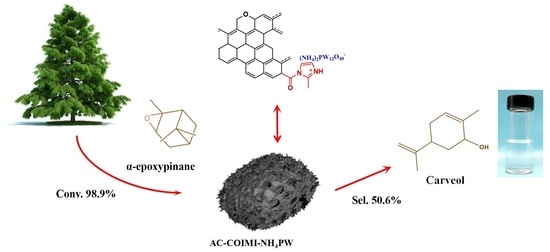
Ammonium Phosphotungstate Bonded on Imidazolized Activated Carbon for Selective Catalytic Rearrangement of α-Epoxypinane to Carveol
Abstract
1. Introduction
2. Results and Discussion
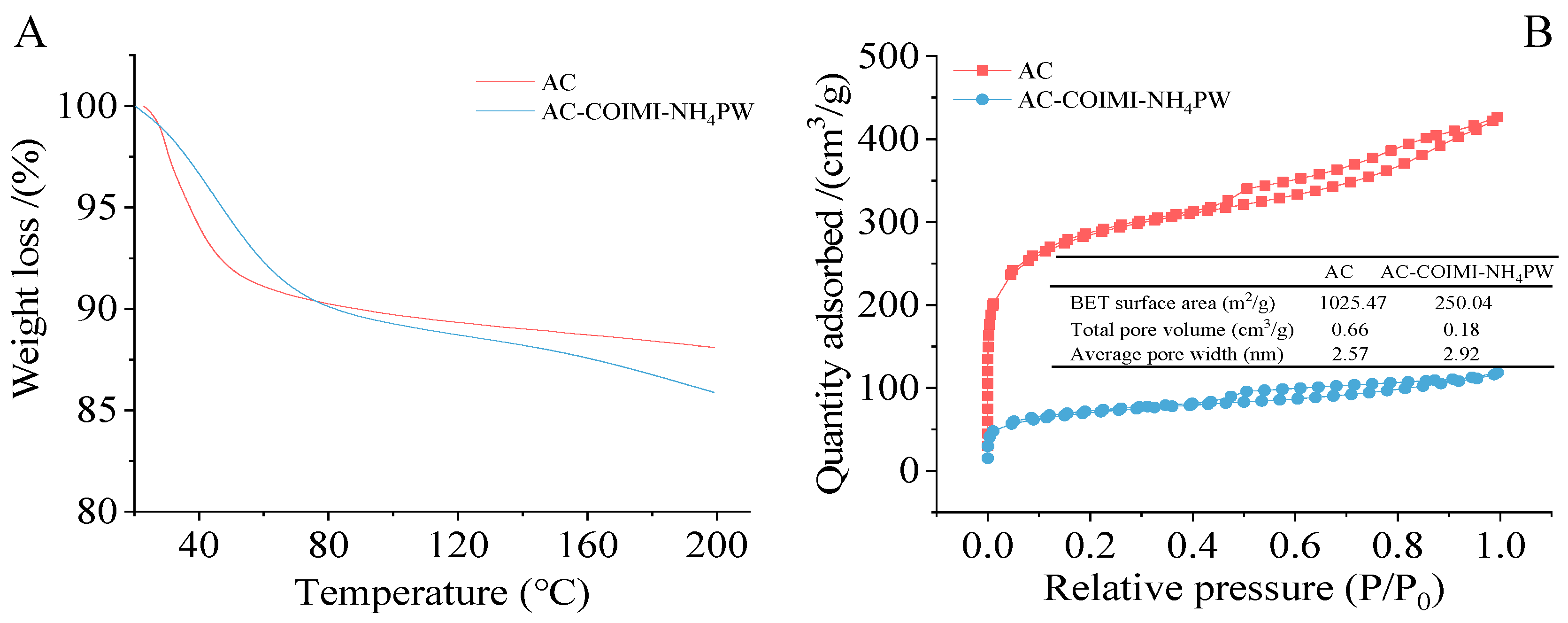
2.1. Structure and Texture Characteristics of Catalysts
2.2. Catalytic Conversion of α-Epoxypinane Rearrangement to Carveol
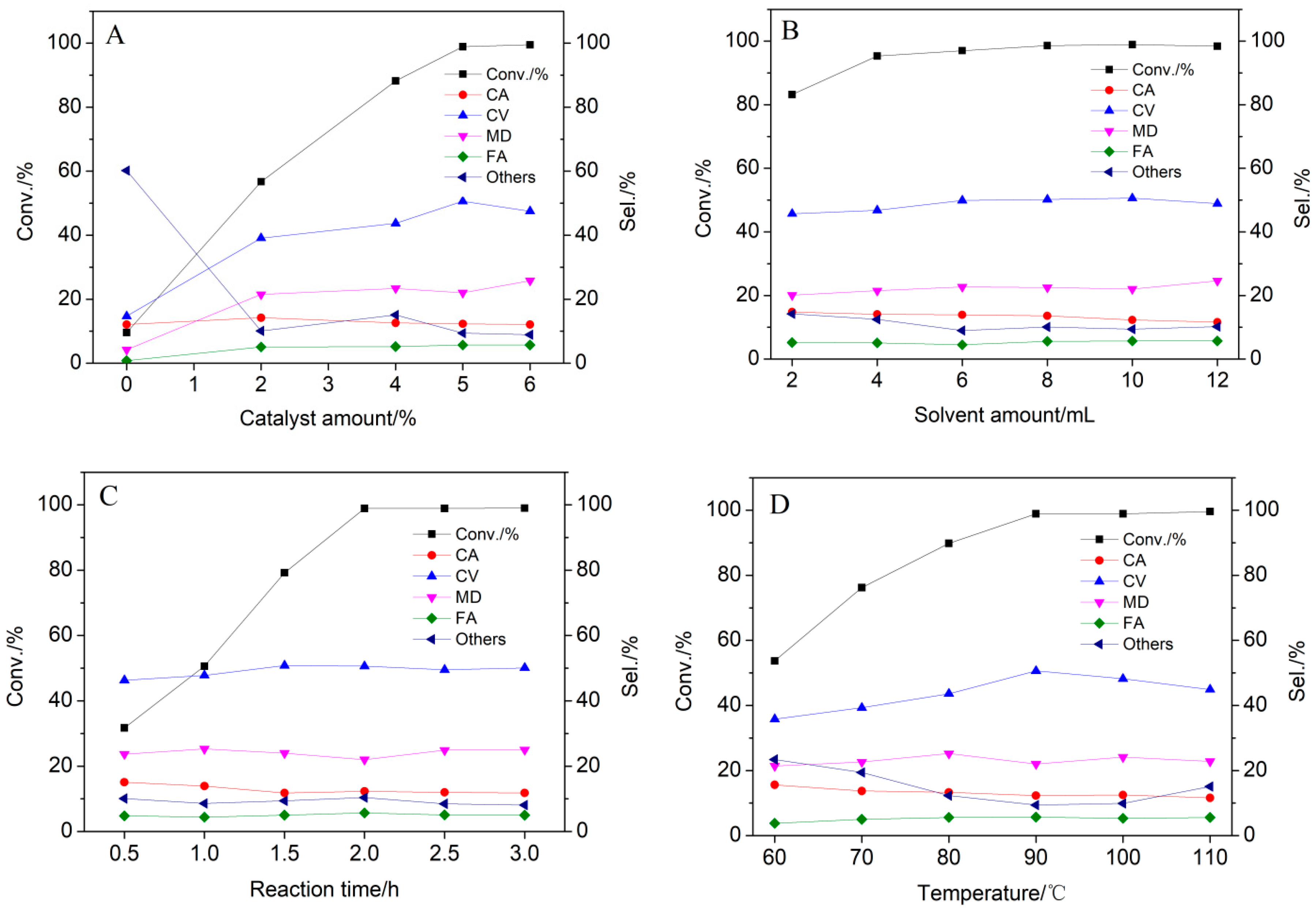
2.2.1. Effect of Catalysts
2.2.2. Solvent Modification
2.2.3. Effect of Reaction Conditions
2.3. Catalytic Stability
2.4. Analysis of the Literature Data on Other Ways of Obtaining Carveol
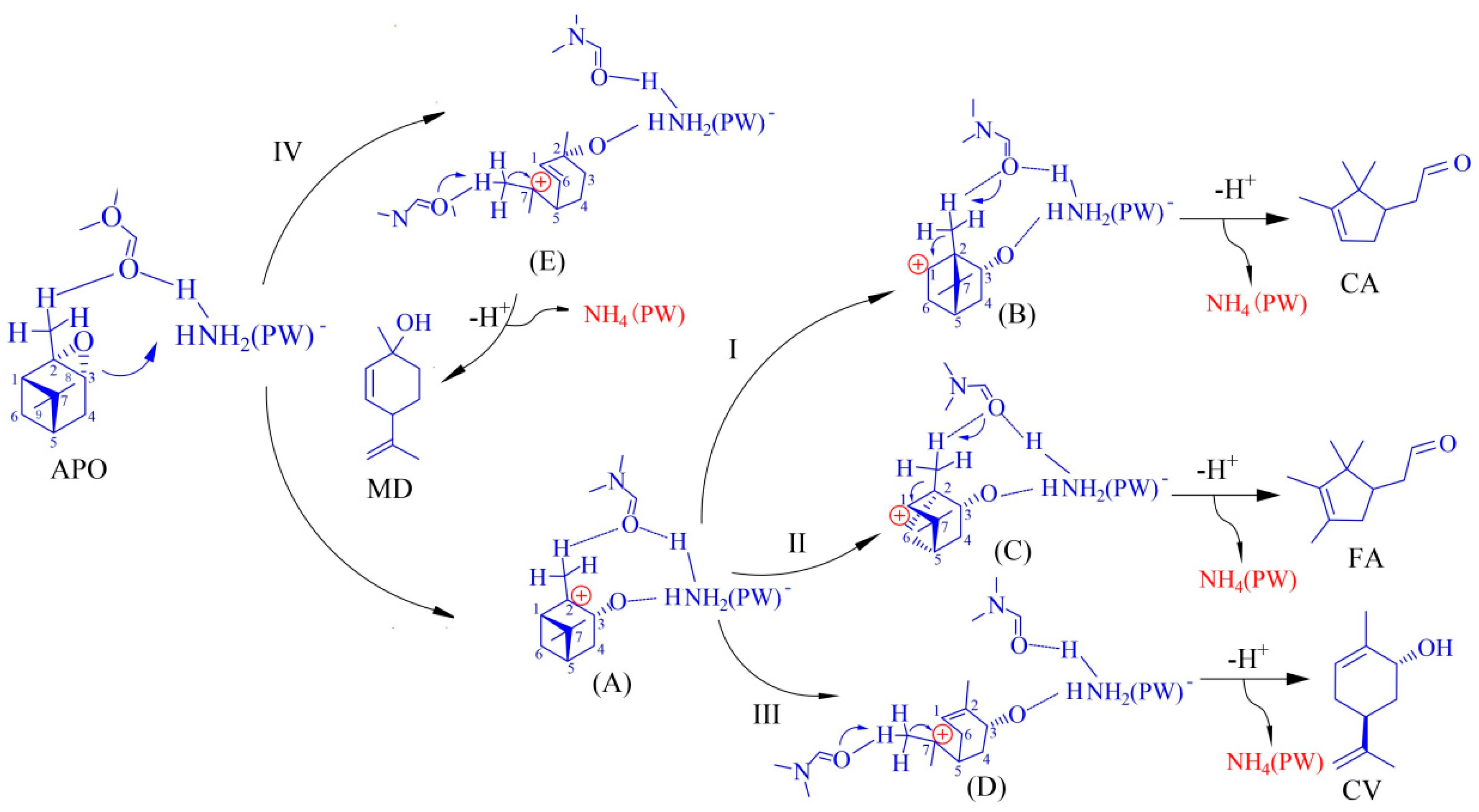
2.5. Plausible Mechanism of α-Epoxypinane Rearrangement
3. Materials and Methods
3.1. Reagent and Materials
3.2. Preparation and Characterization of AC-COIMI-NH4PW Catalysts
3.3. Rearrangement of α-Epoxypinane
3.4. Characterization of AC-COIMI-NH4PW
3.5. Reusing of the Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on carveol. Food Chem. Toxicol. 2008, 46, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Alattar, A.; Alvi, M.A.; Rashid, S.; Hussain, N.; Gul, M.; Ikram, M.; Khalil, A.A.K.; Alshaman, R.; Shah, F.A.; Li, S.; et al. Carveol ameliorates mercury-induced oxidative stress, neuroinflammation, and neurodegeneration in a mouse brain. NeuroToxicology 2022, 92, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Noma, Y.; Asakawa, Y. Enantio and diastereoselectivity in the biotransformation of carveols by Euglena gracilis. Z. Phytochemistry 1992, 31, 2009–2011. [Google Scholar] [CrossRef]
- Ain, R.; Elmar, A.; Anne, O.; Tiiu, K.; Mati, M. Composition of the essential oil of Levisticum officinale WDJ Koch from some European countries. J. Essent. Oil Res. 2008, 20, 318–322. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Khan, A.U.; Kury, L.T.A.; Shah, F.A. Computational and pharmacological evaluation of carveol for antidiabetic potential. Front. Pharmacol. 2020, 11, 1677–1684. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, S.; Yi, X.; Wang, J.; Zhao, Z.; Jiang, J. High value-added application of turpentine as a potential renewable source for the synthesis of heterocyclic schiff base derivatives of cis-1,8-p-menthane-diamine serving as botanical herbicides. Ind. Crops Prod. 2018, 115, 111–116. [Google Scholar] [CrossRef]
- Salvador, V.T.; Silva, E.S.; Gonçalves, P.G.C.; Cella, R. Biomass transformation: Hydration and isomerization reactions of turpentine oil using ion exchange resins as catalyst. Sustain. Chem. Pharm. 2020, 15, 100214. [Google Scholar] [CrossRef]
- Ljunggren, J.; Bylund, D.; Jonsson, B.G.; Edman, M.; Hedenström, E. Antifungal efficiency of individual compounds and evaluation of non-linear effects by recombining fractionated turpentine. Microchem. J. 2020, 153, 104325. [Google Scholar] [CrossRef]
- Merghni, A.; Lassoued, M.A.; Rasoanirina, B.N.V.; Moumni, S.; Mastouri, M. Characterization of Turpentine nanoemulsion and assessment of its antibiofilm potential against methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2022, 166, 105530. [Google Scholar] [CrossRef]
- Patil, M.V.; Yadav, M.K.; Jasra, R.V. Catalytic epoxidation of α-pinene with molecular oxygen using cobalt (II)-exchanged zeolite Y-based heterogeneous catalysts. J. Mol. Catal. A Chem. 2007, 277, 72–80. [Google Scholar] [CrossRef]
- Raupp, Y.S.; Yildiz, C.; Kleist, W.; Meier, M.A.R. Aerobic oxidation of α-pinene catalyzed by homogeneous and MOF-based Mn catalysts. Appl. Catal. A Gen. 2017, 546, 1–6. [Google Scholar] [CrossRef]
- Maharana, T.; Nath, N.; Pradhan, H.C.; Mantri, S.; Routaray, A.; Sutar, A.K. Polymer-supported first-row transition metal schiff base complexes: Efficient catalysts for epoxidation of alkenes. React. Funct. Polym. 2022, 171, 105142. [Google Scholar] [CrossRef]
- Costa, V.V.; Rocha, K.A.S.; Sousa, L.F.; Dutenhefner, P.A.R.; Gusevskaya, E.V. Isomerization of α-pinene oxide over cerium and tin catalysts: Selective synthesis of carveol and trans-sobrerol. J. Mol. Catal. A Chem. 2011, 345, 69–74. [Google Scholar] [CrossRef]
- Ribeiro, C.J.A.; Pereira, M.M.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V.; Rocha, K.A.S.R. Heteropoly acid catalysts in upgrading of biorenewables: Synthesis of para-menthenic fragrance compounds from α-pinene oxide. Catal. Today 2020, 344, 166–170. [Google Scholar] [CrossRef]
- Singh, A.S.; Naikwadi, D.R.; Ravi, K.; Biradar, A.V. Chemoselective isomerization of α-pinene oxide to carveol by robust and mild Brønsted acidic zirconium phosphate catalyst. Mol. Catal. 2022, 521, 112189. [Google Scholar] [CrossRef]
- Barakov, R.; Shcherban, N.; Mäki-Arvela, P.; Yaremov, P.; Bezverkhyy, I.; Wärnå, J.; Murzin, D.Y. Hierarchical Beta zeolites as catalysts in α-pinene oxide isomerization. ACS Sustain. Chem. Eng. 2022, 10, 6642–6656. [Google Scholar] [CrossRef]
- Valvekens, P.; Bloch, E.D.; Long, J.R.; Ameloot, R.; Vos, D.E. Counteranion effects on the catalytic activity of copper salts immobilized on the 2,2′-bipyridine-functionalized metal-organic framework MOF-253. Catal. Today 2015, 246, 55–59. [Google Scholar] [CrossRef]
- Sundaravel, B.; Babu, C.M.; Vinodh, R.; Cha, W.S.; Jang, H.T. Synthesis of campholenic aldehyde from α-pinene using bi-functional PrAlPO-5 molecular sieves. J. Taiwan. Inst. Chem. Eng. 2016, 63, 157–165. [Google Scholar] [CrossRef]
- Sidorenko, A.Y.; Aho, A.; Ganbaatar, J.; Batsuren, D.; Utenkova, D.B.; Sen’kov, G.M.; Wärnå, J.; Murzin, D.Y.; Agabekov, V.E. Catalytic isomerization of α-pinene and 3-carene in the presence of modified layered aluminosilicates. Mol. Catal. 2017, 443, 193–202. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Agudelo-Cifuentes, A.; Villa, A.L. Kinetics of the isomerization of α-pinene epoxide over Fe supported MCM-41 and SBA-15 materials. React. Kinet. Mech. Catal. 2019, 128, 1005–1028. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Gelves, J.F.; Márquez, M.A.; Dorkis, L.; Villa, A.L. Catalytic isomerization of α-pinene epoxide over a natural zeolite. Catal. Lett. 2020, 150, 3132–3148. [Google Scholar] [CrossRef]
- Singh, A.S.; Advani, J.H.; Biradar, A.V. Phosphonate functionalized carbon spheres as Brønsted acid catalysts for the valorization of bio-renewable α-pinene oxide to carveol. Dalton Trans. 2020, 21, 7210–7217. [Google Scholar] [CrossRef] [PubMed]
- Vrbková, E.; Vyskocilová, E.; Lhotka, M.; Cervený, L. Solvent influence on selectivity in α-pinene oxide isomerization using MoO3-Modified aeolite BETA. Catalysts 2020, 10, 1244. [Google Scholar] [CrossRef]
- Zheng, M.; He, H.; Li, X.Z.; Yin, D.L. Imidazolized activated carbon anchoring phosphotungstic acid as recyclable catalyst for oxidation of alcohols with aqueous hydrogen peroxide. Front. Chem. 2022, 10, 925622. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, X.Z.; Xun, Y.Y.; Wang, J.H.; Yin, D.L. Selective catalytic epoxidation–hydration of α-pinene with hydrogen peroxide to sobrerol by durable ammonium phosphotungstate immobilized on imidazolized activated carbon. Nanomaterials 2023, 13, 1554. [Google Scholar] [CrossRef] [PubMed]
- Advani, J.H.; Singh, A.S.; Khan, N.U.; Bajaj, H.C.; Biradar, A.V. Black yet green: Sulfonic acid functionalized carbon as an efficient catalyst for highly selective isomerization of α-pinene oxide to carveol. Appl. Catal. B Environ. 2020, 268, 118456. [Google Scholar] [CrossRef]
- Liu, W.Z.; Guo, R.K.; Peng, G.M.; Yin, D.L. Sulfuric acid immobilized on activated carbon aminated with ethylenediamine: An efficient reusable catalyst for the synthesis of acetals (ketals). Nanomaterials 2022, 12, 1462. [Google Scholar] [CrossRef]
- He, H.T.; Zheng, M.; Liu, Q.; Liu, J.; Zhao, J.; Zhuang, Y.T.; Liu, X.X.; Xu, Q.; Steven, R.K.; Yin, D.L. Hydroxyl-assisted selective epoxidation of perillyl alcohol with hydrogen peroxide by vanadium-substituted phosphotungstic acid hinged on imidazolyl activated carbon. New J. Chem. 2022, 46, 6636–6645. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Wang, Y.; Wang, H.; Song, H. Preparation of highly active g-C3N4 supported amphiphilic quaternary ammonium phosphotungstate catalyst for solvent-free oxidative desulfurization of benzothiophene. React. Kinet. Mech. Catal. 2022, 135, 219–231. [Google Scholar] [CrossRef]
- Toledo, R.B.C.; Aragón-Tobar, C.F.; Gámez, S.; Torre, E. Reactivation process of activated carbons: Effect on the mechanical and adsorptive properties. Molecules 2020, 25, 1681. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Zhao, J.; He, H.; Jiang, D.; Kirk, S.R.; Xu, Q.; Liu, X.; Yin, D. Selectively catalytic isomerization of β-pinene oxide to perillyl alcohol enhanced by tetra-imidazole nitrate ionic liquid. Chemistryopen 2021, 10, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Maki-Arvela, P.; Shcherban, N.; Lozachmeur, C.; Russo, V.; Warna, J.; Murzin, D.Y. Isomerization of α-pinene oxide: Solvent effects, kinetics and thermodynamics. Catal. Lett. 2019, 149, 203–214. [Google Scholar] [CrossRef]
- Shcherban, N.D.; Barakov, R.Y.; Mäki-Arvela, P.; Sergiienko, S.A.; Bezverkhyy, I.; Eränen, K.; Murzin, D.Y. Isomerization of α-pinene oxide over ZSM-5 based micro-mesoporous materials. Appl. Catal. A Gen. 2018, 560, 236–247. [Google Scholar] [CrossRef]
- Sidorenko, A.Y.; Kravtsova, A.V.; Aho, A.; Heinmaa, I.; Kuznetsova, T.F.; Murzin, D.Y.; Agabekov, V.E. Catalytic isomerization of α-pinene oxide in the presence of acid-modified. Mol. Catal. 2018, 448, 18–29. [Google Scholar] [CrossRef]
- Sanchez-Velamdia, J.E.; Villa, A.L. Selective synthesis of high-added value chemicals from α-pinene epoxide and limonene epoxide isomerization over mesostruc- tured catalysts: Effect of the metal loading and solvent. Catal. Today 2021, 394–396, 208–218. [Google Scholar] [CrossRef]
- Rocha, K.S.; Hoehne, J.L.; Gusevskaya, E.V. Phosphotungstic acid as a versatile catalyst for the synthesis of fragrance compounds by α-pinene oxide isomerization: Solvent-induced chemoselectivity. Chem.-A Eur. J. 2008, 14, 6166–6172. [Google Scholar] [CrossRef]
- Coelho, J.V.; Meireles, A.L.P.M.; Rocha, K.A.S.; Pereira, M.C.; Oliveira, L.C.A.; Gusevskaya, E.V. Isomerization of α-pinene oxide catalyzed by iron-modified mesoporous silicates. Appl. Catal. A Gen. 2012, 443–444, 125–132. [Google Scholar] [CrossRef]
- Ravi, K.; Naikwadi, D.R.; Bankar, B.D.; Biradar, A.V. Sustainable isomerization of α-pinene oxide to trans-carveol using formic acid/aniline system at room temperature. Adv. Sustain. Syst. 2021, 5, 00212. [Google Scholar] [CrossRef]






| Catalyst | Conv. (%) | Sel. (%) | ||||
|---|---|---|---|---|---|---|
| CA | CV | MD | FA | Others | ||
| AC | 23.8 | 11.9 | 32.8 | 17.1 | 6.3 | 27.9 |
| AC-COIMI-HNO3 | 89.3 | 10.2 | 40.8 | 24.7 | 6.4 | 17.9 |
| (NH4)3PW | 98.0 | 12.7 | 44.3 | 28.2 | 5.8 | 9.0 |
| AC-COIMI-NH4PW | 98.9 | 12.3 | 50.6 | 22.0 | 5.7 | 9.4 |
| AC-COIMI-HPW | 99.0 | 9.1 | 17.4 | 12.6 | 6.2 | 54.7 |
| Solvent | B.P. (°C) | Dielectric Constant | Conv. (%) | Sel. (%) | ||||
|---|---|---|---|---|---|---|---|---|
| CA | CV | MD | FA | Others | ||||
| Toluene | 111 | 2.4 | 1.6 | 7.5 | 12.2 | 13.8 | 0 | 66.5 |
| a Trichloromethane | 61 | 4.7 | 5.8 | 8.9 | 9.8 | 17.6 | 0 | 63.7 |
| a Ethyl acetate | 77 | 6.0 | 3.3 | 7.8 | 12.4 | 16.5 | 0 | 63.3 |
| a Acetone | 57 | 20.7 | 7.9 | 21.7 | 11.7 | 6.2 | 0 | 60.4 |
| a Acetonitrile | 82 | 37.5 | 75.2 | 25.9 | 33.2 | 17.5 | 0 | 23.4 |
| Nitromethane | 101 | 38.6 | 96.1 | 29.8 | 29.5 | 10.7 | 0 | 30.0 |
| DMF | 153 | 38.7 | 98.9 | 12.3 | 50.6 | 22.0 | 5.7 | 9.4 |
| NMP | 203 | 48.9 | 95.4 | 20.5 | 43.1 | 17.6 | 4.3 | 14.5 |
| Substrate/Catalyst | T/°C | t/h | Conv.% | Sel. a/% | Times b | Ref. |
|---|---|---|---|---|---|---|
| AC-COIMI-NH4PW | 90 | 2 | 98.9 | 50.6 | 6 | This work |
| Ce/SiO2 | 140 | 98 | 98 | 73 | 1 | [13] |
| CsPW | 25 | 5 | 100 | 22 | 1 | [14] |
| Zirconium phosphate | 160 | 5 | 100 | 76 | 1 | [15] |
| ZSM-5 | 140 | 2 | 82.0 | 42.5 | 1 | [33] |
| Acidic clays | 50 | 2 | 99.9 | 15.7 | 1 | [34] |
| Fe/MCM-41 | 70 | 2.5 | 57.5 | 26.8 | 1 | [35] |
| HPW | 140 | 3 | 100 | 90 | 1 | [36] |
| Fe2+-MCM | 40 | 3 | 100 | 64 | 1 | [37] |
| Formic Acid/Aniline | RT | 1 | 100 | 76 | 1 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Li, X.; Yin, D.; Kirk, S.R.; Li, H.; Zhou, P.; Yang, Y. Ammonium Phosphotungstate Bonded on Imidazolized Activated Carbon for Selective Catalytic Rearrangement of α-Epoxypinane to Carveol. Catalysts 2024, 14, 36. https://doi.org/10.3390/catal14010036
Zheng M, Li X, Yin D, Kirk SR, Li H, Zhou P, Yang Y. Ammonium Phosphotungstate Bonded on Imidazolized Activated Carbon for Selective Catalytic Rearrangement of α-Epoxypinane to Carveol. Catalysts. 2024; 14(1):36. https://doi.org/10.3390/catal14010036
Chicago/Turabian StyleZheng, Min, Xiangzhou Li, Dulin Yin, Steven R. Kirk, Hui Li, Peng Zhou, and Yanhong Yang. 2024. "Ammonium Phosphotungstate Bonded on Imidazolized Activated Carbon for Selective Catalytic Rearrangement of α-Epoxypinane to Carveol" Catalysts 14, no. 1: 36. https://doi.org/10.3390/catal14010036
APA StyleZheng, M., Li, X., Yin, D., Kirk, S. R., Li, H., Zhou, P., & Yang, Y. (2024). Ammonium Phosphotungstate Bonded on Imidazolized Activated Carbon for Selective Catalytic Rearrangement of α-Epoxypinane to Carveol. Catalysts, 14(1), 36. https://doi.org/10.3390/catal14010036