Investigation of the Performances of TiO2 and Pd@TiO2 in Photocatalytic Hydrogen Evolution and Hydrogenation of Acetylenic Compounds for Application in Photocatalytic Transfer Hydrogenation
Abstract
1. Introduction
2. Results and Discussion
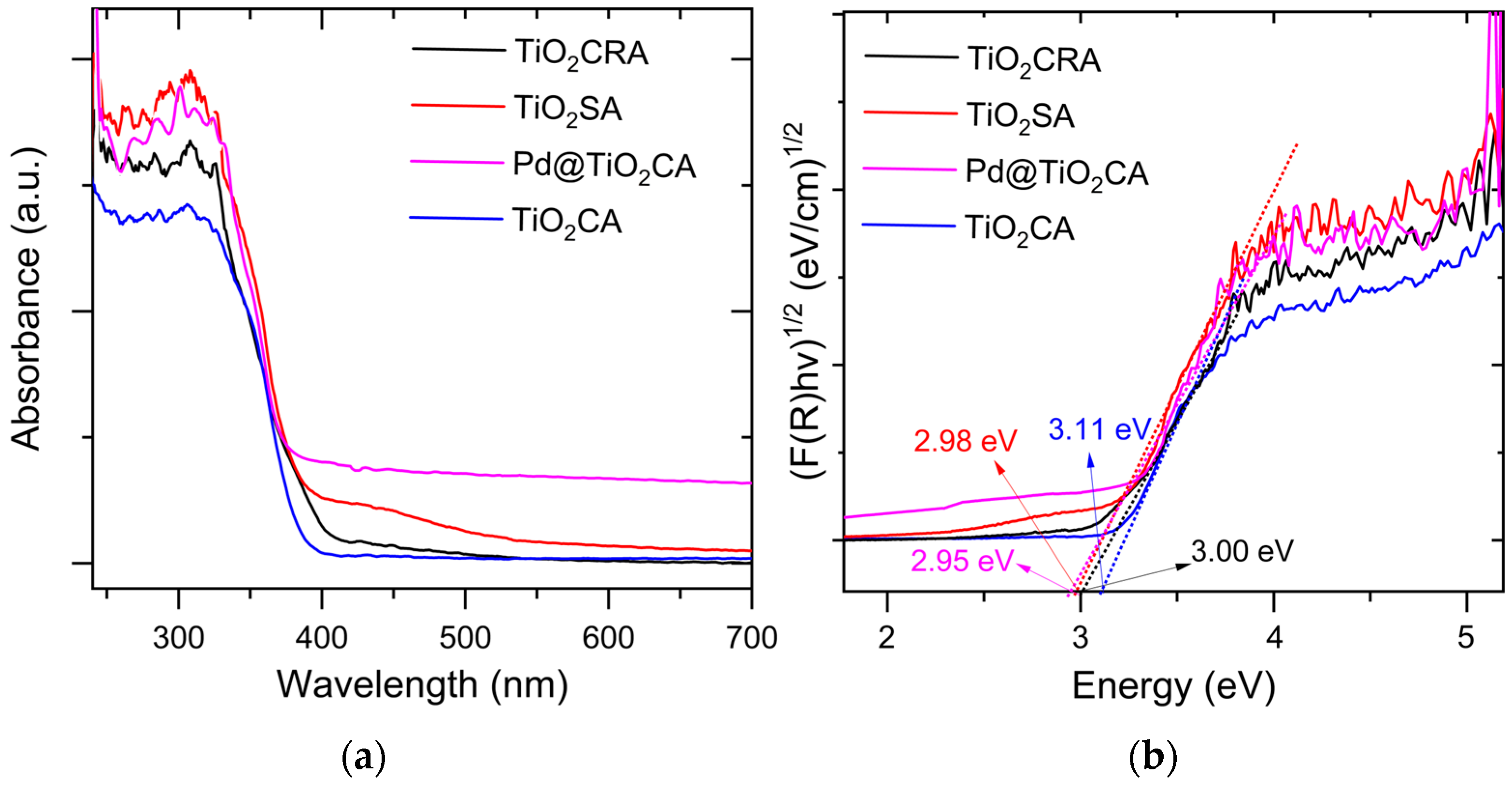
2.1. Characterization of TiO2 Samples and Pd Catalysts Based on Them
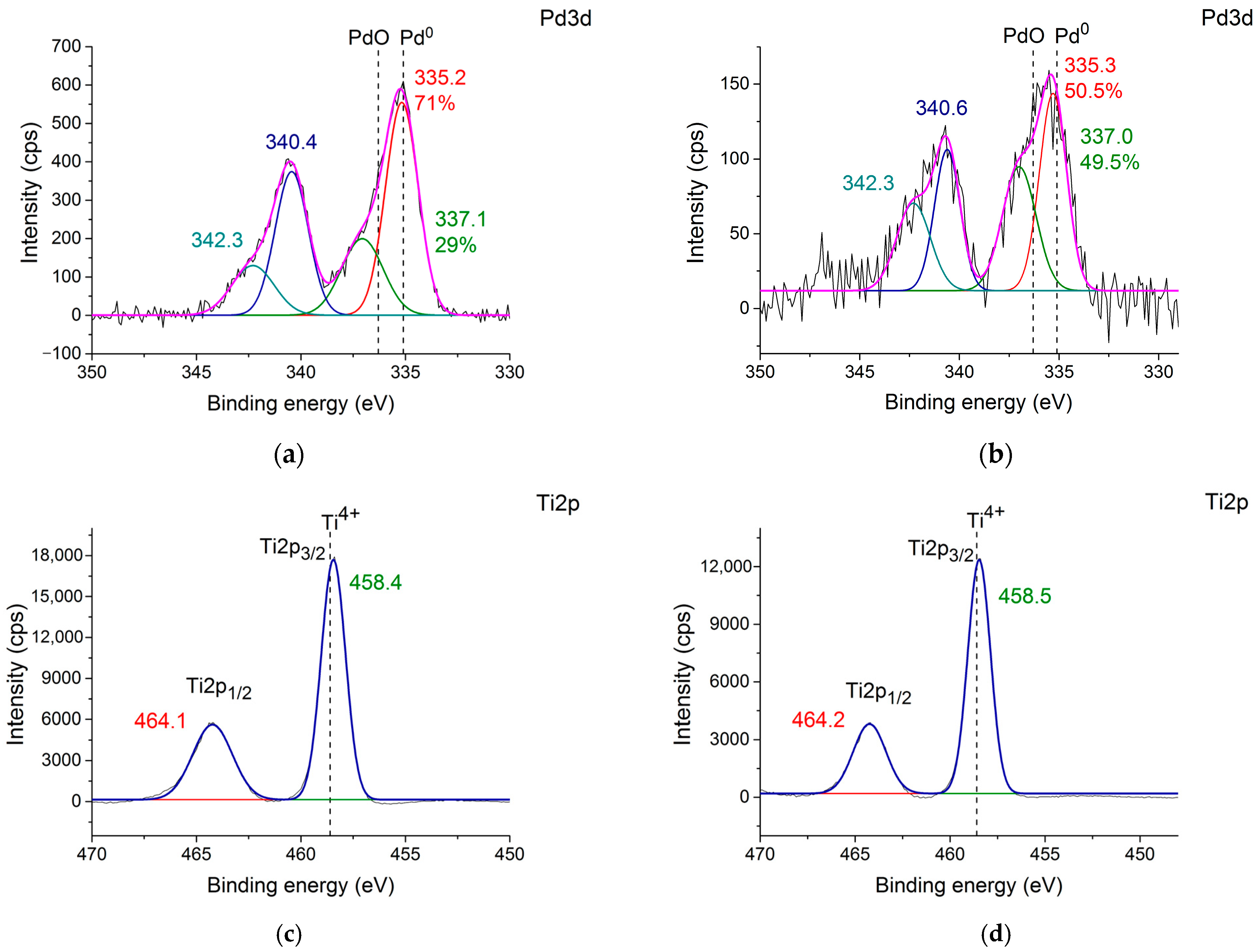
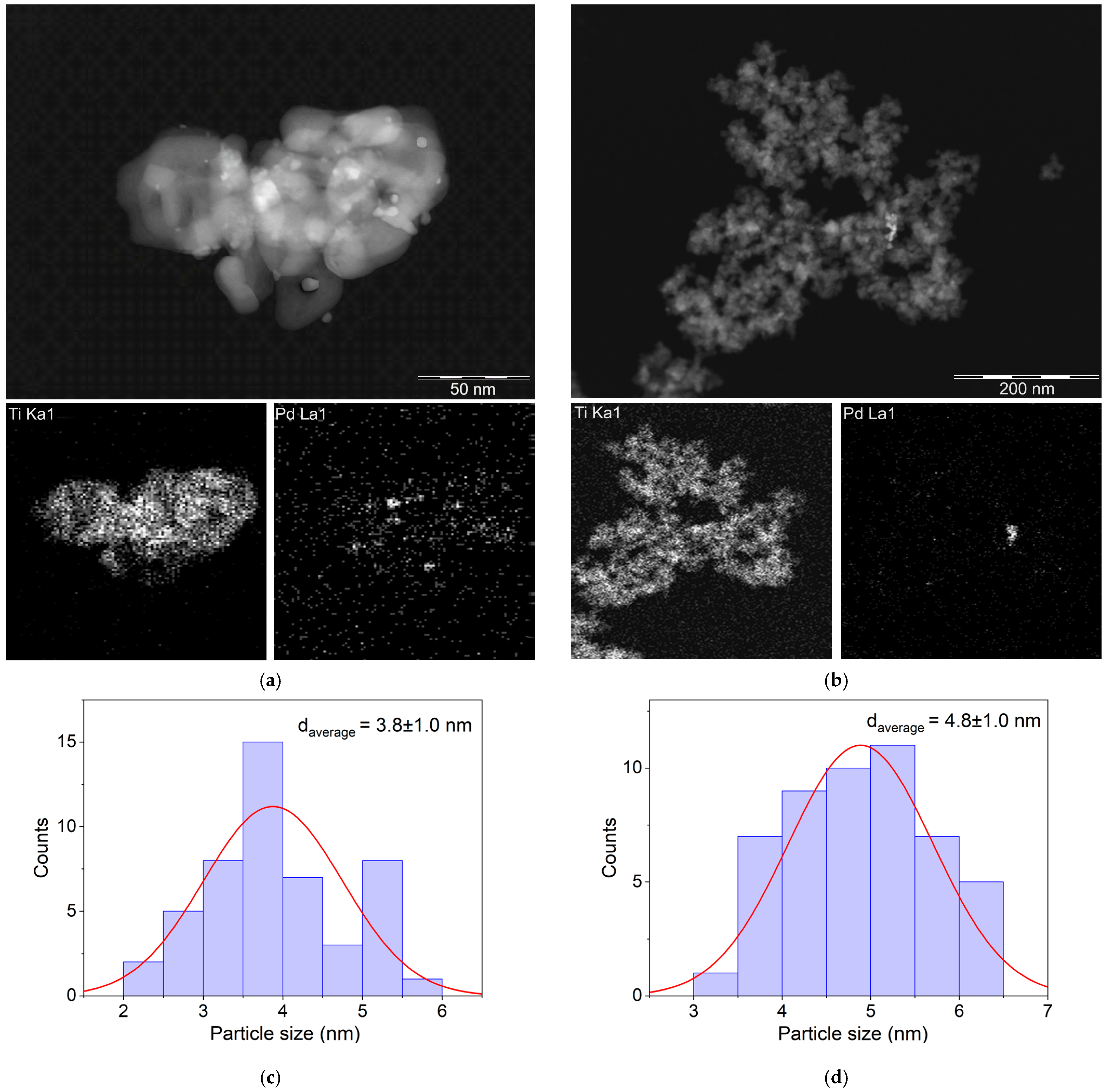
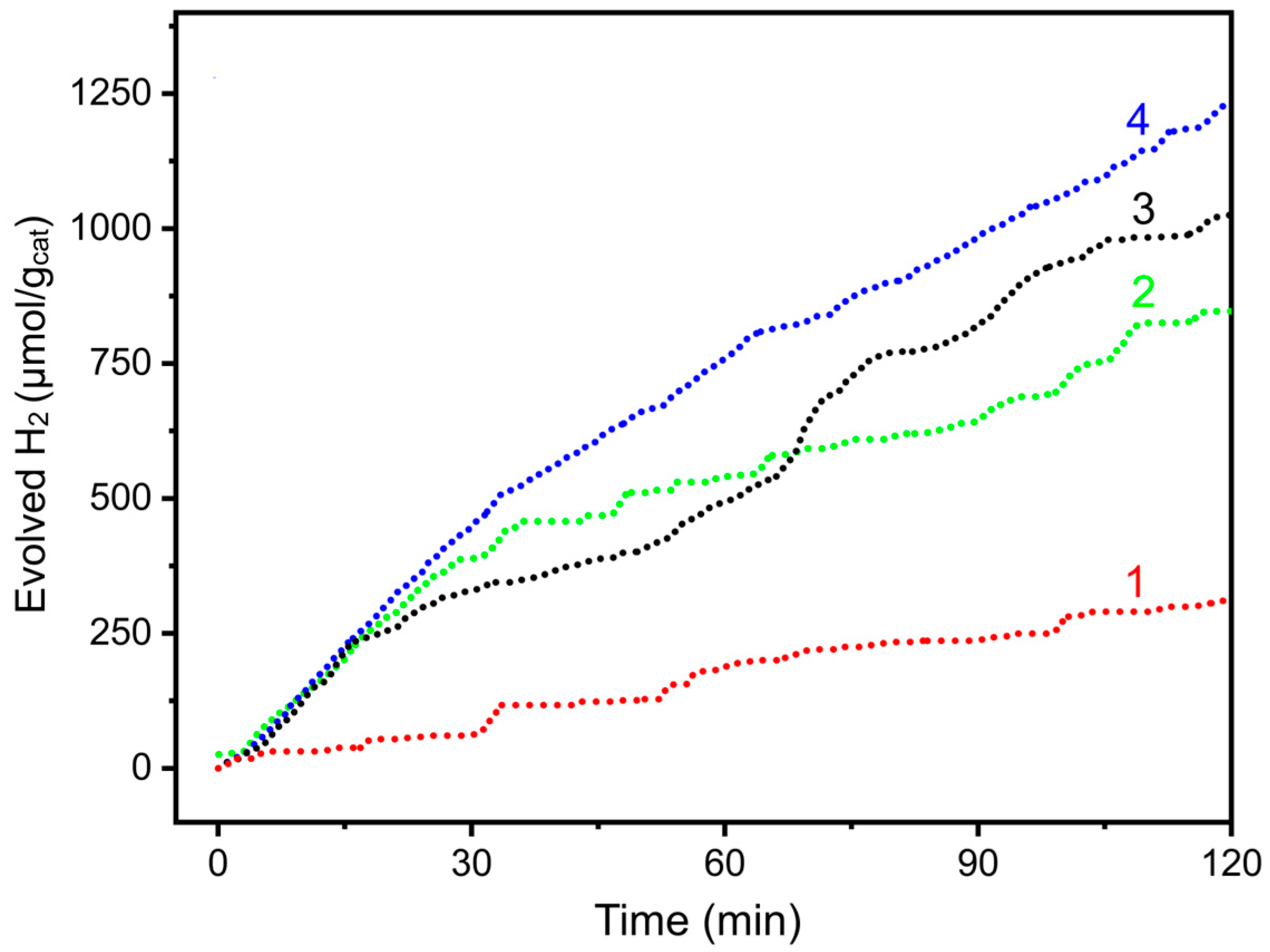
2.2. Photocatalytic H2 Production
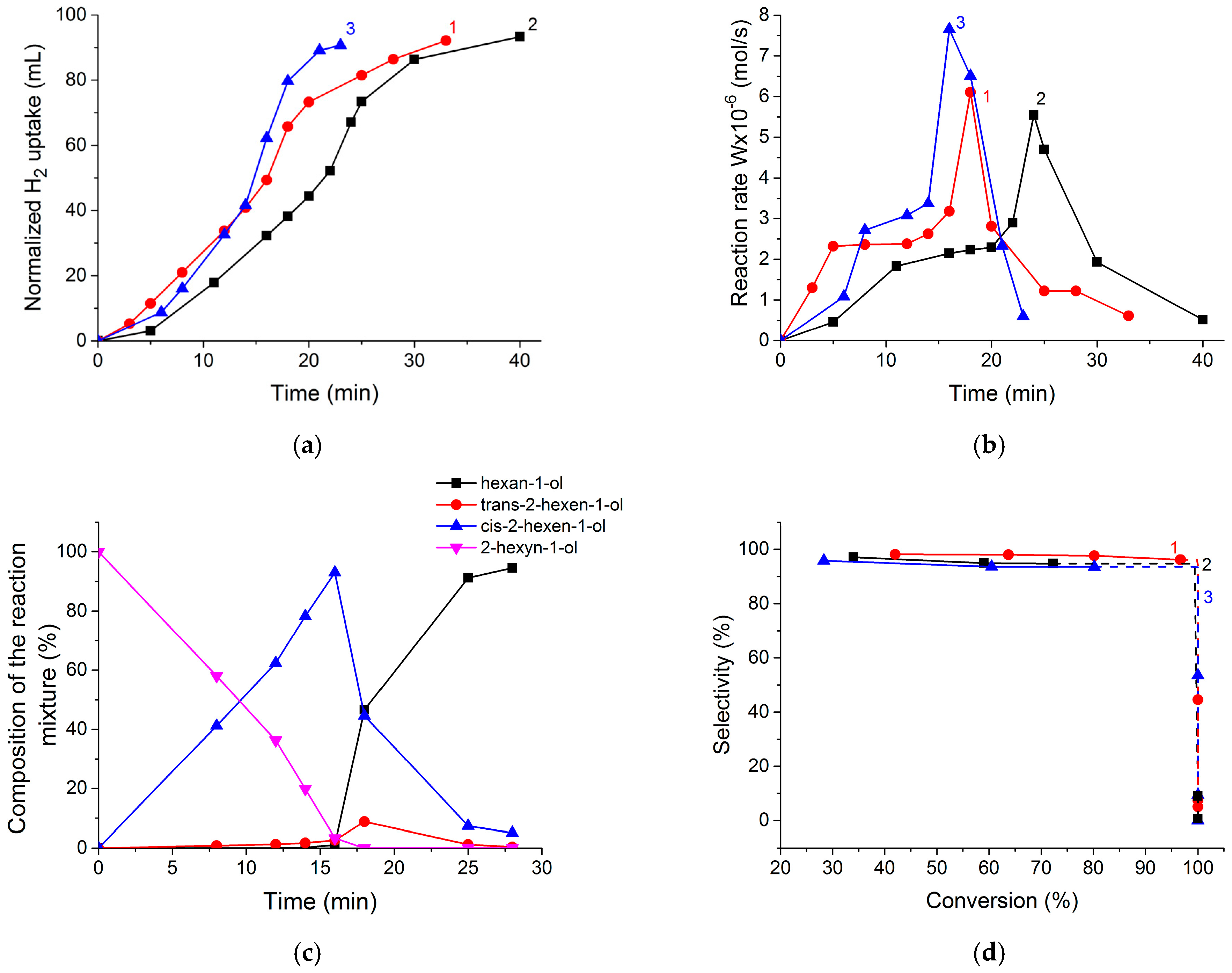
2.3. Catalytic Activity of Pd@TiO2 Catalysts in the Hydrogenation Process
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of Titanium Dioxide (TiO2SA)
3.3. Preparation of Palladium Catalysts
3.4. Characterization of the Composites and Catalysts
3.5. Photocatalytic H2 Evolution Experiments
3.6. Hydrogenation of Acetylenic Compounds with Molecular H2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective Hydrogenation over Supported Metal Catalysts: From Nanoparticles to Single Atoms. Chem. Rev. 2020, 120, 683–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, D.; Zhou, Y.; Qian, J.; Wen, X.; Jiang, P.; Ma, L.; Lu, C.; Feng, F.; Li, X. Preparation of Heteroatom-Doped Carbon Materials and Applications in Selective Hydrogenation. Chem. Sel. 2022, 7, e202102581. [Google Scholar] [CrossRef]
- Audevard, J.; Navarro-Ruiz, J.; Bernardin, V.; Tison, Y.; Corrias, A.; Rosal, I.D.; Favre-Réguillon, A.; Philippe, R.; Gerber, I.C.; Serp, P. Adjustment of the Single Atom/Nanoparticle Ratio in Pd/CNT Catalysts for Phenylacetylene Selective Hydrogenation. Chem. Cat. Chem. 2023, 15, e202300036. [Google Scholar] [CrossRef]
- Zharmagambetova, A.; Auyezkhanova, A.; Talgatov, E.; Jumekeyeva, A.; Buharbayeva, F.; Akhmetova, S.; Myltykbayeva, Z.; Lopez Nieto, J.M. Synthesis of polymer protected Pd–Ag/ZnO catalysts for phenylacetylene hydrogenation. J. Nanopart. Res. 2022, 24, 236. [Google Scholar] [CrossRef]
- Deng, X.; Wang, J.; Guan, N.; Li, L. Catalysts and mechanisms for the selective heterogeneous hydrogenation of cabon-carbon triple bonds. Cell Rep. Phys. Sci. 2022, 3, 101017. [Google Scholar] [CrossRef]
- Albani, D.; Shahrokhi, M.; Chen, Z.; Mitchell, S.; Hauert, R.; López, N.; Pérez-Ramírez, J. Selective ensembles in supported palladium sulfide nanoparticles for alkyne semi-hydrogenation. Nat. Commun. 2018, 9, 2634. [Google Scholar] [CrossRef]
- Montsch, T.; Heuchel, M.; Traa, Y.; Klemm, E.; Stubenrauch, C. Selective hydrogenation of 3-Hexyn-1-ol with Pd nanoparticles synthesized via microemulsions. Appl. Catal. A Gen. 2017, 539, 19–28. [Google Scholar] [CrossRef]
- Liu, K.; Wang, A.; Mao, Y.; Jia, Z.; Su, Y.; Wen, X. Synthesis of ultrafine Pd nanoparticles encapsulated in imidazolium-based porous polymers for semi-hydrogenation of alkynes. Mol. Catal. 2023, 543, 113130. [Google Scholar] [CrossRef]
- Pal, N.; Bhaumik, A. Mesoporous materials: Versatile supports in heterogeneous catalysis for liquid phase catalytic transformations. RSC Adv. 2015, 5, 24363–24391. [Google Scholar] [CrossRef]
- Parapat, Y.R.; Saputra, O.H.I.; Ang, A.P.; Schwarze, M.; Schomäcker, R. Support effect in the preparation of supported metal catalysts via microemulsion. RSC Adv. 2014, 4, 50955–50963. [Google Scholar] [CrossRef]
- Gao, M.; Lyalin, A.; Taketsugu, T. Role of the Support Effects on the Catalytic Activity of Gold Clusters: A Density Functional Theory Study. Catalysts 2011, 1, 18–39. [Google Scholar] [CrossRef]
- Kovtunov, K.V.; Barskiy, D.A.; Salnikov, O.G.; Burueva, D.B.; Khudorozhkov, A.K.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Gerasimov, E.Y.; Bukhtiyarov, V.I.; Koptyug, I.V. Strong metal-support interactions for palladium supported on TiO2 catalysts in the heterogeneous hydrogenation with parahydrogen. Chem. Cat. Chem 2015, 7, 2581–2584. [Google Scholar] [CrossRef]
- Byun, M.Y.; Park, D.-W.; Lee, M.S. Effect of Oxide Supports on the Activity of Pd Based Catalysts for Furfural Hydrogenation. Catalysts 2020, 10, 837. [Google Scholar] [CrossRef]
- Han, Y.; Peng, D.; Xu, Z.; Wan, H.; Zheng, S.; Zhu, D. TiO2 supported Pd@Ag as highly selective catalysts for hydrogenation of acetylene in excess ethylene. Chem. Commun. 2013, 49, 8350–8352. [Google Scholar] [CrossRef] [PubMed]
- Kuai, L.; Chen, Z.; Liu, S.; Kan, E.; Yu, N.; Ren, Y.; Fang, C.; Li, X.; Li, Y.; Geng, B. Titania supported synergistic palladium single atoms and nanoparticles for room temperature ketone and aldehydes hydrogenation. Nat. Commun. 2020, 11, 48. [Google Scholar] [CrossRef]
- Sittikun, J.; Boonyongmaneerat, Y.; Weerachawanasak, P.; Praserthdam, P.; Panpranot, J. Pd/TiO2 catalysts prepared by electroless deposition with and without SnCl2 sensitization for the liquid-phase hydrogenation of 3-hexyn-1-ol. React. Kinet. Mech. Catal. 2014, 111, 123–135. [Google Scholar] [CrossRef]
- Riyapan, S.; Boonyongmaneerat, Y.; Mekasuwandumrong, O.; Yoshida, H.; Fujita, S.-I.; Arai, M.; Panpranot, J. Improved catalytic performance of Pd/TiO2 in the selective hydrogenation of acetylene by using H2-treated sol–gel TiO2. J. Mol. Catal. A Chem. 2014, 383–384, 182–187. [Google Scholar] [CrossRef]
- Volhard, M.-F.; Christ, J.J.; Blank, L.M.; Jüstel, T. Seawater activated TiO2 photocatalyst for degradation of organic compounds. Sustain. Chem. Pharm. 2020, 16, 100251. [Google Scholar] [CrossRef]
- Rafique, M.; Hajra, S.; Irshad, M.; Usman, M.; Imran, M.; Assiri, M.A.; Ashraf, W.M. Hydrogen Production Using TiO2-Based Photocatalysts: A Comprehensive Review. ACS Omega 2023, 8, 25640–25648. [Google Scholar] [CrossRef]
- De Benedetto, A.; De Luca, A.; Pellegrino, P.; Rinaldi, R.; De Matteis, V.; Cascione, M. The Application of Nano Titanium Dioxide for Hydrogen Production and Storage Enhancement. Appl. Sci. 2023, 13, 12521. [Google Scholar] [CrossRef]
- Chen, Y.; Soler, L.; Armengol-Profitós, M.; Xie, C.; Crespo, D.; Llorca, J. Enhanced photoproduction of hydrogen on Pd/TiO2 prepared by mechanochemistry. Appl. Catal. B Environ. 2022, 309, 121275. [Google Scholar] [CrossRef]
- Banerjee, A.N.; Hamnabard, N.; Joo, S.W. A comparative study of the effect of Pd-doping on the structural, optical, and photocatalytic properties of sol–gel derived anatase TiO2 nanoparticles. Ceram. Int. 2016, 42, 12010–12026. [Google Scholar] [CrossRef]
- Ma, D.; Zhai, S.; Wang, Y.; Liu, A.; Chen, C. TiO2 Photocatalysis for Transfer Hydrogenation. Molecules 2019, 24, 330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Zhang, W.; Dong, L.; Zbořil, R.; Chen, Z. Photocatalytic Transfer Hydrogenation Reactions Using Water as the Proton Source. ACS Catal. 2023, 13, 7557–7567. [Google Scholar] [CrossRef]
- Lavorato, C.; Argurio, P.; Molinari, R. TiO2 and Pd/TiO2 as Photocatalysts for Hydrogenation of Ketones and Perspective of Membrane Application. Int. J. Adv. Res. Chem. Sci. 2019, 6, 33–41. [Google Scholar] [CrossRef]
- Imamura, K.; Okubo, Y.; Ito, T.; Tanaka, A.; Hashimoto, K.; Kominami, H. Photocatalytic hydrogenation of alkenes to alkanes in alcoholic suspensions of palladium-loaded titanium (IV) oxide without the use of hydrogen gas. RSC Adv. 2014, 4, 19883. [Google Scholar] [CrossRef]
- Takada, Y.; Caner, J.; Kaliyamoorthy, S.; Naka, H.; Saito, S. Photocatalytic Transfer Hydrogenolysis of Allylic Alcohols on Pd/TiO2: A Shortcut to (S)-(+)-Lavandulol. Chem. Eur. J. 2017, 23, 18025–18032. [Google Scholar] [CrossRef]
- Lv, S.; Liu, H.; Zhang, J.; Wu, Q.; Wang, F. Water promoted photocatalytic transfer hydrogenation of furfural to furfural alcohol over ultralow loading metal supported on TiO2. J. Energy Chem. 2022, 73, 259–267. [Google Scholar] [CrossRef]
- Mascolo, M.; Pei, Y.; Ring, T. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Hosseinzadeh, F.; Zardari, P.; Mermer, O. Pd-Ni nanoparticle supported on reduced graphene oxide and multi-walled carbon nanotubes as electrocatalyst for oxygen reduction reaction. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 675–687. [Google Scholar] [CrossRef]
- Thogersen, A.; Mayandi, J.; Vines, L.; Sunding, M.F.; Olsen, A.; Diplas, S.; Mitome, M.; Bando, Y. Composition and structure of Pd nanoclusters in SiOx thin film. J. Appl. Phys. 2011, 109, 084329. [Google Scholar] [CrossRef]
- Rinaudo, M.G.; Beltrán, A.M.; Fernández, A.; Cadús, L.E.; Morales, M.R. Pd supported on defective TiO2 polymorphic mixtures: Effect of metal-support interactions upon glycerol selective oxidation. Results Eng. 2022, 16, 100737. [Google Scholar] [CrossRef]
- Xu, Q.; Knezevic, M.; Laachachi, A.; Franger, S.; Colbeau-Justin, C.; Ghazzal, M.N. Insight into Interfacial Charge Transfer during Photocatalytic H2 Evolution through Fe, Ni, Cu and Au Embedded in a Mesoporous TiO2@SiO2 Core-shell. Chem. Cat. Chem. 2022, 14, e202200102. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Reddy, G.K.; Peck, T.C.; Roberts, C.A. “PdO vs. PtO”— The Influence of PGM Oxide Promotion of Co3O4 Spinel on Direct NO Decomposition Activity. Catalysts 2019, 9, 62. [Google Scholar] [CrossRef]
- Tanişik, İ.; Uğuz, Ö.; Akyüz, D.; Zunain Ayaz, R.M.; Sarioğlu, C.; Karaca, F.; Özkaya, A.R.; Koca, A. Solar-hydrogen production with reduced graphene oxide supported CdxZn1-xS photocatalysts. Int. J. Hydrogen Energy 2020, 45, 34845–34856. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.-D.; Maurino, V. Influence of Agglomeration and Aggregation on the Photocatalytic Activity of TiO2 Nanoparticles. Appl. Catal. B Environ. 2017, 216, 80–87. [Google Scholar] [CrossRef]
- Gaikwad, A.; Betty, C.; Kumar, A.; Sasikala, R. Microflowers of Pd doped ZnS for visible light photocatalytic and photoelectrochemical applications. Mater. Sci. Semicond. Process. 2018, 86, 139–145. [Google Scholar] [CrossRef]
- Zhen, W.; Ning, X.; Wang, M.; Wu, Y.; Lu, G. Enhancing hydrogen generation via fabricating peroxide decomposition layer over NiSe/MnO2-CdS catalyst. J. Catal. 2018, 367, 269–282. [Google Scholar] [CrossRef]
- Akyüz, D.; Ozkaya, A.R.; Koca, A. Photocatalytic and photoelectrochemical performances of Mo, Ni and Cu decorated metal chalcogenides. Mater. Sci. Semicond. Process. 2020, 116, 105127. [Google Scholar] [CrossRef]
- Navarro-Ruiz, J.; Cornu, D.; López, N. Prevalence of trans-Alkenes in Hydrogenation Processes on Metal Surfaces: A Density Functional Theory Study. J. Phys. Chem. C 2018, 122, 25339–25348. [Google Scholar] [CrossRef]
- Zharmagambetova, A.; Talgatov, E.; Auyezkhanova, A.; Tumabayev, N.; Bukharbayeva, F. Behavior of Pd-supported catalysts in phenylacetylene hydrogenation: Effect of combined use of polyvinylpyrrolidone and NaOH for magnetic support modification. Polym. Adv. Technol. 2021, 7, 2735–2743. [Google Scholar] [CrossRef]
- Ma, L.; Yuan, S.; Jiang, T.; Zhu, X.; Lu, C.; Li, X. Pd4S/SiO2: A Sulfur-Tolerant Palladium Catalyst for Catalytic Complete Oxidation of Methane. Catalysts 2019, 9, 410. [Google Scholar] [CrossRef]
- Tombácz, E. pH-dependent surface charging of metal oxides. Period. Polytech. Chem. Eng. 2009, 53, 77–86. [Google Scholar] [CrossRef]
- Canadell, E.; Badia, J.H.; Soto, R.; Tejero, J.; Bringué, R.; Ramírez, E. One-Pot Liquid-Phase Synthesis of Methyl Isobutyl Ketone Over Bifunctional Ion-Exchange Resins: Unravelling the Role of Resins Structure and Active Pd or Cu Phases on Sintering, Leaching and Catalytic Activity. Top Catal. 2024. [Google Scholar] [CrossRef]
- Talgatov, E.T.; Naizabayev, A.A.; Bukharbayeva, F.U.; Kenzheyeva, A.M.; Yersaiyn, R.; Auyezkhanova, A.S.; Akhmetova, S.N.; Zhizhin, E.V.; Brodskiy, A.R. Pd Catalysts Supported on Mixed Iron and Titanium Oxides in Phenylacetylene Hydrogenation: Effect of TiO2 Content in Magnetic Support Material. Nanomaterials 2024, 14, 1392. [Google Scholar] [CrossRef]




| Samples | Crystalline Size (XRD), nm | Surface Area, m2/g | Surface Blocked (calc.), % | |
|---|---|---|---|---|
| SXRD | SBET | |||
| TiO2SA | 8 | 192 | 98 | 50 |
| TiO2CRA | 15 | 103 | 61 | 40 |
| TiO2CA | 16 | 96 | 66 | 30 |
| Pd@TiO2CA | 16 | 96 | 67 | 30 |
| Catalyst | Amount of Pd in Mother Liquor, ×10−5 Mol | The Degree of Deposition, % | Pd Content in a Catalyst, %wt. | ||
|---|---|---|---|---|---|
| before Deposition | after Deposition | PEC | EDX * | ||
| Pd@TiO2SA | 9.5 | 0.4 | 96 | 1.0 | 1.0 |
| Pd@TiO2CA | 9.5 | 0.1 | 99 | 1.0 | 1.0 |
| Pd@TiO2CRA | 9.5 | 0.3 | 97 | 1.0 | 1.0 |
| Photocatalysts | Amount of the Photocatalyst, mg | Sacrificial Agent | Light Intensity | PCHE Rate (μmol/h gcat) | Ref. |
|---|---|---|---|---|---|
| 5%Pd–ZnS | 50 | Na2S/Na2SO3 | 100 mW cm−2 | 940 | [38] |
| NiSe/MnO2–CdS | 100 | H2O | 100 mW cm−2 | 455 | [39] |
| RGO-Cd0.60Zn0.40S-0.5%Mo | 70 | Na2S/Na2SO3 | Solar Simulator (1000 Wm−2) | 1543 | [40] |
| Pd@TiO2CA | 70 | Na2S/Na2SO3 | Solar Simulator (1000 Wm−2) | 760 * | This work |
| Catalyst | W × 10−6, mol/s | WC≡C:WC=C | Selectivity *, % | Conversion **, % | |
|---|---|---|---|---|---|
| C≡C | C=C | ||||
| 2-hexyn-1-ol | |||||
| Pd@TiO2SA | 2.2 | 5.5 | 1:2.5 | 95 | 86 |
| Pd@TiO2CRA | 3.1 | 7.7 | 1:2.5 | 94 | 80 |
| Pd@TiO2CA | 2.6 | 6.1 | 1:2.3 | 96 | 97 |
| 2-hexyne | |||||
| Pd@TiO2SA | 27.0 | 5.2 | 5.2:1 | 92 | 61 |
| Pd@TiO2CRA | 29.5 | 8.1 | 3.6:1 | 88 | 62 |
| Pd@TiO2CA | 35.7 | 10.0 | 3.6:1 | 96 | 92 |
| 5-hexyn-1-ol | |||||
| Pd@TiO2SA | 6.0 | 13.8 | 1:2.3 | 96 | 77 |
| Pd@TiO2CRA | 6.1 | 13.6 | 1:2.2 | 92 | 76 |
| Pd@TiO2CA | 9.0 | 12.7 | 1:1.4 | 94 | 90 |
| Solvent | W × 10−6, mol/s | WC≡C:WC=C | Selectivity, % | Conversion *, % | |
|---|---|---|---|---|---|
| C≡C | C=C | ||||
| Ethanol | 5.7 | 16.5 | 1:2.9 | 92 | 81 |
| Water | 4.1 | 11.6 | 1:2.8 | 93 | 79 |
| NaOH in water | 4.0 | 9.9 | 1:2.5 | 96 | 77 |
| NaOH in ethanol | 7.7 | 11.3 | 1:1.5 | 97 | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talgatov, E.T.; Naizabayev, A.A.; Kenzheyeva, A.M.; Myltykbayeva, Z.K.; Koca, A.; Bukharbayeva, F.U.; Akhmetova, S.N.; Yersaiyn, R.; Auyezkhanova, A.S. Investigation of the Performances of TiO2 and Pd@TiO2 in Photocatalytic Hydrogen Evolution and Hydrogenation of Acetylenic Compounds for Application in Photocatalytic Transfer Hydrogenation. Catalysts 2024, 14, 665. https://doi.org/10.3390/catal14100665
Talgatov ET, Naizabayev AA, Kenzheyeva AM, Myltykbayeva ZK, Koca A, Bukharbayeva FU, Akhmetova SN, Yersaiyn R, Auyezkhanova AS. Investigation of the Performances of TiO2 and Pd@TiO2 in Photocatalytic Hydrogen Evolution and Hydrogenation of Acetylenic Compounds for Application in Photocatalytic Transfer Hydrogenation. Catalysts. 2024; 14(10):665. https://doi.org/10.3390/catal14100665
Chicago/Turabian StyleTalgatov, Eldar T., Akzhol A. Naizabayev, Alima M. Kenzheyeva, Zhannur K. Myltykbayeva, Atıf Koca, Farida U. Bukharbayeva, Sandugash N. Akhmetova, Raiymbek Yersaiyn, and Assemgul S. Auyezkhanova. 2024. "Investigation of the Performances of TiO2 and Pd@TiO2 in Photocatalytic Hydrogen Evolution and Hydrogenation of Acetylenic Compounds for Application in Photocatalytic Transfer Hydrogenation" Catalysts 14, no. 10: 665. https://doi.org/10.3390/catal14100665
APA StyleTalgatov, E. T., Naizabayev, A. A., Kenzheyeva, A. M., Myltykbayeva, Z. K., Koca, A., Bukharbayeva, F. U., Akhmetova, S. N., Yersaiyn, R., & Auyezkhanova, A. S. (2024). Investigation of the Performances of TiO2 and Pd@TiO2 in Photocatalytic Hydrogen Evolution and Hydrogenation of Acetylenic Compounds for Application in Photocatalytic Transfer Hydrogenation. Catalysts, 14(10), 665. https://doi.org/10.3390/catal14100665






