Highly Stable Photocatalytic Dry and Bi-Reforming of Methane with the Role of a Hole Scavenger for Syngas Production over a Defective Co-Doped g-C3N4 Nanotexture
Abstract
:1. Introduction
2. Results and Discussion
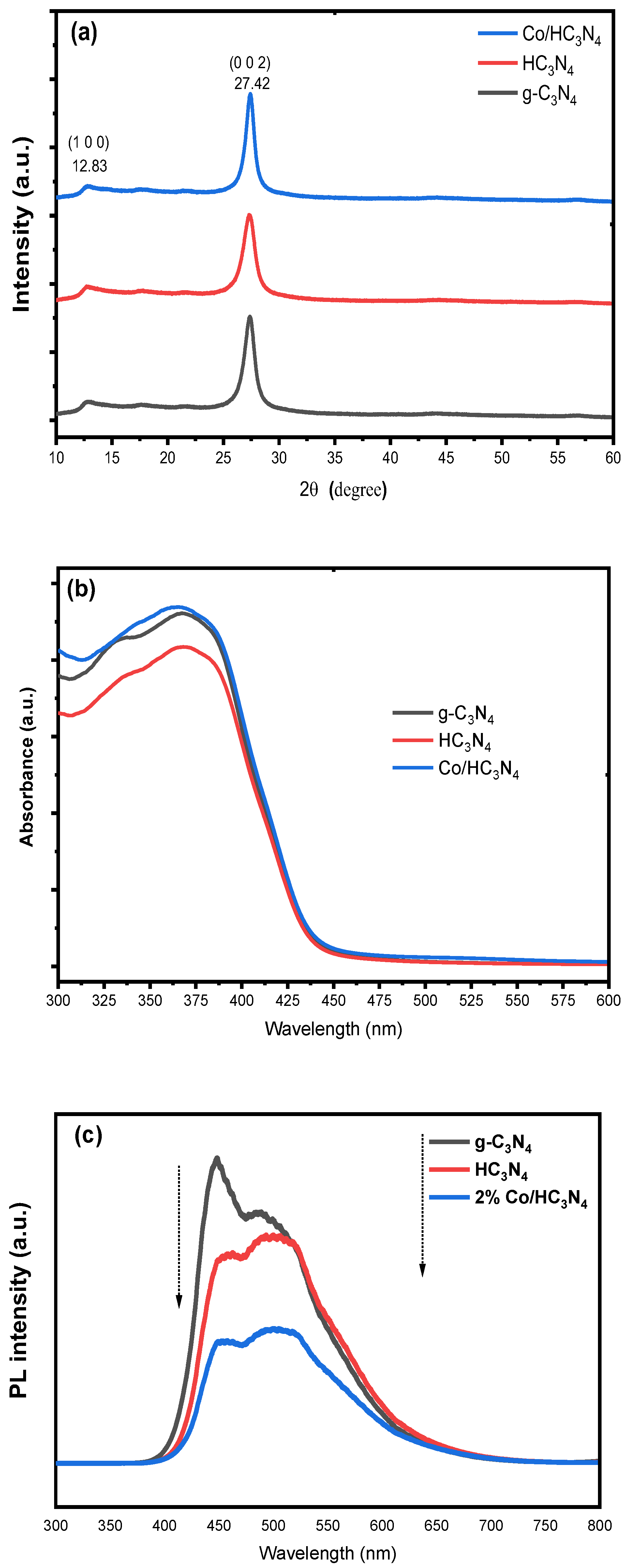
2.1. Characterization of Materials
2.2. Photocatalytic Dry Reforming of Methane
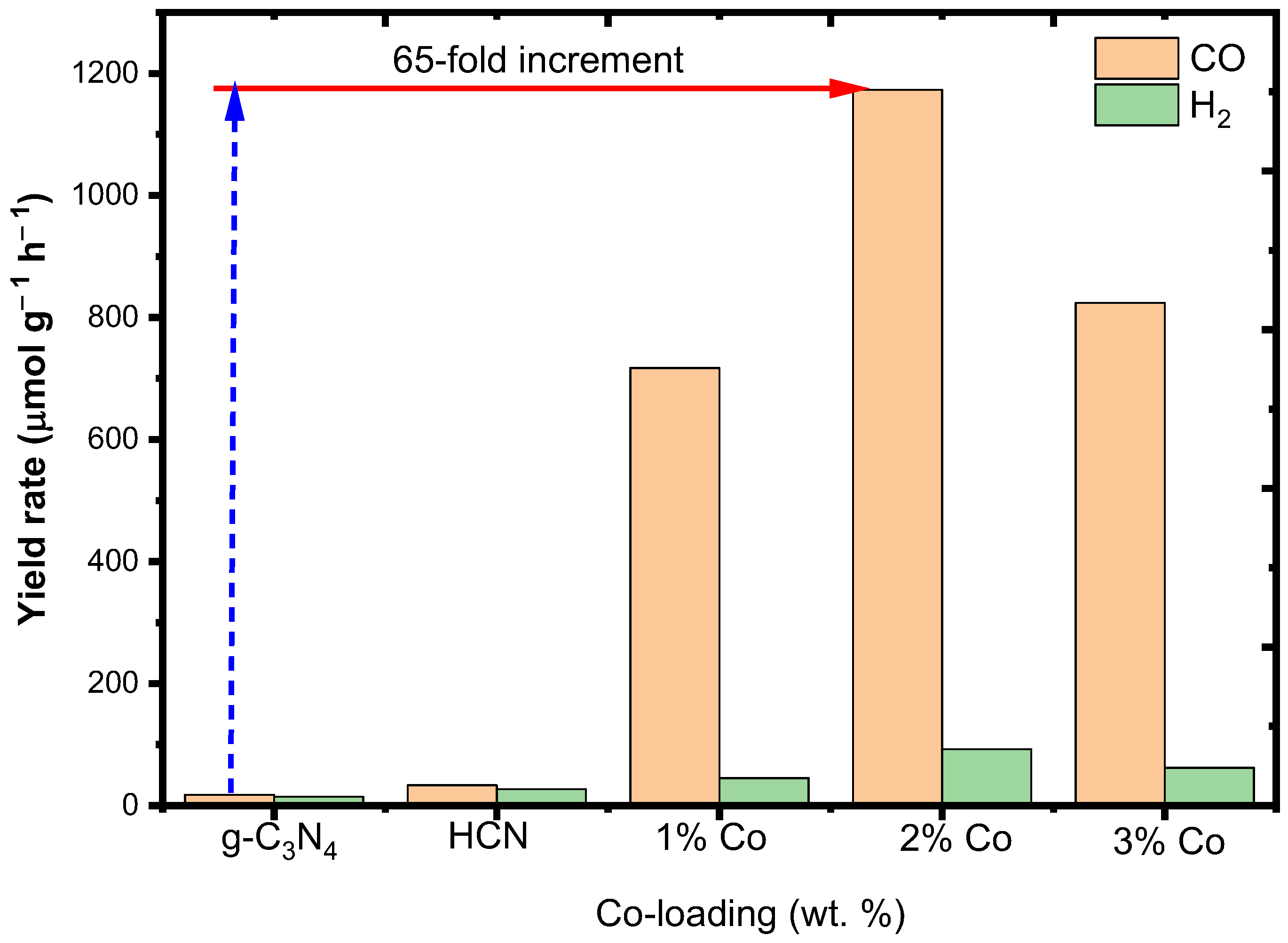
2.2.1. The Effect of Co-Loading on HC3N4 Photoactivity
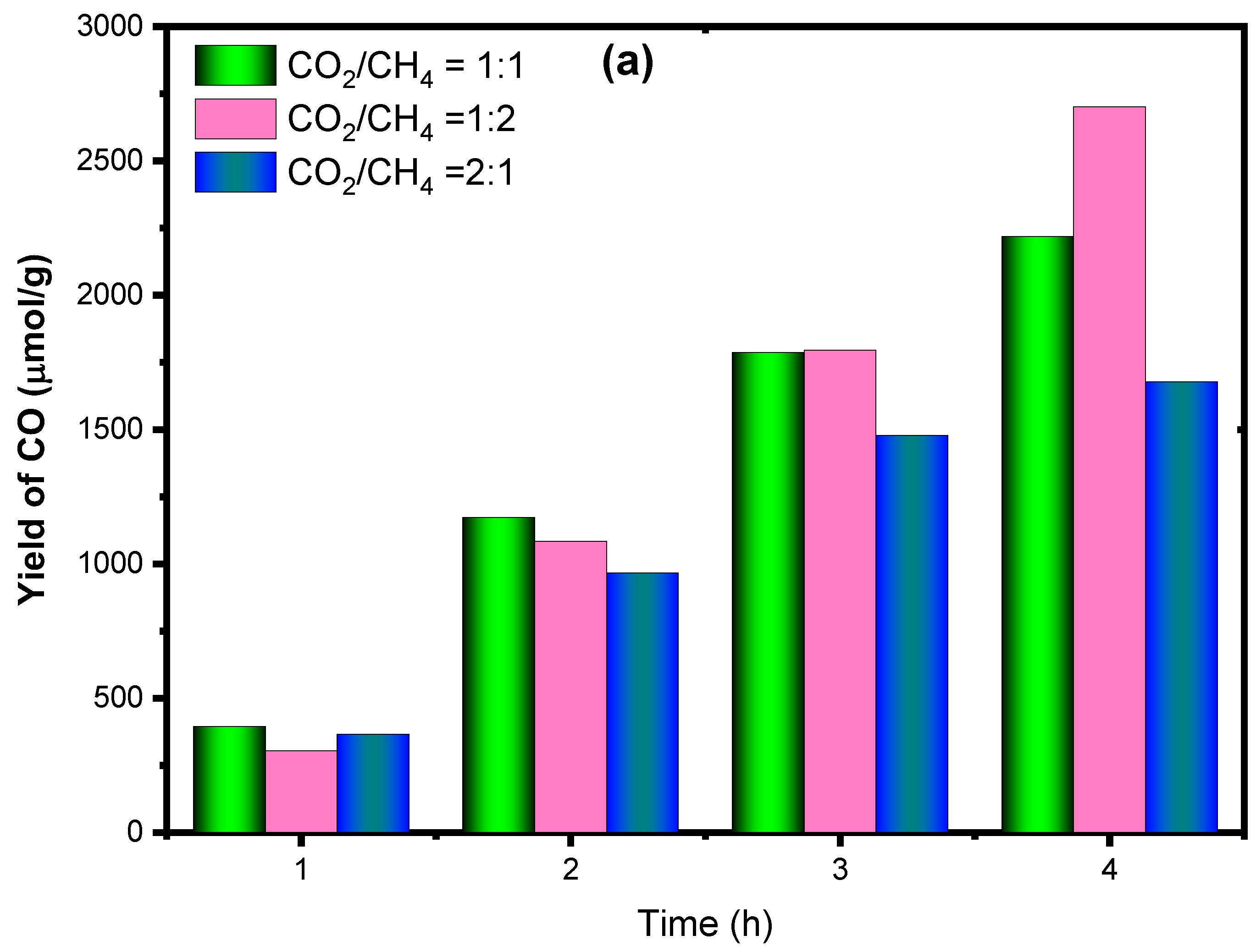
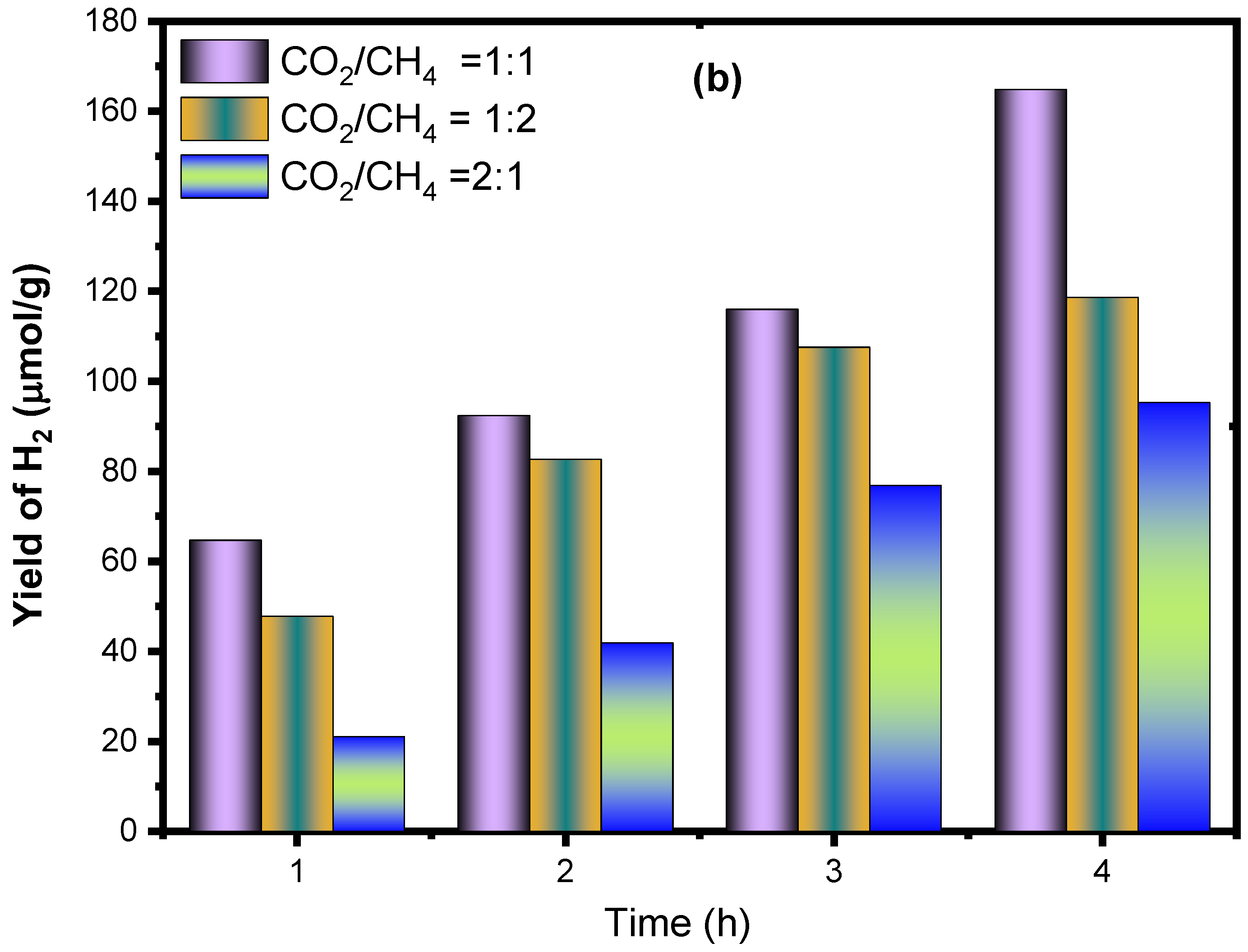
2.2.2. The Effect of CH4/CO2 Feed Ratio for CO and H2 Production
2.2.3. Photocatalytic CO2 Bi-Reforming of Methane and Methanol
2.2.4. Performance Analysis
2.3. Stability Analysis
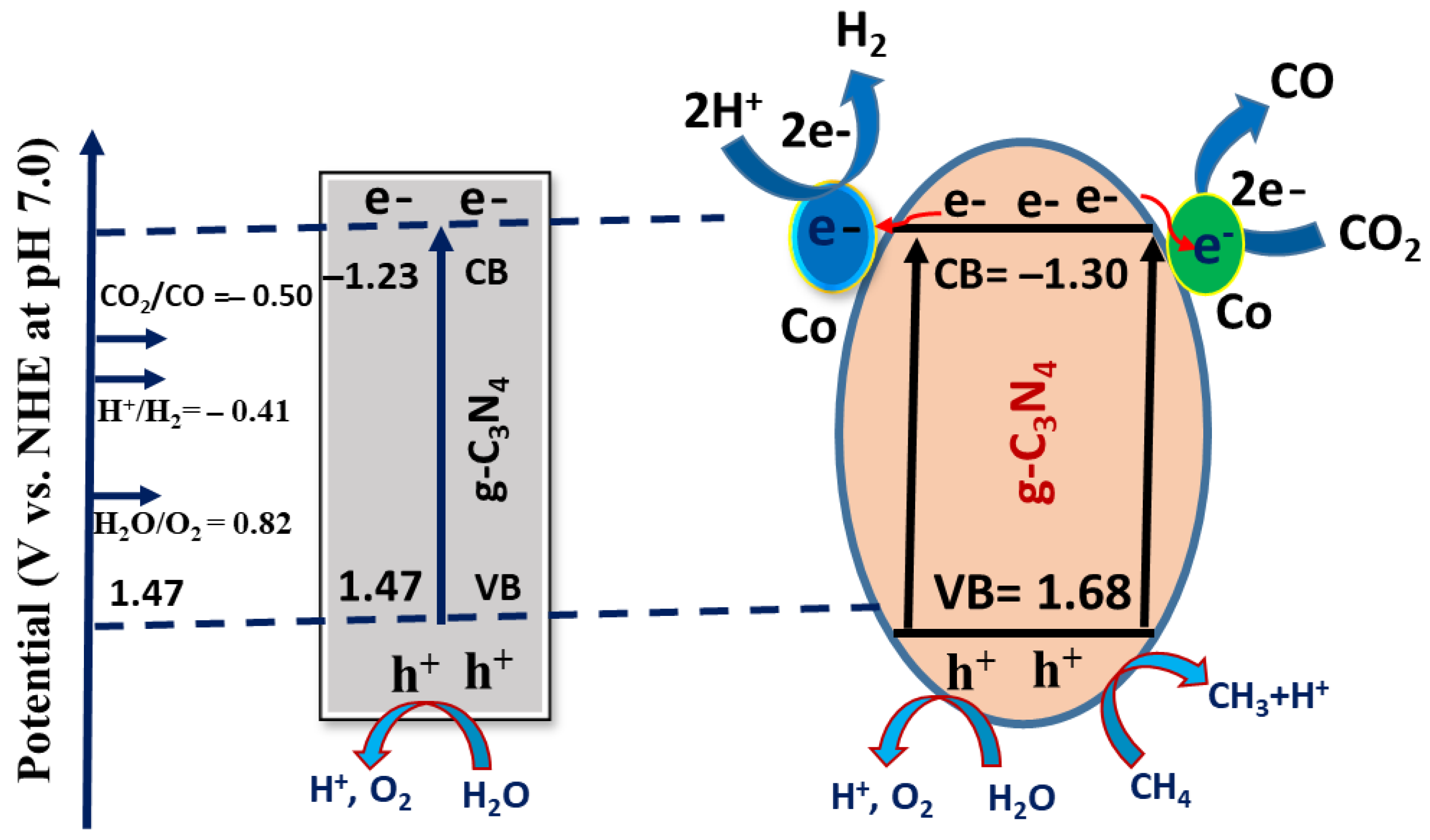
2.4. Proposed Mechanism
3. Experimental
3.1. Synthesis of Hierarchical Graphitic Carbon Nitride (HC3N4)
3.2. Synthesis of Co-Doped g-C3N4 (Co/HC3N4)
3.3. Characterization
3.4. Photocatalytic Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, A.A.; Tahir, M. Recent advancements in engineering approach towards design of photo-reactors for selective photocatalytic CO2 reduction to renewable fuels. J. CO2 Util. 2019, 29, 205–239. [Google Scholar] [CrossRef]
- Waris, M.; Ra, H.; Yoon, S.; Kim, M.-J.; Lee, K. Efficient and Stable Ni/SBA-15 Catalyst for Dry Reforming of Methane: Effect of Citric Acid Concentration. Catalysts 2023, 13, 916. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Dry reforming of methane using different dielectric materials and DBD plasma reactor configurations. Energy Convers. Manag. 2017, 144, 262–274. [Google Scholar] [CrossRef]
- Moral, A.; Reyero, I.; Alfaro, C.; Bimbela, F.; Gandia, L.M. Syngas production by means of biogas catalytic partial oxidation and dry reforming using Rh-based catalysts. Catal. Today 2018, 299, 280–288. [Google Scholar] [CrossRef]
- Goscianska, J.; Pietrzak, R.; Matos, J. Catalytic performance of ordered mesoporous carbons modified with lanthanides in dry methane reforming. Catal. Today 2018, 301, 204–216. [Google Scholar] [CrossRef]
- Huang, N.; Su, T.; Qin, Z.; Ji, H. Nickel Supported on Multilayer Vanadium Carbide for Dry Reforming of Methane. ChemistrySelect 2022, 7, e202203873. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Hu, Q.; Wu, J.; Zhang, Q. Photothermocatalytic Dry Reforming of Methane for Efficient CO2 Reduction and Solar Energy Storage. ACS Sustain. Chem. Eng. 2021, 9, 11635–11651. [Google Scholar] [CrossRef]
- Shi, D.; Feng, Y.; Zhong, S. Photocatalytic conversion of CH4 and CO2 to oxygenated compounds over Cu/CdS-TiO2/SiO2 catalyst. Catal. Today 2004, 98, 505–509. [Google Scholar] [CrossRef]
- Han, B.; Wei, W.; Chang, L.; Cheng, P.; Hu, Y.H. Efficient Visible Light Photocatalytic CO2 Reforming of CH4. ACS Catal. 2015, 6, 494–497. [Google Scholar] [CrossRef]
- László, B.; Baán, K.; Varga, E.; Oszkó, A.; Erdőhelyi, A.; Kónya, Z.; Kiss, J. Photo-induced reactions in the CO2-methane system on titanate nanotubes modified with Au and Rh nanoparticles. Appl. Catal. B Environ. 2016, 199, 473–484. [Google Scholar] [CrossRef]
- Kushida, M.; Yamaguchi, A.; Miyauchi, M. Photocatalytic dry reforming of methane by rhodium supported monoclinic TiO2-B nanobelts. J. Energy Chem. 2022, 71, 562–571. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M.; Zakaria, Z.Y. Synergistic effect of anatase/rutile TiO2 with exfoliated Ti3C2TR MXene multilayers composite for enhanced CO2 photoreduction via dry and bi-reforming of methane under UV–visible light. J. Environ. Chem. Eng. 2021, 9, 105244. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- He, Y.; Jiang, G.; Jiang, Y.; Tang, L.; Zhang, C.; Guo, W.; Li, F.; You, L.; Li, S.; Liu, X. In-situ nanoarchitectonics of noble-metal-free g-C3N4@C-Ni/Ni2P cocatalyst with core-shell structure for efficient photocatalytic H2 evolution. J. Alloys Compd. 2022, 895, 162583. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B. In-situ growth of TiO2 imbedded Ti3C2TA nanosheets to construct PCN/Ti3C2TA MXenes 2D/3D heterojunction for efficient solar driven photocatalytic CO2 reduction towards CO and CH4 production. J. Colloid Interface Sci. 2021, 591, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, F.; Sun, H.; Shi, Y.; Shi, W. Well-designed three-dimensional hierarchical hollow tubular g-C3N4/ZnIn2S4 nanosheets heterostructure for achieving efficient visible-light photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 607 Pt 2, 1391–1401. [Google Scholar] [CrossRef]
- Tahir, M. Investigating the Influential Effect of Etchant Time in Constructing 2D/2D HCN/MXene Heterojunction with Controlled Growth of TiO2 NPs for Stimulating Photocatalytic H2 Production. Energy Fuels 2021, 35, 6807–6822. [Google Scholar] [CrossRef]
- Qi, H.; Wang, C.; Shen, L.; Wang, H.; Lian, Y.; Zhang, H.; Ma, H.; Zhang, Y.; Zhang, J.Z. α-NiS/g-C3N4 Nanocomposites for Photocatalytic Hydrogen Evolution and Degradation of Tetracycline Hydrochloride. Catalysts 2023, 13, 983. [Google Scholar] [CrossRef]
- Muhammad, A.; Tahir, M.; Al-Shahrani, S.S.; Mahmood Ali, A.; Rather, S.U. Template free synthesis of graphitic carbon nitride nanotubes mediated by lanthanum (La/g-CNT) for selective photocatalytic CO2 reduction via dry reforming of methane (DRM) to fuels. Appl. Surf. Sci. 2020, 504, 144177. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Zakaria, Z.Y.; Muhammad, A. Enhanced photocatalytic carbon dioxide reforming of methane to fuels over nickel and montmorillonite supported TiO2 nanocomposite under UV-light using monolith photoreactor. J. Clean. Prod. 2019, 213, 451–461. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tahir, M. Facile fabrication of well-designed 2D/2D porous g-C3N4–GO nanocomposite for photocatalytic methane reforming (DRM) with CO2 towards enhanced syngas production under visible light. Fuel 2021, 305, 121558. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Ag-La loaded protonated carbon nitrides nanotubes (pCNNT) with improved charge separation in a monolithic honeycomb photoreactor for enhanced bireforming of methane (BRM) to fuels. Appl. Catal. B 2019, 248, 167–183. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Photo-induced CO2 reduction by CH4/H2O to fuels over Cu-modified g-C3N4 nanorods under simulated solar energy. Appl. Surf. Sci. 2017, 419, 875–885. [Google Scholar] [CrossRef]
- Yang, P.; Wang, R.; Tao, H.; Zhang, Y.; Titirici, M.-M.; Wang, X. Cobalt Nitride Anchored on Nitrogen-Rich Carbons for Efficient Carbon Dioxide Reduction with Visible Light. Appl. Catal. B 2021, 280, 119454. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Recent advances on cobalt metal organic frameworks (MOFs) for photocatalytic CO2 reduction to renewable energy and fuels: A review on current progress and future directions. Energy Convers. Manag. 2022, 253, 115180. [Google Scholar] [CrossRef]
- Yang, X.; Chen, W.; Liu, Y.; Liang, B.; Hu, J.; Huang, H. Dual-Z-scheme Co3O4/CoO-CNNS heterojunction for facilitating charge transport toward improving photocatalytic hydrogen generation. J. Alloys Compd. 2023, 939, 168807. [Google Scholar] [CrossRef]
- Zhang, W.; Descorme, C.; Valverde, J.L.; Giroir-Fendler, A. Effect of Calcination Conditions on Co3O4 Catalysts in the Total Oxidation of Toluene and Propane. Catalysts 2023, 13, 992. [Google Scholar] [CrossRef]
- An, Z.; Wang, W.; Dong, S.; He, J. Well-distributed cobalt-based catalysts derived from layered double hydroxides for efficient selective hydrogenation of 5-hydroxymethyfurfural to 2,5-methylfuran. Catal. Today 2019, 319, 128–138. [Google Scholar] [CrossRef]
- Abbas, T.; Tahir, M. Tri-metallic Ni–Co modified reducible TiO2 nanocomposite for boosting H2 production through steam reforming of phenol. Int. J. Hydrogen Energy 2021, 46, 8932–8949. [Google Scholar] [CrossRef]
- Chen, W.; Liu, M.; Wang, Y.; Gao, L.; Dang, H.; Mao, L. Non-noble metal Co as active sites on TiO2 nanorod for promoting photocatalytic H2 production. Mater. Res. Bull. 2019, 116, 16–21. [Google Scholar] [CrossRef]
- Peng, S.; Huang, X.; Cao, Y.; Zuo, J.; Huang, Y.; Xu, J.; Li, Y. Modification of carbon nitride with noble-metal-free cobalt phosphide for effective photocatalytic hydrogen evolution. Appl. Surf. Sci. 2022, 584, 152610. [Google Scholar] [CrossRef]
- Ansari, H.M.; Wang, W.; Lei, L.; Bao, K.; Chang, X.; Raza, A.; Chen, Y.; Mehboob, A.; Zhong, Q.; Srivastava, A.; et al. Gas bubbling exfoliation strategy towards 3D g-C3N4 hierarchical architecture for superior photocatalytic H2 evolution. J. Alloys Compd. 2022, 919, 165794. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Hu, T.; Lian, Q.; Liu, Y.; Hou, J.; Yu, Y.; Cao, Y.; Ma, F. In2S3/g-C3N4/CoZnAl-LDH composites with the lamellar dual S-scheme heterostructure and its enhanced photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130744. [Google Scholar] [CrossRef]
- Liang, S.; Sui, G.; Guo, D.; Luo, Z.; Xu, R.; Yao, H.; Li, J.; Wang, C. g-C3N4-wrapped nickel doped zinc oxide/carbon core-double shell microspheres for high-performance photocatalytic hydrogen production. J. Colloid Interface Sci. 2023, 635, 83–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; Lu, J. Fabricating 1D/2D Co3O4/ZnIn2S4 core–shell heterostructures with boosted charge transfer for photocatalytic hydrogen production. Appl. Surf. Sci. 2023, 610, 155272. [Google Scholar] [CrossRef]
- Madi, M.; Tahir, M. Fabricating V2AlC/g-C3N4 nanocomposite with MAX as electron moderator for promoting photocatalytic CO2-CH4 reforming to CO/H2. Int. J. Energy Res. 2022, 46, 7666–7685. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Tailoring performance of La-modified TiO2 nanocatalyst for continuous photocatalytic CO2 reforming of CH4 to fuels in the presence of H2O. Energy Convers. Manag. 2018, 159, 284–298. [Google Scholar] [CrossRef]
- Tahir, M.; Mansoor, R. Constructing a stable 2D Ti3AlC2 MnAXm cocatalyst-modified gC3N4/CoAl-LDH/Ti3AlC2 heterojunction for efficient dry and bireforming of methane for photocatalytic syngas production. J. Alloys Compd. 2023, 947, 169457. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S.; Muhammad, A. Photocatalytic CO2 methanation over NiO/In2O3 promoted TiO2 nanocatalysts using H2O and/or H2 reductants. Energy Convers. Manag. 2016, 119, 368–378. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Silver loaded protonated graphitic carbon nitride (Ag/pg-C3N4) nanosheets for stimulating CO2 reduction to fuels via photocatalytic bi-reforming of methane. Appl. Surf. Sci. 2019, 493, 18–31. [Google Scholar] [CrossRef]
- Ali Khan, A.; Tahir, M. Constructing S-Scheme Heterojunction of CoAlLa-LDH/g-C3N4 through Monolayer Ti3C2-MXene to Promote Photocatalytic CO2 Re-forming of Methane to Solar Fuels. ACS Appl. Energy Mater. 2021, 5, 784–806. [Google Scholar] [CrossRef]
- Yin, S.-Y.; Li, Z.; Hu, Y.; Luo, X.; Li, J. A novel metal-free porous covalent organic polymer for efficient room-temperature photocatalytic CO2 reduction via dry-reforming of methane. Green Energy Environ. 2023; in press. [Google Scholar]
- Tahir, M.; Tahir, B.; Amin, N.S. Photocatalytic CO2 reduction by CH4 over montmorillonite modified TiO2 nanocomposites in a continuous monolith photoreactor. Mater. Res. Bull. 2015, 63, 13–23. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M.; Mohamed, A.R. Constructing S-scheme heterojunction of carbon nitride nanorods (g-CNR) assisted trimetallic CoAlLa LDH nanosheets with electron and holes moderation for boosting photocatalytic CO2 reduction under solar energy. Chem. Eng. J. 2022, 433, 133693. [Google Scholar] [CrossRef]


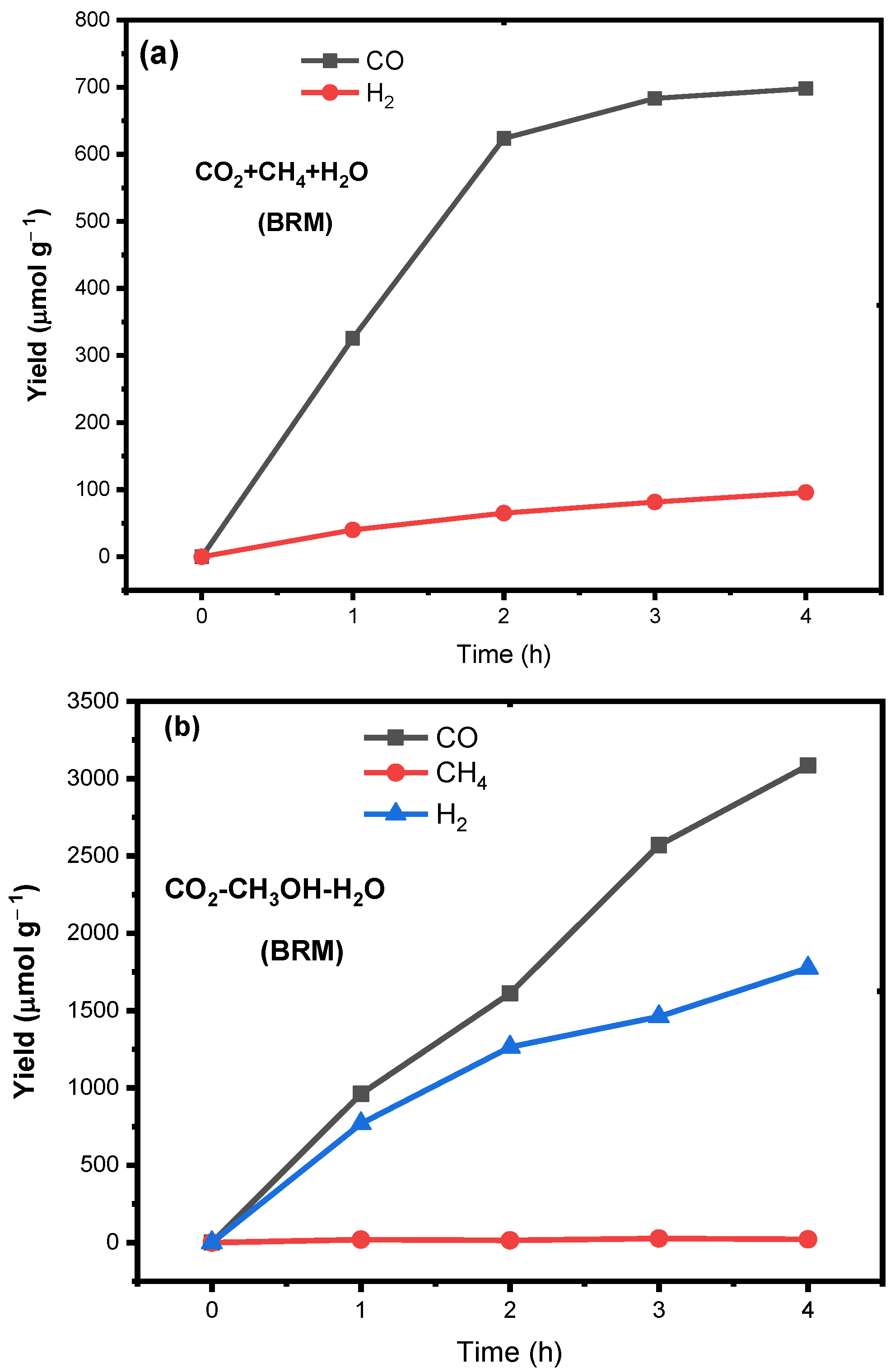

| Catalyst | Feed Ratio | Production Rate (µmol g−1 h−1) * | Selectivity (%) | ||||
|---|---|---|---|---|---|---|---|
| CH4 | CO | H2 | CO | H2 | CH4 | ||
| HC3N4 | CH4/CO2 = 1.0 | - | 30.36 | 23.69 | 56.17 | 43.83 | |
| 2%Co/HC3N4 | CH4/CO2 = 1.0 | - | 555 | 41.2 | 93.09 | 6.91 | |
| 2%Co/HC3N4 | CH4/CO2: 2:1 | - | 676 | 29.7 | 95.79 | 4.21 | |
| 2%Co/HC3N4 | CH4/CO2: 1:2 | - | 420 | 23.8 | 94.64 | 5.36 | |
| 2%Co/HC3N4 | CO2-CH4-H2O | - | 175 | 24 | 87.9 | 12.1 | |
| 2%Co/HC3N4 | CO2-CH3OH-H2O | 4.42 | 771 | 444 | 63.5 | 36.5 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, M.; Ali Khan, A.; Bafaqeer, A.; Kumar, N.; Siraj, M.; Fatehmulla, A. Highly Stable Photocatalytic Dry and Bi-Reforming of Methane with the Role of a Hole Scavenger for Syngas Production over a Defective Co-Doped g-C3N4 Nanotexture. Catalysts 2023, 13, 1140. https://doi.org/10.3390/catal13071140
Tahir M, Ali Khan A, Bafaqeer A, Kumar N, Siraj M, Fatehmulla A. Highly Stable Photocatalytic Dry and Bi-Reforming of Methane with the Role of a Hole Scavenger for Syngas Production over a Defective Co-Doped g-C3N4 Nanotexture. Catalysts. 2023; 13(7):1140. https://doi.org/10.3390/catal13071140
Chicago/Turabian StyleTahir, Muhammad, Azmat Ali Khan, Abdullah Bafaqeer, Naveen Kumar, Mohammad Siraj, and Amanullah Fatehmulla. 2023. "Highly Stable Photocatalytic Dry and Bi-Reforming of Methane with the Role of a Hole Scavenger for Syngas Production over a Defective Co-Doped g-C3N4 Nanotexture" Catalysts 13, no. 7: 1140. https://doi.org/10.3390/catal13071140
APA StyleTahir, M., Ali Khan, A., Bafaqeer, A., Kumar, N., Siraj, M., & Fatehmulla, A. (2023). Highly Stable Photocatalytic Dry and Bi-Reforming of Methane with the Role of a Hole Scavenger for Syngas Production over a Defective Co-Doped g-C3N4 Nanotexture. Catalysts, 13(7), 1140. https://doi.org/10.3390/catal13071140









