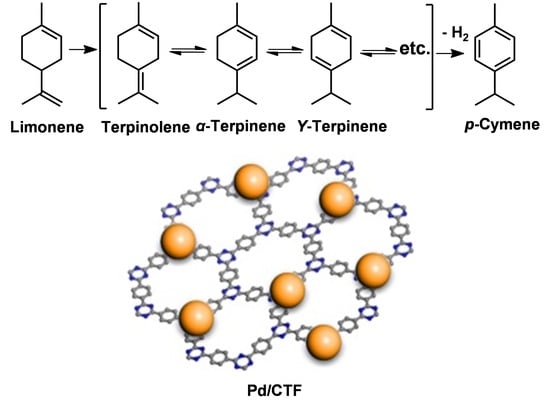
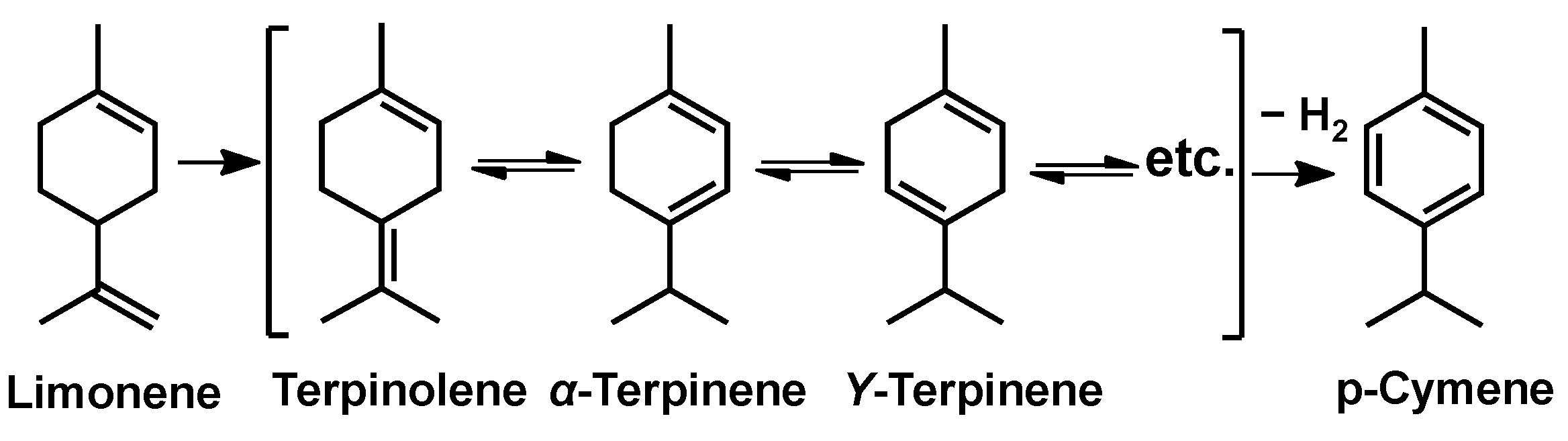
Construction of Palladium Nanoparticles Modified Covalent Triazine Frameworks towards Highly-Efficient Dehydrogenation of Dipentene for p-Cymene Production
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Catalysts
3.2.1. Synthesis of CTF
3.2.2. Synthesis of x% Pd/CTF
3.3. Measurements and Instruments
3.4. Catalytic Dehydrogenation of Industrial Dipentene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weyrich, P.A.; Hölderich, W.; van Daelen, M.A.; Gorman, A.M. Theoretical and experimental study on the selectivity of dehydrogenation of α-limonene in ZSM-5 and zeolite-Y. Catal. Lett. 1998, 52, 7–12. [Google Scholar] [CrossRef]
- Grau, R.J.; Zgolicz, P.D.; Gutierrez, C.; Taher, H.A. Liquid phase hydrogenation, isomerization and dehydrogenation of limonene and derivatives with supported palladium catalysts. J. Mol. Catal. A Chem. 1999, 148, 203–214. [Google Scholar] [CrossRef]
- Cattaneo, S.; Stucchi, M.; Villa, A.; Prati, L. Gold Catalysts for the Selective Oxidation of Biomass-Derived Products. ChemCatChem 2019, 11, 309–323. [Google Scholar] [CrossRef]
- Ozturk, B.; Winterburn, J.; Gonzalez-Miquel, M. Orange peel waste valorisation through limonene extraction using bio-based solvents. Biochem. Eng. J. 2019, 151, 107298. [Google Scholar] [CrossRef]
- Zhang, Q.-G.; Bi, L.-W.; Zhao, Z.-D.; Chen, Y.-P.; Li, D.-M.; Gu, Y.; Wang, J.; Chen, Y.-X.; Bo, C.-Y.; Liu, X.-Z. Application of ultrasonic spraying in preparation of p-cymene by industrial dipentene dehydrogenation. Chem. Eng. J. 2010, 159, 190–194. [Google Scholar] [CrossRef]
- Catrinescu, C.; Fernandes, C.; Castilho, P.; Breen, C. Influence of exchange cations on the catalytic conversion of limonene over Serra de Dentro (SD) and SAz-1 clays - Correlations between acidity and catalytic activity/selectivity. Appl. Catal. A Gen. 2006, 311, 172–184. [Google Scholar] [CrossRef]
- Fernandes, C.; Catrinescu, C.; Castilho, P.; Russo, P.A.; Carrott, M.R.; Breen, C. Catalytic conversion of limonene over acid activated Serra de Dentro (SD) bentonite. Appl. Catal. A Gen. 2007, 318, 108–120. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, J.D.; Russo, D.; Lapkin, A.A.; Bull, S.D. Efficient Syntheses of Biobased Terephthalic Acid, p-Toluic Acid, and p-Methylacetophenone via One-Pot Catalytic Aerobic Oxidation of Monoterpene Derived Bio-p-cymene. ACS Sustain. Chem. Eng. 2021, 9, 8642–8652. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H.; Macquarrie, D.J.; Sherwood, J. A quantitative comparison between conventional and bio-derived solvents from citrus waste in esterification and amidation kinetic studies. Green Chem. 2012, 14, 90–93. [Google Scholar] [CrossRef]
- Ye, L.; Thompson, B.C. p-Cymene: A Sustainable Solvent that is Highly Compatible with Direct Arylation Polymerization (DArP). ACS Macro Lett. 2021, 10, 714–719. [Google Scholar] [CrossRef]
- Granato, A.V.; Santos, A.G.; dos Santos, E.N. p-Cymene as Solvent for Olefin Metathesis: Matching Efficiency and Sustainability. ChemSusChem 2017, 10, 1832–1837. [Google Scholar] [CrossRef]
- Roberge, D.M.; Buhl, D.; Niederer, J.P.M.; Hölderich, W.F. Catalytic aspects in the transformation of pinenes to p-cymene. Appl. Catal. A Gen. 2001, 215, 111–124. [Google Scholar] [CrossRef]
- Martin-Luengo, M.A.; Yates, M.; Rojo, E.S.; Huerta Arribas, D.; Aguilar, D.; Ruiz Hitzky, E. Sustainable p-cymene and hydrogen from limonene. Appl. Catal. A Gen. 2010, 387, 141–146. [Google Scholar] [CrossRef]
- Tavera Ruiz, C.P.; Gauthier-Maradei, P.; Capron, M.; Pirez, C.; Gardoll, O.; Katryniok, B.; Dumeignil, F. Transformation of dl Limonene into Aromatic Compounds Using Supported Heteropolyacid Catalysts. Catal. Lett. 2019, 149, 328–337. [Google Scholar] [CrossRef]
- Satira, A.; Espro, C.; Paone, E.; Calabro, P.S.; Pagliaro, M.; Ciriminna, R.; Mauriello, F. The Limonene Biorefinery: From Extractive Technologies to Its Catalytic Upgrading into p-Cymene. Catalysts 2021, 11, 387. [Google Scholar] [CrossRef]
- Makarouni, D.; Lycourghiotis, S.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Dourtoglou, V. Transformation of limonene into p-cymene over acid activated natural mordenite utilizing atmospheric oxygen as a green oxidant: A novel mechanism. Appl. Catal. B Environ. 2018, 224, 740–750. [Google Scholar] [CrossRef]
- Rachwalik, R.; Hunger, M.; Sulikowski, B. Transformations of monoterpene hydrocarbons on ferrierite type zeolites. Appl. Catal. A Gen. 2012, 427–428, 98–105. [Google Scholar] [CrossRef]
- Lycourghiotis, S.; Makarouni, D.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Dourtoglou, V. Activation of natural mordenite by various acids: Characterization and evaluation in the transformation of limonene into p-cymene. Mol. Catal. 2018, 450, 95–103. [Google Scholar] [CrossRef]
- Retajczyk, M.; Wroblewska, A. The Isomerization of Limonene over the Ti-SBA-15 Catalyst-The Influence of Reaction Time, Temperature, and Catalyst Content. Catalysts 2017, 7, 273. [Google Scholar] [CrossRef]
- Retajczyk, M.; Wróblewska, A. Isomerization and Dehydroaromatization of R(+)-Limonene Over the Ti-MCM-41 Catalyst: Effect of Temperature, Reaction Time and Catalyst Content on Product Yield. Catalysts 2019, 9, 508. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, J.; Luo, Z.; Zhao, C. Mechanisms into dehydroaromatization of bio-derived limonene to p-cymene over Pd/HZSM-5 in the presence and absence of H2. RSC Adv. 2016, 6, 66695–66704. [Google Scholar] [CrossRef]
- Yılmazoğlu, E.; Akgün, M. p-Cymene production from orange peel oil using some metal catalyst in supercritical alcohols. J. Supercrit. Fluids 2018, 131, 37–46. [Google Scholar] [CrossRef]
- Du, J.M.; Xu, H.L.; Shen, J.; Huang, J.J.; Shen, W.; Zhao, D.Y. Catalytic dehydrogenation and cracking of industrial dipentene over M/SBA-15 (M=A1, Zn) catalysts. Appl. Catal. A Gen. 2005, 296, 186–193. [Google Scholar] [CrossRef]
- Kamitsou, M.; Panagiotou, G.D.; Triantafyllidis, K.S.; Bourikas, K.; Lycourghiotis, A.; Kordulis, C. Transformation of α-limonene into p-cymene over oxide catalysts: A green chemistry approach. Appl. Catal. A Gen. 2014, 474, 224–229. [Google Scholar] [CrossRef]
- Buhl, D.; Roberge, D.M.; Holderich, W.F. Production of p-cymene from α-limonene over silica supported Pd catalysts. Appl. Catal. A Gen. 1999, 188, 287–299. [Google Scholar] [CrossRef]
- Alsharif, A.; Smith, N.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Dehydroisomerisation of α-Pinene and Limonene to p-Cymene over Silica-Supported ZnO in the Gas Phase. Catalysts 2021, 11, 1245. [Google Scholar] [CrossRef]
- Alsharif, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Selective dehydroisomerization of cyclic monoterpenes to p-cymene over silica-supported CdO. Appl. Catal. B Environ. 2023, 325, 122362. [Google Scholar] [CrossRef]
- Weyrich, P.A.; Hölderich, W.F. Dehydrogenation of α-limonene over Ce promoted, zeolite supported Pd catalysts. Appl. Catal. A Gen. 1997, 158, 145–162. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Y.; Wang, L.; Tan, M.; Xiao, Y.; Gao, B.; Lin, B. Metal-free 2D/2D heterostructured photocatalyst of black phosphorus/covalent triazine-based frameworks for water splitting and pollutant degradation. Sustain. Energy Fuels 2020, 4, 3739–3746. [Google Scholar] [CrossRef]
- Li, L.; Fang, W.; Zhang, P.; Bi, J.; He, Y.; Wang, J.; Su, W. Sulfur-doped covalent triazine-based frameworks for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. A 2016, 4, 12402–12406. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Yu, H.; Wang, W.; Shen, L.-L.; Zhang, G.-R.; Mei, D. Covalent Triazine Framework Encapsulated Ultrafine PdAu Alloy Nanoclusters as Additive-Free Catalysts for Efficient Hydrogen Production from Formic Acid. ACS Catal. 2023, 13, 5135–5146. [Google Scholar] [CrossRef]
- Li, W.-J.; Chen, Y.-X.; Kang, S.-L.; Mo, L.-P.; Zhang, Z.-H. Design, Synthesis and Characterization of Palladium-Functionalized Covalent Organic Framework and Its Application as Heterogeneous Catalysis for C-H Arylation of Azoles. Chemistry 2023, 29, e202301310. [Google Scholar] [CrossRef]
- Chen, X.; Luo, L.; Zhang, Y.; Zhao, X. Theoretical Screening of Highly Efficient Single-Atom Catalysts Based on Covalent Triazine Frameworks for Oxygen Reduction. Langmuir 2023, 39, 6905–6913. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Wu, W.; Ge, Q.; Ni, S.; Song, C.; Huang, K. Ultrafine Palladium Embedded in N-doped Porous Carbon Material from Carbazole-covalent Triazine Polymer for Green Suzuki-Miyaura Coupling Reaction. Chemnanomat 2022, 8, e202200215. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Xu, W.; Yu, H.; Zhang, W.; Huang, H.; Zhang, G.-R.; Mei, D. Covalent triazine framework encapsulated Pd nanoclusters for efficient hydrogen production via ammonia borane hydrolysis. J. Catal. 2022, 411, 72–83. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Li, Z.; Di, M.; Zhang, Y.; Zhang, B.; Zhang, Z.; Zhang, Z.; Li, A.; Qiao, S. Covalent Triazine Frameworks with Palladium Nanoclusters as Highly Efficient Heterogeneous Catalysts for Styrene Oxidation. ACS Appl. Polym. Mater. 2022, 4, 1047–1054. [Google Scholar] [CrossRef]
- Bi, J.; Fang, W.; Li, L.; Wang, J.; Liang, S.; He, Y.; Liu, M.; Wu, L. Covalent Triazine-Based Frameworks as Visible Light Photocatalysts for the Splitting of Water. Macromol. Rapid Comm. 2015, 36, 1799–1805. [Google Scholar] [CrossRef]
- Lan, Z.-A.; Wu, M.; Fang, Z.; Zhang, Y.; Chen, X.; Zhang, G.; Wang, X. Ionothermal Synthesis of Covalent Triazine Frameworks in a NaCl-KCl-ZnCl2 Eutectic Salt for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202201482. [Google Scholar] [CrossRef]
- Huang, J.; Cui, W.-R.; Wang, Y.-G.; Yan, R.-H.; Jiang, W.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. Rational designed molecularly imprinted triazine-based porous aromatic frameworks for enhanced palladium capture via three synergistic mechanisms. Chem. Eng. J. 2022, 430, 132962. [Google Scholar] [CrossRef]
- Lu, Y.; He, J.; Chen, Y.; Wang, H.; Zhao, Y.; Han, Y.; Ding, Y. Effective Acetylene/Ethylene Separation at Ambient Conditions by a Pigment-Based Covalent-Triazine Framework. Macromol. Rapid Comm. 2018, 39, 1700468. [Google Scholar] [CrossRef] [PubMed]
- Bulushev, D.A.; Golub, F.S.; Trubina, S.V.; Zvereva, V.V.; Bulusheva, L.G.; Gerasimov, E.Y.; Navlani-Garcia, M.; Krot, A.D.; Jena, H.S. Single-Atom Pd Catalysts Supported on Covalent Triazine Frameworks for Hydrogen Production from Formic Acid. ACS Appl. Nano Mater. 2022, 5, 12887–12896. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Liu, X.; Shang, N.; Gao, S.; Feng, C.; Wang, C.; Wang, Z. Pd nanoparticles supported on a covalent triazine-based framework material: An efficient and highly chemoselective catalyst for the reduction of nitroarenes. New J. Chem. 2018, 42, 9684–9689. [Google Scholar] [CrossRef]
- Raza, A.A.; Ravi, S.; Tajudeen, S.S.; Sheriff, A.K.I. Sulfonated covalent triazine polymer loaded with Pd nanoparticles as a bifunctional catalyst for one pot hydrogenation esterification reaction. J. Solid State Chem. 2021, 302, 122417. [Google Scholar] [CrossRef]
- Guo, S.; Xiao, Y.; Jiang, B. Encapsulation of Pd Nanoparticles in Covalent Triazine Frameworks for Enhanced Photocatalytic CO2 Conversion. ACS Sustain. Chem. Eng. 2021, 9, 12646–12654. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Zhuo, H.-Y.; Pan, Y.; Liang, J.-X.; Liu, C.-G.; Li, J. Triazine COF-supported single-atom catalyst (Pd-1/trzn-COF) for CO oxidation. Sci. China Mater. 2021, 64, 1939–1951. [Google Scholar] [CrossRef]
- He, T.; Liu, L.; Wu, G.; Chen, P. Covalent triazine framework-supported palladium nanoparticles for catalytic hydrogenation of N-heterocycles. J. Mater. Chem. A 2015, 3, 16235–16241. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, S.; Zhu, G.; Liu, M.; Gao, J.; Xu, J. Au-Pd alloy cooperates with covalent triazine frameworks for the catalytic oxidative cleavage of beta-O-4 linkages. Green Chem. 2019, 21, 6707–6716. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Wang, P.-L.; Yin, G.-L.; Zhang, X.; Lu, G. Energy transfer in covalent organic frameworks for visible-light-induced hydrogen evolution. Int. J. Hydrogen Energ. 2019, 44, 11872–11876. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, W.D.; Li, F.; Sun, X.; Li, B.; Song, Q.; Kou, J.; Ma, K.; Ren, X.; Dong, Z. Facile preparation of ultrafine Pd nanoparticles anchored on covalent triazine frameworks catalysts for efficient N-alkylation. J. Colloid Interface Sci. 2022, 606, 1340–1351. [Google Scholar] [CrossRef]
- Guo, P.; Zhan, Y.; Yang, Y.; Lu, T. Preparation of COFs Supported Pd as an Efficient Catalyst for the Hydrogenation of Aromatic Nitro. Catal. Lett. 2022, 152, 3725–3732. [Google Scholar] [CrossRef]
- Li, J.H.; Yu, Z.W.; Gao, Z.; Li, J.Q.; Tao, Y.; Xiao, Y.X.; Yin, W.H.; Fan, Y.L.; Jiang, C.; Sun, L.J.; et al. Ultralow-Content Palladium Dispersed in Covalent Organic Framework for Highly Efficient and Selective Semihydrogenation of Alkynes. Inorg. Chem. 2019, 58, 10829–10836. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-C.; Kan, J.-L.; Chen, G.-J.; Chen, C.-X.; Dong, Y.-B. Pd NPs-Loaded Homochiral Covalent Organic Framework for Heterogeneous Asymmetric Catalysis. Chem. Mater. 2017, 29, 6518–6524. [Google Scholar] [CrossRef]
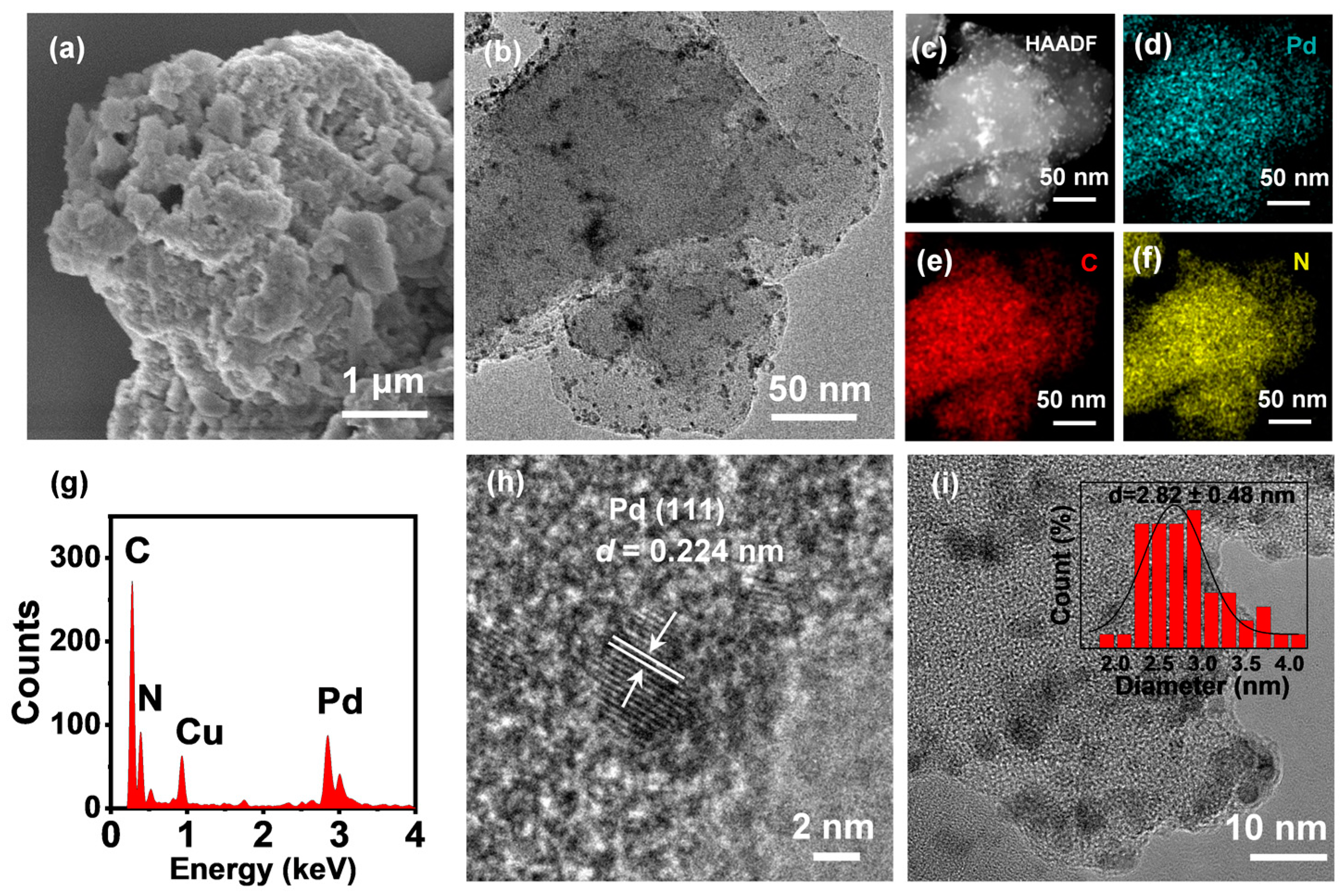


| Entry | Retention Time/Min | Content/ % | Molecular Formula | Relative Molecular Mass | Chemical Compound |
|---|---|---|---|---|---|
| 1 | 7.345 | 0.37 | C10H16 | 136 | Camphene |
| 2 | 9.263 | 0.53 | C10H16 | 136 | 3-Carene |
| 3 | 9.477 | 9.82 | C10H16 | 136 | α-Terpinene |
| 4 | 9.761 | 9.72 | C10H14 | 134 | p-Cymene |
| 5 | 9.921 | 36.52 | C10H16 | 136 | D-Limonene |
| 6 | 11.147 | 7.45 | C10H16 | 136 | γ-Terpinene |
| 7 | 12.402 | 35.59 | C10H16 | 136 | Terpinolene |
| Entry | Retention Time/Min | Content/ % | Molecular Formula | Relative Molecular Mass | Chemical Compound |
|---|---|---|---|---|---|
| 1 | 8.117 | 4.15 | C10H20 | 140 | p-Menthane |
| 2 | 9.771 | 95.85 | C10H14 | 134 | p-Cymene |
| Entry | Catalyst | Reaction Temperature (°C) | Conversion of Dipentene (%) | Selectivity of p-Cymene (%) | Content of p-Cymene (%) |
|---|---|---|---|---|---|
| 1 | 5% Pd/CTF | 220 | 100 | 95.85 | 95.85 |
| 2 | 0.5% Pd/CTF | 220 | 91.25 | 66.60 | 72.99 |
| 3 | 1% Pd/CTF | 220 | 100 | 85.61 | 85.61 |
| 4 | 3% Pd/CTF | 220 | 100 | 86.75 | 86.75 |
| 5 | 7% Pd/CTF | 220 | 100 | 86.04 | 86.04 |
| Entry | Catalyst | Reaction Temperature (°C) | Conversion of Dipentene (%) | Selectivity of p-Cymene (%) | Content of p-Cymene (%) |
|---|---|---|---|---|---|
| 1 | 5% Pd/CTF | 220 | 100 | 95.85 | 95.85 |
| 2 | 10% Pd/AC | 220 | 100 | 82.26 | 82.26 |
| 3 | H-ZSM-5(Si/Al = 30) | 220 | 96.51 | 58.36 | 60.47 |
| 4 | Raney Ni | 220 | 68.63 | 28.30 | 41.24 |
| 5 | 10%Pd/AC+ Raney Ni (1:1) | 220 | 85.04 | 61.08 | 71.83 |
| 6 | M-CTF | 220 | 50.31 | 7.94 | 15.79 |
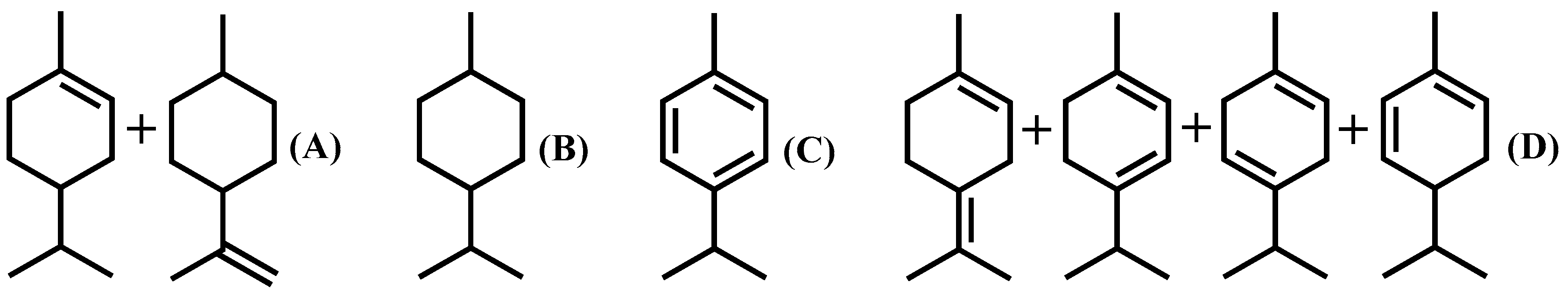
| Entry | Catalyst | Reaction Temperature (°C) | Conversion of Dipentene (%) | Selectivity of p-Cymene (%) | Yield (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||||
| 1 | 5% Pd/CTF | 220 | 100 | 95.85 | 4.15 | 95.85 | |||
| 2 | 5% Pd/CTF | 160 | 96.25 | 77.05 | 4.55 | 6.59 | 80.05 | 5.06 | |
| 3 | 5% Pd/CTF | 180 | 97.75 | 80.57 | 4.87 | 6.90 | 82.42 | 3.56 | |
| 4 | 5% Pd/CTF | 200 | 100 | 83.14 | 5.58 | 7.58 | 83.14 | 3.70 | |
| 5 | 5% Pd/CTF | 240 | 100 | 88.16 | 11.84 | 88.16 | |||
| Entry | Catalyst | Reaction Temperature (°C) | Conversion of Dipentene (%) | Selectivity of p-Cymene (%) | Content of p-Cymene (%) |
|---|---|---|---|---|---|
| 1 | 5% Pd/CTF (First run) | 220 | 100 | 95.85 | 95.85 |
| 2 | 5% Pd/CTF (Second run) | 220 | 100 | 95.54 | 95.54 |
| 3 | 5% Pd/CTF (Third run) | 220 | 100 | 95.02 | 95.02 |
| 4 | 5% Pd/CTF (Forth run) | 220 | 100 | 94.58 | 94.58 |
| Entry | Catalyst | Reaction Temperature (°C) | Conversion of Dipentene (%) | Selectivity of p-Cymene (%) | Content of p-Cymene (%) |
|---|---|---|---|---|---|
| 1 | 10% Pd/AC (First run) | 220 | 100 | 82.26 | 82.26 |
| 2 | 10% Pd/AC (Second run) | 220 | 100 | 74.69 | 74.69 |
| 3 | 10% Pd/AC (Third run) | 220 | 100 | 67.22 | 67.22 |
| 4 | 10% Pd/AC (Forth run) | 220 | 95.39 | 60.99 | 63.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Y.; Wang, Y.; Xiao, Z.; Chen, Y.; Jiang, J.; Rao, X.; Zheng, Y. Construction of Palladium Nanoparticles Modified Covalent Triazine Frameworks towards Highly-Efficient Dehydrogenation of Dipentene for p-Cymene Production. Catalysts 2023, 13, 1248. https://doi.org/10.3390/catal13091248
Liu Y, Chen Y, Wang Y, Xiao Z, Chen Y, Jiang J, Rao X, Zheng Y. Construction of Palladium Nanoparticles Modified Covalent Triazine Frameworks towards Highly-Efficient Dehydrogenation of Dipentene for p-Cymene Production. Catalysts. 2023; 13(9):1248. https://doi.org/10.3390/catal13091248
Chicago/Turabian StyleLiu, Yanni, Yonghui Chen, Yikai Wang, Zijie Xiao, Yilin Chen, Jianchun Jiang, Xiaoping Rao, and Yun Zheng. 2023. "Construction of Palladium Nanoparticles Modified Covalent Triazine Frameworks towards Highly-Efficient Dehydrogenation of Dipentene for p-Cymene Production" Catalysts 13, no. 9: 1248. https://doi.org/10.3390/catal13091248