Investigating Layered Topological Magnetic Materials as Efficient Electrocatalysts for the Hydrogen Evolution Reaction under High Current Densities
Abstract
1. Introduction
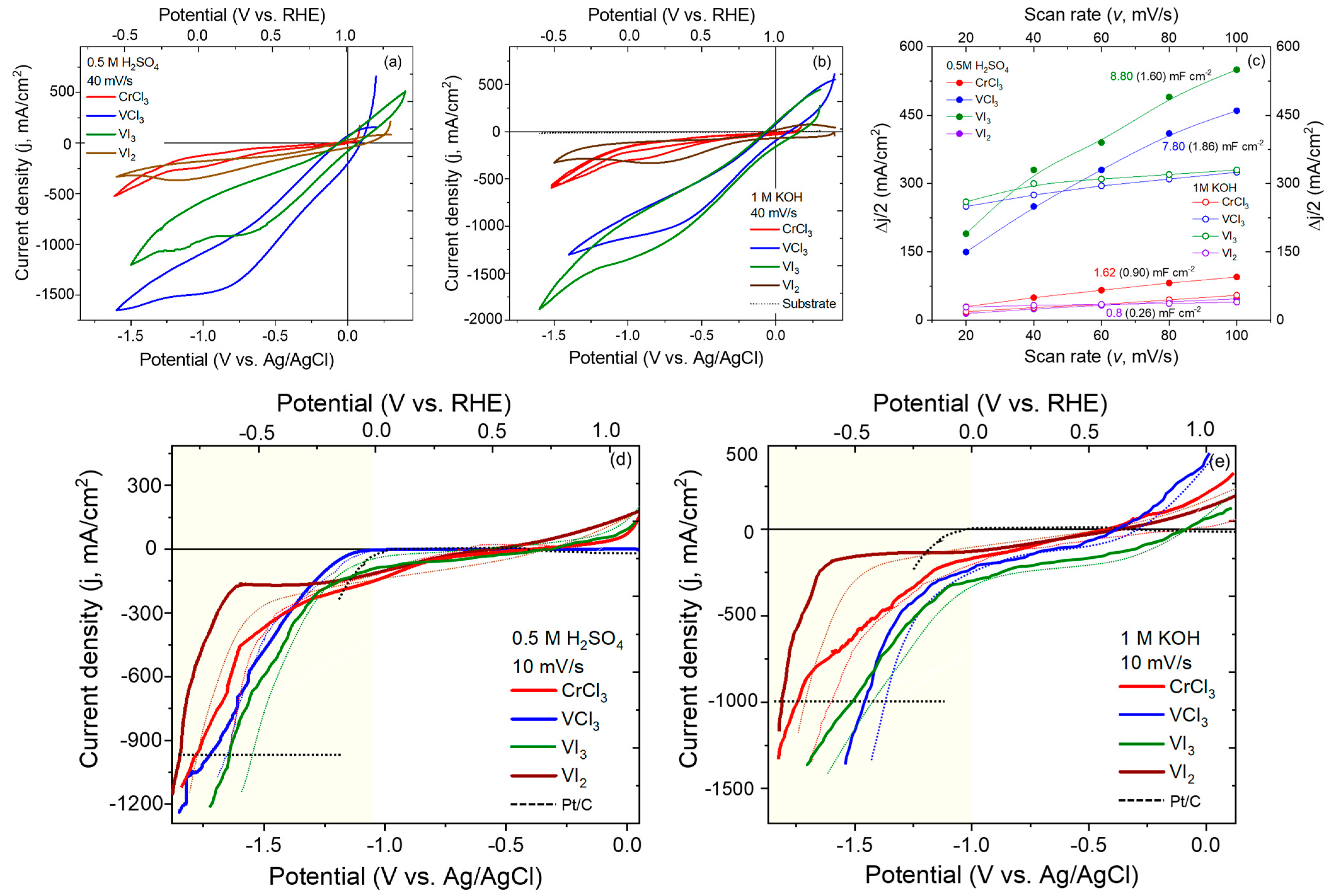
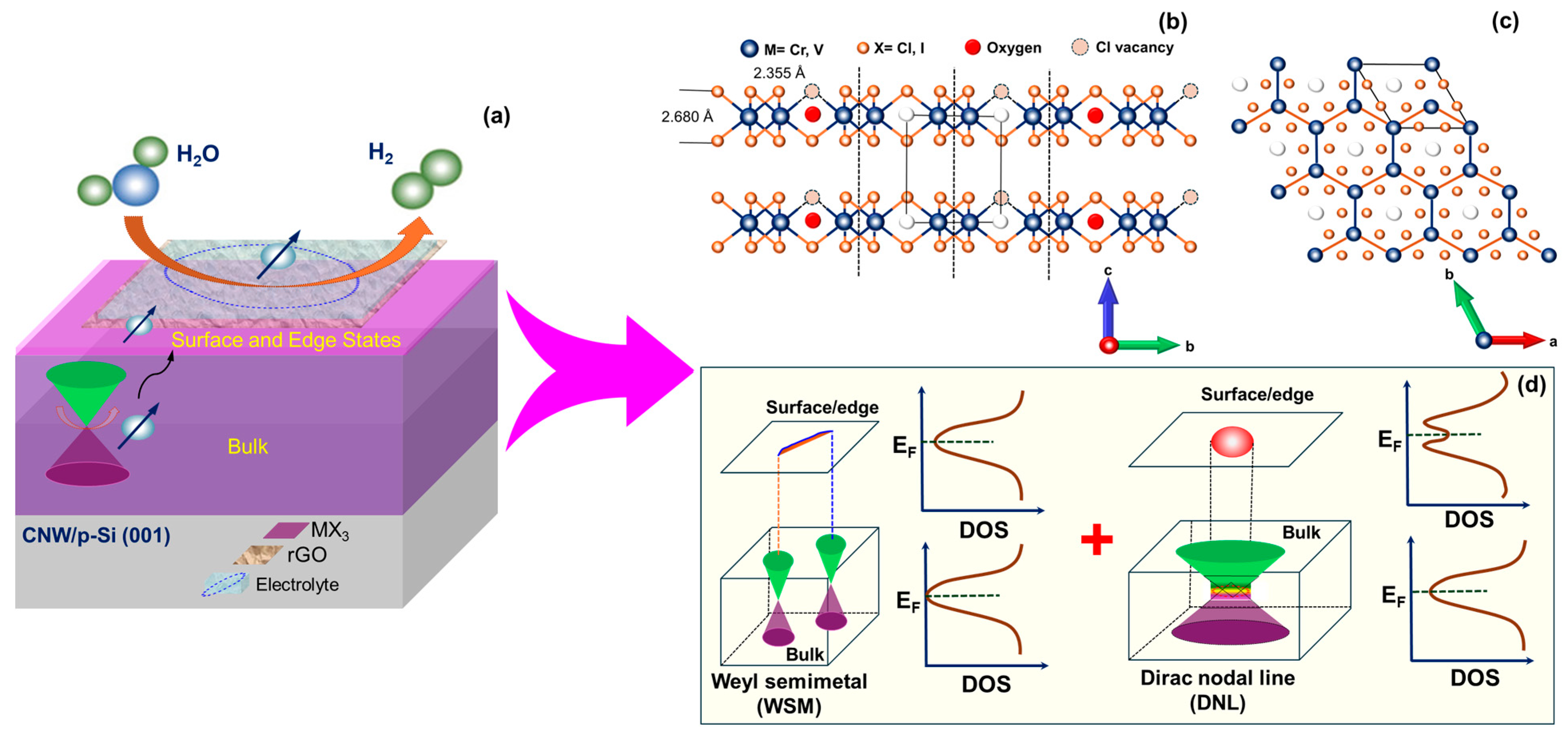
2. Results and Discussion
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, T.N.V.; Barbir, F. Hydrogen: The wonder fuel. Int. J. Hydrogen Energy 1992, 17, 391–400. [Google Scholar]
- Faber, M.S.; Jin, S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- Singh, A.K.; Xu, Q. Synergistic catalysis over bimetallic alloy nanoparticles. ChemCatChem Catal. 2013, 5, 652–676. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sakamoto, H.; Sugano, Y.; Ichikawa, S.; Hirai, T. Pt–Cu bimetallic alloy nanoparticles supported on anatase TiO2: Highly active catalysts for aerobic oxidation driven by visible light. ACS Nano 2013, 7, 9287–9297. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Veziroglu, T.N.; Sahin, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.X.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Guo, W.; Xia, B.Y. Emerging two-dimensional nanocatalysts for electrocatalytic hydrogen production. Chin. Chem. Lett. 2022, 33, 1831–1840. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Z.; Lin, Y.; Chong, F.; Qi, Y. Design Strategies for Large Current Density Hydrogen Evolution Reaction. Front. Chem. 2022, 10, 866415. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Sun, K.; Zhang, K.; Wu, Y.; Du, Y.; Zhao, Q.; Liu, G.; Chen, C.; Sun, Y.; Li, J. Stable hydrogen evolution reaction at high current densities via designing the Ni single atoms and Ru nanoparticles linked by carbon bridges. Nat. Commun. 2024, 15, 2218. [Google Scholar] [CrossRef]
- Zhao, J.; Urrego-Ortiz, R.; Liao, N.; Calle-Vallejo, F.; Luo, J. Rationally designed Ru catalysts supported on TiN for highly efficient and stable hydrogen evolution in alkaline conditions. Nat. Commun. 2024, 15, 6391. [Google Scholar] [CrossRef]
- Li, D.; Wang, R.; Yi, L.; Wei, Y.; Li, J.; Zhao, D.; Sun, W.; Hu, W. High current density hydrogen evolution on heterostructured Ni/Cr bimetallic sulfide catalyst in alkaline media. Int. J. Hydrogen Energy 2024, 49 Pt C, 67–74. [Google Scholar] [CrossRef]
- Zheng, Z. Boosting hydrogen evolution on MoS2 via co-confining selenium in surface and cobalt in inner layer. Nat. Commun. 2020, 11, 3315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Robinson, T.; Dimakis, N. Electrochemically desulfurized molybdenum disulfide (MoS2) and reduced graphene oxide aerogel composites as efficient electrocatalysts for hydrogen evolution. J. Nanosci. Nanotechnol. 2020, 20, 6191–6214. [Google Scholar] [CrossRef]
- Dimakis, N.; Vadodariab, O.; Ruiz, K.; Gupta, S. Molybdenum disulfide monolayer electronic structure information as explored using density functional theory and quantum theory of atoms in molecules. Appl. Surf. Sci. 2021, 555, 149545. [Google Scholar] [CrossRef]
- Arandiyan, H.; Mofarah, S.S.; Wang, Y.; Cazorla, C.; Jampaiah, D.; Garbrecht, M.; Wilson, K.; Lee, A.F.; Zhao, C.; Maschmeyer, T. Impact of surface defects on LaNiO3 perovskite electrocatalysts for oxygen evolution reaction. Chem. A Eur. J. 2021, 27, 14418–14426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Arpino, K.E.; Yang, Q.; Kikugawa, N.; Sokolov, D.A.; Hicks, C.W.; Liu, J.; Felser, C.; Li, G. Observation of a robust and active catalyst for hydrogen evolution under high current densities. Nat. Commun. 2022, 13, 7784. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, R.; Hartmann, R.; Mercaldo, M.T.; Komori, S.; Bjørlig, A.; Kyung, W.; Yasui, Y.; Miyoshi, T.; Olthof, L.A.B.O.; Garcia, C.M.P.; et al. Unveiling unconventional magnetism at the surface of Sr2RuO4. Nat. Commun. 2021, 12, 5792. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tahini, H.A.; Hu, Z.; Dai, J.; Chen, Y.; Sun, H.; Zhou, W.; Liu, M.; Smith, S.C.; Wang, H.; et al. Unusual synergistic effect in layered Ruddlesden–Popper oxide enables ultrafast hydrogen evolution. Nat. Commun. 2019, 10, 149. [Google Scholar] [CrossRef]
- Luo, H.; Yu, P.; Li, G.; Yan, K. Topological quantum materials for energy conversion and storage. Nat. Rev. Phys. 2022, 4, 611–624. [Google Scholar] [CrossRef]
- Yan, B.; Felser, C. Topological Materials: Weyl Semimetals. Annu. Rev. Condens. Matter Phys. 2017, 8, 337–354. [Google Scholar] [CrossRef]
- Xiao, J.; Kou, L.; Yam, C.-Y.; Fraunheim, T.; Yan, B. Toward Rational Design of Catalysts Supported on a Topological Insulator Substrate. ACS Catal. 2015, 5, 7063–7067. [Google Scholar] [CrossRef]
- Rajamathi, C.R.; Gupta, U.; Pal, K.; Kumar, N.; Yang, H.; Sun, Y.; Shekar, C.; Yan, B.; Parkin, S.S.P.; Waghmare, U.V.; et al. Photochemical Water Splitting by Bismuth Chalcogenide Topological Insulators. ChemPhysChem 2017, 18, 2322–2327. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Li, M.; Meng, W.; Liu, Y.; Dai, X.; Liu, G.; Gu, Y.; Liu, J.; Kou, L. Topological surface state: Universal catalytic descriptor in topological catalysis. Mater. Today 2023, 67, 23–32. [Google Scholar] [CrossRef]
- Yu, X.; Cao, X.; Kang, W.; Chen, S.; Jiang, A.; Luo, Y.; Deng, W. Efficient hydrogen production over Bi2Te3-modified TiO2 catalysts: A first principles study. Surf. Sci. 2024, 739, 122401. [Google Scholar] [CrossRef]
- Sakaushi, K. Quantum electrocatalysts: Theoretical picture, electrochemical kinetic isotope effect analysis, and conjecture to understand microscopic mechanisms. Phys. Chem. Chem. Phys. 2020, 22, 11219–11243. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Yang, B.; Li, S.; Jiang, S.; Jin, C.; Tao, Z.; Nichols, G.; Sfgakis, F.; Zhong, S.; Li, C.; et al. Evolution of interlayer and intralayer magnetism in three atomically thin chromium trihalides. Proc. Natl. Acad. Sci. USA 2019, 166, 11131–11136. [Google Scholar] [CrossRef]
- Wang, Z.; Gutiérrez-Lezama, I.; Ubrig, N.; Kroner, M.; Gibertini, M.; Taniguchi, T.; Watanabe, K.; Imamoglu, A.; Giannini, E.; Morpurgo, A.F. Very large tunneling magnetoresistance in layered magnetic semiconductor CrI3. Nat. Commun. 2018, 9, 2516. [Google Scholar] [CrossRef]
- Huang, B.; Clark, G.; Navarro-Moratalla, E.; Klein, D.R.; Cheng, R.; Seyler, K.L.; Zhong, D.; Schmidgall, E.; McGuire, M.A.; Cobden, D.H.; et al. Layer-dependent ferromagnetism in a van der Waals crystal down to the monolayer limit. Nature 2017, 546, 270–273. [Google Scholar] [CrossRef]
- Mastrippolito, D.; Ottaviano, L.; Wang, J.; Yang, J.; Gao, F.; Ali, M.; D’Olimpio, G.; Politano, A.; Palleschi, S.; Kazim, S.; et al. Emerging oxidized and defective phases in low-dimensional CrCl3. Nanoscale Adv. 2021, 3, 4756–4766. [Google Scholar] [CrossRef]
- Kazim, S.; Mastrippolito, D.; Moras, P.; Jugovac, M.; Klimczuk, T.; Ali, M.; Ottaviano, L.; Gunnella, R. Synchrotron radiation photoemission spectroscopy of the oxygen modified CrCl3 surface. Phys. Chem. Chem. Phys. 2023, 25, 3806–3814. [Google Scholar] [CrossRef]
- Kazim, S.; Ali, M.; Palleschi, S.; D’Olimpio, G.; Mastrippolito, D.; Politano, A.; Gunnella, R.; Di Cicco, A.; Renzelli, M.; Moccia, G.; et al. Mechanical Exfoliation and Layer Number Identification of Single Crystal Monoclinic CrCl3. Nanotechnology 2020, 31, 395706. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Chen, Q.; Cao, J.; Zhang, C. Recent progress of electrochemical hydrogen evolution over 1T-MoS2 catalysts. Front. Chem. 2022, 10, 1000406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liao, X.; Pan, X.; Yan, M.; Li, Y.; Tian, X.; Zhao, Y.; Xu, L.; Mai, L. Superior Hydrogen Evolution Reaction Performance in 2H-MoS2 to that of 1T Phase. Small 2019, 15, 1900964. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Botari, T.; Tiwary, C.S.; Halder, A. Hydrogen Evolution at the In Situ MoO3/MoS2 Heterojunctions Created by Nonthermal O2 Plasma Treatment. ACS Appl. Energy Mater. 2020, 3, 5333–5342. [Google Scholar] [CrossRef]
- Sobaszek, M.; Siuzdak, K.; Ryl, J.; Sawczak, M.; Gupta, S.; Carrizosa, S.B.; Ficek, M.; Dec, B.; Darowicki, K.; Bogdanowicz, R. Diamond phase (sp3–C) rich boron-doped carbon nanowalls (sp2–C): Physicochemical and electrochemical properties. J. Phys. Chem. C 2017, 121, 20821–20833. [Google Scholar] [CrossRef]
- Paolucci, V.; Mastrippolito, D.; Ricci, V.; Świątek, H.; Klimczuk, T.; Ottaviano, L.; Cantalini, C. Two-Dimensional CrCl3-Layered Trihalide Nanoflake Sensor for the Detection of Humidity, NO2, and H2. ACS Appl. Nano Mater. 2024, 7, 3679–3690. [Google Scholar] [CrossRef]
- Wang, Z.; Gibertini, M.; Dumcenco, D.; Taniguchi, T.; Watanabe, K.; Giannini, E.; Marpurgo, A.F. Determining the phase diagram of atomically thin layered antiferromagnet CrCl3. Nat. Nanotechnol. 2019, 14, 1116–1122. [Google Scholar] [CrossRef]
- Morosin, B.; Narath, A. X-ray Diffraction and Nuclear Quadrupole Resonance Studies of Chromium Trichloride. J. Chem. Phys. 1964, 40, 1958–1967. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, J.-F.; Li, C.; Ying, T.; Li, S.; Zhang, X.; Liu, K.; Lei, H. Ferromagnetic van der Waals Crystal VI3. J. Am. Chem. Soc. 2019, 141, 5326–5333. [Google Scholar] [CrossRef]
- Munro, J.M.; Latimer, K.; Horton, M.K.; Dwaraknath, S.; Persson, K.A. An improved symmetry-based approach to reciprocal space path selection in band structure calculations. Npj Comput. Mater. 2020, 6, 112. [Google Scholar] [CrossRef]
- Mastrippolito, D.; Camerano, L.; Świątek, H.; Šmíd, B.; Klimczuk, T.; Ottaviano, L.; Profeta, G. Polaronic and Mott insulating phase of layered magnetic vanadium trihalide VCl3. Phys. Rev. B 2023, 108, 045126. [Google Scholar] [CrossRef]
- Glamazda, A.; Lemmens, P.; Do, S.H.; Kwon, S.Y.; Choi, K.Y. Relation between Kitaev magnetism and structure in α–RuCl3. Phys. Rev. B 2017, 95, 174429. [Google Scholar] [CrossRef]
- Avram, C.; Gruia, A.; Brik, M.; Barb, A. Calculations of the electronic levels, spin-hamiltonian parameters and vibrational spectra for the CrCl3 layered crystals. Phys. B Condens. Matter 2015, 478, 31–35. [Google Scholar] [CrossRef]
- Kong, T.; Stolze, K.; Timmons, E.I.; Tao, J.; Ni, D.; Guo, S.; Yang, Z.; Prozorov, R.; Cava, R.J. Ferromagnetic Semiconductors: VI3–a New Layered Ferromagnetic Semiconductor. Adv. Mater. 2019, 31, 1970126. [Google Scholar] [CrossRef]
- Suhan, S.; Matthew, J.C.; Nahyun, L.; Jonghyeon, K.; Yun, K.T.; Hayrullo, H.; Hwanbeom, C.; Cheng, L.; David, M.J.; Philip, A.C.B.; et al. Bulk properties of the van der Waals hard ferromagnet VI3. Phys. Rev. B 2019, 99, 041402. [Google Scholar]
- He, B.-G.; Yang, Z.; Cheng, Z.-P.; Zhang, W.-B.; Li, H. Raman Spectrum of layered Ferromagnetic Material VI3 from First-principles. In Proceedings of the 9th IEEE International Symposium on Next-Generation Electronics, Changsha, China, 10–12 July 2021. [Google Scholar] [CrossRef]
- Hong, M.; Dai, L.; Hu, H.; Zhang, X.; Li, C.; He, Y. Pressure-Induced Structural Phase Transition and Metallization of CrCl3 under Different Hydrostatic Environments up to 50.0 GPa. Inorg. Chem. 2022, 61, 4852–4864. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.A.; Conway, B.E. ac Impedance of Faradaic reactions involving electrosorbed intermediates—I. Kinetic theory. Electrochim. Acta 1987, 32, 1703–1712. [Google Scholar] [CrossRef]
- Krstajić, N.; Popović, M.; Grgur, B.; Vojnović, M.; Šepa, D. On the kinetics of the hydrogen evolution reaction on nickel in alkaline solution: Part I. Mech. J. Electroanal. Chem. 2001, 512, 16–26. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the Electrochemistry of the Hydrogen-Evolution Reaction through Combining Experiment and Theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, P.; Sheng, W. Hydrogen Evolution and Oxidation: Mechanistic Studies and Material Advances. Adv. Mater. 2019, 31, 1808066. [Google Scholar] [CrossRef]
- de Chialvo, M.R.G.; Chialvo, A.C. Existence of two sets of kinetic parameters in the correlation of the hydrogen electrode reaction. J. Electrochem. Soc. 2000, 147, 1619–1622. [Google Scholar] [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H.A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef]
- Vliekar, S.A.; Fishtik, I.; Datta, R. Kinetics of the hydrogen electrode reaction. J. Electrochem. Soc. 2010, 157, B1040–B1050. [Google Scholar] [CrossRef]
- Parsons, R. The rate of electrolytic hydrogen evolution and the heat of adsorption of hydrogen. Trans. Farad. Soc. 1958, 54, 1053–1063. [Google Scholar] [CrossRef]
- Li, G.; Fu, C.; Shi, W.; Jiao, L.; Wu, J.; Yang, Q.; Saha, R.; Kamminga, M.E.; Srivastava, A.K.; Liu, E.; et al. Dirac nodal arc semimetal PtSn4: An ideal platform for understanding surface properties and catalysis for hydrogen evolution. Angew. Chem. Int. Ed. 2019, 58, 13107–13112. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, A.L. Interplay between p− and d− orbitals yields multiple Dirac states in one- and two-dimensional CrB4. 2D Mater. 2018, 5, 035041. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Świątek, H.; Sawczak, M.; Klimczuk, T.; Bogdanowicz, R. Investigating Layered Topological Magnetic Materials as Efficient Electrocatalysts for the Hydrogen Evolution Reaction under High Current Densities. Catalysts 2024, 14, 676. https://doi.org/10.3390/catal14100676
Gupta S, Świątek H, Sawczak M, Klimczuk T, Bogdanowicz R. Investigating Layered Topological Magnetic Materials as Efficient Electrocatalysts for the Hydrogen Evolution Reaction under High Current Densities. Catalysts. 2024; 14(10):676. https://doi.org/10.3390/catal14100676
Chicago/Turabian StyleGupta, Sanju, Hanna Świątek, Mirosław Sawczak, Tomasz Klimczuk, and Robert Bogdanowicz. 2024. "Investigating Layered Topological Magnetic Materials as Efficient Electrocatalysts for the Hydrogen Evolution Reaction under High Current Densities" Catalysts 14, no. 10: 676. https://doi.org/10.3390/catal14100676
APA StyleGupta, S., Świątek, H., Sawczak, M., Klimczuk, T., & Bogdanowicz, R. (2024). Investigating Layered Topological Magnetic Materials as Efficient Electrocatalysts for the Hydrogen Evolution Reaction under High Current Densities. Catalysts, 14(10), 676. https://doi.org/10.3390/catal14100676







