Steam Treatment Promotion on the Performance of Pt/CeO2 Three-Way Catalysts for Emission Control of Natural Gas-Fueled Vehicles
Abstract
:1. Introduction
2. Results and Discussions
2.1. Catalyst Performance
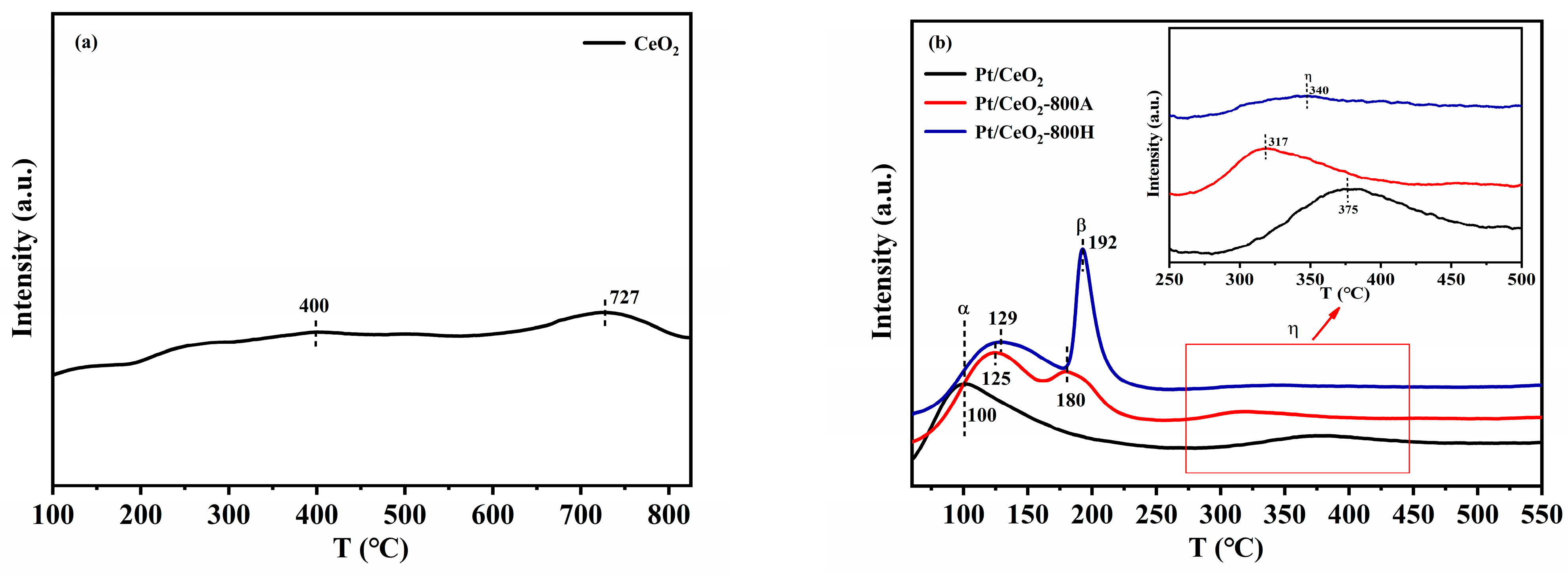
2.2. Catalyst Characterization
2.3. General Assessment
3. Conclusions
4. Experimental Section
4.1. Preparation of Catalysts
4.2. Catalyst Characterization
4.3. Catalytic Activity Evaluation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, H.; Liu, Z.; Zhao, F.; Li, W. Natural gas as vehicle fuel in China: A review. Renew. Sust. Energ. Rev. 2016, 62, 521–533. [Google Scholar] [CrossRef]
- Thiruvengadam, A.; Besch, M.; Padmanaban, V.; Pradhan, S.; Demirgok, B. Natural gas vehicles in heavy-duty transportation—A review. Energy Policy 2018, 122, 253–259. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Tang, X.; Shao, C.; You, Y.; Wang, L.; Zhan, W.; Guo, Y.; Zhao, Y.; Guo, Y. Insight into the roles of Pd state and CeO2 property in C3H8 catalytic oxidation on Pd/CeO2. Appl. Surf. Sci. 2022, 605, 154675. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, J.; Deng, J.; Jiang, X.; Jiao, Y.; Chen, Y. Synthesis of high stability nanosized Rh/CeO2-ZrO2 three-way automotive catalysts by Rh chemical state regulation. J. Energy Inst. 2020, 93, 2325–2333. [Google Scholar] [CrossRef]
- Rood, S.; Eslava, S.; Manigrasso, A.; Bannister, C. Recent advances in gasoline three-way catalyst formulation: A review. Int. J. Automot. Eng. 2019, 234, 936–949. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, G.; Ting, K.W.; Maeno, Z.; Oshima, K.; Satokawa, S.; Nagaoka, S.; Shimizu, K.-I.; Toyao, T. Roles of the basic metals La, Ba, and Sr as additives in Al2O3-supported Pd-based three-way catalysts. J. Catal. 2021, 400, 387–396. [Google Scholar] [CrossRef]
- Huang, C.; Shan, W.; Lian, Z.; Zhang, Y.; He, H. Recent advances in three-way catalysts of natural gas vehicles. Catal. Sci. Technol. 2020, 10, 6407–6419. [Google Scholar] [CrossRef]
- Fan, J.; Chen, Y.; Jiang, X.; Yao, P.; Jiao, Y.; Wang, J.; Chen, Y. A simple and effective method to synthesize Pt/CeO2 three-way catalysts with high activity and hydrothermal stability. J. Environ. Chem. Eng. 2020, 8, 104236. [Google Scholar] [CrossRef]
- Fan, J.; Chen, L.; Li, S.; Mou, J.; Zeng, L.; Jiao, Y.; Wang, J.; Chen, Y. Insights into the promotional effect of alkaline earth metals in Pt-based three-way catalysts for NO reduction. J. Catal. 2023, 418, 90–99. [Google Scholar] [CrossRef]
- Vlachou, M.C.; Marchbank, H.R.; Brooke, E.; Kolpin, A. Challenges and opportunities for platinum in the modern three-way catalyst: Flexibility and performance in gasoline emissions control. Johnson Matthey Technol. Rev. 2023, 67, 219–229. [Google Scholar] [CrossRef]
- Kim, D.Y.; Bae, W.B.; Byun, S.W.; Kim, Y.J.; Yoon, D.Y.; Jung, C.; Kim, C.H.; Kang, D.; Hazlett, M.J.; Kang, S.B. Pt substitution in Pd/Rh three-way catalyst for improved emission control. Korean J. Chem. Eng. 2023, 40, 1606–1615. [Google Scholar] [CrossRef]
- Lee, J.; Young Kim, M.; Hong Jeon, J.; Lee, D.H.; Rao, K.N.; Oh, D.G.; Jeong Jang, E.; Kim, E.; Na, S.C.; Han, H.S.; et al. Effect of Pt pre-sintering on the durability of PtPd/Al2O3 catalysts for CH4 oxidation. Appl. Catal. B 2020, 260, 118098. [Google Scholar] [CrossRef]
- Carrillo, C.; Johns, T.R.; Xiong, H.; DeLaRiva, A.; Challa, S.R.; Goeke, R.S.; Artyushkova, K.; Li, W.; Kim, C.H.; Datye, A.K. Trapping of mobile Pt species by PdO nanoparticles under oxidizing conditions. J. Phys. Chem. Lett. 2014, 5, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; DeLaRiva, A.; Xiong, H.; Peterson, E.J.; Spilde, M.N.; Kunwar, D.; Goeke, R.S.; Wiebenga, M.; Oh, S.H.; Qi, G.; et al. Regenerative trapping: How Pd improves the durability of Pt diesel oxidation catalysts. Appl. Catal. B 2017, 218, 581–590. [Google Scholar] [CrossRef]
- Lan, L.; Huang, X.; Zhou, W.; Li, H.; Xiang, J.; Chen, S.; Chen, Y. Development of a thermally stable Pt catalyst by redispersion between CeO2 and Al2O3. RSC Adv. 2021, 11, 7015–7024. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Qiu, J.; Liang, Y.; Zhao, M.; Wang, J.; Chen, Y. New Insights into excellent catalytic performance of the Ce-modified catalyst for NO oxidation. Ind. Eng. Chem. Res. 2019, 58, 7876–7885. [Google Scholar] [CrossRef]
- Shinjoh, H.; Hatanaka, M.; Nagai, Y.; Tanabe, T.; Takahashi, N.; Yoshida, T.; Miyake, Y. Suppression of noble metal sintering based on the support anchoring effect and its application in automotive three-way catalysis. Top. Catal. 2009, 52, 1967–1971. [Google Scholar] [CrossRef]
- Kunwar, D.; Carrillo, C.; Xiong, H.; Peterson, E.; DeLaRiva, A.; Ghosh, A.; Qi, G.; Yang, M.; Wiebenga, M.; Oh, S.; et al. Investigating anomalous growth of platinum particles during accelerated aging of diesel oxidation catalysts. Appl. Catal. B. 2020, 266, 118598. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide-support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Kunwar, D.; Zhou, S.; DeLaRiva, A.; Peterson, E.J.; Xiong, H.; Pereira-Hernández, X.I.; Purdy, S.C.; ter Veen, R.; Brongersma, H.H.; Miller, J.T.; et al. Stabilizing high metal loadings of thermally stable platinum single atoms on an industrial catalyst support. ACS Catal. 2019, 9, 3978–3990. [Google Scholar] [CrossRef]
- Nie, L.; Mei, D.; Xiong, H.; Ren, Z.; Hernandez, X.I.P.; DeLaRiva, A.; Wang, M.; Engelhard, M.H.; Kovarik, L.; Datye, A.K.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Takahashi, N.; Tanabe, T.; Nagai, Y.; Dohmae, K.; Aoki, Y.; Yoshida, T.; Shinjoh, H. Ideal Pt loading for a Pt/CeO2-based catalyst stabilized by a Pt-O-Ce bond. Appl. Catal. B 2010, 99, 336–342. [Google Scholar] [CrossRef]
- Salaün, M.; Kouakou, A.; Da Costa, S.; Da Costa, P. Synthetic gas bench study of a natural gas vehicle commercial catalyst in monolithic form: On the effect of gas composition. Appl. Catal. B 2009, 88, 386–397. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, J.; Wu, Y.; Hu, W.; Qu, P.; Xiao, X.; Zhang, G.; Liu, X.; Jiao, Y.; Zhong, L.; et al. Particle size effects in stoichiometric methane combustion: Structure-activity relationship of Pd catalyst supported on gamma-alumina. ACS Catal. 2020, 10, 10339–10349. [Google Scholar] [CrossRef]
- Amirante, R.; Distaso, E.; Di Iorio, S.; Sementa, P.; Tamburrano, P.; Vaglieco, B.M.; Reitz, R.D. Effects of natural gas composition on performance and regulated, greenhouse gas and particulate emissions in spark-ignition engines. Energy Convers. Manag. 2017, 143, 338–347. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Y.; Hu, W.; Qu, P.; Zhang, G.; Granger, P.; Zhong, L.; Chen, Y. New insights into the role of Pd-Ce interface for methane activation on monolithic supported Pd catalysts: A step forward the development of novel PGM three-way catalysts for natural gas fueled engines. Appl. Catal. B 2020, 264, 118475. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.N.T.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Zhang, F.; Chan, S.-W.; Spanier, J.E.; Apak, E.; Jin, Q.; Robinson, R.D.; Herman, I.P. Cerium oxide nanoparticles: Size-selective formation and structure analysis. Appl. Phys. Lett. 2002, 80, 127–129. [Google Scholar] [CrossRef]
- Zhou, X.D.; Huebner, W. Size-induced lattice relaxation in CeO2 nanoparticles. Appl. Phys. Lett. 2001, 79, 3512–3514. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Y.; Hu, W.; Qu, P.; Zhang, G.; Jiao, Y.; Zhong, L.; Chen, Y. Evolution of Pd species for the conversion of methane under operation conditions. Ind. Eng. Chem. Res. 2019, 58, 6255–6265. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Y.; Hu, W.; Qu, P.; Liu, X.; Yuan, R.; Zhong, L.; Chen, Y. Insights into the role of Pt on Pd catalyst stabilized by magnesia-alumina spinel on gama-alumina for lean methane combustion: Enhancement of hydrothermal stability. Mol. Catal. 2020, 496, 111185. [Google Scholar] [CrossRef]
- Holmgren, A.; Andersson, B.; Duprez, D. Interactions of CO with Pt/ceria catalysts. Appl. Catal. B 1999, 22, 215–230. [Google Scholar] [CrossRef]
- Chen, J.; Hu, W.; Huang, F.; Li, G.; Yuan, S.; Gong, M.; Zhong, L.; Chen, Y. Catalytic performance of a Pt-Rh/CeO2-ZrO2-La2O3-Nd2O3 three-way compress nature gas catalyst prepared by a modified double-solvent method. J. Rare Earths 2017, 35, 857–866. [Google Scholar] [CrossRef]
- Boaro, M.; Vicario, M.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. The use of temperature-programmed and dynamic/transient methods in catalysis: Characterization of ceria-based, model three-way catalysts. Catal. Today 2003, 77, 407–417. [Google Scholar] [CrossRef]
- Gatica, J.M.; Gómez, D.M.; Hernández-Garrido, J.C.; Calvino, J.J.; Cifredo, G.A.; Vidal, H. Experimental evidences of the relationship between reducibility and micro- and nanostructure in commercial high surface area ceria. Appl. Catal A-Gen. 2014, 479, 35–44. [Google Scholar] [CrossRef]
- Lee, J.; Tieu, P.; Finzel, J.; Zang, W.; Yan, X.; Graham, G.; Pan, X.; Christopher, P. How Pt Influences H2 Reactions on High Surface-Area Pt/CeO2 Powder Catalyst Surfaces. JACS Au 2023, 3, 2299–2313. [Google Scholar] [CrossRef]
- Wang, G.; Jing, Y.; Ting, K.W.; Maeno, Z.; Zhang, X.; Nagaoka, S.; Shimizu, K.-I.; Toyao, T. Effect of oxygen storage materials on the performance of Pt-based three-way catalysts. Catal. Sci. Technol. 2022, 12, 3534–3548. [Google Scholar] [CrossRef]
- Lan, L.; Chen, S.; Li, H.; Liu, D.; Li, D.; Wang, J.; Chen, Y. Optimally designed synthesis of advanced Pd-Rh bimetallic three-way catalyst. Can. J. Chem. Eng. 2019, 97, 2516–2526. [Google Scholar] [CrossRef]
- Pereira-Hernandez, X.I.; DeLaRiva, A.; Muravev, V.; Kunwar, D.; Xiong, H.; Sudduth, B.; Engelhard, M.; Kovarik, L.; Hensen, E.J.M.; Wang, Y.; et al. Tuning Pt-CeO2 interactions by high-temperature vapor-phase synthesis for improved reducibility of lattice oxygen. Nat. Commun. 2019, 10, 1358. [Google Scholar] [CrossRef]
- Anandan, C.; Bera, P. Growth, characterization and interfacial reaction of magnetron sputtered Pt/CeO2 thin films on Si and Si3N4 substrates. Surf. Interface Anal. 2015, 47, 777–784. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Chen, Y.; Jiao, Y.; Jiang, X.; Fan, J. Effect of Different Alkali-assisted Deposition Precipitation Methods on the Durability of Three-way Catalysts. J. Inorg. Mater. 2021, 36, 659–664. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Zhao, Y.; Deng, J.; Wang, J.; Chen, Y. Correlation between the morphology of NH4Al(OH)2CO3 and the properties of CeO2–ZrO2/Al2O3 material. Mater. Chem. Phys. 2021, 266, 124552. [Google Scholar] [CrossRef]
- Maurer, F.; Jelic, J.; Wang, J.; Gänzler, A.; Dolcet, P.; Wöll, C.; Wang, Y.; Studt, F.; Casapu, M.; Grunwaldt, J.-D. Tracking the formation, fate and consequence for catalytic activity of Pt single sites on CeO2. Nat. Catal. 2020, 3, 824–833. [Google Scholar] [CrossRef]
- Sun, M.; Hu, W.; Yuan, S.; Zhang, H.; Cheng, T.; Wang, J.; Chen, Y. Effect of the loading sequence of CeO2 and Pd over Al2O3 on the catalytic performance of Pd-only close-coupled catalysts. Mol. Catal. 2020, 482, 100332. [Google Scholar] [CrossRef]
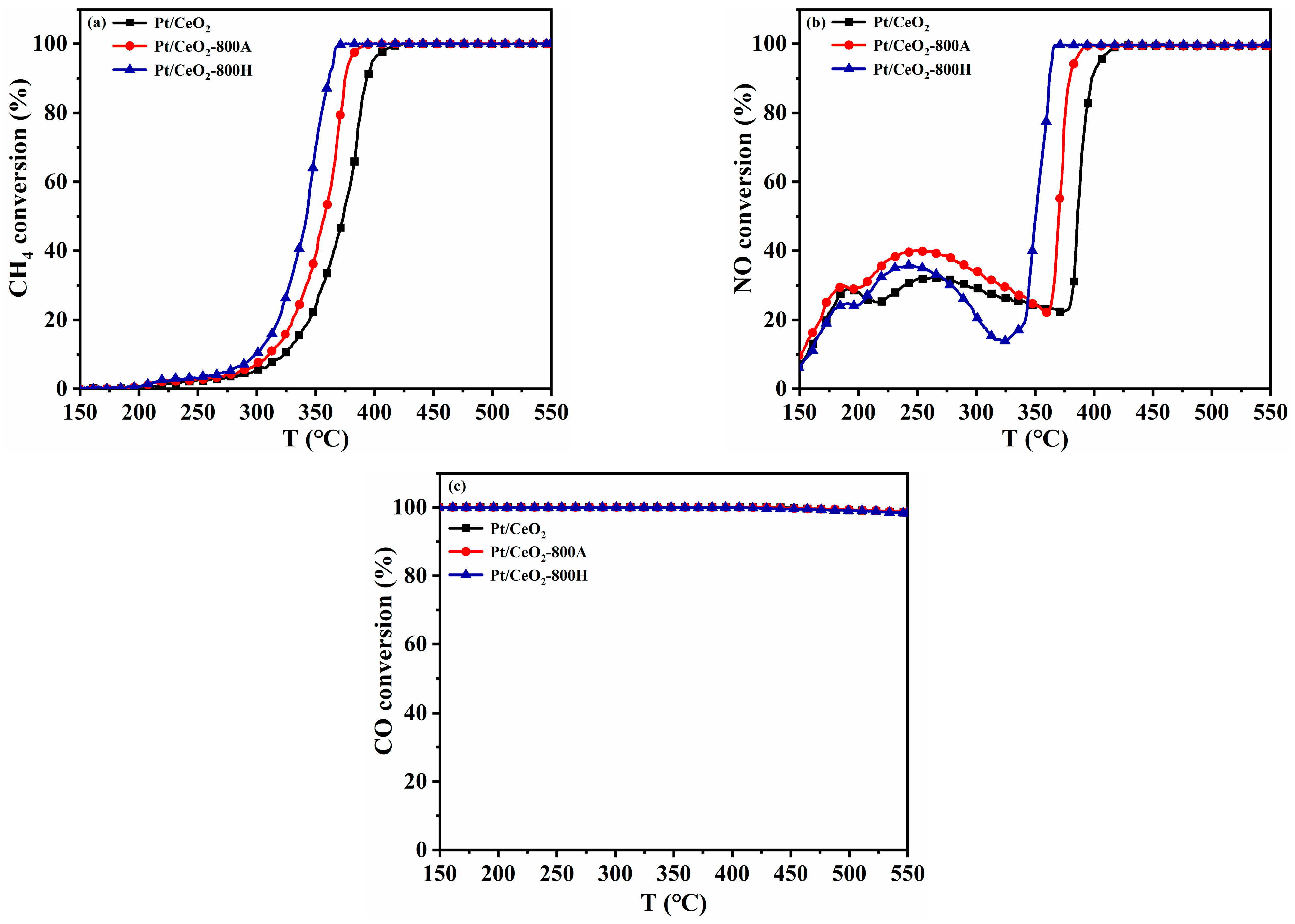
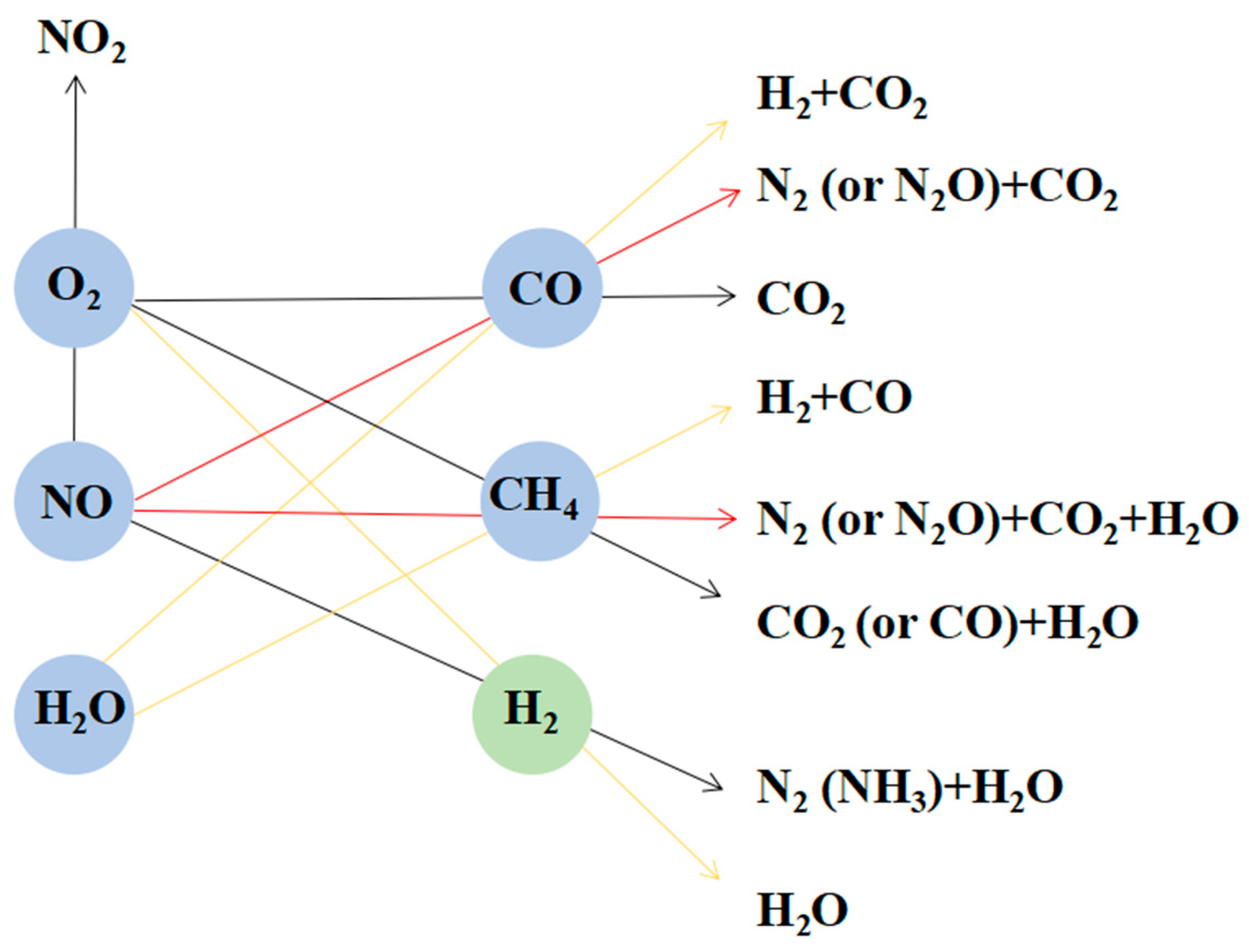
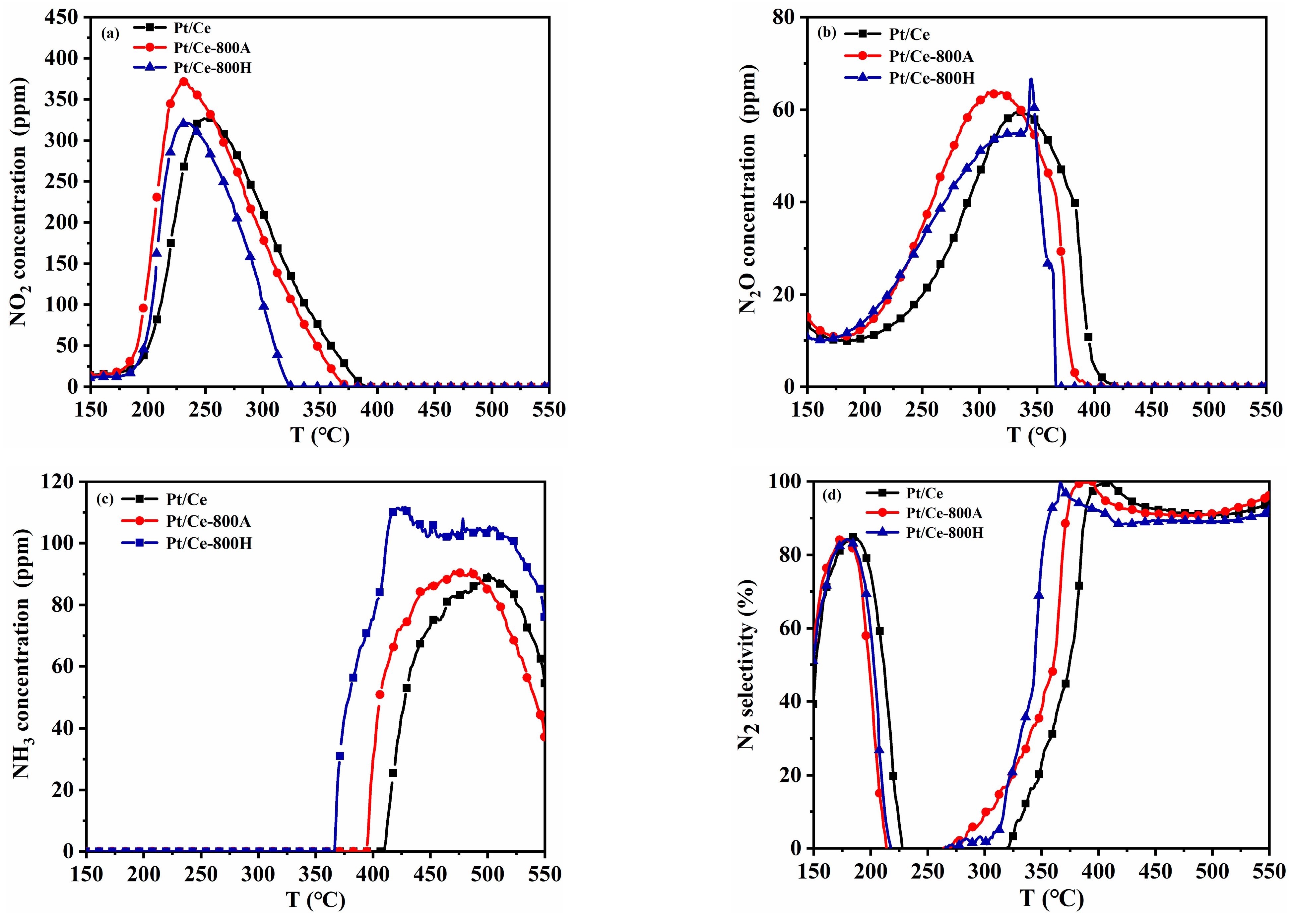


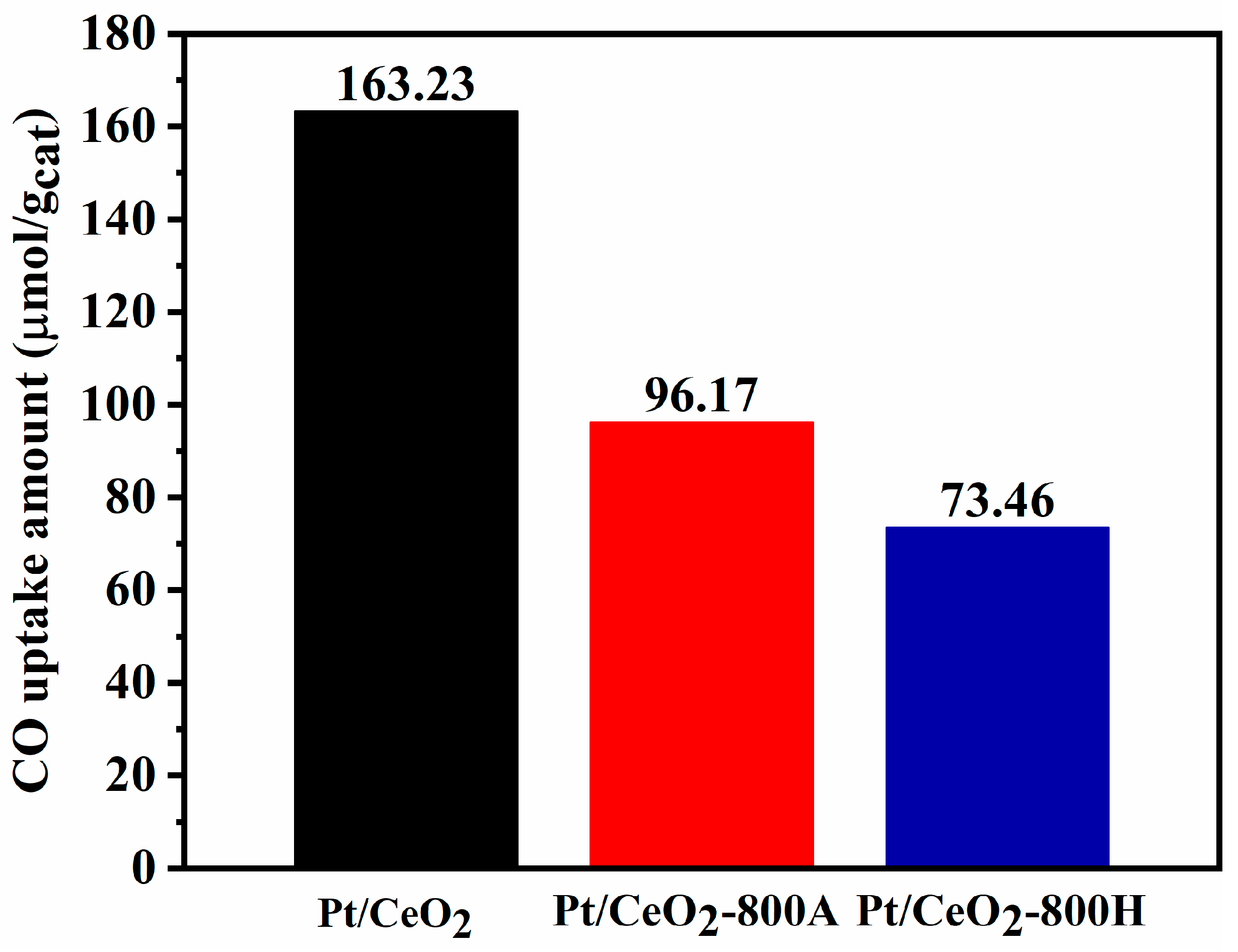

| Samples | T50 (°C) | T90 (°C) | ||||
|---|---|---|---|---|---|---|
| CH4 | NO | CO | CH4 | NO | CO | |
| Pt/CeO2 | 373 | 386 | <150 | 393 | 397 | <150 |
| Pt/CeO2-800A | 358 | 370 | <150 | 375 | 379 | <150 |
| Pt/CeO2-800H | 342 | 350 | <150 | 361 | 362 | <150 |
| Sample | Pt Content a (wt%) | SBET b (m2/g) | CeO2 Crystallite Size c (nm) | CO ad. d (μmol/gcat) |
|---|---|---|---|---|
| CeO2 | - | 51 | 7.0 | - |
| Pt/CeO2 | 2.11 | 44 | 7.2 | 163.23 |
| Pt/CeO2-800A | 2.00 | 41 | 11.1 | 96.17 |
| Pt/CeO2-800H | 2.03 | 45 | 10.8 | 73.46 |
| Sample | Relative Surface Composition (at.%) | Pt2+/Pt (%) | Pt4+/Pt (%) | Ce3+/Ce (%) | Ce4+/Ce (%) | |
|---|---|---|---|---|---|---|
| Pt | Ce | |||||
| Pt/CeO2 | 4.47 | 95.53 | 90.66 | 9.34 | 27.04 | 72.96 |
| Pt/CeO2-800A | 4.26 | 95.74 | 93.55 | 6.45 | 30.79 | 69.21 |
| Pt/CeO2-800H | 4.31 | 95.69 | 93.34 | 6.66 | 35.35 | 64.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Shao, Y.; Ren, X.; Dong, A.; Li, K.; Zhou, B.; Yang, C.; Liu, Y.; Li, Z. Steam Treatment Promotion on the Performance of Pt/CeO2 Three-Way Catalysts for Emission Control of Natural Gas-Fueled Vehicles. Catalysts 2024, 14, 17. https://doi.org/10.3390/catal14010017
Liu X, Shao Y, Ren X, Dong A, Li K, Zhou B, Yang C, Liu Y, Li Z. Steam Treatment Promotion on the Performance of Pt/CeO2 Three-Way Catalysts for Emission Control of Natural Gas-Fueled Vehicles. Catalysts. 2024; 14(1):17. https://doi.org/10.3390/catal14010017
Chicago/Turabian StyleLiu, Xi, Yuankai Shao, Xiaoning Ren, Anqi Dong, Kaixiang Li, Bingjie Zhou, Chunqing Yang, Yatao Liu, and Zhenguo Li. 2024. "Steam Treatment Promotion on the Performance of Pt/CeO2 Three-Way Catalysts for Emission Control of Natural Gas-Fueled Vehicles" Catalysts 14, no. 1: 17. https://doi.org/10.3390/catal14010017





