochemical performance, and can withstand high current densities. | High preparation costs, and the treatment effect of some organic wastewater types is not good. | [107] |
| PbO2-SnO2 electrode | High catalytic activity, good corrosion resistance, and a long service life. | High preparation costs, and the treatment effect of some organic wastewater types is not good. | [108,109,110] |
| ZnO/CuO electrode | High catalytic activity, good chemical stability, and low manufacturing costs. | The requirements in terms of water quality are relatively high (e.g., ion content, pH value). | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, X.; Wang, X.; Li, X.; Wang, D.; Xu, H.; Yan, W. Progress in the Preparation of Metal Oxide Electrodes for the Electrochemical Treatment of Organic Wastewater: A Short Review. Catalysts 2023, 13, 1096. https://doi.org/10.3390/catal13071096
Jing X, Wang X, Li X, Wang D, Xu H, Yan W. Progress in the Preparation of Metal Oxide Electrodes for the Electrochemical Treatment of Organic Wastewater: A Short Review. Catalysts. 2023; 13(7):1096. https://doi.org/10.3390/catal13071096
Chicago/Turabian StyleJing, Xiaosheng, Xinyu Wang, Xiaoliang Li, Dongqi Wang, Hao Xu, and Wei Yan. 2023. "Progress in the Preparation of Metal Oxide Electrodes for the Electrochemical Treatment of Organic Wastewater: A Short Review" Catalysts 13, no. 7: 1096. https://doi.org/10.3390/catal13071096