Upgrading of Rice Straw Bio-Oil Using 1-Butanol over ZrO2-Fe3O4 Bimetallic Nanocatalyst Supported on Activated Rice Straw Biochar to Butyl Esters
Abstract
1. Introduction
2. Results and Discussions
2.1. Catalyst Characterization
2.2. Physical Characterization of Raw and Esterified Upgraded Bio-Oils
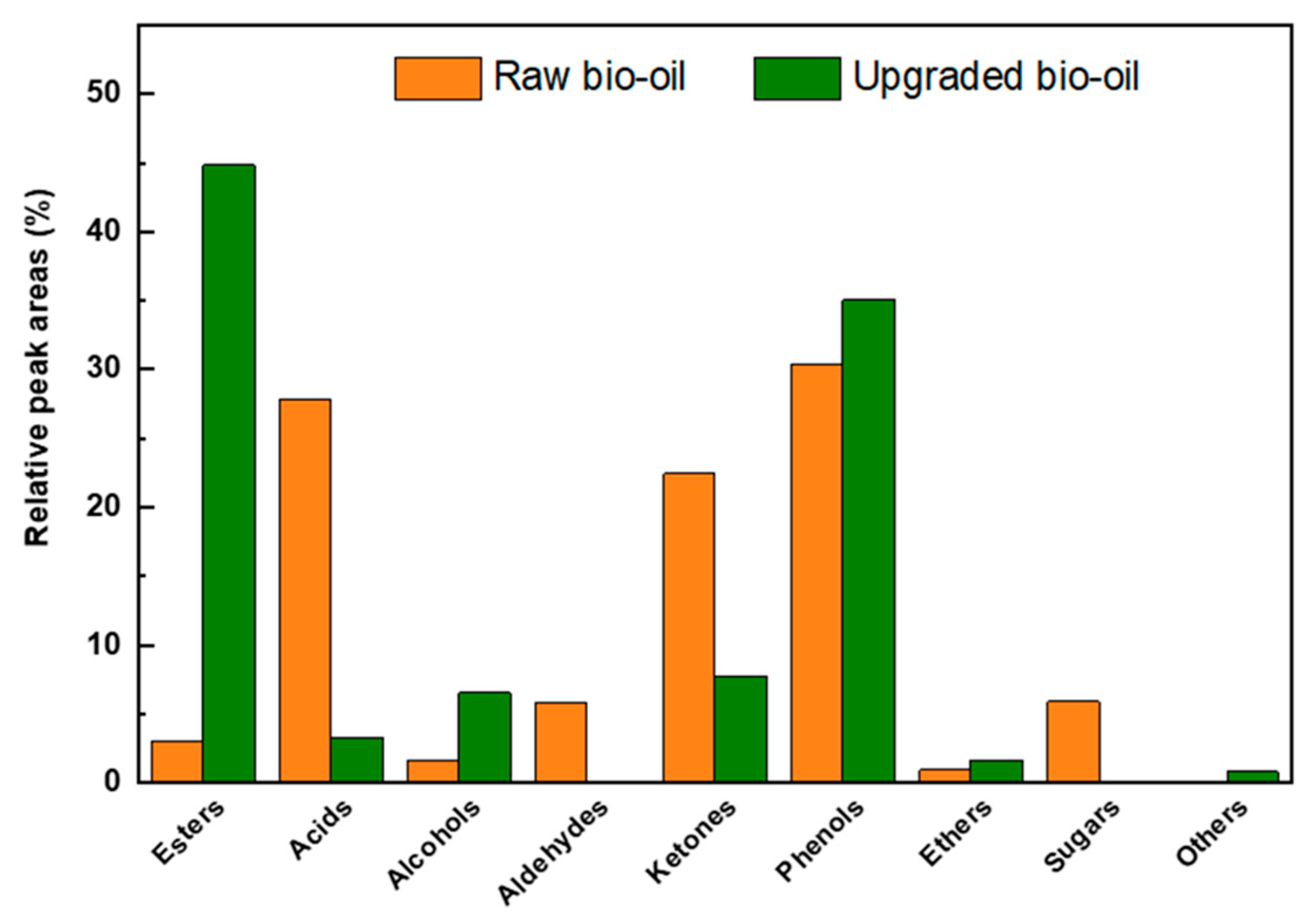
2.3. Chemical Characterization of Raw and Esterified Upgraded Bio-Oils
2.4. RSM Analysis of Ester Yield %
2.5. Optimization of Reaction Conditions by RSM Analysis of Ester Yield %
2.5.1. Effect of Reaction Time on Upgrading of Rice Straw Bio-Oil in Butanol
2.5.2. Effect of Temperature on Upgrading of Rice Straw Bio-Oil in Butanol
2.5.3. Effect of Catalyst on Upgrading of Rice Straw Bio-Oil in Butanol
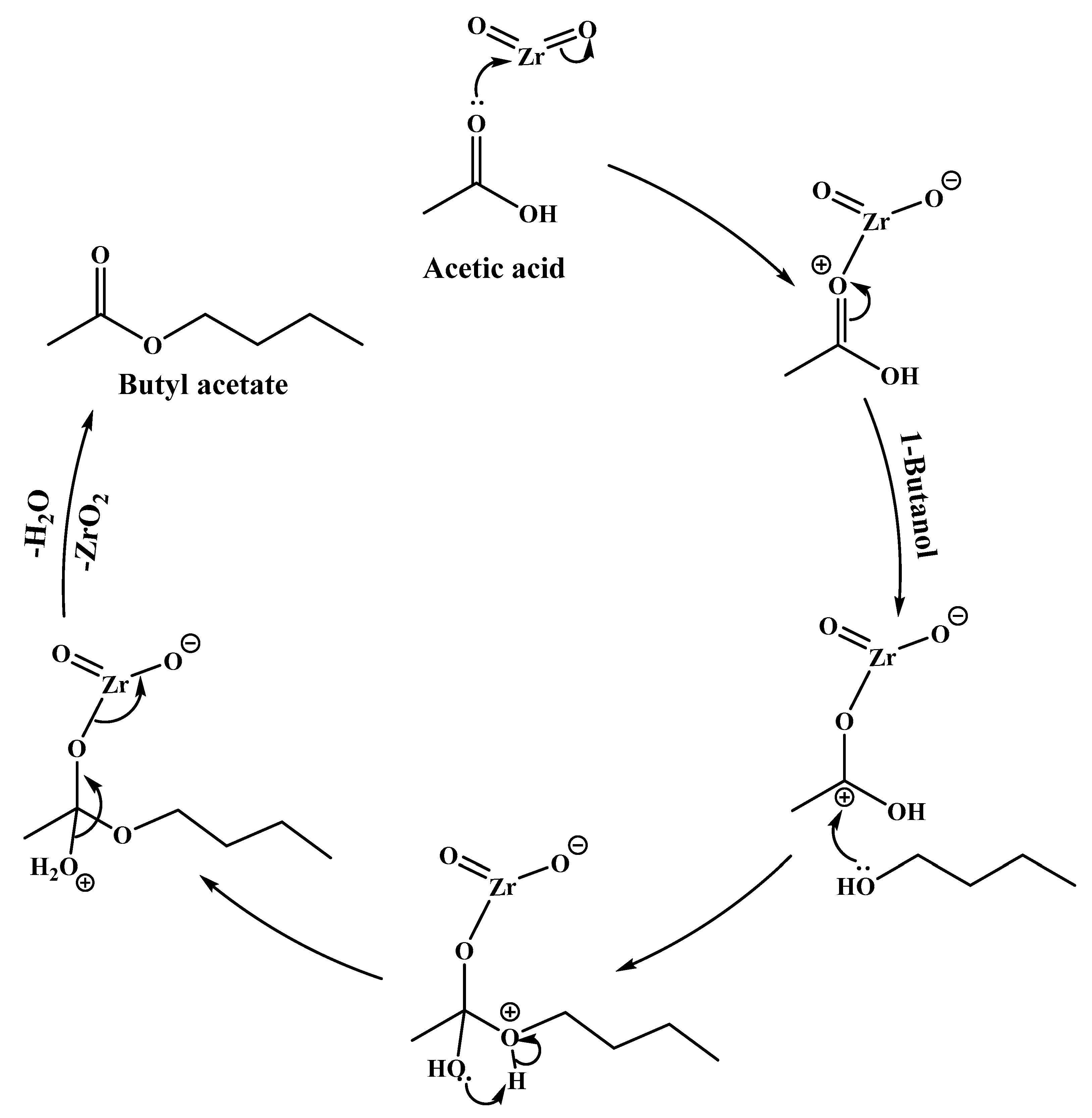
2.5.4. Proposed Esterification Reaction Mechanism
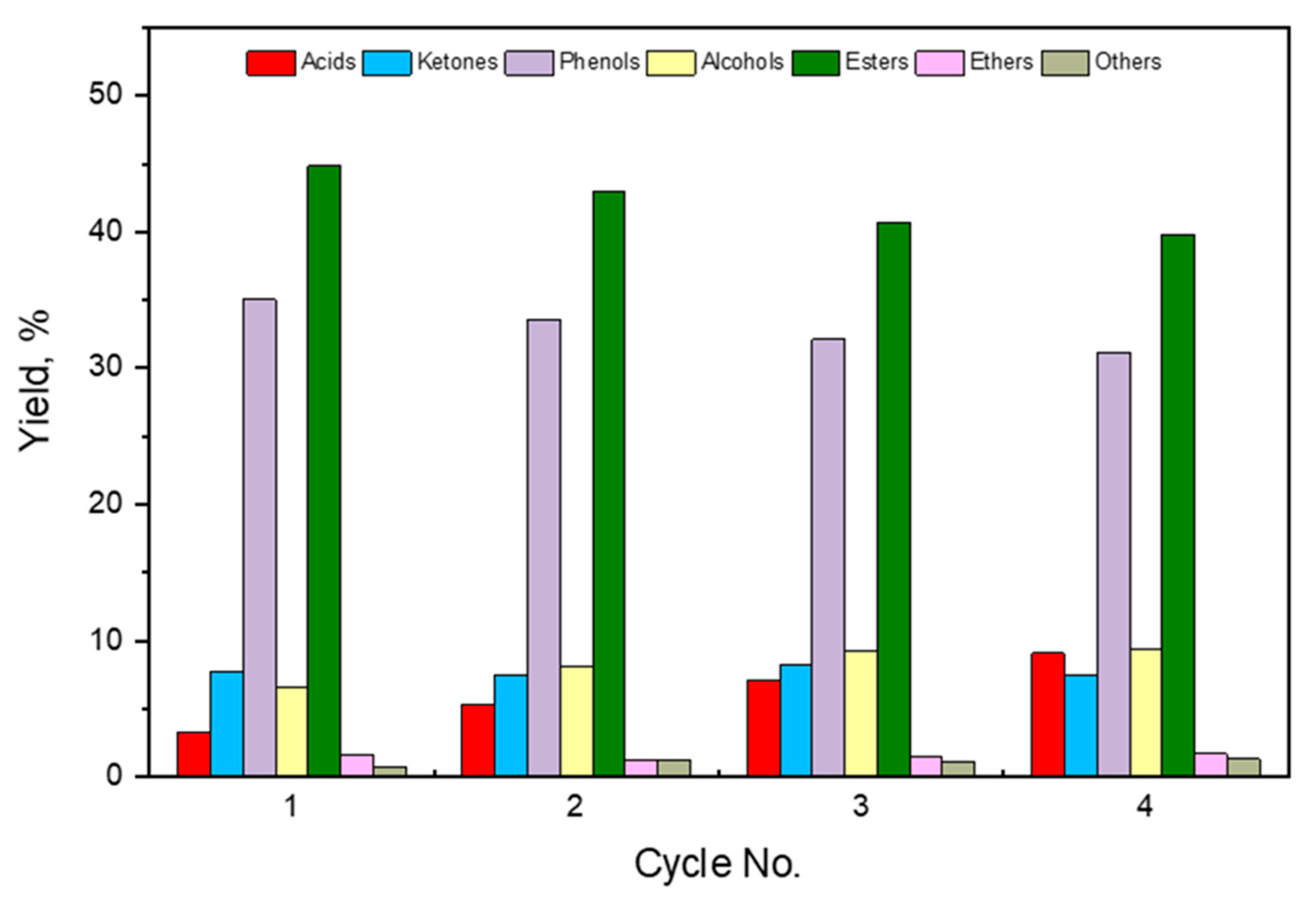
2.6. Catalyst’s Stability
3. Materials and Methods
3.1. Materials
3.2. Pyrolysis of Rice Straw and Activation of Rice Straw Biochar
3.3. Preparation of ZrO2-Fe3O4/AcB
3.4. Catalyst Characterization
3.5. Bio-Oil Upgrading Process in Butanol
3.6. Physical and Chemical Characterization of Raw and Esterified Bio-Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Y.; Hu, S.; Zhang, Z.; Zhu, B.; Bai, D. The path to carbon neutrality in China: A paradigm shift in fossil resource utilization. Resour. Chem. Mater. 2022, 1, 129–135. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, G.; Arya, S.K. Biofuel from rice straw. J. Clean. Prod. 2020, 277, 124101. [Google Scholar] [CrossRef]
- Global Primary Energy Demand by Fuel Type 2022–2045. Statista Research Department. Available online: https://www.statista.com/statistics/282801/primary-energy-demand-by-source-world/ (accessed on 25 September 2024).
- Lucia, L.A. Lignocellulosic biomass: A potential feedstock to replace petroleum. BioResources 2008, 3, 981–982. [Google Scholar] [CrossRef]
- Wyman, C.E. Introduction Overview: World energy resources and the need for biomass for energy and lower fossil carbon dioxide emissions. In Plant Biotechnology for Sustainable Production of Energy and Co-Products; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–23. [Google Scholar]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef] [PubMed]
- Rathour, R.K.; Devi, M.; Dahiya, P.; Sharma, N.; Kaushik, N.; Kumari, D.; Kumar, P.; Baadhe, R.R.; Walia, A.; Bhatt, A.K. Recent trends, opportunities and challenges in sustainable management of rice straw waste biomass for green biorefinery. Energies 2023, 16, 1429. [Google Scholar] [CrossRef]
- Chum, H.L.; Overend, R.P. Biomass and renewable fuels. Fuel Process. Technol. 2001, 71, 187–195. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Shadangi, K.P.; Srivastava, R.K. Chapter 5—Agricultural waste to fuels and chemicals. In Bioenergy Engineering; Woodhead Publishing: Cambridge, MA, USA, 2023; pp. 87–98. [Google Scholar]
- Ahmad, R.; Hamidin, N. Bio-oil Product from Non-catalytic and Catalytic Pyrolysis of Rice Straw. Aust. J. Basic Appl. Sci. 2013, 7, 61–65. [Google Scholar]
- Oasmaa, A.; Lehto, J.; Solantausta, Y.; Kallio, S. Historical Review on VTT Fast Pyrolysis Bio-oil Production and Upgrading. Energy Fuels 2021, 35, 5683–5695. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.G.; Murthy Konda, N.V.S.N.; Garcia, M.C.; Wang, L.; Hallett, J.P.; Shah, N. The multi-scale challenges of biomass fast pyrolysis and bio-oil upgrading: Review of the state of art and future research directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Khosravanipour Mostafazadeh, A.; Solomatnikova, O.; Drogui, P.; Tyagi, R.D. A review of recent research and developments in fast pyrolysis and bio-oil upgrading. Biomass Convers. Biorefinery 2018, 8, 739–773. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Eschenbacher, A.; Fennell, P.; Jensen, A.D. A Review of Recent Research on Catalytic Biomass Pyrolysis and Low-Pressure Hydropyrolysis. Energy Fuels 2021, 35, 18333–18369. [Google Scholar] [CrossRef]
- Kim, T.-S.; Kim, J.-Y.; Kim, K.-H.; Lee, S.; Choi, D.; Choi, I.-G.; Choi, J.W. The effect of storage duration on bio-oil properties. J. Anal. Appl. Pyrolysis 2012, 95, 118–125. [Google Scholar] [CrossRef]
- Maneechakr, P.; Karnjanakom, S. Improving the Bio-Oil Quality via Effective Pyrolysis/Deoxygenation of Palm Kernel Cake over a Metal (Cu, Ni, or Fe)-Doped Carbon Catalyst. ACS Omega 2021, 6, 20006–20014. [Google Scholar] [CrossRef]
- Carpenter, D.L.; Westover, T.L.; Czernik, S.; Jablonski, W.S. Biomass feedstocks for renewable fuel production: A review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green Chem. 2014, 16, 384–406. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Yin, R.; Mei, Y. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renew. Sustain. Energy Rev. 2013, 24, 66–72. [Google Scholar] [CrossRef]
- Morris, M. Production of bio-oils via catalytic pyrolysis. In Handbook of Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2011; pp. 349–389. [Google Scholar]
- Hassan, E.B.M.; Steele, P.H.; Ingram, L. Characterization of fast pyrolysis bio-oils produced from pretreated pine wood. Appl. Biochem. Biotechnol. 2009, 154, 182–192. [Google Scholar] [CrossRef]
- Kazmi, W.W.; Park, J.-Y.; Amini, G.; Lee, I.-G. Upgrading of esterified bio-oil from waste coffee grounds over MgNiMo/activated charcoal in supercritical ethanol. Fuel Process. Technol. 2023, 250, 107915. [Google Scholar] [CrossRef]
- Ibrahim, A.; Elsayed, I.; Hassan, E.B. Catalytic Upgrading of Rice Straw Bio-Oil via Esterification in Supercritical Ethanol over Bimetallic Catalyst Supported on Rice Straw Biochar. Energies 2024, 17, 407. [Google Scholar] [CrossRef]
- Yamanaka, N.; Shimazu, S. Conversion of Biomass-Derived Molecules into Alkyl Levulinates Using Heterogeneous Catalysts. Reactions 2023, 4, 667–678. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; dos Santos, I.A.; Arcanjo, M.R.A.; Cavalcante, C.L.; de Luna, F.M.T.; Fernandez-Lafuente, R.; Vieira, R.S. Production of Jet Biofuels by Catalytic Hydroprocessing of Esters and Fatty Acids: A Review. Catalysts 2022, 12, 237. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, I.-G.; Park, J.-Y.; Lee, K.-Y. Efficient upgrading of pyrolysis bio-oil over Ni-based catalysts in supercritical ethanol. Fuel 2019, 241, 207–217. [Google Scholar] [CrossRef]
- Shafaghat, H.; Kim, J.M.; Lee, I.-G.; Jae, J.; Jung, S.-C.; Park, Y.-K. Catalytic hydrodeoxygenation of crude bio-oil in supercritical methanol using supported nickel catalysts. Renew. Energy 2019, 144, 159–166. [Google Scholar] [CrossRef]
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Riaz, S.; Konstas, K.; Mubarak, N.M.; Srinivasan, M.P.; Griffin, G.J. Catalytic upgradation of bio-oil over metal supported activated carbon catalysts in sub-supercritical ethanol. J. Environ. Chem. Eng. 2021, 9, 105059. [Google Scholar] [CrossRef]
- Bagnato, G.; Sanna, A.; Paone, E.; Catizzone, E. Recent catalytic advances in hydrotreatment processes of pyrolysis bio-oil. Catalysts 2021, 11, 157. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jeon, W.; Lee, J.-H.; Nam, B.; Lee, I.-G. Effects of supercritical fluids in catalytic upgrading of biomass pyrolysis oil. Chem. Eng. J. 2019, 377, 120312. [Google Scholar] [CrossRef]
- Chen, W.; Luo, Z.; Yu, C.; Li, G.; Yang, Y.; Zhang, H. Upgrading of bio-oil in supercritical ethanol: Catalysts screening, solvent recovery and catalyst stability study. J. Supercrit. Fluids 2014, 95, 387–393. [Google Scholar] [CrossRef]
- Petra, P.; Daniel, M.; Mojmir, R.; Michaela, L.; Petr, F.; Zlata, M.; Jakub, L.; Leona, P.; Milan, P.; Gustav, S.; et al. Perspectives of Biobutanol Production and Use. In Biofuel’s Engineering Process Technology; Marco Aurélio dos Santos, B., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 11. [Google Scholar]
- Jin, C.; Yao, M.; Liu, H.; Chia-fon, F.L.; Ji, J. Progress in the production and application of n-butanol as a biofuel. Renew. Sustain. Energy Rev. 2011, 15, 4080–4106. [Google Scholar] [CrossRef]
- Angelici, C.; Weckhuysen, B.M.; Bruijnincx, P.C. Chemocatalytic conversion of ethanol into butadiene and other bulk chemicals. ChemSusChem 2013, 6, 1595–1614. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Gutierrez, A.; Honkela, M.L.; Krause, A.O.I.; Heeres, H.J. Hydrotreatment of wood-based pyrolysis oil using zirconia-supported mono-and bimetallic (Pt, Pd, Rh) catalysts. Appl. Catal. A Gen. 2011, 407, 56–66. [Google Scholar] [CrossRef]
- Davidson, M.; Ji, Y.; Leong, G.J.; Kovach, N.C.; Trewyn, B.G.; Richards, R.M. Hybrid mesoporous silica/noble-metal nanoparticle materials—Synthesis and catalytic applications. ACS Appl. Nano Mater. 2018, 1, 4386–4400. [Google Scholar] [CrossRef]
- Ren, X.-Y.; Cao, J.-P.; Zhao, X.-Y.; Yang, Z.; Liu, T.-L.; Fan, X.; Zhao, Y.-P.; Wei, X.-Y. Catalytic upgrading of pyrolysis vapors from lignite over mono/bimetal-loaded mesoporous HZSM-5. Fuel 2018, 218, 33–40. [Google Scholar] [CrossRef]
- Yamanaka, N.; Abe, D.; Miwaka-Saiga, M.; Yasunaga, K.; Yamada, H.; Shimazu, S. One-pot two-step synthesis of alkyl levulinates directly from furfural by combining Ni3Sn2 alloy nanoparticles and montmorillonite K10. Sustain. Energy Fuels 2022, 6, 5153–5159. [Google Scholar] [CrossRef]
- Mabate, T.P.; Maqunga, N.P.; Ntshibongo, S.; Maumela, M.; Bingwa, N. Metal oxides and their roles in heterogeneous catalysis: Special emphasis on synthesis protocols, intrinsic properties, and their influence in transfer hydrogenation reactions. SN Appl. Sci. 2023, 5, 196. [Google Scholar] [CrossRef]
- Pirkanniemi, K.; Sillanpää, M. Heterogeneous water phase catalysis as an environmental application: A review. Chemosphere 2002, 48, 1047–1060. [Google Scholar] [CrossRef]
- Nava, R.; Pawelec, B.; Castaño, P.; Álvarez-Galván, M.C.; Loricera, C.V.; Fierro, J.L.G. Upgrading of bio-liquids on different mesoporous silica-supported CoMo catalysts. Appl. Catal. B Environ. 2009, 92, 154–167. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Zacher, A.H.; Wang, L.; Ren, S.; Liang, J.; Wei, Y.; Liu, Y.; Tang, J.; Zhang, Q.; et al. A review of catalytic hydrodeoxygenation of lignin-derived phenols from biomass pyrolysis. Bioresour. Technol. 2012, 124, 470–477. [Google Scholar] [CrossRef]
- Kaewmeesri, R.; Nonkumwong, J.; Kiatkittipong, W.; Laosiripojana, N.; Faungnawakij, K. Deoxygenations of palm oil-derived methyl esters over mono- and bimetallic NiCo catalysts. J. Environ. Chem. Eng. 2021, 9, 105128. [Google Scholar] [CrossRef]
- Lopes, R.P.; Astruc, D. Biochar as a support for nanocatalysts and other reagents: Recent advances and applications. Coord. Chem. Rev. 2021, 426, 213585. [Google Scholar] [CrossRef]
- Védrine, J.C. Metal Oxides in Heterogeneous Oxidation Catalysis: State of the Art and Challenges for a More Sustainable World. ChemSusChem 2019, 12, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Rahman, M.M.; Sarker, M.; Chai, M.; Li, C.; Cai, J. A review on the catalytic pyrolysis of biomass for the bio-oil production with ZSM-5: Focus on structure. Fuel Process. Technol. 2020, 199, 106301. [Google Scholar]
- Sihombing, J.L.; Herlinawati, H.; Pulungan, A.N.; Simatupang, L.; Rahayu, R.; Wibowo, A.A. Effective hydrodeoxygenation bio-oil via natural zeolite supported transition metal oxide catalyst. Arab. J. Chem. 2023, 16, 104707. [Google Scholar] [CrossRef]
- Ruan, C.; Akutsu, R.; Yang, K.; Zayan, N.M.; Dou, J.; Liu, J.; Bose, A.; Brody, L.; Lamb, H.H.; Li, F. Hydrogenation of bio-oil-derived oxygenates at ambient conditions via a two-step redox cycle. Cell Rep. Phys. Sci. 2023, 4, 101506. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, B.; Ge, Y.; Li, Z. Efficient depolymerization of alkali lignin to monophenols using one-step synthesized Cu–Ni bimetallic catalysts inlaid in homologous biochar. Biomass Bioenergy 2023, 175, 106873. [Google Scholar] [CrossRef]
- Zhang, M.; Han, X.; Wang, H.; Zeng, Y.; Xu, C.C. Hydrodeoxygenation of Pyrolysis Oil in Supercritical Ethanol with Formic Acid as an In Situ Hydrogen Source over NiMoW Catalysts Supported on Different Materials. Sustainability 2023, 15, 7768. [Google Scholar] [CrossRef]
- Cai, T.; Liu, X.; Zhang, J.; Tie, B.; Lei, M.; Wei, X.; Peng, O.; Du, H. Silicate-modified oiltea camellia shell-derived biochar: A novel and cost-effective sorbent for cadmium removal. J. Clean. Prod. 2021, 281, 125390. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Ichii, M.; Wu, G.F. A rice mutant defective in Si uptake. Plant Physiol. 2002, 130, 2111–2117. [Google Scholar] [CrossRef]
- Nejati, B.; Adami, P.; Bozorg, A.; Tavasoli, A.; Mirzahosseini, A.H. Catalytic pyrolysis and bio-products upgrading derived from Chlorella vulgaris over its biochar and activated biochar-supported Fe catalysts. J. Anal. Appl. Pyrolysis 2020, 152, 104799. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, D.; Wei, L.; Shen, Q.; Ji, Z.; Chen, Y.; Zou, X.; Xu, C.; Zhou, J. Bio-oil production from hydrogenation liquefaction of rice straw over metal (Ni, Co, Cu)-modified CeO2 catalysts. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 200–206. [Google Scholar] [CrossRef]
- Chen, D.; Ma, Q.; Wei, L.; Li, N.; Shen, Q.; Tian, W.; Zhou, J.; Long, J. Catalytic hydroliquefaction of rice straw for bio-oil production using Ni/CeO2 catalysts. J. Anal. Appl. Pyrolysis 2018, 130, 169–180. [Google Scholar] [CrossRef]
- Serna, P.; Gates, B.C. Molecular metal catalysts on supports: Organometallic chemistry meets surface science. Acc. Chem. Res. 2014, 47, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Sapchenko, S.A.; Barsukova, M.O.; Belosludov, R.V.; Kovalenko, K.A.; Samsonenko, D.G.; Poryvaev, A.S.; Sheveleva, A.M.; Fedin, M.V.; Bogomyakov, A.S.; Dybtsev, D.N. Understanding hysteresis in carbon dioxide sorption in porous metal–organic frameworks. Inorg. Chem. 2019, 58, 6811–6820. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Orsini, A.; Plaisant, A.; Pettinau, A. Pyrolysis of coal, biomass and their blends: Performance assessment by thermogravimetric analysis. Bioresour. Technol. 2014, 171, 433–441. [Google Scholar] [CrossRef]
- Héroguel, F.; Rozmysłowicz, B.; Luterbacher, J.S. Improving heterogeneous catalyst stability for liquid-phase biomass conversion and reforming. Chimia 2015, 69, 582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, G.Q.; Jiang, H.F.; Lin, C.; Liao, S.J. Shape- and size-controlled electrochemical synthesis of cupric oxide nanocrystals. J. Cryst. Growth 2007, 303, 400–406. [Google Scholar] [CrossRef]
- Elsayed, I.; Jackson, M.A.; Hassan, E.B. Hydrogen-free catalytic reduction of biomass-derived 5-hydroxymethylfurfural into 2,5-bis (hydroxymethyl) furan using copper–iron oxides bimetallic nanocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 1774–1785. [Google Scholar] [CrossRef]
- Kurakhmedov, A.E.; Morzabayev, A.K.; Tleubay, I.; Berguzinov, A.; Kozlovskiy, A.L. Study of the Mechanisms of Polymorphic Transformations in Zirconium Dioxide upon Doping with Magnesium Oxide, as Well as Establishing the Relationship between Structural Changes and Strength Properties. Ceramics 2023, 6, 1164–1178. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Elsayed, I.; Mashaly, M.; Eltaweel, F.; Jackson, M.A. Dehydration of glucose to 5-hydroxymethylfurfural by a core-shell Fe3O4@ SiO2-SO3H magnetic nanoparticle catalyst. Fuel 2018, 221, 407–416. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Zhai, Y.; Liu, Y.; Zhang, R.; Tang, X. Upgrading of bio-oil using supercritical 1-butanol over a Ru/C heterogeneous catalyst: Role of the solvent. Energy Fuels 2014, 28, 4611–4621. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Tripathi, P.; Pittman, C.U., Jr. Catalytic upgrading of bio-oil using 1-octene and 1-butanol over sulfonic acid resin catalysts. Green Chem. 2011, 13, 940–949. [Google Scholar] [CrossRef]
- Yang, Z.; Kumar, A.; Huhnke, R.L. Review of recent developments to improve storage and transportation stability of bio-oil. Renew. Sustain. Energy Rev. 2015, 50, 859–870. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Oberg, A.L.; Vitek, O. Statistical design of quantitative mass spectrometry-based proteomic experiments. J. Proteome Res. 2009, 8, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Yetilmezsoy, K.; Demirel, S.; Vanderbei, R.J. Response surface modeling of Pb (II) removal from aqueous solution by Pistacia vera L.: Box–Behnken experimental design. J. Hazard. Mater. 2009, 171, 551–562. [Google Scholar] [CrossRef]
- Colquhoun, D. The reproducibility of research and the misinterpretation of p-values. R. Soc. Open Sci. 2017, 4, 171085. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Wang, H.; Li, H.; Han, X.; Zeng, Y.; Xu, C.C. A review of bio-oil upgrading by catalytic hydrotreatment: Advances, challenges, and prospects. Mol. Catal. 2021, 504, 111438. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Yang, H.; Zhu, Y.; Zhao, L.; Li, M. Effects of solid acid and base catalysts on pyrolysis of rice straw and wheat straw biomass for hydrocarbon production. J. Energy Inst. 2022, 101, 140–148. [Google Scholar] [CrossRef]
- Villo, P.; Dalla-Santa, O.; Szabó, Z.; Lundberg, H. Kinetic Analysis as an Optimization Tool for Catalytic Esterification with a Moisture-Tolerant Zirconium Complex. J. Org. Chem. 2020, 85, 6959–6969. [Google Scholar] [CrossRef]
- Guvenc, C.; Alan, E.; Degirmencioglu, P.; Ozcan, M.C.; Karaman, B.P.; Oktar, N. Catalytic upgrading of bio-oil model mixtures in the presence of microporous HZSM-5 and γ-Al2O3 based Ni, Ta and Zr catalysts. Fuel 2023, 350, 128870. [Google Scholar] [CrossRef]



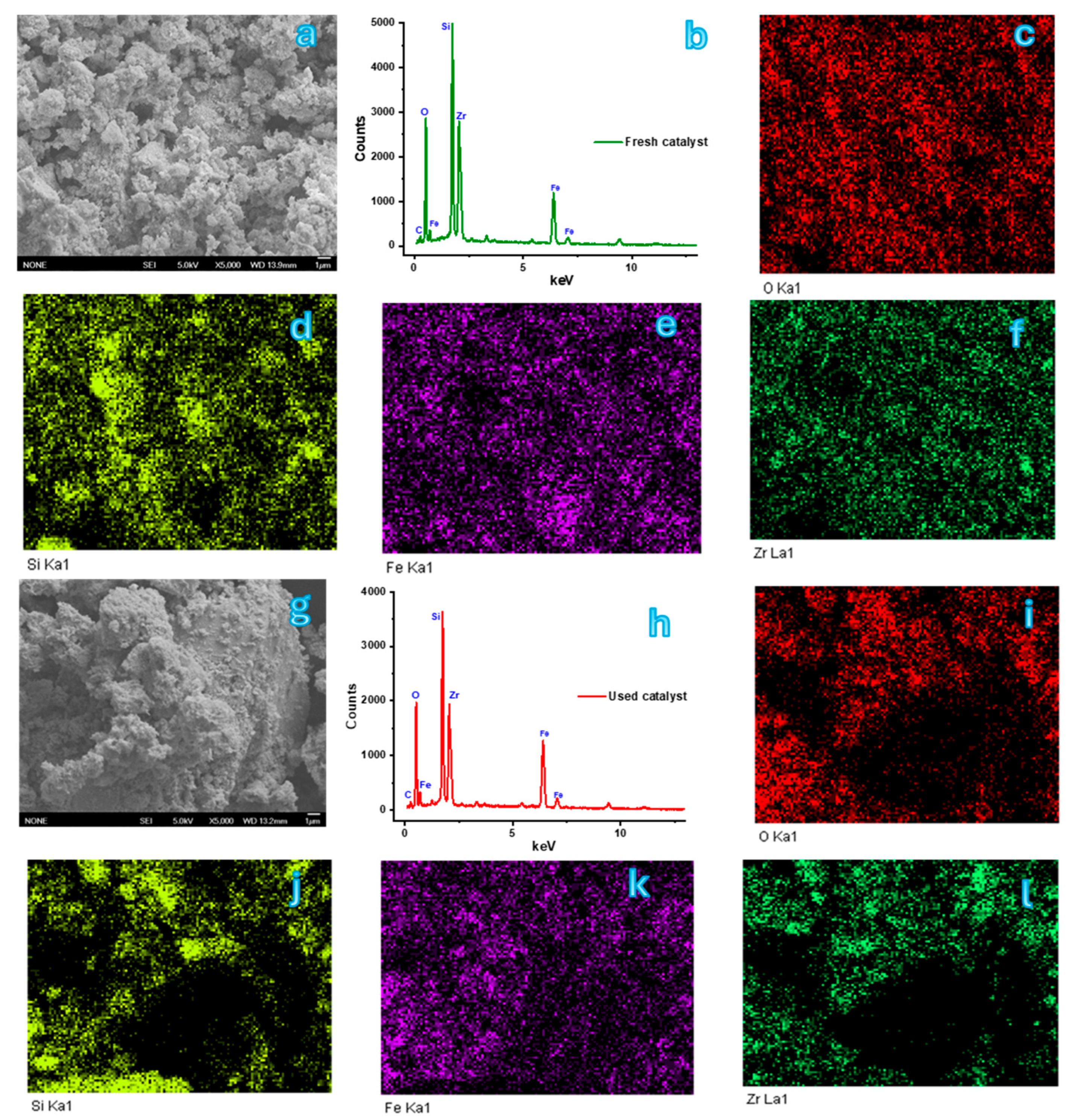


| Catalyst | BET Surface Area (m2/g) | Pore Volume (cc/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| AcB support | 129.48 | 0.311 | 9.60 |
| Fresh ZrO2-Fe3O4/AcB | 38.03 | 0.172 | 18.11 |
| Used ZrO2-Fe3O4/AcB | 34.58 | 0.125 | 19.47 |
| Exp. No. | Catalyst (wt./g) | Temperature (°C) | Time (h) | Yields (wt.%) | |||
|---|---|---|---|---|---|---|---|
| Oil | Aqueous | Solid | Gas | ||||
| Control | 0 | 300 | 2 | 52.04 | 37.36 | 0.69 | 9.91 |
| 1 | 1.80 | 250 | 3.00 | 47.09 | 39.74 | 3.21 | 9.96 |
| 2 | 1.80 | 350.00 | 1.00 | 27.43 | 35.5 | 2.25 | 34.82 |
| 3 | 0.60 | 300.00 | 1.00 | 46.02 | 39.42 | 1.12 | 13.44 |
| 4 | 1.80 | 300.00 | 2.00 | 44.74 | 36.01 | 2.88 | 16.37 |
| 5 | 1.80 | 350.00 | 3.00 | 27.49 | 32.28 | 3.1 | 37.13 |
| 6 | 3.00 | 250.00 | 2.00 | 49.09 | 38.16 | 3.22 | 9.53 |
| 7 | 3.00 | 300.00 | 1.00 | 41.61 | 39.89 | 3.25 | 15.25 |
| 8 | 3.00 | 300.00 | 3.00 | 40.2 | 40.56 | 3.71 | 15.53 |
| 9 | 1.80 | 250.00 | 1.00 | 49.57 | 39.11 | 2.51 | 8.81 |
| 10 | 3.00 | 350.00 | 2.00 | 23.57 | 36.05 | 3.25 | 37.13 |
| 11 | 0.60 | 250.00 | 2.00 | 49.05 | 38.91 | 3.22 | 8.82 |
| 12 | 0.60 | 350.00 | 2.00 | 27.07 | 33.07 | 3.75 | 36.11 |
| 13 | 0.60 | 300.00 | 3.00 | 44.39 | 37.98 | 2.74 | 14.89 |
| 14 | 1.80 | 300.00 | 2.00 | 44.06 | 37.43 | 2.25 | 16.26 |
| 15 | 1.80 | 300.00 | 2.00 | 44.84 | 35.8 | 3.11 | 16.25 |
| EXP. No. | Elemental Analysis (wt.%) | HHV (MJ/kg) | |||
|---|---|---|---|---|---|
| C | H | N | O | ||
| Raw bio-oil | 54.10 | 7.73 | 1.85 | 36.32 | 21.34 |
| 1 | 63.77 | 11.00 | 0.76 | 24.47 | 32.15 |
| 2 | 61.26 | 11.11 | 0.64 | 26.99 | 31.21 |
| 3 | 64.12 | 11.16 | 0.88 | 23.84 | 31.81 |
| 4 | 66.61 | 11.16 | 0.94 | 21.29 | 33.10 |
| 5 | 62.62 | 10.36 | 0.72 | 26.3 | 31.69 |
| 6 | 63.3 | 11.05 | 0.61 | 25.04 | 30.93 |
| 7 | 64.58 | 10.9 | 0.91 | 23.61 | 31.79 |
| 8 | 64.65 | 11.27 | 0.82 | 23.26 | 31.80 |
| 9 | 61.13 | 11.11 | 0.77 | 26.99 | 29.63 |
| 10 | 62.23 | 10.00 | 0.49 | 27.28 | 31.41 |
| 11 | 61.13 | 10.67 | 0.48 | 27.72 | 29.59 |
| 12 | 60.1 | 10.95 | 0.71 | 28.24 | 30.55 |
| 13 | 64.51 | 10.98 | 0.56 | 23.95 | 31.82 |
| 14 | 65.39 | 10.44 | 0.64 | 23.53 | 32.70 |
| 15 | 65.87 | 11.17 | 0.79 | 22.17 | 32.88 |
| EXP. No. | Water Content | Viscosity at 40 °C Cst | Density at 40 °C (g/cm3) | pH | TAN (mg KOH/g) |
|---|---|---|---|---|---|
| Raw bio-oil | 32.5 | 1.88 | 0.92 | 2.9 | 83.5 |
| Control | 12.04 | 3.98 | 1.00 | 3.01 | 78.5 |
| 1 | 11.66 | 7.69 | 0.97 | 3.47 | 69.7 |
| 2 | 9.39 | 5.85 | 0.93 | 3.94 | 61.5 |
| 3 | 10.84 | 7.46 | 0.96 | 3.69 | 65.6 |
| 4 | 9.59 | 7.69 | 0.96 | 3.64 | 66.5 |
| 5 | - | - | - | 4.34 | 59.8 |
| 6 | 10.96 | 8.01 | 0.97 | 3.48 | 70.6 |
| 7 | 10.23 | 6.52 | 0.95 | 3.91 | 61.9 |
| 8 | 10.03 | 6.87 | 0.94 | 3.74 | 64.7 |
| 9 | 11.53 | 7.30 | 0.97 | 3.30 | 73.4 |
| 10 | - | - | - | 4.02 | 60.2 |
| 11 | 11.48 | 8.14 | 0.97 | 3.50 | 69.2 |
| 12 | 9.95 | - | - | 3.92 | 60.9 |
| 13 | 9.62 | 7.23 | 0.95 | 3.71 | 65.2 |
| 14 | 9.97 | 7.25 | 0.95 | 3.57 | 66.2 |
| 15 | 10.47 | 7.17 | 0.95 | 3.55 | 66.4 |
| Factors | Unit | Symbol Coded | Levels in Box-Behnken Design | ||
|---|---|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |||
| Temperature | °C | T | 250 | 300 | 350 |
| Catalyst loading | g | C | 0.6 | 1.8 | 3 |
| Time | min | M | 1 | 2 | 3 |
| Std Order | T | M | C | Experimental Ester Yield % | Predicted Ester Yield % | Error |
|---|---|---|---|---|---|---|
| 1 | 250 | 3 | 1.8 | 43.41 | 43.2 | 0.21 |
| 2 | 350 | 1 | 1.8 | 40.52 | 40.73 | −0.21 |
| 3 | 300 | 1 | 0.6 | 40.25 | 39.98 | 0.27 |
| 4 | 300 | 2 | 1.8 | 44.83 | 44.7 | 0.13 |
| 5 | 350 | 3 | 1.8 | 39.06 | 39.01 | 0.05 |
| 6 | 250 | 2 | 3 | 42.15 | 42.09 | 0.07 |
| 7 | 300 | 1 | 3 | 41.07 | 41.09 | −0.02 |
| 8 | 300 | 3 | 3 | 40.95 | 41.22 | −0.27 |
| 9 | 250 | 1 | 1.8 | 38.86 | 38.91 | −0.05 |
| 10 | 350 | 2 | 3 | 37.81 | 37.59 | 0.23 |
| 11 | 250 | 2 | 0.6 | 38.59 | 38.82 | −0.23 |
| 12 | 350 | 2 | 0.6 | 40.88 | 40.95 | −0.07 |
| 13 | 300 | 3 | 0.6 | 42.44 | 42.42 | 0.02 |
| 14 | 300 | 2 | 1.8 | 44.59 | 44.7 | −0.11 |
| 15 | 300 | 2 | 1.8 | 44.68 | 44.7 | −0.02 |
| Source | DF | Sum of Squares | Mean Square | F Value | Prob > F | Remarks |
|---|---|---|---|---|---|---|
| T | 1 | 2.80845 | 2.80845 | 37.06546 | 0.00173 | Significant |
| M | 1 | 3.3282 | 3.3282 | 43.92504 | 0.00118 | Significant |
| C | 1 | 0.00405 | 0.00405 | 0.05345 | 0.82633 | Not significant |
| T*T | 1 | 23.84275 | 23.84275 | 314.6727 | 1.04 × 10−5 | Highly significant |
| M*M | 1 | 6.27714 | 6.27714 | 82.8447 | 2.68 × 10−4 | Highly significant |
| C*C | 1 | 15.72577 | 15.72577 | 207.54619 | 2.91 × 10−5 | Highly significant |
| T*M | 1 | 9.03003 | 9.03003 | 119.17679 | 1.12 × 10−4 | Highly significant |
| T*C | 1 | 10.98922 | 10.98922 | 145.03398 | 6.97 × 10−5 | Highly significant |
| M*C | 1 | 1.33402 | 1.33402 | 17.60624 | 0.00852 | Significant |
| Error | 5 | 0.37885 | 0.07577 | |||
| Lack of fit | 3 | 0.34945 | 0.11648 | 7.92404 | 0.11412 | Not significant |
| Pure error | 2 | 0.0294 | 0.0147 | |||
| Total | 14 | 73.71849 | ||||
| R2 = 0.9948 | Adj R2 = 0.9856 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.; Elsayed, I.; Hassan, E.B. Upgrading of Rice Straw Bio-Oil Using 1-Butanol over ZrO2-Fe3O4 Bimetallic Nanocatalyst Supported on Activated Rice Straw Biochar to Butyl Esters. Catalysts 2024, 14, 666. https://doi.org/10.3390/catal14100666
Ibrahim A, Elsayed I, Hassan EB. Upgrading of Rice Straw Bio-Oil Using 1-Butanol over ZrO2-Fe3O4 Bimetallic Nanocatalyst Supported on Activated Rice Straw Biochar to Butyl Esters. Catalysts. 2024; 14(10):666. https://doi.org/10.3390/catal14100666
Chicago/Turabian StyleIbrahim, Alhassan, Islam Elsayed, and El Barbary Hassan. 2024. "Upgrading of Rice Straw Bio-Oil Using 1-Butanol over ZrO2-Fe3O4 Bimetallic Nanocatalyst Supported on Activated Rice Straw Biochar to Butyl Esters" Catalysts 14, no. 10: 666. https://doi.org/10.3390/catal14100666
APA StyleIbrahim, A., Elsayed, I., & Hassan, E. B. (2024). Upgrading of Rice Straw Bio-Oil Using 1-Butanol over ZrO2-Fe3O4 Bimetallic Nanocatalyst Supported on Activated Rice Straw Biochar to Butyl Esters. Catalysts, 14(10), 666. https://doi.org/10.3390/catal14100666








