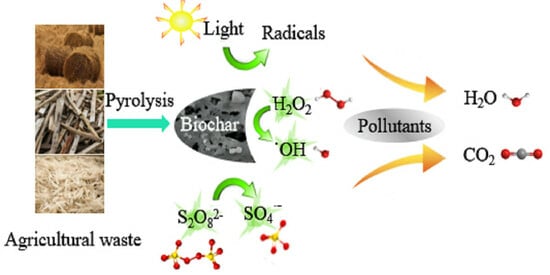
Customized High-Value Agricultural Residue Conversion: Applications in Wastewater Treatment
Abstract
:1. Introduction
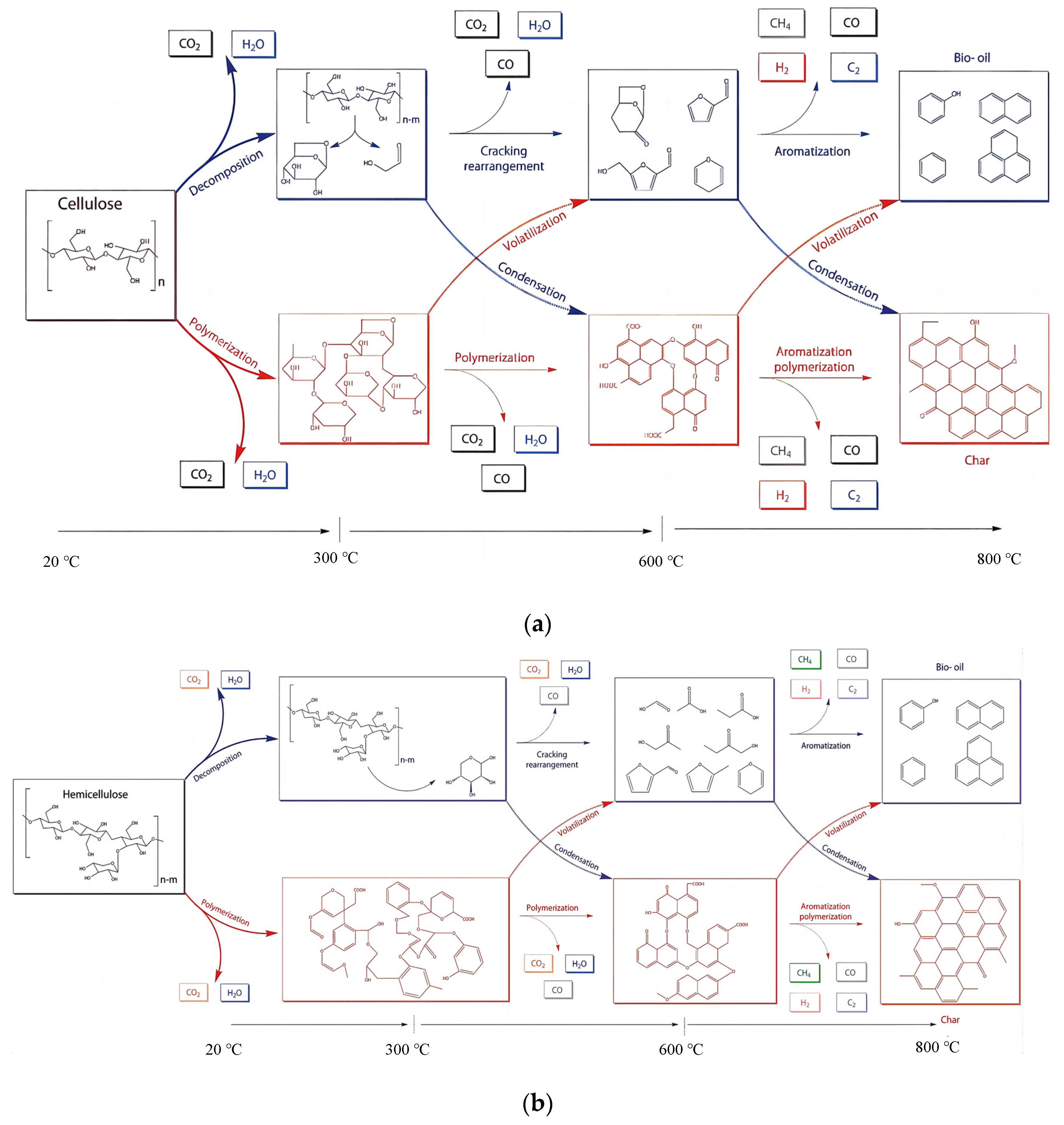
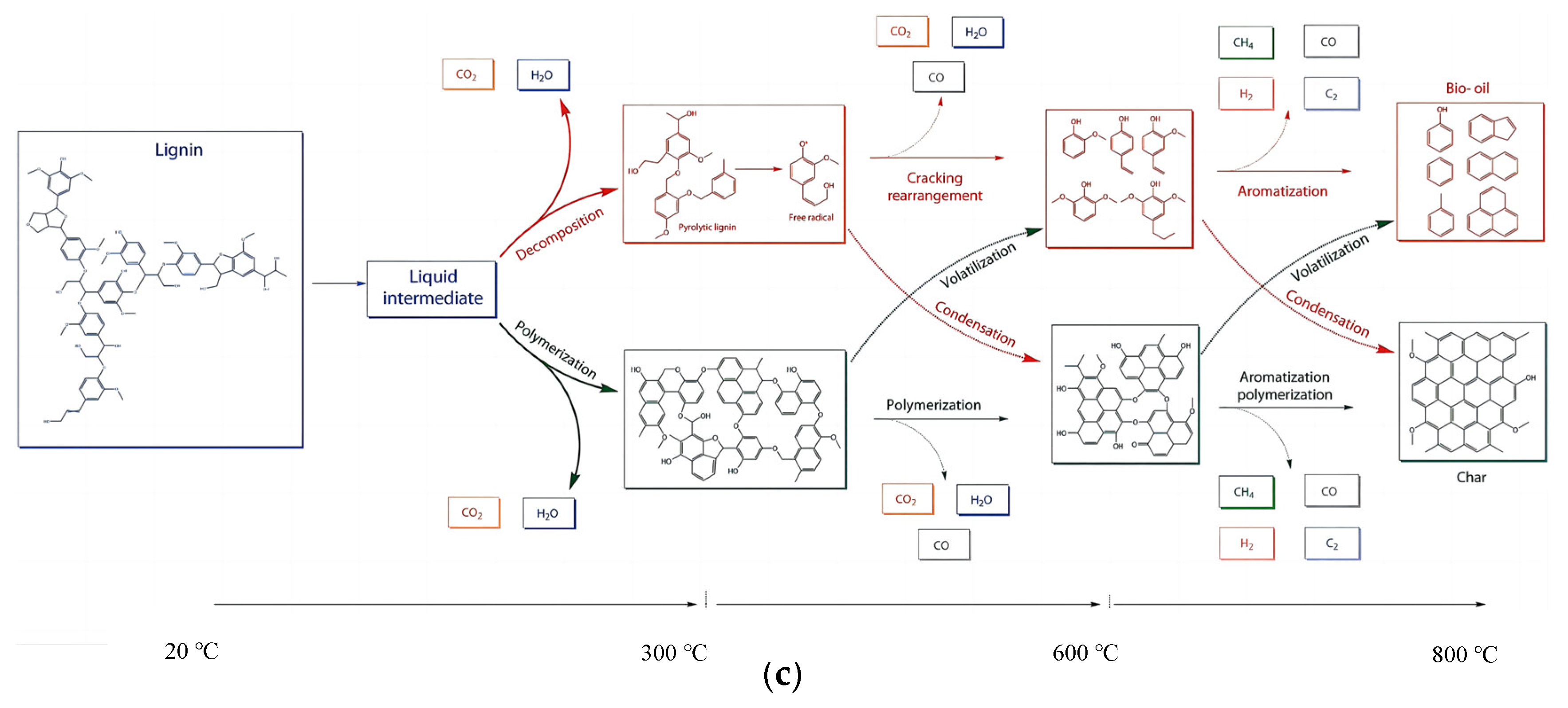
2. Biochemical Composition of Agricultural Residue
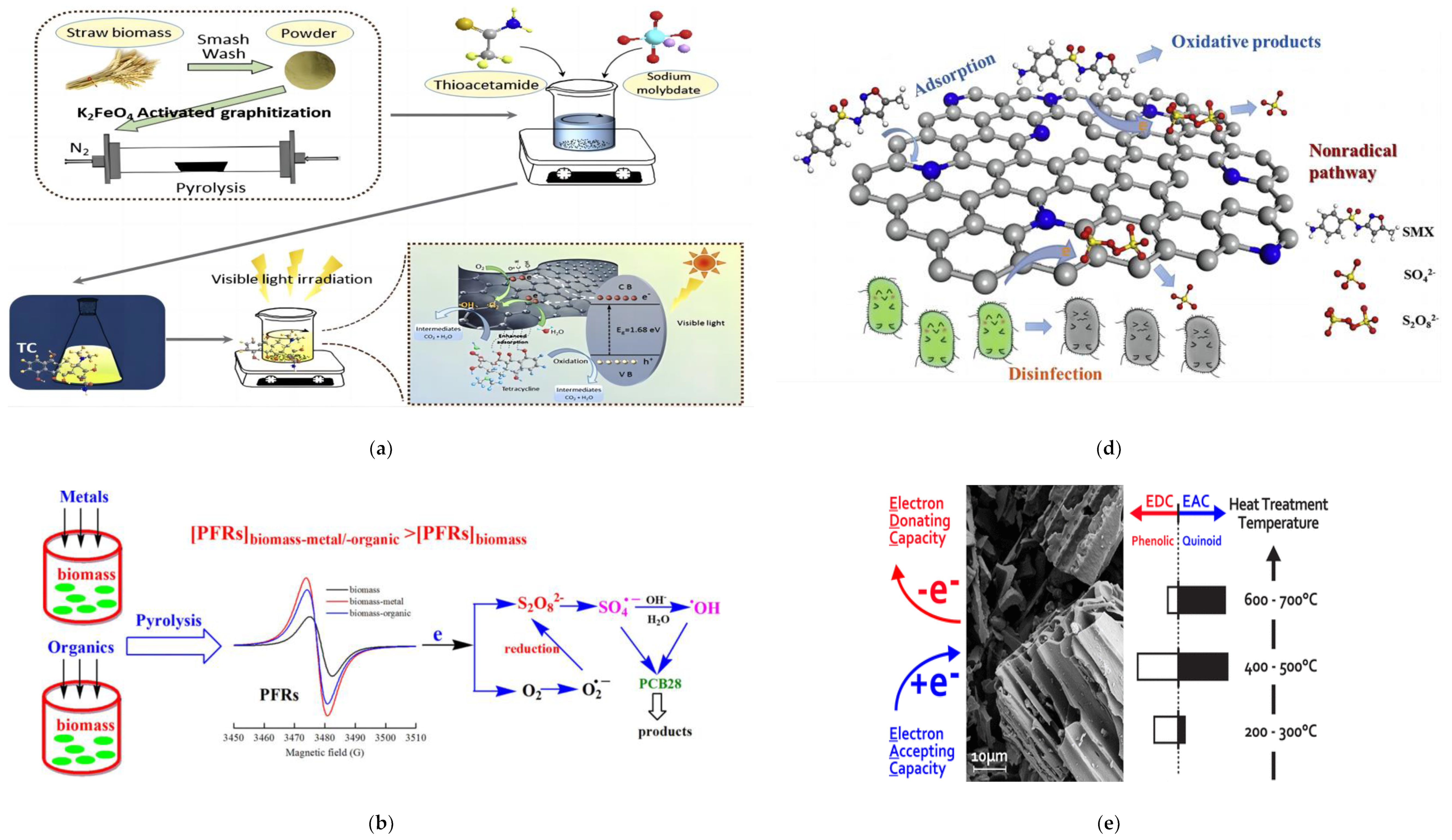
3. Removal of Emerging Pollutants from Sewage
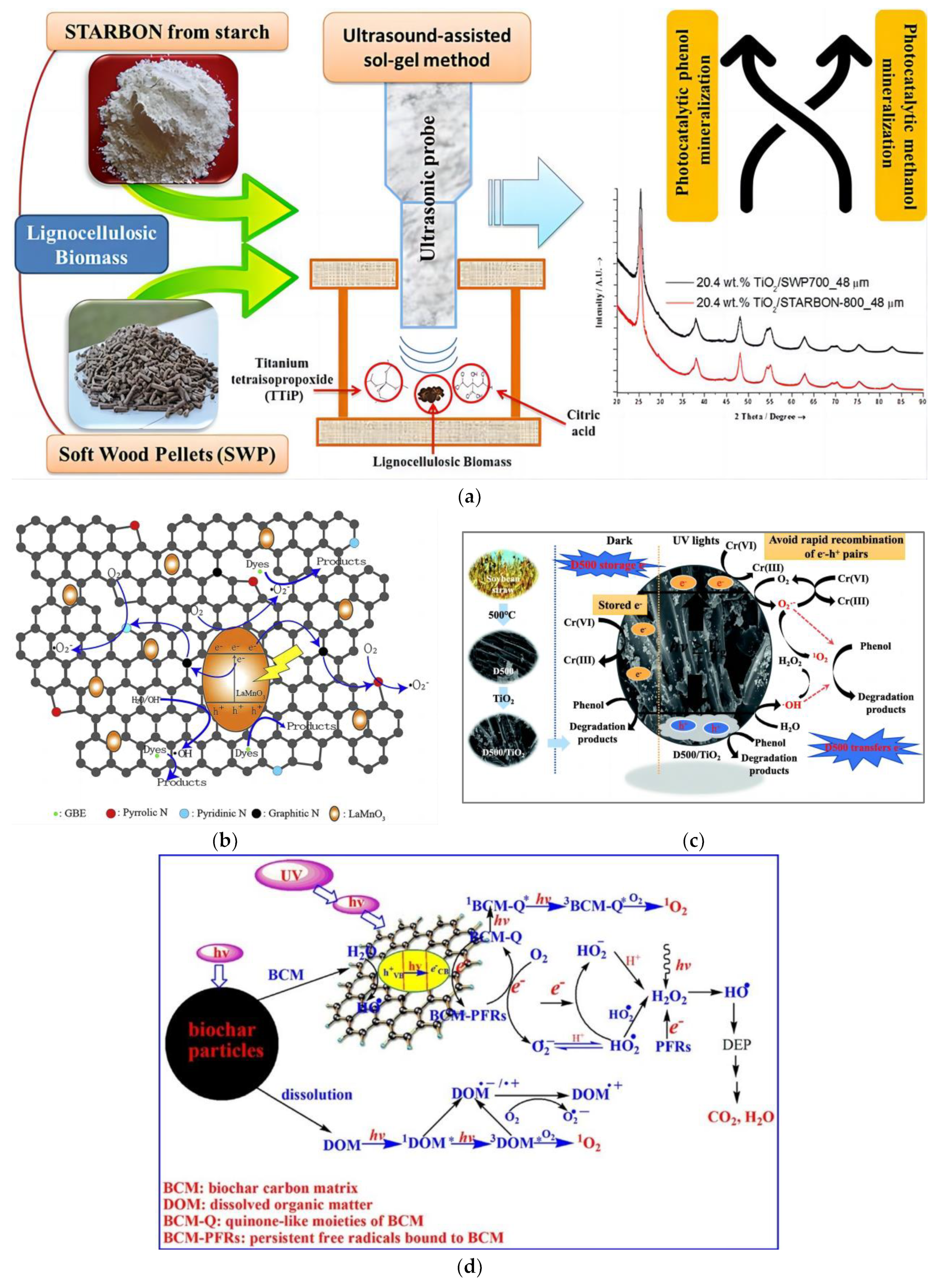
3.1. Photocatalysis
3.2. Redox Catalysis
3.3. Electrocatalysis and Other Joint Technologies
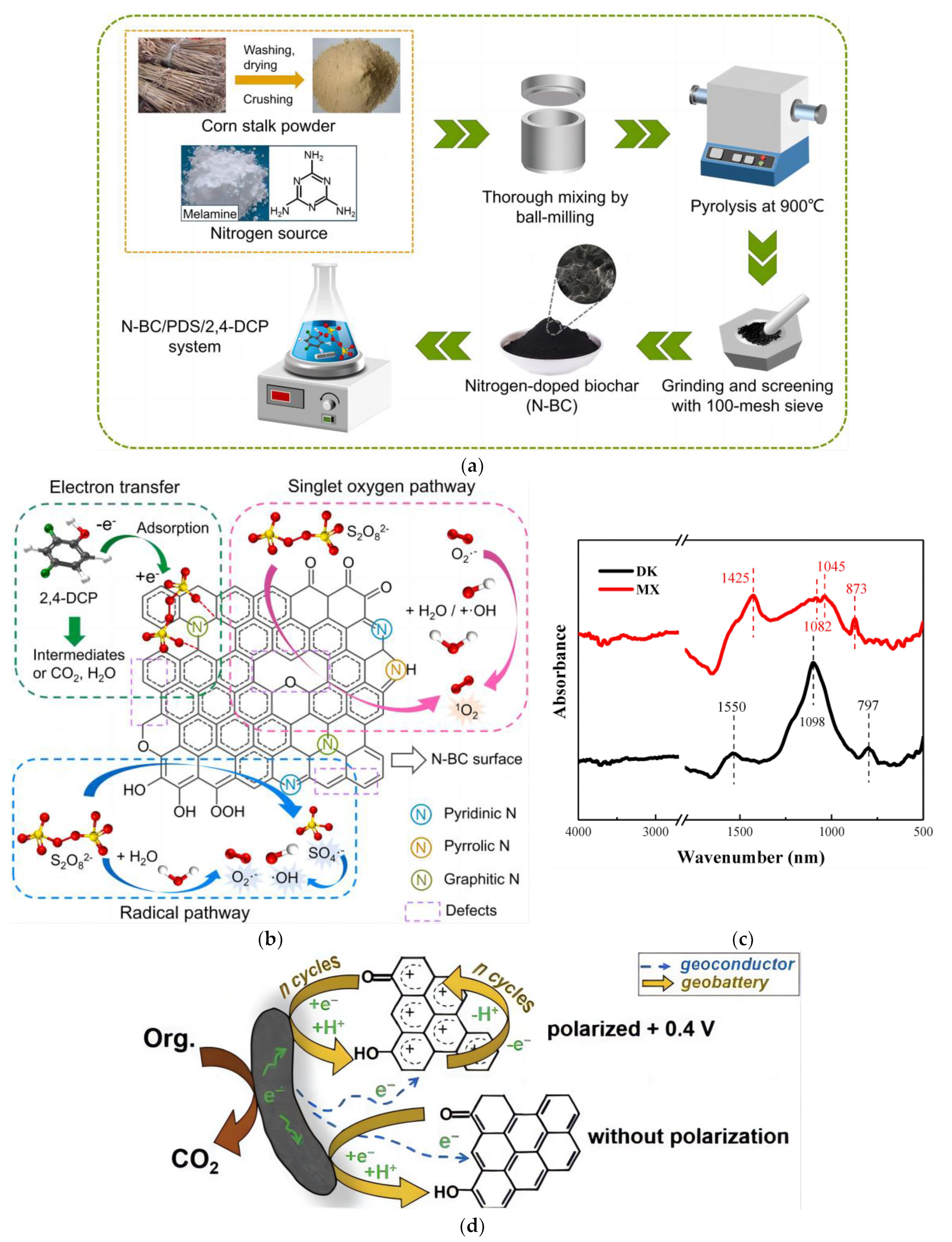
4. Key Factors and Mechanism of Catalysis
4.1. Active Substance and Mechanism Analysis
4.2. Persistent Free Radical
4.3. Electrical Conductivity and Electron Reservoir

5. Conclusions
- (1)
- Biochar has the characteristics of being low-cost, having a simple preparation, a large specific surface area, a rich pore structure, and being rich in various functional groups. It is an important catalytic material for the removal of refractory organic pollutants;
- (2)
- The content of lignin is high in agricultural residue, whereas the prepared biochar will have a higher ash content. Moreover, the particle-agglomeration reaction will lead to a decrease in pore volume;
- (3)
- The content of cellulose is the highest in agricultural residue, whereas the prepared biochar has a high thermal stability and provides the basis of a carbon-fixation framework. Although the biochar prepared from cellulose has less functional groups on the surface, it has more pores and surface area, due to which it has a better adsorption effect;
- (4)
- The protein content is the highest in agricultural residue. As the raw material of endogenous N-doping, it introduces nitrogen vacancies and changes the electronic state of atoms, increasing the number of redox-active sites;
- (5)
- It has been shown that biochar prepared at 400 °C can be induced by PFR-based oxidation reactions, while increasing the temperature to 700–900 °C leads to singlet oxidation and nonradical pathways in defective graphitic regions through surface PDS complexes;
- (6)
- The higher the conductivity and degree of graphitization, the better the electron-transfer-mediated activation of electron acceptors;
- (7)
- Due to the changes in functional groups and chemical structures, biochar treated at a high temperature has redox activity.
6. Future Perspectives
- (1)
- The surface properties of biochar prepared from agricultural residues are adjustable. However, the complex physicochemical properties of its raw materials are still the main obstacle to further optimize its performance. Therefore, an in-depth understanding of the mechanisms of the strengths and weaknesses of specific biochars can validate mission-appropriate principles and improve engineering strategies to more effectively exploit their advantages;
- (2)
- The nature of the active sites should be determined by analyzing the chemical composition of the waste sample. Moreover, its kinetic characteristics should also be analyzed under different conditions. The distribution of active sites to characterize their distribution in the waste needs to be studied. Through an in-depth study of the nature and distribution of active sites, utilization strategies for different wastes can be proposed;
- (3)
- Most of the studies are devoted to the catalytic removal of singular pollutants. However, actual wastewater is often a complex mixture of multiple pollutants. Therefore, it is necessary to improve the efficiency and effectiveness of wastewater treatment through the comprehensive design of catalysts, the study of reaction mechanisms, the development of multistage treatment systems, and the consideration of environmental factors to achieve more sustainable and environment-friendly wastewater treatment methods;
- (4)
- The stability and recyclability of catalysts are the key factors to determine the feasibility of their practical applications. At present, most of the research is limited to the laboratory scale, ignoring the problems faced in practical applications. However, a comprehensive assessment must be made considering all factors, including the feasibility of large-scale preparations, stability, and recovery and reuse.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Makanda, K.; Nzama, S.; Kanyerere, T. Assessing the Role of Water Resources Protection Practice for Sustainable Water Resources Management: A Review. Water 2022, 14, 3153. [Google Scholar] [CrossRef]
- Kumar, M.; Shekhar, S.; Kumar, R.; Kumar, P.; Govarthanan, M.; Chaminda, T. Drinking water treatment and associated toxic byproducts: Concurrence and urgence. Environ. Pollut. 2023, 320, 121009–121026. [Google Scholar] [CrossRef] [PubMed]
- Reinl, K.L.; Harris, T.D.; Elfferich, I.; Coker, A.; Zhan, Q.; Domis, L.N.D.S.; Morales-Williams, A.M.; Bhattacharya, R.; Grossart, H.-P.; North, R.L.; et al. The role of organic nutrients in structuring freshwater phytoplankton communities in a rapidly changing world. Water Res. 2022, 219, 118573–118591. [Google Scholar] [CrossRef]
- An, Y.-C.; Gao, X.-X.; Jiang, W.-L.; Han, J.-L.; Ye, Y.; Chen, T.-M.; Ren, R.-Y.; Zhang, J.-H.; Liang, B.; Li, Z.-L.; et al. A critical review on graphene oxide membrane for industrial wastewater treatment. Environ. Res. 2023, 223, 115409. [Google Scholar] [CrossRef]
- Guo, Q.; Qi, F.; Mu, R.; Yu, G.; Ma, G.; Meng, Q. Advances in sustainable wastewater treatment: Microalgal-bacterial consortia process, greenhouse gas reduction and energy recovery technologies. Water Environ. J. 2023, 37, 192–205. [Google Scholar] [CrossRef]
- Li, D.; Wang, Z.; Yang, Y.; Liu, H.; Fang, S.; Liu, S. Research Status and Development Trend of Wastewater Treatment Technology and Its Low Carbonization. Appl. Sci. 2023, 13, 1400. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Z.; Luo, Y.; Bai, Y.; Fan, J. Bioremediation of phenolic pollutants by algae-current status and challenges. Bioresour. Technol. 2022, 350, 126930–126940. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Pang, Z.; Yang, S.; Lin, Q.; Song, S.; Li, C.; Ma, X.; Nie, S. Improving Wastewater Treatment by Triboelectric-Photo/Electric Coupling Effect. ACS Nano 2022, 16, 3449–3475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ahmed, A.I.S.; Malik, M.Z.; Ali, N.; Khan, A.; Ali, F.; Hassan, M.O.; Mohamed, B.A.; Zdarta, J.; Bilal, M. Cellulose/inorganic nanoparticles-based nano-biocomposite for abatement of water and wastewater pollutants. Chemosphere 2023, 313, 137483–137501. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, F.; Zhao, S.; Zhu, J.; Yin, C. Application of Heterogeneous Catalytic Ozonation in Wastewater Treatment: An Overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Lin, J.; Tian, W.; Guan, Z.; Zhang, H.; Duan, X.; Wang, H.; Sun, H.; Fang, Y.; Huang, Y.; Wang, S. Functional Carbon Nitride Materials in Photo-Fenton-like Catalysis for Environmental Remediation. Adv. Funct. Mater. 2022, 32, 2201743–2201774. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Azhar, Q.M.; Kumar, P.S.; Yusuf, A.A.; Al-Buriahi, A.K.; Mohamed, R.M.S.R.; Al-Shaibani, M.M. Sustainable approaches for removing Rhodamine B dye using agricultural waste adsorbents: A review. Chemosphere 2022, 287, 132080–132090. [Google Scholar] [CrossRef] [PubMed]
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; González-López, C. Microalgae based wastewater treatment coupled to the production of high value agricultural products: Current needs and challenges. Chemosphere 2022, 291, 132968–132980. [Google Scholar] [CrossRef] [PubMed]
- Zan, F.; Iqbal, A.; Lu, X.; Wu, X.; Chen, G. “Food waste-wastewater-energy/resource” nexus: Integrating food waste management with wastewater treatment towards urban sustainability. Water Res. 2022, 211, 118089–118102. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, Y.; Li, J.; Patel, A.K.; Dong, C.-D.; Jin, X.; Gu, C.; Yip, A.C.; Tsang, D.C.; Ok, Y.S. Recent advancements and challenges in emerging applications of biochar-based catalysts. Biotechnol. Adv. 2023, 67, 108181–108260. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Yang, Y.; Han, Y.; Wang, T.; Chen, J.; Tsang, D.C. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 2020, 383, 121240–121248. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Zheng, C.-H.; Huang, Y.-Y. Removal of chlortetracycline from water using spent tea leaves-based biochar as adsorption-enhanced persulfate activator. Chemosphere 2022, 286, 131770–131777. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Xiao, R.; Tafti, N.; DeLaune, R.D.; Seo, D.-C. Degradation of Orange G by Fenton-like reaction with Fe-impregnated biochar catalyst. Bioresour. Technol. 2018, 249, 368–376. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613–123624. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Zhu, Y.; Li, J. Enhanced catalytic degradation of tetracycline antibiotic by persulfate activated with modified sludge bio-hydrochar. Chemosphere 2020, 247, 125854–125861. [Google Scholar] [CrossRef]
- Hou, J.; He, X.; Zhang, S.; Yu, J.; Feng, M.; Li, X. Recent advances in cobalt-activated sulfate radical-based advanced oxidation processes for water remediation: A review. Sci. Total Environ. 2021, 770, 145311–145325. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Jiang, Y.; Wu, Z.; Yao, G.; Lai, B. Synthesis strategies and emerging mechanisms of metal-organic frameworks for sulfate radical-based advanced oxidation process: A review. Chem. Eng. J. 2021, 421, 127863–127894. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Kakavandi, B.; Lin, K.Y.; Ghanbari, F. Acetaminophen removal from aqueous solutions through peroxymonosulfate activation by CoFe2O4/mpg-C3N4 nanocomposite: Insight into the performance and degradation kinetics. Environ. Technol. Innov. 2020, 20, 101127–101140. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Biomass-Based Adsorbents for Removal of Dyes from Wastewater: A Review. Front. Environ. Sci. 2021, 9, 558. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, Y.; Zhang, S.; Zhuang, L.; Hu, B.; Wang, S.; Chen, J.; Wang, X. Application of biochar-based photocatalysts for adsorption-(photo) degradation/reduction of environmental contaminants: Mechanism, challenges and perspective. Biochar 2022, 4, 45–68. [Google Scholar] [CrossRef]
- Hou, N.; Li, X.; Jiang, X.; Zhang, N.; Wang, R.; Li, D. The role of biochar in the photocatalytic treatment of a mixture of Cr(VI) and phenol pollutants: Biochar as a carrier for transferring and storing electrons. Sci. Total Environ. 2022, 844, 157145–157156. [Google Scholar] [CrossRef]
- Kumar, M.; Ambika, S.; Hassani, A.; Nidheesh, P. Waste to catalyst: Role of agricultural waste in water and wastewater treatment. Sci. Total Environ. 2023, 858, 159762–159781. [Google Scholar] [CrossRef]
- Wan, J.; Liu, L.; Ayub, K.S.; Zhang, W.; Shen, G.; Hu, S.; Qian, X. Characterization and adsorption performance of biochars derived from three key biomass constituents. Fuel 2020, 269, 117142–117148. [Google Scholar] [CrossRef]
- Huang, X.; Ren, J.; Ran, J.-Y.; Qin, C.-L.; Yang, Z.-Q.; Cao, J.-P. Recent advances in pyrolysis of cellulose to value-added chemicals. Fuel Process. Technol. 2022, 229, 107175–107192. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824–131839. [Google Scholar]
- Li, D.; Li, C.; Fan, M.; Shao, Y.; Sun, Y.; Zhang, L.; Zhang, S.; Huang, Y.; Li, B.; Wang, S.; et al. Investigation of property of biochar in staged pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2023, 172, 105999–106011. [Google Scholar] [CrossRef]
- Zhong, M.; Li, J.; Zhou, L.; Wang, T.; Liu, J.; Mei, M.; Chen, S. Co-pyrolysis of cellulose and polyethylene terephthalate by TG-MS: Pyrolysis behavior, conventional gas and solid phase product characteristics. J. Anal. Appl. Pyrolysis 2023, 172, 106002–106011. [Google Scholar] [CrossRef]
- Cao, X.; Luo, Q.; Song, F.; Liu, G.; Chen, S.; Li, Y.; Li, X.; Lu, Y. Effects of oxidative torrefaction on the physicochemical properties and pyrolysis products of hemicellulose in bamboo processing residues. Ind. Crops Prod. 2023, 191, 106002–106011. [Google Scholar] [CrossRef]
- Jia, Z.; Ji, N.; Diao, X.; Li, X.; Zhao, Y.; Lu, X.; Li, C. Highly Selective Hydrodeoxygenation of Lignin to Naphthenes over Three-Dimensional Flower-like Ni2P Derived from Hydrotalcite. ACS Catal. 2022, 12, 1338–1356. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, H.; Liu, Z.; Chen, P.; Chen, Y.; Wang, X.; Chen, H.; Wang, S. Pyrolysis of boron-crosslinked lignin: Influence on lignin softening and product properties. Bioresour. Technol. 2022, 355, 127218–127225. [Google Scholar] [CrossRef]
- Yuan, J.-M.; Li, H.; Xiao, L.-P.; Wang, T.-P.; Ren, W.-F.; Lu, Q.; Sun, R.-C. Valorization of lignin into phenolic compounds via fast pyrolysis: Impact of lignin structure. Fuel 2022, 319, 123758–123767. [Google Scholar] [CrossRef]
- Fan, Y.; Lei, M.; Han, Y.; Zhang, Z.; Kong, X.; Xu, W.; Zhang, H.; Xiao, R.; Liu, C. Elucidating radical-mediated pyrolysis behaviors of preoxidized lignins. Bioresour. Technol. 2022, 350, 126908–126915. [Google Scholar] [CrossRef]
- Anto, S.; Sudhakar, M.; Ahamed, T.S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 2021, 285, 119205–119212. [Google Scholar] [CrossRef]
- Chen, Y.-D.; Liu, F.; Ren, N.-Q.; Ho, S.-H. Revolutions in algal biochar for different applications: State-of-the-art techniques and future scenarios. Chin. Chem. Lett. 2020, 31, 2591–2602. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Zhang, S.; Chen, Y.-D.; Wang, R.; Ho, S.-H. Tailoring a novel hierarchical cheese-like porous biochar from algae residue to boost sulfathiazole removal. Environ. Sci. Ecotechnol. 2022, 10, 100168–100176. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.-H.; Chen, W.-H.; Xie, Y.; Liu, Z.; Chang, J.-S. Torrefaction performance and energy usage of biomass wastes and their correlations with torrefaction severity index. Appl. Energy 2018, 220, 598–604. [Google Scholar] [CrossRef]
- Praveen, S.; Jegan, J.; Pushpa, T.B.; Gokulan, R.; Bulgariu, L. Biochar for removal of dyes in contaminated water: An overview. Biochar 2022, 4, 10–15. [Google Scholar] [CrossRef]
- Bhavani, P.; Hussain, M.; Park, Y.K. Recent advancements on the sustainable biochar based semiconducting materials for photocatalytic applications: A state of the art review. J. Clean. Prod. 2022, 330, 129899–129915. [Google Scholar] [CrossRef]
- Qiu, M.; Hu, B.; Chen, Z.; Yang, H.; Zhuang, L.; Wang, X. Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar 2021, 3, 117–123. [Google Scholar] [CrossRef]
- Lisowski, P.; Colmenares, J.C.; Mašek, O.; Lisowski, W.; Lisovytskiy, D.; Grzonka, J.; Kurzydłowski, K. Design and Fabrication of TiO2/Lignocellulosic Carbon Materials: Relevance of Low-Temperature Sonocrystallization to Photocatalysts Performance. Chemcatchem 2018, 10, 3469–3480. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Lu, B.; Wang, X.; Huang, H. LaMnO3 nanoparticles supported on N doped porous carbon as efficient photocatalyst. Vacuum 2019, 159, 59–68. [Google Scholar] [CrossRef]
- Li, F.; Wei, Z.; He, K.; Blaney, L.; Cheng, X.; Xu, T.; Liu, W.; Zhao, D. A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst. Water Res. 2020, 185, 116219. [Google Scholar] [CrossRef]
- Shi, L.; Yin, Y.; Zhang, L.-C.; Wang, S.; Sillanpää, M.; Sun, H. Design and engineering heterojunctions for the photoelectrochemical monitoring of environmental pollutants: A review. Appl. Catal. B Environ. 2019, 248, 405–422. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Ma, C.; Liu, Y.; Dong, H.; Ma, W.; Liu, Z.; Wei, M.; Li, C.; Yan, Y. A two step hydrothermal process to prepare carbon spheres from bamboo for construction of core-shell non-metallic photocatalysts. New J. Chem. 2018, 42, 6515–6524. [Google Scholar] [CrossRef]
- Fang, G.; Liu, C.; Wang, Y.; Dionysiou, D.D.; Zhou, D. Photogeneration of reactive oxygen species from biochar suspension for diethyl phthalate degradation. Appl. Catal. B Environ. 2017, 214, 34–45. [Google Scholar] [CrossRef]
- Antonietti, M.; Lopez-Salas, N.; Primo, A. Adjusting the Structure and Electronic Properties of Carbons for Metal-Free Carbocatalysis of Organic Transformations. Adv. Mater. 2019, 31, 1805719–1805770. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Ren, X.; Dong, W.; Chen, H.; Cai, T.; Zeng, W.; Li, W.; Tang, L. Soybean residue based biochar prepared by ball milling assisted alkali activation to activate peroxydisulfate for the degradation of tetracycline. J. Colloid Interface Sci. 2021, 599, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, H.; Zhang, S.; Su, X. Non-radical mechanism of N-doped porous biochar derived from corn stalks for efficient peroxydisulfate activation. J. Environ. Chem. Eng. 2023, 11, 109123–109132. [Google Scholar] [CrossRef]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, Y.-D.; Li, R.; Zhang, C.; Ge, Y.; Cao, G.; Ma, M.; Duan, X.; Wang, S.; Ren, N.-Q. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019, 159, 77–86. [Google Scholar] [CrossRef]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. Oxidation of Sulfamethoxazole by Rice Husk Biochar-Activated Persulfate. Catalysts 2021, 11, 850. [Google Scholar] [CrossRef]
- Li, F.; Duan, F.; Ji, W.; Gui, X. Biochar-activated persulfate for organic contaminants removal: Efficiency, mechanisms and influencing factors. Ecotoxicol. Environ. Saf. 2020, 198, 110653–110660. [Google Scholar] [CrossRef]
- Babel, K.; Jurewicz, K. KOH activated lignin based nanostructured carbon exhibiting high hydrogen electrosorption. Carbon 2008, 46, 1948–1956. [Google Scholar] [CrossRef]
- Saha, D.; Li, Y.; Bi, Z.; Chen, J.; Keum, J.K.; Hensley, D.K.; Grappe, H.A.; Meyer, H.M.; Dai, S.; Paranthaman, M.P.; et al. Studies on Supercapacitor Electrode Material from Activated Lignin-Derived Mesoporous Carbon. Langmuir 2014, 30, 900–910. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene—An overview. Environ. Sci. Pollut. R. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Huang, G.; Kong, Q.; Yao, W.; Wang, Q. Poly tannic acid carbon rods as anode materials for high performance lithium and sodium ion batteries. J. Colloid Interface Sci. 2023, 629, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Dong, Y.; Ding, Y.; Yang, W.; Han, J.; Jiang, S.; He, S. Nitrogen-doped carbon layer on cellulose derived free-standing carbon paper for high-rate supercapacitors. Appl. Surf. Sci. 2023, 608, 155144–155156. [Google Scholar] [CrossRef]
- Rao, L.; Zhu, Y.; Duan, Z.; Xue, T.; Duan, X.; Wen, Y.; Kumar, A.S.; Zhang, W.; Xu, J.; Hojjati-Najafabadi, A. Lotus seedpods biochar decorated molybdenum disulfide for portable, flexible, outdoor and inexpensive sensing of hyperin. Chemosphere 2022, 301, 134595–134604. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zheng, J.; Feng, L.; Du, C.; Jian, S.; Yang, W.; Wu, Y.A.; Jiang, S.; He, S.; Chen, W. Wood-derived biochar as thick electrodes for high-rate performance supercapacitors. Biochar 2022, 4, 50–68. [Google Scholar] [CrossRef]
- Lin, R.; Li, Y.; Yong, T.; Cao, W.; Wu, J.; Shen, Y. Synergistic effects of oxidation, coagulation and adsorption in the integrated fenton-based process for wastewater treatment: A review. J. Environ. Manag. 2022, 306, 114460–114474. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M. Limitations and future directions of application of the Fenton-like process in micropollutants degradation in water and wastewater treatment: A critical review. Chemosphere 2022, 296, 134041–134054. [Google Scholar] [CrossRef]
- Prado, A.; Berenguer, R.; Esteve-Nunez, A. Electroactive biochar outperforms highly conductive carbon materials for biodegrading pollutants by enhancing microbial extracellular electron transfer. Carbon 2019, 146, 597–609. [Google Scholar] [CrossRef]
- Do Minh, T.; Song, J.; Deb, A.; Cha, L.; Srivastava, V.; Sillanpää, M. Biochar based catalysts for the abatement of emerging pollutants: A review. Chem. Eng. J. 2020, 394, 124856–124880. [Google Scholar] [CrossRef]
- Xin, S.; Huo, S.; Zhang, C.; Ma, X.; Liu, W.; Xin, Y.; Gao, M. Coupling nitrogen/oxygen self-doped biomass porous carbon cathode catalyst with CuFeO2/biochar particle catalyst for the heterogeneous visible-light driven photo-electro-Fenton degradation of tetracycline. Appl. Catal. B Environ. 2022, 305, 121024–121039. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Suvarna, M.; Pan, L.; Tabatabaei, M.; Ok, Y.S.; Wang, X. Wet wastes to bioenergy and biochar: A critical review with future perspectives. Sci. Total Environ. 2022, 817, 152921–152943. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Pohořelý, M.; Meers, E.; Skoblia, S.; Moško, J.; Jeremiáš, M. Potential of coupling anaerobic digestion with thermochemical technologies for waste valorization. Fuel 2021, 294, 120533–120554. [Google Scholar] [CrossRef]
- Liu, H.; Ye, M.; Dong, X.; Ren, Z.; Long, S.; Lichtfouse, E. Removal of humic substances by the synergistic effect of biochar adsorption and activation of persulfate. J. Water Process. Eng. 2021, 44, 102428–102436. [Google Scholar] [CrossRef]
- Feng, D.; Lü, J.; Guo, S.; Li, J. Biochar enhanced the degradation of organic pollutants through a Fenton process using trace aqueous iron. J. Environ. Chem. Eng. 2021, 9, 104677–104686. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, H.; Lei, X.; Lian, Q.; Holmes, W.E.; Fei, L.; Zappi, M.E.; Gang, D.D. Synergistic adsorption and degradation of sulfamethoxazole from synthetic urine by hickory-sawdust-derived biochar: The critical role of the aromatic structure. J. Hazard. Mater. 2021, 418, 126366–126374. [Google Scholar] [CrossRef] [PubMed]
- Mazarji, M.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Fedorenko, A.; Bauer, T.; Soldatov, A.; Barakhov, A.; Dudnikova, T. Biochar-assisted Fenton-like oxidation of benzo a pyrene-contaminated soil. Environ. Geochem. Health 2022, 44, 195–206. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Khan, A.; Zhang, Y. Photochemistry of biochar during ageing process: Reactive oxygen species generation and benzoic acid degradation. Sci. Total Environ. 2021, 765, 144630–144640. [Google Scholar] [CrossRef]
- Rubeena, K.; Reddy, P.H.P.; Laiju, A.; Nidheesh, P. Iron impregnated biochars as heterogeneous Fenton catalyst for the degradation of acid red 1 dye. J. Environ. Manag. 2018, 226, 320–328. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, B.; Shen, J.; Yan, P.; Kang, J.; Wang, W.; Chen, Z. Preparation of novel N-doped biochar and its high adsorption capacity for atrazine based on pi-pi electron donor-acceptor interaction. J. Hazard. Mater. 2022, 432, 128757–128768. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Deng, S.; Zheng, Y.; Du, Y.; Li, L.; Yang, S.; Zhang, G.; Du, L.; Wang, G.; Cheng, M.; et al. The application of transition metal-modified biochar in sulfate radical based advanced oxidation processes. Environ. Res. 2022, 212, 113340–113354. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liang, Y.; Xia, W.; Almatrafi, E.; Song, B.; Wang, Z.; Zeng, Y.; Yang, Y.; Shang, Y.; Wang, C.; et al. Single atom Mn anchored on N-doped porous carbon derived from spirulina for catalyzed peroxymonosulfate to degradation of emerging organic pollutants. J. Hazard. Mater. 2023, 441, 129871–129880. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Bian, Y.; Wang, F.; Herzberger, A.; Yang, X.; Gu, C.; Jiang, X. Effects of biochar on dechlorination of hexachlorobenzene and the bacterial community in paddy soil. Chemosphere 2017, 186, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Zain, M.M.; Minggu, L.J.; Kassim, M.B.; Amin, N.A.S.; Salleh, W.W.; Salehmin, M.N.I.; Nasir, M.F.M.; Hir, Z.A.M. Constructing bio-templated 3D porous microtubular C-doped g-C3N4 with tunable band structure and enhanced charge carrier separation. Appl. Catal. B Environ. 2018, 236, 265–279. [Google Scholar] [CrossRef]
- Chen, N.; Huang, Y.; Hou, X.; Ai, Z.; Zhang, L. Photochemistry of Hydrochar: Reactive Oxygen Species Generation and Sulfadimidine Degradation. Environ. Sci. Technol. 2017, 51, 11278–11287. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, J.; Han, Y.; Zhu, J.; Zhou, L.; Lan, Y. Insights into the mechanism of persulfate activated by rice straw biochar for the degradation of aniline. Chemosphere 2018, 200, 373–379. [Google Scholar] [CrossRef]
- Ding, K.; Xu, W.Q. Black carbon facilitated dechlorination of DDT and its metabolites in the presence of sulfides. Environ. Sci. Technol. 2016, 252, 12976–12983. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pignatello, J.J.; Pan, B.; Xing, B. Degradation of p-Nitrophenol by Lignin and Cellulose Chars: H2O2-Mediated Reaction and Direct Reaction with the Char. Environ. Sci. Technol. 2017, 51, 8972–8980. [Google Scholar] [CrossRef]
- Ye, S.; Yan, M.; Tan, X.; Liang, J.; Zeng, G.; Wu, H.; Song, B.; Zhou, C.; Yang, Y.; Wang, H. Facile assembled biochar-based nanocomposite with improved graphitization for efficient photocatalytic activity driven by visible light. Appl. Catal. B Environ. 2019, 250, 78–88. [Google Scholar] [CrossRef]
- Yan, J.; Han, L.; Gao, W.; Xue, S.; Chen, M. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef]
- Hussain, I.; Li, M.; Zhang, Y.; Li, Y.; Huang, S.; Du, X.; Liu, G.; Hayat, W.; Anwar, N. Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol. Chem. Eng. J. 2017, 311, 163–172. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Recent progress in biochar-supported photocatalysts: Synthesis, role of biochar, and applications. RSC Adv. 2018, 8, 14237–14248. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Zhang, J.; Huang, K.; Liang, Y.; Hu, H.; Xu, X.; Chen, D.; Chang, M.; Wang, Y. Dense and uniform growth of TiO2 nanoparticles on the pomelo-peel-derived biochar surface for efficient photocatalytic antibiotic degradation. J. Environ. Chem. Eng. 2023, 11, 109358–109368. [Google Scholar] [CrossRef]
- Rajput, V.D.; Chernikova, N.; Minkina, T.; Gorovtsov, A.; Fedorenko, A.; Mandzhieva, S.; Bauer, T.; Tsitsuashvili, V.; Beschetnikov, V.; Wong, M.H. Biochar and metal-tolerant bacteria in alleviating ZnO nanoparticles toxicity in barley. Environ. Res. 2023, 220, 115243–115253. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Seo, Y.-D.; Ryu, K.-S.; Park, D.-J.; Lee, S.-H. Redox and catalytic properties of biochar-coated zero-valent iron for the removal of nitro explosives and halogenated phenols. Environ. Sci. Process. Impacts 2017, 19, 711–719. [Google Scholar] [CrossRef]
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef]
- Fang, G.; Liu, C.; Gao, J.; Dionysiou, D.D.; Zhou, D. Manipulation of Persistent Free Radicals in Biochar to Activate Persulfate for Contaminant Degradation. Environ. Sci. Technol. 2015, 49, 5645–5653. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Liu, C.; Dionysiou, D.D.; Wang, Y.; Zhou, D. Key Role of Persistent Free Radicals in Hydrogen Peroxide Activation by Biochar: Implications to Organic Contaminant Degradation. Environ. Sci. Technol. 2014, 48, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-Q.; Qi, J.-Y.; Wang, Y.-P.; Liu, Y.-L.; Wang, L.; Ma, J. Heterogeneous catalytic ozonation of atrazine with Mn-loaded and Fe-loaded biochar. Water Res. 2021, 193, 116860–116871. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R. Highly dispersed iron-doped biochar derived from sawdust for Fenton-like degradation of toxic dyes. J. Clean. Prod. 2021, 297, 126681–126691. [Google Scholar] [CrossRef]
- Tan, H.L.; Abdi, F.F.; Ng, Y.H. Heterogeneous photocatalysts: An overview of classic and modern approaches for optical, electronic, and charge dynamics evaluation. Chem. Soc. Rev. 2019, 48, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, S.; Chen, H.; He, Z.; Cao, G.; Wang, K.; Ho, S.H. Enhancing Biochar-Based Nonradical Persulfate Activation Using Data-Driven Techniques. Environ. Sci. Technol. 2023, 57, 4050–4059. [Google Scholar] [CrossRef]
- Li, M.; Fu, L.; Deng, L.; Hu, Y.; Yuan, Y.; Wu, C. A tailored and rapid approach for ozonation catalyst design. Environ. Sci. Ecotechnol. 2023, 15, 100244–100253. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Feng, Y.; Zhao, Q.; Wang, X.; Liu, J.; Li, N. Electrosynthesis of H2O2 through a two-electron oxygen reduction reaction by carbon based catalysts: From mechanism, catalyst design to electrode fabrication. Environ. Sci. Ecotechnol. 2022, 11, 100170–100200. [Google Scholar] [CrossRef]
- Luo, H. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2018, 361, 353–363. [Google Scholar]
- Ding, L.; Guo, X.; Du, S.; Cui, F.; Zhang, Y.; Liu, P.; Zhu, L. Insight into the Photodegradation of Microplastics Boosted by Iron (Hydr)oxides. Environ. Sci. Technol. 2022, 56, 17785–17794. [Google Scholar] [CrossRef]
- Sun, P.; Li, Y.; Meng, T.; Zhang, R.; Song, M.; Ren, J. Removal of sulfonamide antibiotics and human metabolite by biochar and biochar/H2O2 in synthetic urine. Water Res. 2018, 147, 91–100. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Wu, Q.; Luo, H.; Zhao, Q.; Si, Q.; Sseguya, F.; Ren, N. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-D.; Duan, X.; Zhang, C.; Wang, S.; Ren, N.-Q.; Ho, S.-H. Graphitic biochar catalysts from anaerobic digestion sludge for nonradical degradation of micropollutants and disinfection. Chem. Eng. J. 2020, 384, 123244–123344. [Google Scholar] [CrossRef]
- Qin, Y.; Li, G.; Gao, Y.; Zhang, L.; Ok, Y.S.; An, T. Persistent free radicals in carbon-based materials on transformation of refractory organic contaminants (ROCs) in water: A critical review. Water Res. 2018, 137, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Lieke, T.; Zhang, X.; Steinberg, C.E.; Pan, B. Overlooked Risks of Biochars: Persistent Free Radicals trigger Neurotoxicity in Caenorhabditis elegans. Environ. Sci. Technol. 2018, 52, 7981–7987. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Prévoteau, A.; Ronsse, F.; Cid, I.; Boeckx, P.; Rabaey, K. The electron donating capacity of biochar is dramatically underestimated. Sci. Rep. 2016, 6, 32870. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, W.; Yin, R.; Du, J.; Wu, Q.; Luo, H.; Liu, B.; Sseguya, F.; Ren, N. Biochar-induced Fe(III) reduction for persulfate activation in sulfamethoxazole degradation: Insight into the electron transfer, radical oxidation and degradation pathways. Chem. Eng. J. 2019, 362, 561–569. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Shao, Z.; Wang, S. Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Appl. Catal. B Environ. 2018, 224, 973–982. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lim, T.-T. Design and application of heterogeneous catalysts as peroxydisulfate activator for organics removal: An overview. Chem. Eng. J. 2019, 358, 110–133. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Pandit, B.; Dang, V.D.; Doong, R.-A. Agricultural waste to real worth biochar as a sustainable material for supercapacitor. Sci. Total Environ. 2023, 869, 161441–161459. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, H.; Xu, W.; Tian, L.; Huang, J.; Liang, C.; Zhang, Y. Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors. Biosensors 2022, 12, 377. [Google Scholar] [CrossRef]
- Mehdi, R.; Khoja, A.H.; Naqvi, S.R.; Gao, N.; Amin, N.A.S. A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications. Catalysts 2022, 12, 798. [Google Scholar] [CrossRef]
| Biomass | Pollutant | Operating Conditions | Removal Efficiency (%) | Reference |
|---|---|---|---|---|
| Soybean dregs | Green BE | Green BE = 20 mg/L, pH = 10, Pyrolysis Temp. = 800 °C | 90%, 3 h | [45] |
| Soybean stalks | Cr(VI) and phenol | Cr(VI) = 50 mg/L, phenol = 100 mg/L, pH = 2, Pyrolysis Temp. = 500 °C | 77%, 80.4%, 1 h | [26] |
| Bamboo | Tetracycline | tetracycline = 10 mg/L, Pyrolysis Temp. = 200 °C | 90%, 1 h | [47] |
| Pine needles | Diethyl phthalate | diethyl phthalate = 20 mg/L, pH = 2, Pyrolysis Temp. = 200 °C | 60%, 8 h | [48] |
| Wheat straws | Diethyl phthalate | diethyl phthalate = 20 mg/L, pH = 2, Pyrolysis Temp. = 200 °C | 45%, 8 h | [48] |
| Corn stover | 2,4-dichlorophenol | 2,4-dichlorophenol = 100 mg/L, pH = 3–9 | 98.4, 40 min | [51] |
| Rice husk (RH) | Sulfamethoxazole (SMX) | PS = 500 mg/L, RH = 100 mg/L, pH = 4–6, Pyrolysis Temp. = 850 °C | 96%, 120 min | [54] |
| Rice husk (RH) | Acid orange 7 (AO7) | AO7 = 50 mg/L, Pyrolysis Temp. = 700 °C, PS/AO7 = 40/1, pH = 3 | 100%, 2 h | [57] |
| Sawdust (SD) | AO7 | AO7 = 50 mg/L, Pyrolysis Temp. = 700 °C, PS/AO7 = 40/1, pH = 3 | 100%, 2 h | [57] |
| Corn stalk biochar | Humic acid | CB = 0.5 g/L, PS = 1 mM, Pyrolysis Temp. = 900 °C, HA = 100 mL, pH = 5.0 | 84.3%, 180 min | [72] |
| Cereal residue | 2,4-dichlorophenoxyacetic acid | [H2O2] = 5 mmol/L, [BC] = 3 g/L, [2,4-D] = 20 mg/L, pH = 3 | 94.8%, 60 min | [73] |
| Peanut shell biochar | Sulfamethoxazole | [H2O2] = 1 mM, [BC] = 0.5 mg/L, pH = 9, Pyrolysis Temp. = 700 °C | 56.9% | [74] |
| Sunflower husk | Benzo[a]pyrene | [H2O2] = 1.25 M, [BC] = 5 % w/w, hematite = 2 mg/g, Pyrolysis Temp. = 500 °C | 95% | [75] |
| Rice husk | Benzoic acid | [BA] = 1 mM, [BC] = 10 g/L, pH = 3, Pyrolysis Temp. = 550 °C | 95.2%, 6 h | [76] |
| Rice husk | Acid red1 | [AR1] = 50 mg/L, [H2O2] = 16 mM, [BC] = 5 g/L, pH = 3 | 97.6%, 2 h | [77] |
| Biomass | Pollutant | Removal Performance | Driving Factor | References |
|---|---|---|---|---|
| Wheat straw | Hexachlorobenzene, Dehalococcoidaceae; | Up to 56% | Carbon-centered PFRs | [82] |
| Kapok-derived | Bisphenol A | 91% | Direct h+ oxidation; large surface junctions; promoted separation and transfer of e−/h+ pairs | [83] |
| Pine needles, wheat straw | Diethyl phthalate | 76% (2 h) | e− transfer; conjugated network-confined PFRs; photo-Fenton; secondary formation of ROSs. | [47] |
| Soybean dreg char@LaMnO3 | Direct Green BE | 80%; Reuse: 4 cycles | Separation of e−/h+ pairs; h+ oxidation; O2∙− attack | [44] |
| Platanus acerifolia | Sulfadimidine, | 40% (in dark), 50% (daylight) | PFRs, H2O2 and secondary ROSs (mainly ∙OH) | [84] |
| Rice straw | Tetracycline | 40% TOC removal in 1 h | g-MoS2-enhanced surface adsorption and graphitization, Mo6+-induced PFR formation; h+ and ∙OH are predominant active species | [78] |
| Pine needles | 2,4,4′-Trichlorobiphenyl, PDS | 70–100% | PFRs-activated SO4∙− formation via e− transfer; metal species activators | [81] |
| Rice straw | Aniline, PDS | 94.1% | Generation of h+ | [85] |
| Oak wood | DDT, DDD, DDE, sulfide | e− transfer; carbon–sulfide intermediates | [86] | |
| Lignin and cellulose | p-Nitrophenol | 60% | PFRs direct reaction; nonradical sites | [87] |
| Rice straw | TNT, RDX, DBP, DFP, DCP | 99% (TNT); 70% (RDX); 65% (DBP, 24 h); 70% (DFP, 24 h) | Large SBET; redox potential of Fe(0) | [88] |
| Rice hull | Trichloroethylene, PDS | 99.4% | e− transfer and redox iron species | [89] |
| Rice husk | Nonylphenol, PDS | 96.2%; 73.4% mineralized; reuse: 5 cycles | e− transfer and redox iron species | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Wang, H.; Guo, X.; Ho, S.-H. Customized High-Value Agricultural Residue Conversion: Applications in Wastewater Treatment. Catalysts 2023, 13, 1247. https://doi.org/10.3390/catal13091247
Tan X, Wang H, Guo X, Ho S-H. Customized High-Value Agricultural Residue Conversion: Applications in Wastewater Treatment. Catalysts. 2023; 13(9):1247. https://doi.org/10.3390/catal13091247
Chicago/Turabian StyleTan, Xuefei, Huiwen Wang, Xiaoyan Guo, and Shih-Hsin Ho. 2023. "Customized High-Value Agricultural Residue Conversion: Applications in Wastewater Treatment" Catalysts 13, no. 9: 1247. https://doi.org/10.3390/catal13091247