Solid Acid–Base Catalysts Based on Layered Double Hydroxides Applied for Green Catalytic Transformations
Abstract
:1. Introduction
2. Chemical Composition, Preparation, and Acid–Base Properties of LDHs
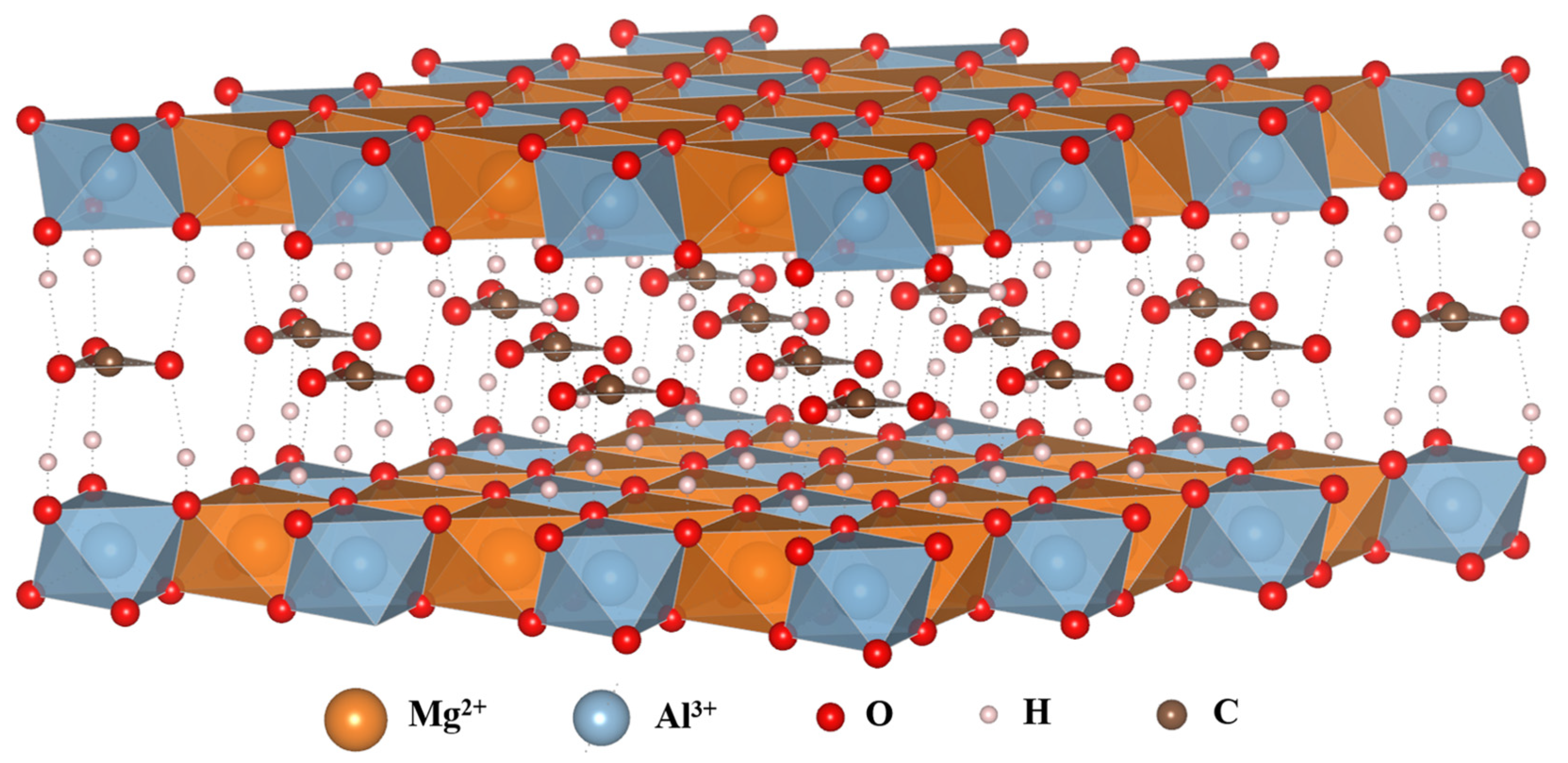
2.1. Chemical Composition
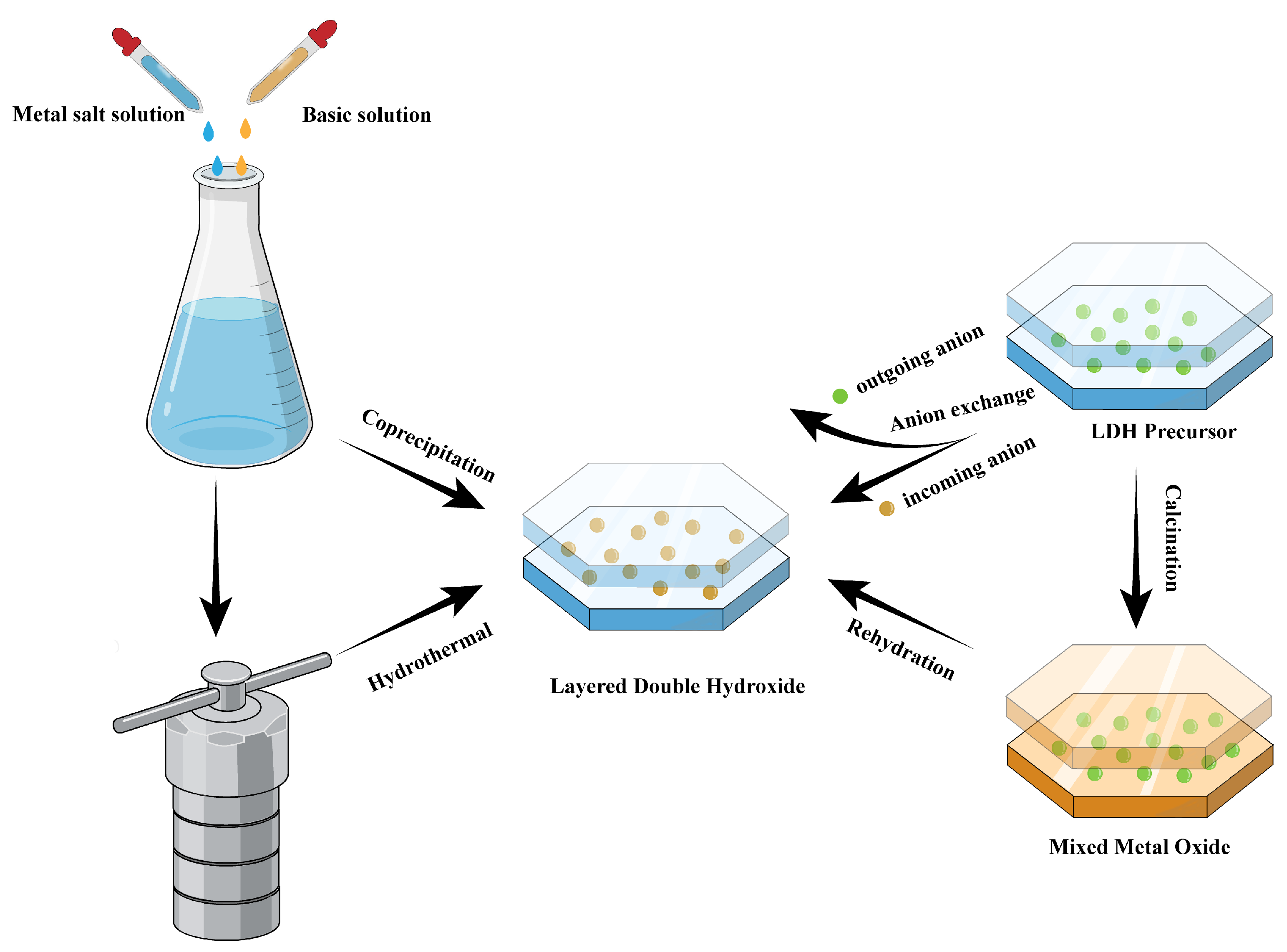
2.2. Preparation Methods
2.3. Acid–Base Properties
3. Application of LDH-Based Catalysts in the Green Acid–Base Catalytic Transformation
3.1. LDH Catalysts
3.1.1. Original LDH Catalysts
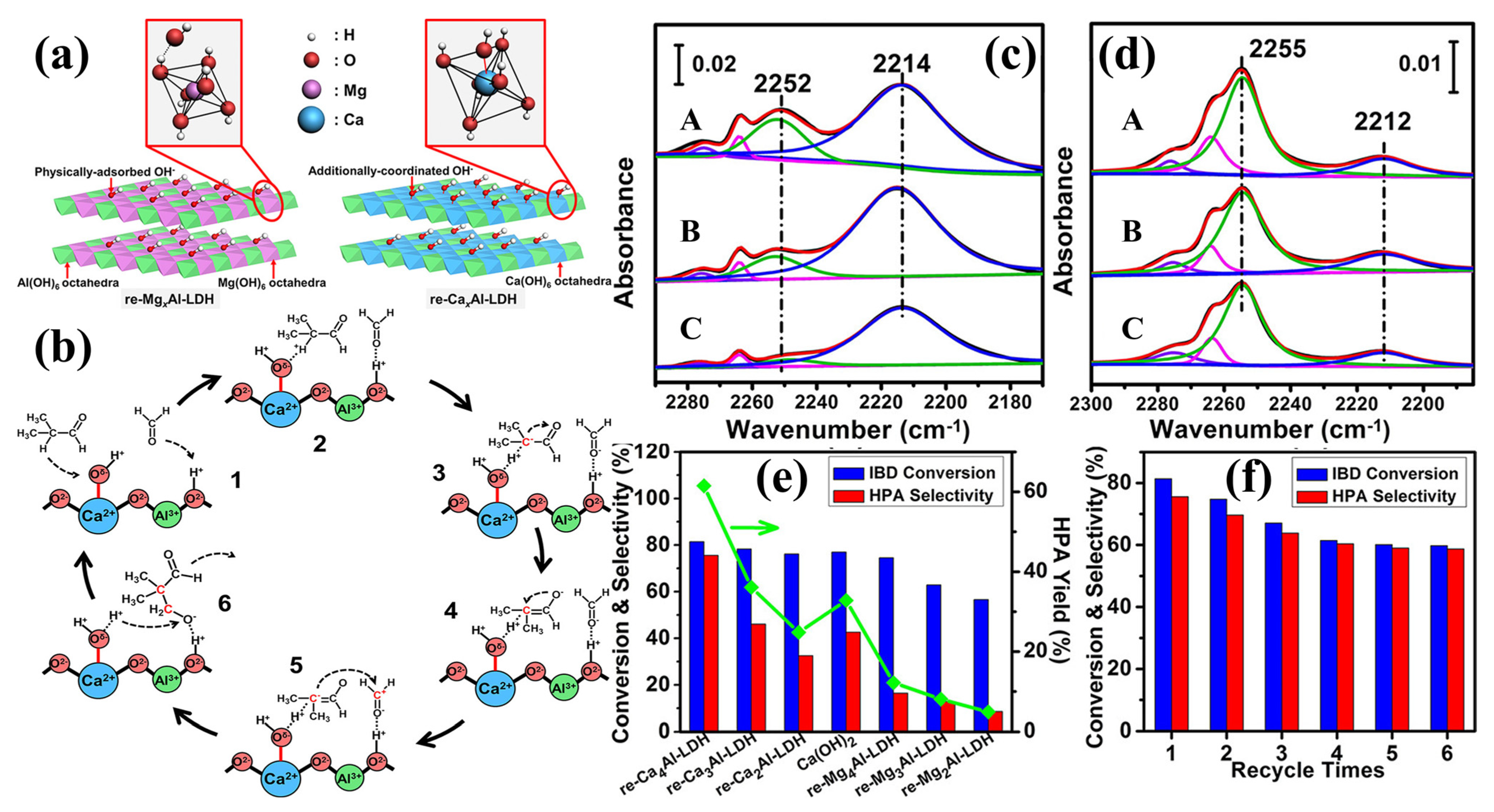
3.1.2. Rehydrated LDH Catalysts
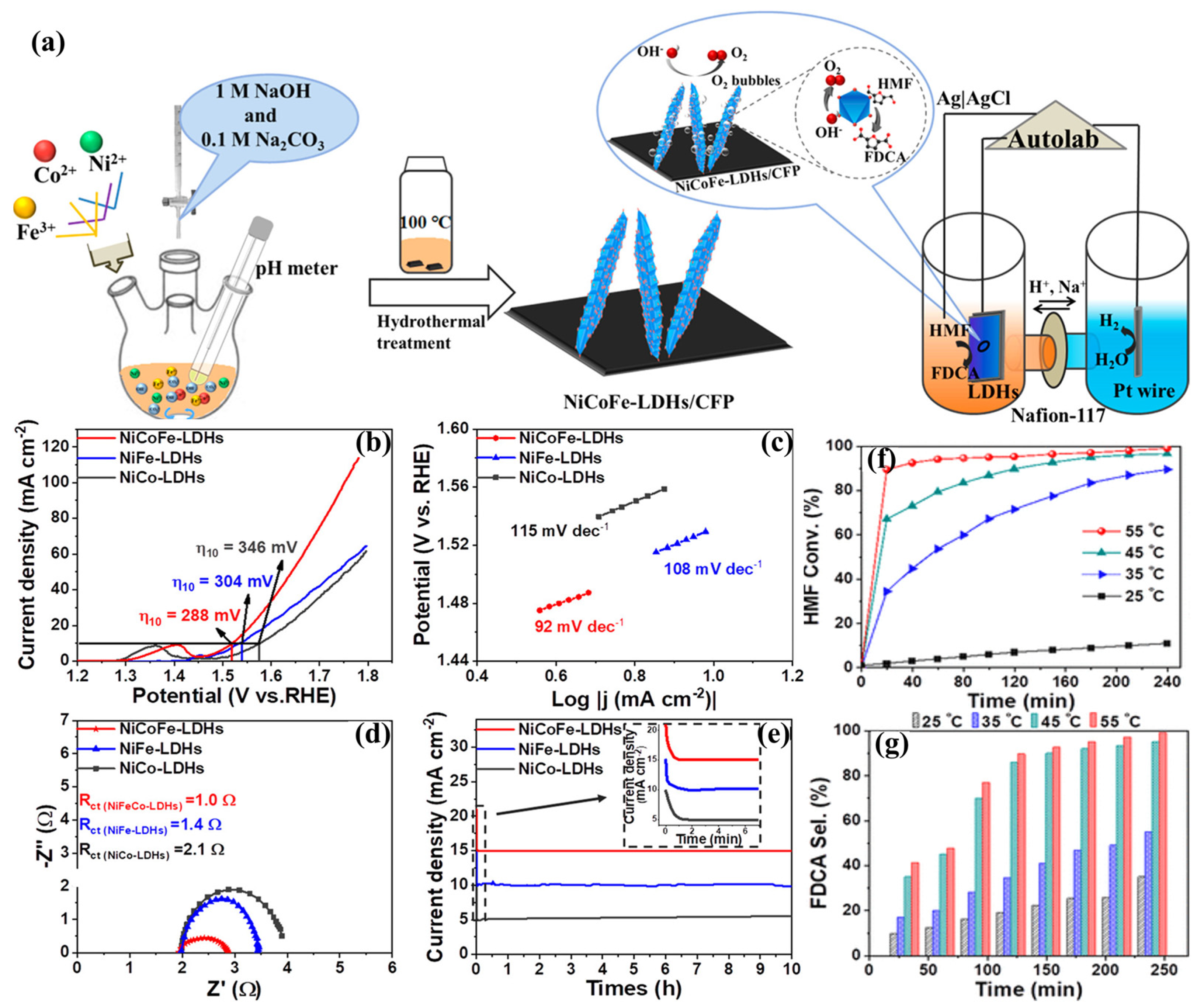
3.2. LDH-Based Metal Nanocatalysts
3.3. LDH-Based Mixed Metal Oxide Catalysts
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Su, F.; Guo, Y.H. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Liu, F.J.; Zheng, A.M.; Noshadi, I.; Xiao, F.S. Design and synthesis of hydrophobic and stable mesoporous polymeric solid acid with ultra strong acid strength and excellent catalytic activities for biomass transformation. Appl. Catal. B Environ. 2013, 136, 193–201. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Alinezhad, H. Brønsted acidic ionic liquids: Green catalysts for essential organic reactions. J. Mol. Liq. 2016, 218, 95–105. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Fattahi, A. A study on the catalytic activity and theoretical modeling of a novel dual acidic mesoporous silica. RSC Adv. 2014, 4, 16647–16654. [Google Scholar] [CrossRef]
- Manríquez-Ramírez, M.; Gómez, R.; Hernández-Cortez, J.G.; Zúñiga-Moreno, A.; Reza-San Germán, C.M.; Flores-Valle, S.O. Advances in the transesterification of triglycerides to biodiesel using MgO–NaOH, MgO–KOH and MgO–CeO2 as solid basic catalysts. Catal. Today 2013, 212, 23–30. [Google Scholar] [CrossRef]
- Suwannakarn, K.; Lotero, E.; Goodwin, J.G. Solid bronsted acid catalysis in the gas-phase esterification of acetic acid. Ind. Eng. Chem. Res. 2007, 46, 7050–7056. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.; Dai, W.-L. Recent advances in silver-based heterogeneous catalysts for green chemistry processes. Appl. Catal. B Environ. 2014, 160–161, 730–741. [Google Scholar] [CrossRef]
- Patial, S.; Raizada, P.; Aslam Parwaz Khan, A.; Singh, A.; Van Le, Q.; Huy Nguyen, V.; Selvasembian, R.; Mustansar Hussain, C.; Singh, P. Emerging new-generation covalent organic frameworks composites as green catalysts: Design, synthesis and solar to fuel production. Chem. Eng. J. 2022, 433, 134594. [Google Scholar] [CrossRef]
- Liu, K.-G.; Sharifzadeh, Z.; Rouhani, F.; Ghorbanloo, M.; Morsali, A. Metal-organic framework composites as green/sustainable catalysts. Coord. Chem. Rev. 2021, 436, 213827. [Google Scholar] [CrossRef]
- Yadav, G.; Yadav, N.; Ahmaruzzaman, M. Microwave-assisted synthesis of biodiesel by a green carbon-based heterogeneous catalyst derived from areca nut husk by one-pot hydrothermal carbonization. Sci. Rep. 2022, 12, 21455. [Google Scholar] [CrossRef]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Synthesis and acid catalysis of cellulose-derived carbon-based solid acid. Solid State Sci. 2010, 12, 1029–1034. [Google Scholar] [CrossRef]
- Elmekawy, A.A.; Shiju, N.R.; Rothenberg, G.; Brown, D.R. Environmentally Benign Bifunctional Solid Acid and Base Catalysts. Ind. Eng. Chem. Res. 2014, 53, 18722–18728. [Google Scholar] [CrossRef]
- Yan, Q.; Hou, X.; Liu, G.; Li, Y.; Zhu, T.; Xin, Y.; Wang, Q. Recent advances in layered double hydroxides (LDHs) derived catalysts for selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2020, 400, 123260. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-L.; Liang, S.; Mo, L.-Z.; Su, H.-H.; Huang, J.-S.; Zhang, P.-J.; Qin, J.-N. Conversion of biomass-derived monosaccharide into furfural over Cr–Mg-LDO@bagasse catalysts. Sustain. Chem. Pharm. 2023, 32, 101013. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, K.; Li, B.; Shim, H.; Huang, Y. Key Considerations on the Industrial Application of Lignocellulosic Biomass Pyrolysis toward Carbon Neutrality. Engineering 2023, 29, 35–38. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, S.; Ke, L.; Wu, Q.; Zhang, Q.; Cui, X.; Dai, A.; Xu, C.; Cobb, K.; Liu, Y.; et al. Research progress on pyrolysis of nitrogen-containing biomass for fuels, materials, and chemicals production. Sci. Total Environ. 2023, 872, 162214. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M.; Khan, N. Recent Developments in Titanium Carbide (Ti3C2)-Based Layered Double Hydroxide (LDH) Nanocomposites for Energy Storage and Conversion Applications: A Minireview and Perspectives. Energy Fuels 2022, 36, 9821–9843. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Li, C.; Zhang, C. Recent Progress of Hydrogenation and Hydrogenolysis Catalysts Derived from Layered Double Hydroxides. Catalysts 2022, 12, 1484. [Google Scholar] [CrossRef]
- Zhu, J.; Li, T.; Wang, S.; Chen, Y.; Ge, F.; Xu, Y. Lattice-distortion active sites of Ni-doped CuMgFe LDH for benzotraizole degradation. J. Environ. Chem. Eng. 2022, 10, 107903. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Panda, H.S.; Srivastava, R.; Bahadur, D. Synthesis and in situ mechanism of nuclei growth of layered double hydroxides. Bull. Mater. Sci. 2011, 34, 1599–1604. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Mertens, M.; Witte, K.; Tendeloo, G.; Cool, P.; Vansant, E.F. Zn–Al layered double hydroxides: Synthesis, characterization and photocatalytic application. Microporous Mesoporous Mater. 2008, 113, 296–304. [Google Scholar] [CrossRef]
- Morel-Desrosiers, N.; Pisson, J.; Israëli, Y.; Taviot-Guého, C.; Besse, J.-P.; Morel, J.-P. Intercalation of dicarboxylate anions into a Zn–Al–Cl layered double hydroxide: Microcalorimetric determination of the enthalpies of anion exchange. J. Mater. Chem. 2003, 13, 2582–2585. [Google Scholar] [CrossRef]
- Zou, W.; Guo, W.; Liu, X.; Luo, Y.; Ye, Q.; Xu, X.; Wang, F. Anion Exchange of Ni-Co Layered Double Hydroxide (LDH) Nanoarrays for a High-Capacitance Supercapacitor Electrode: A Comparison of Alkali Anion Exchange and Sulfuration. Chem. Eur. J 2018, 24, 19309–19316. [Google Scholar] [CrossRef]
- Das, J.; Parida, K.M. Heteropoly acid intercalated Zn/Al HTlc as efficient catalyst for esterification of acetic acid using n-butanol. J. Mol. Catal. A Chem. 2007, 264, 248–254. [Google Scholar] [CrossRef]
- Wei, Y.; Li, F.; Liu, L. Liquid exfoliation of Zn–Al layered double hydroxide using NaOH/urea aqueous solution at low temperature. RSC Adv. 2014, 4, 18044–18051. [Google Scholar] [CrossRef]
- Liu, H.; Yu, T.; Su, D.; Tang, Z.; Zhang, J.; Liu, Y.; Yuan, A.; Kong, Q. Ultrathin Ni-Al layered double hydroxide nanosheets with enhanced supercapacitor performance. Ceram. Int. 2017, 43, 14395–14400. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, X. Carbon fiber/Ni-Co layered double hydroxide@NiMoO4/graphene oxide sandwich structure flexible electrode materials: Facile synthesis and high supercapacitor performance. J. Alloys Compd. 2019, 794, 13–20. [Google Scholar] [CrossRef]
- Erickson, K.L.; Bostrom, T.E.; Frost, R.L. A study of structural memory effects in synthetic hydrotalcites using environmental SEM. Mater. Lett. 2005, 59, 226–229. [Google Scholar] [CrossRef]
- Gao, Z.; Sasaki, K.; Qiu, X. Structural Memory Effect of Mg–Al and Zn–Al layered Double Hydroxides in the Presence of Different Natural Humic Acids: Process and Mechanism. Langmuir 2018, 34, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Z.; Shi, E.W.; Li, W.J.; Zheng, Y.Q.; Wu, N.C.; Zhong, W.Z. Particle size comparison of hydrothermally synthesized cobalt and zinc aluminate spinels. J. Am. Ceram. Soc. 2002, 85, 2949–2955. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Jia, X.D.; Chen, G.B.; Shang, L.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T.R. Ultrafine NiO Nanosheets Stabilized by TiO2 from Monolayer NiTi-LDH Precursors: An Active Water Oxidation Electrocatalyst. J. Am. Chem. Soc. 2016, 138, 6517–6524. [Google Scholar] [CrossRef]
- Dubnova, L.; Danhel, R.; Meinhardova, V.; Korolova, V.; Smolakova, L.; Kondratowicz, T.; Kikhtyanin, O.; Capek, L. Reconstruction of the ZnAl Mixed Oxides Into the Layered Double Hydroxide Catalysts Active in the Aldol Condensation of Furfural: The Role of ZnO Particles. Front. Chem. 2021, 9, 803764. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, M.N.; Kapustin, A.E.; Panchenko, V.N.; Butenko, E.O.; Krupskaya, V.V.; Gil, A.; Vicente, M.A. Synthetic and natural materials with the brucite-like layers as high active catalyst for synthesis of 1-methoxy-2-propanol from methanol and propylene oxide. J. Mol. Catal. A Chem. 2016, 423, 22–30. [Google Scholar] [CrossRef]
- Terzi, C.M.; dos Santos, E.H.; Carvalho, C.; Prevot, V.; Wypych, F.; Forano, C.; Nakagaki, S. MgAl and ZnAl layered double hydroxides modified with molybdate and tungstate anions as catalysts for oxidation of cyclohexane. Catal. Today 2023, 422, 114221. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, G.; Guo, Y.; Guo, Y.; Gong, X.-Q. Effect of One-Pot Rehydration Process on Surface Basicity and Catalytic Activity of MgyAl1-aREEaOx Catalyst for Aldol Condensation of Citral and Acetone. ACS Sustain. Chem. Eng. 2016, 4, 1591–1601. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, W.; Liang, J.; Shen, J.; Fu, X.; He, H.; Yan, S.; Ren, X. Enhanced catalytic phenol hydroxylation by CuZnFeAl layered double hydroxides: Synergistic effects of Cu+ and oxygen vacancies. New J. Chem. 2021, 45, 179–188. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Z.; Liu, Y.; Zhang, M.; Wang, Y.; Wan, Y.; Yan, K. Efficient electrooxidation of biomass-derived aldehydes over ultrathin NiV-layered double hydroxides films. J. Energy Chem. 2023, 78, 412–421. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Liu, B.; Chen, Z.; Xu, H.; Yan, K. Trimetallic NiCoFe-Layered Double Hydroxides Nanosheets Efficient for Oxygen Evolution and Highly Selective Oxidation of Biomass-Derived 5-Hydroxymethylfurfural. ACS Catal. 2020, 10, 5179–5189. [Google Scholar] [CrossRef]
- Wang, T.; Hu, A.; Wang, H.; Xia, Y. Catalytic transfer hydrogenation of furfural into furfuryl alcohol over Ni–Fe-layered double hydroxide catalysts. J. Chin. Chem. Soc. 2019, 66, 1610–1618. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Chen, Y.; Liu, W.; Feng, H.; Wang, B.; Zhang, X.; Wei, M. The selective hydrogenation of furfural over intermetallic compounds with outstanding catalytic performance. Green Chem. 2019, 21, 5352–5362. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Ma, X.; An, Z.; Guo, S.; Shu, X.; Song, H.; Xiang, X.; He, J. A gradient reduction strategy to produce defects-rich nano-twin Cu particles for targeting activation of carbon-carbon or carbon-oxygen in furfural conversion. J. Catal. 2020, 389, 78–86. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Z.; Feng, J.; Fornasiero, P.; He, Y.; Li, D. Insight into the Effect of Dual Active Cu0/Cu+ Sites in a Cu/ZnO–Al2O3 Catalyst on 5-Hydroxylmethylfurfural Hydrodeoxygenation. ACS Sustain. Chem. Eng. 2020, 8, 15288–15298. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, J.; Zheng, L.; Wang, B.; Bi, R.; He, Y.; Liu, H.; Li, D. Interfacial Structure-Determined Reaction Pathway and Selectivity for 5-(Hydroxymethyl)furfural Hydrogenation over Cu-Based Catalysts. ACS Catal. 2019, 10, 1353–1365. [Google Scholar] [CrossRef]
- An, Z.; Wang, W.; Dong, S.; He, J. Well-distributed cobalt-based catalysts derived from layered double hydroxides for efficient selective hydrogenation of 5-hydroxymethyfurfural to 2,5-methylfuran. Catal. Today 2019, 319, 128–138. [Google Scholar] [CrossRef]
- Ye, X.; Shi, X.; Xu, H.; Feng, Y.; Jin, B.; Duan, P. Enhanced catalytic activity of layered double hydroxides via in-situ reconstruction for conversion of glucose/food waste to methyl lactate in biorefinery. Sci. Total Environ. 2022, 829, 154540. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Hanif, A.; Tsang, D.C.W.; Shang, J.; Su, Z.; Song, H.; Ok, Y.S.; Poon, C.S. Tuneable functionalities in layered double hydroxide catalysts for thermochemical conversion of biomass-derived glucose to fructose. Chem. Eng. J. 2020, 383, 122914. [Google Scholar] [CrossRef]
- Kwon, D.; Kang, J.Y.; An, S.; Yang, I.; Jung, J.C. Tuning the base properties of Mg–Al hydrotalcite catalysts using their memory effect. J. Energy Chem. 2020, 46, 229–236. [Google Scholar] [CrossRef]
- Yabushita, M.; Shibayama, N.; Nakajima, K.; Fukuoka, A. Selective Glucose-to-Fructose Isomerization in Ethanol Catalyzed by Hydrotalcites. ACS Catal. 2019, 9, 2101–2109. [Google Scholar] [CrossRef]
- Bing, W.; Zheng, L.; He, S.; Rao, D.; Xu, M.; Zheng, L.; Wang, B.; Wang, Y.; Wei, M. Insights on Active Sites of CaAl-Hydrotalcite as a High-Performance Solid Base Catalyst toward Aldol Condensation. ACS Catal. 2017, 8, 656–664. [Google Scholar] [CrossRef]
- Bing, W.; Wang, H.; Zheng, L.; Rao, D.; Yang, Y.; Zheng, L.; Wang, B.; Wang, Y.; Wei, M. A CaMnAl-hydrotalcite solid basic catalyst toward the aldol condensation reaction with a comparable level to liquid alkali catalysts. Green Chem. 2018, 20, 3071–3080. [Google Scholar] [CrossRef]
- Cui, G.; Meng, X.; Zhang, X.; Wang, W.; Xu, S.; Ye, Y.; Tang, K.; Wang, W.; Zhu, J.; Wei, M.; et al. Low-temperature hydrogenation of dimethyl oxalate to ethylene glycol via ternary synergistic catalysis of Cu and acid−base sites. Appl. Catal. B Environ. 2019, 248, 394–404. [Google Scholar] [CrossRef]
- Pérez-Barrado, E.; Pujol, M.C.; Aguiló, M.; Llorca, J.; Cesteros, Y.; Díaz, F.; Pallarès, J.; Marsal, L.F.; Salagre, P. Influence of acid–base properties of calcined MgAl and CaAl layered double hydroxides on the catalytic glycerol etherification to short-chain polyglycerols. Chem. Eng. J. 2015, 264, 547–556. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, W.; Jia, X.; Chen, B.; An, S.; Xie, X.; Huang, L. Ca–Al layered double hydroxides-derived Ni-based catalysts for hydrogen production via auto-thermal reforming of acetic acid. Int. J. Hydrog. Energy 2019, 44, 20007–20016. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Li, C.; Chen, Z.; Wang, D.; Wang, D.; Ibrahim Sharif, M.; Li, J.; Yu, J.; Gao, S. The effect of acid-base synergistic catalysis on upgrading of volatiles from coal pyrolysis over Mg-Al composite oxides. Fuel 2022, 321, 124030. [Google Scholar] [CrossRef]
- Yan, T.; Bing, W.; Xu, M.; Li, Y.; Yang, Y.; Cui, G.; Yang, L.; Wei, M. Acid-base sites synergistic catalysis over Mg–Zr–Al mixed metal oxide toward synthesis of diethyl carbonate. RSC Adv. 2018, 8, 4695–4702. [Google Scholar] [CrossRef]
- Kuljiraseth, J.; Wangriya, A.; Malones, J.M.C.; Klysubun, W.; Jitkarnka, S. Synthesis and characterization of AMO LDH-derived mixed oxides with various Mg/Al ratios as acid–basic catalysts for esterification of benzoic acid with 2-ethylhexanol. Appl. Catal. B Environ. 2019, 243, 415–427. [Google Scholar] [CrossRef]
- Pithakratanayothin, S.; Tongsri, R.; Chaisuwan, T.; Wongkasemjit, S. Influences of M–Sn intermetallics (M = Ni, Cu) prepared by mechanical alloying on phenol hydroxylation. Catal. Sci. Technol. 2017, 7, 5413–5421. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Konnerth, H.; Yeh, J.-Y.; Prechtl, M.H.G.; Wen, C.-Y.; Wu, K.C.W. De novo synthesis of Cr-embedded MOF-199 and derived porous CuO/CuCr2O4 composites for enhanced phenol hydroxylation. Green Chem. 2019, 21, 1889–1894. [Google Scholar] [CrossRef]
- Yue, X.K.; Zhang, L.H.; Sun, L.X.; Gao, S.T.; Gao, W.; Cheng, X.; Shang, N.Z.; Gao, Y.J.; Wang, C. Highly efficient hydrodeoxygenation of lignin-derivatives over Ni-based catalyst. Appl. Catal. B Environ. 2021, 293, 120243. [Google Scholar] [CrossRef]
- Liu, B.Y.; Xu, S.J.; Zhang, M.; Li, X.; Decarolis, D.; Liu, Y.Q.; Wang, Y.C.; Gibson, E.K.; Catlow, C.R.A.; Yan, K. Electrochemical upgrading of biomass-derived 5-hydroxymethylfurfural and furfural over oxygen vacancy-rich NiCoMn-layered double hydroxides nanosheets. Green Chem. 2021, 23, 4034–4043. [Google Scholar] [CrossRef]
- Upare, P.P.; Chamas, A.; Lee, J.H.; Kim, J.C.; Kwak, S.K.; Hwang, Y.K.; Hwang, D.W. Highly Efficient Hydrotalcite/1-Butanol Catalytic System for the Production of the High-Yield Fructose Crystal from Glucose. ACS Catal. 2019, 10, 1388–1396. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic Isomerization of Biomass-Derived Aldoses: A Review. ChemSusChem 2016, 9, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Huang, H.W.; Zhao, Q.; Ma, T.Y.; Wang, L.Z. Thin-Layered Photocatalysts. Adv. Funct. Mater. 2020, 30, 1910005. [Google Scholar] [CrossRef]
- Kong, W.F.; Xing, Z.P.; Fang, B.; Cui, Y.Q.; Li, Z.Z.; Zhou, W. Plasmon Ag/Na-doped defective graphite carbon nitride/NiFe layered double hydroxides Z-scheme heterojunctions toward optimized photothermal-photocatalytic-Fenton performance. Appl. Catal. B Environ. 2022, 304, 120969. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Y.Y.; Chen, D.D.; Li, J.; Wu, H.G.; Meng, Y.; Huang, J.S.; Yuan, J.F.; Su, Y.Q.; Wang, J.Q.; et al. Relay photo/thermal catalysis enables efficient cascade upgrading of sugars to lactic acid: Mechanism study and life cycle assessment. Chem. Eng. J. 2023, 452, 139687. [Google Scholar] [CrossRef]
- Wang, J.Q.; Jiang, J.Q.; Li, Z.H. Efficient one-pot syntheses of secondary amines from nitro aromatics and benzyl alcohols over Pd/NiTi-LDH under visible light. Dalton Trans. 2023, 52, 16935–16942. [Google Scholar] [CrossRef]
- Liu, W.; Sun, J.; Zhang, X.; Wei, M. Supported Ag Catalysts on Mg–Al Oxides toward Oxidant-Free Dehydrogenation Reaction of Benzyl Alcohol. Ind. Eng. Chem. Res. 2018, 57, 15606–15612. [Google Scholar] [CrossRef]
- Valente, J.S.; Pfeiffer, H.; Lima, E.; Prince, J.; Flores, J. Cyanoethylation of alcohols by activated Mg–Al layered double hydroxides: Influence of rehydration conditions and Mg/Al molar ratio on Brönsted basicity. J. Catal. 2011, 279, 196–204. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Tišler, Z.; Velvarská, R.; Kubička, D. Reconstructed Mg–Al hydrotalcites prepared by using different rehydration and drying time: Physico-chemical properties and catalytic performance in aldol condensation. Appl. Catal. A Gen. 2017, 536, 85–96. [Google Scholar] [CrossRef]
- Liang, G.; Wang, A.; Zhao, X.; Lei, N.; Zhang, T. Selective aldol condensation of biomass-derived levulinic acid and furfural in aqueous-phase over MgO and ZnO. Green Chem. 2016, 18, 3430–3438. [Google Scholar] [CrossRef]
- Lee, R.; Vanderveen, J.R.; Champagne, P.; Jessop, P.G. CO2-Catalysed aldol condensation of 5-hydroxymethylfurfural and acetone to a jet fuel precursor. Green Chem. 2016, 18, 5118–5121. [Google Scholar] [CrossRef]
- Zhan, Y.; Song, K.; Shi, Z.; Wan, C.; Pan, J.; Li, D.; Au, C.; Jiang, L. Influence of reduction temperature on Ni particle size and catalytic performance of Ni/Mg(Al)O catalyst for CO2 reforming of CH4. Int. J. Hydrog. Energy 2020, 45, 2794–2807. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Kazi, F.K.; Patel, A.D.; Serrano-Ruiz, J.C.; Dumesic, J.A.; Anex, R.P. Techno-economic analysis of dimethylfuran (DMF) and hydroxymethylfurfural (HMF) production from pure fructose in catalytic processes. Chem. Eng. J. 2011, 169, 329–338. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
- Gao, D.L.; Lin, W.W.; Lin, Q.J.; Dai, F.F.; Xue, Y.X.; Chen, J.H.; Liu, Y.X.; Huang, Y.; Yang, Q. Remarkable adsorption capacity of Cu2+-doped ZnAl layered double hydroxides and the calcined materials toward phosphate. J. Environ. Chem. Eng. 2023, 11, 109472. [Google Scholar] [CrossRef]
- Takehira, K. Recent development of layered double hydroxide-derived catalysts—Rehydration, reconstitution, and supporting, aiming at commercial application. Appl. Clay Sci. 2017, 136, 112–141. [Google Scholar] [CrossRef]
- Teruel, L.; Bouizi, Y.; Atienzar, P.; Fornes, V.; Garcia, H. Hydrotalcites of zinc and titanium as precursors of finely dispersed mixed oxide semiconductors for dye-sensitized solar cells. Energy Environ. Sci. 2010, 3, 154–159. [Google Scholar] [CrossRef]
- Xia, S.; Shao, M.; Zhou, X.; Pan, G.; Ni, Z. Theoretical and experimental investigation into photoelectrocatalytic oxidation and reduction property of ZnFeTi mixed metal oxides. J. Mol. Catal. A Chem. 2015, 406, 127–136. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; He, Y.; Feng, J.; Wu, T.; Li, D. Highly efficient PdAg catalyst using a reducible Mg-Ti mixed oxide for selective hydrogenation of acetylene: Role of acidic and basic sites. J. Catal. 2017, 348, 135–145. [Google Scholar] [CrossRef]
- Salehpour, S.; Dubé, M.A. Towards the Sustainable Production of Higher-Molecular-Weight Polyglycerol. Macromol. Chem. Phys. 2011, 212, 1284–1293. [Google Scholar] [CrossRef]
- Szabados, M.; Adám, A.A.; Traj, P.; Muráth, S.; Baán, K.; Bélteky, P.; Kónya, Z.; Kukovecz, A.; Sipos, P.; Pálinkó, I. Mechanochemical and wet chemical syntheses of CaIn-layered double hydroxide and its performance in a transesterification reaction compared to those of other Ca2M(III) hydrocalumites (M: Al, Sc, V, Cr, Fe, Ga) and Mg(II)-, Ni(II)-, Co(II)- or Zn(II)-based hydrotalcites. J. Catal. 2020, 391, 282–297. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C. Effect of Preparation Method on the Catalytic Property of Calcined Ca–Al Hydrotalcite for the Synthesis of Ethyl Methyl Carbonate. ACS Omega 2021, 6, 5056–5060. [Google Scholar] [CrossRef]
- Wei, B.; Jin, L.; Wang, D.; Shi, H.; Hu, H. Catalytic upgrading of lignite pyrolysis volatiles over modified HY zeolites. Fuel 2020, 259, 116234. [Google Scholar] [CrossRef]
- Çakirca, E.E.; Akin, A.N. Study on heterogeneous catalysts from calcined Ca riched hydrotalcite like compounds for biodiesel production. Sustain. Chem. Pharm. 2021, 20, 100378. [Google Scholar] [CrossRef]
- Liu, H.R.; Ding, R.; Zhang, Y.G.; Li, H.S.; Li, S.Z. Magnesium promoted hydrocalumite derived nickel catalysts for ethanol steam reforming. Int. J. Hydrog. Energy 2023, 48, 13804–13813. [Google Scholar] [CrossRef]

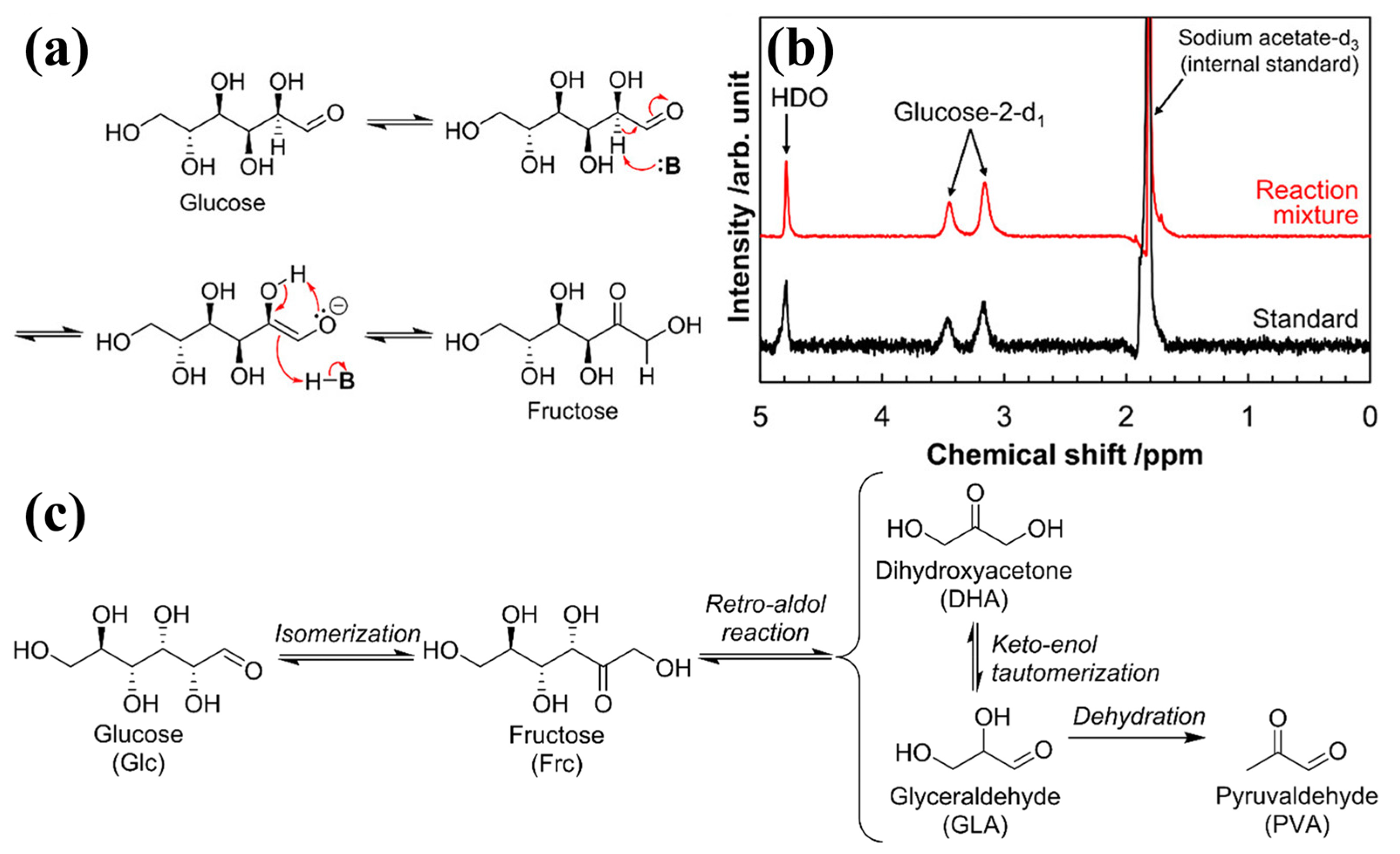
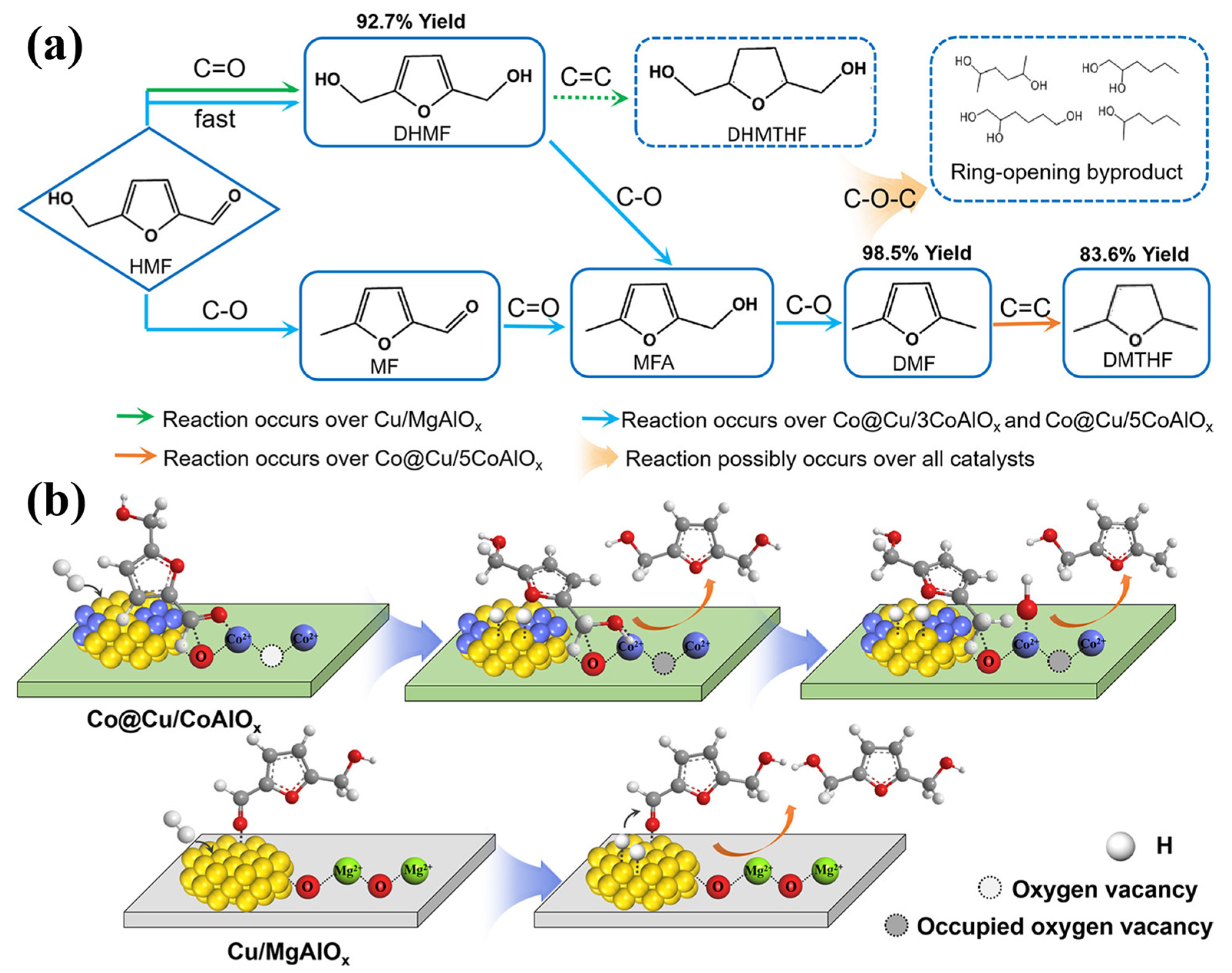
| Reaction Type | Catalysts | Reaction Conditions * | Yield (Y) or Conversion (C) | Ref. |
|---|---|---|---|---|
| Phenol hydroxylation | CuZnFeAl LDHs | T = 60 °C, P = 1 MPa | C = 66.9% | [39] |
| Electrocatalytic aldehyde oxidation | NiV LDHs | ED = 200 mA cm−2 | Y = 77.6% | [40] |
| NiCoFe LDHs | ED = 30 mA cm−2 | C = 95% | [41] | |
| Furfural hydrogenation | NiFe (3:1) LDHs | T = 140 °C, RT = 5 h | Y = 90.2% | [42] |
| NiSn IMCs@Al2O3 | T = 100 °C, P = 2 MPa H2 | C = 100% | [43] | |
| Cu0–Zn (Al)(Zr)O-10 | T = 160 °C, P = 2.5 MPa H2 | C = 100% | [44] | |
| Hydrogenation of pentahydroxymethylfurfural | Cu/ZnAl MMO | T = 180 °C, P = 1.2 MPa H2 | C = 100% | [45] |
| Cu/CoAlOx | T = 150 °C, P = 1.5 Mpa H2, RT = 5 h | C = 100% | [46] | |
| Co–(ZnO–ZnAl2O4) | T = 130 °C, P = 0.7 MPa H2 | Y = 74.2% | [47] | |
| Conversion of glucose to methyl lactate | MgAl (5:1) LDH | T = 150 °C, RT = 2 h | Y = 47.6% | [48] |
| Glucose isomerization | MgAl LDH | T = 120 °C, RT = 5 min | Y = 25 mol% | [49] |
| re-MgAl LDH | T = 100 °C, RT = 5 h | C > 40% | [50] | |
| MgAl (3:1) LDH | T = 90 °C, RT = 2 h, in ethanol | Y = 56% | [51] | |
| Aldehyde-alcohol condensation | re-CaAl LDH | T = 70 °C, RT = 6 h | Y = 61.5% | [52] |
| re-CaMnAl LDH | T = 70 °C, RT = 8 h | Y = 70.3% | [53] | |
| Dimethyl oxalate hydrogenation | CuMgAl−MMO | T = 165 °C | 94.4% | [54] |
| Glycerol etherification | CaAl–MMO | T = 235 °C | Y = 59% | [55] |
| Ethanol vapor reconstitution | Ni/CaO–Al2O3 | T = 650 °C, RT = 9 h | C = 100% | [56] |
| Low-order coal pyrolysis upgrading | MgxAl–LDOs | T = 450 °C | Y = 63.0 wt% | [57] |
| Synthesis of diethylcarbonate | MgZrAl–MMO | T = 200 °C, RT = 5 h | Y = 37.6% | [58] |
| Benzoate conversion | AMO–MgxAlO | T = 110 °C, RT = 10 h | C = 63% | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, X.; Chen, L.; He, S.; Zhang, G. Solid Acid–Base Catalysts Based on Layered Double Hydroxides Applied for Green Catalytic Transformations. Catalysts 2024, 14, 28. https://doi.org/10.3390/catal14010028
You X, Chen L, He S, Zhang G. Solid Acid–Base Catalysts Based on Layered Double Hydroxides Applied for Green Catalytic Transformations. Catalysts. 2024; 14(1):28. https://doi.org/10.3390/catal14010028
Chicago/Turabian StyleYou, Xiaolu, Lishi Chen, Shan He, and Guiju Zhang. 2024. "Solid Acid–Base Catalysts Based on Layered Double Hydroxides Applied for Green Catalytic Transformations" Catalysts 14, no. 1: 28. https://doi.org/10.3390/catal14010028
APA StyleYou, X., Chen, L., He, S., & Zhang, G. (2024). Solid Acid–Base Catalysts Based on Layered Double Hydroxides Applied for Green Catalytic Transformations. Catalysts, 14(1), 28. https://doi.org/10.3390/catal14010028







