Cu/MgO as an Efficient New Catalyst for the Non-Oxidative Dehydrogenation of Ethanol into Acetaldehyde
Abstract
1. Introduction
2. Results and Discussion
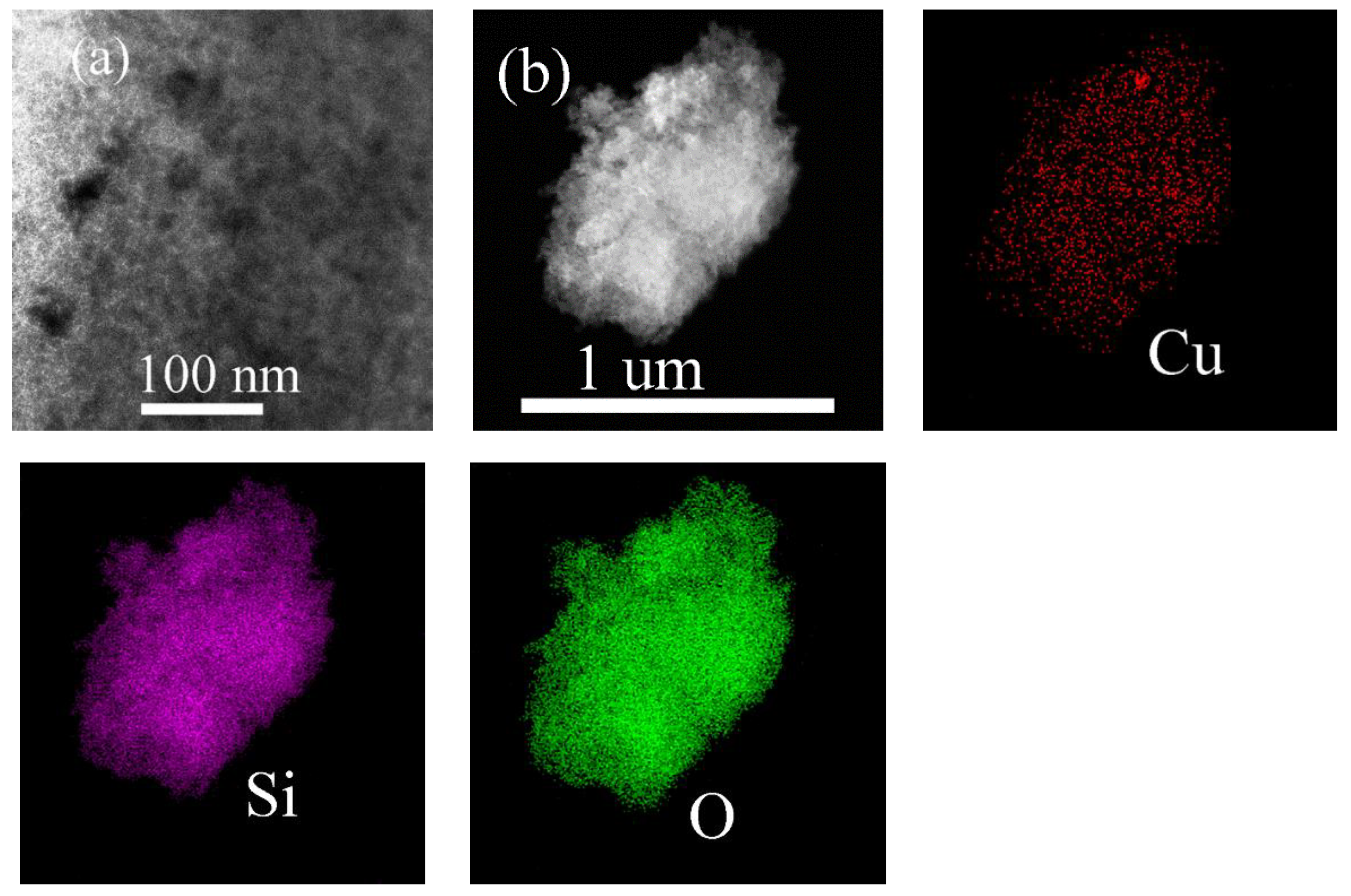
2.1. Structural and Textural Properties
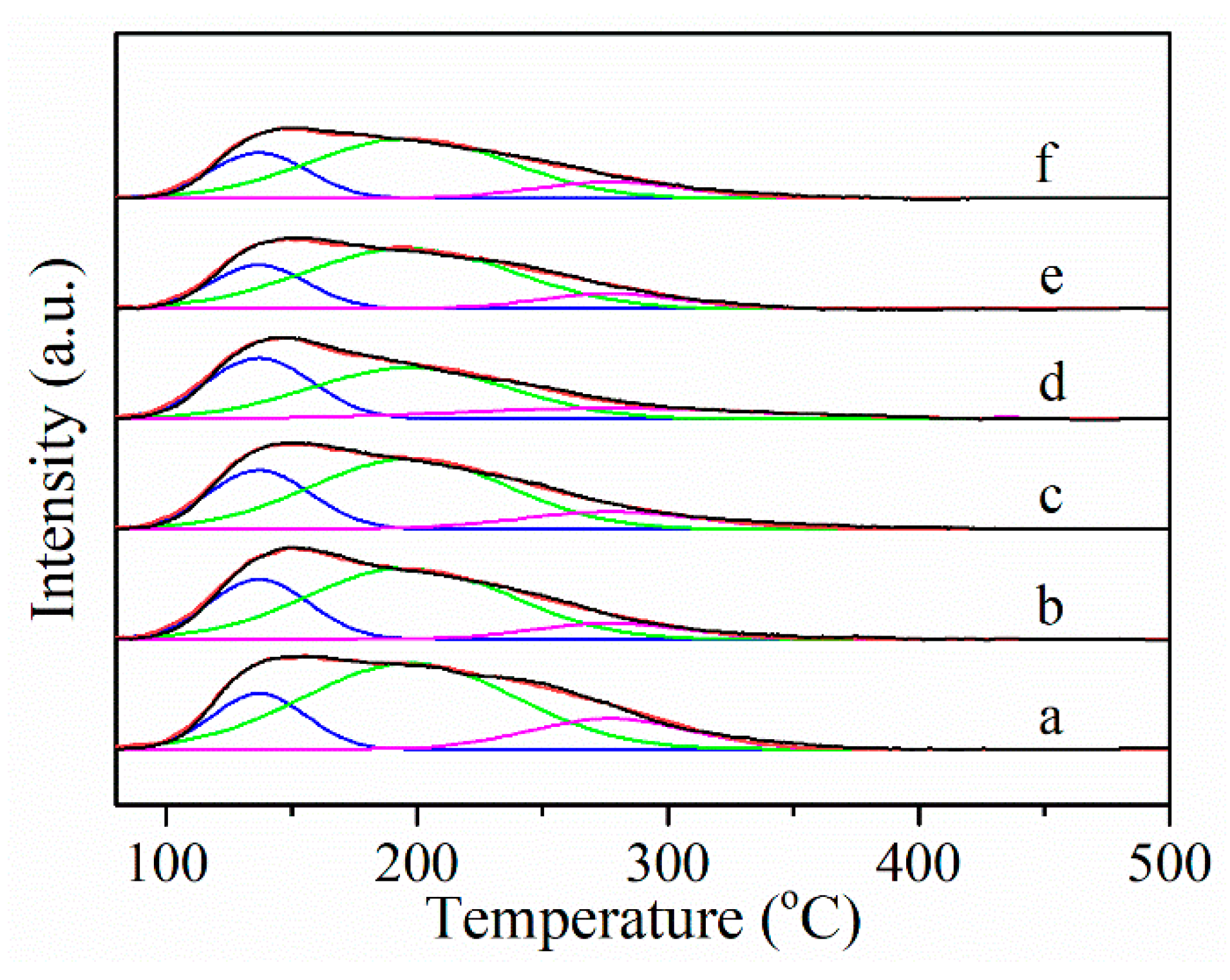
2.2. Basic Properties
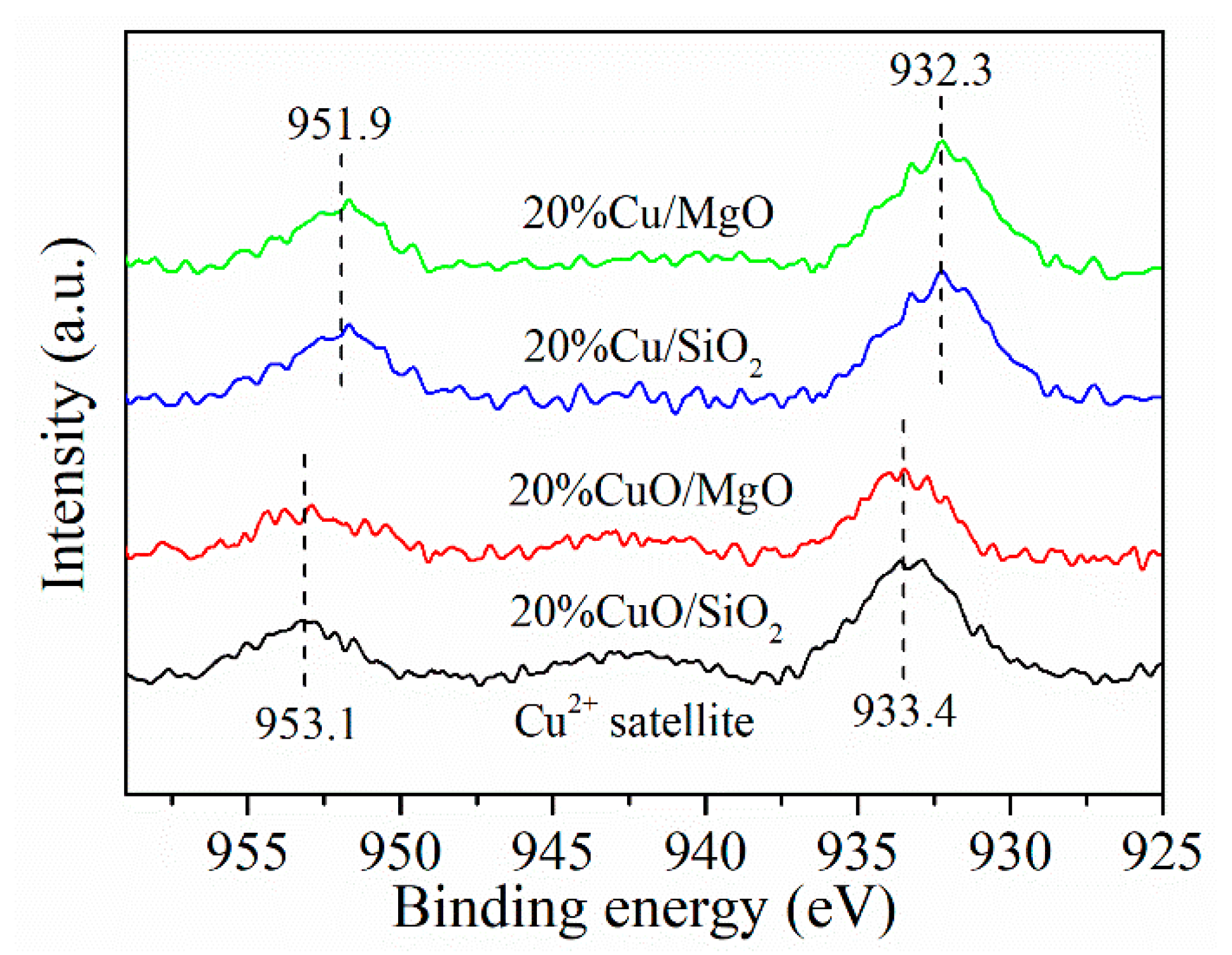
2.3. XPS and TPR
2.4. Catalytic Performance
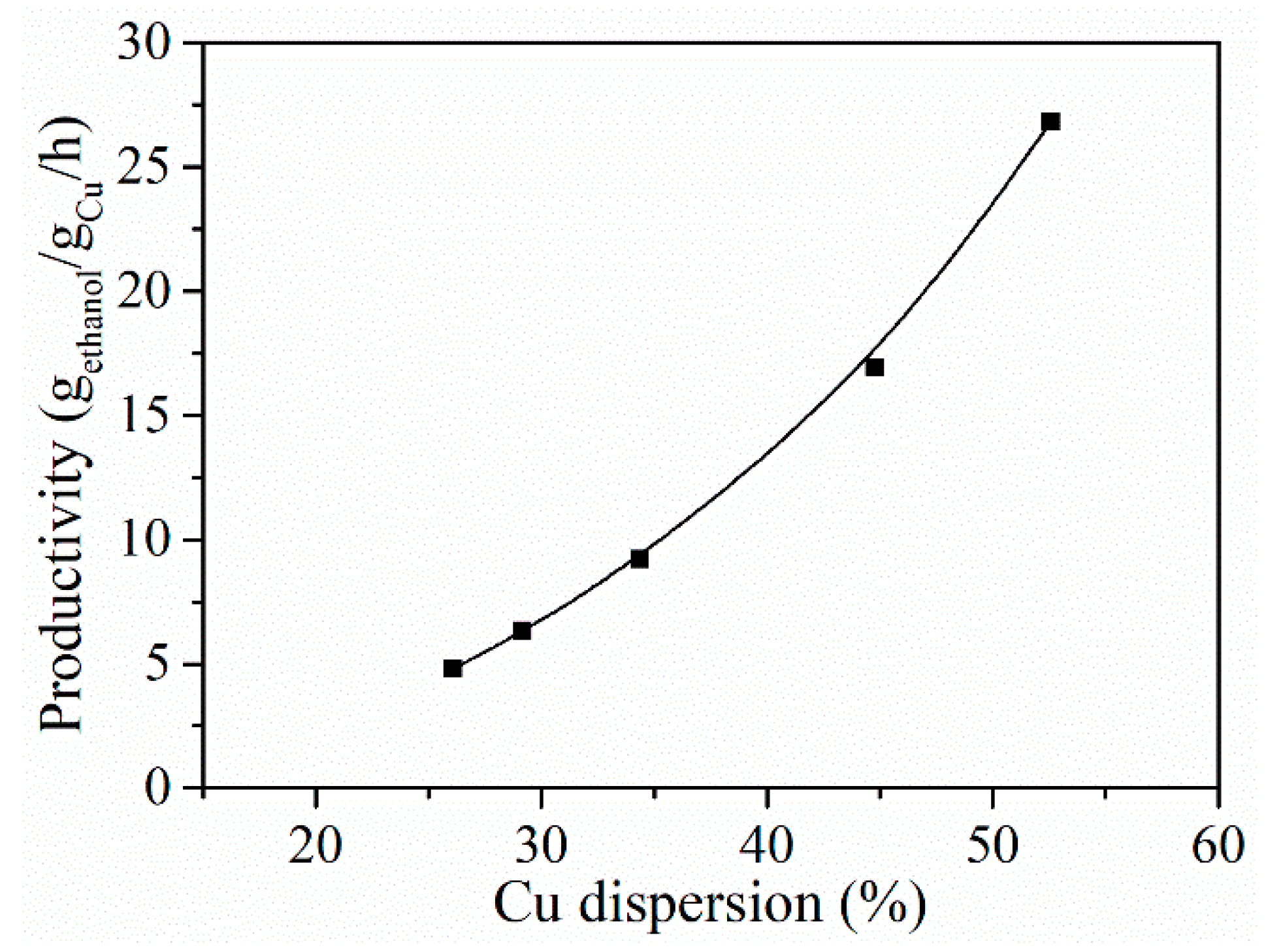
2.4.1. Effect of Cu Loading and Reaction Temperature
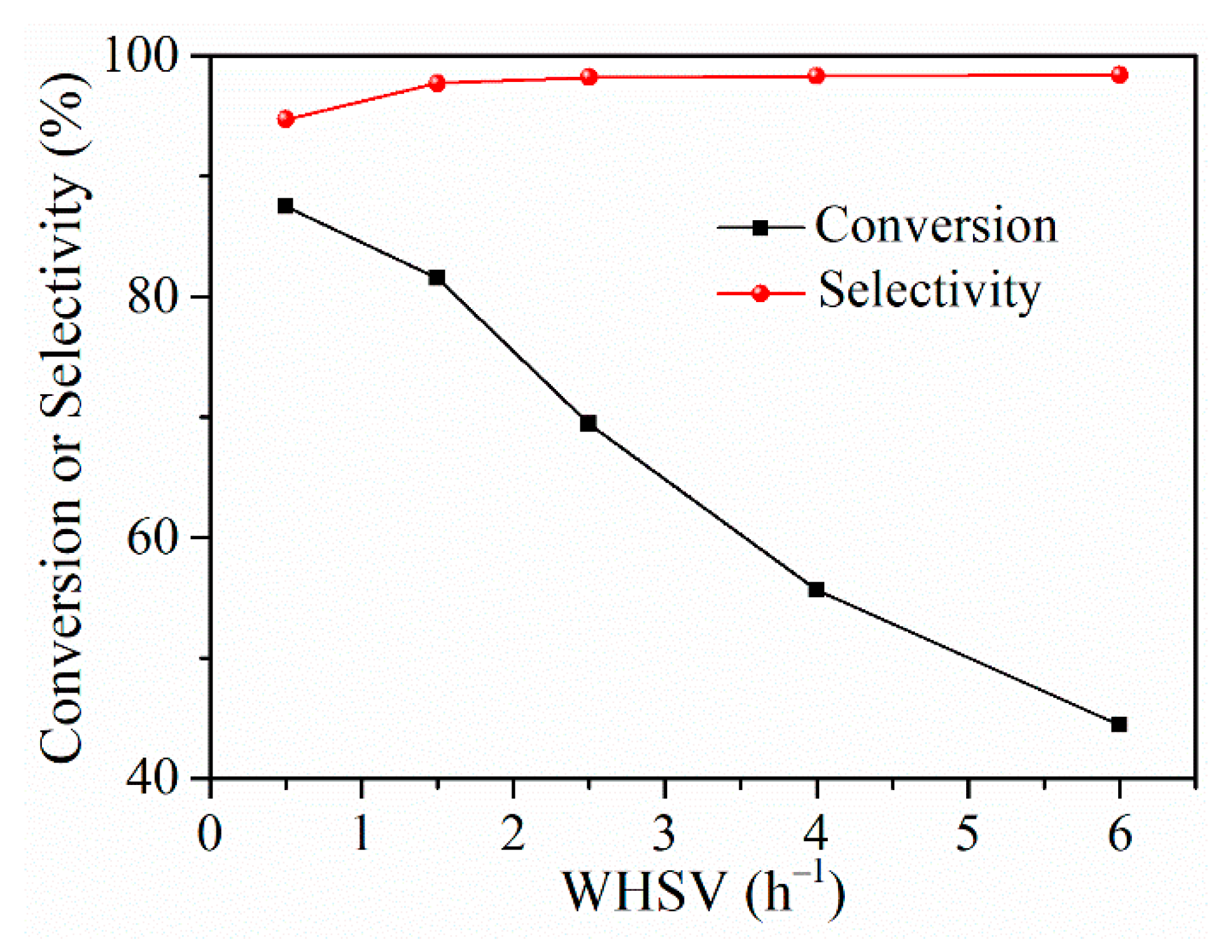
2.4.2. Effect of WHSV
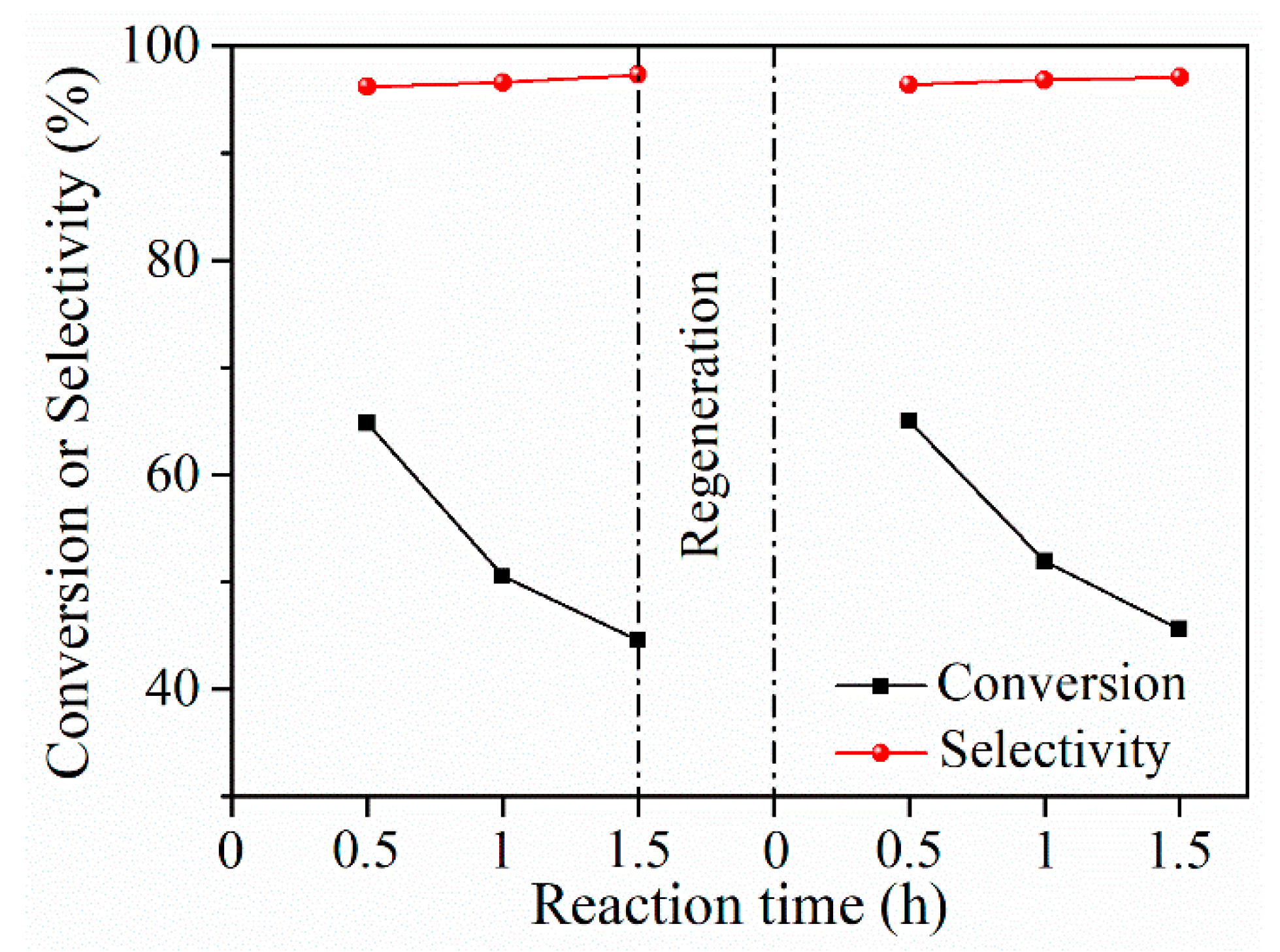
2.4.3. Comparison of Performance for Cu/MgO with Cu/SiO2 in a Prolonged Time
3. Materials and Methods
3.1. Reagent and Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Wang, Y. Recent advances in catalytic conversion of ethanol to chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Li, X.; Kant, A.; He, Y.; Thakkar, H.V.; Atanga, M.A.; Rezaei, F.; Ludlow, D.K.; Rownaghi, A.A. Light olefins from renewable resources: Selective catalytic dehydration of bioethanol to propylene over zeolite and transition metal oxide catalysts. Catal. Today 2016, 276, 62–77. [Google Scholar] [CrossRef]
- Iwamoto, M. Selective catalytic conversion of bio-ethanol to propene: A review of catalysts and reaction pathways. Catal. Today 2015, 242, 243–248. [Google Scholar] [CrossRef]
- Pomalaza, G.; Capron, M.; Ordomsky, V.; Dumeignil, F. Recent breakthroughs in the conversion of ethanol to butadiene. Catalysts 2016, 6, 203. [Google Scholar] [CrossRef]
- Wu, X.; Fang, G.; Tong, Y.; Jiang, D.; Liang, Z.; Leng, W.; Liu, L.; Tu, P.; Wang, H.; Ni, J.; et al. Catalytic upgrading of ethanol to n-butanol: Progress in catalyst development. ChemSusChem 2018, 11, 71–85. [Google Scholar] [CrossRef]
- Masih, D.; Rohani, S.; Kondo, J.; Tatsumi, T. Catalytic dehydration of ethanol-to-ethylene over Rho zeolite under mild reaction conditions. Microporous Mesoporous Mater. 2019, 282, 91–99. [Google Scholar] [CrossRef]
- Wang, Q.; Weng, X.; Zhou, B.; Lv, S.; Miao, S.; Zhang, D.; Han, Y.; Scott, S.L.; Schüth, F.; Lu, A. Direct, selective production of aromatic alcohols from ethanol using a tailored bifunctional cobalt–hydroxyapatite catalyst. ACS Catal. 2019, 9, 7204–7216. [Google Scholar] [CrossRef]
- Li, X.; Pang, J.; Wang, C.; Li, L.; Pan, X.; Zheng, M.; Zhang, T. Conversion of ethanol to 1,3-butadiene over high-performance Mg-ZrOx/MFI nanosheet catalysts via the two-step method. Green Chem. 2020, 22, 2852–2861. [Google Scholar] [CrossRef]
- Pang, J.; Yin, M.; Wu, P.; Li, X.; Li, H.; Zheng, M.; Zhang, T. Advances in catalytic dehydrogenation of ethanol to acetaldehyde. Green Chem. 2021, 23, 7902–7916. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, B.; Yuan, H.; Sun, Y.; Yang, D.; Cui, X.; Shi, F. The catalytic dehydrogenation of ethanol by heterogeneous catalysts. Catal. Sci. Technol. 2021, 11, 1652–1664. [Google Scholar] [CrossRef]
- Phung, T.K. Copper-based catalysts for ethanol dehydrogenation and dehydrogenative coupling into hydrogen, acetaldehyde and ethyl acetate. Int. J. Hydrogen Energy 2022, 47, 42234–42249. [Google Scholar] [CrossRef]
- He, L.; Zhou, B.; Sun, D.; Li, W.; Lv, W.; Wang, J.; Liang, Y.; Lu, A. Catalytic conversion of ethanol to oxygen-containing value-added chemicals. ACS Catal. 2023, 13, 11291–11304. [Google Scholar] [CrossRef]
- Jin, H.; Ding, X.; Tian, C.; Yue, Y.; Hua, W.; Gao, Z. Insights into promoting effect of Sm on catalytic performance of the CeO2/Beta catalyst in direct conversion of bioethanol to propylene. Chem. Res. Chin. Univ. 2022, 38, 1547–1552. [Google Scholar] [CrossRef]
- Jin, H.; Miao, C.; Yue, Y.; Tian, C.; Hua, W.; Gao, Z. Sm-CeO2/zeolite bifunctional catalyst for direct and highly selective conversion of bioethanol to propylene. Catalysts 2022, 12, 407. [Google Scholar] [CrossRef]
- Jin, H.; Yue, Y.; Miao, C.; Tian, C.; Hua, W.; Gao, Z. Direct and highly selective conversion of bioethanol to propylene over Y-CeO2 and zeolite Beta composite. Catal. Lett. 2023, 153, 230–238. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; de Freitas, I.C.; Longo, E.; Bueno, J.M.C. Site-selective ethanol conversion over supported copper catalysts. Catal. Commun. 2012, 26, 122–126. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, H.R.; Jaenicke, S.; Chuah, G.K. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. J. Catal. 2020, 389, 19–28. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Wang, C.; Yang, X.; Liu, H.; Liu, X.; Sun, J.; Wang, Y.; Zhang, T. Hierarchical echinus-like Cu-MFI catalysts for ethanol dehydrogenation. ACS Catal. 2020, 10, 13624–13629. [Google Scholar] [CrossRef]
- Pang, J.; Yin, M.; Wu, P.; Song, L.; Li, X.; Zhang, T.; Zheng, M. Redispersion and stabilization of Cu/MFI catalysts by the encapsulation method for ethanol dehydrogenation. ACS Sustain. Chem. Eng. 2023, 11, 3297–3305. [Google Scholar] [CrossRef]
- Finger, P.H.; Osmari, T.A.; Cabral, N.M.; Bueno, J.M.C.; Marcel, J.; Gallo, R. Direct synthesis of Cu supported on mesoporous silica: Tailoring the Cu loading and the activity for ethanol dehydrogenation. Catal. Today 2021, 381, 26–33. [Google Scholar] [CrossRef]
- Lin, L.; Cao, P.; Pang, J.; Wang, Z.; Jiang, Q.; Su, Y.; Chen, R.; Wu, Z.; Zheng, M.; Luo, W. Zeolite-encapsulated Cu nanoparticles with enhanced performance for ethanol dehydrogenation. J. Catal. 2022, 413, 565–574. [Google Scholar] [CrossRef]
- Liu, H.; Chang, Z.; Fu, J.; Hou, Z. A CuZn-BTC derived stable Cu/ZnO@SiO2 catalyst for ethanol dehydrogenation. Appl. Catal. B 2023, 324, 122194. [Google Scholar] [CrossRef]
- Campisano, I.S.P.; Rodella, C.B.; Sousa, Z.S.B.; Henriques, C.A.; da Silva, V.T. Influence of thermal treatment conditions on the characteristics of Cu-based metal oxides derived from hydrotalcite-like compounds and their performance in bio-ethanol dehydrogenation to acetaldehyde. Catal. Today 2018, 306, 111–120. [Google Scholar] [CrossRef]
- Santos, R.M.M.; Briois, V.; Martins, L.; Santilli, C.V. Insights into the preparation of copper catalysts supported on layered double hydroxide derived mixed oxides for ethanol dehydrogenation. ACS Appl. Mater. Interfaces 2021, 13, 26001–26012. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, Y.; Zhou, R.; Chang, Z.; Hou, Z. Co-production of hydrogen and acetaldehyde from ethanol over a highly dispersed Cu catalyst. Fuel 2022, 321, 123980. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, L.; Lu, A. Highly selective copper catalyst supported on mesoporous carbon for the dehydrogenation of ethanol to acetaldehyde. ChemCatChem 2015, 7, 2846–2852. [Google Scholar] [CrossRef]
- Klinthongchai, Y.; Prichanont, S.; Praserthdam, P.; Jongsomjit, B. Synthesis, characteristics and application of mesocellular foam carbon (MCF-C) as catalyst for dehydrogenation of ethanol to acetaldehyde. J. Environ. Chem. Eng. 2020, 8, 103752. [Google Scholar] [CrossRef]
- Shi, R.; Wang, F.; Mu, X.; Ta, N.; Li, Y.; Huang, X.; Shen, W. Transfer dehydrogenation of 1-octanol to 1-octanal over Cu/MgO catalyst: Effect of Cu particle size. Chin. J. Catal. 2010, 31, 626–630. [Google Scholar] [CrossRef]
- Wang, F.; Shi, R.; Liu, Z. Highly efficient dehydrogenation of primary aliphatic alcohols catalyzed by Cu nanoparticles dispersed on rod-shaped La2O2CO3. ACS Catal. 2013, 3, 890–894. [Google Scholar] [CrossRef]
- Xia, S.; Nie, R.; Lu, X.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol over Cu0.4/Zn5.6−xMgxAl2O8.6 catalysts: The role of basicity and hydrogen spillover. J. Catal. 2012, 296, 1–11. [Google Scholar] [CrossRef]
- Hincapié, G.; López, D.; Moreno, A. Infrared analysis of methanol adsorption on mixed oxides derived from Mg/Al hydrotalcite catalysts for transesterification reactions. Catal. Today 2018, 302, 277–285. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Gervasini, A.; Manzoli, M.; Martra, G.; Ponti, A.; Ravasio, N.; Sordelli, L.; Zaccheria, F. Dependence of copper species on the nature of the support for dispersed CuO catalysts. J. Phys. Chem. B 2006, 110, 7851–7861. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Shengping Wang, S.; Ma, X. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0–Cu+ sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Amokrane, S.; Boualouache1, A.; Simon, P.; Capron, M.; Otmanine1, G.; Allam, D.; Hocine, S. Effect of adding transition metals to copper on the dehydrogenation reaction of ethanol. Catal. Lett. 2021, 151, 2864–2883. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; Meira, D.M.; Damyanova, S.; Longo, E.; Bueno, J.M.C. Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J. Catal. 2013, 307, 1–17. [Google Scholar] [CrossRef]
- Wang, Q.N.; Shi, L.; Li, W.; Li, W.C.; Si, R.; Schüth, F.; Lu, A.H. Cu supported on thin carbon layer-coated porous SiO2 for efficient ethanol dehydrogenation. Catal. Sci. Technol. 2018, 8, 472–479. [Google Scholar] [CrossRef]
- Cheng, S.Q.; Weng, X.F.; Wang, Q.N.; Zhou, B.C.; Li, W.C.; Li, M.R.; He, L.; Wang, D.Q.; Lu, A.H. Defect-rich BN-supported Cu with superior dispersion for ethanol conversion to aldehyde and hydrogen. Chin. J. Catal. 2022, 43, 1092–1100. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, D.; Liu, W.; Zhang, M.; Lou, Y.; Wang, Z.; Tang, Q.; Yang, J. Uniformly dispersed Cu nanoparticles over mesoporous silica as a highly selective and recyclable ethanol dehydrogenation catalyst. Catalysts 2022, 12, 1049. [Google Scholar] [CrossRef]
- Chen, L.F.; Guo, P.J.; Qiao, M.H.; Yan, S.R.; Li, H.X.; Shen, W.; Xu, H.L.; Fan, K.N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Cui, X.; Wang, G.; Cen, Y.; Deng, T.; Yan, W.; Gao, J.; Zhu, S.; Olsbye, U.; et al. A highly stable copper-based catalyst for clarifying the catalytic roles of Cu0 and Cu+ species in methanol dehydrogenation. Angew. Chem. Int. Ed. 2018, 57, 1836–1840. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, G.; Zhao, Y.; Liu, P.; Duan, X.; Gu, L.; Fu, G.; Yuan, Y.; Zheng, N. Interfacing with silica boosts the catalysis of copper. Nat. Commun. 2018, 9, 3367. [Google Scholar] [CrossRef]
- Ding, Y.; Tian, J.; Chen, W.; Guan, Y.; Xu, H.; Li, X.; Wu, H.; Wu, P. One-pot synthesized core/shell structured zeolite@copper catalysts for selective hydrogenation of ethylene carbonate to methanol and ethylene glycol. Green Chem. 2019, 21, 5414–5426. [Google Scholar] [CrossRef]
- Tu, Y.J.; Chen, Y.W. Effects of alkali metal oxide additives on Cu/SiO2 catalyst in the dehydrogenation of ethanol. Ind. Eng. Chem. Res. 2001, 40, 5889–5893. [Google Scholar] [CrossRef]
- Dong, X.; Ma, X.; Xu, H.; Ge, Q. Comparative study of silica-supported copper catalysts prepared by different methods: Formation and transition of copper phyllosilicate. Catal. Sci. Technol. 2016, 6, 4151–4158. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Kang, H.; Chen, J.; Zhang, X.; Xia, C. Highly dispersed silica-supported copper nanoparticles prepared by precipitation−gel method: A simple but efficient and stable catalyst for glycerol hydrogenolysis. Chem. Mater. 2008, 20, 5090–5099. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Sugino, K.; Sawabe, K.; Satsuma, A. Oxidant-free dehydrogenation of alcohols heterogeneously catalyzed by cooperation of silver clusters and acid–base sites on alumina. Chem. Eur. J. 2009, 15, 2341–2351. [Google Scholar] [CrossRef]
- Han, Y.; Shen, J.; Chen, Y. Microkinetic analysis of isopropanol dehydrogenation over Cu/SiO2 catalyst. Appl. Catal. A 2001, 205, 79–84. [Google Scholar] [CrossRef]
- Freitas, I.C.; Damyanova, S.; Oliveira, D.C.; Marques, C.M.P.; Bueno, J.M.C. Effect of Cu content on the surface and catalytic properties of Cu/ZrO2 catalyst for ethanol dehydrogenation. J. Mol. Catal. A 2014, 381, 26–37. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Sodesawa, T.; Yuma, K.I.; Obata, Y. Distinction between surface and bulk oxidation of Cu through N2O decomposition. J. Catal. 2000, 196, 195–199. [Google Scholar] [CrossRef]








| Catalyst | SBET | Basicity (mmol/g) | DCu b | Coke c | |||
|---|---|---|---|---|---|---|---|
| (m2/g) | Weak | Medium | Strong | Total | (%) | (g CHx/mmol Cu) | |
| MgO | 95 | 0.028 | 0.098 | 0.027 | 0.153 | − | − |
| 2.5%Cu/MgO | 63 | 0.026 | 0.078 | 0.013 | 0.117 | 52.6 | 0.107 |
| 5%Cu/MgO | 61 | 0.026 | 0.070 | 0.014 | 0.110 | 44.8 | 0.054 |
| 10%Cu/MgO | 59 | 0.028 | 0.068 | 0.013 | 0.109 | 34.4 | 0.025 |
| 15%Cu/MgO | 55 | 0.028 | 0.064 | 0.013 | 0.105 | 29.2 | 0.021 |
| 20%Cu/MgO | 45 | 0.027 | 0.063 | 0.013 | 0.103 | 26.1 | 0.021 |
| 20%Cu/SiO2 | 201 a | 0.013 | 0.017 | 0.006 | 0.036 | 3.6 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, C.; Yue, Y.; Miao, C.; Hua, W.; Gao, Z. Cu/MgO as an Efficient New Catalyst for the Non-Oxidative Dehydrogenation of Ethanol into Acetaldehyde. Catalysts 2024, 14, 541. https://doi.org/10.3390/catal14080541
Tian C, Yue Y, Miao C, Hua W, Gao Z. Cu/MgO as an Efficient New Catalyst for the Non-Oxidative Dehydrogenation of Ethanol into Acetaldehyde. Catalysts. 2024; 14(8):541. https://doi.org/10.3390/catal14080541
Chicago/Turabian StyleTian, Chao, Yinghong Yue, Changxi Miao, Weiming Hua, and Zi Gao. 2024. "Cu/MgO as an Efficient New Catalyst for the Non-Oxidative Dehydrogenation of Ethanol into Acetaldehyde" Catalysts 14, no. 8: 541. https://doi.org/10.3390/catal14080541
APA StyleTian, C., Yue, Y., Miao, C., Hua, W., & Gao, Z. (2024). Cu/MgO as an Efficient New Catalyst for the Non-Oxidative Dehydrogenation of Ethanol into Acetaldehyde. Catalysts, 14(8), 541. https://doi.org/10.3390/catal14080541








