Abstract
A KF/γ-Al2O3 solid base catalyst was prepared by wet impregnation and applied to the synthesis of eugenol ethyl ether (EEE) from eugenol and diethyl carbonate. By measuring the yield of eugenol ethyl ether, we investigated the effects of the catalyst active component, impregnation temperature, KF impregnation concentration, impregnation time, and calcination temperature on catalyst performance. The results showed that the KF/γ-Al2O3 catalyst can adequately facilitate the conversion of eugenol to EEE. The characterizations of the catalysts were examined by means of X-ray diffraction (XRD) and scanning electron microscopy (SEM). The characterizations of EEE were examined by nuclear magnetic resonance spectrometry (H1-NMR), gas chromatography–mass spectrometer (GC-MS) and Fourier-transform infrared spectroscopy (FT-IR). It was found that the KF/Al2O3 catalyst (impregnation temperature 60 °C, KF impregnation concentration 40%, impregnation time 8 h, and calcination temperature 450 °C) showed the best effect. The yield of EEE remained 51.2% after recycling the supported catalyst three times.
1. Introduction
Developing greener and more economical chemical processes is being driven by environmental protection, commercial competition, and the community’s commitment to environmental responsibility. The development of green chemistry for the synthesis of flavors has attracted a great deal of interest. Eugenol ethyl ether (EEE) (C12H16O, CAS No.97-53-0) is gaining interest from researchers as an intermediate for isoeugenol ethyl ether (C12H16O, CAS No.7784-67-0), which is a white crystal with a mild, sweet, balsamic–carnation-like, warm, floral odor with a mildly spicy, vanilla-like flavor [1]. Isoeugenol ethyl ether is widely used in cosmetics and perfumes as it is not expected to be genotoxic, repeat-dose toxic, and skin sensitizing [2]. It was also confirmed that isoeugenol ethyl ether has no induction in humans as a result of repeated insult patch tests with isoeugenol ethyl ether as a fragrance ingredient at safe-use levels [3].
The traditional EEE synthesis method involves O-ethylation of eugenol using toxic, harmful, and environmentally unfriendly ethylating reagents (usually diethyl sulphate and ethyl halide) [4]. As a commonly used alkylating agent, diethyl sulfate is considered a major concern at work because of its toxicity and carcinogenic properties [5]. Diethyl sulfate is a highly toxic product that produces sulfuric acid during the reaction process, which is highly corrosive and increases the equipment requirements for the reaction, as well as making the post-treatment process cumbersome and generating large amounts of waste acid water. Diethyl carbonate (DEC), as one of the important green chemical raw materials in the 21st century, is an important organic carbonate, colorless, and transparent liquid with light toxicity [6,7]. DEC is an alternative for diethyl sulfate and ethyl halides, which can be used in the ethylation reaction of phenolic compounds [8]. Since DEC produces only ethanol and carbon dioxide as by-products, which do not cause emission of volatile organic compounds into the atmosphere, it is an environmentally friendly ethylation reagent [9].
Heterogeneous catalysts applied to the O-ethylation reaction of DEC and phenol have attracted increasing attention. Bing reported the O-ethylation of phenol with DEC over KNO3/NaY catalyst (320 °C and 0.4 MPa), which showed outstanding catalytic performance, with phenol conversions up to 96.1% and phenetole selectivity of 98.3% [10]. Silvana tested the gas phase O-ethylation of phenol using DEC via fly ash-based zeolite as a catalyst, obtaining up to 95% conversion of phenol with a selectivity of over 85% for phenetole [7]. Matthew et al. proved that organocatalyst N-heterocyclic carbene catalyzed the O-ethylation of phenols with DEC at temperatures as low as 160 °C under microwave conditions; the conversion of phenol reached 93%, and the O-ethylation product yield was as high as 90% [11]. In summary, in previous studies, O-ethylation reactions using diethyl carbonate were carried out under harsh conditions, which prevented DEC from being used on a large scale.
In this study, we investigated the production of EEE from eugenol using KF/γ-Al2O3 as the supported catalyst. Firstly, the reaction synthesis process of DEC ethylated eugenol was optimized to improve the utilization rate of DEC. Secondly, the influence of different active components on the catalytic performance of the catalyst was investigated. The impregnation temperature, impregnation concentration, impregnation time, and calcination temperature in the catalyst preparation process were investigated, and the conditions were optimized. Afterwards, the EEE and catalyst were characterized. Lastly, in order to determine the catalysts meet industrial production requirements, repeated experiments were conducted.
2. Results and Discussion
2.1. Comparison of the Effectiveness of Catalysts with Different Active Ingredients
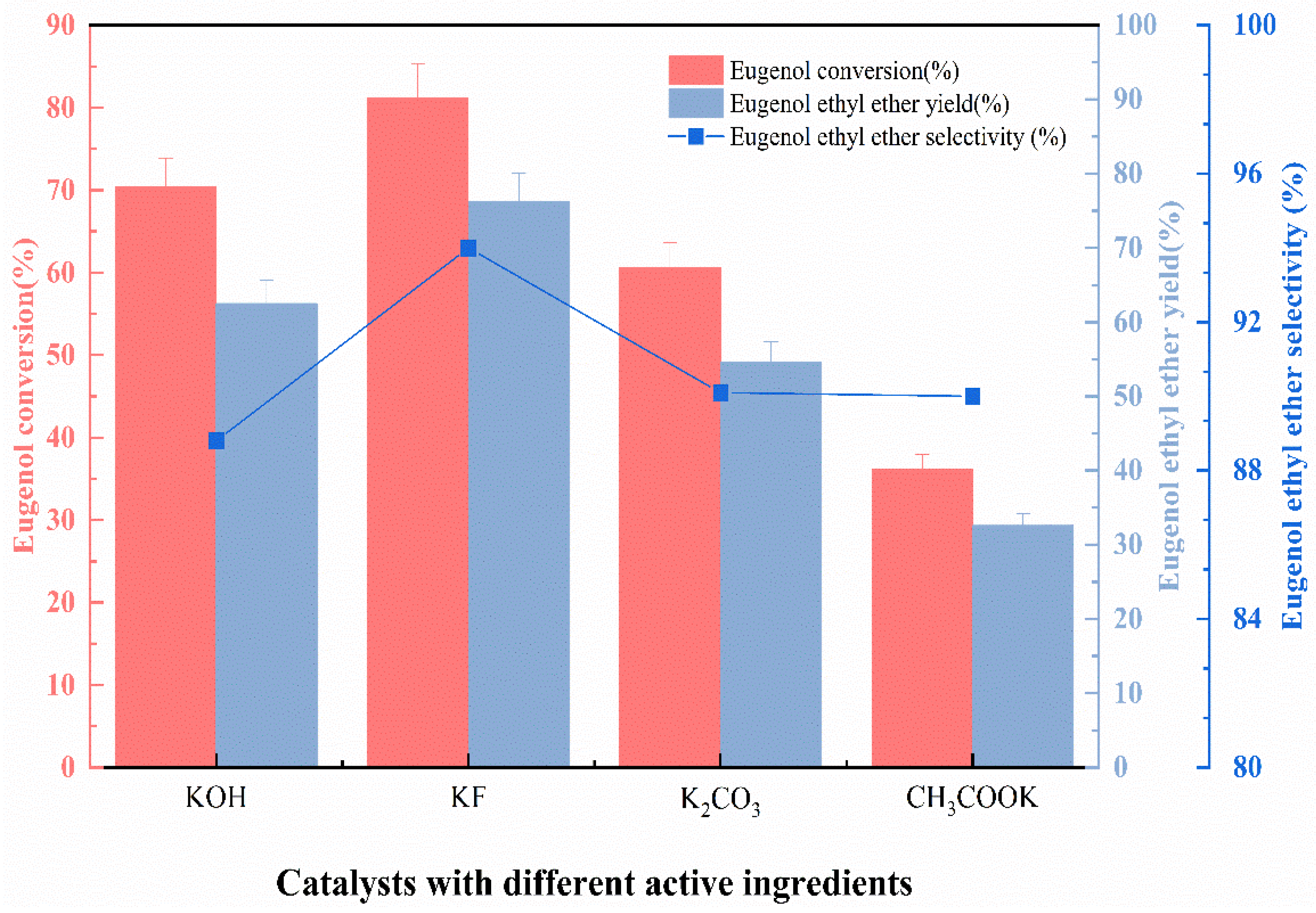
The basic principle of the eugenol O-ethylation reaction can be learned from Section 2.4.3. By reacting the Lewis base with phenolic hydroxyl groups in eugenol, Ar-PhO− anion is formed, which can then be ethylated. In the beginning, different functional ingredients loaded on γ-Al2O3 for O-ethylation reaction was discussed. Several Lewis bases were tested, KF, CH3COOK, KOH, and K2CO3, which were usually present as active ingredients on the surface of the catalyst. The catalysts used in this section here were prepared under the following conditions: impregnation at room temperature, impregnation concentration of 40%, impregnation time of 8 h, and calcination temperature of 450 °C. Based on the catalytic properties of different active ingredients, the active ingredients with the greatest influence on eugenol conversion, EEE yield, and EEE selectivity were selected. The experimental conditions were: fixed n (eugenol):n (DEC) = 1:5, the catalyst with the appropriate ratio (9 wt.%) relative to the EEE was added to the reaction, the reaction temperature was 175 °C, and the reaction time was 4 h. The active ingredients were loaded onto the carrier at a concentration of 40%. The data are presented in Figure 1.
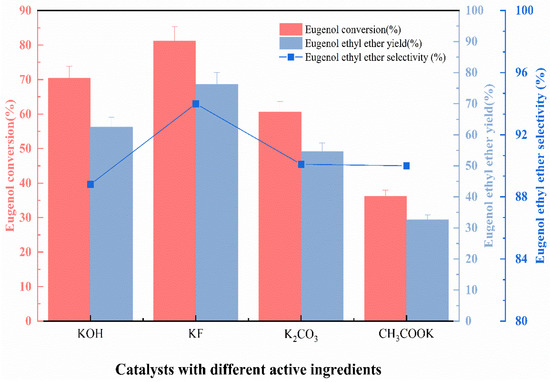
Figure 1.
The effect of catalysts with different active ingredients on catalytic performance.
As can be seen from Figure 1, the catalysts with different active ingredients have various effects on the eugenol conversion, EEE yield, and selectivity under the same conditions. When KOH was applied as the active ingredient, the eugenol conversion reached 70% and the EEE yield reached 55%, but the EEE selectivity was the lowest at 88%, due to the fact that KOH is a strong base that favors the isomerization of allyl groups in eugenol, which ultimately leads to the low selectivity of EEE [12]. An amount of 76.3% of eugenol ether was yielded by the ethylation reaction when KF is the active ingredient, with 94% selectivity for eugenol ether. In the presence of K2CO3 and CH3COOK as active ingredients, the conversion of eugenol, the yield of EEE, and the selectivity of EEE were lower. It is worth noting that the catalysts with K2CO3, KOH, and CH3COOK as active ingredients all performed poorly in the reproducibility experiments, due to the fact that active ingredients, as K2CO3, KOH, and CH3COOK, were depleted during the O-ethylation reaction of eugenol.
2.2. Effect of Catalyst Preparation Conditions on Catalyst Performance
2.2.1. Effect of Impregnation Temperature on Catalyst Performance
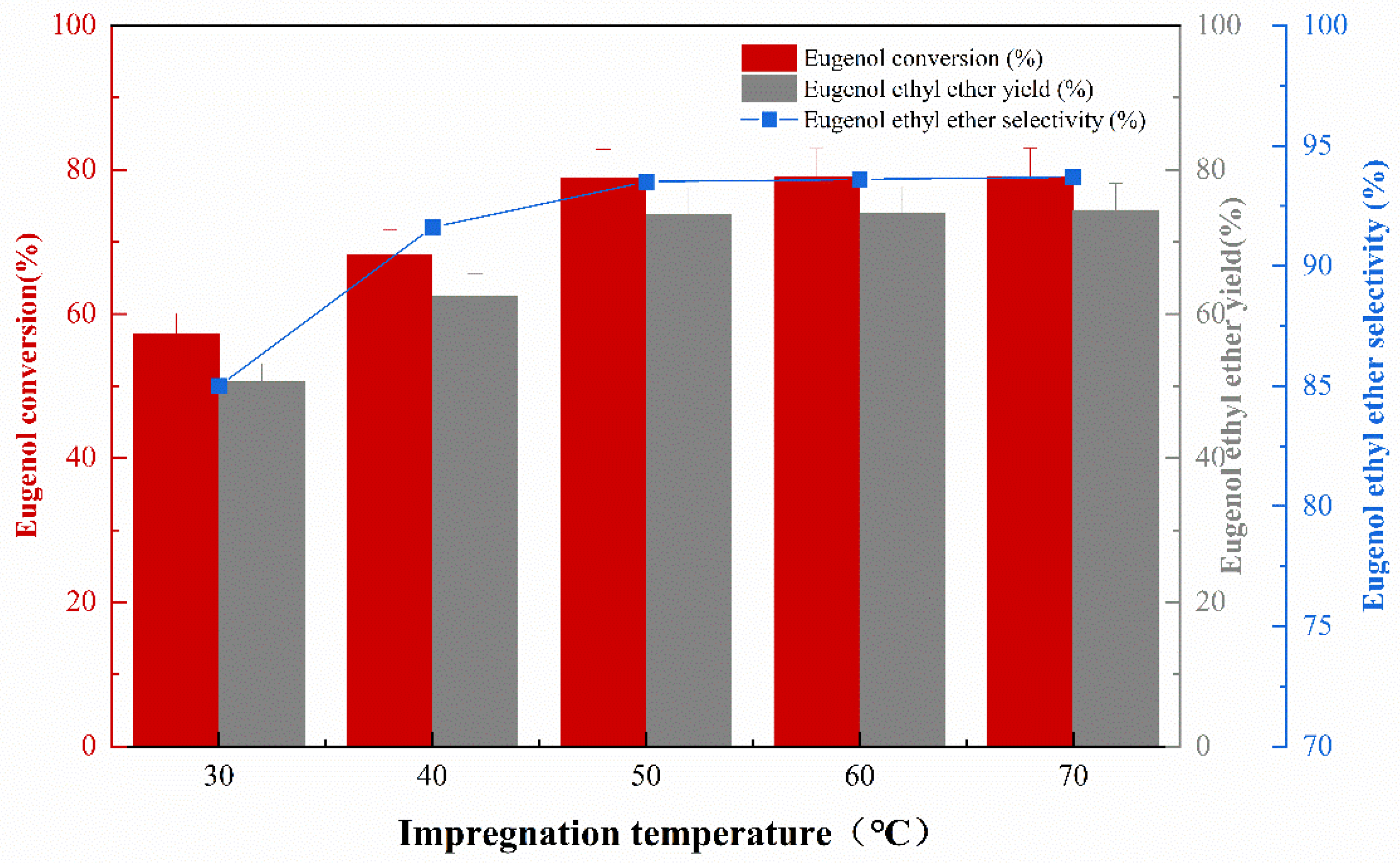
The effect of impregnation temperature on the catalytic activity of the catalyst was studied under the conditions of an impregnation concentration of 30%, impregnation time of 10 h, and calcination temperature of 400 °C. The experimental results are shown in Figure 2. It can be seen that with the increase in impregnation temperature, the eugenol conversion, the EEE yield, and the selectivity gradually increase. By increasing the impregnation temperature from 30 °C to 50 °C, the active component KF impregnated to γ-Al2O3 was more effective, which was reflected in a better O-ethylation reaction and an increase in the conversion of eugenol and the yield of eugenol ethyl ether. Increasing the impregnation temperature from 50 °C to 70 °C did not increase the catalytic effect of the catalyst. It is recommended that an impregnation temperature of 50 °C can be used. Although the effect of catalyst impregnation temperature on catalyst performance has rarely been investigated, this study is consistent with previous γ-Al2O3 catalysts in that the catalyst was impregnated at room temperature, and the catalyst is poorly effective [13].
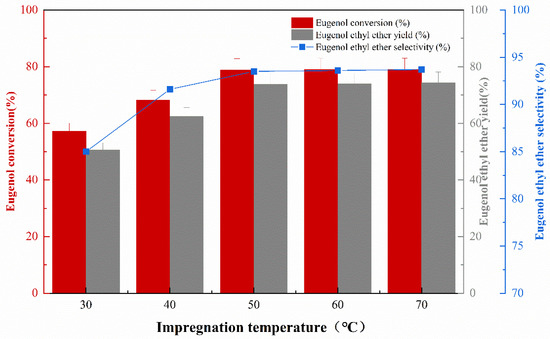
Figure 2.
The effect of different impregnation temperature on catalytic performance.
2.2.2. Effect of Impregnation Concentration on Catalyst Performance
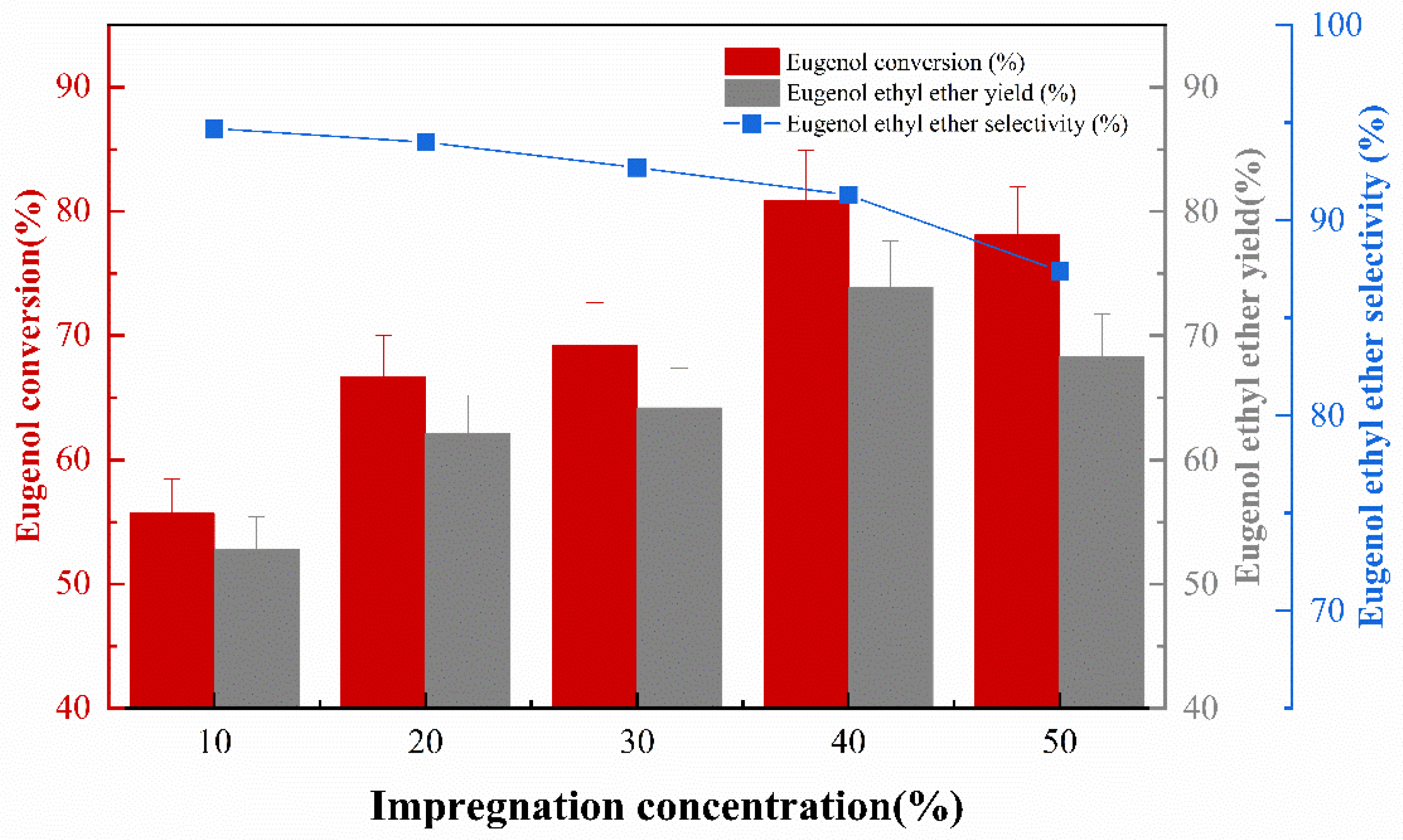
The effect of impregnation concentration on the catalytic activity of catalyst preparation was investigated under the conditions of an impregnation temperature 50 °C, impregnation time 10 h, and calcination temperature of 400 °C. The experimental results are shown in Figure 3. It can be seen that the activity of the catalyst increases with the increase in KF loading within a specific range (10 wt.% to 40 wt.%). This is due to the fact that as the loading of the KF active ingredient on the γ-Al2O3 increases, the effect of the catalyst becomes more pronounced. As the KF concentration continues to increase, the surface of γ-Al2O3 is covered with KF, thus potentially reducing the specific surface area and the contact area between the catalyst and the reactants, and finally the catalytic effect on the O-ethylation reaction decreases [14,15]. Conversely, the EEE selectivity continues to decrease as the KF concentration increases on the catalyst surface, due to the fact that the more basic the catalyst effect is (as the KF concentration increases) in the reaction system, the more isomerization of the allyl groups in eugenol increases. This conclusion is similar to previous studies, where the isomerization of the allyl was correlated with the concentration of the base [16]. In summary, the impregnation concentration of about 40% is optimal.
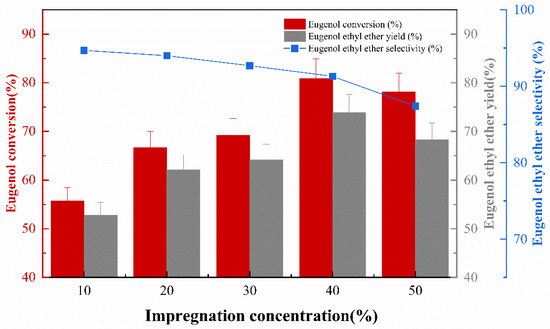
Figure 3.
The effect of impregnation concentration on catalytic performance.
2.2.3. Effect of Impregnation Time on Catalyst Performance
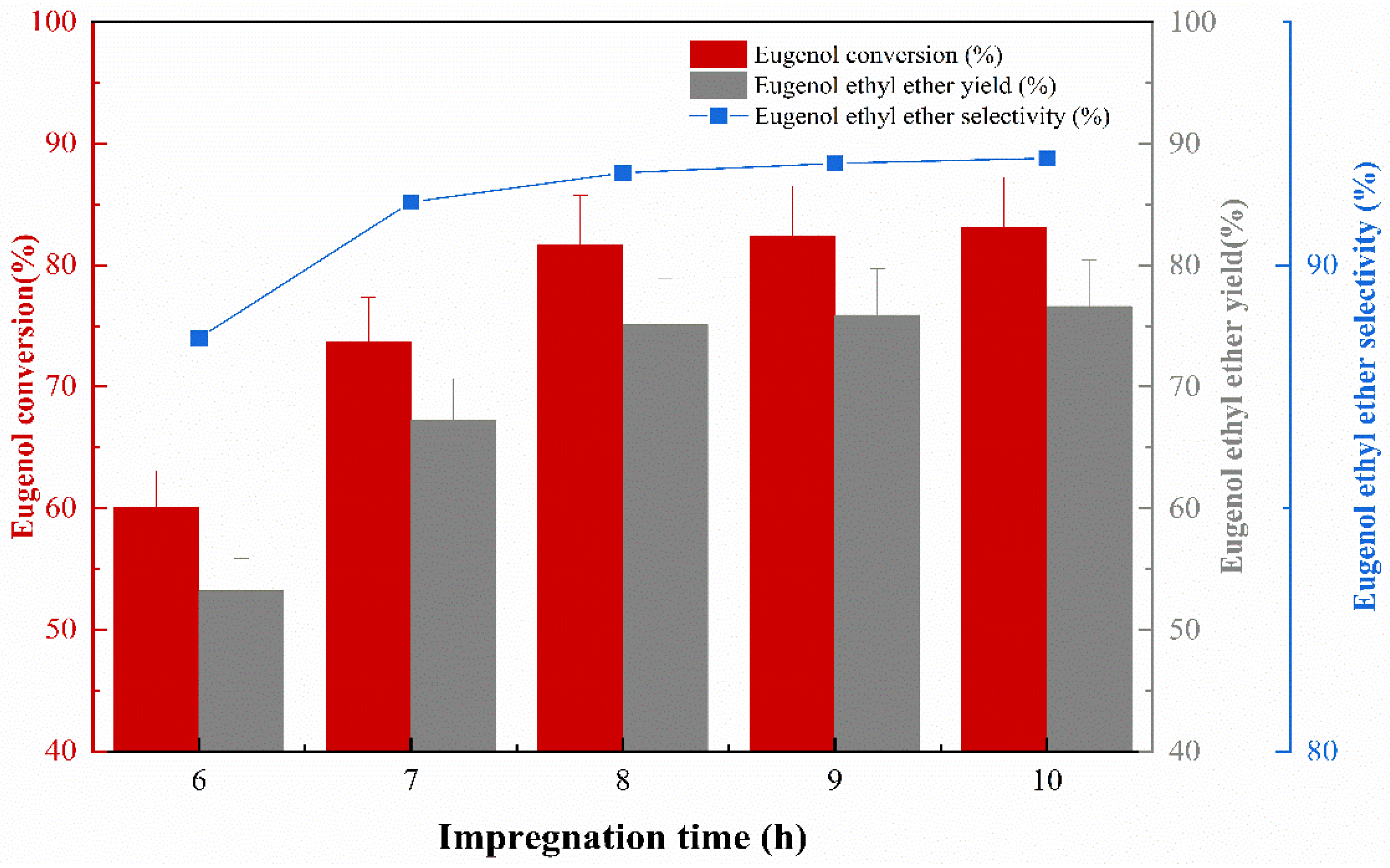
The impregnation concentration was 40%, the impregnation temperature was 50 °C, and the calcination temperature was 400 °C. The effect of impregnation time on the catalytic activity was investigated, and the experimental results are shown in Figure 4. The catalyst performance in terms of eugenol conversion, EEE yield, and EEE selectivity continued to increase as the impregnation time of the catalyst increased, due to the fact that KF could sufficiently adhere to the catalyst surface as the impregnation time increased, improving the performance of the catalyst. When the impregnation time was up to 8 h, the conversion of eugenol was 81.7% and the yield of EEE was 75.1%, while the selectivity of EEE reached 91.9%. However, the performance of the catalyst tends to be steady when the impregnation time exceeds 8 h; this is probably due to the fact that the maximum loading of KF on the γ-Al2O3 surface is 40%; in spite of the increase in impregnation time, the amount of KF on the surface of γ-Al2O3 remained the same. Therefore, an impregnation time of 8 h is most appropriate.
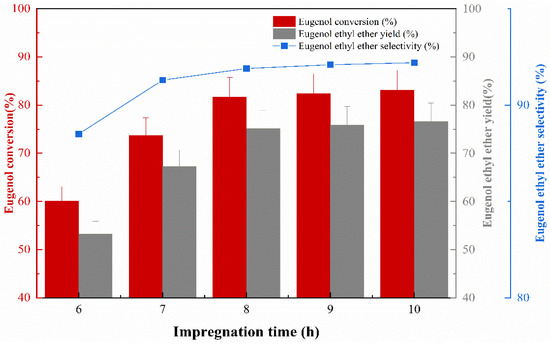
Figure 4.
The effect of impregnation time on catalytic performance.
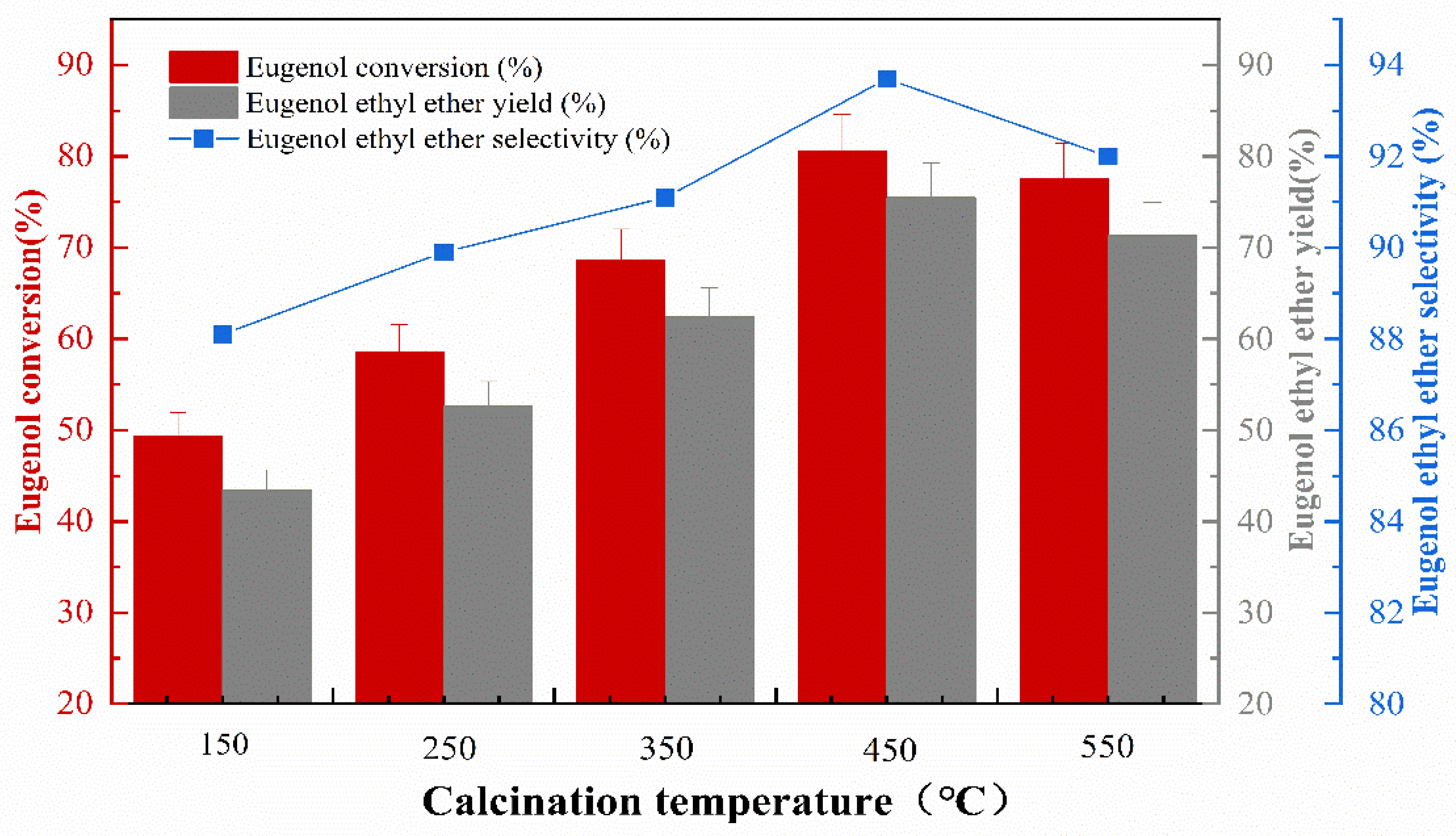
2.2.4. Effect of Calcination Temperature on Catalyst Performance
The impregnation concentration was 40%, the impregnation temperature was 50 °C, and the impregnation time was 8 h. The effect of calcination temperature on the catalytic activity during catalyst preparation was investigated, and the experimental results are shown in Figure 5 that the KF/γ-Al2O3 catalysts were more effective regarding eugenol conversion, EEE yield, and EEE selectivity when the calcination temperature was increased from 150 °C to 450 °C. This is due to the main species containing F in KF/γ-Al2O3 being identified as K3AlF6, formed by the reaction between KF and γ-Al2O3 at around 400 °C [17]. As a result, fluoride ions, the active ingredient, are exposed to give the catalyst a more efficient reaction [18]. The catalyst was most effective at 450 °C, with a maximum eugenol conversion of 80.6%, the highest EEE yield of 75.5%, and the highest selectivity of 93.7%. However, as the calcination temperature increases, the conversion of eugenol, the yield of EEE, and the selectivity of EEE were all significantly reduced. The phenomenon is probably related to the influence of high-temperature calcination on the catalytic system (γ-Al2O3) and subsequent effects on the reaction conditions [19]. Therefore, a calcination temperature of 450 °C is preferred. In addition, experimental comparisons showed that the catalysts after high-temperature calcination performed well in repeatability experiments.
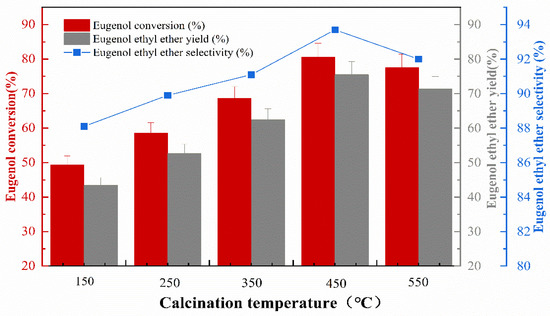
Figure 5.
The effect of calcination temperature on catalytic performance.
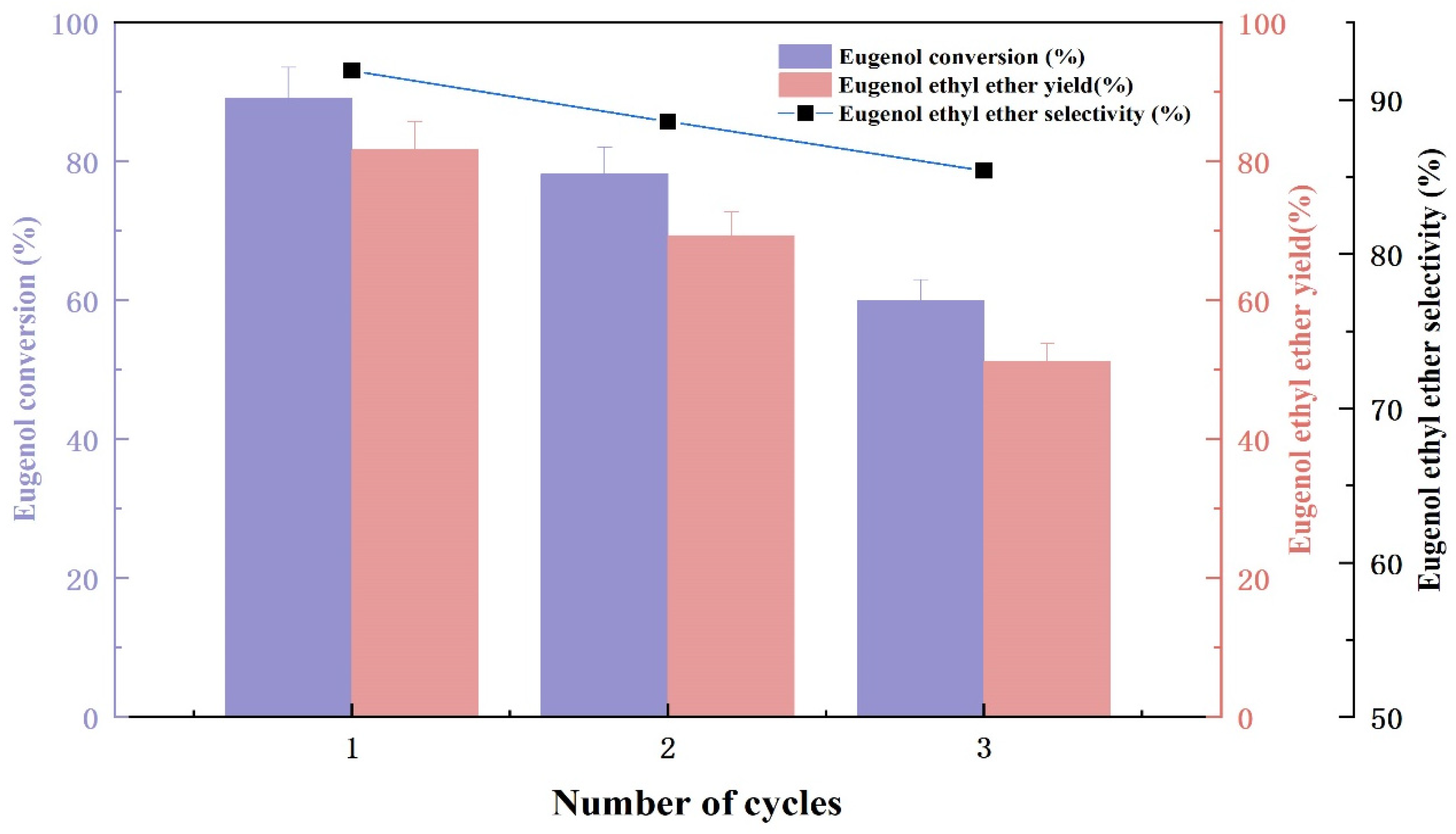
2.3. Catalyst Reusability Test
The stability of the KF/γ-Al2O3 catalyst is a very crucial factor for its industrial application. The active ingredient falls off the catalyst carrier or the product adheres to the catalyst surface, blocking small pores and reducing catalyst activity, which limits the use of catalysts at an industrial level [20]. Since 40% KF/γ-Al2O3 presented high catalytic activity and selectivity in previous experiments, it was subjected to reproducible experiments. Based on the results presented in Figure 6, eugenol conversion and EEE yield decreased with each repeat of the catalyst. The conversion of eugenol and the selectivity of EEE decreased with the increasing number of experimental repetitions, while the yield of EEE decreased from 81.7% to 59.2%. This is due to the catalyst’s active component falling off of the γ-Al2O3 during reaction. In addition, the catalyst surface covered by products (as analyzed in the XRD spectrum); all of this led to a gradual reduction in catalyst activity with the increasing numbers of repetitions. Therefore, optimization of the catalyst preparation process and recycling procedure needs improvement in future studies.
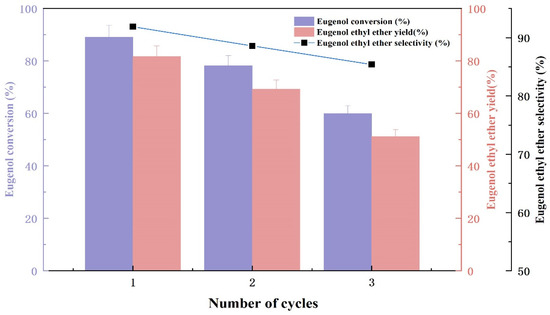
Figure 6.
The result of catalyst reusability test.
2.4. Characterization of the Catalysts
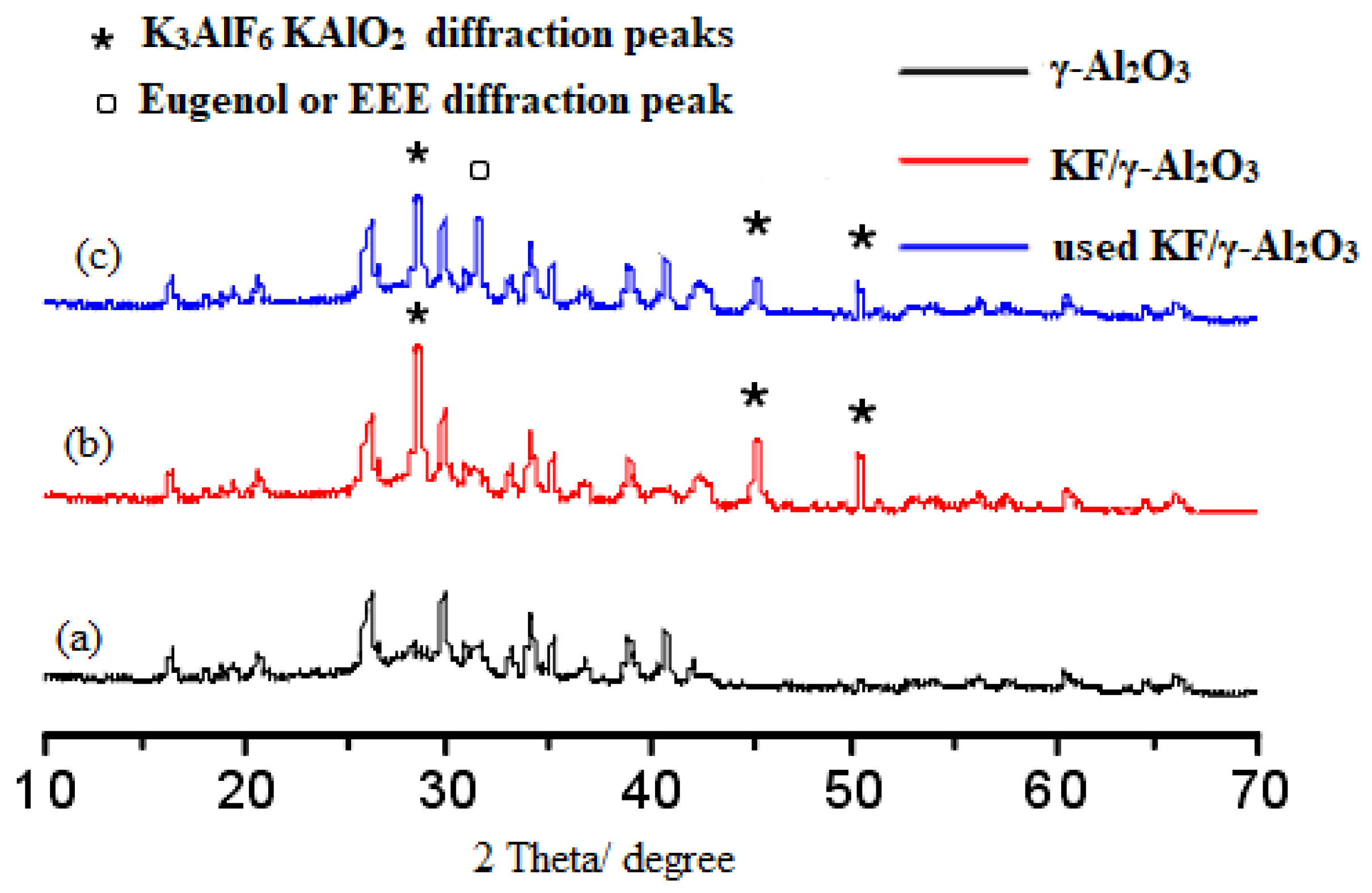
2.4.1. X-ray Diffraction Analysis
It has been demonstrated that the following reactions occur during the calcination process [21].
12KF + Al2O3 + 3H2O → 2K3AlF6 + 6KOH
6KF + 2Al2O3 → K3AlF6 +3KAlO2
Ono et al. reported that there are three basic mechanisms of appearance of the basicity of KF/γ-Al2O3: (1) the presence of active fluoride, (2) the presence of [Al-O−] ion which generates OH- when water is added, (3) the cooperation of F- and [Al-OH] [21]. It is possible that these new phases represent strongly basic centers that provide catalytic activity in O-ethylation reactions. Spectrum b in Figure 7 shows the X-ray diffraction pattern of the supported catalyst KF/γ-Al2O3. When 40wt.% of KF was loaded on γ-Al2O3, the diffraction peaks at 2θ = 29.8°, 52.8° are attributed to K3AlF6. Meanwhile, the peak of KAlO2 appeared at 2θ = 46.0°. As in previous studies, characteristic peaks of K3AlF6 and KAlO2 appear when KF is loaded on γ-Al2O3 [22]. The comparison of the b and c spectrum shows that the diffraction peaks of KAlO2 and K3AlF6 weaken after using KF/γ-Al2O3 catalyst, which may be caused by the loss of active components after using KF/γ-Al2O3 catalyst. Meanwhile, a novel diffraction peak of slightly higher intensity appears on the C spectrum, at 2θ = 32°. The diffraction peak may be due to the adsorption of eugenol or EEE on the catalyst surface. As these absorbed substances accumulate on the catalyst surface, they also clog the pores of the catalyst, which also reduces the catalyst’s effectiveness over time.
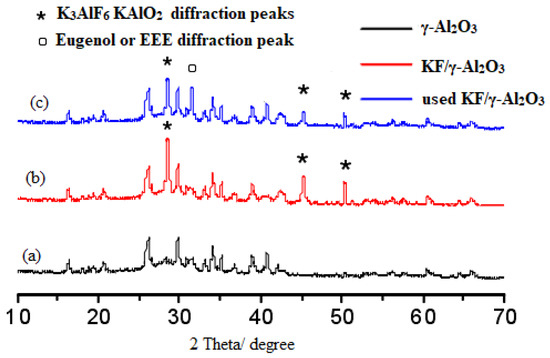
Figure 7.
XRD patterns of γ-Al2O3 (a), KF/γ-Al2O3 (b) and used KF/γ-Al2O3 (c).
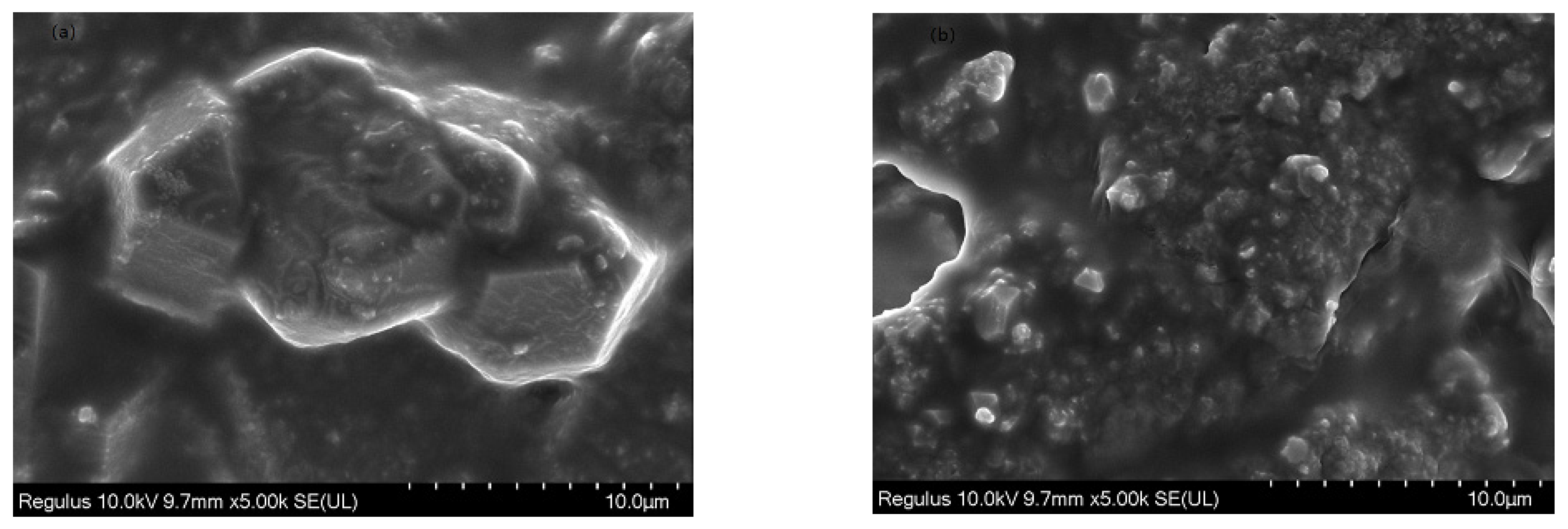
2.4.2. SEM Analysis
It can be seen from Figure 8a in the SEM image of γ-Al2O3 before loading that the surface of the γ-Al2O3 carrier is smooth. Figure 8b SEM photograph showed the γ-Al2O3 surface after loading KF. The increased surface roughness of KF/γ-Al2O3 is related to the homogeneous coverage of KF on the surface of γ-Al2O3, which is caused by the formation of crystalline substances after loading KF. This phenomenon is consistent with the results obtained by SEM analysis and previous research. Wang et al. [23] have demonstrated that the catalyst surface roughness increases with increasing catalyst surfactant concentration. In addition, as the catalytic surface activation point increases, catalytic activity is enhanced. A 40% KF loading on the surface of γ-Al2O3 makes the surface rough and improves the catalytic activity of the catalyst.
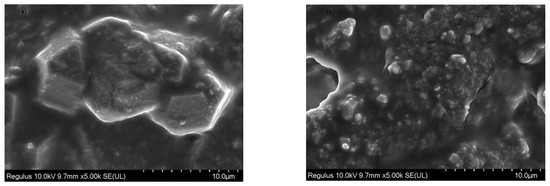
Figure 8.
SEM images of γ-Al2O3 (a) and 40%KF/γ-Al2O3 (b).
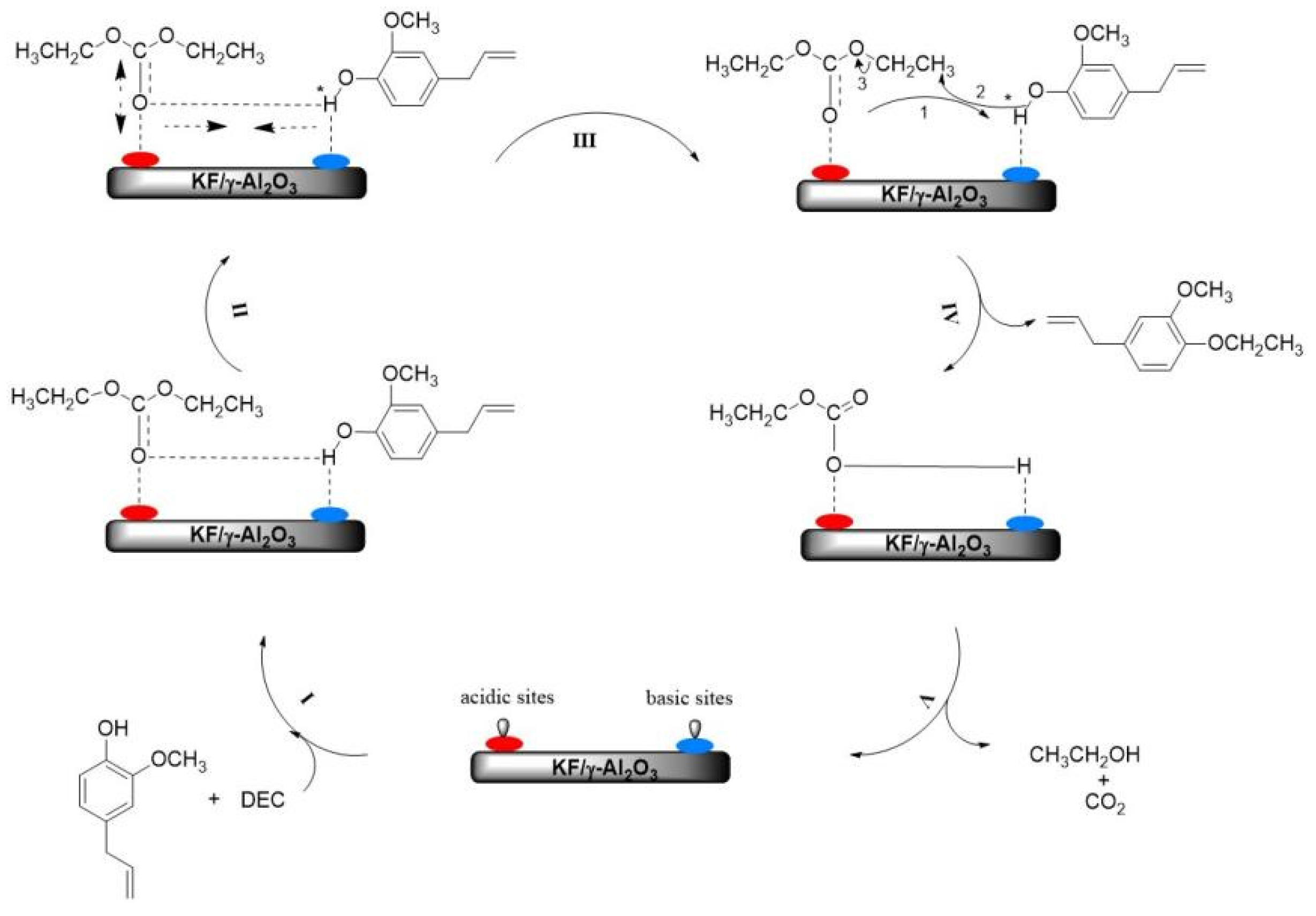
2.4.3. Proposed O-Ethylation Reaction Mechanism
As shown in Figure 9, a preliminary reaction mechanism for the O-ethylation of eugenol with DEC over KF/γ-Al2O3 catalyst was proposed. Firstly, the catalyst is made up of basic and acidic substances, the acidic part being taken up by the Al atoms, which have empty electron orbitals and can accept electrons; the basic part is made up of F atoms, which can provide electrons. The phenolic hydroxyl group of the reactant eugenol is absorbed by the basic part of the catalyst medium. The carbonyl oxygen (I) in the DEC molecule is adsorbed by the acidic site in the catalyst. Once the eugenol molecule reaches the excited state (II), the hydrogen bond length (O-H) between eugenol and DEC decreases; meanwhile, the length of C=O in the DEC molecule increases, which promotes the cleavage of phenolic hydroxyl groups and the formation of nucleophilic anions (Ar-phO−) [24]. Thirdly, the anion Ar-PhO− attacks the ethyl carbon in the DEC molecule, which then produces eugenol ethers (III and IV). Finally, the O-ethylation (V) is completed with the production of ethanol and CO2. By this catalytic mechanism, the rate of the O-ethylation reaction can be accelerated.
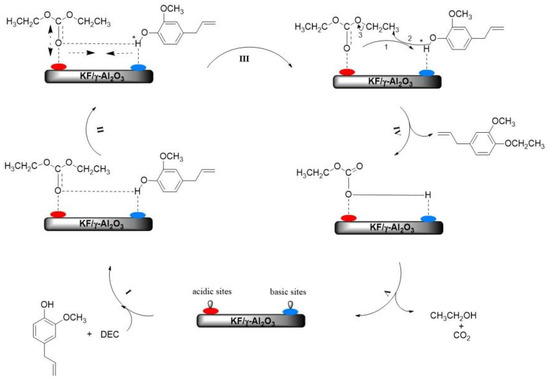
Figure 9.
Schematic diagram of the proposed O-ethylation reaction mechanism.
3. Experimental Section
3.1. Materials
Eugenol (99%) was purchased from Nanjing Eurasian Flavor and Fragrance Co., Ltd. (Nanjing, China). DEC (99.5%) was purchased from Nanjing Fubon Chemical Co., Ltd. (Nanjing, China). Potassium hydroxide (KOH), potassium carbonate (K2CO3), potassium fluoride (KF) and potassium acetate (CH3COOK) were purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. (Shanghai, China). Gamma-phase aluminum oxide (γ-Al2O3) was supplied by Hebei Tianda Tianjiu Chemical Technology Co., Ltd. (Bazhou, China).
3.2. Methods
3.2.1. Catalyst Preparation
The KF catalyst supported on γ-Al2O3 was prepared by the impregnation method. For the preparation of KF/γ-Al2O3 catalysts, (e.g., a loading concentration of 40% KF/γ-Al2O3), 4 g of KF dissolved in 100 mL of water is added to 6 g of γ-Al2O3 at 50 °C for 8 h. The redundant solution was filtered and dried at 100 °C for 2 h. The calcination of the catalyst at 450 °C was performed in a muffle furnace for 3 h. The other catalysts used in this study were prepared following a similar procedure.
3.2.2. Catalyst Characterization
The XRD spectra of the supported catalyst were recorded with a Philips X-ray diffraction spectrometer. The Cu Kα radiation (40 kV, 40 mA) was used as the X-ray source, and the scan data were recorded for 2 s at each step in a step-scan (0.05°/step) over a 2θ range 10–80°. The microstructural property of the catalyst was performed with the aid of scanning electron microscopy analysis (SEM, electron microscope model JEOL JSM-7600E).
3.2.3. Catalytic Reaction
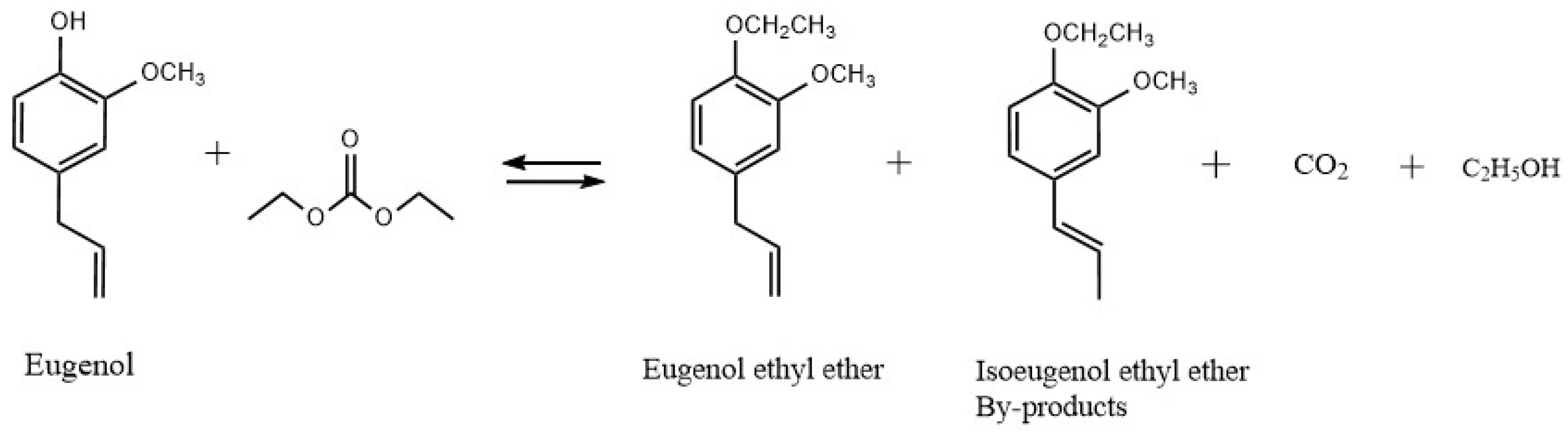
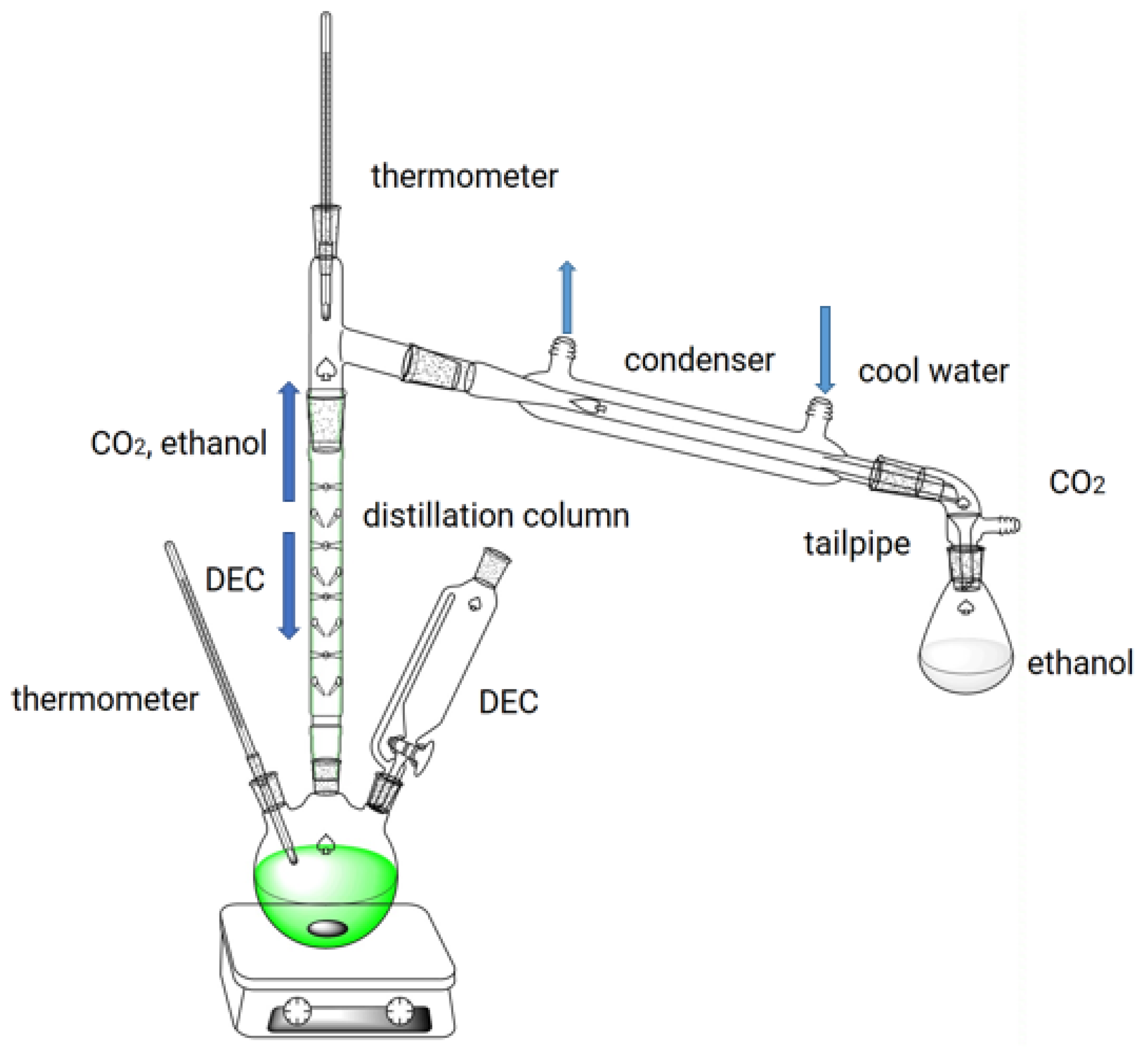
The reaction for the synthesis of EEE from eugenol is shown in Figure 10. Using a 250 mL three-necked flask equipped with a distillation column, thermometer, dropping funnel, and magnetic stirrer, the prescribed amounts of eugenol and KF/γ-Al2O3 were stirred. The synthesis process is shown in Figure 11. For preventing eugenol from oxidizing, the system was purged with nitrogen before heating. Using a drop-in method, the required amount of DEC was added to the flask when the temperature reached a preset degree (170 °C). During O-ethylation of eugenol by DEC, carbon dioxide (CO2) and ethanol are produced. Considering the different boiling points of the two substances, DEC (126 °C) and ethanol (78 °C), the vaporized DEC, ethanol, and gaseous CO2 are separated in a distillation column. The CO2 and ethanol are distilled from the top of the distillation column, while the DEC is refluxed back into the flask for the reaction; through this experimental design, the utilization rate of DEC can be improved. The O-ethylation reaction is complete when no more CO2 is produced and no ethanol is collected in the tailpipe. In order to neutralize the reactants, 5% hydrochloric acid was added to achieve pH = 7, ethyl acetate was used to extract EEE, which was then distilled under reduced pressure, sampled for analysis, and the yields of EEE were calculated.
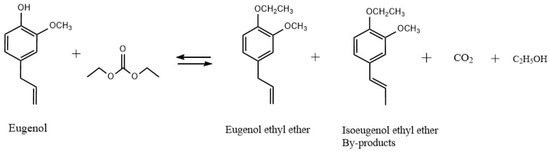
Figure 10.
Synthesis of EEE from eugenol.
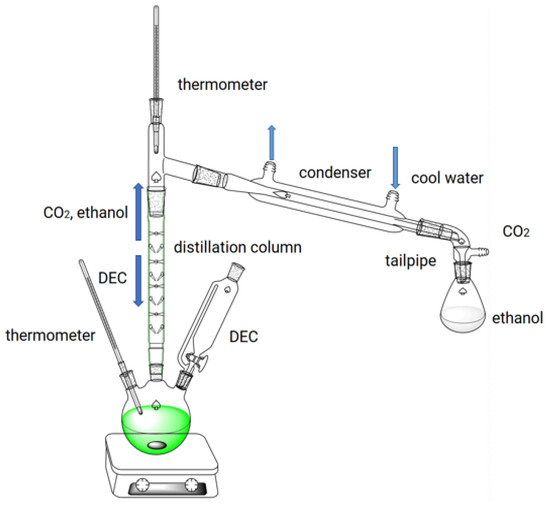
Figure 11.
Process flow diagram for the synthesis of EEE.
3.2.4. Procedure for Recycling the Catalyst
After each reaction, the catalyst was washed twice with a 10 wt.% KOH solution and then once with water. After being dried at 100 °C for 2 h, it was ready to proceed to the next experiment.
3.2.5. Eugenol Ethyl Ether Analysis
The samples were analyzed by a GC-9800 gas chromatograph equipped with a flame ionization detector (FID) and a SE-54 flexible quartz capillary column (30 m × 250 μm × 0.25 μm) (non-polar). Nitrogen was used as a carrier gas, at a flow rate of 0.85 mL/min. The temperatures of the GC column oven, injector, and detector were 70, 270, and 270 °C, respectively. The column temperature was initialed at 70 °C for 5 min and then increased to 270 °C at a rate of 10 °C/min and held at 270 °C for 5 min. The injection volume was 0.2 µL. The yield of eugenol ether in each experiment was calculated based on its content in the composition analyzed by GC. A standard curve method was used to determine the content of eugenol ethyl ether. FT-IR spectra were analyzed on a Nicolet FTIR-360 (Nicolet Instrument Corp., Fitchburg, WI, USA) Fourier-transform infrared spectrophotometer. Potassium bromide (KBr) was used as the infrared beam splitter with coverage of the normal 4000 cm−1 400 cm−1 mid-IR spectral region. The 1HNMR spectra were recorded using Bruker AVANCE 600 MHz with deuterated chloroform (CDCl3) as the solvent.
3.2.6. Eugenol Ethyl Ether Yield Analysis
The conversion, yield, and selectivity of EEE were calculated according to Equations (1)–(3) as follows [25]:
in the formula: m: total product mass, g;
w: content of eugenol ethyl ether in the product, %, detected by GC;
M: relative molecular mass of eugenol ethyl ether, g/mol;
n: theoretical molarity of eugenol ethyl ether, mol.
4. Product Characterization and Analysis
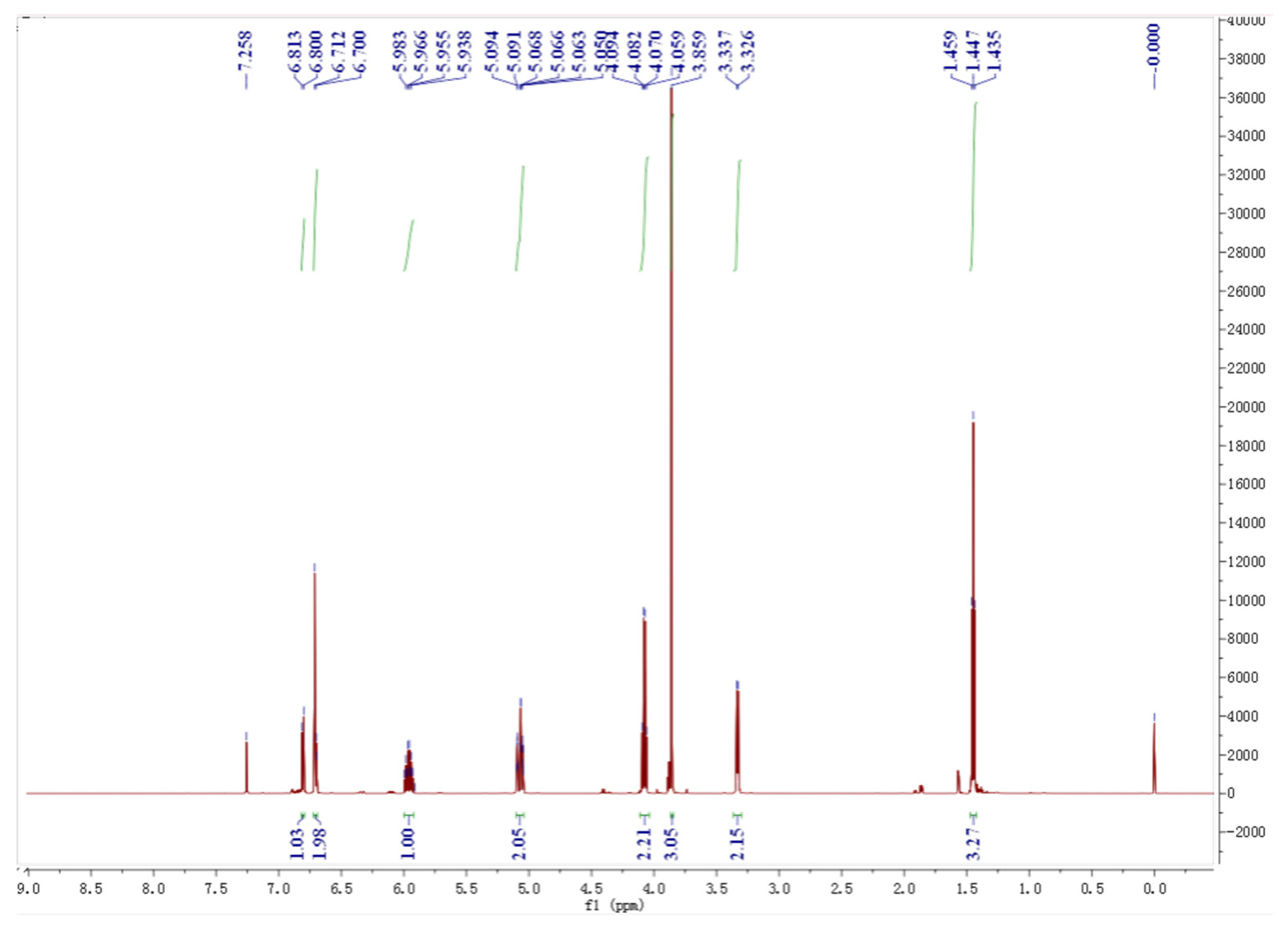
4.1. NMR Analysis of Eugenol Ethyl Ether
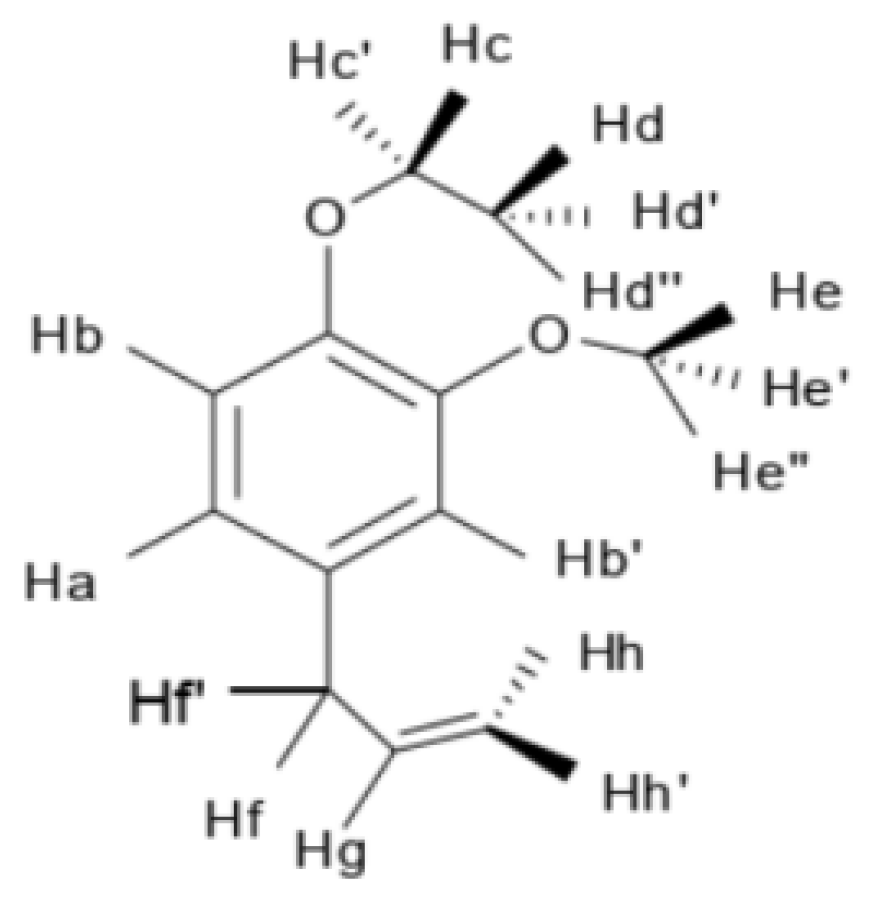
NMR spectrum of EEE and attribution of individual H-atoms in the NMR spectrum of EEE are presented in Figure 12 and Figure 13: 1H NMR (600 MHz, CDC13), δ6.81 (d, J = 8.2 Hz, 1H, Hb), 6.72–6.69 (m, 2H, Ha, Hb′), 5.96 (ddt, J = 16.8, 10.0, 6.7 Hz, 1H, Hg), 5.10–5.04 (m, 2H, Hh, Hh′), 4.08 (q, J = 7.0 Hz, 2H, Hc, Hc′), 3.86 (s, 3H, He, He′, He″), 3.33 (d, J = 6.7 Hz, 2H, Hf, Hf′), and 1.47–1.42 (t, J = 7.2 Hz, 3H, Hd, Hd′, Hd″).
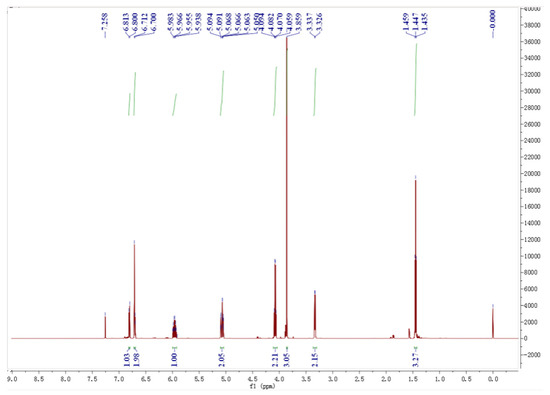
Figure 12.
NMR spectrum of EEE.
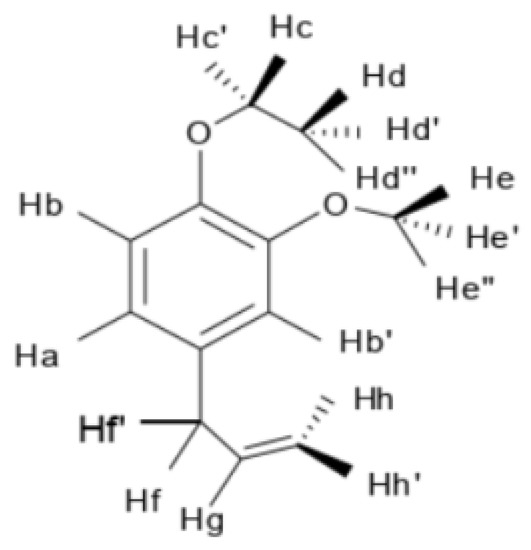
Figure 13.
Attribution of individual H-atoms in the NMR spectrum of EEE.
4.2. MS Analysis
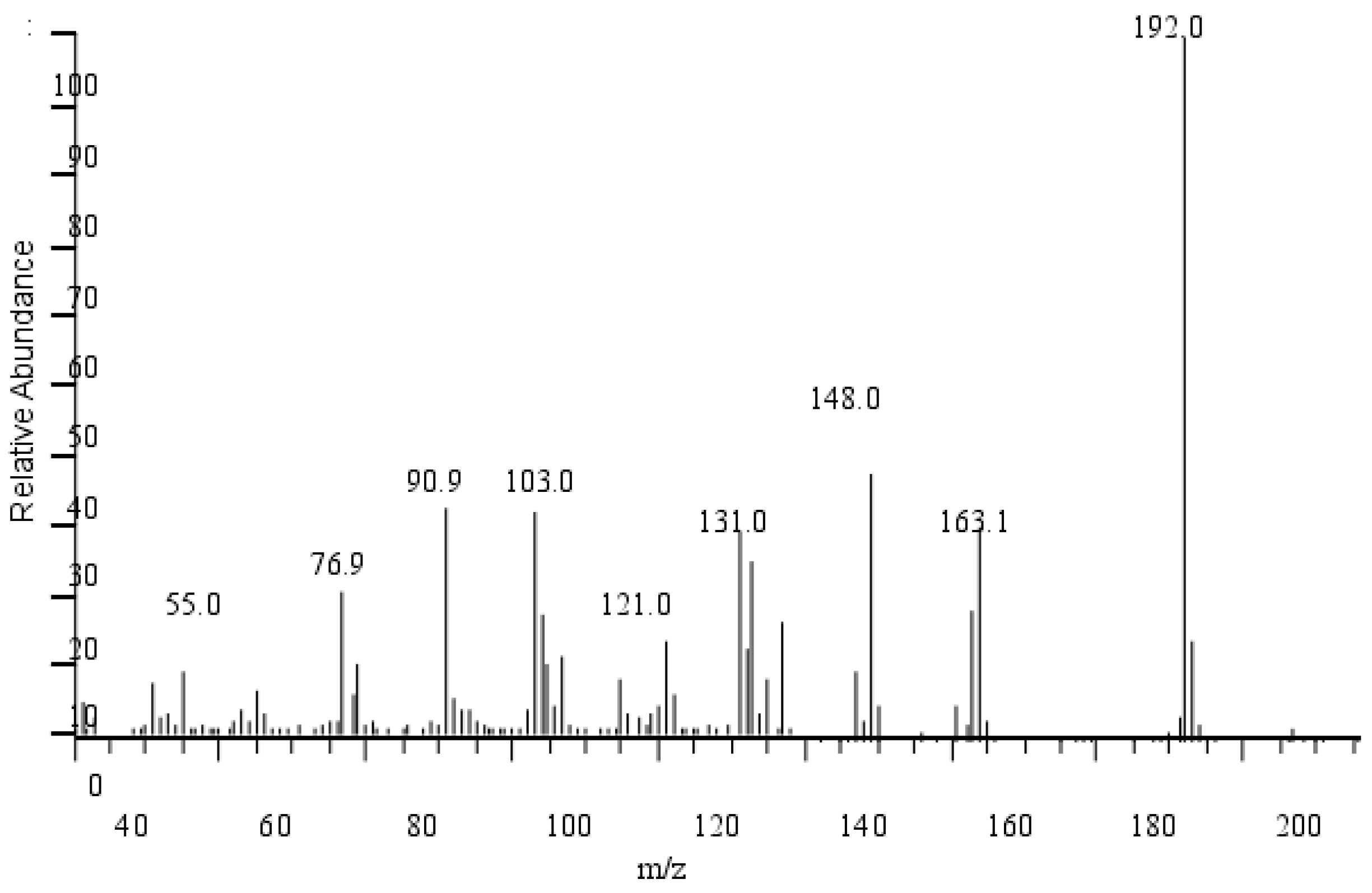
The mass spectra of eugenol ether are shown in Figure 14. It can be seen that, according to MS measurements, the maximum peak of EEE is 192, consistent with EEE’s relative molecular mass. The first fragmentation resulted in the formation of the ion m/z = 163 due to the loss of the ethyl group, C2H5 (m/z = 29). The ion in the spectrum is observed at m/z = 148 ion due to breaking the group CH3 (m/z = 15). Another fragmentation scheme involves breaking the CH=CH2 group (m/z = 27), forming ions m/z = 121, and fragmentation at m/z = 91 and m/z = 77 is typical of the ion peaks of the C6H5CH2+ and C6H5+ groups.
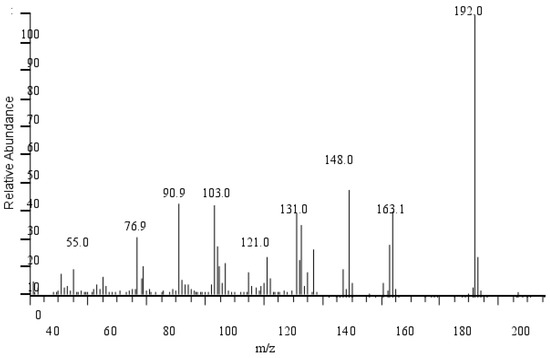
Figure 14.
MS chromatography of eugenol ethyl ether.
4.3. FT-IR Analysis
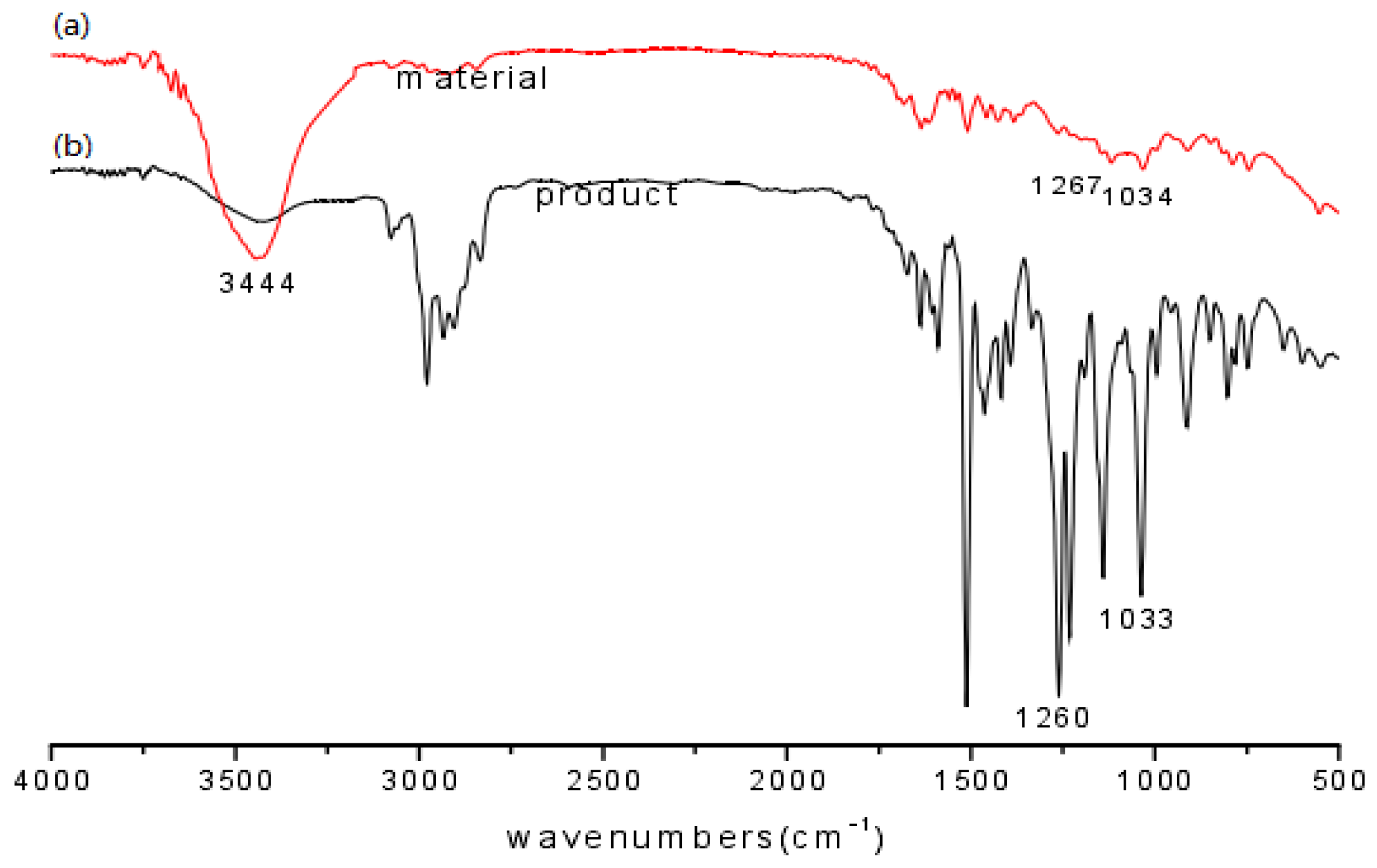
The raw material eugenol and the product EEE were compared using FT-IR spectroscopy as shown in Figure 15. As can be seen from the figure, the (a) spectrum of eugenol has O-H stretching vibrational absorption at 3444 cm−1 and asymmetric and symmetric stretching vibration absorption peaks of Ar-O-C at 1260 cm−1 and 1033 cm−1. The comparison revealed that the O-H characteristic absorption peak of eugenol ether (as shown in b spectrum) is significantly weakened in the eugenol ether spectrum, while the characteristic Ar-O-C absorption peaks of the product at 1260 cm−1 and 1033 cm−1 were enhanced in intensity compared to the corresponding absorption peaks of eugenol, indicating O-ethylation of the phenolic hydroxyl group with DEC.
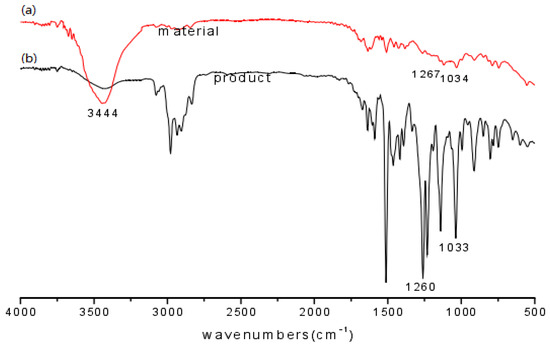
Figure 15.
FT-IR spectra of eugenol (a) and eugenol ethyl ether (b).
5. Conclusions
In this study, a KF/γ-Al2O3 solid base catalyst was prepared and used for the synthesis of EEE from eugenol and green solvent DEC. Firstly, a new experimental procedure had been designed to improve DEC reagent utilization via reflux method. This experimental design increases the utilization of DEC and also allows DEC to be used in large-scale conditions. Subsequently, the active ingredients were screened, and KF was found to be the most effective for the O-ethylation reaction when loaded on the γ-Al2O3 carrier surface. The catalyst preparation process was optimized, and the best performance was achieved at impregnation temperature of 50 °C, impregnation concentration of 40%, impregnation time of 8 h, and calcination temperature of 450 °C. Subsequently, the prepared catalysts were subjected to repeat experiments; the KF/γ-Al2O3 catalyst still can catalyze the eugenol O-ethylation reaction, and the yield of EEE reached 51.2% in three replicate experiments. Finally, the KF/γ-Al2O3 catalysts and EEE were characterized.
Author Contributions
Conceptualization, K.Z. and Z.Z.; methodology, Q.Z.; software, T.D.; validation, J.W.; formal analysis, Z.Z.; data curation, T.D.; writing—original draft preparation, Q.Z.; writing—review and editing, Q.Z., T.D. and J.W.; visualization, T.D.; supervision, K.Z.; project administration, J.L.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (22078227).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We are grateful to the Department of Fine Chemicals, School of Chemical Engineering, Nanjing Forestry University for their help in the catalyst characterization and product purification.
Conflicts of Interest
We declare that we have no competing interest.
References
- Arctander, S. Perfume and Flavor Chemicals: (Aroma Chemicals); Allured Publishing Corporation: Carol Stream, IL, USA, 1969; Volume 2. [Google Scholar]
- Api, A.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G., Jr.; Buschmann, J.; Cancellieri, M.; Dagli, M.; Date, M.; Dekant, W. RIFM fragrance ingredient safety assessment, isoeugenyl ethyl ether, CAS Registry Number 7784-67-0. Food Chem. Toxicol. 2022, 161, 112873. [Google Scholar] [CrossRef]
- Na, M.; Ritacco, G.; O’Brien, D.; Lavelle, M.; Api, A.M.; Basketter, D. Fragrance skin sensitization evaluation and human testing: 30-year experience. Dermatitis 2021, 32, 339–352. [Google Scholar] [CrossRef]
- Weidlich, T.; Pokorný, M.; Padělková, Z.; Růžička, A. Aryl ethyl ethers prepared by ethylation using diethyl carbonate. Green Chem. Lett. Rev. 2007, 1, 53–59. [Google Scholar] [CrossRef]
- Yang, Y.L. Determination of Medial Lethal Dose of Diethyl Sulfate to Silkworm, Bombyx mori. Sci. Seric. 2012, 38, 70–73. [Google Scholar]
- Shukla, K.; Srivastava, V.C. Diethyl carbonate: Critical review of synthesis routes, catalysts used and engineering aspects. RSC Adv. 2016, 6, 32624–32645. [Google Scholar] [CrossRef]
- Gjyli, S.; Korpa, A.; Tabanelli, T.; Trettin, R.; Cavani, F.; Belviso, C. Higher conversion rate of phenol alkylation with diethylcarbonate by using synthetic fly ash-based zeolites. Microporous Mesoporous Mater. 2019, 284, 434–442. [Google Scholar] [CrossRef]
- Raiguel, S.; Gijsemans, L.; Van den Bossche, A.; Onghena, B.; Binnemans, K. Solvent extraction of gold (III) with diethyl carbonate. ACS Sustain. Chem. Eng. 2020, 8, 13713–13723. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Wang, L.; Li, H. Environment-friendly synthesis of diethyl carbonate via ethyl carbamate alcoholysis over cerium oxide catalyst. J. Environ. Manag. 2019, 232, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Jia, K.; Xu, J.; Liu, N.; Liu, P.; Xu, C.; Li, Y. A novel method to prepare KNO3/NaY solid base catalysts and their application in the O-ethylation of phenol with diethyl carbonate. React. Kinet. Mech. Catal. 2012, 107, 435–447. [Google Scholar] [CrossRef]
- Lui, M.Y.; Yuen, A.K.; Masters, A.F.; Maschmeyer, T. Masked N-Heterocyclic Carbene-Catalyzed Alkylation of Phenols with Organic Carbonates. ChemSusChem 2016, 9, 2312–2316. [Google Scholar] [CrossRef]
- Popławski, J.; Łozowicka, B.; Dubis, A.T.; Lachowska, B.; Witkowski, S.; Siluk, D.; Petrusewicz, J.; Kaliszan, R.; Cybulski, J.; Strzałkowska, M. Synthesis and hypolipidemic and antiplatelet activities of α-asarone isomers in humans (in vitro), mice (in vivo), and rats (in vivo). J. Med. Chem. 2000, 43, 3671–3676. [Google Scholar] [CrossRef] [PubMed]
- Triyono, T. Effect of impregnation procedure of Pt/γ-Al2O3 catalysts upon catalytic oxidation of CO. Indones. J. Chem. 2010, 2, 8–11. [Google Scholar] [CrossRef]
- Tang, Y.; Ren, H.; Chang, F.; Gu, X.; Zhang, J. Nano KF/Al 2 O 3 particles as an efficient catalyst for no-glycerol biodiesel production by coupling transesterification. RSC Adv. 2017, 7, 5694–5700. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Zheng, J.; Zhang, H. Synthesis of curcumin catalyzed by KF/Al2O3. Pigment Resin Technol. 2016, 45, 225–233. [Google Scholar] [CrossRef]
- Hassam, M.; Taher, A.; Arnott, G.E.; Green, I.R.; van Otterlo, W.A. Isomerization of allylbenzenes. Chem. Rev. 2015, 115, 5462–5569. [Google Scholar] [CrossRef]
- Hattori, H. Solid base catalysts: Generation, characterization, and catalytic behavior of basic sites. J. Jpn. Pet. Inst. 2004, 47, 67–81. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Feng, L. Fluoridation-induced high-performance catalysts for the oxygen evolution reaction: A mini review. Electrochem. Commun. 2021, 122, 106901. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Chuvilin, A.L.; Kuvshinov, G.G. Decomposition of methane over iron catalysts at the range of moderate temperatures: The influence of structure of the catalytic systems and the reaction conditions on the yield of carbon and morphology of carbon filaments. J. Catal. 2001, 201, 183–197. [Google Scholar] [CrossRef]
- Castro, C.S.; Cardoso, D.; Nascente, P.A.; Assaf, J.M. MgAlLi mixed oxides derived from hydrotalcite for catalytic transesterification. Catal. Lett. 2011, 141, 1316–1323. [Google Scholar] [CrossRef]
- Ono, Y.; Hattori, H. Solid Base Catalysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 101. [Google Scholar]
- Liu, Z.; Wang, J.; Kang, M.; Yin, N.; Wang, X.; Tan, Y.; Zhu, Y. Synthesis of glycerol carbonate by transesterification of glycerol and dimethyl carbonate over KF/γ-Al2O3 catalyst. J. Braz. Chem. Soc. 2014, 25, 152–160. [Google Scholar]
- Wang, Y.; Qi, K.; Wu, S.; Cao, Z.; Zhang, K.; Lu, Y.; Liu, H. Preparation, characterization and catalytic sodium borohydride hydrolysis of nanostructured cobalt–phosphorous catalysts. J. Power Sources 2015, 284, 130–137. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Zheng, Z. Light assisted O-alkylation of phenols to ethers using layered double oxides catalyst under green and mild conditions. J. Photochem. Photobiol. A Chem. 2020, 400, 112695. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Visible-light-mediated photocatalytic aerobic dehydrogenation of N-heterocycles by surface-grafted TiO2 and 4-amino-TEMPO. ACS Catal. 2019, 9, 10694–10704. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).