Tailoring Morphology in Hydrothermally Synthesized CdS/ZnS Nanocomposites for Extraordinary Photocatalytic H2 Generation via Type-II Heterojunction
Abstract
1. Introduction
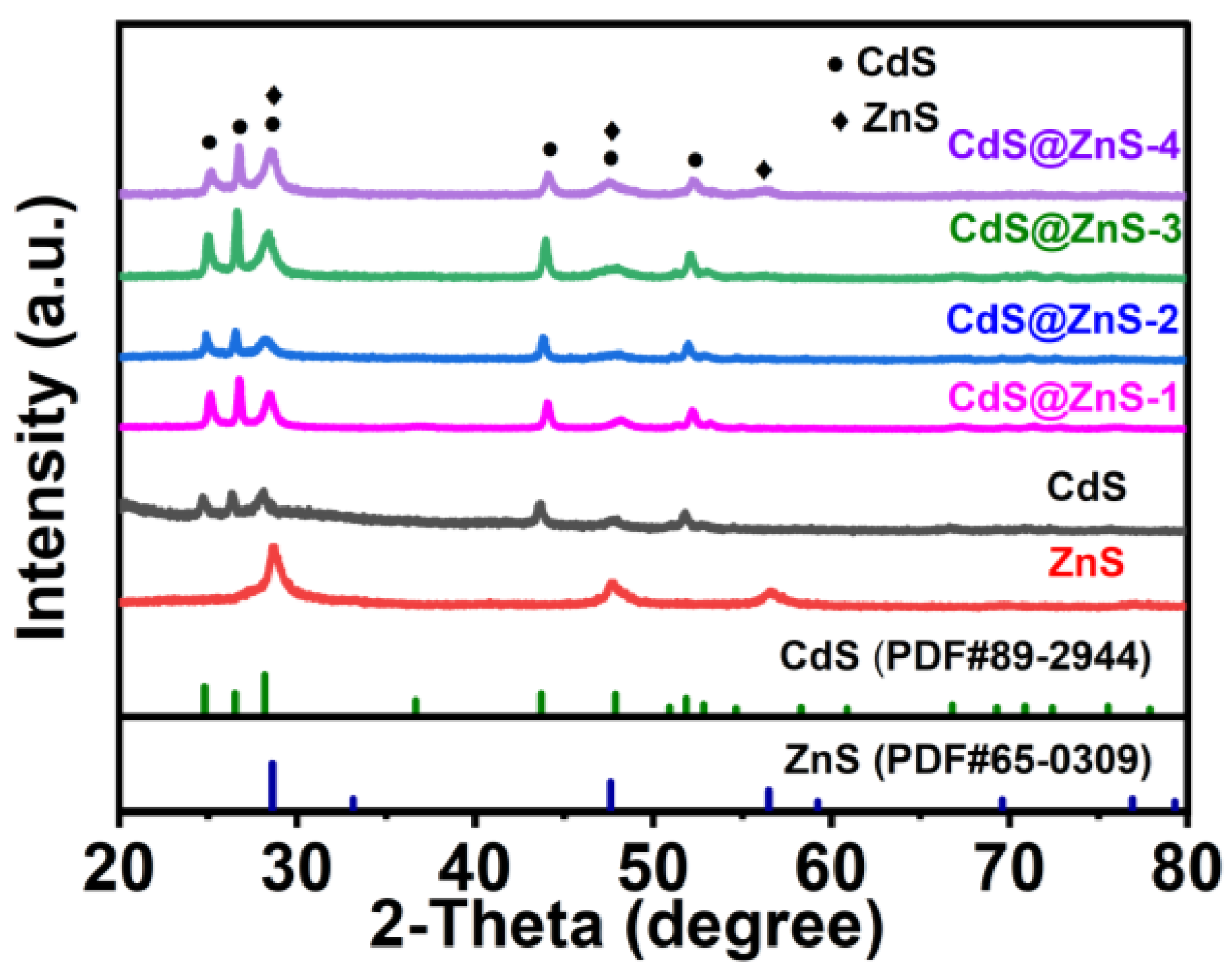
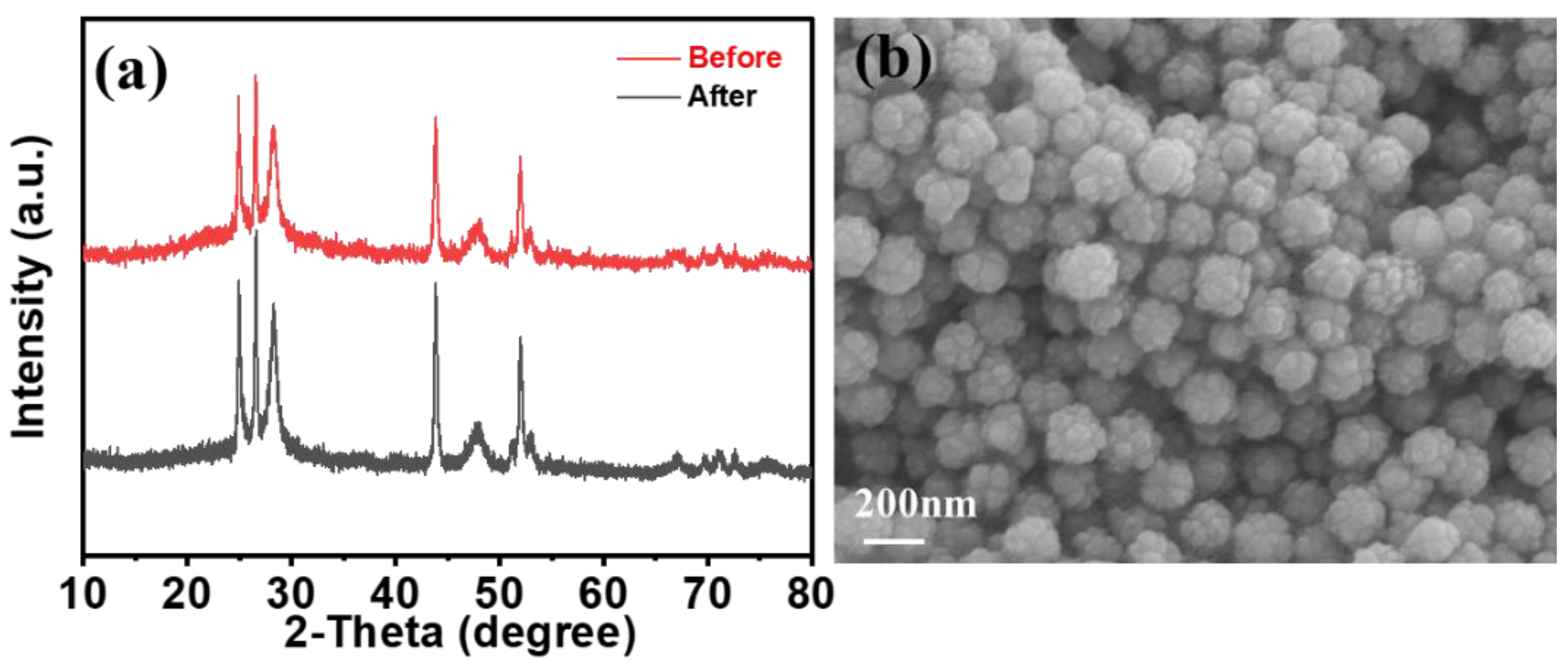
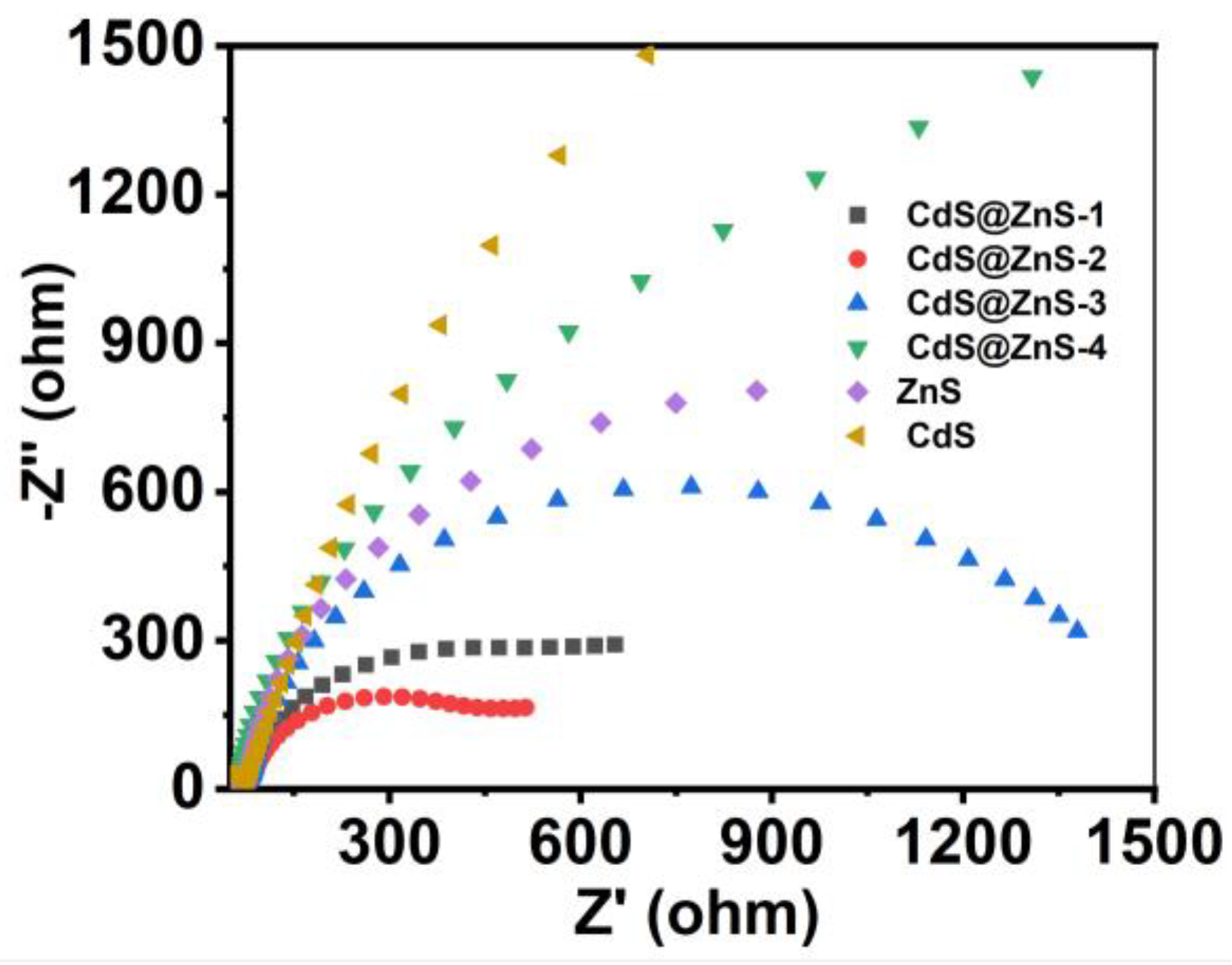
2. Results and Discussion
Characterization of CdS@ZnS Samples
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of CdS
3.2.2. Synthesis of ZnS
3.2.3. Synthesis of CdS@ZnS
3.3. Characterization
3.4. Photoelectrochemical Measurements
3.5. Photocatalytic Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Huang, M.; Yu, M.; Si, R.; Xu, W.; Xu, M.; Xiao, C.; Chen, W.-J.; Sun, L.; Weng, B.; Pan, X. Polar semiconductor photocatalysts with intrinsic electric fields for selective organic transformation. N. J. Chem. 2023, 47, 783–797. [Google Scholar] [CrossRef]
- Li, J.Y.; Li, Y.H.; Qi, M.Y.; Lin, Q.; Tang, Z.R.; Xu, Y.J. Selective Organic Transformations over Cadmium Sulfide-Based Photocatalysts. ACS Catal. 2020, 10, 6262–6280. [Google Scholar] [CrossRef]
- Liu, X.; Sayed, M.; Bie, C.; Cheng, B.; Hu, B.; Yu, J.; Zhang, L. Hollow CdS-based photocatalysts. J. Mater. 2021, 7, 419–439. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Guo, C.; Chen, T.; Guo, C.; Lu, Y.; Wang, J. CdS(ZB)/CdS(WZ)/Ni-BTC photocatalytic selective oxidation of benzyl alcohol to benzaldehyde coupled with hydrogen evolution. Appl. Surf. Sci. 2022, 571, 151284. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based photocatalysts. Ener. Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Peng, Y.-H.; Wen, P.-Y.; Wu, T.; Lin, Y.-W. Enhanced Visible Light Photocatalytic Degradation of Methylene Blue by CdS-ZnS-BiPO4 Nanocomposites Prepared by a Solvent-Assisted Heating Method. Catalysts 2021, 11, 1095. [Google Scholar] [CrossRef]
- Kai, S.; Xi, B.; Liu, X.; Ju, L.; Wang, P.; Feng, Z.; Ma, X.; Xiong, S. An innovative Au-CdS/ZnS-RGO architecture for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 2895–2899. [Google Scholar] [CrossRef]
- Chai, N.N.; Wang, H.X.; Hu, C.X.; Wang, Q.; Zhang, H.L. Well-controlled layer-by-layer assembly of carbon dot/CdS heterojunctions for efficient visible-light-driven photocatalysis. J. Mater. Chem. A 2015, 3, 16613–16620. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, W.; Chen, F.; Wang, P.; Fan, J.; Yu, H. Photoinduced self-stability mechanism of CdS photocatalyst: The dependence of photocorrosion and H2-evolution performance. J. Mater. Sci. Technol. 2022, 121, 19–27. [Google Scholar] [CrossRef]
- Navakoteswara Rao, V.; Lakshmana Reddy, N.; Preethi, V.; Karthik, M.; Yu, Y.-T.; Yang, J.M.; Mamatha Kumari, M.; Shankar, M.V. A critical review on core/shell-based nanostructured photocatalysts for improved hydrogen generation. Int. J. Hydrogen Energy 2023, 48, 11754–11774. [Google Scholar] [CrossRef]
- Qin, D.; Yang, G.; Wang, Y.; Zhang, J.; Zhang, L. Microwave-assisted synthesis of pectin-stabilised CdS/ZnS core/shell nanocrystals and enhanced photocatalytic performance. Micro Nano Lett. 2020, 15, 595–599. [Google Scholar] [CrossRef]
- An, L.; Han, X.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. ZnS–CdS–TaON nanocomposites with enhanced stability and photocatalytic hydrogen evolution activity. J. Sol-gel. Sci. Techn. 2019, 91, 82–91. [Google Scholar] [CrossRef]
- Rangappa, A.P.; Praveen Kumar, D.; Hong, Y.; Jeong, S.; Reddy, D.A.; Song, J.K.; Kim, T.K. Construction of a Highly Efficient and Durable 1D Ternary CdS/ZnS/Pt Nanohybrid Catalyst for Photocatalytic CO2 Reduction into Chemical Fuels under Solar Light Irradiation. Acs Appl. Energy Mater. 2020, 3, 10533–10540. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Cao, H.; Wang, L.; Wan, Z.; Hao, Y.; Wang, X. Facile preparation of ZnS/CdS core/shell nanotubes and their enhanced photocatalytic performance. Int. J. Hydrogen Energy 2017, 42, 17394–17402. [Google Scholar] [CrossRef]
- Su, J.; Zhang, T.; Wang, L.; Shi, J.; Chen, Y. Surface treatment effect on the photocatalytic hydrogen generation of CdS/ZnS core-shell microstructures. Chinese J. Catal. 2017, 38, 489–497. [Google Scholar] [CrossRef]
- Reddy, C.V.; Shim, J.; Cho, M. Synthesis, structural, optical and photocatalytic properties of CdS/ZnS core/shell nanoparticles. J. Phys. Chem. Solids 2017, 103, 209–217. [Google Scholar] [CrossRef]
- Sun, G.; Mao, S.; Ma, D.; Zou, Y.; Lv, Y.; Li, Z.; He, C.; Cheng, Y.; Shi, J.-W. One-step vulcanization of Cd(OH)Cl nanorods to synthesize CdS/ZnS/PdS nanotubes for highly efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2019, 7, 15278–15287. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.; Liu, F. Preparation and characterization of CdS/ZnS core-shell nanoparticles. J. Disper. Sci. Technol. 2019, 41, 725–732. [Google Scholar] [CrossRef]
- Kočí, K.; Praus, P.; Edelmannová, M.; Ambrožová, N.; Troppová, I.; Fridrichová, D.; Słowik, G.; Ryczkowski, J. Photocatalytic Reduction of CO2 Over CdS, ZnS and Core/Shell CdS/ZnS Nanoparticles Deposited on Montmorillonite. J. Nanosci. Nanotechnol. 2017, 17, 4041–4047. [Google Scholar] [CrossRef]
- Huang, M.; Yan, Y.; Feng, W.; Weng, S.; Zheng, Z.; Fu, X.; Liu, P. Controllable Tuning Various Ratios of ZnO Polar Facets by Crystal Seed-Assisted Growth and Their Photocatalytic Activity. Cryst. Growth Des. 2014, 14, 2179–2186. [Google Scholar] [CrossRef]
- Ding, Y.; Hu, Z.; Zhao, Y.; Shi, C.; Zhang, S.; Zhang, Z. Self-assembled nanoplatforms with ZIF-8 as a framework for FRET-based glutathione sensing in biological samples. Analyst 2022, 147, 5775–5784. [Google Scholar] [CrossRef]
- Lu, M.Y.; Hong, M.H.; Ruan, Y.M.; Lu, M.P. Probing the photovoltaic properties of Ga-doped CdS-Cu2S core-shell heterostructured nanowire devices. Chem. Commun. 2019, 55, 5351–5354. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Zhang, S.; Zhang, T. Defect Engineering in Photocatalytic Nitrogen Fixation. ACS Catal. 2019, 9, 9739–9750. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, G.; Wang, Q.; Yu, Y.; Zhou, C.; Wang, Y. Sonochemistry synthesis and enhanced photocatalytic H2-production activity of nanocrystals embedded in CdS/ZnS/In2S3 microspheres. Nanoscale 2012, 4, 2010–2017. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wu, H.; Zhang, J.; Lin, H.; Nie, M.; Zhang, Y. High yield growth of uniform ZnS nanospheres with strong photoluminescence properties. Mater. Sci. Eng. B 2013, 178, 135–141. [Google Scholar] [CrossRef]
- Xie, Y.P.; Yu, Z.B.; Liu, G.; Ma, X.L.; Cheng, H.-M. CdS–mesoporous ZnS core–shell particles for efficient and stable photocatalytic hydrogen evolution under visible light. Ener. Environ. Sci. 2014, 7, 1895–1901. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Zhang, W.; Zhang, G.; Qu, D.; Miao, X.; Sun, S.; Sun, Z. Surface Defects Enhanced Visible Light Photocatalytic H2 Production for Zn-Cd-S Solid Solution. Small 2016, 12, 793–801. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, X.; Ma, C.; Zhou, M.; Huo, P.; Yu, L.; Pan, J.; Shi, W.; Yan, Y. Enhanced photocatalytic degradation of tetracycline antibiotics by reduced graphene oxide–CdS/ZnS heterostructure photocatalysts. N. J. Chem. 2015, 39, 5150–5160. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.-Q.; Tang, Z.-R.; Xu, Y.-J. CdS–graphene nanocomposites as visible light photocatalyst for redox reactions in water: A green route for selective transformation and environmental remediation. J. Catal. 2013, 303, 60–69. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lin, J.; Li, R.; Su, Y.; Zhao, X.; Liu, Y.; Chen, W.-J.; Lian, X.; Chen, X.; Pan, X. Hierarchical ZnO Nanosheet-Reduced Graphene Oxide Composites for Photocatalytic Ethylene Oxidation. Acs Appl. Nano Mater. 2021, 5, 1828–1835. [Google Scholar] [CrossRef]
- Navakoteswara Rao, V.; Reddy, N.L.; Kumari, M.M.; Ravi, P.; Sathish, M.; Kuruvilla, K.M.; Preethi, V.; Reddy, K.R.; Shetti, N.P.; Aminabhavi, T.M.; et al. Photocatalytic recovery of H2 from H2S containing wastewater: Surface and interface control of photo-excitons in Cu2S@TiO2 core-shell nanostructures. Appl. Catal. B-Environ. 2019, 254, 174–185. [Google Scholar]
- Wang, X.; Peng, W.-C.; Li, X.-Y. Photocatalytic hydrogen generation with simultaneous organic degradation by composite CdS–ZnS nanoparticles under visible light. Int. J. Hydrogen Energy 2014, 39, 13454–13461. [Google Scholar] [CrossRef]
- Fu, J.; Bie, C.; Cheng, B.; Jiang, C.; Yu, J. Hollow CoSx Polyhedrons Act as High-Efficiency Cocatalyst for Enhancing the Photocatalytic Hydrogen Generation of g-C3N4. ACS Sustain. Chem. Eng. 2018, 6, 2767–2779. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, W.; Dong, Y.; Su, Y.; Liu, Y.; Chen, W.J.; Xu, M.; Li, R.; Gao, Y.; Chen, X.; et al. Facile Surfactant-Free synthesis of Pd-Sn1.1Nb2O5.5F0.9@SnO2 Core-Shell Nano-Octahedrons for efficient photocatalytic ethylene oxidation. Sep. Purif. Technol. 2022, 297, 121478. [Google Scholar] [CrossRef]
- Zhu, Y.; Wan, T.; Wen, X.; Chu, D.; Jiang, Y. Tunable Type I and II heterojunction of CoOx nanoparticles confined in g-C3N4 nanotubes for photocatalytic hydrogen production. Appl. Catal. B-Environ. 2019, 244, 814–822. [Google Scholar] [CrossRef]
- Reddy, N.L.; Rao, V.N.; Kumari, M.M.; Sathish, M.; Muthukonda Venkatakrishnan, S. Development of high quantum efficiency CdS/ZnS core/shell structured photocatalyst for the enhanced solar hydrogen evolution. Int. J. Hydrogen Energy 2018, 43, 22315–22328. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, X.; Zheng, Y.; Wang, K.; Dai, H. Controlled one-step synthesis of CdS@ZnS core–shell particles for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2017, 42, 2924–2930. [Google Scholar] [CrossRef]
- Kundu, J.; Satpathy, B.K.; Pradhan, D. Composition-Controlled CdS/ZnS Heterostructure Nanocomposites for Efficient Visible Light Photocatalytic Hydrogen Generation. Ind. Eng. Chem. Res. 2019, 58, 22709–22717. [Google Scholar] [CrossRef]
- Yin, X.-L.; Li, L.-L.; Wang, Y.-L.; Yao, L.-H.; Li, X.-Y.; Lu, Y. MoS2/CdS nanosheet-on-nanorod: An efficient photocatalyst for H2 generation from lactic acid decomposition. Int. J. Hydrogen Energy 2022, 47, 20103–20111. [Google Scholar] [CrossRef]
- Shi, X.; Dai, C.; Wang, X.; Hu, J.; Zhang, J.; Zheng, L.; Mao, L.; Zheng, H.; Zhu, M. Protruding Pt single-sites on hexagonal ZnIn2S4 to accelerate photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 1287. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lian, J.; Si, R.; Wang, L.; Pan, X.; Liu, P. Spatial Separation of Electrons and Holes among ZnO Polar {0001} and {10ī0} Facets for Enhanced Photocatalytic Performance. ACS Omega 2022, 7, 26844–26852. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Yu, M.; Si, R.; Zhao, X.; Chen, S.; Liu, K.; Pan, X. Tailoring Morphology in Hydrothermally Synthesized CdS/ZnS Nanocomposites for Extraordinary Photocatalytic H2 Generation via Type-II Heterojunction. Catalysts 2023, 13, 1123. https://doi.org/10.3390/catal13071123
Huang M, Yu M, Si R, Zhao X, Chen S, Liu K, Pan X. Tailoring Morphology in Hydrothermally Synthesized CdS/ZnS Nanocomposites for Extraordinary Photocatalytic H2 Generation via Type-II Heterojunction. Catalysts. 2023; 13(7):1123. https://doi.org/10.3390/catal13071123
Chicago/Turabian StyleHuang, Mianli, Maoqing Yu, Ruiru Si, Xiaojing Zhao, Shuqin Chen, Kewei Liu, and Xiaoyang Pan. 2023. "Tailoring Morphology in Hydrothermally Synthesized CdS/ZnS Nanocomposites for Extraordinary Photocatalytic H2 Generation via Type-II Heterojunction" Catalysts 13, no. 7: 1123. https://doi.org/10.3390/catal13071123
APA StyleHuang, M., Yu, M., Si, R., Zhao, X., Chen, S., Liu, K., & Pan, X. (2023). Tailoring Morphology in Hydrothermally Synthesized CdS/ZnS Nanocomposites for Extraordinary Photocatalytic H2 Generation via Type-II Heterojunction. Catalysts, 13(7), 1123. https://doi.org/10.3390/catal13071123





