The Improved para-Selective C(sp2)-H Borylation of Anisole Derivatives Enabled by Bulky Lewis Acid
Abstract
1. Introduction
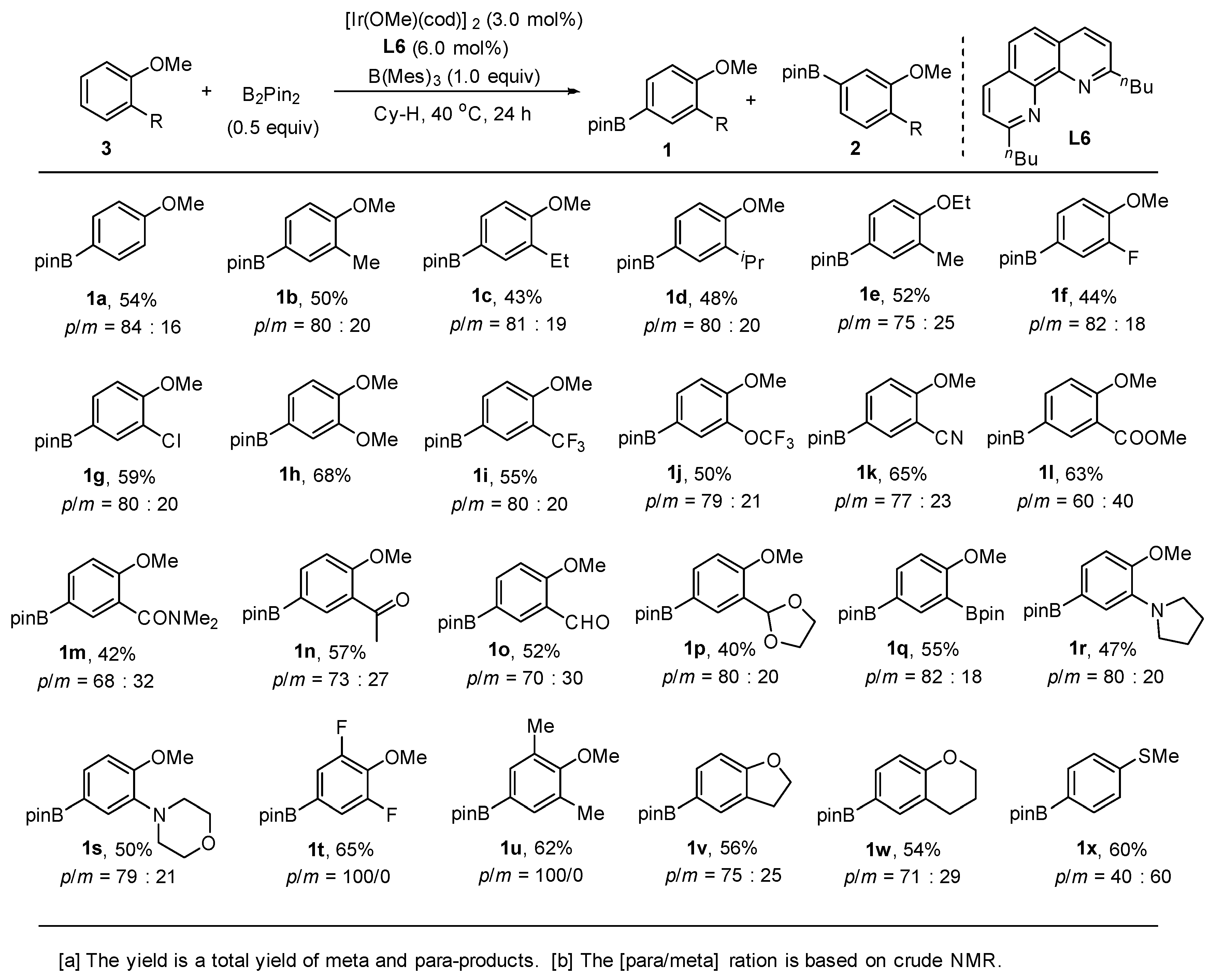
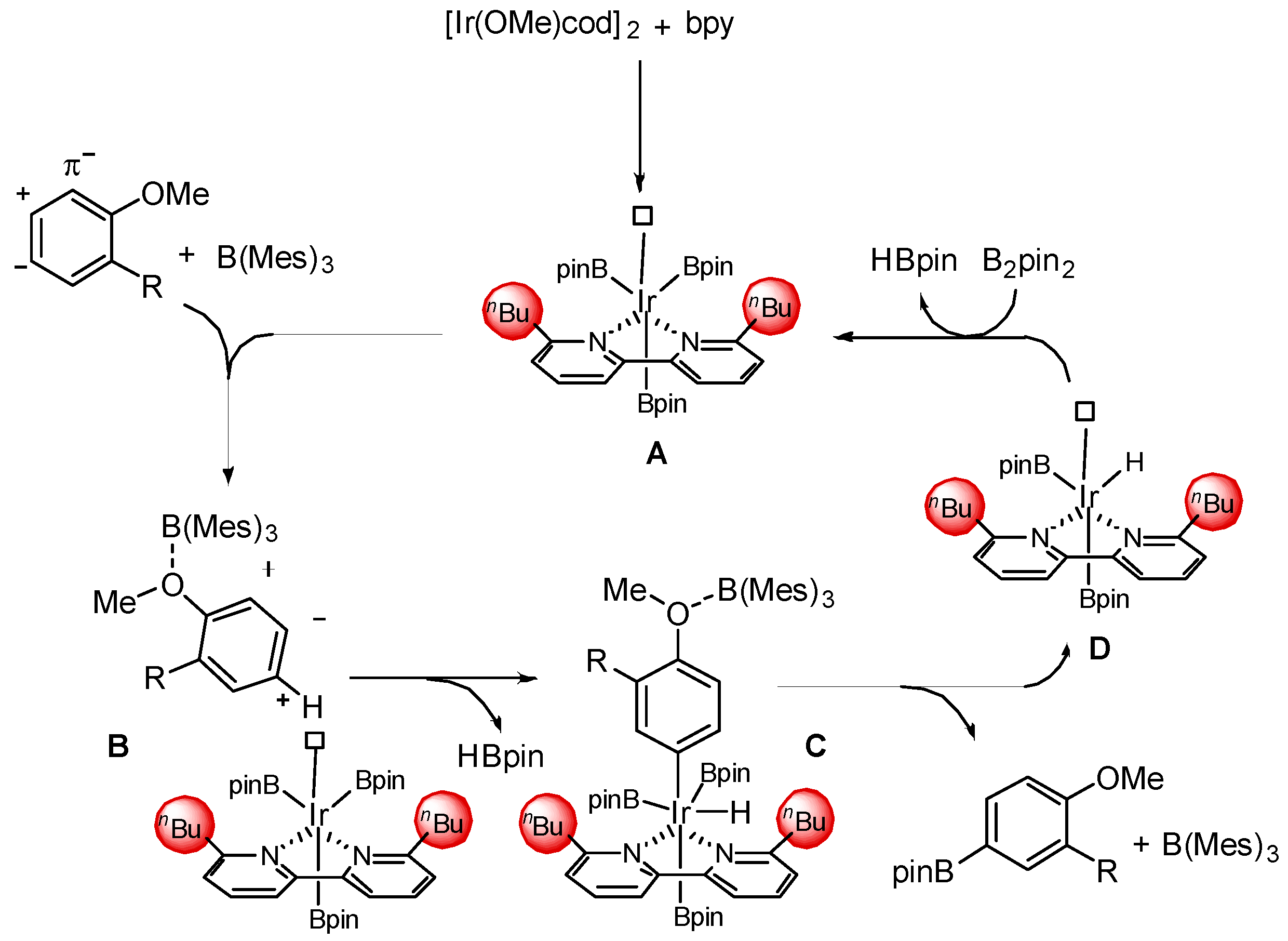
2. Results and Discussion
3. Material and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Lewis Acid (Pentafluorophenyl Borate ArFB-1 to ArFB-3) [44]

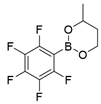


3.2.2. Preparation of 2-alkyl Anisole Derivatives (3c to 3e) [45]
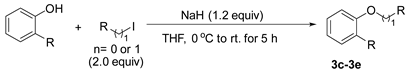
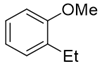
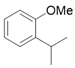

3.2.3. Preparation of 2-Methoxy-N,N-Dimethylbenzamide (3m) [46]
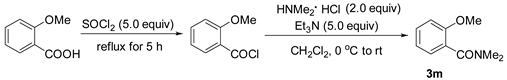
3.2.4. Preparation of 2-(2-Methoxylphenyl)-1,3-dioxolane (3p) [47]
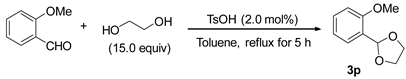
3.2.5. Preparation of 2-heterocycle Substituted Anisole (3r and 3s) [48]
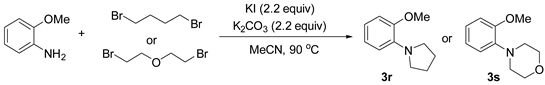

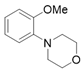
3.2.6. Preparation of Para-Selective C-H Borylation of Anisole Derivatives (1a to 1x)
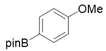


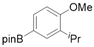
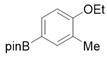


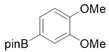


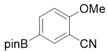
3.2.7. Preparation of PED4 Inhibitor [41]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, S.; Joo, J.-M. Transoition-Metal-Catalyzed Divergent C-H Functionalization of Five-Membered Heteroarenes. Acc. Chem. Res. 2021, 54, 4158. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Yang, K.; Lawrence, B.; Ge, H. Transient Ligand-Enabled Transition Metal-Catayzed C-H Functionalization. ChemSusChem 2019, 13, 2955. [Google Scholar] [CrossRef] [PubMed]
- Britton, L.; Docherty, J.H.; Nichol, G.S.; Dominey, A.P.; Thomas, S.P. Iron-Catalysed C(sp2)-H Borylation with Expanded Functional Group Tolerance. Chin. J. Chem. 2022, 40, 2875–2881. [Google Scholar] [CrossRef]
- Sauermann, N.; Meyer, T.H.; Ackermann, L. Electrochemical Cobalt-Catalyzed C-H Activation. Chem. Eur. J. 2018, 24, 16209. [Google Scholar] [CrossRef]
- Nakanowatari, S.; Müller, T.; Oliveira, J.; Ackermann, L. Bifurcated Nickel-Catalyzed Functionalizations: Heteroarene C-H Activation with Allenes. Angew. Chem. Int. Ed. 2017, 56, 15891. [Google Scholar] [CrossRef]
- Haito, A.; Yamaguchi, M.; Chatani, M. Ru3(CO)12-Catalyzed Carbonylation of C-H Bonds by Triazole-Directed C-H Activation. Asian J. Org. Chem. 2018, 7, 1315. [Google Scholar] [CrossRef]
- Vásquez-Céspedes, S.; Wang, X.M.; Glorius, F. Plausible Rh(V) Intermediates in Catalytic C-H Activation Reaction. ACS Catal. 2018, 8, 242. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, M.X.; Zhou, T.; Han, Y.Q.; Xu, Y.T.; Zhang, K.; Shi, B.F. Pd(II)-Catalyzed Enantioselective Arylation of Unbiased Methylene C(sp3)-H Bonds Enabled by a 3,3’-F2-BINOL Ligand. Chem. Commun. 2021, 57, 5562–5565. [Google Scholar]
- Zhang, Y.F.; Wu, B.; Shi, Z.J. Ir-Catalyzed C-H Amidation of Aldehydes with Stoichiometric/Catalytic Directing Group. Chem. Eur. J. 2016, 22, 17808. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Darby, M.T.; Liu, J.L.; Wimble, J.M.; Lucci, F.R.; Lee, S.; Michaelides, A.; Flytzani-Stephanopoulos, M.; Stamatakis, M.; Sykes, E. Pt/Cu Single-Atom Alloys as Coke-Resistant Catalysts for Efficent C-H Activation. Nat. Chem. 2016, 8, 531. [Google Scholar]
- Kalyani, D.; Deprez, N.R.; Desai, L.V.; Sanford, M.S. Oxidative C−H Activation/C−C Bond Forming Reactions: Synthetic Scope and Mechanistic Insights. J. Am. Chem. Soc. 2005, 127, 7330. [Google Scholar] [CrossRef]
- Kawamorita, S.; Murakami, R.; Iwai, T.; Sawamura, M. Synthesis of Primary and Secondary Alkylboronates through Site-Selectivity C(sp3)-H Activation with Silica-Supported Monophosphine-Ir Catalysts. J. Am. Chem. Soc. 2013, 135, 2947. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhao, H.; Li, Y.; Gao, Q.; Ke, Z.; Xu, S. Chiral Bidentate Boryl Ligand Enabled Iridium-Catalyzed Asymmetric C(sp2)-H Borylation of Diarylmethylamines. J. Am. Chem. Soc. 2019, 141, 5334. [Google Scholar] [CrossRef]
- Daugulis, O.; Roane, J.; Tran, L.D. Bidentate, Monoanionic Auxiliary-Directed Functionalization of Carbon-Hydrogen Bonds. Acc. Chem. Res. 2015, 48, 1053. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.M.; Sharma, S.; Park, J.; Han, S.; Kim, I.S. Recent Advances in Catalytic C(sp2)-H Allylation Reactions. ACS Catal. 2017, 7, 2821. [Google Scholar] [CrossRef]
- Liao, G.; Zhou, T.; Yao, Q.-J.; Shi, B.-F. Recent Advacnes in the Synthesis of Axially Chiral Biaryl via Transition Metal-Catalyzed Asymmetric C-H Functionalization. Chem. Commun. 2019, 55, 8514. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Ikemoto, H.; Matsunaga, S.; Kanai, M. A Cationic High-Valent Cp*CoIII Complex for the Catalytic Generation of Nucleophilic Organometallic Species: Directed C-H Bond Activation. Angew. Chem. Int. Ed. 2013, 52, 2207. [Google Scholar] [CrossRef]
- Chen, X.; Goodhue, C.E.; Yu, J.-Q. Palladium-Catalyzed Alkylation of sp2 and sp3 C-H Bonds with Methylboroxine and Alkylboronic Acids: Two Distinct C-H Activation Pathways. J. Am. Chem. Soc. 2006, 128, 12634. [Google Scholar] [CrossRef]
- Shabashov, D.; Daugulis, O. Auxiliary-Assisted Palladium-Catalyzed Arylation and Alkylation of sp2 and sp3 Carbon-Hydrogen Bonds. J. Am. Chem. Soc. 2010, 132, 3965. [Google Scholar] [CrossRef]
- Cho, J.Y.; Tse, M.K.; Holmes, D.; Maleczka, R.E., Jr.; Smith, M.R., III. Remarkably Selective Iridium Catalysts for the Elaboration of Aromatic C-H Bonds. Science 2002, 295, 305. [Google Scholar] [CrossRef]
- Ishiyama, T.; Miyaura, N.; Hartiwg, J.F. Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate. J. Am. Chem. Soc. 2002, 124, 390. [Google Scholar] [CrossRef]
- Tajuddin, H.; Harrisson, P.; Bitterlich, B.; Collings, J.C.; Sim, N.; Batsanov, A.S.; Cheung, M.S.; Kawamorita, S.; Maxwell, A.C.; Shukla, L.; et al. Iridium-Catalyzed C-H Borylation of Quinolines and Unsymmetrical 1,2-Disubstituted Benzenes: Insights into Steric and Electronic Effects on Selectivity. Chem. Sci. 2012, 3, 3505. [Google Scholar]
- Ishiyama, T.; Takagi, J.; Yonekawa, Y.; Hartwig, J.F.; Miyaura, N. Iridium-Catalyzed Direct Borylation of Five-Membered Heteroarenes by Bis(pinacolato)diboron: Regioselective, Stoichiometric, and Room Temperature Reactions. Adv. Synth. Catal. 2003, 345, 1103. [Google Scholar] [CrossRef]
- Ishiyama, T.; Nobuta, Y.; Hartwig, J.F.; Miyaura, N. Room Temperature Borylation of Arenes and Heteroarenes using Stoichiometric Amounts of Pinacolborane Catalyzed by Iridium Complexes in An Inert Solvent. Chem. Commun. 2003, 2924. [Google Scholar] [CrossRef]
- Saito, Y.; Segawa, Y.; Itami, K. Para-C-H Borylation of Benzene Derivatives by a Bulky Iridium. Catalyst. J. Am. Chem. Soc. 2015, 137, 5193. [Google Scholar] [CrossRef]
- Saito, Y.; Yamanoue, K.; Segawa, Y.; Itami., K. Selective Transformmation of Strychnine and 1,2-Disubstituted Benzenes by C-H Borylation. Chem 2020, 6, 985. [Google Scholar] [CrossRef]
- Yang, L.; Semba, K.; Nakao, Y. Para-Selective C-H Borylation of (Hetero) Arenes by Cooperative Iridium/Aluminum Catalysis. Angew. Chem. Int. Ed. 2017, 56, 4853. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Hartwig, J.F. Rhodium-Catalyzed Intermolecular C-H Sililation of Arenes with High Steric Control. Science 2014, 343, 853. [Google Scholar] [CrossRef]
- Bastidas, J.R.; Chhabra, A.; Feng, Y.; Oleskey, T.J.; Smith, M.R.; Maleczka, R.E. Steric Shielding Effects Induced by Intramolecular C-H-O Hydrogen Bonding: Remote Borylation Directed by Bpin Group. ACS Catal. 2022, 12, 2694. [Google Scholar] [CrossRef]
- Kuninobu, Y.; Ida, H.; Nishi, M.; Kanai, M. A Meta-Selective C–H Borylation Directed by A Secondary Interaction between Ligand and Substrate. Nat. Chem. 2015, 7, 712. [Google Scholar]
- Lu, X.; Yoshigoe, Y.; Ida, H.; Nishi, M.; Kanai, M.; Kuninobu, Y. Hydrogen Bond-Accelerated meta-selective C-H Borylation of Aromatic Compounds and Expression of Functional Group and Substrate Specificites. ACS Catal. 2019, 9, 1705. [Google Scholar] [CrossRef]
- Davis, H.J.; Mihai, M.T.; Phipps, R.J. Ion Pair-Directed Regiocontrol in Transition-Metal Catalysis: A Meta-Selective C-H Borylation of Aromatic Quaternary Ammonium Salts. J. Am. Chem. Soc. 2016, 138, 12759. [Google Scholar] [CrossRef] [PubMed]
- Golding, W.A.; Pearce-Higgins, R.; Phipps, R.J. Site-Selective Cross-Coupling of Remote Chorides Enabled by Electrostatically-Directed Palladium Catalysis. J. Am. Chem. Soc. 2018, 140, 13570. [Google Scholar] [CrossRef]
- Mihai, M.T.; Genov, G.R.; Phipps, R.J. Access to the Meta Position of Arenes Through Transition Metal Catalyzed C-H Bond Functionalization: A focus on metals other than palladium. Chem. Soc. Rev. 2018, 47, 149. [Google Scholar] [CrossRef]
- Chattopadhyay, B.; Dannatt, J.E.; Andujar-De Sanctis, I.L.; Gore, K.A.; Maleczka, E.E.; Singleton, D.A.; Smith, M.R., III. Ir-Catalyzed ortho-Borylation of Phenols Directed by Substrate-Ligand Electrosatic Interactions: A Combined Experimental/in Silico Strategy for Optimizing Weak Interactions. J. Am. Chem. Soc. 2017, 139, 7864. [Google Scholar] [CrossRef]
- Hoque, M.E.; Bisht, R.; Haldar, C. Chattopadhyay, B. Noncovalent Interactions in Ir-Catalyzed C–H Activation: L-Shaped Ligand for Para-Selective Borylation of Aromatic Esters. J. Am. Chem. Soc. 2017, 139, 7745. [Google Scholar] [CrossRef] [PubMed]
- Bisht, R.; Haldar, C.; Hassan, M.; Hoque, M.E.; Chaturvadi, J.; Chattopadhyay, B. Metal-Catalyzed C-H Bond Activation and Borylation. Chem. Soc. Rev. 2022, 51, 5042. [Google Scholar] [CrossRef]
- Li, H.L.; Kuninobu, Y.; Kanai, M. Lewis Acid-Base Interaction-Controlled ortho-Selective C-H Borylation of Aryl Sulfides. Angew. Chem. Int. Ed. 2017, 56, 1495. [Google Scholar] [CrossRef]
- Li, H.L.; Kanai, M.; Kuninobu, Y. Iridium/Bipyridine-Catalyzed ortho-Selective C-H Boylation of Phenol and Aniline Derivatives. Org. Lett. 2017, 19, 5944. [Google Scholar] [CrossRef]
- Wojcieszyk, M.; Knuutila, L.; Kroyan, Y.; Balsemao, M.; Tripathi, R.; Keskivali, J.; Karvo, A.; Santasalo-Aarnio, A.; Blomstedt, O.; Larmi, L. Performance of Anisole and Isobutanol as Gasoline Bio-Blendstocks for Spark Ignition Engines. Sustainability 2021, 13, 8729. [Google Scholar] [CrossRef]
- Prieto, M.; Zurita, E.; Rosa, E.; Munoz, L.; Lloyd-Williams, P.; Giralt, E. Arylboronic Acids and Arylpinacolboronate Esters in Suzuki Coupling Reactions Involving Indoles. Partner Role Swapping and Heterocycle Protection. J. Org. Chem. 2004, 69, 6812. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, P.L.; Bremer, R.E.; Gillette, S.J.; Cho, H.; Nespi, M.; Mamo, S.; Zhang, C.; Artis, D.R.; Lee, B.; Zuckerman, R.L. Bicyclic Heteroaryl PDE4B Inhibitors. WO2006026754, 22 February 2005. [Google Scholar]
- Ajvazi, N.; Stavber, S. Alcohols in direct carbon-carbon and carbon-heteroatom bond-forming reactions: Recent advances. Arkivoc 2018, 2018, 288. [Google Scholar] [CrossRef]
- Adamczyk, A.; Jakubczyk, M.; Jankowski, P.; Sporzynski, A.; Urbánski, P.M. Influence of the Diol Structure on the Lewis Acidity of Phenylboronates. J. Phys. Org. Chem. 2013, 26, 415. [Google Scholar] [CrossRef]
- Kinoshita, H.; Yaguchi, K.; Tohjima, T.; Miura, K. Diisobutylaluminum Hydrode Pomoted Cyclization of Silylated 1,3-dien-5-ynes: Application to Total Synthesis of A 20-Norabietane Derivative. Tetrahedron Lett. 2017, 58, 1607. [Google Scholar] [CrossRef]
- Chen, J.; Lim, J.W.; Ong, D.Y.; Chiba, S. Iterative Addition of Carbon Nucleophiles to N,N-dialkyl Carboxamides for Synthesis of α-Tertiary Amines. Chem. Sci. 2022, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bao, S.H.; Yang, J.G. Synthesis of A Novel Multi-SO3H Functionalized Strong Bronsted Acidic Ionic Liquid and its Catalytic Activities for Acetalization. Chin. Sci. Bull. 2009, 54, 3958. [Google Scholar] [CrossRef]
- Rao, G.A.; Periasamy, M. Cycloaddition of Enamine and Iminium Ion Intermediates Formed in the Reaction of N-Arylpyrrolidines with T-HYDRO. Synth. Lett. 2015, 26, 2231. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.-Y.; Yu, R.-M.; Li, J.-P.; Yang, D.-F.; Pang, Q.; Li, H.-L. The Improved para-Selective C(sp2)-H Borylation of Anisole Derivatives Enabled by Bulky Lewis Acid. Catalysts 2023, 13, 1193. https://doi.org/10.3390/catal13081193
Li D-Y, Yu R-M, Li J-P, Yang D-F, Pang Q, Li H-L. The Improved para-Selective C(sp2)-H Borylation of Anisole Derivatives Enabled by Bulky Lewis Acid. Catalysts. 2023; 13(8):1193. https://doi.org/10.3390/catal13081193
Chicago/Turabian StyleLi, Dai-Yu, Rui-Mu Yu, Jin-Ping Li, Deng-Feng Yang, Qi Pang, and Hong-Liang Li. 2023. "The Improved para-Selective C(sp2)-H Borylation of Anisole Derivatives Enabled by Bulky Lewis Acid" Catalysts 13, no. 8: 1193. https://doi.org/10.3390/catal13081193
APA StyleLi, D.-Y., Yu, R.-M., Li, J.-P., Yang, D.-F., Pang, Q., & Li, H.-L. (2023). The Improved para-Selective C(sp2)-H Borylation of Anisole Derivatives Enabled by Bulky Lewis Acid. Catalysts, 13(8), 1193. https://doi.org/10.3390/catal13081193





