Recent Advances in Novel Catalytic Hydrodeoxygenation Strategies for Biomass Valorization without Exogenous Hydrogen Donors—A Review
Abstract
:1. Introduction
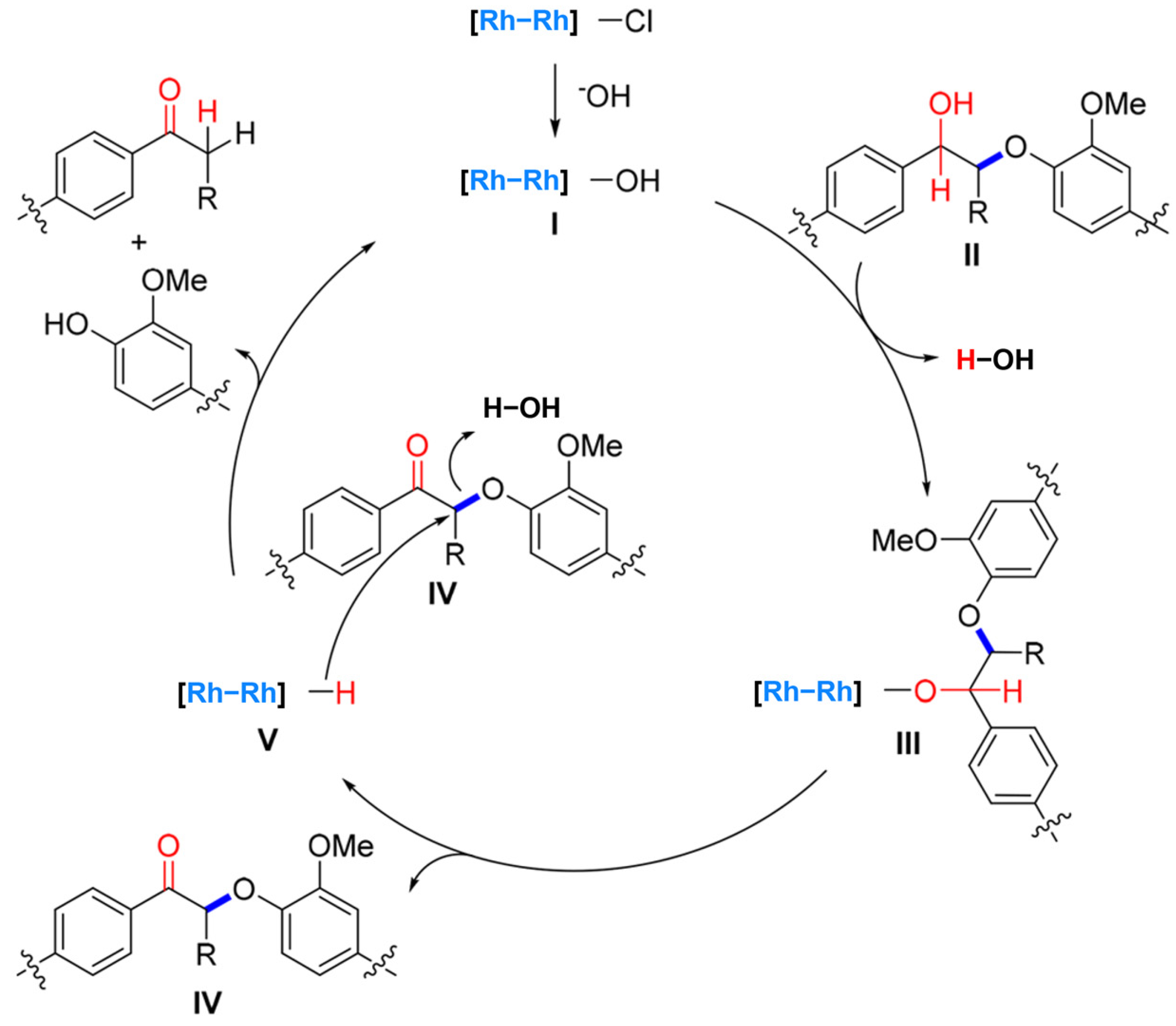
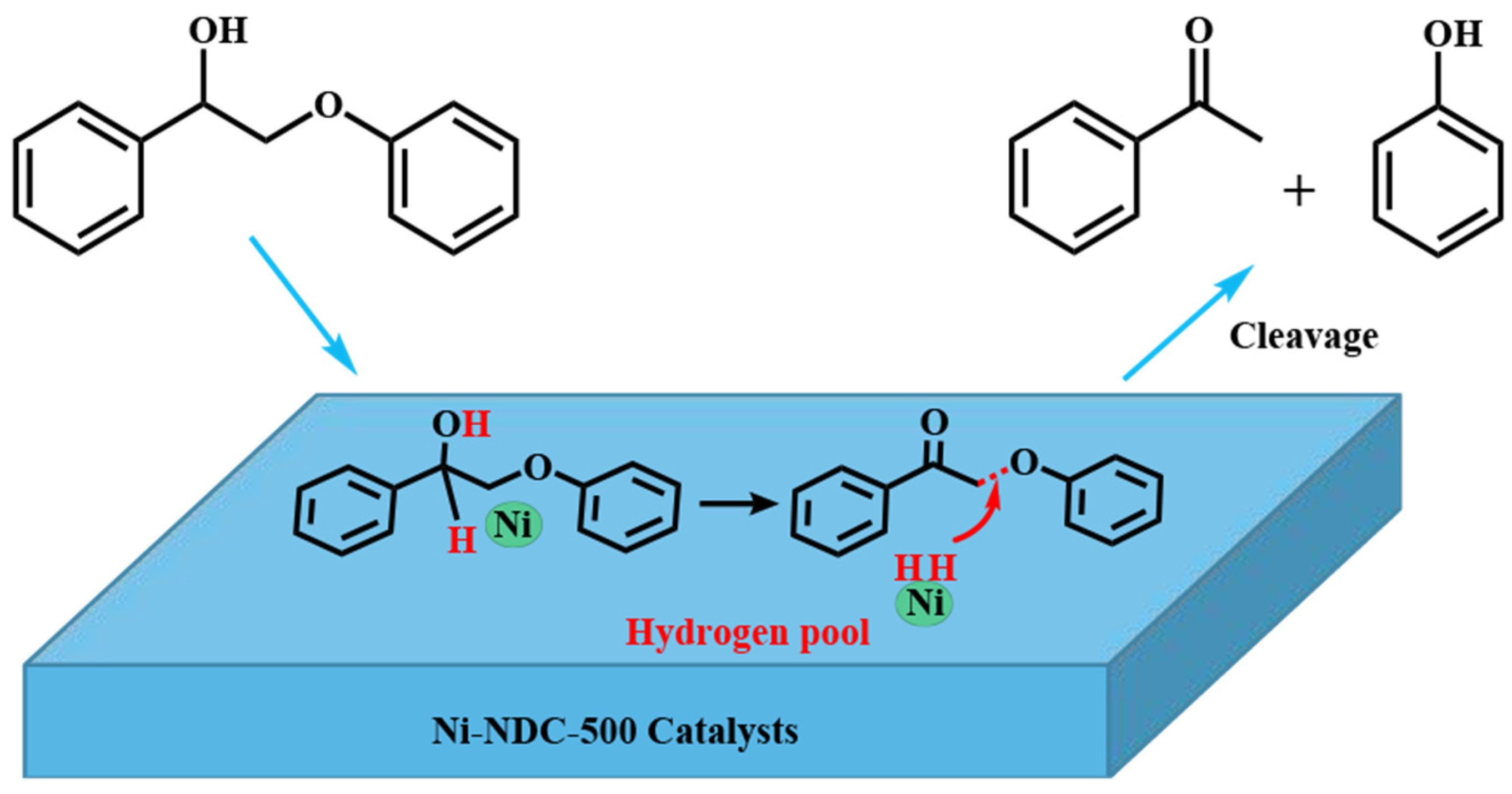
2. Catalytic Self-Transfer Hydrogenolysis with Endogenous Hydrogen
2.1. Effects of Catalysts on Self-Transfer Hydrogenolysis Process
2.2. Self-Reforming-Driven Hydrogenolysis of Lignin
2.3. Self-Hydrogen Supplied Catalytic Fractionation
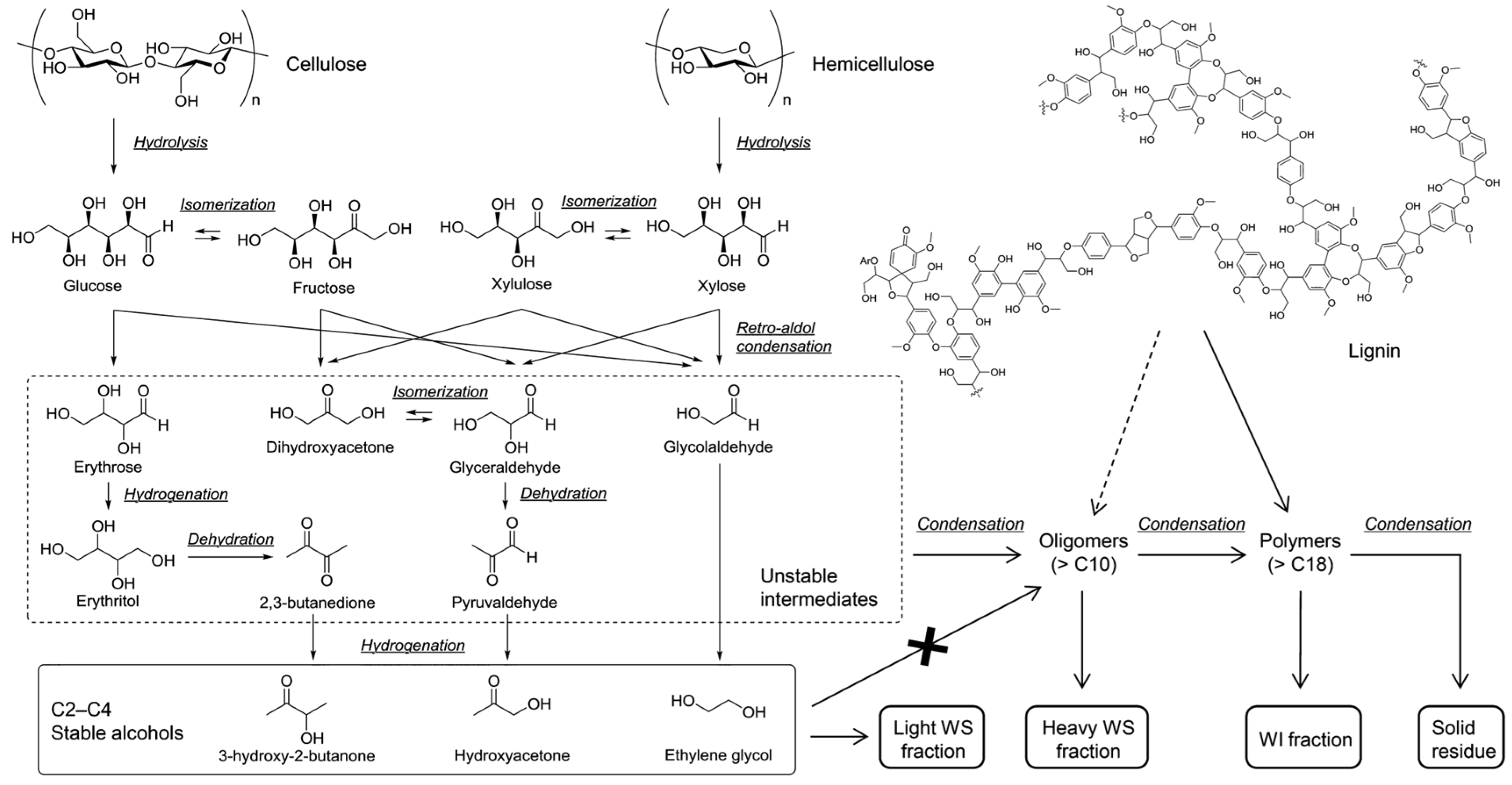
3. In Situ Catalytic Hydrodeoxygenation Employing Water as the Hydrogen Donor
3.1. Application of Multifunctional Catalysts for Water Splitting and Biomass HDO
3.2. Synergistic Utilization of Endogenous Hydrogen Generated from Water Splitting and Biomass Structure
4. In Situ Hydrodeoxygenation Assisted by Zero-Valent Metals
4.1. Promotion Effects and Mechanism of In Situ Hydrogen Induced by Zero-Valent Metals on Biomass Liquefaction/Upgrading20
4.2. Synergistic Effects of Zero-Valent Metals and Hydrodeoxygenation Catalysts on Hydrothermal Liquefaction of Biomass Feedstocks
5. Conclusions and Perspectives
- Exploring cost-effective transition-metal catalysts: identifying and developing cost-effective and abundant transition-metal catalysts as alternatives to precious metal catalysts is crucial for the practical application of catalytic self-transfer hydrogenolysis technology. Especially, innovative designs for transition-metal catalysts that can enhance both the cleavage of ether bonds and the dehydrogenation reactions of hydrogen-donating groups are essential for improving overall HDO efficiency;
- Design of efficient solvent systems: developing solvent systems that can effectively dissolve actual biomass feedstocks and facilitate the interaction of catalytic sites with hydrogen-donating groups is important for improving hydrogen transfer efficiency and HDO reactions. Moreover, employing biomass pretreatment approaches or assisted methods during the liquefaction process to promote the degradation of biomass macromolecular structures can further enhance the effectiveness of catalytic self-hydrogenation technology and improve the HDO catalyst performance;
- Advancing research on complex compounds and real biomass: research should progressively focus on more complex model compounds and real biomass materials to gain a comprehensive understanding of the reaction mechanisms and catalytic HDO pathways involving hydrogen-donating functional groups. This approach will provide a scientific basis for enhancing catalytic performance and advancing the practical application of this technology.
- Investigating the in situ hydrogen supply mechanism of water splitting: a deeper exploration of the water-splitting-based hydrogen supply mechanism and the reaction pathways for active hydrogen transfer is essential for enhancing the efficiency of a water-assisted HDO system. A comprehensive understanding of this mechanism will facilitate the development of more effective HDO catalysts and the design of aqueous liquefaction systems, ultimately improving hydrogen transfer efficiency;
- Combining in situ hydrogenation methods: given the limited hydrogen supply capability of water, integrating water-mediated in situ hydrogenation techniques with other alternative hydrogen supply strategies, such as catalytic self-transfer hydrogenolysis and catalytic reforming processes, can strengthen the stable supply and efficient utilization of internal active hydrogen through multiple pathways. Additionally, developing multifunctional catalysts that can simultaneously improve various in situ hydrogen supply methods will significantly reduce reaction costs and improve the overall HDO efficiency.
- The specific mechanisms between zero-valent metals and HDO catalysts remain inadequately understood. Further investigation is essential to elucidate their reaction pathways and catalytic efficiencies, which is crucial for developing suitable catalysts that work synergistically with zero-valent metals and their oxides during HDO reactions to enhance overall efficiency;
- Future research should also prioritize the development of cost-effective and environmentally friendly recovery technologies for unreacted zero-valent metals and generated metal oxides. This will help mitigate the economic burden and energy consumption associated with the zero-valent metal-assisted HDO process. Additionally, optimizing metal oxide regeneration technologies can further lower production costs for in situ hydrogen and facilitate the cyclic utilization of costly zero-valent metals, thus promoting the practical application of this technology.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pattnaik, F.; Patra, B.R.; Okolie, J.A.; Nanda, S.; Dalai, A.K.; Naik, S. A review of thermocatalytic conversion of biogenic wastes into crude biofuels and biochemical precursors. Fuel 2022, 320, 123857. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Rajendran, S.; Vasseghian, Y.; Dragoi, E.-N. Advanced integrated nanocatalytic routes for converting biomass to biofuels: A comprehensive review. Fuel 2021, 314, 122762. [Google Scholar] [CrossRef]
- Lee, D.; Nam, H.; Seo, M.W.; Lee, S.H.; Tokmurzin, D.; Wang, S.; Park, Y.-K. Recent progress in the catalytic thermochemical conversion process of biomass for biofuels. Chem. Eng. J. 2022, 447, 137501. [Google Scholar] [CrossRef]
- Drugkar, K.; Rathod, W.; Sharma, T.; Sharma, A.; Joshi, J.; Pareek, V.K.; Ledwani, L.; Diwekar, U. Advanced separation strategies for up-gradation of bio-oil into value-added chemicals: A comprehensive review. Sep. Purif. Technol. 2021, 283, 120149. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C.; et al. Applications of catalysts in thermochemical conversion of biomass (pyrolysis, hydrothermal liquefaction and gasification): A critical review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Shahbeik, H.; Panahi, H.K.S.; Dehhaghi, M.; Guillemin, G.J.; Fallahi, A.; Hosseinzadeh-Bandbafha, H.; Amiri, H.; Rehan, M.; Raikwar, D.; Latine, H.; et al. Biomass to biofuels using hydrothermal liquefaction: A comprehensive review. Renew. Sustain. Energy Rev. 2024, 189, 113976. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, Y.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C.C. Recent Advances in Hydroliquefaction of Biomass for Bio-oil Production Using In Situ Hydrogen Donors. Ind. Eng. Chem. Res. 2020, 59, 16987–17007. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, G.-H.; Um, B.-H. Use of an alkaline catalyst with ethanol-water as a co-solvent in the hydrothermal liquefaction of the Korean native kenaf: An analysis of the light oil and heavy oil characteristics. Energy 2022, 249, 123509. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, X.; Rosendahl, L.; Toor, S.S.; Zhang, S.; Sun, Z.; Lu, S.; Zhao, J.; Yang, J.; Chen, G. Fast hydrothermal liquefaction of barley straw: Reaction products and pathways. Biomass-Bioenergy 2022, 165, 106587. [Google Scholar] [CrossRef]
- Jin, W.; Pastor-Pérez, L.; Shen, D.; Sepúlveda-Escribano, A.; Gu, S.; Reina, T.R. Catalytic Upgrading of Biomass Model Compounds: Novel Approaches and Lessons Learnt from Traditional Hydrodeoxygenation—A Review. ChemCatChem 2019, 11, 924–960. [Google Scholar] [CrossRef]
- Xu, H.; Li, H. Alcohol-assisted hydrodeoxygenation as a sustainable and cost-effective pathway for biomass derivatives upgrading. J. Energy Chem. 2022, 73, 133–159. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, E.E.; Kim, Y.T.; Jung, S.; Kim, H.J.; Huber, G.W.; Lee, J. Recent advances in hydrodeoxygenation of biomass-derived oxygenates over heterogeneous catalysts. Green Chem. 2019, 21, 3715–3743. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Miyata, Y.; Sagata, K.; Hirose, M.; Yamazaki, Y.; Nishimura, A.; Okuda, N.; Arita, Y.; Hirano, Y.; Kita, Y. Fe-Assisted Hydrothermal Liquefaction of Lignocellulosic Biomass for Producing High-Grade Bio-Oil. ACS Sustain. Chem. Eng. 2017, 5, 3562–3569. [Google Scholar] [CrossRef]
- Besse, X.; Schuurman, Y.; Guilhaume, N. Reactivity of lignin model compounds through hydrogen transfer catalysis in ethanol/water mixtures. Appl. Catal. B Environ. 2017, 209, 265–272. [Google Scholar] [CrossRef]
- Valentini, F.; Marrocchi, A.; Vaccaro, L. Liquid Organic Hydrogen Carriers (LOHCs) as H-Source for Bio-Derived Fuels and Additives Production. Adv. Energy Mater. 2022, 12, 2103362. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Panagiotopoulou, P.; Mironenko, A.V.; Jenness, G.R.; Vlachos, D.G.; Xu, B. Mechanistic Insights into Metal Lewis Acid-Mediated Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran. ACS Catal. 2015, 5, 3988–3994. [Google Scholar] [CrossRef]
- Xu, H.; Ju, J.; Li, H. Toward efficient heterogeneous catalysts for in-situ hydrodeoxygenation of biomass. Fuel 2022, 320, 123891. [Google Scholar] [CrossRef]
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent Advances in Catalytic Transfer Hydrogenation with Formic Acid over Heterogeneous Transition Metal Catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Guo, J.; Yin, C.K.; Zhong, D.L.; Wang, Y.L.; Qi, T.; Liu, G.H.; Shen, L.T.; Zhou, Q.S.; Peng, Z.H.; Yao, H.; et al. Formic Acid as a Potential On-Board Hydrogen Storage Method: Development of Homogeneous Noble Metal Catalysts for Dehydrogenation Reactions. ChemSusChem 2021, 14, 2655–2681. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.; Tang, Q.; Liang, K.; Sun, Y.; Yu, Y.; Lou, Y.; Liu, Y.; Yu, H. Hydrogen-transfer strategy in lignin refinery: Towards sustainable and versatile value-added biochemicals. ChemSusChem 2024, 17, e202301912. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Zhang, Z.; Wang, M. Self-hydrogen transfer hydrogenolysis of native lignin over Pd-PdO/TiO2. Appl. Catal. B Environ. 2022, 301, 120767. [Google Scholar] [CrossRef]
- Mei, X.; Liu, H.; Wu, H.-H.; Wu, W.; Zheng, B.; Liu, Y.; Zheng, X.; Wang, Y.; Han, W.; Han, B. Catalytic self-transfer hydrogenolysis of lignin over Ni/C catalysts. Green Chem. 2024, 26, 4544–4551. [Google Scholar] [CrossRef]
- Usman, M.A.; Naeem, M.; Saeed, M.; Zaheer, M. Catalytic C–O bond cleavage in a β-O-4 lignin model through intermolecular hydrogen transfer. Inorganica Chim. Acta 2021, 521, 120305. [Google Scholar] [CrossRef]
- Li, L.; Dong, L.; Li, D.; Guo, Y.; Liu, X.; Wang, Y. Hydrogen-Free Production of 4-Alkylphenols from Lignin via Self-Reforming-Driven Depolymerization and Hydrogenolysis. ACS Catal. 2020, 10, 15197–15206. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, C.; Han, B.; Wang, F. Catalytic self-transfer hydrogenolysis of lignin with endogenous hydrogen: Road to the carbon-neutral future. Chem. Soc. Rev. 2022, 51, 1608–1628. [Google Scholar] [CrossRef]
- Zhou, H.; Song, K.; Guo, Y.; Liu, X.; Wang, Y. Selective Production of 4-Propylphenol from Lignin Oil without Exogenous Hydrogen over a RuNi/NiAl2O4 Catalyst. ACS Sustain. Chem. Eng. 2023, 11, 15052–15059. [Google Scholar] [CrossRef]
- Jin, W.; Santos, J.L.; Pastor-Perez, L.; Gu, S.; Centeno, M.A.; Reina, T.R. Noble Metal Supported on Activated Carbon for “Hydrogen Free” HDO Reactions: Exploring Economically Advantageous Routes for Biomass Valorisation. ChemCatChem 2019, 11, 4434–4441. [Google Scholar] [CrossRef]
- Jin, W.; Pastor-Pérez, L.; Villora-Picó, J.J.; Sepúlveda-Escribano, A.; Gu, S.; Reina, T.R. Investigating New Routes for Biomass Upgrading: “H2-Free” Hydrodeoxygenation Using Ni-Based Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 16041–16049. [Google Scholar] [CrossRef]
- Ren, X.; Qiang, Q.; Sun, Z.; Wei, T.; Yu, X.; Rong, Z.; Li, C. Water Splitting Integrated with Self-Transfer Hydrogenolysis for Efficient Demethoxylation of Guaiacols to Phenols over the Ni/MgO Catalyst. ACS Catal. 2024, 14, 5247–5259. [Google Scholar] [CrossRef]
- Liu, W.-J.; Zhang, X.-S.; Qv, Y.-C.; Jiang, H.; Yu, H.-Q. Bio-oil upgrading at ambient pressure and temperature using zero valent metals. Green Chem. 2012, 14, 2226–2233. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Li, J.; Yan, B.; Tao, J.; Cheng, Z.; Chen, G. In-situ hydrodeoxygenation of lignin via hydrothermal liquefaction with water splitting metals: Comparison between autocatalytic and non-autocatalytic processes. Int. J. Hydrogen Energy 2022, 47, 7252–7262. [Google Scholar] [CrossRef]
- Miyata, Y.; Sagata, K.; Yamazaki, Y.; Teramura, H.; Hirano, Y.; Ogino, C.; Kita, Y. Mechanism of the Fe-Assisted Hydrothermal Liquefaction of Lignocellulosic Biomass. Ind. Eng. Chem. Res. 2018, 57, 14870–14877. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Wang, H.; Hu, Y.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C.C. Synergistic effects of metallic Fe and other homogeneous/heterogeneous catalysts in hydrothermal liquefaction of woody biomass. Renew. Energy 2021, 176, 543–554. [Google Scholar] [CrossRef]
- Jin, W.; Pastor-Pérez, L.; Yu, J.; Odriozola, J.; Gu, S.; Reina, T. Cost-effective routes for catalytic biomass upgrading. Curr. Opin. Green Sustain. Chem. 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Jin, X.; Yin, B.; Xia, Q.; Fang, T.; Shen, J.; Kuang, L.; Yang, C. Catalytic Transfer Hydrogenation of Biomass-Derived Substrates to Value-Added Chemicals on Dual-Function Catalysts: Opportunities and Challenges. ChemSusChem 2019, 12, 71–92. [Google Scholar] [CrossRef]
- Manna, S.; Antonchick, A.P. Catalytic Transfer Hydrogenation Using Biomass as Hydrogen Source. ChemSusChem 2018, 12, 3094–3098. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Lu, G.-P.; Cai, C. Self-hydrogen transfer hydrogenolysis of β-O-4 linkages in lignin catalyzed by MIL-100(Fe) supported Pd–Ni BMNPs. Green Chem. 2017, 19, 4538–4543. [Google Scholar] [CrossRef]
- Nichols, J.M.; Bishop, L.M.; Bergman, R.G.; Ellman, J.A. Catalytic C-O Bond Cleavage of 2-Aryloxy-1-arylethanols and Its Application to the Depolymerization of Lignin-Related Polymers. J. Am. Chem. Soc. 2010, 132, 12554–12555. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Miao, W.; Tang, W.; Xue, D.; Li, C.; Zhang, B.; Xiao, J.; Wang, A.; Zhang, T.; et al. Mild Redox-Neutral Depolymerization of Lignin with a Binuclear Rh Complex in Water. ACS Catal. 2019, 9, 4441–4447. [Google Scholar] [CrossRef]
- Meng, Q.; Yan, J.; Liu, H.; Chen, C.; Li, S.; Shen, X.; Song, J.; Zheng, L.; Han, B. Self-supported hydrogenolysis of aromatic ethers to arenes. Sci. Adv. 2019, 5, eaax6839. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yan, J.; Wu, R.; Liu, H.; Sun, Y.; Wu, N.; Xiang, J.; Zheng, L.; Zhang, J.; Han, B. Sustainable production of benzene from lignin. Nat. Commun. 2021, 12, 4534. [Google Scholar] [CrossRef] [PubMed]
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. A critical review on catalyst design for aqueous phase reforming. Int. J. Hydrogen Energy 2022, 47, 151–180. [Google Scholar] [CrossRef]
- Coronado, I.; Stekrova, M.; Reinikainen, M.; Simell, P.; Lefferts, L.; Lehtonen, J. A review of catalytic aqueous-phase reforming of oxygenated hydrocarbons derived from biorefinery water fractions. Int. J. Hydrogen Energy 2016, 41, 11003–11032. [Google Scholar] [CrossRef]
- Jongerius, A.L.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Liquid-phase reforming and hydrodeoxygenation as a two-step route to aromatics from lignin. Green Chem. 2013, 15, 3049–3056. [Google Scholar] [CrossRef]
- Jongerius, A.L.; Copeland, J.R.; Foo, G.S.; Hofmann, J.P.; Bruijnincx, P.C.A.; Sievers, C.; Weckhuysen, B.M. Stability of Pt/γ-Al2O3 Catalysts in Lignin and Lignin Model Compound Solutions under Liquid Phase Reforming Reaction Conditions. ACS Catal. 2013, 3, 464–473. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Guo, Z.; Liu, X.; Guo, Y.; Huang, Y.; Wang, Y. Unraveling the Role of Metal in M/NiAl2O4 (M = Pt, Pd, Ru) Catalyst for the Self-Reforming-Driven Hydrogenolysis of Lignin. Ind. Eng. Chem. Res. 2021, 60, 11699–11706. [Google Scholar] [CrossRef]
- Guo, Z.; Li, L.; Guo, Y.; Liu, X.; Wang, Y. Size effect of Ru particles on the self-reforming-driven hydrogenolysis of a lignin model compound. Catal. Sci. Technol. 2022, 12, 5143–5151. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Kumaniaev, I.; Karacic, A.; Baddigam, K.R.; Hanes, R.J.; Subbotina, E.; Bartling, A.W.; Huertas-Alonso, A.J.; Moreno, A.; Håkansson, H.; et al. Lignin-first biorefining of Nordic poplar to produce cellulose fibers could displace cotton production on agricultural lands. Joule 2022, 6, 1845–1858. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S.M. Selective Route to 2-Propenyl Aryls Directly from Wood by a Tandem Organosolv and Palladium-Catalysed Transfer Hydrogenolysis. ChemSusChem 2014, 7, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.J.; Samec, J.S.M. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. [Google Scholar] [CrossRef]
- Ferrini, P.; Rinaldi, R. Catalytic Biorefining of Plant Biomass to Non-Pyrolytic Lignin Bio-Oil and Carbohydrates through Hydrogen Transfer Reactions. Angew. Chem. Int. Ed. 2014, 53, 8634–8639. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, X.; Guo, Y.; Wang, Y. Self-Hydrogen Supplied Catalytic Fractionation of Raw Biomass into Lignin-Derived Phenolic Monomers and Cellulose-Rich Pulps. JACS Au 2023, 3, 1911–1917. [Google Scholar] [CrossRef]
- Cheng, Y.; Shan, H.; Guo, P.; Li, H. Review and Outlook on Regulation of Catalyst Activity, Selectivity, and Stability for Biomass Hydrodeoxygenation Reaction in an Aqueous Environment. Energy Fuels 2024, 38, 6644–6658. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H. Water-mediated catalytic hydrodeoxygenation of biomass. Fuel 2021, 310, 122242. [Google Scholar] [CrossRef]
- Carrasco-Ruiz, S.; Parrilla-Lahoz, S.; Santos, J.; Penkova, A.; Odriozola, J.; Reina, T.; Pastor-Perez, L. Water-assisted HDO of biomass model compounds enabled by Ru-based catalysts. Fuel Process. Technol. 2023, 249, 107860. [Google Scholar] [CrossRef]
- Hu, X.; Wang, G.; Qin, C.; Xie, X.; Zhang, C.; Xu, W.; Liu, Y. Ligandless nickel-catalyzed transfer hydrogenation of alkenes and alkynes using water as the hydrogen donor. Org. Chem. Front. 2019, 6, 2619–2623. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, X.; Xing, M.; Zhang, P.; Zhao, Q.; Zhang, C. Cu-Catalyzed highly selective reductive functionalization of 1,3-diene using H2O as a stoichiometric hydrogen atom donor. Chem. Commun. 2019, 55, 8651–8654. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Miao, M.; Du, W.; Shi, J.; Liu, Y.; Hou, Z. Selective hydrogenation of C C bond over N-doped reduced graphene oxides supported Pd catalyst. Appl. Catal. B Environ. 2016, 180, 607–613. [Google Scholar] [CrossRef]
- Wang, G.; Cao, Z.; Gu, D.; Pfänder, N.; Swertz, A.; Spliethoff, B.; Bongard, H.; Weidenthaler, C.; Schmidt, W.; Rinaldi, R.; et al. Nitrogen-Doped Ordered Mesoporous Carbon Supported Bimetallic PtCo Nanoparticles for Upgrading of Biophenolics. Angew. Chem. Int. Ed. 2016, 55, 8850–8855. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Pastor-Pérez, L.; Villora-Picó, J.J.; Pastor-Blas, M.M.; Odriozola, J.A.; Sepúlveda-Escribano, A.; Reina, T.R. In-situ HDO of guaiacol over nitrogen-doped activated carbon supported nickel nanoparticles. Appl. Catal. A Gen. 2021, 620, 118033. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Jin, W.; Villora-Picó, J.J.; Wang, Q.; Pastor-Blas, M.M.; Sepúlveda-Escribano, A.; Reina, T.R. “H2-free” demethoxylation of guaiacol in subcritical water using Pt supported on N-doped carbon catalysts: A cost-effective strategy for biomass upgrading. J. Energy Chem. 2020, 58, 377–385. [Google Scholar] [CrossRef]
- Parrilla-Lahoz, S.; Jin, W.; Pastor-Pérez, L.; Duyar, M.; Martínez-Quintana, L.; Dongil, A.; Reina, T.R. Multicomponent graphene based catalysts for guaiacol upgrading in hydrothermal conditions: Exploring “H2-free” alternatives for bio-compounds hydrodeoxygenation. Catal. Today 2023, 423, 114020. [Google Scholar] [CrossRef]
- de Castro, I.B.D.; Graça, I.; Rodríguez-García, L.; Kennema, M.; Rinaldi, R.; Meemken, F. Elucidating the reactivity of methoxyphenol positional isomers towards hydrogen-transfer reactions by ATR-IR spectroscopy of the liquid–solid interface of RANEY® Ni. Catal. Sci. Technol. 2018, 8, 3107–3114. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Yu, G.; Lv, J.; Rong, Z.; Wang, M.; Wang, Y. Selective Hydrodeoxygenation of Guaiacol to Cyclohexanol Catalyzed by Nanoporous Nickel. Catal. Lett. 2019, 150, 837–848. [Google Scholar] [CrossRef]
- Ren, X.; Sun, Z.; Lu, J.; Cheng, J.; Zhou, P.; Yu, X.; Rong, Z.; Li, C. Hydrodeoxygenation of guaiacol to phenol using endogenous hydrogen induced by chemo-splitting of water over a versatile nano-porous Ni catalyst. Green Chem. 2023, 25, 1955–1969. [Google Scholar] [CrossRef]
- Zhong, H.; Jiang, C.; Zhong, X.; Wang, J.; Jin, B.; Yao, G.; Luo, L.; Jin, F. Non-precious metal catalyst, highly efficient deoxygenation of fatty acids to alkanes with in situ hydrogen from water. J. Clean. Prod. 2018, 209, 1228–1234. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Muthukumarappan, K.; Kharel, P.R.; Cao, Y.; Boakye, E.; Raynie, D.; Gu, Z. Hydrodeoxygenation upgrading of pine sawdust bio-oil using zinc metal with zero valency. J. Taiwan Inst. Chem. Eng. 2017, 74, 146–153. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, W.; Li, R.; Li, B.; Kai, X.; Sun, Y. Deoxy-liquefaction of Corn Stalk in Subcritical Water with Hydrogen Generated in Situ via Aluminum–Water Reaction. Energy Fuels 2017, 31, 9605–9612. [Google Scholar] [CrossRef]
- Yang, T.; Shi, L.; Li, R.; Li, B.; Kai, X. Hydrodeoxygenation of crude bio-oil in situ in the bio-oil aqueous phase with addition of zero-valent aluminum. Fuel Process. Technol. 2018, 184, 65–72. [Google Scholar] [CrossRef]
- Li, R.; Li, B.; Kai, X.; Yang, T. Hydro-liquefaction of rice stalk in supercritical ethanol with in situ generated hydrogen. Fuel Process. Technol. 2017, 167, 363–370. [Google Scholar] [CrossRef]
- de Caprariis, B.; Bavasso, I.; Bracciale, M.P.; Damizia, M.; De Filippis, P.; Scarsella, M. Enhanced bio-crude yield and quality by reductive hydrothermal liquefaction of oak wood biomass: Effect of iron addition. J. Anal. Appl. Pyrolysis 2019, 139, 123–130. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, Y.; Qi, L.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C.C. Promotion effects of metallic iron on hydrothermal liquefaction of cornstalk in ethanol-water mixed solvents for the production of biocrude oil. Fuel 2021, 285, 119150. [Google Scholar] [CrossRef]
- de Caprariis, B.; Scarsella, M.; Bavasso, I.; Bracciale, M.P.; Tai, L.; De Filippis, P. Effect of Ni, Zn and Fe on hydrothermal liquefaction of cellulose: Impact on bio-crude yield and composition. J. Anal. Appl. Pyrolysis 2021, 157, 105225. [Google Scholar] [CrossRef]
- Miyata, Y.; Yamazaki, Y.; Hirano, Y.; Kita, Y. Quantitative analysis of the aqueous fraction from the Fe-assisted hydrothermal liquefaction of oil palm empty fruit bunches. J. Anal. Appl. Pyrolysis 2018, 132, 72–81. [Google Scholar] [CrossRef]
- Cheah, K.W.; Yusup, S.; Kyriakou, G.; Ameen, M.; Taylor, M.J.; Nowakowski, D.J.; Bridgwater, A.V.; Uemura, Y. In-situ hydrogen generation from 1,2,3,4-tetrahydronaphthalene for catalytic conversion of oleic acid to diesel fuel hydrocarbons: Parametric studies using Response Surface Methodology approach. Int. J. Hydrogen Energy 2018, 44, 20678–20689. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Alsowij, M.R.; Corbin, F.; Julson, J.; Boakye, E.; Raynie, D. In situ hydrodeoxygenation upgrading of pine sawdust bio-oil to hydrocarbon biofuel using Pd/C catalyst. J. Energy Inst. 2018, 91, 163–171. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Rabnawaz, M. Catalytic liquefaction of pine sawdust and in-situ hydrogenation of bio-crude over bifunctional Co-Zn/HZSM-5 catalysts. Fuel 2018, 223, 252–260. [Google Scholar] [CrossRef]
- Hamidi, R.; Tai, L.; Paglia, L.; Scarsella, M.; Damizia, M.; De Filippis, P.; Musivand, S.; de Caprariis, B. Hydrotreating of oak wood bio-crude using heterogeneous hydrogen producer over Y zeolite catalyst synthesized from rice husk. Energy Convers. Manag. 2022, 255, 115348. [Google Scholar] [CrossRef]
- Tai, L.; de Caprariis, B.; Scarsella, M.; De Filippis, P.; Marra, F. Improved Quality Bio-Crude from Hydrothermal Liquefaction of Oak Wood Assisted by Zero-Valent Metals. Energy Fuels 2021, 35, 10023–10034. [Google Scholar] [CrossRef]
- Guo, B.; Walter, V.; Hornung, U.; Dahmen, N. Hydrothermal liquefaction of Chlorella vulgaris and Nannochloropsis gaditana in a continuous stirred tank reactor and hydrotreating of biocrude by nickel catalysts. Fuel Process. Technol. 2019, 191, 168–180. [Google Scholar] [CrossRef]
- Xu, D.; Liu, L.; He, Z.; Yang, J.; Wu, Z.; Jing, Z. Hydrothermal upgrading of water-insoluble algal biocrude over γ-Al2O3 supported multi-metallic catalysts. J. Anal. Appl. Pyrolysis 2019, 140, 188–194. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Li, R.; Yang, T.; Kai, X. Aluminum-water reactions assisted in situ hydrodeoxygenation of enzymolysis lignin from bioconversion of rice straw over NiMo catalyst. Ind. Crop. Prod. 2020, 154, 112727. [Google Scholar] [CrossRef]
- Hirano, Y.; Miyata, Y.; Taniguchi, M.; Funakoshi, N.; Yamazaki, Y.; Ogino, C.; Kita, Y. Fe-assisted hydrothermal liquefaction of cellulose: Effects of hydrogenation catalyst addition on properties of water-soluble fraction. J. Anal. Appl. Pyrolysis 2020, 145, 104719. [Google Scholar] [CrossRef]
- Tai, L.; Hamidi, R.; Paglia, L.; De Filippis, P.; Scarsella, M.; de Caprariis, B. Lignin-enriched waste hydrothermal liquefaction with ZVMs and metal-supported Al2O3 catalyst. Biomass-Bioenergy 2022, 165, 106594. [Google Scholar] [CrossRef]








| Hydrogen Donation Strategy | Hydrogen Supply Mechanism | Advantages and Limitations |
|---|---|---|
| Catalytic self-transfer hydrogenolysis with endogenous hydrogen | In situ hydrogen production from potential hydrogen-donor groups in biomass structure via catalytic dehydrogenation reactions | Advantages: Reducing the additional costs associated with the utilization of high-pressured hydrogen or exogenous hydrogen donors; reducing the complexity of the reaction system; further enhancing the efficiency of self-transfer hydrogenolysis by integrating with aqueous-phase reforming (APR) reactions; avoiding side reactions caused by liquid hydrogen donors; and reducing the energy consumption in the purification and hydrogen-donor recovery process. |
| Limitations: Low selectivity of hydrogenation products due to the structural heterogeneity of biomass feedstocks and difficult for the potential hydrogen-donor groups to effectively serve as inherent hydrogen donors due to dense cross-linked macromolecular structures of actual biomass and the existence of competing reactions. | ||
| In situ catalytic hydrodeoxygenation employing water as the hydrogen donor | In situ hydrogen production from catalytic water splitting under biomass upgrading conditions | Advantages: A cost-effective, non-toxic, safe, and environmentally friendly hydrodeoxygenation method with only water serving as the hydrogen donor; reducing the complexity of the reaction system; avoiding side reactions caused by liquid hydrogen donors; and reducing the energy consumption in the purification and hydrogen-donor recovery process. |
| Limitations: Unsatisfactory yield and selectivity of hydrogenation products due to limited hydrogen supply capacity of water and relatively harsh reaction conditions. | ||
| In situ hydrodeoxygenation assisted by zero-valent metals | In situ hydrogen production from the reactions between zero-valent metals and sub-critical water | Advantages: High reaction efficiency due to the exothermal property of metal hydrolysis; catalytic effects of the generated metallic oxides for biomass conversion; and avoiding side reactions caused by liquid hydrogen donors. |
| Limitations: Relatively high cost associated with the application of zero-valent metals and high energy input and complexity of the recycling and regenerating procedures of the metallic oxides. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Du, B.; Hu, J.; Huang, Z.; Xu, S.; Chen, Z.; Cheng, D.; Xu, C.C. Recent Advances in Novel Catalytic Hydrodeoxygenation Strategies for Biomass Valorization without Exogenous Hydrogen Donors—A Review. Catalysts 2024, 14, 673. https://doi.org/10.3390/catal14100673
Zhao B, Du B, Hu J, Huang Z, Xu S, Chen Z, Cheng D, Xu CC. Recent Advances in Novel Catalytic Hydrodeoxygenation Strategies for Biomass Valorization without Exogenous Hydrogen Donors—A Review. Catalysts. 2024; 14(10):673. https://doi.org/10.3390/catal14100673
Chicago/Turabian StyleZhao, Bojun, Bin Du, Jiansheng Hu, Zujiang Huang, Sida Xu, Zhengyu Chen, Defang Cheng, and Chunbao Charles Xu. 2024. "Recent Advances in Novel Catalytic Hydrodeoxygenation Strategies for Biomass Valorization without Exogenous Hydrogen Donors—A Review" Catalysts 14, no. 10: 673. https://doi.org/10.3390/catal14100673







_Xu.png)


