Abstract
Heterogeneous catalysis ozonation technology can achieve efficient treatment of refractory organics in industrial wastewater due to its advantages including fast reaction speed, high ozone utilization rate, low catalyst loss and low cost and has a broad application prospect. The development of efficient and stable heterogeneous ozone catalytic materials is the key to promoting the application of this technology in industrial wastewater treatment. Based on this, an Mn/Al2O3 catalyst was successfully prepared by impregnation method using 3~5 mm γ-Al2O3 pellets as the carrier, and the surface morphology characteristics, elemental state and phase composition of the catalyst were investigated by SEM-EDX, XRD and XPS. The results showed that Mn was successfully loaded onto the surface of a γ-Al2O3 carrier. On this basis, intermittent single factor experiments were conducted to systematically investigate the effects of catalyst dosage, pH, and ozone concentration on the catalytic performance of phenol. It was found that under the optimal conditions of a catalyst dosage of 100 g (filling height of 14.2 cm), pH of 7, and ozone concentration of 4 mg/L (gas volume of 1 L/min), the removal efficiencies of 800 mL 100 mg/L of simulated phenol wastewater reached 100% after 60 min of reaction. The removal efficiencies of the catalyst still reached 95.8% within 60 min even after the fifth cycle reaction, indicating excellent reusability of the catalyst. This work provides a facile strategy for the treatment of refractory organics in industrial wastewater.
1. Introduction
Phenol is a common organic pollutant with strong toxicity. It is widely used as a raw material in the chemical industry, causing serious damage to the environment and affecting the life of aquatic organisms. The main sources of phenol wastewater are the chemical industry, such as oil, gas, coking and refining, and industries that use phenol as a raw material or product [1]. Phenol is toxic to humans and aquatic organisms and can cause coagulation and denaturation of proteins. If phenol wastewater is discharged directly, it will do great harm to the aquatic environment [2]. Because of the strong biological toxicity of phenol, conventional biological treatment is not enough to achieve the compliance of wastewater treatment [3]. Therefore, the deep and efficient treatment technology of phenol wastewater has become an urgent problem to be solved.
At present, the common treatment methods for phenol wastewater include adsorption, extraction, biological and Fenton oxidation [4]. The adsorption method has the problem of difficult disposal of used adsorbents, and improper treatment of adsorbents will cause secondary pollution. During the operation of the extraction method, the organic extractant will slightly dissolve in water, resulting in the loss of the extractant and secondary pollution [5]. Due to the strict requirements, the biological method is very strict in the screening of suitable strains, and phenol has strong biological toxicity, which adds a certain difficulty to the selection of strains, and the application scope of phenol wastewater treatment in practical engineering is limited [6]. The Fenton oxidation method will form a large amount of sludge in the actual use process, which increases the cost of sewage treatment, and it also has the disadvantages of too high concentration of total dissolved solids (TDS) in the reuse process [7].
Ozone oxidation method has attracted much attention because of its advantages including high oxidation capacity, fast reaction speed, flexible operation and no secondary pollutants. However, there is a problem of low ozone oxidation rate when using ozone to treat organic wastewater. Compared with the traditional ozone oxidation technology, the catalytic ozone oxidation technology has a higher ozone utilization rate and reaction rate [8,9]. Under the action of catalyst, the oxidation capacity of ozone is greatly improved and the rate of decomposition of organic matter in water is greatly accelerated; thus the decomposition rate and mineralization rate of organic matter are improved. Ozone catalytic oxidation technology has the advantages of wide application range, strong oxidation capacity and no secondary pollution and has a good treatment effect on organic wastewater, so it is favored by researchers [10,11]. In practical application, the heterogeneous ozone catalytic oxidation process has the advantages of high efficiency and low cost, which has been a concern of the industry and researchers. In heterogeneous catalysts, supported transition metal catalysts have been widely used because of the advantages of low cost, simple preparation process and easy operation, which can reduce the production of catalyst waste and toxic substances in industrial production and benefit protection of the environment [12,13].
Sun et al. [14] prepared a Fe-Mn@Bt ozone catalyst by the doping roasting method and applied it to the advanced treatment of biochemical tail water for coal chemical wastewater. It was found that the addition of a Fe-Mn@Bt porous catalyst increased the actual ozone utilization rate from 0.14 kgCOD/kgO3 (single ozone oxidation system) to 0.22 kgCOD/kgO3. Huang et al. [15] successfully synthesized three kinds of MnO2 with mesoporous structure, which were further used as catalysts to degrade phenol wastewater through ozone catalytic oxidation. The results showed that Mn could promote the degradation of phenol and exhibited excellent catalytic activity. Under alkaline conditions, the removal rate of phenol reached higher than 90% within 90 min. Rabia et al. [16] successfully designed and prepared a porous Mn3O4 nano-catalyst with an open pore structure for the catalytic oxidation of phenol. The results showed that 25 mg/L phenol could be completely removed within 30 min with the catalyst dosage of 0.8 g/L and the temperature of 25 °C. Amazingly, no significant loss of catalyst activity was observed after four consecutive cycles. Chaliha et al. [17] synthesized a mesoporous catalyst modified with Mn by immersion calcination method to catalyze the oxidation of phenol. The results showed that the removal efficiencies of phenol reached to 86.5% within 5 h, exhibiting excellent catalytic performance. Wang et al. [18] used chitosan as a raw material and KOH and MnCl2 as activators to synthesize a modified biological carbon material through pyrolysis, which was further a catalyst to catalyze the degradation of phenol. It was found that within 95 min, 30 m/L phenol was completely degraded and the mineralization rate reached higher than 50%.
Al2O3 is a highly dispersed solid material with a large surface area, and its microporous surface has the characteristics required for catalysis, such as adsorption properties, surface activity, excellent thermal stability, etc., so it is widely used as a catalyst carrier. The surface of Al2O3 is rich in hydroxyl groups (OH−), which can effectively promote the generation of hydroxyl radicals [19]. Manganese oxide has the advantages of environmental friendliness, low cost, adjustable oxidation reduction, variable crystal structure and simple preparation, and it can be selected as the active component supported on the catalyst for catalytic ozonation [20,21]. Therefore, in order to degrade phenol wastewater, activated alumina was selected as the catalyst carrier, and the Mn metal active component was loaded on the carrier to synthesize the ozone catalytic oxidation catalyst [22].
In this paper, a heterogeneous catalytic ozonation system was established by preparing a catalyst Mn/Al2O3 with activated alumina as the carrier and manganese as the active component. Scanning electron microscopy-energy dispersive spectrometer (SEM-EDS), X-ray diffraction (XRD) and X-ray Photoelectron Spectroscopy (XPS) were used to investigate the surface morphology and elemental composition of the catalyst. Furthermore, the effects of catalyst dosage, pH, ozone concentration and water substrate on catalytic ozone degradation of phenol were investigated. It was found that Mn/Al2O3 exhibited removal efficiencies of 100% to 800 mL 100 mg/L phenol wastewater within 60 min with a catalyst dosage of 100 g (filling height of 14.2 cm), pH of 7, and ozone concentration of 4 mg/L (gas volume of 1 L/min).
Also, repeated catalyst experiments were conducted to verify the long-term stability of the new catalyst. It was observed that Mn/Al2O3 possessed and 95.8% of removal efficiencies to phenol wastewater in 60 min even after the fifth use cycle, indicating excellent reusability of Mn/Al2O3. This work provides a new strategy for effective treatment of organic wastewater in industry.
2. Results
2.1. Morphology and Structure Characterization
Table 1 lists the element contents. According to the surface scanning results, the main elements on the surface of the blank ball are Al and O, which mainly belong to the theme of alumina. At the same time, it was found that there was a small amount of C element on the surface, which was ascribed to the absorption of CO2 for high surface of γ-Al2O3 in the sample preparation process.

Table 1.
Surface element content comparison of untreated γ-Al2O3.
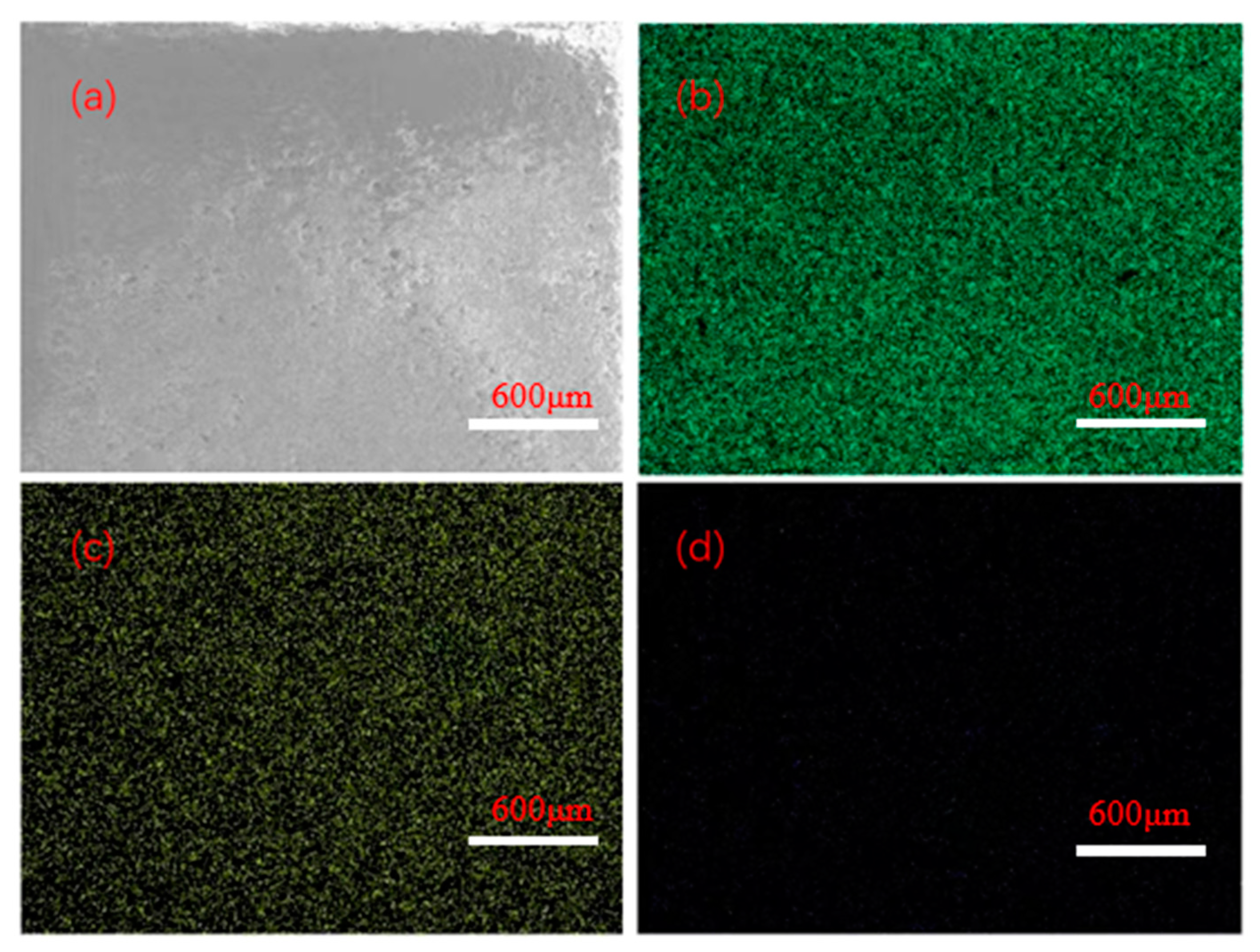
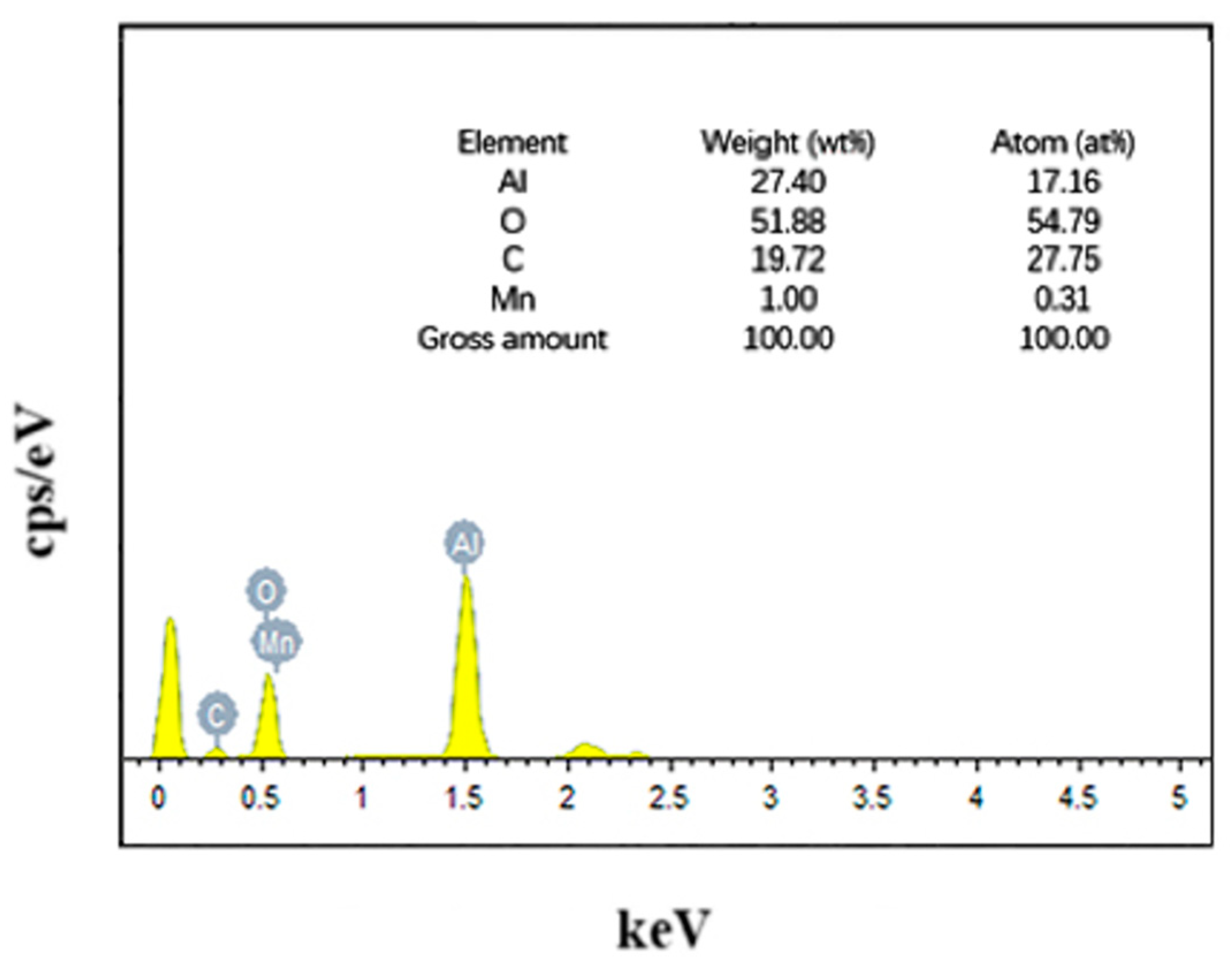
Figure 1 shows the local surface scanning images of the Mn/Al2O3 catalyst loaded with Mn, and the corresponding element compositions are shown in Figure 2. The EDX mapping results showed that the surface elements of the catalyst are composed of O, Al, Mn and C, with mass percentages of 51.88 wt%, 27.40 wt%, 1 wt% and 19.72 wt%, respectively. It can be seen that after Mn treatment, the surface of Mn/Al2O3 contains about 1% Mn element. The presence of Mn element can effectively enhance the catalytic activity of the Mn/Al2O3 catalyst [23].
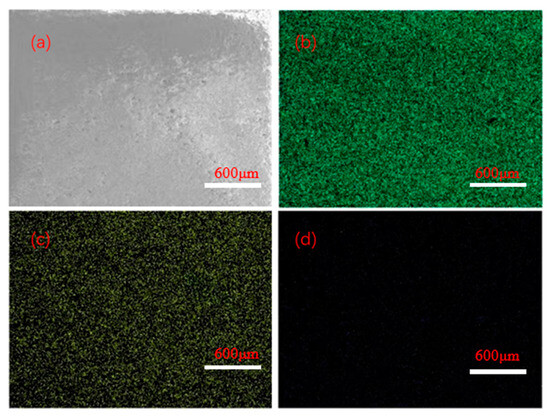
Figure 1.
SEM-EDS images of surface for Mn/Al2O3: (a) SEM image of the scanning area; (b) mapping results of distribution for Al elements; (c) mapping results of distribution for O elements; (d) mapping results of distribution for Mn elements.
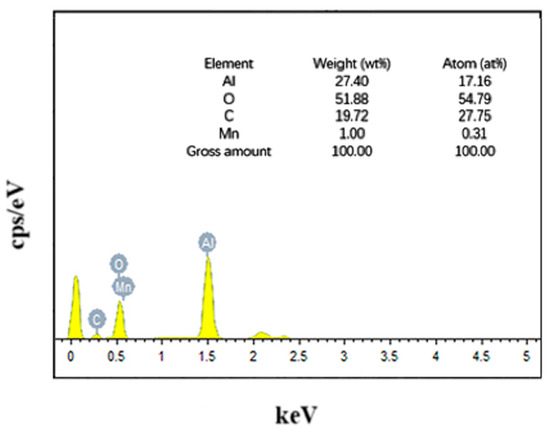
Figure 2.
EDS spectrum image of Mn/Al2O3.
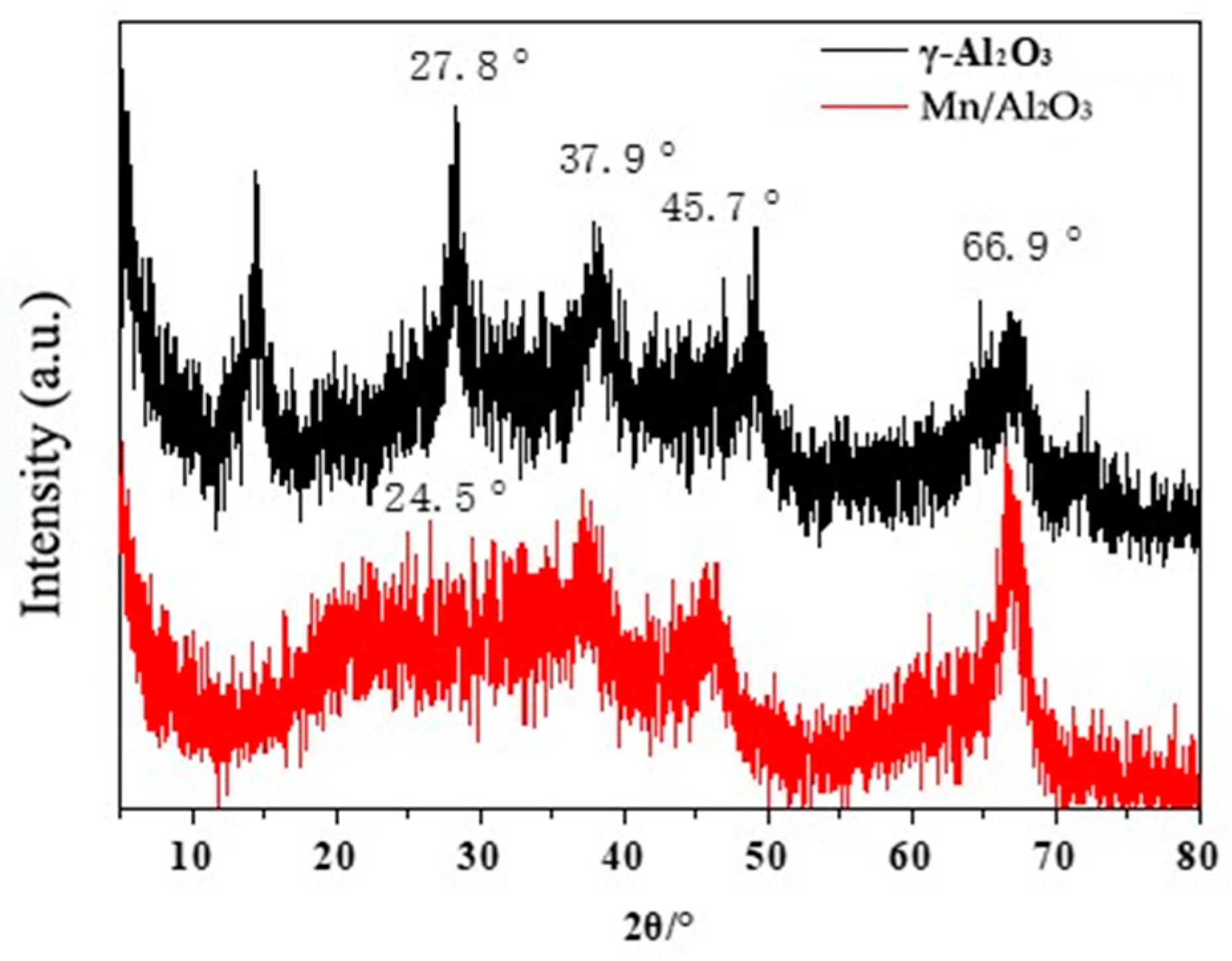
Figure 3 shows the XRD patterns of γ-Al2O3 and Mn/Al2O3. It can be seen that γ-Al2O3 has relatively obvious diffraction peaks at 37.9°, 45.7° and 66.9°, corresponding to the diffraction peaks of the (311), (400) and (440) crystal planes of γ-Al2O3 respectively, which are consistent with the diffraction spectrum of γ-Al2O3 (JCPDF 29-0063) [24]. When Mn was loaded, Mn/Al2O3 exhibited a halo in the area of 20° to 30°, which was also observed in a previous report [25]. This halo suggested the existence of an amorphous phase on MnOx/γ-Al2O3.
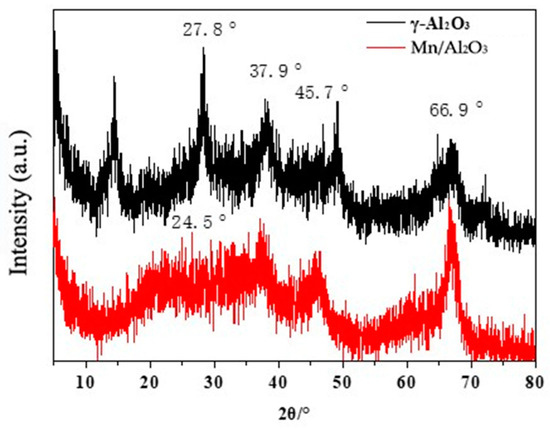
Figure 3.
XRD patterns of γ-Al2O3 and Mn/Al2O3.
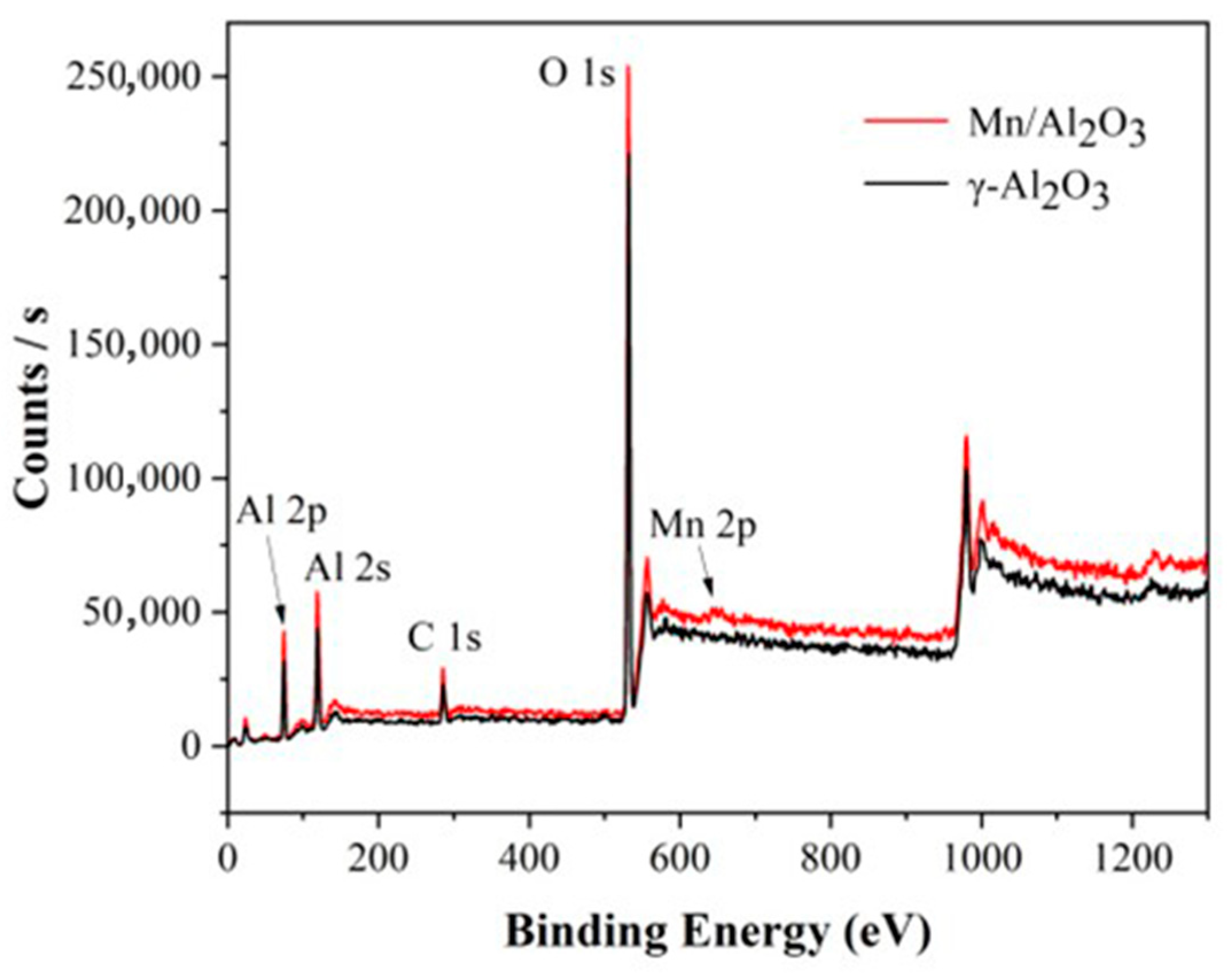
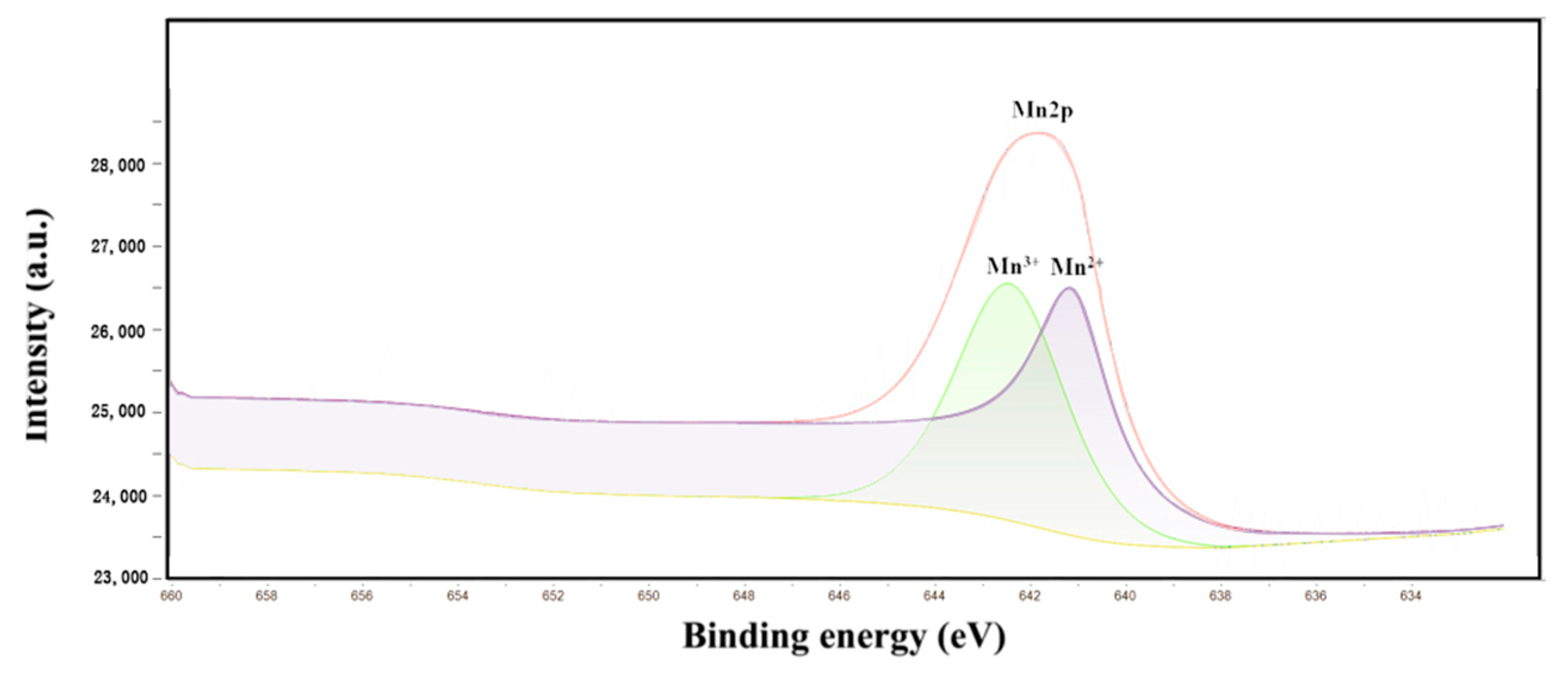
Figure 4 shows the XPS spectra of γ-Al2O3 and Mn/Al2O3. For γ-Al2O3, the peaks at around 75.1 eV and 119.1 eV correspond to Al 2p and Al 2s peaks, respectively. The peak at 531.2 eV can be ascribed to O 1s peak, indicating that the surface of the γ-Al2O3 ball is mainly Al and O elements, which are consistent with EDS results. At the same time, a weak C 1s peak was observed at 285.0 eV, which may be due to the introduction of a small amount of carbonate in the preparation of alumina pellets. This phenomenon is also confirmed by SEM-EDS. Figure 5 presented a peak-fitting of Mn 2p, which confirmed the existence of Mn (II) (641.39 eV) and Mn (III) (642.95 eV) [26], implying that the Mn element is successfully loaded on the surface of γ-Al2O3. The introduction of Mn could significantly enhance the catalytic degradation performance of Mn/Al2O3.
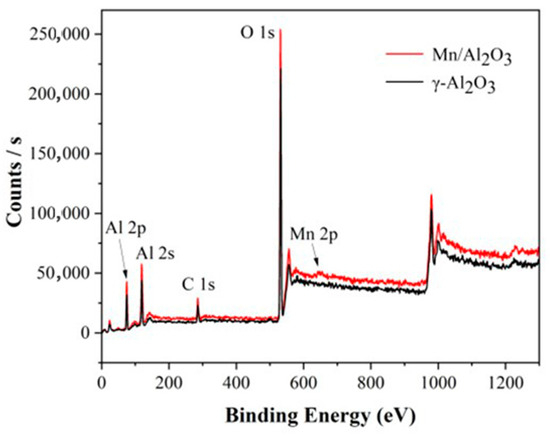
Figure 4.
XPS spectra of γ-Al2O3 and Mn/Al2O3.
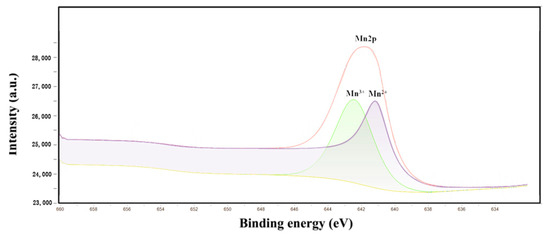
Figure 5.
Peak-fitting of Mn 2p.
2.2. Degradation of Phenol Wastewater by Mn/Al2O3 Catalyst
2.2.1. Absorption Effect of Mn/Al2O3 to Phenol
Phenol was selected as the model pollutant to investigate the catalytic ozonation degradation performance of the Mn-supported active Al2O3 catalysts. Before the catalytic degradation experiment, the adsorption abilities of active Al2O3 and Mn/Al2O3 catalysts to phenol were investigated. The results show that Al2O3 and Mn/Al2O3 have no adsorption effect to phenol within 60 min, indicating that the adsorption capacity of prepared Mn/Al2O3 catalyst is weak.
2.2.2. Effects of Catalyst Dosage on Catalytic Performance
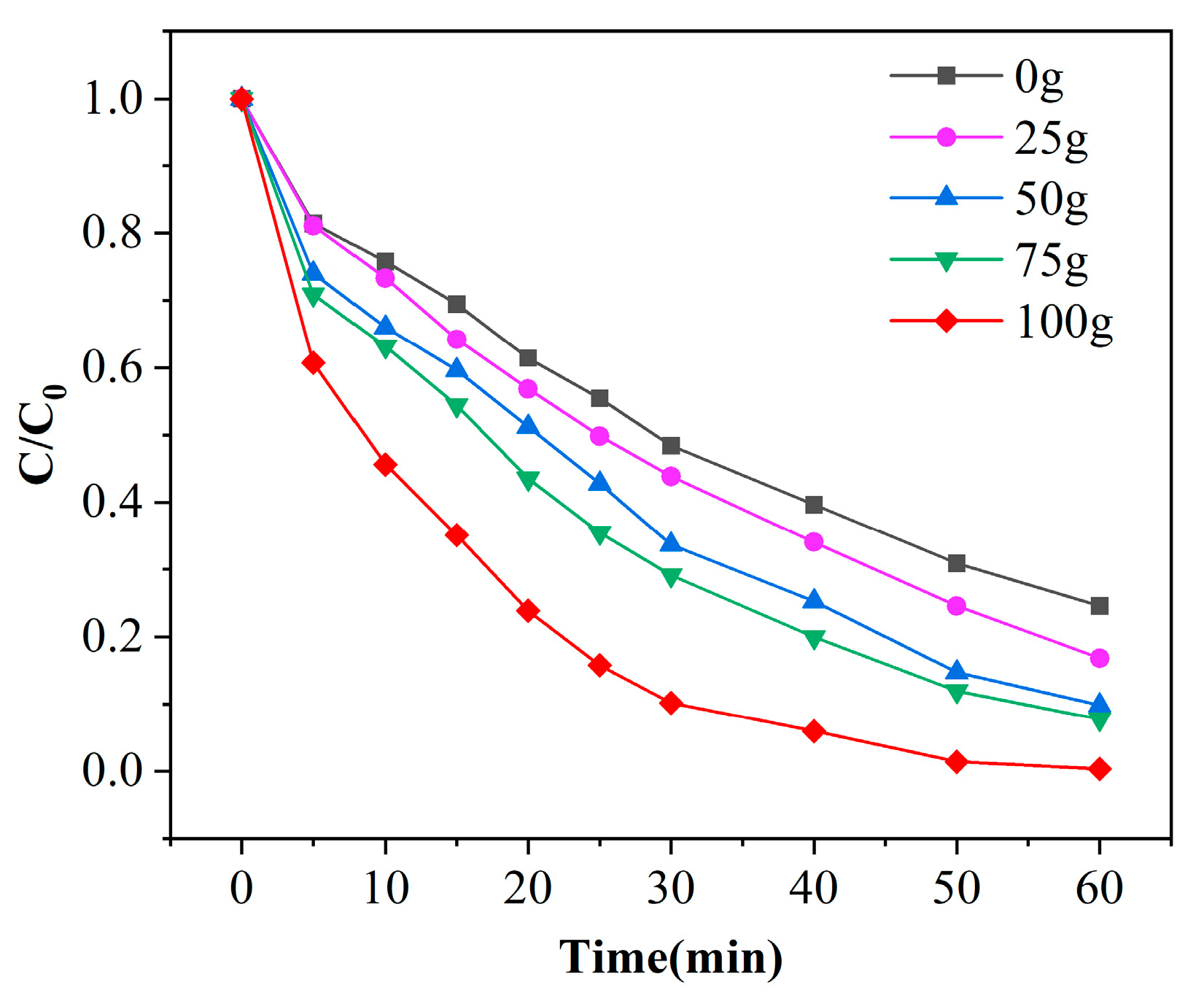
Mn/Al2O3 catalyst has a large specific surface area, which can provide many active sites for the catalytic reaction, and the number of active sites will directly affect the degradation efficiency of phenol [27]. Figure 6 shows the degradation curves of phenol under different catalyst dosages with a pH of 7 and ozone dosage of 4 mg/min. As observed from the figure, the removal efficiencies of phenol increases with the extension of reaction time, and the removal efficiencies slowed down after 30 min. It is also found that with the increase of catalyst dosage, the degradation effect of phenol is improved; this comes from the fact that more catalyst can provide more active sites in the same time. It is observed that γ-Al2O3 has a certain catalytic effect combined with ozone. In the condition of the same reaction time of 60 min, when the catalyst dosage is increased from 0 to 100 g (14.2 cm), the removal efficiencies of phenol areis enhanced from 75.4% to 99.6%. This phenomenon is ascribed that with the increase of the amount of catalyst, the active sites provide ozone oxidation of phenol increase continuously, which enhances the degradation efficiency of phenol per unit time. Therefore, the optimal dosage of catalyst is 100 g.
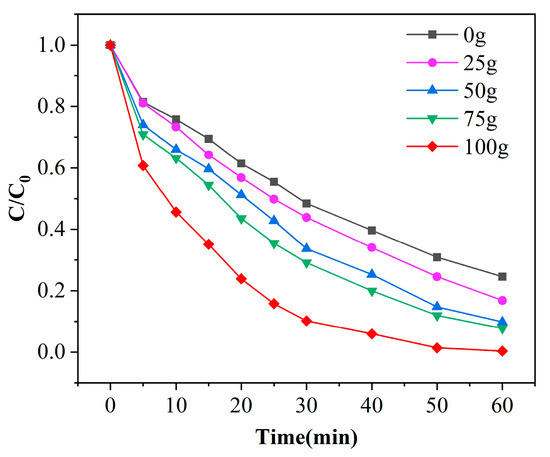
Figure 6.
Experimental results of phenol degradation efficiency with the dosage of catalyst ([phenol] = 100 mg/L, [O3] = 4 mg/L, pH = 7).
2.2.3. Effects of pH on Catalytic Performance
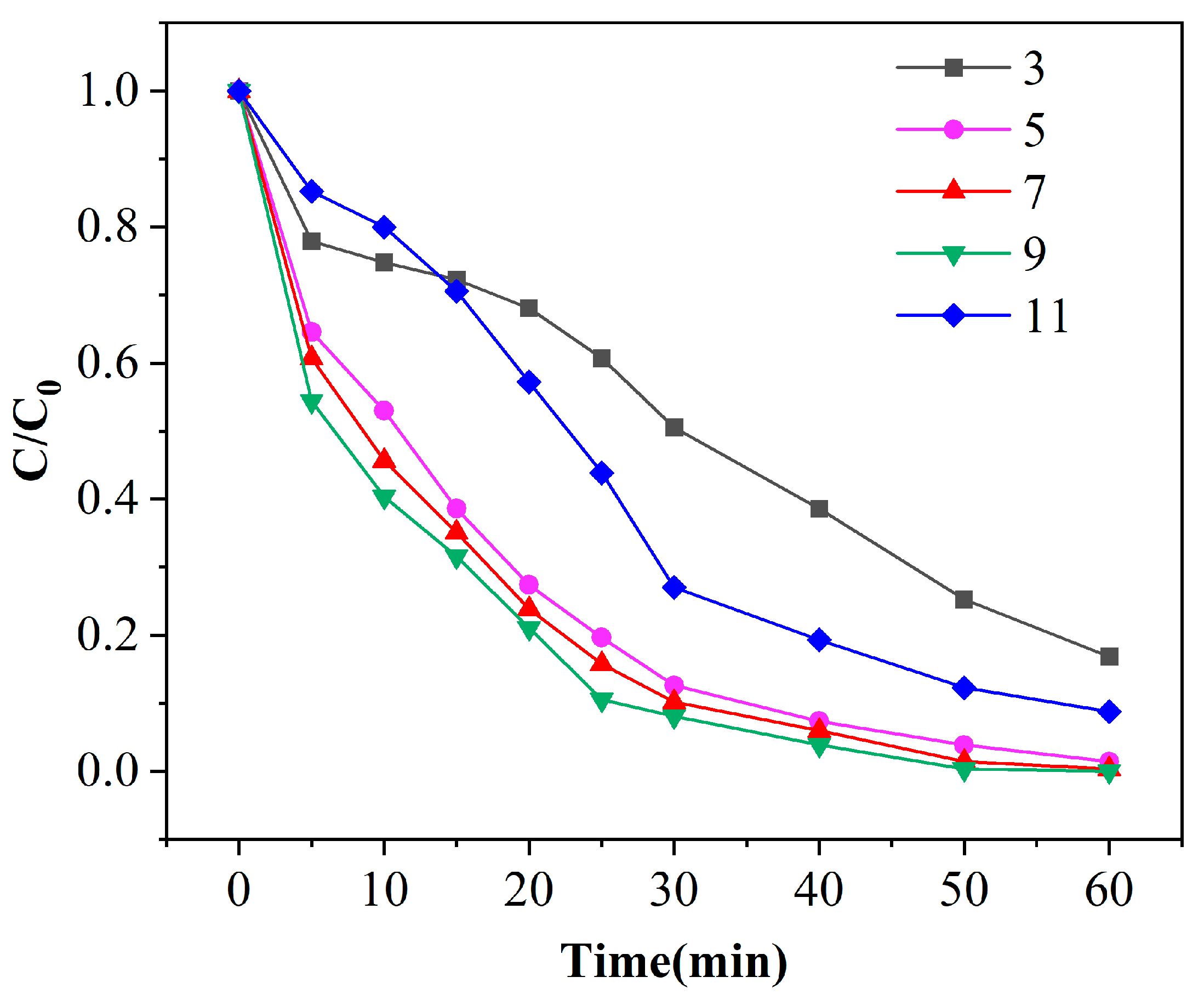
The pH of the solution will affect the decomposition of ozone. When the charge on the surface of the catalyst is higher or lower than its zero charge, the production of·OH in the reaction system and the process of adsorption on the surface of the catalyst will also be affected, thus affecting the degradation of phenol [28]. Figure 7 shows the degradation curves of phenol under different pH conditions with a catalyst dosage of 100 g and ozone dosage of 4 mg/min. When the initial pH of the solution is 3, the degradation effect is the lowest, which is only 83.2% after 60 min of treatment. When the pH is 9, the degradation effect is the highest, which can reach an amazing 100% after 60 min of reaction. When the initial pH values are 5 and 7, the degradation effects of the catalyst to phenol are similar. After 40 min of reaction, the removal efficiencies of phenol are 92.6%, 94.0% and 96.1%, respectively, with a difference of less than 3.5%, and the removal efficiencies are closer to the continuation of the reaction. According to Figure 8, it can be also found that with the continuous increase of the initial pH of the solution, the removal efficiencies of phenol at the same reaction time show a trend of first increasing and then decreasing. This is because, under acidic conditions, ozone molecules become the main oxidant in the reaction system with high selectivity, resulting in incomplete oxidation of organic matter. Under neutral or alkaline conditions, the concentration of OH− in the solution increases, which plays as the initiator of free radical reaction and promotes the adsorption and decomposition of ozone on the surface of the catalyst, thus generating more active free radicals (such as •OH) and increasing the reaction rate. The surface charge of the catalyst is also affected by the pH of the wastewater. However, when the pH continues to increase, the utilization of the initiator in the reaction system reaches saturation. Excess OH− can cause deprotonation of hydroxyl groups on the catalyst surface, changing the charge on the catalyst surface, thereby weakening the free radical reaction and reducing the degradation performance of phenol wastewater. Under the condition of pH 7 and pH 9, the removal efficiencies of phenol are very close, so it can be concluded that a pH of 7 is the best reaction condition.
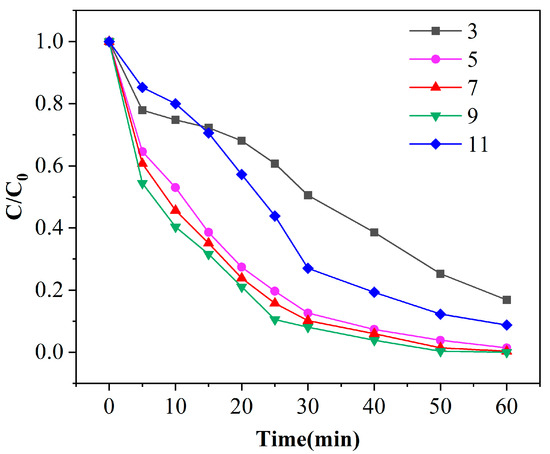
Figure 7.
Experimental results of initial pH value of solution on phenol degradation efficiency ([phenol] = 100 mg/L, [O3] = 4 mg/L, [catalyst] = 125 g/L).
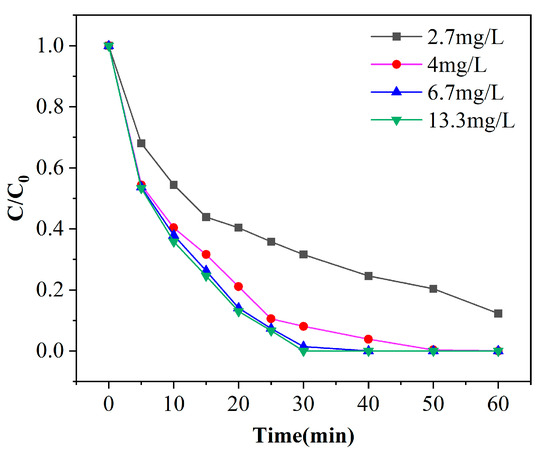
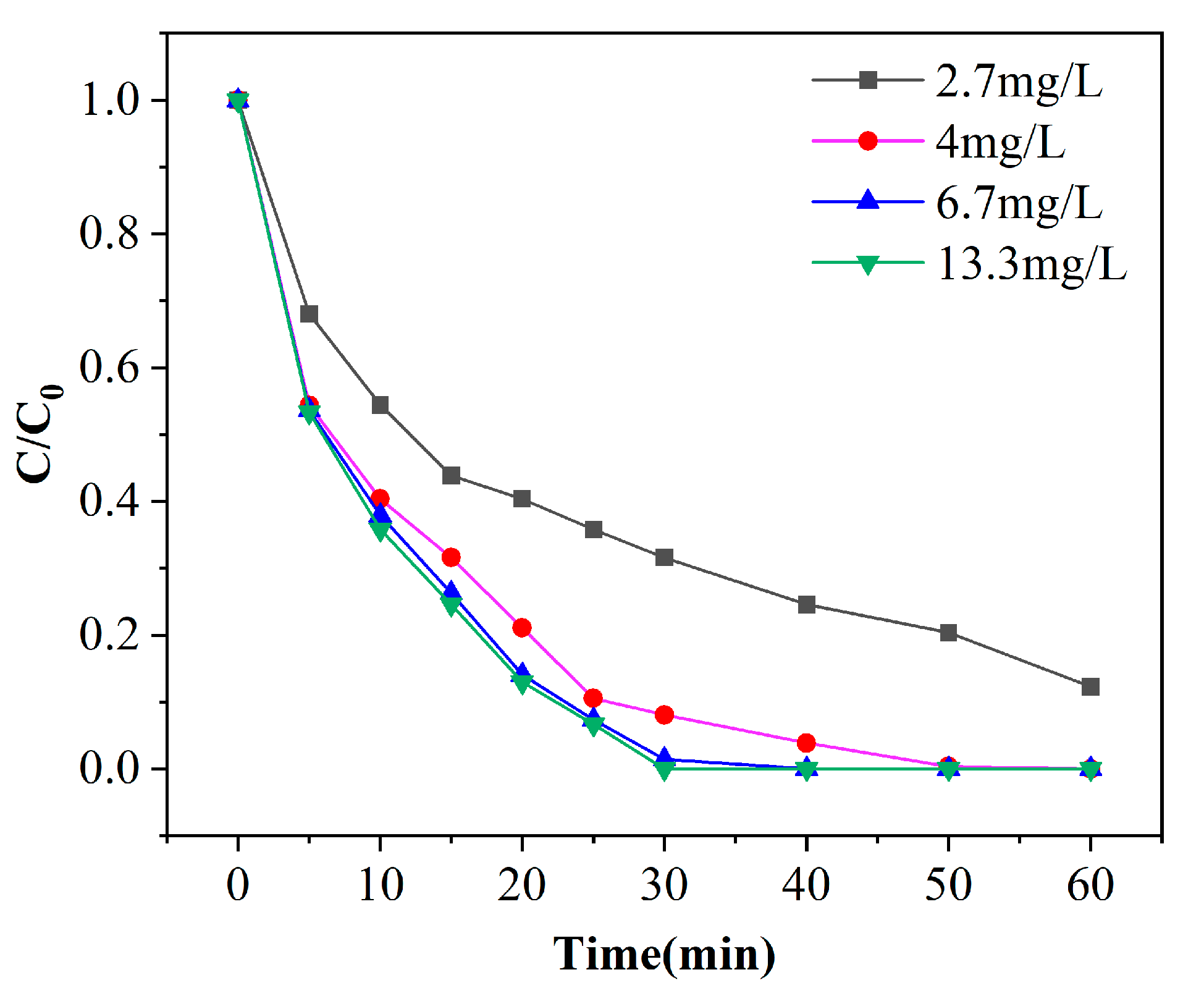
Figure 8.
Experimental results of phenol degradation efficiency by ozone concentration ([phenol] = 100 mg/L, pH = 7, [catalyst] = 125 g/L).
2.2.4. Effects of Ozone Dosage on Catalytic Performance
Ozone is one of the most critical substances in the whole reaction process, which directly or indirectly participates in the degradation process of phenol. In order to achieve the goal of efficient removal of phenol, improve the reaction rate and avoid the waste of ozone, it is necessary to find out the appropriate ozone concentration. Figure 8 shows the degradation curves of phenol in different ozone concentrations with a pH of 7 and catalyst dosage of 100 g. As observed from Figure 8, the removal efficiencies of phenol increases with the increase of ozone concentration. The removal efficiencies of phenol is 87.7% after 60 min when the ozone concentration is 2.7 mg/L. In the other three groups, when the ozone concentration is 4, 6.7 and 13.3 mg/L, respectively, the removal efficiencies of phenol all reached 100% after 60 min of treatment. When the ozone concentration is 13.3 mg/L, the phenol solution (800 mL, 100 mg/L) exhibited the fastest rate and completed degradation within 30 min. When the ozone concentration is 6.7 mg/L, complete degradation required 40 min. The removal efficiencies of phenol can reach 99.6% with ozone concentration of 4 mg/L after a 50-min reaction. It can be seen that the increase of ozone concentration increases the removal efficiencies, because the contact chance of ozone with the catalyst and phenol is increased, and thus the degradation effect is enhanced. When the concentration is increased from 4 mg/L to 13.3 mg/L, there is no significant difference in the degradation of phenol, only a slight improvement. This is because there is a saturated equilibrium concentration of ozone in the solution, and the contact surface area with the catalyst is also limited [29]. As the amount of ozone continues to increase, it cannot participate in the reaction process, and the remaining ozone escapes with the gas. From the point of view of treatment effect and cost, the best reaction condition of ozone concentration is 4 mg/L.
2.2.5. Effects of Water Substrate on Catalytic Performance
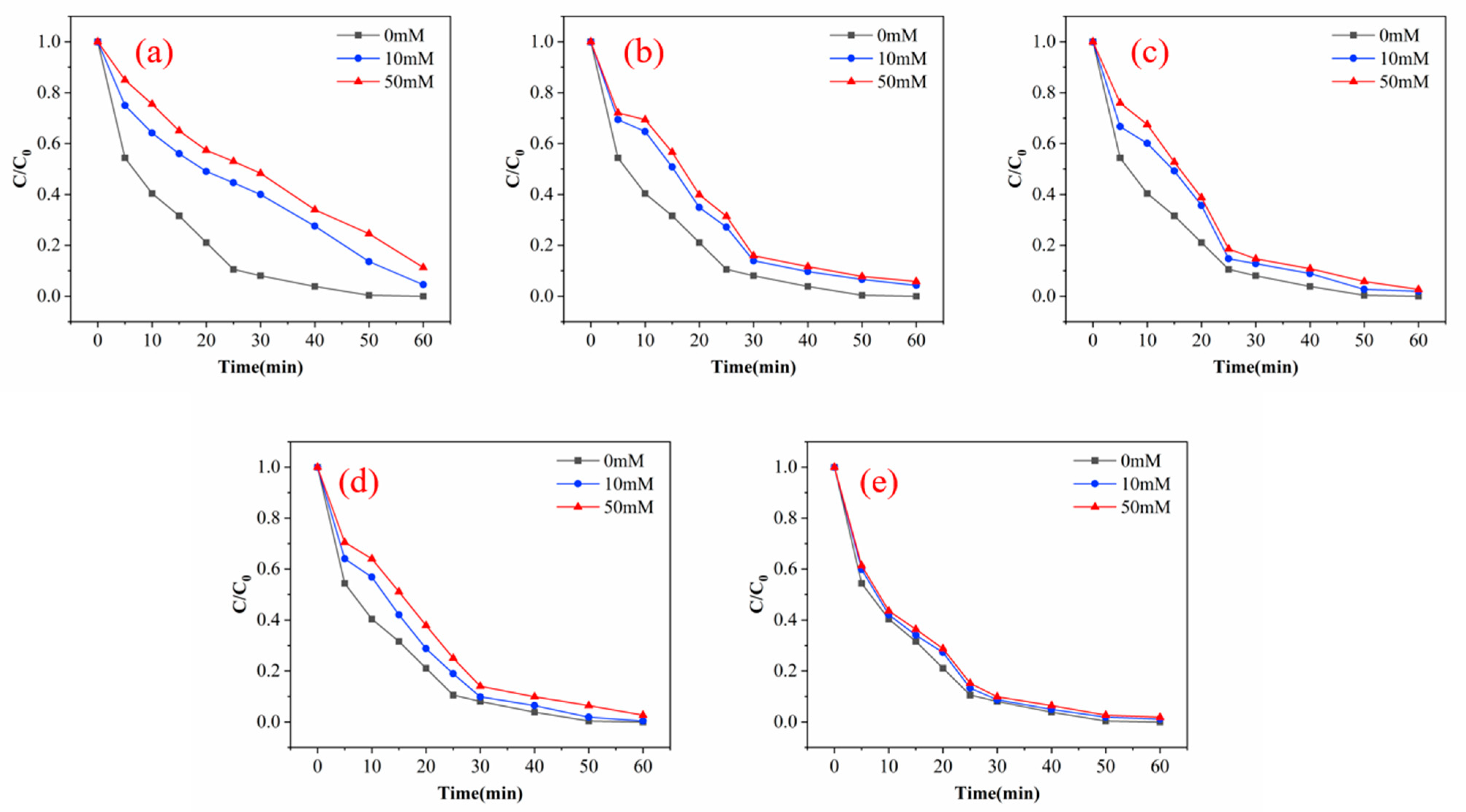
There are many inorganic anions in wastewater, and these anions can affect the degradation of pollutants in the process of ozone oxidation through competition reaction. The specific degree of influence is related to the type of inorganic anion, the type of oxidant, the type of target organic pollutant and the surface physicochemical properties of the catalyst in heterogeneous catalysts [30]. Figure 9 shows the curve of the influence of different anions (Cl−, SO42−, NO3−, CO32−, HPO43−) on the degradation effect of phenol with a pH of 7, catalyst dosage of 100 g and ozone dosage of 4 mg/min. When the concentration of HPO43− increases to 50 mM, the removal efficiencies of phenol decrease to 88.7% after 60 min. When the concentration of CO32− increases to 50 mM at the same condition, the removal efficiencies of phenol decrease to 94.2% after 60 min. In the condition of SO42− concentration of 50 mM, the removal efficiencies of phenol decrease to 97.2% after 60 min. The removal efficiencies of phenol decrease to 97.4% after 60 min when the concentration of NO3− increases to 50 mM. In the condition of 50 mM Cl−, the removal efficiencies of phenol decrease to 98.1%. It can be clearly observed that anion has a certain inhibitory effect on the degradation of phenol, and the inhibitory effect is HPO43− > CO32− > SO42− > NO3− > Cl−. It can be found that SO42−, NO3− and Cl− have a slight inhibitory effect on the removal efficiencies of phenol. The high inhibition effect of HPO43− and CO32− may be partly due to the change in pH during the reaction. The solution pH of all anions except PO43− and CO32− decrease, and the pH of CO32− does not change because it reacts with·OH to form hydroxide ions, which increase the pH of the solution.
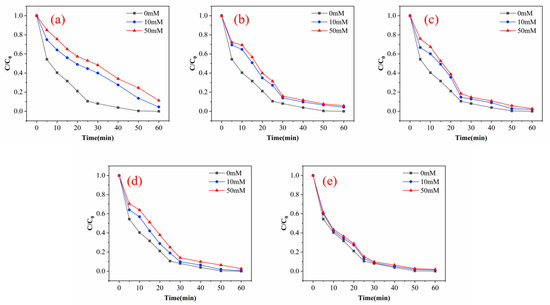
Figure 9.
Experimental results of inorganic anions on the degradation efficiency of phenol: (a) HPO43−; (b) CO32−; (c) SO42−; (d) NO3−; (e) Cl−.
2.3. Stability of Mn/Al2O3 Catalyst
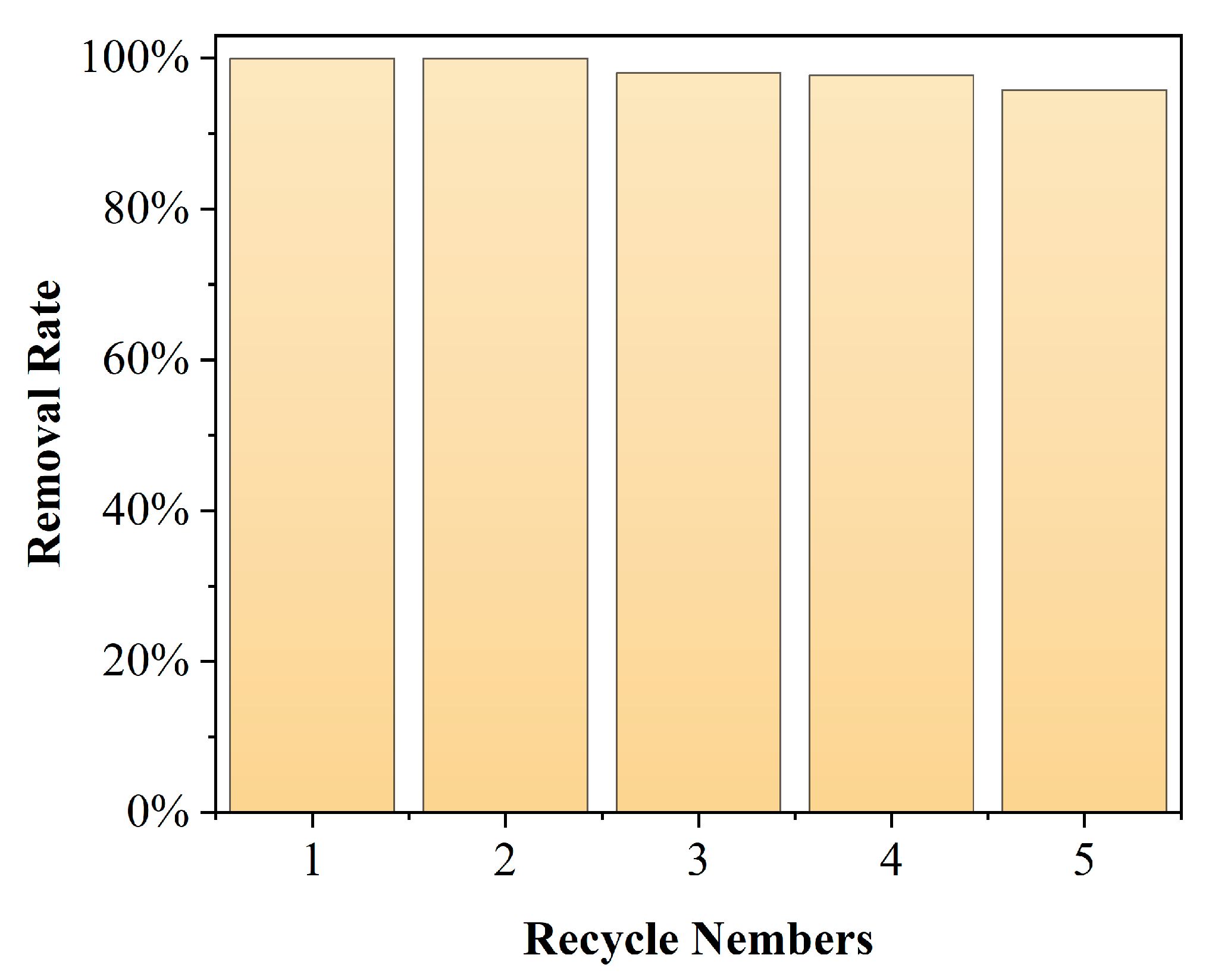
The stability of the catalyst during the catalytic process is critical to the application of the heterogeneous catalysis [31]. To investigate the stability of the Mn/Al2O3 catalyst during catalytic ozonation, cyclic catalytic ozonation degradation experiments were conducted, and the corresponding result is shown in Figure 10 with a pH of 7, catalyst dosage of 100 g and ozone dosage of 4 mg/min. It is observed that the performance of the Mn/Al2O3 catalyst for catalytic ozonation degradation of phenol is not significantly reduced after five repeated cycles. In the fifth repeated experiment, the degradation efficiency of phenol still reaches >95%, indicating that the prepared Mn/Al2O3 catalyst has good stability during the catalytic ozonation process, which is a benefit to a long-time run of the Mn/Al2O3 catalyst.
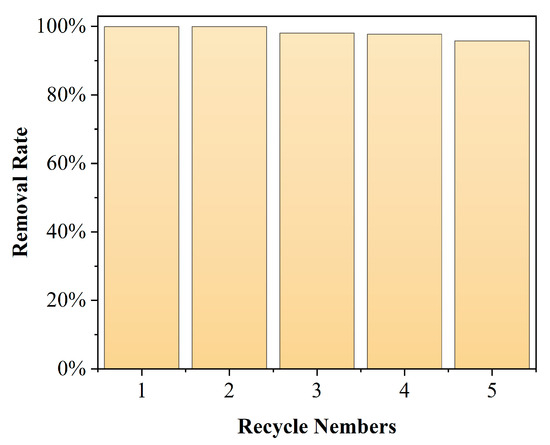
Figure 10.
Cycling runs of the Mn/Al2O3 catalyst for the catalytic ozonation degradation of phenol.
3. Experimental Section
3.1. Experimental Materials
Phenol was purchased from Tianjin Alta Scientific Co., Ltd. (Tianjin, China). Activated alumina particles (γ-Al2O) with a diameter of 3–5 mm were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China); 50 wt% manganese nitrate solution was purchased from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China). All chemical reagents were used without any further purification. Deionized water in the experiment was made in the laboratory. All pieces of glassware were washed by deionized three times before use and dried in a 105 °C oven for 2 h.
3.2. Preparation of the Catalysts
3.2.1. Activation Treatment of γ-Al2O3
The activated γ-Al2O3 particles with a uniform shape were selected and washed three times by deionized water to remove the residual impurities and dust on the surfaces of particles, which were then dried in an oven at 110 °C for 10 h. Then, the dried γ-Al2O3 pellets were cooled to room temperature and stored for future use.
3.2.2. Preparation of Mn Loaded γ-Al2O3 (Mn/Al2O3)
The 50 wt% manganese nitrate solution was used as the impregnating solution, the mass of Mn and γ-Al2O3 particles was added according to the calculated value and deionized water was added according to the saturation water absorption rate and then stirred evenly. The configured carrier γ-Al2O3 particles and metalion solution were added into the water bath with a constant temperature oscillator for 10 h, then the impregnated turbid liquid was poured out and the ball was rinsed with deionized water. The treated Al2O3 particles were put it in the oven and dried at 105 °C for 4 h to content weight. After the dried process, the pellets were cooled to room temperature, which were heated from room temperature to 500 °C and calcined at 500 °C for 4 h, then kept at 500 °C for 3 h. The particles were cooled to room temperature, and Mn/Al2O3 catalyst samples were obtained.
3.3. Characterization of the Catalyst
The microscopic morphology of the catalysts was investigated by NANO SEM430 scanning electron microscopy (SEM) (FEI company, Hillsboro, OR, USA) combined with an Electron Backscattered Diffraction (EBSD) system. Before the observation, the samples were coated with conductive later. The accelerating voltage was 10 kV. The crystal structure of the Mn-loaded activated Al2O3 catalysts (Mn/Al2O3) was analyzed by Bruker D8ADVANCE X-ray diffraction (XRD) (Bruker, Billerica, MA, USA) with a Cu Kα tube and Ni filter (λ = 0.1542 nm). The elemental composition on the catalyst surface was analyzed by a Thermo Scientific Escalab 250Xi X-ray photoelectron spectrometry (XPS) (Thermo Scientific, Waltham, MA, USA).
3.4. Ozone Catalytic Oxidation Experiment
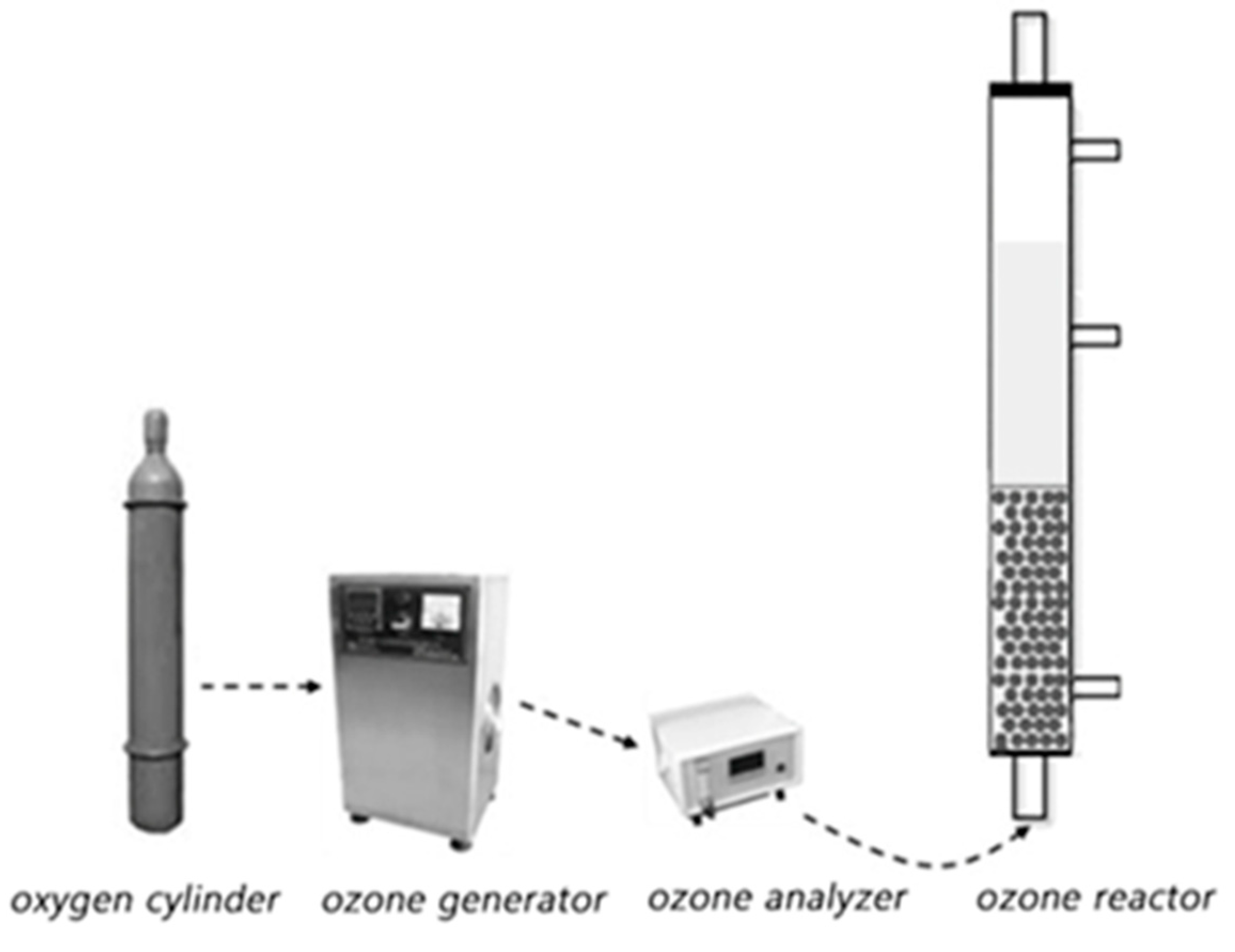
The schematic diagram for the ozone catalytic oxidation experiment is shown in Figure 11. Eight hundred mL phenol simulated wastewater with a concentration of 100 mg/L and a certain mass of solid catalyst was added to the reactor. An ozone generator was used to provide ozone. After a certain reaction time, water samples were taken, which were filtered by a 0.22 μm filter membrane. Then, 100 μL of Na2S2O3 solution (0.8 mol/L) was added to terminate the ozonation reaction. The phenol concentration of treated water sample was detected by UltiMate 3000 high performance liquid chromatograph (Thermo Scientific, Waltham, MA, USA). The comparative experiment was conducted by using the ozone experiment alone without any solid catalyst.
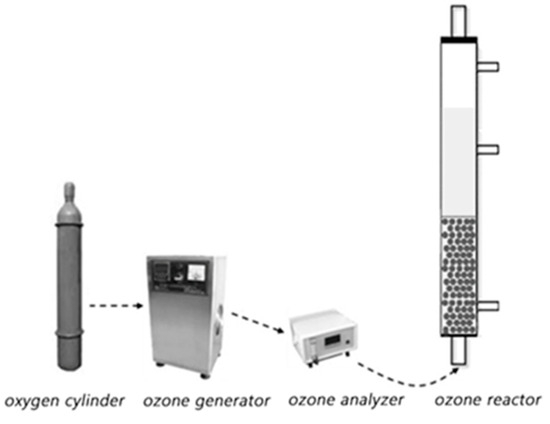
Figure 11.
The schematic diagram of the catalytic ozone oxidation system.
4. Conclusions
In this work, an Mn/Al2O3 catalyst was prepared by the immersion-calcination method. Furthermore, SEM-EDX, XRD and XPS were used to investigate the surface morphology, elemental distribution and structure of the catalyst, which confirmed the successful fabrication of Mn/Al2O3. On this basis, a batch single factor experiment was carried out to systematically investigate the influence of catalyst dosage, pH and ozone concentration on the catalytic effect of phenol. Finally, the stability of the catalyst was judged according to the reuse effect of the catalyst. It was found that when the catalyst was used with a phenol wastewater concentration of 100 mg/L, the degradation effect of phenol reached 99.6% under the conditions of ozone concentration of 4 mg/L, catalyst dosage of 100 g and solution pH of 7. The recycle experiment confirmed that the Mn/Al2O3 catalyst possessed excellent stability, which had a removal efficiency of higher than 95% even after five-times repeated recycling. This work provides a novel strategy for efficient treatment of organic chemical wastewater. The high removal efficiency and stability suggest the prospect of applications of catalytic ozonation for industrial wastewater.
Author Contributions
S.Y.: investigation, conceptualization, methodology, validation, writing—original draft. X.Z.: investigation, data curation, visualization. M.Z.: writing—review and editing, resources, methodology, supervision. H.Z.: project administration, methodology. S.P.: conceptualization, supervision, funding acquisition, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Natural Science Foundation of China (No. 52000102), Provincial Nature Science Foundation of Jiangsu Province (No. BK20190689).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kong, Z.; Li, L.; Xue, Y. Challenges and prospects for the anaerobic treatment of chemical industrial organic wastewater: A review. J. Clean. Prod. 2019, 231, 913–927. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Zhao, H.P. Highly efficient one-step advanced treatment of biologically pretreated coking wastewater by an integration of coagulation and adsorption process. Bioresour. Technol. 2018, 247, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ma, W.; Han, H.; Han, Y.; Ma, W. Catalytic ozonation of quinoline using nano-MgO: Efficacy, pathways, mechanisms and its application to real biologically pretreated coal gasification wastewater. Chem. Eng. J. 2017, 327, 91–99. [Google Scholar] [CrossRef]
- Huang, J.W.; Puyang, C.P.; Wang, Y.W.; Zhang, J.W.; Guo, H. Hydroxylamine activated by discharge plasma for synergetic degradation of tetracycline in water: Insight into performance and mechanism. Sep. Purif. Technol. 2022, 300, 121913. [Google Scholar] [CrossRef]
- Wu, H.Y.; Li, C.T.; Zhao, L.K. Removal of gaseous elemental mercury by cylindrical activated coke loaded with CoOx-CeO2 from simulated coal combustion flue gas. Energy Fuels 2015, 29, 6747–6757. [Google Scholar] [CrossRef]
- Deng, S.; Jothinathan, L.; Cai, Q.; Li, R.; Wu, M.; Ong, S.L.; Hu, J. FeOx@GAC catalyzed microbubble ozonation coupled with biological process for industrial phenolic wastewater treatment: Catalytic performance, biological process screening and microbial characteristics. Water Res. 2021, 190, 116687. [Google Scholar] [CrossRef]
- Tyagi, M.; Rana, A.; Kumari, S.; Jagadevan, S. Adsorptive removal of cyanide from coke oven wastewater onto zero-valent iron: Optimization through response surface methodology, isotherm and kinetic studies. J. Clean. Prod. 2018, 20, 398–407. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wu, C.Y.; Zhou, Y.X.; Wang, Y.N.; He, X.W. Effect of wastewater particles on catalytic ozonation in the advanced treatment of petrochemical secondary effluent. Chem. Eng. J. 2018, 345, 280–289. [Google Scholar] [CrossRef]
- Wu, M.; Kwok, Y.H.; Zhang, Y.; Szeto, W.; Huang, H.; Leung, D.Y.C. Synergetic effect of vacuum ultraviolet photolysis and ozone catalytic oxidation for toluene degradation over MnO2-rGO composite catalyst. Chem. Eng. Sci. 2021, 231, 116288. [Google Scholar] [CrossRef]
- Ghuge, S.P.; Saroha, A.K. Catalytic ozonation for the treatment of synthetic and industrial effluents-Application of mesoporous materials: A review. J. Environ. Manag. 2018, 211, 83–102. [Google Scholar] [CrossRef]
- Hassani, E.K.; Kalnina, D.; Turks, M.; Beakou, B.H.; Anouar, A. Enhanced degradation of an azo dye by catalytic ozonation over Ni-containing layered double hydroxide catalyst. Sep. Purif. Technol. 2019, 210, 764–774. [Google Scholar] [CrossRef]
- Jayanti, M.; Lodh, B.K.; Sharma, R.; Mahata, N.; Shah, M.P.; Mandal, S.; Ghanta, S.; Bhunia, B. Advanced oxidation process for the treatment of industrial wastewater: A review on strategies, mechanisms, bottlenecks and prospects. Chemosphere 2023, 345, 140473. [Google Scholar] [CrossRef]
- Tan, X.Q.; Wan, Y.F.; Huang, Y.J.; He, C.; Zhang, Z.L.; He, Z.Y.; Hu, L.L.; Zeng, J.W.; Shu, D. Three-dimensional MnO2 porous hollow microspheres for enhanced activity as ozonation catalysts in degradation of bisphenol A. J. Hazard. Mater. 2017, 321, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, W.; Ma, L.; Huang, Y.; Wang, H. Characteristics and mechanisms of catalytic ozonation with Fe-shaving-based catalyst in industrial wastewater advanced treatment. J. Clean. Prod. 2019, 222, 174–181. [Google Scholar] [CrossRef]
- Huang, X.; Cui, W.; Yu, J.; Lu, S.; Liao, X. Preparation of Mesoporous MnO2 Catalysts with Different Morphologies for Catalytic Ozonation of Organic Compounds. Catal. Lett. 2022, 152, 1441–1450. [Google Scholar] [CrossRef]
- Yeşil, R.; Çetinkaya, S. Mn3O4/p(DCPD)HIPE nanocomposites as an efficient catalyst for oxidative degradation of phenol. J. Nanoparticle Res. 2020, 22, 198. [Google Scholar] [CrossRef]
- Chaliha, S.; Bhattacharyya, K.G. Using Mn(II)−MCM41 as an Environment-Friendly Catalyst to Oxidize Phenol, 2-Chlorophenol, and 2-Nitrophenol in Aqueous Solution. Ind. Eng. Chem. Res. 2008, 47, 1370–1379. [Google Scholar] [CrossRef]
- Wang, X.P.; Yang, Z.H.; Jiang, Y.Q.; Zhao, P.Q.; Meng, X. Adsorption and catalytic degradation of phenol in water by a Mn, N co-doped biochar via a non-radical oxidation process. Sep. Purif. Technol. 2024, 330, 125267. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Zheng, Y.; Dacquin, J.; Royer, S.; Zhang, H. Mechanism and kinetics of catalytic ozonation for elimination of organic compounds with spinel-type CuAl2O4 and its precursor. Sci. Total Environ. 2019, 651, 2585–2596. [Google Scholar] [CrossRef]
- Bing, J.; Hu, C.; Zhang, L. Enhanced mineralization of pharmaceuticals by surface oxidation over mesoporous γ-Ti-Al2O3 suspension with ozone. Appl. Catal. B Environ. 2017, 202, 118–126. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Pan, Z.; Ma, S.; Li, L. Synthesis of MnOx/SBA-15 for norfloxacin degradation by catalytic ozonation. Sep. Purif. Technol. 2017, 173, 99–104. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, J.; Jiang, Y.; Tang, X.; Zhou, Z.; Zhu, Y. Promotion of catalytic ozonation of aniline with Mn-Ce-Ox/γ-Al2O3. Water Sci. Technol. 2018, 78, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, W.; Tang, Y.; Li, L. Relationship between the structure of Fe-MCM-48 and its activity in catalytic ozonation for diclofenac mineralization. Chemosphere 2018, 206, 615–621. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Luan, P.; Mo, L.; Xu, J.; Li, J.; Zhu, L.; Zeng, J. Mineralization of recalcitrant organic pollutants in pulp and paper mill wastewaters through ozonation catalyzed by Cu-Ce supported on Al2O3. Bioresources 2018, 13, 3686–3703. [Google Scholar] [CrossRef]
- EzhilRaj, A.M.; Victora, S.G.; Jothy, V.B.; Ravidhas, C.; Wollschlager, J.; Suendorf, M.; Neumann, M.; Jayachandran, M.; Sanjeeviraja, C. XRD and XPS characterization of mixed valence Mn3O4 hausmannite thin films prepared by chemical spray pyrolysis technique. Appl. Surf. Sci. 2010, 256, 2920–2926. [Google Scholar] [CrossRef]
- Zhong, S.F.; Zhang, H.C. Mn(III)-ligand complexes as a catalyst in ligand-assisted oxidation of substituted phenols by permanganate in aqueous solution. J. Hazard. Mater. 2020, 384, 121404. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wen, L.; Yu, C.; Li, S.; Tang, J. Activation of peroxymonosulfate by MnFe2O4@BC composite for bisphenol A degradation: The coexisting of free-radical and non-radical pathways. Chem. Eng. J. 2022, 442, 136250. [Google Scholar] [CrossRef]
- Resende, K.; Braga, A.H.; Noronha, F.B.; Hori, C.E. Hydrodeoxygenation of phenol over Ni/Ce1−xNbxO2 catalysts. Appl. Catal. B Environ. 2019, 15, 100–113. [Google Scholar] [CrossRef]
- Uematsu, T.; Miyamoto, Y.M.; Ogasawara, Y.; Suzuki, K.; Yamaguchi, N. Mizuno Molybdenum-doped α-MnO2 as an efficient reusable heterogeneous catalyst for aerobic sulfide oxygenation. Catal. Sci. Technol. 2016, 6, 222–233. [Google Scholar] [CrossRef]
- Ma, G.; Tang, W.; Wang, A.; Zhang, L.; Guan, J.; Han, N.; Chen, Y. Heterogeneity CuO/Cu2O catalyst for highly efficient ozone removal. J. Environ. Sci. 2023, 125, 340–348. [Google Scholar] [CrossRef]
- Wang, W.L.; Hu, H.Y.; Liu, X.; Shi, H.X.; Zhou, T.H.; Wang, C.; Wu, Q.Y. Combination of catalytic ozonation by regenerated granular activated carbon (rGAC) and biological activated carbon in the advanced treatment of textile wastewater for reclamation. Chemosphere 2019, 231, 369–377. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).